The soybean salinity-induced NAC transcription factor GmSIN1 promotes root growth and salt tolerance by simultaneously controlling the production of abscisic acid and reactive oxygen species.
Abstract
Abscisic acid (ABA) and reactive oxygen species (ROS) act as key signaling molecules in the plant response to salt stress; however, how these signals are transduced and amplified remains unclear. Here, a soybean (Glycine max) salinity-induced NAM/ATAF1/2/CUC2 (NAC) transcription factor encoded by SALT INDUCED NAC1 (GmSIN1) was shown to be a key component of this process. Overexpression of GmSIN1 in soybean promoted root growth and salt tolerance and increased yield under salt stress; RNA interference–mediated knockdown of GmSIN1 had the opposite effect. The rapid induction of GmSIN1 in response to salinity required ABA and ROS, and the effect of GmSIN1 on root elongation and salt tolerance was achieved by boosting cellular ABA and ROS contents. GmSIN1 upregulated 9-cis-epoxycarotenoid dioxygenase coding genes in soybean (GmNCED3s, associated with ABA synthesis) and Respiratory burst oxidase homolog B genes in soybean (GmRbohBs, associated with ROS generation) by binding to their promoters at a site that has not been described to date. Together, GmSIN1, GmNCED3s, and GmRbohBs constitute a positive feed-forward system that enables the rapid accumulation of ABA and ROS, effectively amplifying the initial salt stress signal. These findings suggest that the combined modulation of ABA and ROS contents enhances soybean salt tolerance.
INTRODUCTION
High salt concentrations in soils strongly reduce crop performance (Munns and Tester, 2008) , and salt-affected soils currently account for 10% of the world’s total arable land area (Shahid et al., 2018). Soybean (Glycine max) is a major agricultural crop for oil and protein resources, and its production is reported to decrease by 40% with increasing salt stress (Papiernik et al., 2005) . To engineer salt-tolerant soybean varieties, it is crucial to identify the key components of the plant salt-tolerance network. Many soybean genes (Wang et al., 2016), including transcription factor (TF) genes (Chen et al., 2007; Wei et al., 2009; Zhang et al., 2009; Hao et al., 2011; Zhai et al., 2013; Wang et al., 2015a), have been found to confer salt stress tolerance when heterologously expressed in transgenic model plants. In soybean, a cation/H+ exchanger gene contributes to natural variation in salt tolerance (Guan et al., 2014; Qi et al., 2014). However, few of these genes have been demonstrated to affect soybean yield in saline field conditions and little is known about how soybeans respond and adapt to high salinity (Shi et al., 2018; Zhang et al., 2019). This lack of knowledge has greatly inhibited attempts to enhance salt tolerance in crops.
Understanding how plants generate and transduce salt stress signals could provide novel approaches for enhancing salt tolerance. In the plant response to high salinity, the initial signal activates the production of compounds that trigger activity in a number of metabolic and developmental pathways. One of the most important compounds is abscisic acid (ABA; Finkelstein and Gibson, 2002). Under salt or drought stress conditions, the endogenous plant ABA level can rise by ∼40-fold (Verslues et al., 2006).
Activation of ABA biosynthetic enzymes has a key role in salt and drought stress responses. During ABA biosynthesis, the cleavage of 9-cis-epoxycarotenoids to xanthoxin is catalyzed by 9-cis-epoxycarotenoid dioxygenases (NCEDs); this is believed to be the key regulatory step of ABA biosynthesis (Lefebvre et al., 2006). Among the five NCED genes in Arabidopsis (Arabidopsis thaliana), NCED3 plays a key role in ABA biosynthesis during water deficit and salt stress (Iuchi et al., 2001; Tan et al., 2003; Nambara and Marion-Poll, 2005; Barrero et al., 2006). The Arabidopsis TF NGATHA1 induces NCED3 in response to drought stress (Sato et al., 2018); however, the factors that induce NCED genes in response to salt stress remain largely unknown.
Reactive oxygen species (ROS) are also implicated in the salt stress response (Yang and Guo, 2018). ROS participate in a number of signaling pathways and other processes, but ROS over-accumulation is cytotoxic (Mittler, 2017). The ROS superoxide is generated by respiratory burst oxidase homologs (RBOHs), a family of proteins that are well conserved throughout the plant kingdom. The rapid production of ROS generated by NADPH oxidases (NOXs) occurs in response to a number of external stimuli (Marino et al., 2012). Analysis of the Arabidopsis atrbohD mutant has suggested that RbohD is required to generate ROS in plants exposed to salt stress (Xie et al., 2011). ABA influences the production of ROS through its effect on RBOH expression or RBOH activity (Kwak et al., 2003; Lin et al., 2009). Therefore, ABA and salinity signaling are thought to overlap by both affecting RBOH-derived ROS production (Xie et al., 2011).
The relationship between ABA and ROS in regulating salt stress responses and plant growth is far from fully understood (Mittler and Blumwald, 2015; Qi et al., 2018). ABA clearly stimulates the production of ROS in Arabidopsis guard cells (Zhang et al., 2001; Wang and Song, 2008; Jannat et al., 2011) and maize (Zea mays) leaves (Jiang and Zhang, 2002). However, the situation in roots remains unclear as some studies have found that ABA promotes ROS production in roots (Kwak et al., 2003; He et al., 2012; Jiao et al., 2013; Yang et al., 2014), but others show that ABA suppresses ROS production in roots (Almagro et al., 2009; Zhang et al., 2014).
Many attempts have been made to enhance plant stress tolerance by modulating ABA/ROS levels or signaling. Indeed, enhanced ABA or ROS levels or signaling promotes abiotic stress tolerance in transgenic plants (Min et al., 2015; Yang et al., 2015b; Perea-Resa et al., 2016; Qin et al., 2016; Fan et al., 2019). However, in other cases, lower ABA or ROS levels elevate abiotic stress tolerance (Ruggiero et al., 2004; Wu et al., 2014; Shu et al., 2015; Yang et al., 2015a). It is also notable that the enhancement of abiotic stress tolerance in transgenic plants with improved ABA signaling is often coupled with a negative effect on growth (Fujita et al., 2005; Furihata et al., 2006; Wang et al., 2015b). Therefore, the complex interaction between ABA and ROS in abiotic stress responses needs to be clarified to enable improvements of plant stress tolerance by modulating ABA and ROS levels/signaling.
The NAM/ATAF1/2/CUC2 (NAC) genes encode plant-specific TFs (Riechmann et al., 2000) and comprise one of the largest TF families in plants, with 106 NAC genes in Arabidopsis and 226 in soybean (Olsen et al., 2005b; Le et al., 2011) . NAC TFs play important roles in various biological processes including the salt stress response; for example, overexpression of specific NAC TF genes can improve salt tolerance in plants (Jeong et al., 2010; Hao et al., 2011; Han et al., 2015; Huang et al., 2015). Some NAC-specific binding motifs have been shown to mediate the direct transcriptional regulation of their target genes (listed in Supplemental Table 1). This suggests that the diversity of NAC binding motifs is related to the functional specificity of NAC TFs. However, our knowledge of the binding motifs and direct target genes of the NAC TFs in salt stress responses remains limited, particularly in legume crops. Identification of NAC binding sites and NAC target genes will uncover the transcriptional regulatory networks of specific NAC TFs that function in salt stress responses.
Approaches to identify genes related to salt tolerance include examining cultivars that show strong salt tolerance and identifying genes induced by salt stress. To this end, earlier work selected the soybean cv Shengdou No. 9 as the most salt-tolerant accession in a panel of cultivars (Ji et al., 2011). A microarray assay comparing soybean cv Williams 82 with and without salt stress identified a number of salt stress–induced genes; some of these genes were isolated and tested to see whether their overexpression could confer salt tolerance (Song et al., 2012). One highly salt-induced gene (up to 120-fold induction) was cloned from Shengdou No. 9 (Ji et al., 2011) and named SALT INDUCED1 (GmSIN1). GmSIN1 encodes a predicted NAC-domain TF and overexpression of GmSIN1 led to a marked improvement in field-based salinity tolerance. Here, we investigated the effect of overexpressing and knocking down GmSIN1 on root growth and salt tolerance. Based on the results, we propose a feed-forward pathway in which GmSIN1, GmNCED3s, and GmRbohBs collaborate to rapidly amplify the initial salt stress signal.
RESULTS
GmSIN1 Promotes Root Growth and Salt Tolerance
To confirm the function of GmSIN1 in soybean salt tolerance, we generated transgenic soybean lines that overexpressed or silenced GmSIN1. We constructed GmSIN1 overexpression (OE; 35Spro:GmSIN1) and RNA interference (RNAi; 35Spro:GmSIN1 RNAi) transgenic soybean lines in the salt-tolerant cv Wei6823 (Supplemental Figures 1 and 2), and the T4 homozygous OE lines (Supplemental Figure 3) and T2 RNAi lines were examined for their salt tolerance.
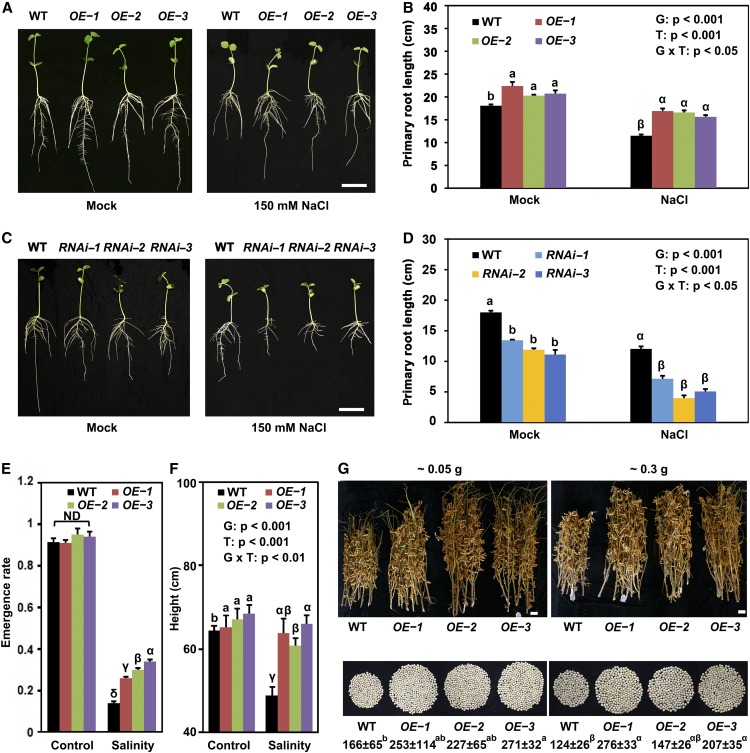
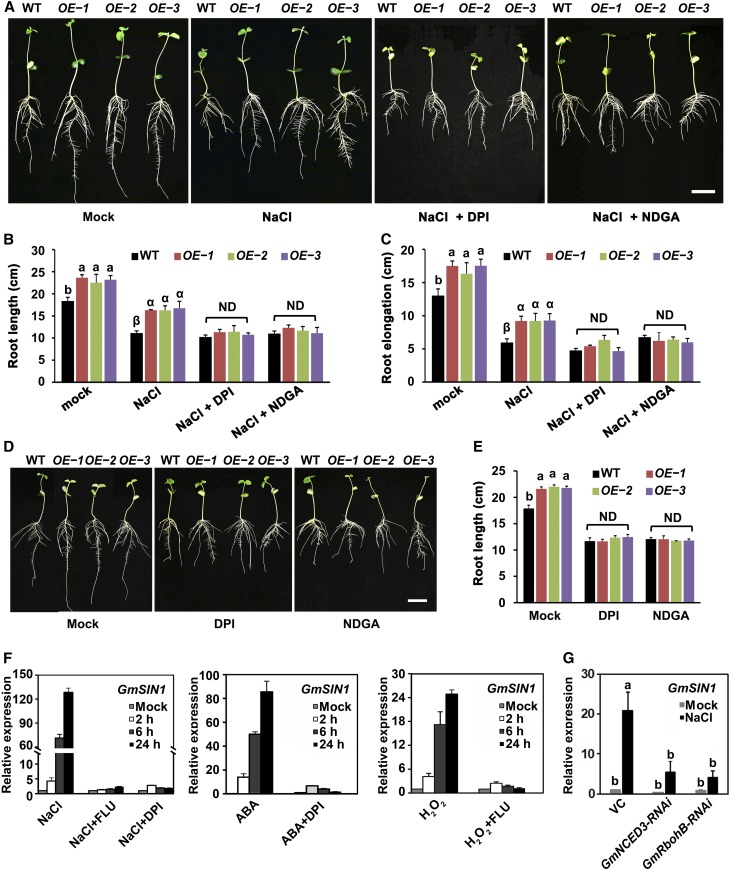
To test the effect of overexpression and silencing of GmSIN1, we first examined the effect on root growth. In bag-grown seedlings grown under normal lab growth conditions, the OE lines produced longer primary roots compared with Wei6823 (Figures 1A and 1B; Supplemental Figure 4), and RNAi lines produced shorter primary roots (Figures 1C and 1D). Under NaCl stress (150 mM, treatment of 3-d-old seedlings for 4 d), the primary roots were longer in OE lines and shorter in RNAi lines compared with the wild type. Therefore, in terms of root growth, the OE lines showed more salt tolerance compared with wild type and the RNAi lines showed less salt tolerance (Figures 1A to 1D; Supplemental Data Set 1).
Figure 1.
GmSIN1 Promoted Root Growth and Salt Tolerance.
(A) to (D) Root length of 7-d-old soybean seedlings challenged with either 0 mM (mock) or 150 mM NaCl. Images were taken 4 d after the stress treatment was initiated (A) and (C). Each column represents the mean ± se length of 20 to 30 roots (B) and (D). The roots were collected from seedlings grown in 10 plastic growth bags with the same treatment. Each bag contained three seedlings each from the wild-type (WT) and three transgenic lines. The data shown are representative of several independent experiments. G, genotype; T, treatment; G × T, genotype × treatment. Bar = 5 cm.
(E) and (F) Emergence rate (see [F]; n = 6 experiments with 40 seeds from each genotype per experiment) and plant height (see [E]; n = 60, 10 plants in each of 6 replicate plots) of the wild type (WT) and three independent GmSIN1 overexpressors (OE-1, OE-2, and OE-3) grown in the field under the same climate environment but with different salinity. For emergence rate, data were collected from the replicate plots. In each plot, 40 seeds were planted for each genotype. For height assays, 10 seedlings of each line from each plot were randomly selected. The salinity of the control field and saline field were 0.15 and 0.35 g of total soluble salts per 100 g of dry soil, respectively. Each column represents the mean emergence rate or plant height ± se. G, genotype; T, treatment; G × T, genotype × treatment.
(G) Representative results of 10 seedlings each for GmSIN1 OE-1, OE-2, OE-3, and the wild type (WT) grown in different fields with different salinity, and the average number of seeds produced per seedling. Numbers indicate the average number of seeds ± se (n = 30). Five seedlings of each line from each plot were randomly selected. Values at the top indicate the soil salinity (total soluble salts amount per 100 g of dry weight of soil). Two-way ANOVA was conducted in (B), (D), and (F) and genotype and NaCl treatment as the two factors. The P-values are shown. Significant differences between samples labeled with different Roman (a, b) or Greek letters (α, β, γ, δ) were determined by one-way ANOVA, P < 0.05. ND, no significant difference (the details of the statistical results are in Supplemental Data Set 1). G, genotype; T, treatment; G × T, genotype × treatment.
We next examined other cellular processes affected by salt stress, specifically photosynthetic parameters and antioxidant defenses. For photosynthesis, we measured the net photosynthesis rate, stomatal conductance, intercellular CO2 concentration, and photosystem II photochemical potential. These were measured in the first trifoliolate compound leaves of 2-week-old Wei6823 and OE plants at 10 d after a 2-d treatment with 50, 75, 100, 125, and 150 mM NaCl. The photosynthesis of OE plants was reduced to a lesser extent than that of the wild type (Supplemental Figures 5A to 5D). Next, we compared the concentrations of Pro and malonaldehyde and the activity of the antioxidant enzymes superoxide dismutase, peroxidase, and catalase under NaCl stress between the OE lines and Wei6823 (Supplemental Figures 5E to 5I). After NaCl treatment, the GmSIN1 OE lines showed higher accumulation of Pro, lower concentrations of malonaldehyde, and higher activities of ROS-scavenging enzymes compared with Wei6823.
We next examined salt tolerance in field conditions. For this, we selected three single-copy GmSIN1 OE homozygous transgenic lines. A comparison of the field performance of the OE lines and the wild type under control (0.15 g of total soluble salts per 100 g of dry soil) and saline (0.35 g of total soluble salts per 100 g of dry soil) conditions (Supplemental Figure 6A) in the same environment showed that for all three OE lines, seedlings emerged more readily than did the wild type (Figure 1E; Supplemental Data Set 1). The height of mature wild-type plants was strongly reduced in saline soil, but the effect on the OE lines was much less severe (Figure 1F; Supplemental Data Set 1). Further yield trials were conducted in three different geographical locations using fields with 0.05, 0.30, and 0.35 g of total soluble salts per 100 g of dry soil. In all fields, the OE plants were taller than the wild type, produced more seeds per plant, and had higher yields (Figure 1G; Supplemental Figure 6B; Supplemental Data Set 1). Over four seasons, the performance of the OE lines under medium-salinity conditions (0.2 to 0.3 g of total soluble salts per 100 g of dry soil) exceeded that of the wild type in terms of plant height, pod number per plant, and the number of seeds per plant (Table 1). Therefore, GmSIN1 overexpression enhanced plant growth and productivity not just under saline conditions but also (albeit to a lesser extent) under nonsaline conditions.
Table 1. Traits of GmSIN1 OE Transgenic Seedlings in Medium-Saline Field.
| Year | Line | Height | Effective Pod No. | Seed No. |
|---|---|---|---|---|
| 2018 | GmSIN1 OE-1 | 68 ± 3*** | 66 ± 3** | 121 ± 6** |
| GmSIN1 OE-2 | 69 ± 3*** | 69 ± 4** | 114 ± 6* | |
| GmSIN1 OE-3 | 69 ± 2*** | 64 ± 3* | 108 ± 5 | |
| WT | 54 ± 2 | 56 ± 2 | 96 ± 6 | |
| GmSIN1 OE-1 | 61 ± 6** | 157 ± 42*** | 276 ± 33*** | |
| 2016 | GmSIN1 OE-2 | 63 ± 10** | 90 ± 17*** | 147 ± 26 |
| GmSIN1 OE-3 | 61 ± 8** | 104 ± 19*** | 207 ± 35*** | |
| WT | 51 ± 8 | 62 ± 11 | 124 ± 26 | |
| 2015 | GmSIN1 OE-1 | 55 ± 6*** | 145 ± 37* | 286 ± 88** |
| GmSIN1 OE-2 | 53 ± 8** | 136 ± 36* | 261 ± 58** | |
| WT | 43 ± 7 | 105 ± 44 | 191 ± 72 | |
| 2014 | GmSIN1 OE-1 | 63 ± 4* | 40 ± 7* | 74 ± 36* |
| GmSIN1 OE-2 | 58 ± 5* | 40 ± 9 | 85 ± 37* | |
| GmSIN1 OE-3 | 57 ± 4 | 45 ± 9* | 80 ± 39* | |
| WT | 43 ± 4 | 32 ± 5 | 66 ± 34 |
The significance of differences between GmSIN1 OE transgenic and the wild-type (WT) seedlings was determined with the Student’s t test (*P < 0.05; **P < 0.01; ***P < 0.001, n = 15 to 45 plants). All data are given as mean ± se. The salinity of the field is ∼0.2 to 0.3 g of soluble salt per 100 g of dry soil.
Taken together, these findings suggested that GmSIN1 influences root growth and salt tolerance in soybean.
Characterization of GmSIN1
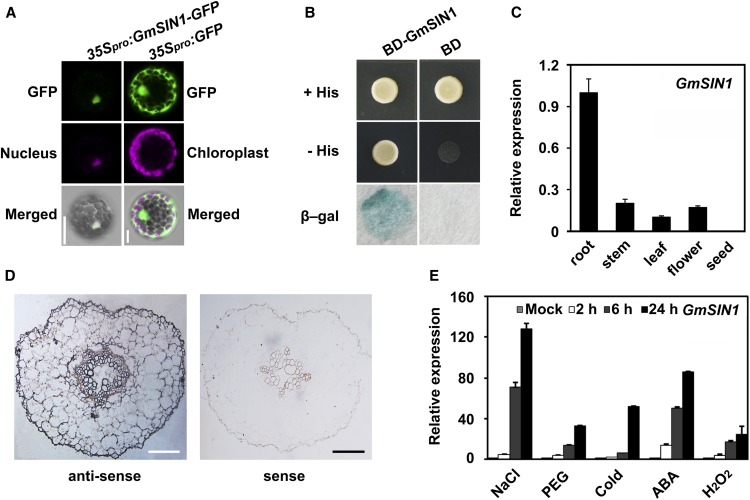
As a preliminary characterization of GmSIN function, we examined its conserved domains and subcellular localization. In silico translation of the GmSIN1 coding sequence predicted a 342-residue polypeptide including a conserved NAC domain (amino acids 14 to 139) in the N terminus and a variable C terminus (Supplemental Figure 7A). GmSIN1 shares a mean identity of 45% with its Arabidopsis homologs (Supplemental Figure 7A). A phylogenetic tree based on polypeptide sequences showed that GmSIN1 is closely related to Glyma.13G279900 and Glyma.12G221500 from soybean and ANAC019, ANAC055, and ANAC072 from Arabidopsis (Supplemental Figure 7B; Supplemental Data Set 2). GmSIN1 shares a mean identity of 45% with its Arabidopsis homologs (Supplemental Figure 7A).
The subcellular distribution of GmSIN1 was investigated by imaging a GmSIN1-green fluorescent protein (GFP) fusion transformed into Arabidopsis protoplasts (Supplemental Figure 8). In these protoplasts, signal was detectable in the nucleus (Figure 2A).
Figure 2.
Characterization of GmSIN1.
(A) GmSIN1-GFP fusion proteins localized to the nucleus in transiently transformed Arabidopsis protoplasts. (Left) Images of a protoplast harboring p35Spro:GmSIN1-GFP and pMDC32-1A BES1n-mCherry (nuclear marker). (Right) Images of a protoplast harboring p35Spro:GFP. The top row shows the GFP signal (green), the middle row shows the nuclear marker (left) and chloroplast autofluorescence (right; magenta), and the bottom row shows the merged images. The images show representative results from more than 30 transformed protoplasts from three independent experiments. More images can be found in Supplemental Figure 8B. Bar = 20 μm.
(B) Ability of yeast transformants to grow on medium lacking His and Leu but containing 10 mM 3-aminotriazole, and the formation of color in the X-β-Gal assay indicates transcriptional activation. The images show representative results from more than five independent yeast transformants. BD, Yeast colony expressing GAL4 DNA binding domain; BD-GmSIN1, Yeast colony expressing BD-GmSIN1 fusion protein.
(C) Expression of GmSIN1 analyzed using RT-qPCR in root, stem, leaf, flower, and seed tissue in cv Shengdou No. 9.
(D) Localization of GmSIN1 mRNA using in situ hybridization. (Left) Transverse section of a root 1 mm from the root tip of 6-d-old cv Shengdou No. 9 plants probed with a GmSIN1 antisense probe. (Right) Profile obtained using a sense strand probe. Bar = 100 μm.
(E) Expression of GmSIN1 analyzed using RT-qPCR in response to NaCl (150 mM), moisture stress (20% [w/v] polyethylene glycol [PEG] 6000), low temperature (4°C), 100 µM ABA, or 1 mM H2O2 treatment in cv Shengdou No.9. The roots of 2-week-old seedlings were collected for RNA extraction. Error bars represent se (n = 3 biological repeats). The details of the sampling methods are shown in “Methods.”
To further characterize GmSIN1 function, we examined its transcriptional activation activity by fusing its coding sequence with the GAL4 DNA binding domain of the yeast expression vector pGBKT7 and tested whether this fusion could activate reporter genes (conferring His auxotrophy and β-galactosidase activity) downstream of the GAL4 binding site. The yeast transformants with pGmSIN1-GBKT7 or the empty vector grew freely on medium lacking Leu (Figure 2B). However, on medium lacking His, cells having the empty vector pGBKT7 did not grow, while those carrying GmSIN1 grew well (Figure 2B). The transactivation activity assay (X-β-Gal assay) gave a consistent result (Figure 2B). Therefore, GmSIN1 represents a soybean gene that encodes a nucleus-localized NAC transcription activator.
We further investigated the expression pattern of GmSIN1 by quantifying the relative abundance of the mRNA in different organs of Shengdou No. 9 plants. The GmSIN1 expression was much higher in roots, but moderate in stems, leaves, and flowers. The transcripts were not detectable in seeds (Figure 2C). Using RNA in situ hybridization, we detected the expression of GmSIN1 predominantly in endodermal cells and cells associated with phloem and xylem in the root (Figure 2D). Similar to other stress-responsive NAC TF genes (Le et al., 2011), the expression of GmSIN1 was rapidly induced by high salinity, osmotic stress (induced by polyethylene glycol treatment), cold, oxidative stress, and ABA treatment in the salt-tolerant cv Shengdou No. 9 (Figure 2E).
Transcriptomic Analysis of 35Spro:GmSIN1 Transgenic Soybeans
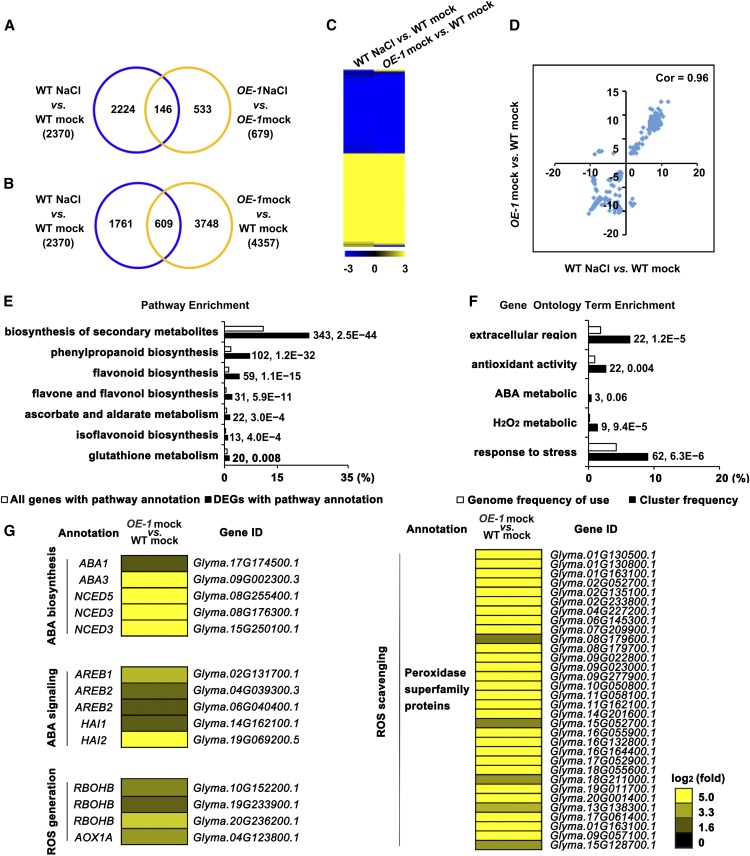
To reveal the effect of the TF GmSIN1 on the soybean transcriptome in response to salt stress, we conducted RNA sequencing (RNA-seq) on the OE and wild-type plants under control conditions (mock treated) and NaCl treatment. We first tested the expression pattern of GmSIN1 in the wild type and OE-1 in response to 150 mM NaCl treatment. In both the wild-type and OE plants, GmSIN1 was induced rapidly in response to NaCl stress and achieved peak expression at 6 h. After that, GmSIN1 expression declined quickly and reached its original level at 48 h. During the treatment, the expression level of GmSIN1 was higher in the OE plants than in the wild-type plants (Supplemental Figure 9A). This suggests that GmSIN1 mainly functions in the early response to salt stress.
Based on the expression of GmSIN1, we used a 6-h NaCl treatment in the RNA-seq experiment and performed two biological replicates (Supplemental Table 2). Following the 6-h NaCl treatment, 2370 genes were differentially expressed (fold change > 2 and P > 0.7) in the wild type relative to mock treatment (Figure 3A; Supplemental Data Set 3). However, NaCl treatment caused a less dramatic transcriptomic change in GmSIN1 OE plants relative to the wild-type plants, with only 363 upregulated genes and 316 downregulated genes (Figure 3A; Supplemental Data Set 3). This suggests that GmSIN1 OE plants are less sensitive to NaCl treatment than the wild-type plants in terms of transcriptomic changes.
Figure 3.
Transcriptomic Analysis of GmSIN1 OE-1 Transgenic Soybean.
(A) and (B) Numbers of genes showing differential expression between GmSIN1 OE-1 transgenic soybeans and Wei6823 (A) or between non-NaCl–stressed and NaCl-stressed seedlings (B). WT, wild type.
(C) Hierarchical cluster analysis of the overlapping genes from (B) differentially expressed in NaCl-treated Wei6823 versus mock-treated Wei6823 and GmSIN1 OE-1 versus Wei6823. The numerical values in the yellow-to-blue gradient bar represent log2-fold change relative to the control sample. WT, wild type.
(D) Correlation of the overlapping genes from (B) differentially expressed in NaCl-treated Wei6823 versus mock-treated Wei6823 and GmSIN1 OE-1 versus Wei6823. Cor, correlation; WT, wild type.
(E) Pathways that were statistically enriched in DEGs in GmSIN1 OE-1 versus Wei6823 RNA-seq data. The numbers near the columns indicate the number of DEGs with corresponding annotation and the P-value, respectively.
(F) GO terms that were statistically enriched in differentially expressed genes in GmSIN1 OE-1 versus Wei6823 RNA-seq assay. The numbers near the columns indicate the number of DEGs with corresponding annotation and the q value, respectively.
(G) Heatmap of differential expression of ABA biosynthesis, ABA signaling, ROS generation, and ROS scavenging-related genes in GmSIN1 OE-1 versus Wei6823. The numerical values for the yellow-to-blue gradient bar represent log2-fold change relative to the control sample. WT, wild type.
The largest number of differentially expressed genes (DEGs), 4357, was found between mock-treated GmSIN1 OE plants and the wild-type plants. This included 609 DEGs in common with DEGs from the comparison of the NaCl-treated and control wild-type plants (Figure 3B; Supplemental Data Set 3). To better understand the relationship of DEGs caused by GmSIN1 overexpression (DEGs in GmSIN1 OE mock versus the wild-type mock) and by NaCl treatment (DEGs in the wild-type NaCl treatment versus the wild-type mock), we analyzed the expression pattern of the 609 DEGs using hierarchical clustering and correlation analysis. This showed that for most DEGs, the expression pattern is exactly the same (Figure 3C) and the correlation is as high as 0.96 (Figure 3D). These results suggest that some of the transcriptional changes caused by salt stress are mediated by GmSIN1.
Biological pathways of secondary metabolites, especially for the biosynthesis of flavonoid, flavone, flavonol, and isoflavonoid compounds, were greatly enriched among the upregulated DEGs in mock-treated GmSIN1 OE plants versus the wild-type plants. Moreover, the
gene ontology (GO) terms responsive to stress, antioxidant activity, hydrogen peroxide metabolic, and ABA metabolic were especially enriched among the upregulated genes (Figures 3E and 3F). This was consistent with the conclusion that GmSIN1 is involved in the salt stress response. Moreover, it suggested a function for GmSIN1 in ABA and ROS homeostasis.
To further explore the effect of GmSIN1 on the transcription of ABA- and ROS-related genes, we identified all the genes that were homologs of Arabidopsis genes annotated as functioning in ABA biosynthesis, ABA signaling, ROS generation, and ROS scavenging. This identified 5 genes in ABA biosynthesis and signaling, 4 genes in ROS generation, and 32 genes in ROS scavenging that were significantly upregulated in GmSIN1 OE-1 plants in comparison with the wild type (fold change > 2 and P > 0.7) under normal conditions (Figure 3G). We then hypothesized that changes in the expression of these genes may affect the ABA and ROS levels and contribute to salt tolerance of GmSIN1 OE soybean. To test this, three ABA- or ROS-responsive genes were selected and their expression was measured using RT-qPCR in GmSIN1 OE-1 and RNAi plants (Supplemental Figure 9B). The selected genes were all upregulated in OE plants and downregulated in RNAi plants (Supplemental Figure 9C). Additionally, they all showed earlier or higher induction in OE plants than in the wild-type plants in response to NaCl treatment (Supplemental Figure 9D). These data further suggest that GmSIN1 affects ABA and ROS pathways at early stages of NaCl treatment.
GmSIN1 Induces the Production of ABA and ROS in Response to Salt Stress
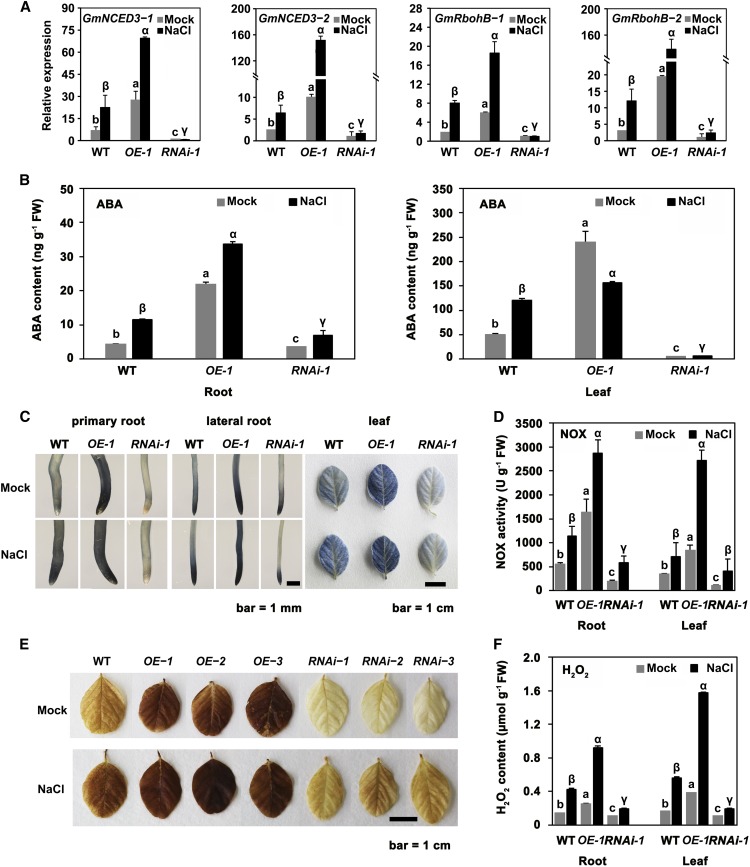
NCED3 and NOX have been suggested to be responsible for the increases in ABA and ROS that occur in response to salt stress in Arabidopsis (Barrero et al., 2006; Kurusu et al., 2015). NCED3 and NOX genes were upregulated in the GmSIN1 overexpression lines, as shown by the RNA-seq data (Figure 3G). Therefore, we tested the effect of GmSIN1 overexpression and suppression on GmNCED3-1 (Glyma.15G250100.1), GmNCED3-2 (Glyma.08G176300.1), GmRbohB-1 (Glyma.10G152200.1), and GmRbohB-2 (Glyma.20G236200.1) transcripts in the presence of salt stress by RT-qPCR of OE and RNAi plants. Under control (nonsaline) conditions, the gene expression in the OE seedlings was higher than in the wild-type seedlings that, in turn, was higher than in RNAi seedlings (Figure 4A). The abundance of each transcript increased in the roots of the wild-type and OE seedlings exposed to 150 mM NaCl for 6 h, but not in the RNAi plants (Figure 4A).
Figure 4.
GmSIN1 Modulated ABA and ROS Contents by Inducing Genes Involved in ABA/ROS Synthesis in Response to Salt Stress.
(A) Transcript levels of GmNCED3-1, GmNCED3-2, GmRbohB-1, and GmRbohB-2 in the roots of 6-d-old seedlings of GmSIN1 OE (OE-1), a GmSIN1 knockdown (RNAi), and the wild type (WT) exposed to either 0 mM (mock) or 150 mM NaCl for 6 h. Data obtained by RT-qPCR.
(B) ABA content of the root and leaf of the 5-d-old seedlings shown in (A). WT, wild type.
(C) NBT staining reveals the superoxide content of primary and lateral roots of 6-d-old and of leaves of 17-d-old GmSIN1 OE (OE-1), a GmSIN1 knockdown (RNAi), and the wild-type (WT) seedlings exposed to either 0 mM (mock) or 150 mM NaCl for 2 h. The staining intensity reflects the concentration of superoxide.
(D) NOX activity in the OE-1, RNAi, and the wild-type (WT) plants shown in (C). FW, fresh weight.
(E) DAB staining reveals the H2O2 content of the leaf of 17-d-old GmSIN1 OE-1, GmSIN1 RNAi, and the wild-type (WT) plants exposed to either 0 mM (mock) or 150 mM NaCl for 2 h. The staining intensity reflects the concentration of H2O2.
(F) The H2O2 content of the root of 6-d-old and the leaf of 17-d-old OE-1, RNAi, and the wild-type (WT) seedlings. Histogram data are given in the form mean ± se (n = 3 for RT-qPCR data and n = 9 to 12 for the other assays). Significant differences between samples labeled with different Roman (a, b, c) or Greek letters (α, β, γ) were determined by one-way ANOVA and Tukey’s test, P < 0.05. The details of the sampling procedures are presented in the “Methods.” FW, fresh weight; ND, no significant difference.
To demonstrate the functionality of GmNCED3-1 and GmRbohB-1 in soybean, the two genes were transiently overexpressed in soybean leaves. Indeed, GmNCED3-1 overexpression had a positive effect on ABA accumulation and GmRbohB-1 overexpression similarly increased ROS generation (Supplemental Figure 10). The effect of altering the abundance of GmNCED3-1, GmNCED3-2, GmRbohB-1, and GmRbohB-2 transcripts on the ABA and ROS contents was explored by analyzing root and leaf samples from GmSIN1 OE and RNAi plants grown in the presence or absence of salt stress. The level of ABA in GmSIN1 OE roots was significantly higher (fivefold and threefold higher for mock and NaCl treatment, respectively) than in the wild-type roots, and in GmSIN1 RNAi roots, the opposite trend was observed (Figure 4B). In leaves, the change of ABA level was similar to that in roots (Figure 4B). Comparing the ABA increase in response to 6-h salt treatment in the wild-type and GmSIN1 RNAi plants showed that the ABA increase was partially suppressed in GmSIN1 RNAi roots and completely suppressed in leaves. This suggested that the induction of ABA under short-term salinity in soybean was at least partially mediated by GmSIN1.
Using nitroblue tetrazolium (NBT) to quantify the production of the ROS superoxide, we observed that the overexpression of GmSIN1 promoted the accumulation of superoxide in the root. The staining intensity was much weaker in the wild-type roots, and even weaker in the RNAi roots compared with the OE roots (Figure 4C). When the plants were exposed to 150 mM NaCl for 2 h, superoxide production was concentrated at the root tip of the wild-type plants but at a lower level than in the OE roots and at a higher level than in the RNAi roots (Figure 4C). Greater NOX activity was seen in the OE tissues than in those of the wild type, and less NOX activity was seen in RNAi tissues (Figure 4D). Finally, the cellular H2O2 content, as measured by 3,3-diaminobenzidine (DAB) staining, was higher in OE than in the wild type and lower in RNAi tissue (Figure 4E). The overexpression of GmSIN1 promoted H2O2 accumulation under saline and nonsaline conditions in both the root and the leaf (Figure 4F). The induction of superoxide, H2O2, and NOX activity in response to NaCl treatment in GmSIN1 RNAi lines was partly suppressed in comparison with the wild type. Overall, this suggests that GmSIN1 contributes to salinity-induced ROS accumulation at early stages in response to salt stress.
GmSIN1 Improves Root Growth and Salt Tolerance by Promoting ABA Accumulation and ROS Generation
To investigate whether altered root growth and salt tolerance in transgenic soybean were due to changes in ABA levels and/or ROS accumulation, we used diphenyliodonium chloride (DPI), an inhibitor of NADPH oxidases, which contribute to the NaCl-induced ROS accumulation (Mazel et al., 2004), and nordihydroguaiaretic acid (NDGA), an inhibitor of lipoxygenase, which catalyzes dioxygenation of polyunsaturated fatty acids and is reported to inhibit carotenoid cleavage dioxygenases including NCED (Creelman et al., 1992) in root elongation assays in GmSIN1 OE and the wild-type plants. Compared with the wild type, GmSIN1 OE lines produced longer primary roots under optimal growth conditions and significantly more relative root elongation after a 4-d treatment with 150 mM NaCl (Figure 1A). Surprisingly, when the NaCl was coupled with DPI or NDGA treatment, the difference of root length and root elongation between OE plants and the wild-type plants was abolished (Figures 5A to 5C). This demonstrates that the salt tolerance conferred by GmSIN1 requires both ROS and ABA.
Figure 5.
ABA and ROS Are Required for GmSIN1 to Enhance Salt Tolerance and Root Growth.
(A) Phenotype of 7-d-old GmSIN1 OE transgenic plants compared with Wei6823 under control, 150 mM NaCl, 150 mM NaCl with 100 µM DPI, or 150 mM NaCl with 50 µM NDGA treatment. The treatments began at 3 d after germination and were maintained for 4 d. WT, wild type.
(B) and (C) Root length (B) and root elongation (C) measured in the seedlings shown in (A). ND, no significant difference; WT, wild type.
(D) Phenotype of 7-d-old GmSIN1 OE transgenic plants compared with Wei6823 under mock, 100 µM DPI, or 50 µM NDGA treatment. The treatments began at 3 d after germination and were maintained for 4 d. WT, wild type.
(E) Root length of seedlings from (D). All data are given as means ± se (n = 20). OE-1, OE-2, and OE-3 are GmSIN1 OE transgenic lines. The roots of each genotype were collected from seedlings grown in 10 plastic growth bags with the same treatment. Each bag included three seedlings each from the wild type (WT) and the three transgenic lines. The data shown are a representative result from several independent experiments. ND, no significant difference.
(F) Expression of GmSIN1 analyzed using RT-qPCR in response to 150 mM NaCl, 1 mM H2O2, and 100 µM ABA treatment. FLU (50 nM) or 100 µM DPI was supplemented or not with NaCl, ABA, or H2O2 in the roots of 6-d-old seedlings of cv Shengdou No. 9.
(G) Expression of GmSIN1 analyzed using RT-qPCR in response to 150 mM NaCl for 48 h in soybean leaves transiently transformed with empty vector pB7GWIWG2(II), p35Spro:GmNCED3-RNAi, or p35Spro:GmRbohB-RNAi. Data are means ± se (n = 3). Significant differences between samples labeled with different Roman (a, b, c) or Greek letters (α, β, γ) were determined by one-way ANOVA and Tukey’s test, P < 0.05. The details of the sampling procedures are presented in the “Methods.”
Furthermore, DPI or NDGA treatment completely rescued the long-root phenotype in GmSIN1 OE plants under optimal conditions (Figures 5D and 5E). Fluridone (FLU), an inhibitor of carotenoid synthesis that has also been linked to depressed levels of endogenous ABA, has a similar effect to NDGA (Figure 5F; Supplemental Figure 11). Therefore, the salt tolerance in root elongation and the promotion of root length in GmSIN1 OE plants required the accumulation of both ABA via NCED activity and ROS via NOX activity. These data also demonstrate that the genes encoding NCED and NOX function genetically downstream of GmSIN1 in this process.
GmSIN1 is transcriptionally induced by NaCl, oxidative stress, and ABA treatment. To determine whether its induction is mediated by ABA or ROS, we used FLU or DPI to inhibit ABA or ROS production in NaCl-treated seedlings, respectively. Indeed, GmSIN1 induction by high salinity was almost completely suppressed by FLU and DPI (Figure 5F). Additionally, the induction of GmSIN1 by ABA was suppressed by DPI treatment and the induction of GmSIN1 by H2O2 was suppressed by FLU treatment (Figure 5F).
In addition, we constructed RNAi vectors that target GmNCED3 and GmRbohB. These two constructs and the control vector were transiently expressed in Shengdou No. 9 leaves, and GmSIN1 expression was measured using RT-qPCR with or without NaCl treatment. The induction of GmSIN1 in response to NaCl treatment was significantly reduced when the expression of GmNCED3 and GmRbohB was suppressed (Figure 5G). The evidence strongly suggests that the high induction of GmSIN1 in response to NaCl treatment is mediated by ABA and ROS, both of which are required for this response.
ABA and ROS Coordinate in Modulation of Root Elongation by GmSIN1
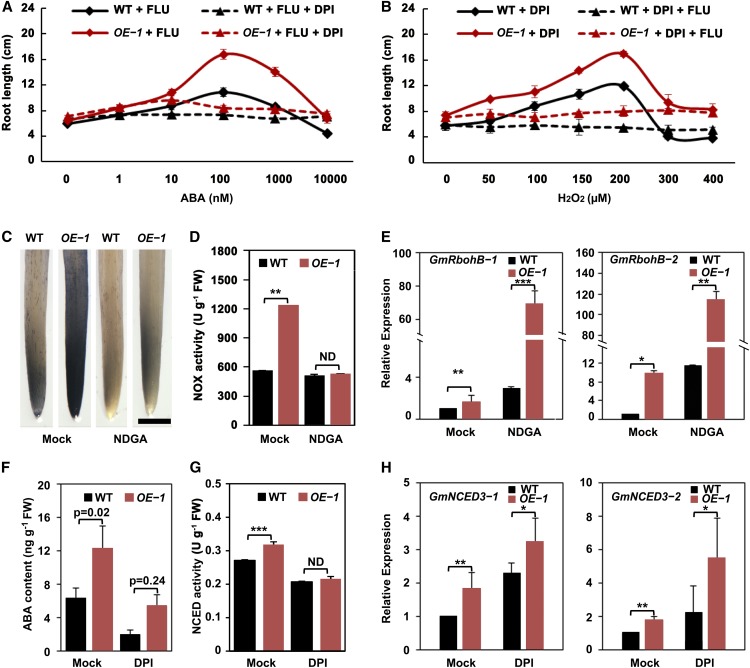
To better understand the interplay of ABA and ROS in GmSIN1-modulated root elongation, we measured root length in the presence of ABA and/or ROS biosynthesis inhibitors. To examine the effect of ABA dosage, the wild-type and GmSIN1 OE seedlings were grown at different ABA concentrations in the presence of FLU to inhibit endogenous ABA synthesis. Consistent with the results shown in Figures 5F and 5G, the root length of the wild-type and GmSIN1 OE seedlings showed no difference with only FLU treatment, but they both showed inverted U curves with the rise in ABA concentration and GmSIN1 OE seedlings had longer roots than the wild type at 100 to 10,000 nM ABA (Figure 6A). The optimal concentration of ABA for root growth seemed to be 100 nM in both GmSIN1 OE and control seedlings (Figure 6A). However, when sufficient DPI was added to inhibit NOX activity, the effect of ABA dosage on root elongation was completely suppressed and the root length difference between the wild-type and GmSIN1 OE seedlings disappeared (Figure 6A). When the root growth assay was performed with DPI treatment and different concentrations of exogenous H2O2 in the wild-type and GmSIN1 OE seedlings with or without FLU treatment, similar phenotypes to that in ABA treatment were observed (Figure 6B).
Figure 6.
Interaction of ABA and ROS in GmSIN1-Modulated Root Elongation.
(A) Root length of 6-d-old seedlings of GmSIN1 OE-1 transgenic and the wild-type (WT) seedlings grown in the presence of 50 nM FLU and different concentrations of ABA with or without 100 µM DPI.
(B) Root length of 6-d-old seedlings of GmSIN1 OE-1 transgenic and the wild-type (WT) seedlings grown with 100 µM DPI and different concentrations of H2O2 with or without 50 nM FLU. The treatments began at 2 d after germination and were maintained for 4 d. Data are means ± se (n = 20 seedlings). The roots of each treatment were collected from seedlings grown in plastic growth bags with the same treatment. Each bag included five seedlings each of WT and OE-1. The data shown are a representative result of several independent experiments.
(C) NBT staining reveals the superoxide content of the root tips of 6-d-old GmSIN1 OE-1 and the wild-type (WT) seedlings exposed to either 0 µM (mock) or 50 µM NDGA. The treatments began at 2 d after germination and were maintained for 4 d. The staining intensity reflects the concentration of superoxide. Bar = 1 mm.
(D) NOX activity in the roots of the GmSIN1 OE-1 and the wild-type (WT) plants as in (C). Data are means ± se (n = 5 biological replicates). FW, fresh weight.
(E) to (G) Transcript abundance of GmRbohB-1 and GmRbohB-2 (E), GmNCED3-1 and GmNCED3-2 (H). Data obtained by RT-qPCR. The roots from seedlings with the same treatments as in (C) and (F) were used for RNA preparation. (F) ABA contents of the roots of 6-d-old GmSIN1 OE-1 and the wild-type (WT) seedlings exposed to either 0 µM (mock) or 100 µM DPI. The treatments began at 2 d after germination and were maintained for 4 d. Error bars denote the se (n = 3 biological replicates). (G) NECD activity in the roots of the GmSIN1 OE and WT plants with the same treatment as in (F). Data are means ± se (n = 5 biological replicates). Asterisks denote significant differences between the mutated promoter and the WT promoter (t test, *P < 0.05, ***P < 0.001). FW, fresh weight; ND, no significant difference.
To better understand the effect of ABA and H2O2 dosage on root elongation, we treated the wild-type and OE plants with different concentrations of ABA or H2O2 without any inhibitor. Low concentrations of ABA or H2O2 promoted root growth in the wild-type seedlings and high concentrations of ABA inhibited root growth. By contrast, in OE plants, all concentrations of exogenous ABA or H2O2 inhibited root growth (Supplemental Figures 12A and 12B). This suggested that the ABA and H2O2 levels are closer to optimal in OE plants than in the wild-type plants; therefore, any exogenous ABA or H2O2 exceeds the optimal level and inhibits growth. In Arabidopsis, a similar dosage effect was observed (Supplemental Figures 12C and 12D). Interestingly, an additive effect of low concentrations of ABA and H2O2 on root elongation was found in Arabidopsis (Supplemental Figure 12E). These results show that both ABA and H2O2 have dosage effects on root growth, and they both are required to maintain optimal root growth. Additionally, a low combined concentration of ABA and H2O2 might be better for root growth. Besides the root elongation, seed germination also required an optimal ABA level in soybean (Supplemental Figure 12F).
To reveal the details of how ABA and ROS act together in this process, the wild-type and GmSIN1 OE seedlings were cultivated with mock or NDGA treatment and the O2⋅− content, NOX activity, and transcript levels of GmRbohB genes were tested. NDGA treatment completely suppressed the increase of O2⋅− and NOX activity in GmSIN1 OE seedlings in comparison with the wild type but promoted the transcription of GmRbohBs in both the wild-type and GmSIN1 OE seedlings (Figures 6C to 6E). These results suggest that ABA is required for GmSIN1 to promote H2O2 production and acts downstream of transcription of GmRbohBs. Furthermore, DPI treatment suppressed the increase of ABA and NCED activity in GmSIN1 OE seedlings in comparison with the wild type but promoted the transcription of GmNCED3s in both the wild-type and GmSIN1 OE seedlings (Figures 6F to 6H). Therefore, ROS are also required for GmSIN1 to promote ABA accumulation and act downstream of transcription of GmNCED3s.
GmSIN1 Binds to a Unique Motif That Differs from the Core NAC Binding Motif
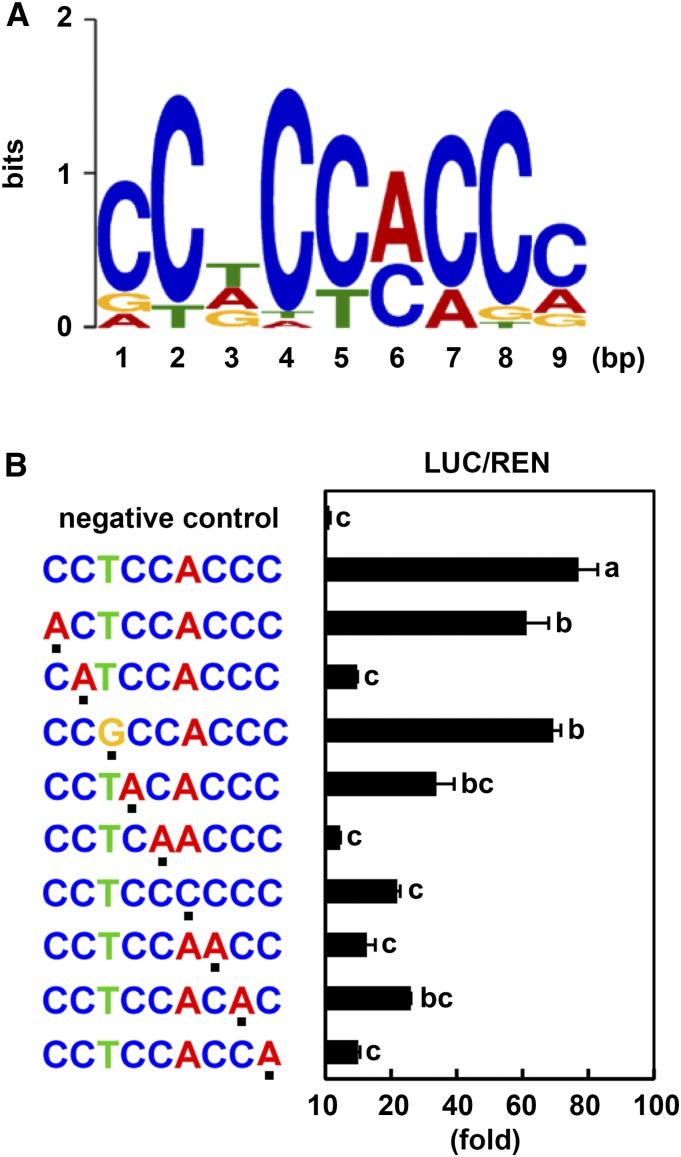
The 4-bp core sequence of the NAC binding motif is CACG (Olsen et al., 2005a); in addition, each NAC transcription factor likely has a specific and distinct binding motif (Olsen et al., 2005a; Zhang and Gan, 2012; De Clercq et al., 2013). To identify the specific binding motif for GmSIN1, we used the Multiple Em for Motif Elicitation (MEME) motif discovery tool (http://meme-suite.org) and our RNA-seq data. We identified a 9-bp consensus sequence, CCTCCACCC, which we named SIN1BM, in 30 of the top 34 genes that were upregulated by both GmSIN1 and NaCl treatment (Table 2; Supplemental Data Set 4).
Table 2. SIN1BMs in Promoters of GmSIN1 and Salt Stress Co-Upregulated Genes.
| Gene Locus | P-Value | Consensus Motif | Homologs in Arabidopsis | Annotations |
|---|---|---|---|---|
| Glyma.14G034200 | 8.00E−08 | TCTTTAGTCA CCGCCACCC AACCAGGACA | AT5G19420.1 | Regulator of chromosome condensation family with FYVE zinc finger domain |
| Glyma.13G333200 | 8.00E−08 | TTAATGAATT CCTCCCCCC CCCCCAAATG | AT3G46130.2 | Myb domain protein 48 |
| Glyma.10G207000 | 2.08E−07 | CAAATCACCC CCTCCACCC TACCACTACT | – | |
| Glyma.12G230300 | 4.29E−07 | CCTTCGGATT CCGCCACCG CCATAACGCC | AT1G16916.1 | |
| Glyma.01G240100 | 5.31E−07 | AGCTACAGTA CCGCCCACC AGATCGGGAG | AT4G21750.2 | MERISTEM LAYER1 |
| Glyma.16G095700 | 8.34E−07 | GTTTTCTACA CCGCCACCA TTAAACAACA | AT4G26470.1 | Calcium-binding EF-hand family protein |
| Glyma.16G005600 | 8.34E−07 | GTCCATGTTG CCGCCACCA TTCGTCGTTA | AT1G77320.1 | MEIOSIS DEFECTIVE1 |
| Glyma.07G196700 | 9.28E−07 | GCTAGAAACT CCACCCCCA ACAACGATCT | AT4G30930.1 | NUCLEAR FUSION DEFECTIVE1 |
| Glyma.11G213200 | 1.81E−06 | CTGAAAATAC CCACCCACC TAACCTTACA | AT2G01990.1 | |
| Glyma.10G294100 | 1.81E−06 | AGGGAGAGTT GCTCCACCC TTTTCCCTCT | AT1G04880.1 | HMG box protein with ARID/BRIGHT DNA-binding domain |
| Glyma.19G003700 | 2.02E−06 | GATTCCATCA CCGCCACGC GCGCCATTAA | AT3G27570.1 | Sucrose/ferredoxin-like family protein |
| Glyma.18G136900 | 2.58E−06 | ACCACATCAA CCACCACCA CAACCATTAC | AT1G23860.3 | RS-containing zinc finger protein21 |
| Glyma.09G010500 | 3.33E−06 | GTGCTCTACG CCTCTACCC TTTAGAATCG | ||
| Glyma.05G016700 | 4.28E−06 | GGAAGGCATA CTTCCCCCC TAACCCTCTA | AT1G73980.1 | Phosphoribulokinase/uridine kinase family |
| Glyma.14G001500 | 6.20E−06 | TCATCTGCGC ACACCACCC TCAACCTCTA | AT4G38350.1 | ATNPC1-2 |
| Glyma.02G260300 | 6.20E-06 | AGACACATAT ACACCACCC ACGTGGTTTT | AT3G56200.1 | Transmembrane amino acid transporter family protein |
| Glyma.11G199700 | 6.88E−06 | TATTGGTAAT CTTCCACCC CTTTCTTAAC | AT5G58300.2 | Leucine-rich repeat protein kinase family protein |
| Glyma.06G098900 | 9.83E−06 | TTCTTTTGTT CCTCCCCTC CTTTGGAAAA | AT1G12680.1 | Phosphoenolpyruvate carboxylase-related kinase 2 |
| Glyma.17G109200 | 1.09E−05 | CAAAACAGAC CCACTCCCA CGGGTCAAGA | AT5G20300.2 | TOC90 |
| Glyma.05G014400 | 1.09E−05 | AAGACCTTCC CCACTCCCA CTTCGTGTTA | AT1G48950.1 | C3HC zinc finger-like |
| Glyma.10G221500 | 1.35E−05 | ATGATATTTT CCTCTCACC TCCTGAGTCC | AT1G22770.1 | GIGANTEA |
| Glyma.13G149600 | 1.46E−05 | GGTAAAAAAT CCACCCCGA ATCCCTACTC | AT3G53000.1 | PHLOEM PROTEIN2-A15 |
| Glyma.16G034400 | 1.94E−05 | GCATTCAACT CCTTCACCC AACCTATTGT | AT1G29320.1 | Transducin/WD40 repeat-like superfamily protein |
| Glyma.05G230700 | 2.22E−05 | TGAAAGGTCT CCAACACCC CAGAGAATGG | AT5G21222.1 | Protein kinase family protein |
| Glyma.09G031100 | 2.78E−05 | TCGCAGCCGT CCACTAACC GTGATTTCCC | AT3G06480.1 | DEAD box RNA helicase family protein |
| Glyma.15G248200 | 2.96E−05 | CATTACTTAA GCTCCCACG CATTTTTTAA | AT1G17110.1 | UBP15 |
| Glyma.10G215300 | 2.96E−05 | GGCTGGAATT GCTCTCCCG AACCCAATTC | AT1G22700.2 | Tetratricopeptide repeat (TPR)-like superfamily protein |
| Glyma.05G135900 | 2.96E−05 | AGCCAGGAAG GCGCCAACG CGAATCAAAA | AT2G02180.1 | TOBAMOVIRUS MULTIPLICATION PROTEIN3 |
| Glyma.15G099100 | 4.70E−05 | AAAATGAATT ATGCCACCC ATTAATAATT | AT5G18760.1 | RING/U-box superfamily protein |
ATNPC, Arabidopsis NIEMANN-PICK DISEASE TYPE C1-2; C3HC, C3HC type zinc finger protein; DEAD, DEAD box helicase domain; EF, EF hand domain; FYVE, FYVE zinc finger domain; HMG, HMG, High mobility group box domain; Myb, myb DNA binding domain; RS, arginine/ serine-rich domain; UBP, ubiquitin C-terminal hydrolases; TOC, translocon at the outer chloroplast membrane; WD40, short ∼40 amino acid motifs, often terminating in a Trp-Asp (W-D) dipeptide. Blank cells or dashes indicate no data.
To determine the key bases in this candidate sequence, we performed point mutation analysis of SIN1BM and tested GmSIN1 binding to the mutated sequences by Firefly luciferase (LUC) assays. Based on the SIN1BM sequence (CCTCCACCC; Figure 7A), we prepared a series of single-nucleotide–substituted sequences and fused them to a DNA fragment of the TUBULIN BETA CHAIN2 (TUB2) promoter, which does not contain a SIN1BM. We first confirmed that GmSIN1 does not bind to the TUB2 promoter (Figure 7B, negative control). Next, we found that point mutations in the second nucleotide (C) and fourth to ninth nucleotides (CCACCC) of the SIN1BM significantly decreased GmSIN1 binding, and mutations of the first (C) and third (T) nucleotide did not significantly reduce the binding (Figure 7B). This suggests that the C (second) and CCACCC (fourth to ninth) bases are the core sequence of the SIN1BM and are essential for the DNA binding of GmSIN1.
Figure 7.
Identification of the GmSIN1 Binding Motif (SIN1BM).
(A) Using MEME analysis, conserved sequences were identified in the promoter regions of genes co-upregulated in GmSIN1 OE plants and the NaCl-treated wild-type plants (Table 2). Position weight matrix of SIN1BM showing the probability of a nucleotide(s) at each position.
(B) Interaction of GmSIN1 with the SIN1BM consensus and its substituted sequences in LUC (Firefly luciferase) assays. The sequences of interest were fused to a promoter fragment of TUB2 that does not contain a SIN1BM. LUC activity and REN (Renilla luciferase) activity were measured. Dots below the sequences indicate the substitution from SIN1BM, which was determined by MEME analysis (shown in [A]). pTUB2pro::LUC and pGreenII 0800-35S-LUC were used for negative control and positive control, respectively. Mean and se of LUC activity/REN activity were obtained from more than four independent transformation experiments. Significant differences between samples labeled with different letters (a, b, c) were determined by one-way ANOVA and Tukey’s test, P < 0.05.
GmSIN1 Binds Directly to the SIN1BM in the Promoters of Genes Associated with ABA Synthesis and ROS Generation
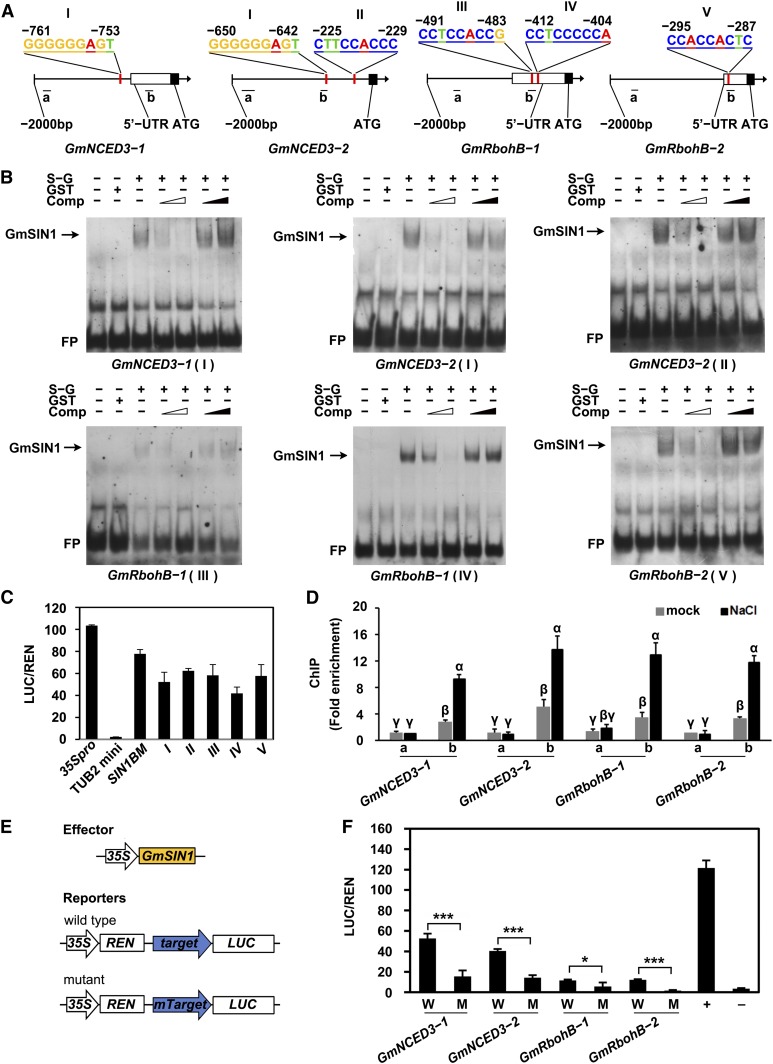
To investigate whether genes involved in ABA synthesis and ROS generation are direct targets of GmSIN1, we looked for candidate SIN1BMs in their promoter regions using FIMO (http://meme-suite.org/tools/fimo). We tested the promoters of GmNCED3-1, GmNCED3-2, GmRbohB-1, and GmRbohB-2 (Supplemental Data Set 5) and found one or two SIN1BMs in each of the promoters of these four genes. For GmRbohB genes, the SIN1BM is located in the 5′ untranslated region (Figure 8A). To examine whether GmSIN1 can bind to these sites in the promoters, we performed electrophoretic mobility shift assays (EMSAs) and LUC assays. The full-length GmSIN1 was fused to glutathione transferase (GST) and used for the EMSA, and DNA probes were biotin-labeled 50-bp promoter fragments of each gene that contains SIN1BM, with the SIN1BM in the center (probe sequences are listed in Supplemental Table 3). GmSIN1 could strongly bind to the SIN1BM in the promoters of GmNCED3-1, GmNCED3-2, GmRbohB-1, and GmRbohB-2, and the binding was efficiently competed off by the unlabeled wild-type probes, but not by unlabeled, SIN1BM-mutated probes (Mutant, Figures 8B), indicating that the binding is specific. The LUC assay used the same method as used in Figure 8B. It revealed that GmSIN1 bound to the SIN1BMs in the promoters of these four genes (Figure 8C).
Figure 8.
GmSIN1 Upregulates the Expression of GmNCED3s and GmRbohBs by Binding to Their Promoters.
(A) Schematic of SIN1BM binding to the promoters of GmNCED3-1, GmNCED3-2, GmRbohB-1, and GmRbohB-2. Red lines show the potential SIN1BM binding site on the promoters, empty boxes indicate the 5′ untranslated region (UTR) of the genes, and black blocks indicate the ATG translation initiation site. Roman numbers indicate the different potential SIN1BMs in the promoters.
(B) EMSA results of GmSIN1 binding with the SIN1BM site in the target gene promoters. (Left to right) Lane 1, free probe (FP; labeled probe with no protein added); lane 2, labeled probe with GST protein as negative control; lane 3, labeled probe with GmSIN1-GST protein; lanes 4 and 5, GmSIN1-GST binding to the labeled probe was competed with 50× or 200× unlabeled wild-type probes (denoted by the empty wedge); lanes 6 and 7, binding was competed with mutant probe sequences (the SIN1BM was mutated; lanes 6 and 7, marked by the filled wedge). The arrows indicate the protein-probe complex. Comp, competitor probe; S-G, GmSIN1-GST fusion protein.
(C) Interaction of GmSIN1 with the potential SIN1BMs in LUC assays. The candidate SIN1BM sequences were fused to a promoter fragment of TUB2 that does not contain a SIN1BM. LUC activity and REN activity was measured (see “Methods”). pTUB2mini:LUC and pGreenII 0800-35S-LUC were used for negative and positive controls, respectively. I to V indicate the potential SIN1BMs shown in (A). Mean and se of LUC activity/REN activity were obtained from more than four independent transformation experiments.
(D) ChIP-qPCR assay showing that GmSIN1 interacts with GmNCED3 and GmRbohB promoters in vivo. Anti-GmSIN1 antibodies were used to precipitate chromatin prepared from 1-week-old Shengdou No. 9 seedlings after 24-h NaCl treatment (150 mM) or mock treatment. The fold enrichment was calculated based on the relative change in anti-GFP samples compared with rabbit serum samples (as mock). The “a” and “b” promoter fragments are indicated in (A). Data are means ± se (n = 3 independent experiments). Significant differences between samples labeled with different Greek letters (α, β, γ) were determined by one-way ANOVA and Tukey’s test, P < 0.05.
(E) Constructs used for transient expression assay. For the constructs, 2-kb fragments of the GmNCED3 and GmRbohB promoters drive LUC expression. Mutant indicates the promoter with the SIN1BM site(s) mutated. The arrow indicates the promoter and the box indicates the coding sequence.
(F) Transient assays for GmNCED3 and GmRbohB expression regulated by GmSIN1. Data are means ± se of four independent biological repeats. Asterisks denote significant differences between the mutated promoter and the wild-type promoter (t test, *P < 0.05, ***P < 0.001), n = 4. +, protoplasts cotransformed with pGreenII-35Spro-LUC and p35Spro:GmSIN1 as positive control. −, protoplasts cotransformed with pGreenII-0800-LUC and p35Spro:GmSIN1 as negative control. M, reporter construct containing a promoter with mutant SIN1BM site; W, reporter construct containing the wild-type target gene promoter driving LUC.
An RT-qPCR–based chromatin immunoprecipitation (ChIP) assay was also deployed to monitor the binding affinity of GmSIN1 to the various gene promoters in 2-week-old Shengdou No. 9 seedlings exposed to either 0 or 150 mM NaCl for 12 h. The quality of GmSIN1 antibody was confirmed by immunoblot in GmSIN1 transgenic Arabidopsis and soybean and the corresponding wild-type plants (Supplemental Figure 2). Fragments of the GmNCED3 and GmRbohB promoters near (b) or far away (a) from SIN1BMs (>1000 bp) were amplified by PCR (Figure 8D). In the ChIP experiment, the “b” fragments were enriched much more than “a” fragments under salt stress, but they were enriched to similar extents under control conditions (Figure 8D), indicating that GmSIN1 can bind these promoters in vivo via the SIN1BM under salinity stress.
To further confirm that GmSIN1 regulates GmNCED3s and GmRbohBs in vivo, we examined whether GmSIN1 can directly regulate the transcription of these genes via the SIN1BMs using a protoplast transient assay system (Figures 8E and 8F). The wild-type and SIN1BM-mutated promoters of these genes were cloned into a reporter vector (pGreen II 0800-LUC) as a transcriptional fusion with the LUC reporter gene, and the effect of GmSIN1 on transcription was assessed by changes in LUC activity (Figure 8E). The reporter constructs or control construct were each cotransfected with the effector construct 35Spro:GmSIN1 into ecotype Columbia-0 (Col-0) protoplasts for a gene transcription activity assay. Overexpression of GmSIN1 in the protoplasts caused a significant increase of LUC expression driven by the wild-type promoters of GmNCED3-1, GmNCED3-2, GmRbohB-1, and GmRbohB-2, relative to the expression driven by the SIN1BM-mutated promoters (Figure 8F). These results suggest that GmSIN1 regulates transcription of these genes through binding to the SIN1BM in their promoters. Thus, GmSIN1 directly targets the key ABA biosynthesis and ROS generation genes GmNCED3-1, GmNCED3-2, GmRbohB-1, and GmRbohB-2 to regulate their expression, thereby modulating the root elongation in response to salt stress in soybean.
DISCUSSION
Finding the balance between environmental stress tolerance and plant growth is an emerging, important research topic (Deng et al., 2017; Xu et al., 2017; Wang et al., 2018). Here, the overexpression of GmSIN1 was shown to promote plant growth, whether or not the environment has high salinity. The underlying mechanism involves a GmSIN1/GmNCED3s/GmRbohBs feed-forward loop that rapidly amplifies the initial stress signal, thereby raising the ABA and ROS contents of the soybean root. This observation not only updates our understanding of the regulatory network involved in the early salinity stress response but also suggests a novel genetic engineering-based strategy to maintain crop productivity under environmentally challenging growth conditions.
The Unique Role of GmSIN1 in Root Elongation and Salt Tolerance in Soybean Differs from Its Homologs in Other Species
NAC transcription factors associate with abiotic stress tolerance (Lu et al., 2012; Mao et al., 2012, 2015; Puranik et al., 2012; Huang et al., 2015; Yang et al., 2015a). However, few NACs simultaneously improve abiotic stress tolerance and plant growth as GmSIN1 does (Figure 1; Table 1). By contrast, some NACs have negative effects on plant growth. For example, ectopic expression of MlNAC5 in Arabidopsis produced dwarfism and late flowering (Yang et al., 2015a). Moreover, ANAC072 overexpression produced shorter plants, fewer flowers, and reduced seed yield (Fujita et al., 2004), and OsNAC6 overexpression caused growth retardation and lower reproductive yields (Nakashima et al., 2007). Finally, heterologous expression of GmNAC11 in hairy roots caused lower root growth (Hao et al., 2011). This suggests that GmSIN1 plays a unique role in promoting salt tolerance as well as growth in soybean, which might make it valuable for crop improvement.
The GmSIN1 gene isolated from Shengdou No. 9 represents a gene locus that differs from its homologs Glyma.12G221500 and Glyma.13G279900 in Williams 82. The genes in Arabidopsis with the closest phylogenetic relationship to GmSIN1 are ANAC019, ANAC055, and ANAC072 (also named RD26). As shown in Supplemental Figure 7A, the products of these six genes shared strong peptide sequence similarity. However, the reported experimental data showed that the function of GmSIN1 might be different from its homologs. For example, ANAC019, ANAC055, and ANAC072 are expressed most in stems (Tran et al., 2004; Jensen et al., 2010) rather than in roots, like GmSIN1 (Figures 2C and 2D). Additionally, their OE transgenic plants were drought tolerant and ABA hypersensitive in root growth and seed germination (Tran et al., 2004; Jensen et al., 2010). By contrast, 35Spro:GmSIN1 transgenic soybeans were salt tolerant and insensitive to ABA in seed germination (Supplemental Figure 13). Glyma.12G221500 (GmNAC004) and its homologs in the legume Caragana intermedia were recently reported to improve lateral root formation and caused insensitivity to ABA in seed germination when overexpressed in transgenic Arabidopsis (Quach et al., 2014; Han et al., 2015). However, improved primary root elongation under normal and salt stress conditions in 35Spro:GmSIN1 transgenic soybeans was not reported (Figure 1). This suggests that GmSIN1 functions differently from its previously reported homologs in other plants including legumes.
A Feed-Forward Pathway Involving GmSIN1, GmNCED3s, and GmRbohBs Functions during the Early Salt Stress Response
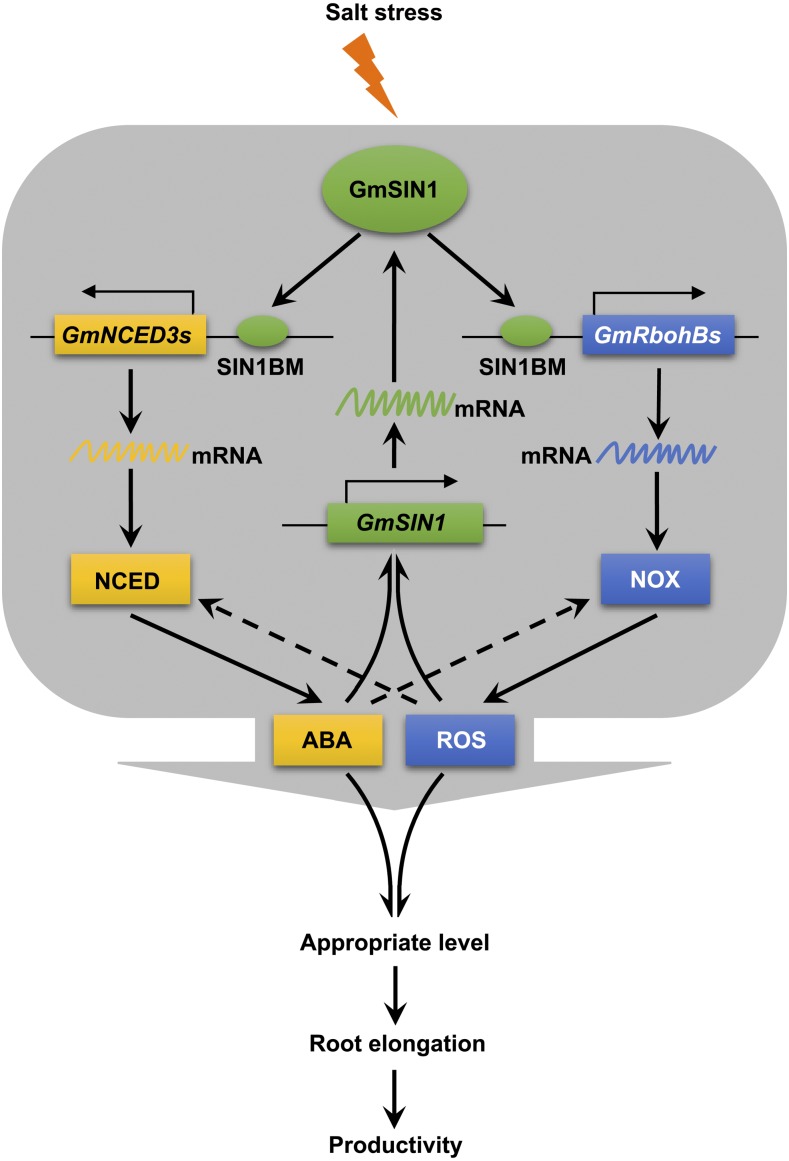
Secondary messengers amplify primary signals, so a fuller understanding of how plants transduce the primary salt stress signal will facilitate efforts to enhance salt tolerance. Two key secondary messengers of salinity stress are ABA and ROS, which induce global changes in gene expression and metabolism (Finkelstein and Gibson, 2002). Despite their large effect, how they rapidly accumulate and coordinate their effects remains unresolved. NCED3 has been implicated as an important contributor to ABA synthesis during episodes of moisture deficit or salt stress (Iuchi et al., 2001; Barrero et al., 2006), while RBOH proteins boost ROS production in response to salt stress (Xie et al., 2011). NCED3 and RBOH are upregulated by salinity stress (Barrero et al., 2006; Xie et al., 2011), although the identity of the TF(s) involved in driving the accumulation of either ABA or ROS in the early salinity response remains unknown. The present experiments have provided evidence that the rapid induction of ABA and ROS in soybean in response to salt stress was mostly dependent on the presence of GmSIN1 (Figure 4) and that GmSIN1 promoted the synthesis of ABA and ROS by binding to the GmNCED3 and GmRbohB promoters and upregulating the expression of these genes (Figure 4 and 8). Moreover, our observations show that GmSIN1 was rapidly induced by salt stress or exogenous ABA or ROS, with the induction requiring both ABA and ROS to be synthesized (Figure 2F, 5F and 5G) and that GmSIN1 mediated part of the early transcriptional response to salt stress in soybean (Figures 3A to 3D). Our major conclusion is that GmSIN1, GmNCED3s, and GmRbohBs work together as a positive feed-forward loop to mediate the rapid accumulation of ABA and ROS, which amplifies the salt stress signal (Figure 9).
Figure 9.
Model of GmSIN1-Modulated Root Elongation under Salt Stress.
Salinity or other abiotic stresses induce the expression of GmSIN1, which promotes the transcription of the ABA biosynthesis gene GmNCED3s and the ROS generation gene GmRbohBs and thus induces the accumulation of ABA and ROS. At the same time, ABA and ROS promote the transcription of GmSIN1. GmSIN1, GmNCED3s, and GmRbohBs work as a positive feedback loop to mediate the rapid accumulation of ABA and ROS in response to salt stress (and maybe other abiotic stresses as well). These factors work interdependently to establish optimal ABA and ROS levels and thus maintain root elongation under salt stress. The arrows and dashed arrows in the gray area indicate direct and indirect regulation, respectively.
ABA and ROS had been thought of as inhibitors of root elongation (Pilet, 1975) . Recently, increasing evidence shows that ABA and ROS also promote root elongation and maintain root growth. For example, root elongation under conditions of low water potential is reduced in ABA-deficient mutants in maize, and elongation can be restored by the addition of ABA (Sharp et al., 2004). Also, ABA biosynthesis and signaling are necessary to promote full growth recovery during salt stress (Geng et al., 2013). The ROS generation genes AtRbohC, AtRbohD, and AtRbohF are required for root hair tip growth (Foreman et al., 2003) and root length in response to ABA (Kwak et al., 2003; Jiao et al., 2013) in Arabidopsis. PvRbohB was reported to promote lateral root elongation in Phaseolus vulgaris (Montiel et al., 2013). These findings suggest that there is an optimal ABA or ROS level for root elongation and that deviating above or below that level reduces the ability of the root to elongate. The existence of this dual threshold has not been directly shown experimentally, and how the thresholds are regulated remains unknown. In this study, we used biosynthesis inhibitors to eliminate endogenous ABA and ROS and supplemented with different concentrations of ABA or ROS to assay the effect of their dosage on root growth. ABA and ROS both have dosage effects on root elongation, and the optimal amounts of ABA and ROS are essential for root growth in soybean (Figure 6).
GmSIN1 also functions in this process, as shown by the following observations. (1) The inhibition of ABA or ROS production can completely rescue the increased root growth phenotype of GmSIN1 OE seedlings (Figures 5D, 5E, 6A and 6B), demonstrating that the effect of GmSIN1 in promoting root growth under both optimal and salt stress conditions was mostly dependent on the ABA and ROS pathway. (2) Overexpression of GmSIN1 altered the sensitivity to ABA and ROS in root elongation (Figures 6A and 6B). This demonstrated that GmSIN1 plays an important role in mediating ABA- and ROS-dependent root elongation. It also suggested that GmSIN1 improved root elongation by promoting ABA and ROS levels and modulating their sensitivity.
The relationship between ABA and ROS in root growth and salinity tolerance is another issue that needs to be clarified. Generally, ROS act downstream of ABA signals in multiple plant species and tissues (Zhang, 2014). However, our results showed that ROS cannot regulate root elongation without ABA (Figure 6B) and the GmSIN1-promoted ROS accumulation required ABA biosynthesis (Figures 6C and 6D), and vice versa; i.e., GmSIN1-promoted ABA accumulation required ROS production (Figures 6A and 6F). This indicates that in root growth in soybean, ABA and ROS have an interdependent relationship, not a simple linear relationship as previously thought.
In the mechanism of ABA-regulated ROS production, RbohF is phosphorylated and activated by OST1 (Sirichandra et al., 2009) and SNF1-related protein kinase 2 (Umezawa et al., 2013), two positive regulators of ABA signaling. Consistent with this, our results showed that the ABA-dependent increase of ROS accumulation in GmSIN1 OE soybean relied on NOX activity rather than transcriptional regulation of GmRbohB genes (Figures 6D and 6E). Notably, we found the increase of ABA level and NCED activity caused by GmSIN1 overexpression was impaired by DPI treatment (Figures 6G and 6H) and this effect occurred downstream of GmNCED3s transcription. This indicated a positive effect of ROS on ABA biosynthesis, which has not been shown in previous studies.
Based on the full set of experimental evidence, we propose a working model to illustrate the mechanism underlying the GmSIN1/GmNCED3/GmRbohB transduction and amplification of the primary salt stress signal, using ABA and ROS as secondary signals. Both ABA and ROS were required to regulate root growth, and an appropriate level of ABA and ROS is optimal for root growth (Figure 9).
GmSIN1 Binds to a cis-Acting Element to Regulate Gene Transcription
Plant genomes encode many NAC TFs, with 226 in soybean (Le et al., 2011) and 106 in Arabidopsis (Riechmann et al., 2000). Identifying their binding motifs will help elucidate the functional specificity of each NAC TF. So far, the direct binding motifs of 25 NACs have been revealed (Supplemental Table 1) and found to be diverse. However, most contain the CGT[GA] sequence (Olsen et al., 2005a; Yabuta et al., 2010; Balazadeh et al., 2011; Yang et al., 2011; Lee et al., 2012; Wu et al., 2012; Zhang and Gan, 2012). These results suggest that CGT[GA] may be the core binding sequence for most NAC proteins, although NAC binding motifs not containing the core sequence have also been identified. The specific DNA binding property of each NAC may contribute to its functional specificity. For example, ANAC016 and ANAC017 have close phylogenetic relationships but diverse functions: ANAC016 regulates drought tolerance and leaf senescence (Kim et al., 2013; Sakuraba et al., 2015), while ANAC017 regulates mitochondrial retrograde signaling (Ng et al., 2013). Their functional differences may be due to their different binding motifs and target genes (Sakuraba et al., 2015).
In this study, we used RNA-seq analysis, the MEME motif discovery program, and mutation analysis to identify SIN1BM, [CA][CT][TAG]CC[AC]CC[AGC] (Figure 7). Notably, SIN1BM does not harbor the CGT[GA] sequence (Figure 7) and differs from the previously identified binding motifs of NAC TFs (Supplemental Table 1). GmSIN1 was further demonstrated to bind to the SIN1BMs in the promoters of GmNCED3s and GmRbohBs and upregulate their transcription (Figure 4A; Figure 8). GmSIN1 has a close phylogenetic relationship and sequence similarity to ANAC019/055/072 (Supplemental Figures 9 and 10), which bind to the core DNA sequence CGT[G/A] (Tran et al., 2004; Jensen et al., 2010). Using EMSA, we found that GmSIN1 could also bind to CGT[GA] as did its homologs in Arabidopsis (Supplemental Figure 14; Tran et al., 2004). This suggested that the binding motif of this specific NAC TF was not unique and the binding motifs of NAC TFs are more diverse than had been previously shown. This divergence likely provides diversity in regulation of their target genes and gives the plant more flexibility in its response to external or internal signals.
Overall, the present study has revealed the relationship between GmSIN1, GmNCED3, and GmRbohB, which constitute a feed-forward loop responsible for the rapid amplification of the salt stress signal, and has shown how GmSIN1 upregulates GmNCED3 and GmRbohB by directly binding to a novel motif in their promoters. Notably, the process was shown to require ABA and ROS, which suggests that the combined manipulation of tissue ABA and ROS contents could represent a viable strategy for improving salt tolerance. GmSIN1 is therefore held to be a promising potential target for genetic intervention aimed at raising the yield of soybean in both non saline and saline environments.
METHODS
Plant Materials and Growth Conditions
The soybean (Glycine max) cv Shengdou No. 9 and cv Wei6823 were used for gene cloning and phenotypic assays, respectively. For germination, soybean seeds were placed on moistened filter paper for 2 d at 20°C. Seedlings of uniform size were transferred to a 16-h photoperiod regime (light/dark temperature, 28°C/20°C) under 800 µmol m−2 s−1 illumination (fluorescent lamp) and a relative humidity of 60% and were grown hydroponically in half-strength Hoagland solution (for RNA preparation assay) or were grown in plastic root growth bags soaked with water (for phenotype assay). In root growth phenotype assays, to minimize position effects, several parallel bags were used for each treatment, and all genotypes were included in each bag in the same number. Among different bags for the same treatment, the materials were placed in different sequences to avoid the position effects. Abiotic stress was applied to soybean seedlings by the addition of 100 or 150 mM NaCl, 100 µM ABA, or 1 mM H2O2 to the hydroponic solution.
To test the agronomic traits of field-grown soybeans, seeds were planted using a randomized complete block design with six repetitions. Each plot included four rows in random arrangement with the wild-type and three OE lines and the six plots were randomly distributed in the field. Every genotype had the same number of seeds planted in every row within the plot. The spacing between rows was 0.5 m, the plot length was 2 m, and the spacing between plants was 5 cm. The seeds were planted at the end of May and harvested at the beginning of October in north China. The soil salinity is defined as the weight of total soluble salt per 100 g of dry soil. The soil from ∼20 cm to the surface in each plot was collected for salinity assays at ∼1 month before sowing. The average salinity was used as the field salinity.
Phylogenetic Analysis
The ClustalW-based alignment of GmSIN1 and other NAC protein polypeptide sequences used a gap open penalty of 10 and a gap extension penalty of 0.2, as implemented within MEGA6 software (Tamura et al., 2013). The resulting phylogenetic tree was derived by the same software and used the neighbor-joining method based on the Jones-Taylor-Thornton amino acid substitution model. The Glyma.01G051300.1 sequence was adopted as the out-group in the phylogenetic analysis, which encodes a NAC family protein. In total, 1000 bootstrap replicates were included to allow for the assigning of confidence levels to each node. The sequences used in phylogenetic analysis are listed in Supplemental Data Set 2.
Generation of Transgenic Soybean Plants
To generate the 35Spro:GmSIN1 construct for overexpression in soybean, the GmSIN1 coding DNA sequence (CDS) was amplified with primers GmSIN1-OE-F and GmSIN1-OE-R, and the PCR product was then ligated into the Gateway pDONR221 vector (Invitrogen) via a BP recombination reaction and then were transferred into the pB2GW7 binary vector (under the control of the 35S promoter; Plant Systems Biology [PSB], Ghent University, Belgium) via an LR recombination reaction. The RNAi construct was designed to target the middle of the GmSIN1 CDS. A 402-bp fragment was amplified using primers GmSIN1-RNAi-F and GmSIN1-RNAi-R and inserted in reverse orientation into the binary vector pB7GWIWG2(II) under the control of the 35S promoter (PSB) to knock down GmSIN1. The binary plasmids were transferred into the Agrobacterium tumefaciens strain GV3101 using the freeze-thaw method. Soybean plants were transformed following the protocol described previously (Cui et al., 2013). DNA gel blotting was performed following the manufacturer’s manual (DIG High Prime DNA Labeling and Detection Starter Kit II, Roche). A DNA fragment from the 35S promoter region present in pB2GW7 and pB7GWIWG2(II) was amplified with primers 35S-F and 35S-R and then labeled as probe. The primer sequences used are listed in Supplemental Table 4.
cDNA Synthesis and RT-qPCR
The cDNA synthesis, RT-qPCR, and data analysis were conducted as described previously by Li et al. (2016). Gene-specific primers (sequences given in Supplemental Table 4) were designed using Beacon Designer v7.90 (http://www.premierbiosoft.com). GmTUB was used as the internal reference gene for profiling across the plant (Song et al., 2014), Gm60S for H2O2 treatments, and GmELF1b for the salinity and ABA treatments (Le et al., 2012). Tissues from three to five seedlings under the same treatment were pooled for RNA extraction as one biological replicate. Three biological replicates from independent experiments were included for each treatment. For qPCR, each sample was amplified in three parallel reactions as technical replicates. The average cycle threshold (Ct) value of three technical replicates was assigned as the final Ct value of each biological replicate. The relative gene expression level was calculated using the 2–∆∆CT method (Livak and Schmittgen, 2001).
Subcellular Localization of GmSIN1 Protein
The GmSIN1 CDS was fused to GFP and inserted into the pART27-GFP plant expression vector (kindly provided by Shuqing Cao, Hefei University of Technology, Hefei, China), containing the GFP coding sequence under the control of the 35S promoter. This generated the p35Spro:GmSIN1-GFP construct. The Arabidopsis protoplast transformation and GFP signal observation were conducted as described previously (Li et al., 2016). The p35Spro:GFP (Li et al., 2011) transgenic protoplasts were used as localization controls for expression in the cytoplasm/nucleus. The pMDC32-1A BES1n-mCherry was used as an indicator of the nucleus (Liang et al., 2015).
Transcriptional Activation Activity Assay
The GmSIN1 CDS in Gateway pDONR221 vector was transferred into the pDEST32 vector (Invitrogen) via an LR recombination reaction, and the transcriptional activation activity assay was performed as described previously (Li et al., 2016).
Transient Transformation of Soybean Leaves
For RNAi constructs, a 252-bp cDNA fragment of GmNCED3-1 (Glyma.15G250100) and a 218-bp fragment of GmRbohB-3 (Glyma.19G233900.1) were PCR amplified using primers provided in Supplemental Table 4 and inserted into the binary vector pB7GWIWG2(II) (under the control of the 35S promoter, PSB). These constructs were introduced into the A. tumefaciens strain GV3101. The strains carrying pB7GWIWG2(II) (as vector control), p35Spro:GmNCED3-RNAi, or p35Spro:GmRbohB-RNAi, together with the strain carrying p35Spro:p19, were co-infiltrated into leaf epidermal cells of 2-week old soybean plants (Shengdou No. 9), following a protocol described previously (Hanano and Goto, 2011). After infiltration for 24 h, the seedlings were subject to 150 mM NaCl treatment or mock treatment for another 48 h. The transformed leaves were used for RNA extraction and RT-qPCR assay. Three transformed leaves from different seedlings were pooled as one biological repeat, and three biological repeats were included for each transformation.
RNA-Seq Assay
Total RNA was extracted from mock-treated and NaCl-treated 2-week-old seedlings (Wei6823 and GmSIN1 OE-1) using the RNeasy Plant Mini kit (Qiagen) according to the manufacturer’s instructions. The total RNA from roots and leaves was isolated separately and combined in an equal mixture. A pool of tissues from at least three seedlings was considered a biological replicate. The mRNA sequencing libraries were constructed with barcodes using the TrueSeq RNA Sample Preparation kit (Illumina). Two biological replicates were sequenced on an Illumina HiSeq 2000 system by BGI-Tech (Shenzhen, China), resulting in 16 to 18 million 49-bp single-end reads per sample. We used BWA (Li and Durbin, 2009) to map clean reads to the soybean genome (Glycine max Wm82.a2.v1 in Phytozome v11.0 database; https://phytozome.jgi.doe.gov/). DEG identification was based on the Noiseq method (Tarazona et al., 2011) with fold change ≥ 2 and diverge probability ≥ 0.7. The RNA-seq data sets used in this study have been deposited at Gene Expression Omnibus under accession number GSE93322 (secure token for reviewers: wpitymcihbcjbqx). Hierarchical clustering was performed using Cluster v3.0 software (Human Genome Center, University of Tokyo) and visualized by Java TreeView v1.1.1 (Saldanha, 2004).
KEGG Pathway and GO Function Enrichment Analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG; Kanehisa et al., 2008), the major public pathway-related database, was used to perform pathway enrichment analysis of DEGs. This analysis identifies significantly enriched metabolic pathways or signal transduction pathways in DEGs compared with the whole-genome background. The calculated P-value was subjected to Bonferroni correction (Abdi, 2007), taking a corrected P-value ≤ 0.05 as a threshold for significance.
GO term enrichment analysis of the gene sets of interest was performed to identify enriched GO terms. The calculation of P-value was conducted by the same method as that in KEGG pathway enrichment analysis. GO terms fulfilling this condition are defined as significantly enriched GO terms in DEGs.
DAB and NBT Staining
The whole seedlings of 6-d-old (for root stain) or 17-d-old (for leaf stain) soybean seedlings were treated with 0 or 100 mM NaCl in half-strength Hoagland solution for 2 h before staining. The treated 6-d-old whole seedlings were stained to see the signals in roots, and the detached leaves from 17-d-old plants were stained directly. For DAB staining, the samples were submerged in the DAB solution (CSB-K09758AB-2, CUSABIO) for 2 h and then in 95% (v/v) ethanol to decolor. For NBT staining, the samples were submerged in NBT staining solution (AR-0632, DINGGUO) until the dark blue color appeared (∼10 min for roots and 3 h for leaves). Next, the samples were cleared of chlorophyll with 95% (v/v) ethanol. The photographs were taken with a stereomicroscope (SZX16, Olympus).
Quantification of ABA Content, H2O2 Content, NOX Activity, and NCED Activity
Tissue ABA contents were measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS-8030 Plus triple quadrupole mass spectrometer, Shimadzu) as follows. In total, 200 mg of liquid nitrogen–frozen fresh sample was ground with a small glass pestle in a 2-mL vial. Following the addition of 1.0 mL of 80% (v/v) methanol, homogenates were well mixed in an ultrasonic bath and then kept overnight at 4°C. After being centrifuged at 15,200g for 10 min at 4°C, the supernatant was collected and then vacuumed to dryness in an RCT-60 concentrator (Jouan). Dried extract was dissolved in 200 μL of sodium phosphate solution (0.1 mol/L, pH 7.8) and then passed through a Sep-Pak C18 cartridge (Waters). The cartridge was eluted with 1500 μL of 80% (v/v) methanol, and the eluate was vacuumed to dryness again. After being dissolved in 50 μL of 10% (v/v) methanol, 5 μL of the solution was injected into the LC-MS/MS system. LC was performed using a 2.1 × 100-mm AcquityUltra Performance Liquid Chromatograph Ethylene Bridged Hybrid column (1.7 μm, Waters) with a column temperature of 40°C. The mobile phase composed of solvent A (0.02% [v/v] aqueous acetic acid) and solvent B (100% [v/v] acetonitrile) was used in a gradient mode (time/A concentration/B concentration [min/%/%] for 0/90/10, 4/30/70, 5/0/100, 6/90/10) at an eluant flow rate of 0.25 mL/min. The system was set to multiple reaction monitoring mode using electrospray ionization in negative ion mode with operation conditions as follows: nebulizing gas flow of 3 L/min, drying gas flow of 15 L/min, desolvation temperature of 250°C, and a heat block temperature of 480°C. Deuterium-labeled ABA (2H6-ABA, Olchemim) was used as an internal standard. For ABA, the ionization conditions pre-bias voltage of 19 V for quadrupole 1 and 28 V for quadrupole 3, collision energy of 10 eV, and mass-to-charge ratio (m/z) of 263/153.2 were used. For 2H6-ABA, the ionization conditions pre-bias voltage of 20 V for quadrupole 1 and 15 V for quadrupole 3, collision energy of 11 eV, and m/z of 269/159.2 were used. The sample ABA contents were calculated according to the calibration curve established using the internal standard of 2H6-ABA.
H2O2 content was determined following the protocol as described by Liu et al. (2014). For the NOX activity assay, a 100-mg sample of fresh tissue was snap frozen in liquid nitrogen and powdered and then treated with an NADPH oxidase assay kit (Nanjing Jiancheng Bioengineering Institute), following the manufacturer’s protocol.
The NCED activity was measured with the Plant NCED ELISA kit (USCN Life Science) according to the manufacturer’s manual.
Tissues from several seedlings (more than three) under the same treatment were pooled as one biological replicate. Three or more biological replicates were included for each assay. For roots, the 1-cm root tips were collected. For leaves, the fully expanded true leaves were collected.
MEME Analysis
For MEME analysis, sequences corresponding to the promoter region (1500 to 0 from ATG codon) of the upregulated genes (Supplemental Data Set 6) were obtained from Phytozome 11 (https://phytozome.jgi.doe.gov/pz/portal.html). Upstream DNA sequences were submitted to the MEME program and analyzed using the previously described parameters (http://meme.nbcr.net/meme/cgi-bin/meme.cgi; Bailey and Elkan, 1994).
Transient Assays of Gene Transcription
To study whether GmSIN1 can bind to specific motifs or promoters to regulate target gene expression, the pGreen II 0800-LUC vector system (Hellens et al., 2005) was used and the promoters were cloned into the vector to generate reporter constructs, respectively. The primers used to clone the promoters or to generate the mutation are listed in Supplemental Table 4. Each reporter construct, together with either 35Spro:GmSIN1 construct, was cotransformed into Arabidopsis (Col-0) protoplasts using the same method as described in “Subcellular Localization of GmSIN1 Protein” for transcription activity assay. The signals of Firefly and Renilla LUC were assayed using the Dual-Luciferase Reporter Assay System (Promega). A transformation of ∼2 × 104 protoplasts in one tube was considered one biological replicate. For qPCR, each sample was tested in three parallel reactions for LUC activity as technical replicates. The average relative LUC activity value of three technical replicates was assigned as the final value of each biological replicate. Four or more biological repeats were conducted to obtain the final results.
Expression of Recombinant GmSIN1 Protein and EMSA
Full-length GmSIN1 protein fused with GST was expressed in the vector pGEX-T in the Escherichia coli strain BL21. The protein extraction and EMSA were conducted as described previously (Yu et al., 2016). The oligonucleotide probe sequences are listed in Supplemental Table 4.
ChIP-qPCR Assays
The synthetic peptides of GmSIN1 (CGGGHVGTSVPQK) were injected into rabbits to generate the corresponding polyclonal antibodies by MW BIOTECH (HK) LIMITED. The specificities of anti-GmSIN1 polyclonal antibodies were determined by immunoblot analysis using the wild-type soybeans, 35Spro:GmSIN1 transgenic Arabidopsis, and wild-type Arabidopsis (Col-0; Supplemental Figure 2). ChIP was conducted as described previously (Yu et al., 2016). The plants of 2-week-old Shengdou No. 9 seedlings were treated with 150 mM NaCl or H2O as mock-treated control for 12 h. Briefly, 3 g of whole seedlings was fixed by immersion in 1% (v/v) formaldehyde and then the chromatin was sheared by sonication to a size range of ∼100 to 1000 bp. After centrifugation at 13,000 rpm for 10 min at 4°C, the complex was immunoprecipitated with a GmSIN1-specific antibody at dilution 1:600 or rabbit serum protein (as mock sample). Primers for promoters of candidate GmSIN1 target genes or negative control genes were used to detect the corresponding promoters in the ChIP products and the primers used in this study are listed in Supplemental Table 4. The enrichment percentages were calculated based on the relative change in anti-GmSIN1 compared with input samples. The mock samples did not generate enough PCR product to detect. The mean and sd are shown for three independent ChIP experiments, and the significance of differences between means was assessed with one-way analysis of variance (ANOVA) and Tukey’s test. Each immunoprecipitation sample with a pool of several seedlings is considered one biological replicate. Three biological repeats were conducted.
Statistical Methods
For comparisons between two sample groups, the Student’s t test was applied. For multiple comparison, significance analysis was performed with one-way ANOVA followed by post hoc tests. Tukey’s test or Dunnett’s t test were used in the post hoc tests. Two-way ANOVA was conducted to test the interaction between two factors. The statistical analysis was performed using IBM SPSS Statistics software version 25 (IBM). Details of statistical results are in Supplemental Data Set 1.
Accession Numbers
Sequence data from this article can be found in the Phytozome or GenBank databases under the following accession numbers: GmSIN1 (KY661969), GmRbohB-1 (Glyma.10G152200), GmRbohB-2 (Glyma.20G236200), GmNCED3-1 (Glyma.15G250100), GmNCED3-2 (Glyma.08G176300), GmTUB (Glyma.08G014200), Gm60S (Glyma.13G318800); GmELF1b (Glyma.02G276600), AtTUB2 (AT5G62690).
The RNA-seq data sets used in this study have been deposited at the Gene Expression Omnibus under accession number GSE93322.
Supplemental Data
Supplemental Figure 1. Identification of GmSIN1 overexpression and RNAi transgenic soybeans.
Supplemental Figure 2. Confirmation of the presence of GmSIN1 protein in soybean plants overexpressing GmSIN1.
Supplemental Figure 3. Fixation of the transgene in three soybean lines overexpressing GmSIN1.
Supplemental Figure 4. Normal root growth in transgenic soybean harboring an empty vector.
Supplemental Figure 5. The effect of GmSIN1 overexpression on salt tolerance.
Supplemental Figure 6. Yield of GmSIN1 OE and the wild-type plants grown in fields with different salinity.
Supplemental Figure 7. GmSIN1 and its homologs in Arabidopsis.
Supplemental Figure 8. Subcellular localization of GmSIN1.
Supplemental Figure 9. Expression GmSIN1 and selected ABA- or ROS- responsive genes.
Supplemental Figure 10. GmRbohB-1 and GmNCED3-1 overexpression in transiently transformed soybean leaves.
Supplemental Figure 11. FLU inhibits root growth.
Supplemental Figure 12. The effect of ABA and H2O2 on root elongation in Arabidopsis and soybean and germination in soybean.
Supplemental Figure 13. Germination of 35Spro:GmSIN1 transgenic soybeans.
Supplemental Figure 14. GmSIN1 binds to CGT[AG] sites in the GmRbohB-1 promoter.
Supplemental Table 1. NAC proteins and their binding motifs.
Supplemental Table 2. The design of the RNA-Seq experiment.
Supplemental Table 3. Probe sequences.
Supplemental Table 4. Primer sequences.
Supplemental Data Set 1. Statistical results.
Supplemental Data Set 2. Polypeptide sequence of GmSIN1 and its homologs in soybean and A. thaliana.
Supplemental Data Set 3. Differentially transcribed genes derived from the RNA-Seq experiment.
Supplemental Data Set 4. The GmSIN1 binding motif predicted by MEME analysis.
Supplemental Data Set 5. Promoter sequences of genes related to ABA synthesis or ROS generation.
Supplemental Data Set 6. Promoter sequences used for MEME analysis.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
This research was financially supported by the National Key Research and Development Program of China (grant 2016YFD0101902), National Transgenic Project of China (grants 2018ZX08009-14B and 2016ZX08010002-002), the Major Program of Shandong Province Natural Science Foundation (grant ZR2018ZC0334), National Natural Science Foundation of China (grants 31770317, 31471515, 31201269, and 30970243), the National Special Science Research Program of China (grant 2013CB967300), and the National High Technology Research and Development Program “863” (grant 2013AA102602-4).
AUTHOR CONTRIBUTIONS
S.L. and F.X. supervised and designed the experiments; N.W., D.J., and W.Z. performed most of the experiments; S.L., Y.W., Y.Y., M.L., S.Z., J.Y., W.Z., J.T., L.X., Y.Z., L.W., X.W., and Z.L. performed part of the experiments; M.B. analyzed part of the data; S.L. analyzed the data and wrote the article; and F.X. supervised and assisted with the writing.
Footnotes
Articles can be viewed without a subscription.
References
- Abdi H. (2007). The Bonferroni and Sidák corrections for multiple comparisons. In Encyclopedia of Measurement and Statistics, Salkind N.J., ed (Thousand Oaks, CA: Sage; ). [Google Scholar]
- Almagro L., Gómez Ros L.V., Belchi-Navarro S., Bru R., Ros Barceló A., Pedreño M.A. (2009). Class III peroxidases in plant defence reactions. J. Exp. Bot. 60: 377–390. [DOI] [PubMed] [Google Scholar]
- Bailey T.L., Elkan C. (1994). Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2: 28–36. [PubMed] [Google Scholar]
- Balazadeh S., Kwasniewski M., Caldana C., Mehrnia M., Zanor M.I., Xue G.P., Mueller-Roeber B. (2011). ORS1, an H2O2-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana. Mol. Plant 4: 346–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero J.M., Rodríguez P.L., Quesada V., Piqueras P., Ponce M.R., Micol J.L. (2006). Both abscisic acid (ABA)-dependent and ABA-independent pathways govern the induction of NCED3, AAO3 and ABA1 in response to salt stress. Plant Cell Environ. 29: 2000–2008. [DOI] [PubMed] [Google Scholar]
- Chen M., Wang Q.Y., Cheng X.G., Xu Z.S., Li L.C., Ye X.G., Xia L.Q., Ma Y.Z. (2007). GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 353: 299–305. [DOI] [PubMed] [Google Scholar]
- Creelman R.A., Bell E., Mullet J.E. (1992). Involvement of a lipoxygenase-like enzyme in abscisic acid biosynthesis. Plant Physiol. 99: 1258–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Barampuram S., Stacey M.G., Hancock C.N., Findley S., Mathieu M., Zhang Z., Parrott W.A., Stacey G. (2013). Tnt1 retrotransposon mutagenesis: A tool for soybean functional genomics. Plant Physiol. 161: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq I., et al. (2013). The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 25: 3472–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., et al. (2017). Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 355: 962–965. [DOI] [PubMed] [Google Scholar]
- Fan W., Xu J.M., Wu P., Yang Z.X., Lou H.Q., Chen W.W., Jin J.F., Zheng S.J., Yang J.L. (2019). Alleviation by abscisic acid of Al toxicity in rice bean is not associated with citrate efflux but depends on ABI5-mediated signal transduction pathways. J. Integr. Plant Biol. 61: 140–154. [DOI] [PubMed] [Google Scholar]
- Finkelstein R.R., Gibson S.I. (2002). ABA and sugar interactions regulating development: Cross-talk or voices in a crowd? Curr. Opin. Plant Biol. 5: 26–32. [DOI] [PubMed] [Google Scholar]
- Foreman J., Demidchik V., Bothwell J.H., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D., Davies J.M., Dolan L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446. [DOI] [PubMed] [Google Scholar]
- Fujita M., Fujita Y., Maruyama K., Seki M., Hiratsu K., Ohme-Takagi M., Tran L.S., Yamaguchi-Shinozaki K., Shinozaki K. (2004). A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 39: 863–876. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Fujita M., Satoh R., Maruyama K., Parvez M.M., Seki M., Hiratsu K., Ohme-Takagi M., Shinozaki K., Yamaguchi-Shinozaki K. (2005). AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17: 3470–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furihata T., Maruyama K., Fujita Y., Umezawa T., Yoshida R., Shinozaki K., Yamaguchi-Shinozaki K. (2006). Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl. Acad. Sci. USA 103: 1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Wu R., Wee C.W., Xie F., Wei X., Chan P.M., Tham C., Duan L., Dinneny J.R. (2013). A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 25: 2132–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R., et al. (2014). Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 80: 937–950. [DOI] [PubMed] [Google Scholar]
- Han X., Feng Z., Xing D., Yang Q., Wang R., Qi L., Li G. (2015). Two NAC transcription factors from Caragana intermedia altered salt tolerance of the transgenic Arabidopsis. BMC Plant Biol. 15: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano S., Goto K. (2011). Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 23: 3172–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y.J., et al. (2011). Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 68: 302–313. [DOI] [PubMed] [Google Scholar]
- He J., Duan Y., Hua D., Fan G., Wang L., Liu Y., Chen Z., Han L., Qu L.J., Gong Z. (2012). DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 24: 1815–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Wang Y., Li B., Chang J., Chen M., Li K., Yang G., He G. (2015). TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 15: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Kobayashi M., Taji T., Naramoto M., Seki M., Kato T., Tabata S., Kakubari Y., Yamaguchi-Shinozaki K., Shinozaki K. (2001). Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 27: 325–333. [DOI] [PubMed] [Google Scholar]
- Jannat R., Uraji M., Morofuji M., Islam M.M., Bloom R.E., Nakamura Y., McClung C.R., Schroeder J.I., Mori I.C., Murata Y. (2011). Roles of intracellular hydrogen peroxide accumulation in abscisic acid signaling in Arabidopsis guard cells. J. Plant Physiol. 168: 1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.K., Kjaersgaard T., Nielsen M.M., Galberg P., Petersen K., O’Shea C., Skriver K. (2010). The Arabidopsis thaliana NAC transcription factor family: Structure-function relationships and determinants of ANAC019 stress signalling. Biochem. J. 426: 183–196. [DOI] [PubMed] [Google Scholar]
- Jeong J.S., Kim Y.S., Baek K.H., Jung H., Ha S.H., Do Choi Y., Kim M., Reuzeau C., Kim J.K. (2010). Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 153: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D.D., Liu C., Cao Q.C., Xiang F.N. (2011). Comparison of the salt tolerance at sprout and seedling in different soybean. Journal of Shandong Polytechnic University 25: 4–7. [Google Scholar]
- Jiang M., Zhang J. (2002). Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 53: 2401–2410. [DOI] [PubMed] [Google Scholar]
- Jiao Y., Sun L., Song Y., Wang L., Liu L., Zhang L., Liu B., Li N., Miao C., Hao F. (2013). AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. J. Exp. Bot. 64: 4183–4192. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., Katayama T., Kawashima S., Okuda S., Tokimatsu T., Yamanishi Y. (2008). KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36 (Database): D480–D484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S., Sakuraba Y., Han S.H., Yoo S.C., Paek N.C. (2013). Mutation of the Arabidopsis NAC016 transcription factor delays leaf senescence. Plant Cell Physiol. 54: 1660–1672. [DOI] [PubMed] [Google Scholar]
- Kurusu T., Kuchitsu K., Tada Y. (2015). Plant signaling networks involving Ca(2+) and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 6: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.M., Mori I.C., Pei Z.M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D., Schroeder J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D.T., Nishiyama R., Watanabe Y., Mochida K., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.S. (2011). Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 18: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D.T., Aldrich D.L., Valliyodan B., Watanabe Y., Ha C.V., Nishiyama R., Guttikonda S.K., Quach T.N., Gutierrez-Gonzalez J.J., Tran L.S., Nguyen H.T. (2012). Evaluation of candidate reference genes for normalization of quantitative RT-PCR in soybean tissues under various abiotic stress conditions. PLoS One 7: e46487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Seo P.J., Lee H.J., Park C.M. (2012). A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 70: 831–844. [DOI] [PubMed] [Google Scholar]
- Lefebvre V., North H., Frey A., Sotta B., Seo M., Okamoto M., Nambara E., Marion-Poll A. (2006). Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 45: 309–319. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang N., Ji D., Xue Z., Yu Y., Jiang Y., Liu J., Liu Z., Xiang F. (2016). Evolutionary and functional analysis of membrane-bound NAC transcription factor genes in soybean. Plant Physiol. 172: 1804–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang X., Yang Y., Li R., He Q., Fang X., Luu D.T., Maurel C., Lin J. (2011). Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 23: 3780–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Li H., Zhou F., Li H., Liu J., Hao Y., Wang Y., Zhao H., Han S. (2015). Subcellular distribution of NTL transcription factors in Arabidopsis thaliana. Traffic 16: 1062–1074. [DOI] [PubMed] [Google Scholar]
- Lin F., Ding H., Wang J., Zhang H., Zhang A., Zhang Y., Tan M., Dong W., Jiang M. (2009). Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. J. Exp. Bot. 60: 3221–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Liu S., Wang M., Wei T., Meng C., Wang M., Xia G. (2014). A wheat SIMILAR TO RCD-ONE gene enhances seedling growth and abiotic stress resistance by modulating redox homeostasis and maintaining genomic integrity. Plant Cell 26: 164–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lu M., Ying S., Zhang D.F., Shi Y.S., Song Y.C., Wang T.Y., Li Y. (2012). A maize stress-responsive NAC transcription factor, ZmSNAC1, confers enhanced tolerance to dehydration in transgenic Arabidopsis. Plant Cell Rep. 31: 1701–1711. [DOI] [PubMed] [Google Scholar]
- Mao H., Wang H., Liu S., Li Z., Yang X., Yan J., Li J., Tran L.S., Qin F. (2015). A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 6: 8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Zhang H., Qian X., Li A., Zhao G., Jing R. (2012). TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J. Exp. Bot. 63: 2933–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D., Dunand C., Puppo A., Pauly N. (2012). A burst of plant NADPH oxidases. Trends Plant Sci. 17: 9–15. [DOI] [PubMed] [Google Scholar]
- Mazel A., Leshem Y., Tiwari B.S., Levine A. (2004). Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol. 134: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J.H., Chung J.S., Lee K.H., Kim C.S. (2015). The CONSTANS-like 4 transcription factor, AtCOL4, positively regulates abiotic stress tolerance through an abscisic acid-dependent manner in Arabidopsis. J. Integr. Plant Biol. 57: 313–324. [DOI] [PubMed] [Google Scholar]
- Mittler R. (2017). ROS are good. Trends Plant Sci. 22: 11–19. [DOI] [PubMed] [Google Scholar]
- Mittler R., Blumwald E. (2015). The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel J., Arthikala M.K., Quinto C. (2013). Phaseolus vulgaris RbohB functions in lateral root development. Plant Signal. Behav. 8: e22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R., Tester M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59: 651–681. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Tran L.S., Van Nguyen D., Fujita M., Maruyama K., Todaka D., Ito Y., Hayashi N., Shinozaki K., Yamaguchi-Shinozaki K. (2007). Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 51: 617–630. [DOI] [PubMed] [Google Scholar]
- Nambara E., Marion-Poll A. (2005). Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 56: 165–185. [DOI] [PubMed] [Google Scholar]
- Ng S., et al. (2013). A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 25: 3450–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A.N., Ernst H.A., Leggio L.L., Skriver K. (2005a). DNA-binding specificity and molecular functions of NAC transcription factors. Plant Sci. 169: 785–797. [Google Scholar]
- Olsen A.N., Ernst H.A., Leggio L.L., Skriver K. (2005b). NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 10: 79–87. [DOI] [PubMed] [Google Scholar]
- Papiernik S.K., Grieve C.M., Lesch S.M., Yates S.R. (2005). Effects of salinity, imazethapyr, and chlorimuron application on soybean growth and yield. Commun. Soil Sci. Plant Anal. 36: 951–967. [Google Scholar]
- Perea-Resa C., Carrasco-López C., Catalá R., Turečková V., Novak O., Zhang W., Sieburth L., Jiménez-Gómez J.M., Salinas J. (2016). The LSM1-7 complex differentially regulates Arabidopsis tolerance to abiotic stress conditions by promoting selective mRNA decapping. Plant Cell 28: 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet P.E. (1975). Abscisic acid as a root growth inhibitor: Physiological analyses. Planta 122: 299–302. [DOI] [PubMed] [Google Scholar]
- Puranik S., Sahu P.P., Srivastava P.S., Prasad M. (2012). NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 17: 369–381. [DOI] [PubMed] [Google Scholar]
- Qi X., et al. (2014). Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing. Nat. Commun. 5: 4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Song C.P., Wang B., Zhou J., Kangasjärvi J., Zhu J.K., Gong Z. (2018). Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 60: 805–826. [DOI] [PubMed] [Google Scholar]
- Qin C., Cheng L., Shen J., Zhang Y., Cao H., Lu D., Shen C. (2016). Genome-wide identification and expression analysis of the 14-3-3 family genes in Medicago truncatula. Front. Plant Sci. 7: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach T.N., Tran L.S., Valliyodan B., Nguyen H.T., Kumar R., Neelakandan A.K., Guttikonda S.K., Sharp R.E., Nguyen H.T. (2014). Functional analysis of water stress-responsive soybean GmNAC003 and GmNAC004 transcription factors in lateral root development in Arabidopsis. PLoS One 9: e84886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann J.L., et al. (2000). Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 290: 2105–2110. [DOI] [PubMed] [Google Scholar]
- Ruggiero B., Koiwa H., Manabe Y., Quist T.M., Inan G., Saccardo F., Joly R.J., Hasegawa P.M., Bressan R.A., Maggio A. (2004). Uncoupling the effects of abscisic acid on plant growth and water relations. Analysis of sto1/nced3, an abscisic acid-deficient but salt stress-tolerant mutant in Arabidopsis. Plant Physiol. 136: 3134–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y., Kim Y.S., Han S.H., Lee B.D., Paek N.C. (2015). The Arabidopsis transcription factor NAC016 promotes drought stress responses by repressing AREB1 transcription through a trifurcate feed-forward regulatory loop involving NAP. Plant Cell 27: 1771–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha A.J. (2004). Java Treeview--extensible visualization of microarray data. Bioinformatics 20: 3246–3248. [DOI] [PubMed] [Google Scholar]
- Sato H., Takasaki H., Takahashi F., Suzuki T., Iuchi S., Mitsuda N., Ohme-Takagi M., Ikeda M., Seo M., Yamaguchi-Shinozaki K., Shinozaki K. (2018). Arabidopsis thaliana NGATHA1 transcription factor induces ABA biosynthesis by activating NCED3 gene during dehydration stress. Proc. Natl. Acad. Sci. USA 115: E11178–E11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid S.A., Zaman M., Heng L. (2018). Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques., Zaman M., Shahid S.A., and Heng L., eds (Cham, Switzerland: Springer; ), pp. 43–53. [Google Scholar]
- Sharp R.E., Poroyko V., Hejlek L.G., Spollen W.G., Springer G.K., Bohnert H.J., Nguyen H.T. (2004). Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 55: 2343–2351. [DOI] [PubMed] [Google Scholar]
- Shi W.Y., Du Y.T., Ma J., Min D.H., Jin L.G., Chen J., Chen M., Zhou Y.B., Ma Y.Z., Xu Z.S., Zhang X.H. (2018). The WRKY transcription factor GmWRKY12 confers drought and salt tolerance in soybean. Int. J. Mol. Sci. 19: E4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y.J., Song L.L., Zhang J., Liu Y., Guo C.H. (2015). Genome-wide identification and characterization of the Dof gene family in Medicago truncatula. Genet. Mol. Res. 14: 10645–10657. [DOI] [PubMed] [Google Scholar]
- Sirichandra C., Gu D., Hu H.C., Davanture M., Lee S., Djaoui M., Valot B., Zivy M., Leung J., Merlot S., Kwak J.M. (2009). Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583: 2982–2986. [DOI] [PubMed] [Google Scholar]
- Song H., Yin Z., Chao M., Ning L., Zhang D., Yu D. (2014). Functional properties and expression quantitative trait loci for phosphate transporter GmPT1 in soybean. Plant Cell Environ. 37: 462–472. [DOI] [PubMed] [Google Scholar]
- Song Y., Ji D., Li S., Wang P., Li Q., Xiang F. (2012). The dynamic changes of DNA methylation and histone modifications of salt responsive transcription factor genes in soybean. PLoS One 7: e41274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B.C., Joseph L.M., Deng W.T., Liu L., Li Q.B., Cline K., McCarty D.R. (2003). Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 35: 44–56. [DOI] [PubMed] [Google Scholar]
- Tarazona S., García-Alcalde F., Dopazo J., Ferrer A., Conesa A. (2011). Differential expression in RNA-seq: a matter of depth. Genome Res. 21: 2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L.S., Nakashima K., Sakuma Y., Simpson S.D., Fujita Y., Maruyama K., Fujita M., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. (2004). Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16: 2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T., Sugiyama N., Takahashi F., Anderson J.C., Ishihama Y., Peck S.C., Shinozaki K. (2013). Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci. Signal. 6: rs8. [DOI] [PubMed] [Google Scholar]
- Verslues P.E., Agarwal M., Katiyar-Agarwal S., Zhu J., Zhu J.K. (2006). Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 45: 523–539. [DOI] [PubMed] [Google Scholar]
- Wang F., et al. (2015a). GmWRKY27 interacts with GmMYB174 to reduce expression of GmNAC29 for stress tolerance in soybean plants. Plant J. 83: 224–236. [DOI] [PubMed] [Google Scholar]
- Wang J., et al. (2018). A single transcription factor promotes both yield and immunity in rice. Science 361: 1026–1028. [DOI] [PubMed] [Google Scholar]
- Wang P., Song C.P. (2008). Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 178: 703–718. [DOI] [PubMed] [Google Scholar]
- Wang N., Zhao S.Z., Lv M.H., Xiang F.N., Li S. (2016). Research progress on identification of QTLs and functional genes involved in salt tolerance in soybean. Yi Chuan 38: 992–1003. [DOI] [PubMed] [Google Scholar]
- Wang T., Tohge T., Ivakov A., Mueller-Roeber B., Fernie A.R., Mutwil M., Schippers J.H., Persson S. (2015b). Salt-related MYB1 coordinates abscisic acid biosynthesis and signaling during salt stress in Arabidopsis. Plant Physiol. 169: 1027–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Huang J., Hao Y.J., Zou H.F., Wang H.W., Zhao J.Y., Liu X.Y., Zhang W.K., Ma B., Zhang J.S., Chen S.Y. (2009). Soybean GmPHD-type transcription regulators improve stress tolerance in transgenic Arabidopsis plants. PLoS One 4: e7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., et al. (2012). JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24: 482–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Gaffney B., Hunt A.G., Li Q.Q. (2014). Genome-wide determination of poly(A) sites in Medicago truncatula: Evolutionary conservation of alternative poly(A) site choice. BMC Genomics 15: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.J., Xu S., Han B., Wu M.Z., Yuan X.X., Han Y., Gu Q., Xu D.K., Yang Q., Shen W.B. (2011). Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant J. 66: 280–292. [DOI] [PubMed] [Google Scholar]
- Xu G., Yuan M., Ai C., Liu L., Zhuang E., Karapetyan S., Wang S., Dong X. (2017). uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature 545: 491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta Y., Morishita T., Kojima Y., Maruta T., Nishizawa-Yokoi A., Shigeoka S. (2010). Identification of recognition sequence of ANAC078 protein by the cyclic amplification and selection of targets technique. Plant Signal. Behav. 5: 695–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Guo Y. (2018). Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 60: 796–804. [DOI] [PubMed] [Google Scholar]
- Yang L., Zhang J., He J., Qin Y., Hua D., Duan Y., Chen Z., Gong Z. (2014). ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet. 10: e1004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.D., Seo P.J., Yoon H.K., Park C.M. (2011). The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell 23: 2155–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wang X., Ji L., Yi Z., Fu C., Ran J., Hu R., Zhou G. (2015a). Overexpression of a Miscanthus lutarioriparius NAC gene MlNAC5 confers enhanced drought and cold tolerance in Arabidopsis. Plant Cell Rep. 34: 943–958. [DOI] [PubMed] [Google Scholar]
- Yang Y., Sun T., Xu L., Pi E., Wang S., Wang H., Shen C. (2015b). Genome-wide identification of CAMTA gene family members in Medicago truncatula and their expression during root nodule symbiosis and hormone treatments. Front. Plant Sci. 6: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Liu Z., Wang L., Kim S.G., Seo P.J., Qiao M., Wang N., Li S., Cao X., Park C.M., Xiang F. (2016). WRKY71 accelerates flowering via the direct activation of FLOWERING LOCUS T and LEAFY in Arabidopsis thaliana. Plant J. 85: 96–106. [DOI] [PubMed] [Google Scholar]
- Zhai Y., Wang Y., Li Y., Lei T., Yan F., Su L., Li X., Zhao Y., Sun X., Li J., Wang Q. (2013). Isolation and molecular characterization of GmERF7, a soybean ethylene-response factor that increases salt stress tolerance in tobacco. Gene 513: 174–183. [DOI] [PubMed] [Google Scholar]
- Zhang D.P. (2014). Abscisic Acid: Metabolism, Transport and Signaling. (Dordrecht, The Netherlands: Springer; ). [Google Scholar]
- Zhang W., et al. (2019). A cation diffusion facilitator, GmCDF1, negatively regulates salt tolerance in soybean. PLoS Genet. 15: e1007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Gan S.S. (2012). An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol. 158: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Bousquet A., Harris J.M. (2014). Abscisic acid and lateral root organ defective/NUMEROUS INFECTIONS AND POLYPHENOLICS modulate root elongation via reactive oxygen species in Medicago truncatula. Plant Physiol. 166: 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Chen M., Li L., Xu Z., Chen X., Guo J., Ma Y. (2009). Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 60: 3781–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang L., Dong F., Gao J., Galbraith D.W., Song C.P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126: 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]