Abstract
This study was designed to correlate clinical findings with the extent of pathologic a-synuclein (aSyn) in the brain using the Unified Staging System for Lewy Body disorders (USSLB). Data from 280 cases from the Arizona Study of Aging and Neurodegenerative Disorders are presented. Each case had a complete USSLB staging and at least 1 full research clinical assessment, including subspecialty neurologist-administered movement and cognitive evaluation. Of the 280, 25.7% were cognitively normal, 8.6% had mild cognitive impairment, and 65.7% had dementia. All cases could be categorized into 1 of 5 USSLB stages (8.6% stage I—olfactory bulb only; 15.4% IIa—brainstem predominant; 13.6% IIb—limbic predominant; 31.8% III—brainstem and limbic; and 30.7% IV—neocortical) yet using the Braak staging system 70 cases (25.3%) could not be classified. Those with USSLB stages III and IV died at a younger age. Multiple measures of motor parkinsonism, cognitive impairment, hyposmia, and probable RBD were significantly correlated with increasing USSLB stage. We conclude that the USSLB is the most comprehensive staging system for all Lewy body disorders and allows for categorization and ranking of all brains with significant correlations to many motor and nonmotor clinical signs and symptoms.
Keywords: a-Synuclein, Braak staging system, Dementia with Lewy bodies, Incidental Lewy body disease, Lewy body, Parkinson disease, Unified Staging System for Lewy Body Disorders
INTRODUCTION
There are multiple neuropathological staging systems for neurodegenerative disorders characterized by Lewy bodies, including Parkinson disease (PD) and dementia with Lewy bodies (DLB) (1–13). However, due to either overly restrictive or overly permissive classification rules, none (6, 14–16) has allowed for the unambiguous classification of all such cases until the development of the Unified Staging System for Lewy Body Disorders (USSLB) (3). In particular, other staging systems neither adequately address pathological a-synuclein (aSyn) staining in the olfactory bulb only (2) nor the cases with aSyn sparing the brainstem and predominating in the amygdala and other limbic regions (4, 5).
Aside from offering unambiguous classification of subjects, the critical test of any pathology-based staging system is its ability to predict clinical status. Clinicopathological correlations in the initial USSLB study (3) were based on scores on the Mini Mental State Examination (MMSE) and the Unified Parkinson’s Disease Rating Scale (UPDRS). Since that time, autopsied subjects with more comprehensive standardized clinical assessment data have become available. The current study assessed clinicopathological associations of the USSLB with motor and nonmotor findings in prospectively characterized and autopsied subjects (3, 17).
MATERIALS AND METHODS
The Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) database (17) was queried for autopsied subjects with aSyn and a full clinical research assessment antemortem, with autopsies between January 1, 1997 and December 31, 2015 in the Banner Sun Health Research Institute Brain and Body Donation Program (www.brainandbodydonationprogram.org, Accessed August 8, 2019). All subjects signed written informed consent approved by either the Banner Sun Health Institutional Review Board or the Western IRB (Seattle, WA).
All included subjects had at least 1 annual movement and cognitive evaluation as well as autopsy, as previously described (17, 18). All data presented are for the last evaluation prior to death. Final clinical diagnoses, including motor and cognitive diagnoses, were based on review of all clinical information available prior to autopsy, as previously described (18, 19). The final clinicopathological diagnoses were made by reviewing the final pathological findings with the clinical findings.
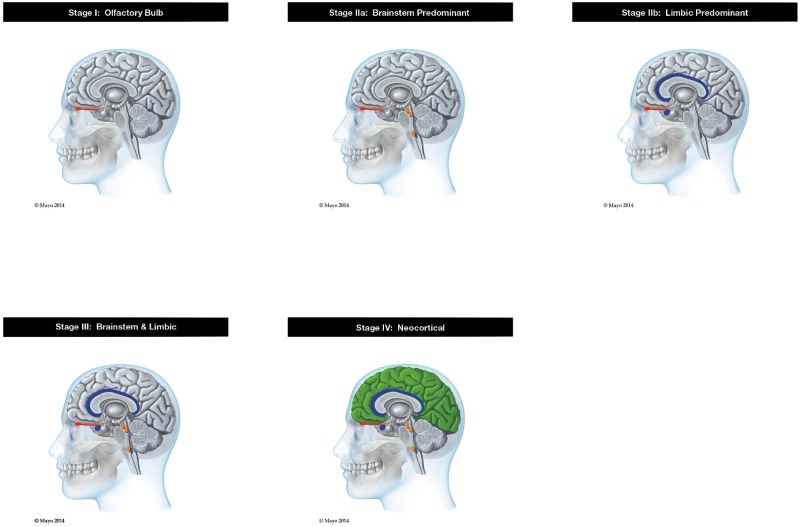
Postmortem microscopic examinations were performed blinded to clinical diagnosis by a single neuropathologist (T.G.B.). The postmortem diagnosis of PD was made based on previously reported neuropathological criteria together with a clinical diagnosis of parkinsonism (3, 17, 20). The postmortem diagnosis of DLB and Alzheimer disease (AD were made based on previously reported Dementia with Lewy Bodies Consortium (DLBC) “intermediate” or “high” consensus clinicopathological criteria (5, 17). Incidental Lewy body disease (ILBD) was diagnosed if an autopsied subject with pathological aSyn had no evidence for parkinsonism or dementia at last assessment. All subjects were staged according to the USSLB while the neuropathologist was blinded to clinical diagnoses (3). Staging requires the joint consideration of aSyn density scores from 10 brain regions; all but the olfactory bulb is as specified in the 2005 DLBC publication (5). Brainstem regions include medulla at the level of the IX and X cranial nerves, pons at the level of the locus ceruleus and midbrain at the level of the IIIrd cranial nerve. Limbic regions include the amygdala through its midpoint, transentorhinal area adjacent to the amygdala and anterior cingulate gyrus. Neocortical regions include the middle temporal gyrus, middle frontal gyrus and inferior parietal lobule. The USSLB stages (Fig. 1) are: I. Olfactory Bulb Only; IIa. Brainstem Predominant; IIb. Limbic Predominant; III. Brainstem and Limbic; IV. Neocortical (3). In brief, aSyn density scores (0–4) for each region were derived using the published DLBC grading templates for each of the 10 brain regions examined (5). These templates depict medium-magnification photomicrographs of gray matter neuropil with successively greater densities of puncta, short fibers and perikaryal neuronal inclusions immunoreactive with an immunohistochemical method for pathological aSyn. For stage IV (neocortical) the neocortical score must be at least 2 in at least 1 of the 3 neocortical regions (temporal, frontal, parietal) examined. Separation between brainstem predominant and limbic predominant stages is based on the relative density scores within each set of component brain regions. For example, a subject is classed as brainstem predominant if the highest brainstem area score range (1–2 or 3–4) is higher than the highest limbic score range (0 or 1–2, respectively). When the highest brainstem and limbic scores are equivalent in range (both in the 1–2 range or both in the 3–4 range), the subject is classed as stage III: brainstem and limbic. For the olfactory bulb-only stage I, all other regional scores must be 0. Otherwise, the olfactory bulb score does not affect scoring for any other stage. See Table 1 for examples of the density measurements and for further details of scoring, see the original USSLB report (3).
FIGURE 1.
Graphic depiction of the Unified Staging System for Lewy Body Disorders. Red is olfactory bulb and tract; orange is brainstem nuclei-substantia nigra, dorsal motor nucleus of the vagus, and locus coeruleus; blue is limbic regions-amygdala and cingulate cortex; and green is neocortex.
TABLE 1.
Unified Staging System for Lewy Body Disorders (USSLB) Staging Rules and Examples
| Staging Rules and Examples | Olfactory Bulb Score | Brainstem Score(Any Single Area) | Limbic Score(Any Single Area) | Neocortical Score(Any Single Area) |
|---|---|---|---|---|
| Olfactory Bulb-Only Staging Rule | Score 1–4 | Score 0 | Score 0 | Score 0 |
| Olfactory Bulb Example | 3 | 0 | 0 | 0 |
| Brainstem Predominant Staging Rule | Score 0–4 | Match a or b BrainstemWith a and b Limbic | Scores 0–1 | |
| a. Scores 1–2b. Scores 3–4 | a. Score 0b. Scores 1–2 | |||
| Brainstem Example a | 2 | 1 | 0 | 0 |
| Brainstem Example b | 4 | 3 | 1 | 0 |
| Limbic Predominant Staging Rule | Score 0–4 | Match a or b BrainstemWith a and b Limbic | Scores 0–1 | |
| a. Score 0b. Scores 1–2 | a. Scores 1–2b. Scores 3–4 | |||
| Limbic Example a | 4 | 0 | 1 | 0 |
| Limbic Example b | 3 | 2 | 4 | 1 |
| Brainstem & LimbicStaging Rule | Score 0–4 | Either a or ba. Scores 0–2b. Scores 3–4 | Either a or ba. Scores 0–2b. Scores 3–4 | Scores 0–1 |
| Brainstem & LimbicExample a | 4 | 2 | 2 | 0 |
| Brainstem & LimbicExample b | 4 | 4 | 3 | 1 |
| NeocorticalStaging Rule | Score 0–4 | Score 0–4 | Score 0–4 | Score > 1 |
| Neocortical Example a | 3 | 3 | 4 | 2 |
| Neocortical Example b | 4 | 4 | 4 | 3 |
Several individual cases’ regional density scores are given to illustrate how density scores are translated into USSLB stages. For example, a subject would be scored as brainstem predominant if the highest brainstem area score range (1–2 or 3–4) was higher than the highest limbic score range (0–1 or 1–2, respectively). Additionally, to qualify as any stage less than neocortical, the highest neocortical area score would have to be 0 or 1. For olfactory bulb-only stage, all other regional scores must all be 0. Otherwise, the olfactory bulb score does not affect scoring for any other stage.
For comparison purposes, the cases were also classified using the Braak Lewy body staging system (7) with awarding of stages using a simplified binary rule (any aSyn in a region was counted as involvement of that region) an adaptation of Braak staging applied by Muller et al (12) and BrainNet Europe (13).
As the USSLB utilizes semiquantitative density scoring in the 10 brain regions scored, with a total possible score of 40, statistical analyses included correlations of the summary brain aSyn density scores with clinical measures. Peripheral nervous system synuclein pathology was not considered in this study or used for classification purposes and readers are referred for this to prior published studies (21–23).
Statistical Analysis
Associations between clinical measures and USSLB stages were made using analysis of variance when comparing continuous measures among groups. Chi-square test, or Fisher exact test were used when comparing proportions among groups (Fisher test used when expected cell count <5). UPDRS scores were dichotomized by using 2 as the cutoff into abnormal (≥2) versus normal (<2). Linear regression (for continuous outcomes) or logistic regression (for binary outcomes) adjusted for age at exam were used to test if there were significant tendencies for clinical findings to increase with increasing USSLB stage and summary Lewy-type synucleinopathy density scores. Because there is no inherent ascendancy to either USSLB Stage IIa or IIb, and as the clinical findings didn’t significantly differ between the 2 groups (data not shown), these stages were combined into a single stage II group for the trend test analysis. A p value <0.05 was considered significant for each test. False discovery rate (FDR) was used to control for multiple comparisons (24).
RESULTS
In the designated time frame there were a total of 641 autopsied subjects with at least 1 full clinical research assessment (movement exam and cognitive testing). Of these, 361 cases were excluded for the following reasons: 327 cases did not have aSyn (USSLB stage 0) and 34 had other significant brain diseases (19 with vascular dementia, 2 each with Huntington disease, normal pressure hydrocephalus, corticobasal degeneration, and 9 with brain tumors). We did not initially exclude cases with a concurrent neuropathological diagnosis of AD. Of the remaining 280 cases, all were classified by USSLB: 8.6% Stage I, 15.4% IIa, 13.6% IIb, 31.8% III, and 30.7% IV. Using a modified Braak staging system with simplifications to increase the percentage of classifiable cases (12, 13), of 277 cases in the initial cohort (3 cases could not be classified due to missing brain regions), 70 (25.3%) could not be classified due to nonsequential involvement of brain regions, including 21 cases that were olfactory bulb only.
As the presence of diagnostic levels of concomitant AD pathology obscures the clinical expression of aSyn pathology (as previously reported by the DLB Consortium [5] and by ourselves [3]), clinicopathological analyses were performed only in the subset of 145 cases that had concomitant AD excluded (Table 2). General demographics were not statistically different for the AD-excluded group. The mean age of death was 82.5 (7.7) and 60.7% were male. Age at death was younger for the stage III and IV cases (Table 2). Clinically, 49.7% of this group of 145 cases were cognitively normal, 16.6% had mild cognitive impairment, and 33.8% had dementia. The distribution by USSLB stage was as follows: 6.9% stage I; 22.1% IIa; 8.3% IIb; 43.4% III; and 19.3% IV. The clinicopathologic diagnoses were 31.0% ILBD, 64.1% PD, and only 1.4% DLB (because all but 2 DLB cases had concomitant diagnostic levels of AD pathology). There were 4 cases with PSP that had aSyn that did not meet neuropathological criteria for PD (1 olfactory bulb only and 3 stage III) and 1 case with dementia having no clear neuropathological cause. Of the 93 autopsy confirmed PD cases, 10 (10.8%) were Stage IIa, 5 (5.4%) IIb, 50 (53.8%) III, and 28 (30.1%) Stage IV.
TABLE 2.
Demographics, Clinicopathological, and Pathological Findings in 145 aSyn Cases Without Concomitant Alzheimer Disease, Grouped by USSLB Stage
| I | IIa | IIb | III | IV | Total | p | |
|---|---|---|---|---|---|---|---|
| n (% of total) | 10 (6.9%) | 32 (22.1%) | 12 (8.3%) | 63 (43.4%) | 28 (19.3%) | 145 | |
| Male, n (%) | 6 (60%) | 20 (62.5%) | 6 (50.0%) | 42 (66.7%) | 14 (50.0%) | 88 (60.7%) | 0.5738* |
| Age at death (mean + SD) | 86.1 + 5.9 | 86.3 + 7.3 | 87.3 + 10.2 | 79.8 + 6.5 | 80.6 + 7.2 | 82.5 +7.7 | <0.0001† |
| Time interval between last clinical assessment and autopsy (days)(mean + SD) | 342.1 (203.0) | 302.6 (253.4) | 468.9 (194.5) | 225.0 (202.1) | 335.1 (243.1) | 292.0 (230.2) | 0.0065† |
| Final cognitive status | 0.0013‡ | ||||||
| CogNL | 7 (70.0%) | 22 (68.8%) | 8 (66.7%) | 29 (46.0%) | 6 (21.4%) | 72 (49.7%) | |
| MCI | 2 (20.0%) | 5 (15.6%) | 3 (25.0%) | 8 (12.7%) | 6 (21.4%) | 24 (16.6%) | |
| Dementia | 1 (10.0%) | 5 (15.6%) | 1 (8.3%) | 26 (41.3%) | 16 (57.1%) | 49 (33.8%) | |
| Final clinicopathologic diagnosis | <0.0001‡ | ||||||
| ILBD | 8 (80.0%) | 22 (68.8%) | 7 (58.3%) | 8 (12.7%) | 0 (0%) | 45 (31.0%) | |
| PD | 0 (0.0%) | 10 (31.2%) | 5 (41.7%) | 50 (79.4%) | 28 (100%) | 93 (64.1%) | |
| DLB | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (3.2%) | 0 (0.0%) | 2 (1.4%) | |
| PSP | 1 (10%) | 0 (0.0%) | 0 (0.0%) | 3 (4.8%) | 0 (0.0%) | 4 (2.8%) | |
| Dementia NOS | 1 (10%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | |
CogNL, cognitively normal; MCI, mild cognitive impairment; ILBD, incidental Lewy body disease; Parkinson’s disease; DLB, dementia with Lewy bodies; PSP, progressive supranuclear palsy; Dementia NOS, no clear pathology to explain the dementia.
Chi-square p value.
ANOVA F-test p value.
Fisher exact p value.
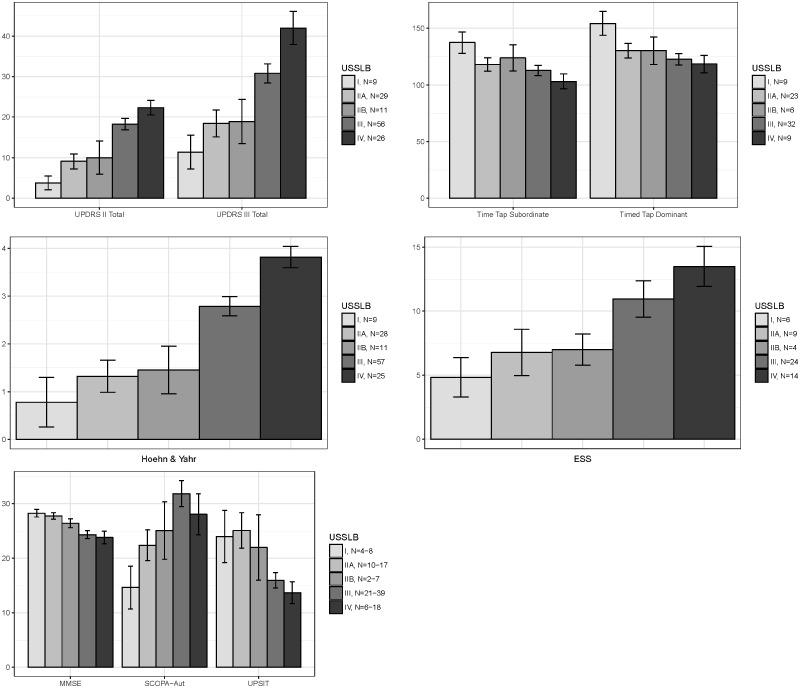
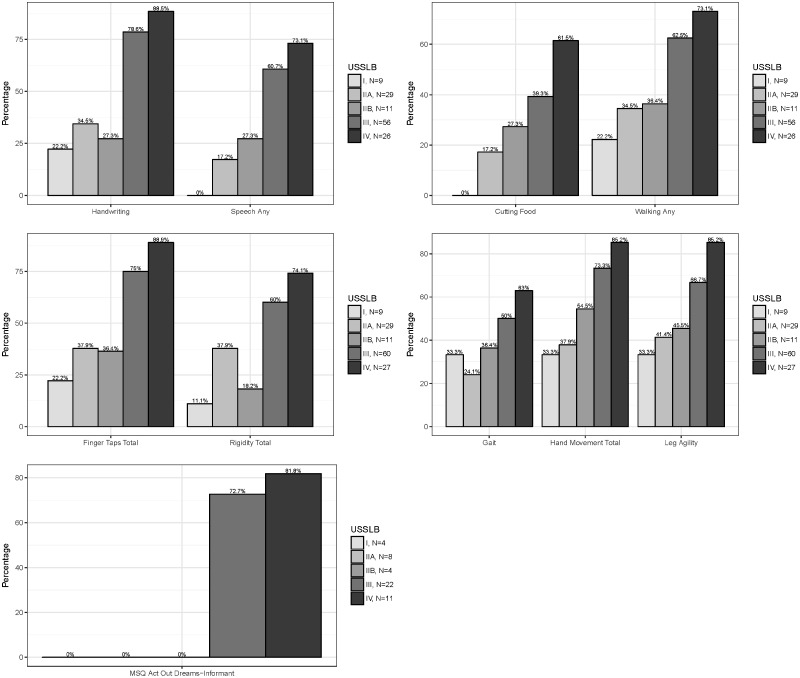
USSLB stage was significantly correlated with multiple clinical measures, including UPDRS part I scores of intellectual impairment, thought disorder, and apathy as well as UPDRS part II and part III total scores (Fig. 2) and most individual scores. Significant correlations were also found for Hoehn and Yahr stage and timed tap testing (Fig. 2). The nonmotor questionnaires for autonomic function (SCOPA-Aut) and the Epworth Sleepiness Scale had a positive correlation with USSLB stage while a negative correlation was found for MMSE and UPSIT (Fig. 2). When data were dichotomized, defining abnormality as a score of ≥2 for each UPDRS part I, II, and III score, USSLB stage had similar correlations (Fig. 3). For those cases that had informant data for the Mayo Sleep Questionnaire, probable REM sleep behavior disorder (RBD) was correlated with USSLB stage, being present in 0/4 stage I cases, 0/12 stage II (both IIa and IIb), 16/22 (72.7%) stage III, and 9/11 (81.8%) stage IV cases.
FIGURE 2.
Correlation of USSLB stages with UPDRS parts II and III total scores, timed tap scores, Hoehn and Yahr staging scale, Epworth Sleepiness Scale scores, MMSE, SCOPA-Aut, and the UPSIT for the 145 cases without concomitant AD. Data displayed as mean ± standard error of the mean (SEM).
FIGURE 3.
The percentage of 145 cases, without concomitant AD, at each USSLB stage with a UPDRS part II (handwriting, speech, cutting food, and walking) or part III (finger taps, rigidity, gait, hand movements, leg agility) score of ≥2.
Correlation results using the USSLB summary brain aSyn density scores were very similar to those using the USSLB stages (Table 3). The odds ratio for an increase in density is shown for all of those UPDRS parts II and III items that were significant (Table 3). Density scoring has further utility in that it allows, across and within stages, ranking of subjects and more precise testing of clinicopathological correlations.
TABLE 3.
Odds Ratios (OR) for Individual Items in the UPDRS With a Score of ≥2 Correlating With the Sum Lewy Body Density Score for the Items That Had a p Value <0.05, for the 145 Cases Without Concomitant Alzheimer Disease
| Outcomes | Sum Lewy Body Density OR for Every Increase of 10 in LB Density * | 95% Confidence Interval | p Value From Logistic Regression † |
|---|---|---|---|
| UPDRS part I | |||
| Intellectual impairment | 2.83 | 1.58–5.07 | 0.0005 |
| Thought disorder | 2.12 | 1.18–3.83 | 0.0124 |
| UPDRS part II | |||
| Speech | 2.84 | 1.65–4.87 | 0.0002 |
| Handwriting | 2.67 | 1.63–4.37 | 0.0001 |
| Cutting food | 2.41 | 1.37–4.23 | 0.0022 |
| Dressing | 2.66 | 1.62–4.39 | 0.0001 |
| Hygiene | 2.14 | 1.35–3.38 | 0.0012 |
| Turning in bed | 2.04 | 1.27–3.28 | 0.0033 |
| Walking | 1.87 | 1.21–2.90 | 0.0051 |
| UPDRS part III | |||
| Facial expression | 2.47 | 1.46–4.18 | 0.0007 |
| Rigidity, total | 1.84 | 1.18–2.85 | 0.0068 |
| Finger taps, total | 2.73 | 1.62–4.59 | 0.0002 |
| Hand movement, total | 1.80 | 1.15–2.84 | 0.0107 |
| RAM hands, total | 2.07 | 1.32–3.27 | 0.0017 |
| Leg agility, total | 1.58 | 1.03–2.43 | 0.0359 |
| Gait | 1.65 | 1.08–2.54 | 0.0212 |
| Postural stability | 1.79 | 1.15–2.80 | 0.0101 |
| Body bradykinesia | 2.68 | 1.59–4.51 | 0.0002 |
The interpretation for the OR estimate is that for every 10 unit increase in sum Lewy body density score, the odds of having abnormal clinical outcome will increase by X times.
The findings with p value <0.0359 is equivalent to controlling for FDR at 0.05 level.
For the cohort of cases with AD excluded, all cases could be staged using the USSLB, but 31/142 (21.8%) could not be classified using the modified Braak staging system (12, 13). This included 9 olfactory bulb only, 5 where aSyn skipped the medulla, 2 that skipped the LC, 1 that skipped both medulla and LC, 6 that skipped the SN, 2 that skipped the entire brainstem, 1 that skipped both medulla and amygdala, and 5 that skipped the transentorhinal area.
DISCUSSION
The USSLB was developed to improve the classification of autopsied subjects with all types of Lewy body disorders (LBD). Prior staging systems were specifically designed for single disease states (PD [2] or DLB [4–6]) and were thus poorly suited for the broad range of subjects with brain aSyn that come to autopsy. The USSLB has been independently validated as a logical classifier in 3 separate cohorts of autopsied subjects (25, 26) but the ultimate test of a classification system is its ability to predict clinical status.
The data presented here clearly show that all cases with Lewy bodies, even those with concomitant AD, were classified using the USSLB. This has been shown in an independent sample as well (26). Additionally, the data show a clear association between USSLB stage and both motor and nonmotor clinical signs and symptoms. While not all individual measures correlated with USSLB stage, this was possibly due to small group sizes, treatment effects (even though exams were performed in the practically defined off state when possible), or lack of progression of some signs such as rest tremor. As previously reported, cognitive impairment correlated with the presence of aSyn in the limbic and neocortical regions (25, 27). As noted by others, a higher USSLB stage also correlated with younger age of death, even when concomitant AD was excluded (28). The tendency for concomitant AD to obscure the typical clinical findings of LBD has been noted previously by others (5) as well as ourselves (3) and hence clinicopathological correlations with any synuclein staging system are not well served in this setting.
This strong association of USSLB stage with clinical findings (subjective and objective), and the ability of the system to unambiguously assign stages to all subjects with brain aSyn, supports the use of the USSLB rather than the Braak system. The USSLB may be used for the pathological classification of all LBDs, being applicable to autopsy studies assessing ILBD, PD, DLB, ADLB, or any other neurodegenerative disorder cases with aSyn that don’t meet PD or DLB criteria. While the Braak staging system (1, 2) was designed for use with PD subjects and subjects with prodromal PD, in the present study, when AD was excluded, even though 95% of the remaining subjects were PD or possible prodromal PD (ILBD), there were still 21.8% that could not be classified using the Braak system. Very similar results were recently published (26) in a study of 324 autopsied subjects from the Honolulu-Asia Aging Study, with 17% of cases being unclassifiable by the Braak system while 100% were classified by the USSLB.
The greater usefulness of the USSLB, as compared with the Braak system, is not solely classification ability. Stage IIa and IIb subjects, who are only defined by the USSLB, appear to have fundamental biological differences. There is a marked tendency for peripheral aSyn occurrence to segregate with Stage IIa rather than Stage IIb cases (21, 23). As both Stage IIa and IIb generally have lighter aSyn density loads, both may represent earlier disease states along different disease trajectories. Stage IIa would appear to be consistent with prodromal and early PD while Stage IIb may represent prodromal or early DLB (21). Whether an individual proceeds down the USSLB Stage IIa or IIB pathway may be dependent on genetic background, environmental exposure, or even on random generation or spread of aSyn within the differing component brain regions (29), but the origin of aSyn pathology in the olfactory bulb is surprisingly uniform.
The presence of a stage devoted to aSyn restricted to the olfactory bulb is an advantage of the USSLB and is not adequately addressed in other staging systems (1, 2, 4–6). The olfactory bulb may have unique features that contribute to its apparent primacy, across LBD, in the initiation of aSyn and the prodromal clinical finding of hyposmia (30).
A further advantage of the USSLB system is its semiquantitative aSyn density score assignment. While some groups have favored simple presence-or-absence pathology annotation for both AD and DLB, due to reportedly poor interobserver agreement on ordinal semiquantitative density scoring, others, including a group sponsored by the NIA-AA in the setting of AD lesions, have found interobserver histopathological density score agreement to be reliable and useful (9). The results of the present study indicate that summary brain USSLB a aSyn density score is equivalent to USSLB staging as a predictor of clinical measures. Furthermore, density scoring allows severity ranking of all subjects and testing of clinicopathological correlations within stages. From a brain banking perspective, density scoring data allow researchers to correlate molecular changes with pathology density within individual brain regions.
Graphic depiction of the USSLB is seen in Figure 1 and was recently published (31). As previously proposed (3), synucleinopathies appear, by inference from regional prevalence at death, to most often first appear in the olfactory bulb, followed by either the brainstem or limbic regions, and then the peripheral nervous system. It must be emphasized that autopsy studies cannot determine whether aSyn physically spreads from neuron to neuron or whether the observed regional predilections are instead due to regional probabilistic variability in the spontaneous generation of aSyn. Whether the progression of PD and DLB differ, with initial affection of the brainstem region for PD and of the limbic region for DLB, is unproven, given that autopsied cases do not allow for longitudinal assessment of synuclein progression, but it is a reasonable conjecture (21, 29). The nearly complete co-existence of DLB with AD, combined with the very common presence of less-than-diagnostic levels of limbic-predominant aSyn in ADLB, suggests that stage IIb is likely, in at least some subjects, to be an early pathological stage of DLB.
While the number of autopsied cases with informant data for the Mayo Sleep Questionnaire was limited, it was surprising that no stage I or II cases (n = 16) had probable RBD. As RBD is known to occur in prodromal disease and is a risk factor for the development of PD and DLB, it might have been expected that early brain aSyn involvement, particularly of brainstem regions known to be involved in regulating sleep, would be associated with RBD (32–36). Additionally, multiple studies of idiopathic RBD have shown olfactory deficits (35, 37), yet not all of the 16 stage I or II cases, all with olfactory bulb aSyn, had hyposmia. As olfactory bulb and brainstem aSyn density both rise with USSLB stage, it seems likely that a threshold density may be necessary for hyposmia and RBD to occur and that this threshold is not often reached at lower USSLB stages. Alternatively, as hypothesized for AD, the sleep disorder may precede, and predispose to, the molecular pathology, instead of being a result of the pathology (38, 39).
This study has some limitations. First, the number of cases with stage I or stage IIb that did not also have AD was small, as was the number of cases with UPSIT, SCOPA-Aut, and MSQ. Strengths of the study included the prospective longitudinal collection of motor and nonmotor assessments, the large number of subjects entered into the cohort that did not initially have parkinsonism or dementia, and the assessment of all subjects by a small group of fellowship-trained movement disorder and behavioral neurologists and neuropsychologists. Neuropathological examination and staging by a single neuropathologist might be regarded as a strength in that these were done in a consistent manner over the 20 years of this study. It also could be considered a weakness in that the generalizability of the method to other neuropathologists may need study. However, there are 2 independent published studies using the USSLB that have concluded that the USSLB could stage all cases studied and had good interobserver agreement (25, 26). The AZSAND makes a data set available to qualified investigators, at www.brainandbodydonationprogram.org, Accessed August 8, 2019.
This study provides further evidence that the USSLB staging system provides a more complete and useful classification of autopsied cases with aSyn pathology than the major alternative system devised by Braak and modified by others (2, 5). Wider use of the USSLB staging system would help standardize research performed on all LBD. The system provides clear rules for classification, eliminating the arbitrary case attribution of borderline subjects that is inevitable with other systems. Finally, the clinical correlates of the USSLB system have been much more thoroughly demonstrated than has been done with the Braak system or other systems, both here and in our original publication (3). Additional validation by other centers would be valuable in confirming the utility of the USSLB.
ACKNOWLEDGMENTS
We are grateful to the subjects who volunteered to participate in the Arizona Study of Aging and Neurodegenerative Disorders. The authors thank Mr. Bruce Peterson for developing and maintaining the database.
This study was funded by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901, and 1001 to the Arizona Parkinson's Disease Consortium), the Michael J. Fox Foundation for Parkinson’s Research, and Mayo Clinic Foundation.
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Braak H, Del Tredici K, Bratzke H, et al. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages). J Neurol 2002;249(Suppl. 3):III/1–5 [DOI] [PubMed] [Google Scholar]
- 2. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]
- 3. Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 2009;117:613–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies: Report of the consortium on DLB international workshop. Neurology 1996;47:1113–24 [DOI] [PubMed] [Google Scholar]
- 5. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005;65:1863–72 [DOI] [PubMed] [Google Scholar]
- 6. Leverenz J, Hamilton R, Tsuang DW, et al. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol 2008;1:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braak H, Bohl JR, Muller CM, et al. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord 2006;21:2042–51 [DOI] [PubMed] [Google Scholar]
- 8. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saito Y, Ruberu NN, Sawabe M, et al. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol 2004;63:742–9 [DOI] [PubMed] [Google Scholar]
- 11. Marui W, Iseki E, Nakai T, et al. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci 2002;195:153–9 [DOI] [PubMed] [Google Scholar]
- 12. Muller CM, de Vos RA, Maurage CA, et al. Staging of sporadic Parkinson disease-related alpha-synuclein pathology: Inter- and intra-rater reliability. J Neuropathol Exp Neurol 2005;64:623–8 [DOI] [PubMed] [Google Scholar]
- 13. Alafuzoff I, Parkkinen L, Al-Sarraj S, et al. Assessment of alpha-synuclein pathology: A study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol 2008;67:125–43 [DOI] [PubMed] [Google Scholar]
- 14. Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 2008;116:1–16 [DOI] [PubMed] [Google Scholar]
- 15. Kalaitzakis ME, Graeber MB, Gentleman SM, et al. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: A critical analysis of alpha-synuclein staging. Neuropathol Appl Neurobiol 2008;34:284–95 [DOI] [PubMed] [Google Scholar]
- 16. Parkkinen L, Pirttila T, Alafuzoff I.. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 2008;115:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beach TG, Adler CH, Sue LI, et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 2015;35:354–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: Clinicopathologic study. Neurology 2014;83:406–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord 2007;22:1272–7 [DOI] [PubMed] [Google Scholar]
- 20. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: Refining the diagnostic criteria. Lancet Neurol 2009;8:1150–7 [DOI] [PubMed] [Google Scholar]
- 21. Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010;119:689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beach TG, Adler CH, Dugger BN, et al. Submandibular gland biopsy for the diagnosis of Parkinson's disease. J Neuropathol Exp Neurol 2013;72:130–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beach TG, Adler CH, Serrano G, et al. Prevalence of submandibular gland synucleinopathy in Parkinson's disease, dementia with Lewy bodies and other Lewy body disorders. JPD 2016;6:153–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc 1995;57:289–300 [Google Scholar]
- 25. Toledo JB, Gopal P, Raible K, et al. Pathological alpha-synuclein distribution in subjects with coincident Alzheimer's and Lewy body pathology. Acta Neuropathol 2016;131:393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coughlin DG, Petrovitch H, White LR, et al. Most cases with Lewy pathology in a population-based cohort adhere to the Braak progression pattern but failure to fit is highly dependent on staging system applied. Park Relat Disord 2019;[Epub ahead of print] 10.1016/j.parkreldis.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dickson DW, Fujishiro H, Orr C, et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 2009;15(Suppl. 3):S1–5 [DOI] [PubMed] [Google Scholar]
- 28. Graff-Radford J, Aakre J, Savica R, et al. Duration and pathologic correlates of Lewy body disease. JAMA Neurol 2017;74:310–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frigerio R, Fujishiro H, Ahn TB, et al. Incidental Lewy body disease: Do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol Aging 2011;32:857–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Driver-Dunckley E, Adler CH, Hentz JG, et al. Olfactory dysfunction in incidental Lewy body disease and Parkinson's disease. Park Relat Disord 2014;20:1260–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adler CH, Beach TG.. Neuropathological basis of nonmotor manifestations of Parkinson's disease. Mov Disord 2016;31:1114–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boeve BF, Silber MH, Ferman TJ, et al. REM sleep behavior disorder and degenerative dementia: An association likely reflecting Lewy body disease. Neurology 1998;51:363–70 [DOI] [PubMed] [Google Scholar]
- 33. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75:494–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol 2013;12:443–53 [DOI] [PubMed] [Google Scholar]
- 35. Mahlknecht P, Iranzo A, Hogl B, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 2015;84:654–8 [DOI] [PubMed] [Google Scholar]
- 36. Postuma RB, Gagnon JF, Vendette M, et al. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009;72:1296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Postuma RB, Lang AE, Massicotte-Marquez J, et al. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 2006;66:845–51 [DOI] [PubMed] [Google Scholar]
- 38. Lucey BP, Bateman RJ.. Amyloid-beta diurnal pattern: Possible role of sleep in Alzheimer's disease pathogenesis. Neurobiol Aging 2014;35(Suppl. 2):S29–34 [DOI] [PubMed] [Google Scholar]
- 39. Varga AW, Wohlleber ME, Gimenez S, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Abeta42 levels in cognitively normal elderly. Sleep 2016;39:2041–8 [DOI] [PMC free article] [PubMed] [Google Scholar]