Abstract
Many animal taxa live in groups to increase foraging and reproductive success and aid in predator avoidance. For fish, a large proportion of species spend all or part of their lives in groups, with group coordination playing an important role in the emergent benefits of group-living. Group cohesion can be altered by an array of factors, including exposure to toxic environmental contaminants. Oil spills are one of the most serious forms of pollution in aquatic systems, and while a range of effects of acute oil exposure on animal physiology have been demonstrated, sub-lethal effects on animal behavior are relatively under-studied. Here we used an open-field behavioral assay to explore influence of acute oil exposure on social behavior in a gregarious fish native to the Gulf of Mexico, Atlantic croaker (Micropogonias undulatus). We used two oil concentrations (0.7% and 2% oil dilution, or 6.0 ± 0.9 and 32.9 ± 5.9 μg l−1 ΣPAH50 respectively) and assays were performed when all members of a group were exposed, when only one member was exposed, and when no individuals were exposed. Shoal cohesion, as assessed via mean neighbor distance, showed significant impairment following acute exposure to 2% oil. Fish in oil-exposed groups also showed reduced voluntary movement speed. Importantly, overall group cohesion was disrupted when even one fish within a shoal was exposed to 2% oil, and the behavior of unexposed in mixed groups, in terms of movement speed and proximity to the arena wall, was affected by the presence of these exposed fish. These results demonstrate that oil exposure can have adverse effects on fish behavior that may lead to reduced ecological success.
Subject terms: Environmental impact, Marine biology
Introduction
Animals living in groups often must coordinate movements with group mates in space or time, with varying degrees of synchronicity1,2. This type of social behavior is particularly important, as group living may be beneficial for predator avoidance, foraging, and reproductive success3,4. This is especially true for fish, in which a large range of species spend some or all of their life within a group-living scenario5,6. Additionally, group movements allow individuals to reduce the energetic costs of swimming by taking advantage of vortices produced by groupmates7–9. In a shoal of fish, the series of individual decisions that result in cohesive collective behaviors are influenced by a range of abiotic and biotic factors, including food availability, water flow rate7, turbidity10, predator abundance11, and a suite of social cues12. In addition, social behaviors in fish are affected by various forms of anthropogenic environmental disturbance, including human-induced hypoxic episodes13,14, ocean acidification15, and anthropogenic noise16. Environmental contaminants such as industrial pollutants and heavy metals can also adversely impact fish social behavior, such as schooling and courtship17,18. This is particularly relevant as group decision making, social learning, and group responses to potential threats19,20 are all dependent on the behaviors of individual group members, with key individuals often influencing the behavior of an entire group21,22.
One of the most serious forms of anthropogenic disturbances is pollution from the release of petroleum products into aquatic environments23,24. Uncontrolled oil spills are the most well known form of crude oil pollution, which was epitomized by the Deepwater Horizon spill that released over 700 million L of crude oil into the Gulf of Mexico over a period of 84 days25,26. However, oil is also routinely released into aquatic habitats via shipping, terrestrial runoff, and dumping. Oil toxicity is driven by a class of chemicals known as polycyclic aromatic hydrocarbons (PAHs), and marine fish are particularly susceptible to these chemicals. Embryonic exposure can result in craniofacial and cardiac deformities that have been linked to mortality at very low concentrations (μg/L)27–31. In juvenile and adult life stages, acute oil exposure impairs cardiac performance32–36, which leads to reductions in swimming performance, maximum metabolic rate and aerobic scope37–40. Similarly, acute oil exposure can also cause changes in routine metabolism41,42. Importantly, the sub-lethal effects of an acute exposure event lasting only 24 hours can persist in the animal for multiple weeks39,40, which raises concern about the long-term ecological performance of exposed individuals.
Despite well-known physiological effects of oil pollution, there is surprisingly little known about the negative impacts of oil exposure on fish social behaviors. Behavioral characteristics are crucial when extending organismal toxicology to ecologically relevant population-level effects, as described in the adverse outcomes pathway framework43. Sociability, or an animal’s tendency to interact with conspecifics44, plays an important role in shoal behaviors. Shoals comprised of more social individuals have higher shoal cohesion, though exhibit a reduction in average swim speed and social alignment45. Further, individuals with lower sociability are more likely to swim faster and act as a leader within a school, with these individuals effectively initiating group movements45. Sociability is in turn influenced by individual metabolism with less social animals having higher standard metabolic rates46. Therefore, the previous mentioned negative impacts of oil exposure on metabolism37–40 and cardiac performance32–34, could imply a direct pathway for oil exposure to influence shoal cohesion. This was highlighted recently in a study of coral reef fish following acute oil exposure, whereby exposed individuals showed a suite of behavioral changes – habitat usage, thigmotaxis, and basic aspects of shoaling behavior – that significantly increased predation rates47. Further, sensory abilities, such as vision48,49, olfaction50, and input from the lateral line51, influence shoal cohesion, grouping choices, and coordinated movements. Any change in sociability, sensory ability, or locomotor capacity in any or all group members due to oil exposure could therefore disrupt overall group function with important ecological consequences.
To this end, the current study examined the effects of environmentally relevant levels of crude oil exposure on social behavior and group cohesion in Atlantic croaker (Micropogonias undulatus). This gregarious fish species is prevalent in the Gulf of Mexico and depends on estuarine environments, which can be particularly impacted by oil pollution. Specifically, we aimed to answer the following questions: 1) under acute oil exposure is shoal cohesion, or individual exploratory behaviors, different in an open-field when compared to non-exposed groups? and, 2) if a single individual is acutely exposed is the cohesion of the shoal, or the exploratory behaviors of other individuals altered?
Results
Oil chemistry analysis
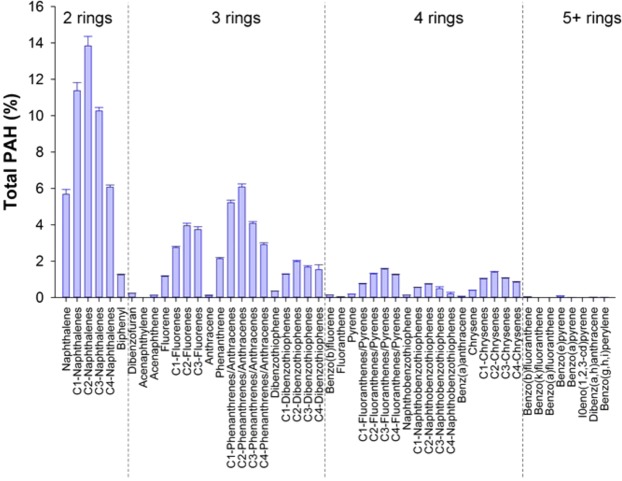
HEWAFS from both the low and high concentrations were analyzed for 50 individual PAHs. As expected, for both HEWAFs, 2 ring PAHs were highest in abundance (47% and 53% in the low and high concentrations respectively) followed by 3 ring PAHs (41% and 36%), with the remaining being 4 and 5 ring PAHs (Fig. 1). Using initial and final concentrations, the average geometric mean (±SEM) for the low concentration was 6.0 ± 0.9 μg l−1 ΣPAH50 and for the high concentration was 32.9 ± 5.9 μg l−1 ΣPAH50.
Figure 1.
Relative composition of polycyclic aromatic hydrocarbons in the low and high concentration HEWAFs. 50 individual PAHs were measured and are shown on the X-axis. Dashed lines denote subclasses of PAHs.
Effects of oil on group behavior
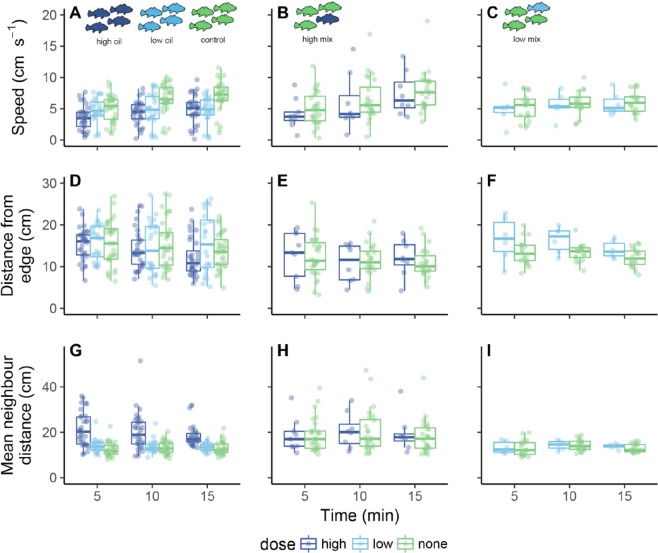
While fish tended to increase their movement speed over the course of each 15 min trial, individuals in HO groups showed decreased speed of movement as compared to fish in all other treatments (Fig. 2a). Fish in HM groups were closer to the arena wall when compared to all other treatment groups (Table 1). Fish in HO and HM groups showed increased mean neighbor distances (Fig. 2g; Table 1). Within a given treatment there was generally large among-group variation for all behavioral indices, and overall, values for model R2C was higher than R2M (Tables 1 and S1).
Figure 2.
Responses to high (32.9 ± 5.9 μg l−1 ΣPAH50) or low (6.0 ± 0.8 μg l−1 ΣPAH50) oil exposure in juvenile Atlantic croaker in groups of four individuals swimming within an open field (n = 15 groups in total). Groups were composed of either control fish (that were not oil exposed; green); fish exposed to a high concentration of oil (dark blue); fish exposed to a low concentration of oil (light blue); three fish not exposed to oil plus one fish exposed to a high concentration of oil; or three fish not exposed to oil plus one fish exposed to a low concentration of oil. Each data point overlaid on the boxplots represents one fish within a group. Refer to Supplemental Tables 1, 2 and 3 for statistical comparisons among treatments.
Table 1.
Results of a linear mixed effects models examining the factors influencing behavior in groups of fish receiving various levels of oil exposure.
| Estimate | SEM | df | t | p | R2m | R2c | |
|---|---|---|---|---|---|---|---|
| Speed (cm s −1 ) | |||||||
| intercept | 1.65 | 0.999 | 146.54 | 1.653 | 0.101 | 0.2 | 0.832 |
| mass | 0.133 | 0.04 | 141.97 | 3.302 | 0.001 | ||
| time | 0.153 | 0.013 | 289.13 | 11.947 | <0.0001 | ||
| treatment | |||||||
| high oil | −1.719 | 0.556 | 141.73 | −3.093 | 0.002 | ||
| low oil | −1.115 | 0.606 | 141.74 | −1.841 | 0.068 | ||
| high mix | 0.078 | 0.539 | 143.59 | 0.145 | 0.885 | ||
| low mix | −0.397 | 0.599 | 141.73 | −0.663 | 0.508 | ||
| Distance to arena edge (cm) | |||||||
| intercept | 16.319 | 1.899 | 148.7 | 8.592 | <0.0001 | 0.085 | 0.721 |
| mass | 0.011 | 0.076 | 141.51 | 0.144 | 0.886 | ||
| time | −0.167 | 0.03 | 289.57 | −5.482 | <0.0001 | ||
| treatment | |||||||
| high oil | −1.041 | 1.053 | 141.19 | −0.989 | 0.325 | ||
| low oil | 0.461 | 1.147 | 141.19 | 0.402 | 0.688 | ||
| high mix | −3.14 | 1.023 | 143.71 | −3.069 | 0.003 | ||
| low mix | −1.242 | 1.133 | 141.18 | −1.096 | 0.275 | ||
| Mean distance to neighbors (cm) | |||||||
| intercept | 1.078 | 0.048 | 151.7 | 22.453 | <0.0001 | 0.283 | 0.78 |
| mass | 0.001 | 0.002 | 141.6 | 0.634 | 0.527 | ||
| time | −0.001 | 0.001 | 290.5 | −0.617 | 0.538 | ||
| treatment | |||||||
| high oil | 0.019 | 0.026 | 141.2 | 7.052 | <0.0001 | ||
| low oil | 0.035 | 0.003 | 141.2 | 1.218 | 0.225 | ||
| high mix | 0.152 | 0.026 | 144.3 | 5.908 | <0.0001 | ||
| low mix | 0.027 | 0.029 | 141.2 | 0.952 | 0.343 | ||
For the fixed effect of “treatment”, control groups, which received no oil exposure, are the reference level. Models included individual nested within group as a random effect.
Effects of oil-exposed individuals on non-exposed individuals within the same group
Within LM groups, treated individuals tended to be further from the arena walls than untreated fish (Fig. 2f; Tables S2 and S3). While there were no differences in distance from the wall between treated and untreated individuals within the HM groups, this is likely because even untreated fish within high mixed groups were closer to the arena walls than fish in control groups (Table S2). Untreated fish in HM groups showed increased mean neighbor distances compared to fish in control groups comprised entirely of unexposed fish (Fig. 2h; Tables S2 and S3).
Discussion
The results here suggest that environmentally relevant oil exposure scenarios cause decreased cohesion in fish social groups. At the highest level of exposure, groups comprised of oil-exposed fish had increased distances between neighboring fish within groups. This effect was also observed in groups that contained a single high oil-exposed fish in a shoal of four, whereby untreated fish within the same group showed disruptions to normal behavior and greater distances between neighbors. Overall, these results indicate that exposure to oil pollution in aquatic environments has the potential to negatively affect fish social behaviors and group functioning.
The data presented here clearly demonstrate that an acute oil exposure of 32.9 ± 5.9 µg l−1 ΣPAH50 results in a less cohesive shoal and alters overall group behavior. While acute oil exposure has a well-documented suite of sub-lethal effects on fishes, the severity of sub-lethal endpoints on overall ecological performance can be difficult to ascertain. The concept of ecological death is useful in this context, as it draws an equivalency between immediate mortality and any toxicological impairment that reduces the ability to perform in the environment and produce offspring17. Shoaling for fish is a particularly noteworthy behavior in this regard as it has been shown to have positive effects in the context of foraging and predator avoidance52. For example, during predator attacks group cohesiveness allows groupmates to react to danger even when they themselves did not observe the attack19, and coordinated movements of prey fish during predator-prey interactions can serve to confuse predators. Any reductions in shoal cohesion and coordination will preclude individuals from experiencing the benefits of group living, and therefore put the individuals at greater risk.
The overall reduced speed of movement among groups of oil exposed fish could also reduce foraging ranges and the likelihood of encountering food while exploring a given environment53. Fish in swimming schools can also position themselves relative to groupmates such that they can reduce their own costs of movement by taking advantage of the vortices produced by other fish within the school8,9,54. Individual fish also prefer specific spatial positions within schools (e.g. front versus back, edge versus center) in relation to their own physiological and behavioral traits55,56, possibly contributing to the establishment of leader-follower dynamics and formation of characteristic social networks57. Reduced group cohesion may impact the tendency of fish to occupy their preferred or optimal positions within moving groups; however, further work is needed to examine the extent to which oil exposure may impair the ability of individual fish to occupy their preferred spatial position within social groups.
Individual speed of movement is also a determinant of leadership during directed group behaviors, with individuals being attracted to more active conspecifics and those that display directional, linear movements16,58,59. Reduced spontaneous activity therefore suggests that leadership capacity could be compromised in oil-exposed fish. Interestingly, however, exposed and unexposed fish in the HM groups all displayed decreased distances from the arena wall as compared to fish in other treatment groups. Although their ability to lead may be diminished, it is possible that exposed fish in HM groups were nonetheless altering the behavior of unexposed groupmates in an emergent manner that is not possible when all fish in a group are oil-exposed. A tendency to remain closer to the wall in an open field test is generally interpreted as a reduction in boldness or risk-taking behavior60 and it is possible that abnormal behavior in oil exposed fish elicited increased timidity in unexposed groupmates. This is the opposite effect to previous observations in larval red drum and coral reef species, both of which demonstrated an decrease in anxiety-like behavior individually61 and in groups47. Clearly, additional work is needed to investigate interactions between leader-follower dynamics and resultant levels of risk experienced by groupmates among fish with varying levels of oil exposure within the same social group.
It is notable that the negative effects on shoal cohesion occurred when even a single individual within the group was exposed to oil. It is important to remember that oil spills are heterogeneous events, and that the physiological effects of acute 24 h exposures have been shown to persist over prolonged time scales39,40. As such, it is plausible that in the wild, fish with varying levels of oil exposure will interact as they migrate or otherwise move within their environment. The results here demonstrate that in smaller shoals, the presence of a minority of exposed individuals can disrupt the behavior of the entire group. In other contexts, it has been observed that the presence of key individuals within a group can have a beneficial effect on group function21. For example, social learning can allow naïve groupmates to more quickly gain knowledge of foraging patches or danger from more informed individuals20,58. The ecological relevance of the influence of oil-exposed fish on the unexposed groupmates warrants further study, but this appears to be an example whereby one individual has a disproportionate effect on the behavior of the entire group. It has previously been speculated that individuals with particular behavioral or physiological traits may act as “keystone” individuals that have a disproportionate effect on the behavior or success of entire groups21,57,62. The current results also suggest that individual sensitivity or exposure to pollutants, and other adverse environmental conditions, could induce similar effects on social groups stemming from disrupted behavior in a minority of individuals.
In the current study, groups were relatively small, and so the particular behavioral tendencies of one individual would likely have a large influence on overall group behavior, even in groups of fish receiving equal oil exposures (and including the control groups). It is notable that for mean neighbor distance, groups of fish receiving high oil exposures (for all fish or one fish within the group) showed greatly increased among-group variation compared to all other group types. Additional research is required to understand how the effects observed in this study scale up to larger group sizes, and whether larger groups have reduced among-group variation in the behavior they display. In addition, fish social groups often display among-group assortment based on various morphological and possibly physiological characteristics14,63. An interesting area for future work would be to determine how groups of fish with varying phenotypic composition may show differential group-level responses to oil exposure.
The underlying cause for the oil induced behavioral changes is not immediately clear. The established paradigm for oil toxicity in fish is that sensitivity is driven by cardiac malformations caused by 3 ringed PAHs64,65. It is possible that the observed changes stem from altered metabolic characteristics, as a recent study demonstrated that similar oil exposure scenarios significantly reduced maximum metabolic rate and aerobic scope in this species66. Prior work has demonstrated that individuals with a higher metabolic rate can be less social, presumably because they prioritize food acquisition over the safety of being in a group14; however, such observations have not been extended to aerobic scope. It seems more likely that the mechanism relates to neurological or sensory impairment, both of which have recently been shown to be impacted in yolk-sac larval fish during developmental exposure67,68. This hypothesis was previously posited to explain the changes in anti-predator behavior observed in coral reef fish species47, and the reduced prevalence of thigmotaxis (i.e. anxiety behavior) in larval red drum61. Such developmental effects could impact the ability to detect and respond to shoal-inducing sensory stimuli, or the tendency to generate such stimuli.
In our experimental protocol, we ran control groups first during each experimental day to eliminate risk of oil contamination between trials. It is therefore conceivable that temporal variation in groups or trial order may have contributed to the observed differences among treatments. Several lines of evidence, however, suggest that if such effects occurred they are small relative to the direct effects of oil exposure. Firstly, the direction of temporal effects on a given behavioral measure (e.g. movement speed, which increased with time in the control group) was opposite the effects of the oil exposure treatments. This suggests that even if there were temporal effects on behavior it would have only served to reduce the magnitude of the observed effects from oil exposure. Furthermore, the HO and LO groups were run at the same time of day and yet still displayed differences in behavior. Individuals in LO groups behaved more similarly to control groups than those in the HO exposed groups, indicating that changes observed in the HO treatment were the result of the exposure level and not time of day or trial order. Additionally, the total time for all trials to be completed was relatively short (e.g. within 2 hours), reducing the potential of a temporal or hunger effect on behavior. It’s also important to note that in the HM groups, the unexposed individuals behaved differently than the oil-exposed fish within their group, again indicating that exposure level and not time of day or trial order was responsible for the observed changes in behavior.
In summary, this study demonstrates that oil exposure alters social cohesion in sub-adult Atlantic croaker. Indeed, exposure of a single individual can alter the behavior of the group. Importantly, the exposure scenarios used here are comparable to ΣPAH50 concentrations found in the Gulf of Mexico following the Deepwater Horizon spill69. Social behaviors are key to foraging, predator-avoidance, migration, and reproduction in most fish species, and so reduction in shoal cohesion is likely to have a range of adverse effects on these aspects of species’ ecology. Although additional work is required to more fully ascertain the mechanisms by which oil exposure alters behavior, the data presented here provide added support for the recently described phenomenon of oil induced behavioral impairments in fish47, while also highlighting novel effects of ecological significance. Future examinations of individual variation in sensitivity to oil exposure in a social context would provide further understanding of the selective effects of oil pollution on fish populations, and the potential implications for evolutionary trajectories70,71. Within-generation effects of pollution on behavioral plasticity, and indeed any anthropogenic stressor, are likely to have consequences for important ecological phenomena such as fish migrations, spawning aggregations, and survival during the juvenile stages, in which fish frequently use shoaling as an anti-predator strategy.
Methods
Oil preparation
To test the effects of oil exposure, oil was prepared following previously described standard protocols for high-energy water accommodated fractions (HEWAF)28,30. Briefly, non-weathered oil collected from the source of a Massachusetts pipeline, an appropriate surrogate for Deepwater Horizon source oil, was loaded with seawater (35ppt) at a rate of 1 g per l. Oil was blended in a heavy-duty blender (Waring Commercial, Connecticut, USA) at a low setting for 30 s, and then placed into a Teflon 1 L sieve funnel for 60 minutes. The lower 85% of the WAF was removed and used to generate oil exposures. All HEWAFs were prepared fresh for exposures. Oil was delivered under proper chain of custody and was stored at 4 °C until for roughly a year before used.
Fish and exposure
To determine the effect of oil exposure on social behaviors we obtained 200 juvenile Atlantic croaker (9.8–15.1 cm standard length) from a commercial supplier. Fish were acquired in groups of 50 but were randomly split into smaller groups of 25 for acclimation. During acclimation, fish were held in 76 cm × 99 cm tanks, filled to 350L, at 24 ± 1 °C, and maintained with sterilized seawater (35 ppt) for two weeks before experiments began. Holding tanks were large enough to maintain water quality for groups of 25 or less. To maintain fish at conditions that were consistent to their native environment in the Gulf of Mexico, filtered seawater was piped directly from the Gulf into the lab.
Twenty-four h prior to exposure, 12 fish were randomly selected from each group, anaesthetized with 250 mg l−1 of MS222 (buffered with 500 mg l−1 NaHCO3), weighed, measured for total and standard length, and fitted with either a yellow, orange, green or purple 7 × 6 mm plastic bead for identification purposes. The 12 fish were divided into groups of four, which would be used for the behavioral trials. Selection for groups was random though there was an effort to group together fish with a similar body mass (±9.0 g). Fish were then allowed to recover (observed for return to equilibrium) for one h in an aerated opaque tank and returned to the original holding tank where they were food-deprived for 24 h prior to oil exposure. The following day the tagged fish were moved to individual aerated, 6.14 L tanks (24 × 16 × 16 cm), which were filled with fresh seawater, and exposed to one of three nominal concentrations of HEWAF (0, 0.7 and 2%) for 24 h. Concentrations were chosen based on levels recorded shortly after the Deepwater Horizon spill in 201069,72, and have been used previously on this species66.
The behaviors of the following group compositions were tested: control (N = 9), low-oil exposure (LO; N = 7), high-oil exposure (HO; N = 8), low-mixed exposure (LM; N = 7) and high-mixed exposure (LM; N = 9). The mixed groups contained three control fish and one exposed fish of the respective oil dose. Each individual within the group had a unique colored bead, to allow for individual tracking and identification. A group of four fish was used to allow for the tracking of individuals within the group while also allowing all fish to move freely within the arena without constrained movement.
During the oil exposure protocol, standard water quality parameters were monitored, and seawater samples were taken for PAH analysis at the beginning and end of three exposures for each oil dose. Samples were taken throughout the course of the study. PAH analysis was performed commercially by ALS environmental under extraction protocol EPA 3510 C and measurement protocol 8270D SIM. Samples were spiked with fluorine-d10, fluoranthene-d10 and terphenyl-d14 to assess extraction efficiency, with general recovery of >80%, >90% and >90%, respectively. Detection limits ranged from 4.5–20.5 ng l−1 depending on the specific PAH. All samples were stored at 4 °C and delivered within one week of collection under proper chain of custody.
Behavioral assays
To determine if exposure to oil influenced the exploration or group interactions of Atlantic croaker, the activity of groups of four fish were monitored in open-field tests. The arena for the open field consisted of a circular solid plastic tank with a diameter of 91.5 cm and filled with 7 cm of fresh UV sterilized seawater from the same source used to fill holding tanks. To visually contrast between the fish and the background, a white vinyl covering was used to line the arena. The fish were all caught with a dip net from the exposure tank, released into a 3 L container, where they were rinsed with fresh seawater, and transferred as a group to an opaque cylindrical holding arena (30 cm diameter), located within the center of the arena. After 10 minutes of acclimation the container was lifted, and fish were allowed to swim freely for 15 min, filmed from above by stationary GoPro Hero 4 (GoPro, California, USA) at 30 frames per second. To avoid contamination, the control group was always released into the arena first, followed by the mixed group and the oil only group last. At the end of each day the arena was drained, rinsed and allowed to dry before being refilled one h before the next assay began. All open field assays were completed within two h between 9 AM and 12 PM, with no more than 3 groups assessed per day. Owing to logistical constraints, water changes were not done between groups. Importantly, however, we believe that the effects of any olfactory cues accumulating across trials are minimal or non-existent. Firstly, the total time between the first and last trial done each day was relatively short (less than two h), with times that fish were in the arena being equal to only 60 min maximum before the last trial. Secondly, fish were fasted and so no feces were left in the tanks after testing that could potentially affect fish behavior. Additionally, there was no difference in the behavior of the untreated fish in the control and LM groups, indicating that even if there were residual cues, chemical signals, and scents left behind by the previous group, it was not enough to affect the fish in the latter groups. Finally, there were several days in which only two trials were performed, with either a mixed or oil treatment group being performed either first or second during the day. Among these groups, there was no effect of trial order on the behavior of fish in any treatment (linear mixed effect models, p > 0.50 for all behaviors in all cases).
Videos were analyzed using Ethovison (Version 10; Noldus, Wageningen, Netherlands), which was able to track fish based on the unique colored beads. The following variables were quantified for each fish within each shoal: (1) average speed; (2) average distance from the arena wall; and (3) average mean distance between the focal fish and all other fish within the shoal. All variables were measured continuously throughout each video but then aggregated using mean values within 5 min time bins throughout the trial (see Data Analysis, below).
This research was approved by the Institutional Animal Care and Use Committee of the university at which the research took place (reference number AUP-2015-00147) and followed to the ASAB/ABS Guidelines for the Use of Animals in Research. Of the 200 fish obtained for experimental purposes only two did not survive post anesthesia, neither of these fish were of the groups exposed to oil.
Data analysis
All analyses were conducted using R v. 3.4.0 (R Development Core Team 2017) using the function lmer in package lme473 for linear mixed effect models, MuMIn 1.9.13 for determining model effect sizes (marginal and conditional R2)74; (http://CRAN.R-project.org/package=MuMIn). All plots were created using the package ggplot275. An initial set of linear mixed effects models (LMEs) were fitted using restricted maximum likelihood estimation, with a separate model using each of speed, distance from arena wall, and mean neighbor distance as the response variable. The models also included fish body mass and group exposure treatment (control, HO, HM, LO, LM) as categorical fixed effects. To account for any shifts in behavior over the course of each trial, videos were split into 3 different bins (5 min each) so that time over the course of the study could be included as a fixed effect. Individual nested within group was included as a random effect. To compare behaviors between exposed and unexposed fish within the mixed groups, and to compare unexposed fish within mixed groups to the control fish, a second set of LMEs were constructed with a given behavioral index as the response variable, fish body mass as a continuous fixed effect, and individual treatment (control, low exposed, low unexposed, high exposed, high unexposed) as a categorical fixed effect, and individual nested with group as a random effect. For all models, interactions between treatment and time were included, but dropped when not significant and the models re-run. Model assumptions were verified by visual examination of residual-fit plots. Significance testing (α = 0.05) was employed to provide some indication of the strength of evidence for observed patterns, along with model R2 values. This included marginal R2 (R2M) and conditional R2 (R2C) which indicate the variance explained by fixed factors and by both fixed and random factors, respectively76.
Supplementary information
Acknowledgements
This research was made possible by a grant from The Gulf of Mexico Research Initiative awarded to AJE, Grant No: SA-1520; Name: Relationship of Effects of Cardiac Outcomes in fish for Validation of Ecological Risk (RECOVER). SSK was supported by NERC Advanced Fellowship NE/J019100/1 and European Research Council Starting Grant no. 640004. Data are publicly available through the Gulf of Mexico Research Initiative Information & Data Cooperative (GRIIDC) at https://data.gulfresearchinitiative.org (DOI: 10.7266/N77D2SNH and 10.7266/N73N21WQ).
Author Contributions
T.A., A.J.K., S.S.K. and A.J.E. contributed to experimental design. T.A. and A.J.K. conducted experiments. T.A., A.J.K., S.S.K. and H.F. analyzed data. S.S.K., T.A., and A.J.K. wrote the main portion of the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tiffany Armstrong and Alexis J. Khursigara contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49994-1.
References
- 1.Chapman BB, Ward AJW, Krause J. Schooling and learning: early social environment predicts social learning ability in the guppy, Poecilia reticulata. Animal Behaviour. 2008;76:923–929. doi: 10.1016/j.anbehav.2008.03.022. [DOI] [Google Scholar]
- 2.Wark Abigail R., Greenwood Anna K., Taylor Elspeth M., Yoshida Kohta, Peichel Catherine L. Heritable Differences in Schooling Behavior among Threespine Stickleback Populations Revealed by a Novel Assay. PLoS ONE. 2011;6(3):e18316. doi: 10.1371/journal.pone.0018316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause, J. & Ruxton, G. D. Living in groups. Living in groups (2002).
- 4.Ward, A. & Webster, M. Sociality: The Behaviour of Group-Living Animals (2016).
- 5.Brown C, Irving E. Individual personality traits influence group exploration in a feral guppy population. Behavioral Ecology. 2014;25:95–101. doi: 10.1093/beheco/art090. [DOI] [Google Scholar]
- 6.Song Z, Boenke MC, Rodd FH. Interpopulation Differences in Shoaling Behaviour in Guppies (Poecilia reticulata): Roles of Social Environment and Population Origin. Ethology. 2011;117:1009–1018. doi: 10.1111/j.1439-0310.2011.01952.x. [DOI] [Google Scholar]
- 7.Killen SS, Marras S, Steffensen JF, Mckenzie DJ. Aerobic capacity influences the spatial position of individuals within fish schools. Proceedings of the Royal Society B: Biological Sciences. 2012;279:357–364. doi: 10.1098/rspb.2011.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marras S, et al. Fish swimming in schools save energy regardless of their spatial position. Behavioral Ecology and Sociobiology. 2015;69:219–226. doi: 10.1007/s00265-014-1834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weihs D. Hydromechanics of Fish Schooling. Nature. 1973;241:290–291. doi: 10.1038/241290a0. [DOI] [Google Scholar]
- 10.Kimbell HS, Morrell LJ. Turbidity in fluences individual and group level responses to predation in guppies, Poecilia reticulata. Animal Behaviour. 2015;103:179–185. doi: 10.1016/j.anbehav.2015.02.027. [DOI] [Google Scholar]
- 11.Ryan MR, Killen SS, Gregory RS, Snelgrove PVR. Predators and distance between habitat patches modify gap crossing behaviour of juvenile Atlantic cod (Gadus morhua, L. 1758) Journal of Experimental Marine Biology and Ecology. 2012;422–423:81–87. doi: 10.1016/j.jembe.2012.04.017. [DOI] [Google Scholar]
- 12.Tien JH, Levin SA, Rubenstein DI. Dynamics of fish schools: identifying key decision rules. Evolutionary Ecology Research. 2004;6:555–565. [Google Scholar]
- 13.Domenici Paolo, Steffensen John F., Marras Stefano. The effect of hypoxia on fish schooling. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1727):20160236. doi: 10.1098/rstb.2016.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Killen, S. S., Marras, S., Nadler, L., Domenici, P. & Killen, S. S. The role of physiological traits in assortment among and within fish shoals. Philosophical Transactions of the Royal Society B: Biological Sciences372 (2017). [DOI] [PMC free article] [PubMed]
- 15.Nadler LE, Killen SS, Mccormick MI, Watson S-A, Munday PL. Effect of elevated carbon dioxide on shoal familiarity and metabolism in a coral reef fish. Conservation. Physiology. 2016;4:1–13. doi: 10.1093/conphys/cow052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbert-Read, J. E., Kremer, L., Bruintjes, R., Radford, A. N. & Ioannou, C. C. Anthropogenic noise pollution from pile-driving disrupts the structure and dynamics of fish shoals. Proceedings of the Royal Society B284 (2017). [DOI] [PMC free article] [PubMed]
- 17.Scott GR, Sloman KA. The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquatic Toxicology. 2004;68:369–392. doi: 10.1016/j.aquatox.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Ward AJW, Duff AJ, Horsfall JS, Currie S. Scents and scents-ability: pollution disrupts chemical social recognition and shoaling in fish. Proceedings of the Royal Society B. 2008;274:101–105. doi: 10.1098/rspb.2007.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marras Stefano, Domenici Paolo. Schooling Fish Under Attack Are Not All Equal: Some Lead, Others Follow. PLoS ONE. 2013;8(6):e65784. doi: 10.1371/journal.pone.0065784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reebs SG. Can a minority of informed leaders determine the foraging movements of a fish shoal? Animal Behaviour. 2000;59:403–409. doi: 10.1006/anbe.1999.1314. [DOI] [PubMed] [Google Scholar]
- 21.Modlmeier AP, Keiser CN, Watters JV, Sih A, Pruitt JN. The keystone individual concept: an ecological and evolutionary overview. Animal Behaviour. 2014;89:53–62. doi: 10.1016/j.anbehav.2013.12.020. [DOI] [Google Scholar]
- 22.Keiser, C. N. & Pruitt, J. N. Personality composition is more important than group size in determining collective foraging behaviour in the wild. Proceedings of the Royal Society B281 (2014). [DOI] [PMC free article] [PubMed]
- 23.Douben, P. E. T. PAHs: An Ecotoxicological (2003).
- 24.Readman JW, et al. Petroleum and PAH contamination of the Black Sea. Marine Pollution Bulletin. 2002;44:48–62. doi: 10.1016/S0025-326X(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 25.Crone TJ, Tolstoy M. Magnitude of the 2010 Gulf of Mexico Oil Leak. Science. 2010;330:634. doi: 10.1126/science.1195840. [DOI] [PubMed] [Google Scholar]
- 26.McNutt MK, et al. Review of flow rate estimates of the Deepwater Horizon oil spill. Proc Natl Acad Sci USA. 2012;109:20260–20267. doi: 10.1073/pnas.1112139108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmunds RC, et al. Corresponding morphological and molecular indicators of crude oil toxicity to the developing hearts of mahi mahi. Scientific Reports. 2015;5:17326. doi: 10.1038/srep17326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esbaugh AJ, et al. The effects of weathering and chemical dispersion on Deepwater Horizon crude oil toxicity to mahi-mahi (Coryphaena hippurus) early life stages. Science of the Total Environment. 2016;543:644–651. doi: 10.1016/j.scitotenv.2015.11.068. [DOI] [PubMed] [Google Scholar]
- 29.Khursigara AJ, Perrichon P, Martinez Bautista N, Burggren WW, Esbaugh AJ. Cardiac function and survival are affected by crude oil in larval red drum, Sciaenops ocellatus. Sci Total Environ. 2017;579:797–804. doi: 10.1016/j.scitotenv.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Incardona JP, et al. Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proceedings of the National Academy of Sciences. 2014;111:E1510–E1518. doi: 10.1073/pnas.1320950111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stieglitz JD, et al. A novel system for embryo-larval toxicity testing of pelagic fish: Applications for impact assessment of Deepwater Horizon crude oil. Chemosphere. 2016;162:261–268. doi: 10.1016/j.chemosphere.2016.07.069. [DOI] [PubMed] [Google Scholar]
- 32.Brette F, et al. Excitation-Contraction Coupling in Fish. Science. 2014;1681:772–776. doi: 10.1126/science.1242747. [DOI] [PubMed] [Google Scholar]
- 33.Brette, F. et al. A Novel Cardiotoxic Mechanism for a Pervasive Global Pollutant. Scientific Reports, 1–9, 10.1038/srep41476 (2017). [DOI] [PMC free article] [PubMed]
- 34.Nelson D, et al. Effects of crude oil on in situ cardiac function in young adult mahi–mahi (Coryphaena hippurus) Aquatic Toxicology. 2016;180:274–281. doi: 10.1016/j.aquatox.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Nelson D, et al. Cardio-respiratory function during exercise in the cobia, Rachycentron canadum: The impact of crude oil exposure. Comparative Biochemistry and Physiology Part - C: Toxicology and Pharmacology. 2017;201:58–65. doi: 10.1016/j.cbpc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Cox GK, et al. Oil Exposure Impairs in Situ Cardiac Function in Response to β-Adrenergic Stimulation in Cobia (Rachycentron canadum) Environmental Science and Technology. 2017;51:14390–14396. doi: 10.1021/acs.est.7b03820. [DOI] [PubMed] [Google Scholar]
- 37.Claireaux G, et al. Influence of oil exposure on the physiology and ecology of the common sole Solea solea: Experimental and field approaches. Aquat. Living Resour. 2004;17:335–351. doi: 10.1051/alr:2004043. [DOI] [Google Scholar]
- 38.Claireaux G, et al. Effects of oil exposure and dispersant use upon environmental adaptation performance and fitness in the European sea bass, Dicentrarchus labrax. Aquat Toxicol. 2013;130–131:160–170. doi: 10.1016/j.aquatox.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Johansen JL, Esbaugh AJ. Sustained impairment of respiratory function and swim performance following acute oil exposure in a coastal marine fish. Aquatic Toxicology. 2017;187:82–89. doi: 10.1016/j.aquatox.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Mager EM, et al. Acute embryonic or juvenile exposure to Deepwater Horizon crude oil impairs the swimming performance of mahi-mahi (Coryphaena hippurus) Environ Sci Technol. 2014;48:7053–7061. doi: 10.1021/es501628k. [DOI] [PubMed] [Google Scholar]
- 41.Davison W, Franklin CE, Mckenzie JC, Dougan MCR. The effects of acute exposure to the water soluble fraction of diesel fuel oil on survival and metabolic rate in antartic fish (Pagothenia Borchgrevinki) Comparative Biochemistry and Physiology. 1992;102:185–188. doi: 10.1016/0300-9629(92)90032-L. [DOI] [Google Scholar]
- 42.Klinger DH, et al. Exposure to Deepwater Horizon weathered crude oil increases routine metabolic demand in chub mackerel, Scomber japonicus. Marine Pollution Bulletin. 2015;98:259–266. doi: 10.1016/j.marpolbul.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 43.Ankley GT, et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 44.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biological Reviews. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- 45.Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. Consistent Individual Differences Drive Collective Behavior and Group Functioning of Schooling Fish. Current Biology. 2017;27:2862–2868.e2867. doi: 10.1016/j.cub.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Killen SS, et al. The relationship between metabolic rate and sociability is altered by food deprivation. Functional Ecology. 2016;30:1358–1365. doi: 10.1111/1365-2435.12634. [DOI] [Google Scholar]
- 47.Johansen Jacob L., Allan Bridie J. M., Rummer Jodie L., Esbaugh Andrew J. Oil exposure disrupts early life-history stages of coral reef fishes via behavioural impairments. Nature Ecology & Evolution. 2017;1(8):1146–1152. doi: 10.1038/s41559-017-0232-5. [DOI] [PubMed] [Google Scholar]
- 48.Kowalko JE, et al. Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms. Current Biology. 2013;23:1874–1883. doi: 10.1016/j.cub.2013.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahn AK, et al. Parasitic infection of the eye lens affects shoaling preferences in three-spined stickleback. Biological Journal of the Linnean Society. 2018;123:377–387. doi: 10.1093/biolinnean/blx155. [DOI] [Google Scholar]
- 50.Ward AJW, Axford S, Krause J. Mixed-species shoaling in fish: The sensory mechanisms and costs of shoal choice. Behavioral Ecology and Sociobiology. 2002;52:182–187. doi: 10.1007/s00265-002-0505-z. [DOI] [Google Scholar]
- 51.Faucher K, Parmentier E, Becco C, Vandewalle N, Vandewalle P. Fish lateral system is required for accurate control of shoaling behaviour. Animal Behaviour. 2010;79:679–687. doi: 10.1016/j.anbehav.2009.12.020. [DOI] [Google Scholar]
- 52.Ward AJW, Sumpter DJT, Couzin ID, Hart PJB, Krause J. Quorum decision-making facilitates information transfer in fish shoals. Proceedings of the National Academy of Sciences. 2008;105:6948–6953. doi: 10.1073/pnas.0710344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruxton GD, Hall SJ, Gurney WSC. Attraction Toward Feeding Conspecifics when Food Patches are Exhaustible. The American Naturalist. 2018;145:653–660. doi: 10.1086/285760. [DOI] [Google Scholar]
- 54.Killen SS, Marras S, Mckenzie DJ. Fuel, fasting, fear: Routine metabolic rate and food deprivation exert synergistic effects on risk-taking in individual juvenile European sea bass. Journal of Animal Ecology. 2011;80:1024–1033. doi: 10.1111/j.1365-2656.2011.01844.x. [DOI] [PubMed] [Google Scholar]
- 55.Burns ALJ, Herbert-Read JE, Morrell LJ, Ward AJW. Consistency of Leadership in Shoals of Mosquitofish (Gambusia holbrooki) in Novel and in Familiar Environments. PLoS One. 2012;7:1–6. doi: 10.1371/journal.pone.0036567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mclean S, Persson A, Norin T, Killen SS. Metabolic Costs of Feeding Predictively Alter the Spatial Distribution of Individuals in Fish Schools Report Metabolic Costs of Feeding Predictively Alter the Spatial Distribution of Individuals in Fish Schools. Current Biology. 2018;28:1144–1149. doi: 10.1016/j.cub.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 57.Laskowski, K. L. & Pruitt, J. N. Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proceedings of the Royal Society B281 (2014). [DOI] [PMC free article] [PubMed] [Retracted]
- 58.Ioannou CC, Singh M, Couzin ID. Potential Leaders Trade Off Goal-Oriented and Socially Oriented Behavior in Mobile Animal Groups. The American Naturalist. 2015;186:284–293. doi: 10.1086/681988. [DOI] [PubMed] [Google Scholar]
- 59.Faria JJ, et al. A novel method for investigating the collective behaviour of fish: introducing ‘Robofish’. Behavioral Ecology and Sociobiology. 2010;64:1211–1218. doi: 10.1007/s00265-010-0988-y. [DOI] [Google Scholar]
- 60.Conrad JL, Weinersmith KL, Brodin T, Saltz JB, Sih A. Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. J Fish Biol. 2011;78:395–435. doi: 10.1111/j.1095-8649.2010.02874.x. [DOI] [PubMed] [Google Scholar]
- 61.Rowsey, L. E., Johansen, J. L., Khursigara, A. J. & Esbaugh, A. J. Oil exposure impairs predator-prey dynamics in larval red drum (Sciaenops ocellatus). Marine and Freshwater Research (2019).
- 62.Pruitt JN, Keiser CN. The personality types of key catalytic individuals shape colonies’ collective behaviour and success. Animal Behaviour. 2014;93:87–95. doi: 10.1016/j.anbehav.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones KA, Croft DP, Ramnarine IW, Godin J-GJ. Size-Assortative Shoaling in the Guppy (Poecilia reticulata): The Role of Active Choice. Ethology. 2009;116:147–154. doi: 10.1111/j.1439-0310.2009.01727.x. [DOI] [Google Scholar]
- 64.Buskey, E. J., White, H. J. & Esbaugh, A. J. Impacts of Oil Spills on Marine Life in the Gulf of Mexico. Oceanography (2016).
- 65.Collier TK, et al. Effects on Fish of Polycyclic Aromatic Exposures. Fish. Physiology: Organic Chemical Toxicology of Fishes. 2014;33:195–255. [Google Scholar]
- 66.Pan YK, Khursigara AJ, Johansen JL, Esbaugh AJ. The effects of oil induced respiratory impairment on two indices of hypoxia tolerance in Atlantic croaker (Micropogonias undulatus) Chemosphere. 2018;200:143–150. doi: 10.1016/j.chemosphere.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 67.Xu EG, et al. Larval Red Drum (Sciaenops ocellatus) Sublethal Exposure to Weathered Deepwater Horizon Crude Oil: Developmental and Transcriptomic Consequences. Environ Sci Technol. 2017;51:10162–10172. doi: 10.1021/acs.est.7b02037. [DOI] [PubMed] [Google Scholar]
- 68.Xu, E. G. et al. Time- and Oil-Dependent Transcriptomic and Physiological Responses to Deepwater Horizon Oil in Mahi-Mahi (Coryphaena hippurus) Embryos and Larvae. Environmental Science & Technology, acs.est.6b02205 (2016). [DOI] [PubMed]
- 69.Diercks AR, et al. Characterization of subsurface polycyclic aromatic hydrocarbons at the Deepwater Horizon site. Geophysical Research Letters. 2010;37:1–6. doi: 10.1029/2010GL045046. [DOI] [Google Scholar]
- 70.Killen SS, Adriaenssens B, Marras S, Claireaux G, Cooke SJ. Context dependency of trait repeatability and its relevance for management and conservation of fish populations. Conserv Physiol. 2016;4:cow007. doi: 10.1093/conphys/cow007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mckenzie DJ, Belaõ TC, Killen SS, Rantin FT. To boldly gulp: standard metabolic rate and boldness have context-dependent influences on risktaking to breathe air in a catfish. Journal of Experimental Biology. 2016;218:3762–3770. doi: 10.1242/jeb.138842. [DOI] [PubMed] [Google Scholar]
- 72.Bejarano AC, Levine E, Mearns AJ. Effectiveness and potential ecological effects of offshore surface dispersant use during the Deepwater Horizon oil spill: a retrospective analysis of monitoring data. Environ Monit Assess. 2013;185:10281–10295. doi: 10.1007/s10661-013-3332-y. [DOI] [PubMed] [Google Scholar]
- 73.Bates, D. et al. Package ‘lme4’. In R Package Version 11–10, 2016 (2016).
- 74.Barton K. MuMIn: Multi-Model Inference. In R Package Version. 2015;1151:2015. [Google Scholar]
- 75.Wickham, H., Chang, W. & Package, W. M. H. Package ‘ggplot2’ (2013).
- 76.Nakagawa S, Schielzeth H. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biological Reviews. 2010;85:935–956. doi: 10.1111/j.1469-185X.2010.00141.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.