Abstract
Background
p66Shc, a Src homologue and collagen homologue (Shc) adaptor protein, mediates oxidative stress signaling. The p66Shc-null mice have increased lifespan and enhanced resistance to oxidative stress. Studies have also indicated its potential role in inner ear aging, which can lead to deafness.
Objective
The aim of this study was to determine the effects of p66Shc down-regulation on the marginal cells (MCs) of the inner ear stria vascularis.
Methods
Primary MCs were isolated from neonatal rats and treated with glucose oxidase to induce oxidative stress. The cells were transduced with adenovirus expressing siRNA, and the knockdown was verified by Western blotting. The reactive oxygen species (ROS) levels and apoptosis were analyzed using the DCFH-DA probe and Annexin-V/7-AAD staining respectively. The ultrastructure of the differentially-treated cells was examined by transmission electron microscopy (TEM).
Results: The in vitro oxidative stress model was established successfully in rat MCs. Knockdown of p66Shc alleviated the high ROS levels and apoptosis in the glucose oxidase-treated cells. In addition, glucose oxidase significantly increased the number of peroxisomes in the MCs, which was decreased by p66Shc inhibition.
Conclusion
Oxidative stress increases p66Shc levels in the marginal cells of the inner ear, which aggravates ROS production and cellular injury. Blocking p66Shc expression can effectively reduce oxidative stress and protect the MCs.
Keywords: p66Shc, marginal cells of stria vascularis, oxidative stress, peroxisome
Introduction
Oxidative stress is a critical driver of cellular dysfunction, and is associated with numerous pathological conditions, including deafness.1 Studies show increased oxidative stress levels during aging, noise and drug-induced hearing impairment.2–4 A globally aging population has increased the incidence of aging-related disorders like presbycusis, which seriously affect the quality of life. According to the theory of oxidative stress in aging, excessive production of reactive oxygen species (ROS) and/or impaired antioxidant defense system disrupts the cellular redox balance, resulting in accumulation of free radicals, macromolecular damage and cellular dysfunction.5 Mitochondria are the main sites of ROS generation. Mitochondrial aging significantly increases ROS levels, resulting in cellular senescence, mitochondrial dysfunction and an increase in the number of peroxisomes.6
ShcA is a member of the proto-oncogene Shc family, and encodes proteins p46shc, p52shc and p66Shc.7 All three proteins have highly conserved N-terminal phosphotyrosine binding (PTB), central proline enrichment region 1 (CHl) and C-terminal Src homology region 2 (SH2) domains.8 P46shc and p52shc are involved in signal transduction during proliferation and mitosis, whereas p66Shc has inhibitory effects on both.9 p66Shc has an additional collagen-homology domain (CH2) with oxidoreductase function, which regulates cellular oxidative stress levels.10 Downregulation of p66Shc is associated with lower ROS levels and increased resistance to oxidative stress, and the life span of p66Shc knockout mice is prolonged by 30%.
The marginal cells (MCs) of the stria vascularis form the innermost layer of the cochlear lateral wall, and are adjacent to the endolymphatic fluid and the microvasculature. MCs participate in the formation of endolymphatic fluid and ion transport, and maintain the high osmotic pressure, high potassium levels and high cochlear potential of cochlear endolymphatic fluid, which are necessary for electro-mechanical signal transduction.11 Numerous studies12–15 have shown that the oxidative damage of striatum cells can lead to hearing loss, indicating that reversing oxidative stress can potentially treat deafness.
We isolated MCs from the stria vascularis of neonatal rats, and established an in vitro model of oxidative stress using glucose oxidase, which oxidizes glucose to produce low levels of hydrogen peroxide.16,17 The oxidative stress induced by glucose oxidase was accompanied by increased levels of p66Shc, and knocking down the latter alleviated the high ROS and apoptosis levels.
Materials and methods
The neonatal (3 days old) Sprague Dawley (SD) rats were obtained from the department of laboratory animals, central south university. the department of laboratory animals, central south university has qualification for raising and breeding animals. Animals were fed according to the standard protocols approved by the Laboratory Animal Management Statute of the China. All of theanimal experiments followed the instructions of the Laboratory Animal Management Statute of China. The protocol was approved by the Center for Medical Ethics Central South University (Permit Number: 201403088).
Isolation and primary culture of MCs
Six neonatal (3 days old) SD rats were anesthetized with ethylether, and disinfected by swabbing with 75% ethanol. The bilateral temporal bones were removed, and the stria vascularis from the apical turn to basal turn was dissected. The tissues were chopped into 0.5 mm thick pieces and digested with 0.1% type II collagenase for 30 min at 37 °C. After centrifuging for 5 min at 1000 rpm, the cells were harvested and re-suspended in Epithelial Cell Medium-animal (EpiCM-animal; ScienCell, USA). The MCs were seeded in tissue culture plates, and cultured at 37 °C under 5% CO2. Cells of the third to the tenth passages were used
Immunofluorescence
The MCs were seeded onto glass slides and once a monolayer was formed, the culture medium was discarded and the cells were washed with PBS. After fixing with 4% paraformaldehyde for 15 min at room temperature, the MCs were rinsed thrice with PBS, permeabilized in 0.3% Triton x-100 (Sigma, USA) for 20 min, and washed again. The samples were blocked with 5% BSA (bovine serum albumin) for 10 min, and incubated overnight with anti-CK18 antibody (1:500; Abcam, USA). After washing thrice with PBS, the cells were incubated with anti-rabbit IgG (diluted 1:100 to 1:200 with 1% BSA) for 30 min. The slides were suitably processed, mounted and observed under a fluorescence electron microscope.
Establishment of MCs oxidative stress model
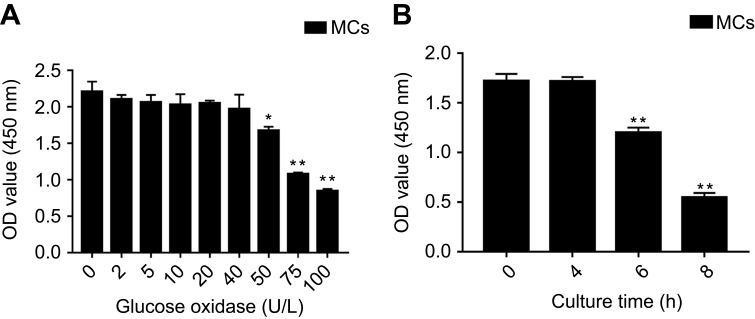
To establish the MCs oxidative stress model using glucose oxidase, the optimum concentration of the enzyme was first determined. Briefly, MC monolayers were harvested and the cells were re-suspended and seeded into 96-well plates. After allowing the cells to adhere, they were treated with 0, 2, 5, 10, 20, 40, 50, 75 and 100 U/L glucose oxidase (Sigma, USA) for 48 h, and each concentration was tested in triplicates. To determine the viability of the cells, 20 μl of CCK8 test solution (Cell Counting Kit-8; Dojindo, Japan) was added to each well, and the cells were incubated for another 1.5 h. The absorbance at 450 nm was then measured using a microplate reader. Based on the results, the cells were then treated with 50 U/L glucose oxidase for 0, 2, 4 and 8 h in triplicates to determine the optimum treatment duration.
Adenoviral transfection and experimental grouping
The MCs were seeded in 6-well plates at the density of 5×105 cells/well, and allowed to adhere and form a monolayer. The cells were randomized into the (A) transduced + oxidative stress, (B) transduced, (C) oxidative stress, and (D) untreated negative control groups. The group A and B cells were transduced with recombinant adenovirus p66Shc-RNAi, and 12 h later the group A and C cells were treated with 50 U/L glucose oxidase for 4 h.The recombinant adenovirus p66Shc-RNAi concentration is 4×106 PFU/mL.
Western blotting
The treated MCs were harvested, washed with PBS, and homogenized in ice-cold RIPA lysis buffer (Beyotime, China) for a few seconds. The lysates were precipitated by centrifuging at 12,000x g at 4 °C for 10 min, and the supernatants were quantified using the BCA reagent (Beyotime, China). Equal amounts of protein per sample (20 µg) was mixed with the loading buffer (1/4th the volume of supernatants) and denatured by boiling for 10 min at 100 °C.The samples were resolved by SDS-PAGE (Bio-Rad, USA), and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). The membranes were blocked with 5% skimmed milk dissolved in TBS with 0.1% Tween 20 (TBST), and incubated overnight with the primary antibody (dilution) at 4 °C. After washing with TBST, the HRP-conjugated secondary antibody (1:10,000) was added and the blots were incubated at room temperature for 1 h. The membranes were washed again with TBST, and the protein bands were detected using the ECL reagent (Beyotime, China).
Annexin V/7-AAD double staining
The suitably-treated MCs were rinsed twice with cold PBS, and harvested using trypsin. The detached cells were centrifuged at 1000 rpm for 5 min, and the pellet was washed twice with cold PBS. Around 1×105 cells per sample were re-suspended in 100 μl of 1× binding buffer containing 5 μl Annexin-APC and 10 μl 7-AAD, and incubated for 15 min at room temperature in the dark. The stained cells were washed once, and analyzed by flow cytometry (BD Biosciences, Carlsbad, CA).
DCFH-DA staining
The MCs were harvested and washed as described above, and incubated with DCFH-DA for 20 min in the dark, with periodic mixing every 3–5 min. The stained cells were centrifuged to remove the dye, washed twice with serum-free medium, and analyzed by flow cytometry.
Transmission electron microscopy (TEM) observation
The cells were harvested and washed as described, and the pellets were fixed overnight with glutaraldehyde. The fixed cells were rinsed thrice with PBS, immobilized with 1% osmium fixative, and dehydrated through the following gradient: 50% acetone, 70% acetone, 80% acetone, 90% acetone twice (each 10 min), 100% acetone twice (each 15 min). The dehydrated samples were embedded in epoxy resin, and cut into ultrathin sections that were stained with uranium acetate. The stained sections (3 per sample) was examined using TEM (HT7700, Hitachi, Japan) to determine cellular ultrastructure.
Statistical analyses
Statistical analysis was conducted using GraphPad Prism 5.0. Data were presented as the mean ± S.E.M of three independent experiments. The data in Figure 2 were analyzed by Student’s unpaired or unpaired t-test with Welch’s correction. The data in Figure 3–5 were analyzed by one-way ANOVA followed by Student-Newman-Keuls multiple comparison test. and P-values less than 0.05 were considered statistically significant.
Figure 2.
Viability of glucose oxidase-treated MCs. OD450 of MCs treated with (A) different concentrations of glucose oxidase for 48 h (n=5), and (B) 50 U/L glucose oxidase for varying durations (n=5). The data are presented as the means ± SEM. *p<0.05 vs control group; **p<0.01 vs control group.
Figure 3.
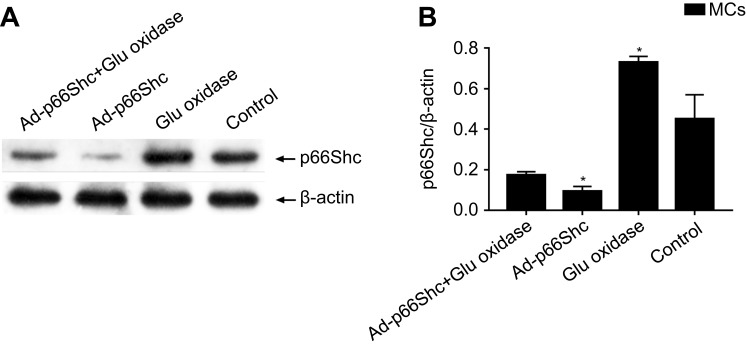
The expression levels of p66Shc protein in MCs. The data are presented as the means ± SEM (n=3). One-way ANOVA followed by Student-Newman-Keuls multiple comparison test, *p<0.05 vs control group.
Figure 5.
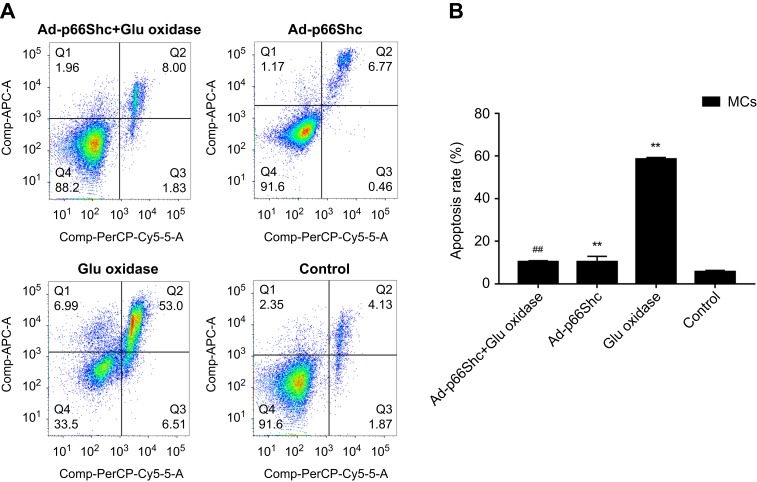
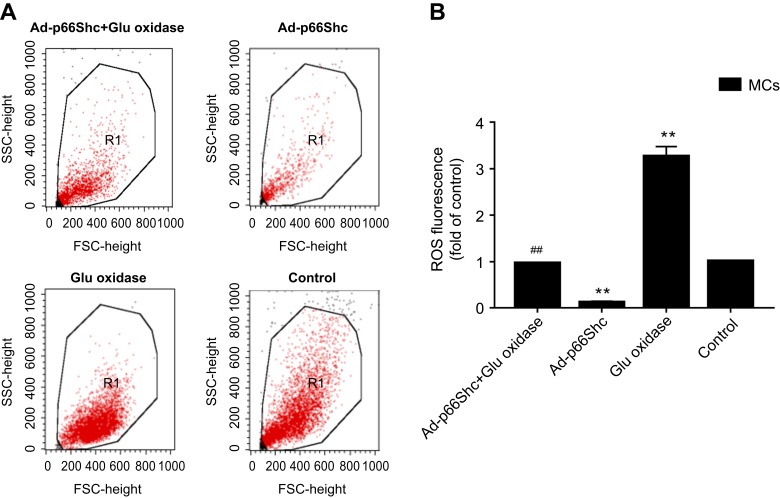
Apoptosis and oxidative stress in differentially-treated MCs. (A) Detection of apoptosis rate by flow cytometry. (B) Detection of ROS levels. Data are presented as the mean ± SEM (n=3). One-way ANOVA followed by Student-Newman-Keuls multiple comparison test, **p<0.01 vs control group; ##p<0.01 vs glucose oxidase group.
Results
The in vitro model of oxidative stress was successfully established using rat MCs
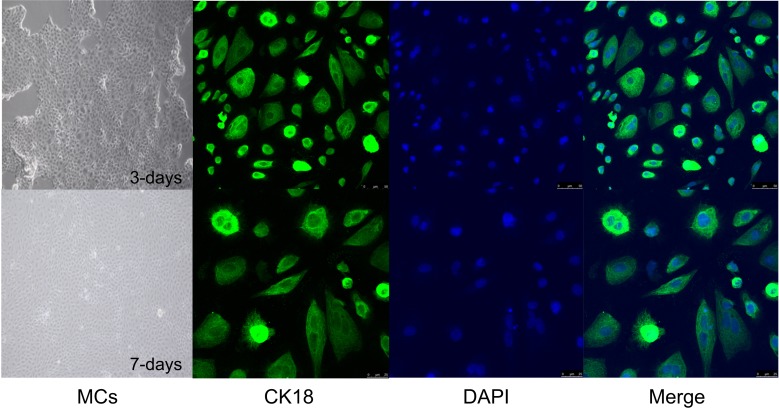
As shown in Figure 1, the isolated primary MCs formed a compact “paved stone” monolayer by the 3rd day of culture, and formed the characteristic dome structure on the 7th day, and were characterized by high expression levels of CK-18. Glucose oxidase significantly decreased the viability of the MCs is a concentration and time-dependent manner (p<0.05; Figure 2A and B respectively), and in subsequent experiments the cells were treated with 50 U/L glucose oxidase for 4 h. The oxidative stress was evaluated on the basis of p66Shc expression levels, ROS levels and apoptosis rates. As shown in Figure 3, glucose oxidase significantly increased p66Shc protein levels relative to the untreated controls (p<0.05, SNK-q test). In addition, both intracellular ROS levels (Figure 4) and percentage of apoptotic cells (Figure 5) were significantly higher in the glucose oxidase-treated cells compared to untreated controls. Taken together, glucose oxidase induced oxidative stress in the MCs, which was accompanied by upregulation of p66Shc.
Figure 1.
Characterization of MCs isolated from the striae of neonatal rats. Morphology of MCs on day 3 and 7 of culture (100x magnification). This picture shows the marginal cells, which present as polygons with a pleomorphic growth pattern and are dark with clear boundaries. Representative fluorescence images showing in situ CK-18 (green) in the cytoplasm of primary MCs on day 3 and 7 of culture. Because CK18 is mainly expressed in cytoplasm, the green fluorescence is distributed in the cytoplasm. The nuclei are stained with DAPI (blue).
Figure 4.
Detection of ROS levels. (A) Representative images showing the fluorescent intensity of DCFH-DA in the treated MCs. (B) Bar graph showing ROS levels. The data are presented as the means ± SEM (n=3). One-way ANOVA followed by Student-Newman-Keuls multiple comparison test, **p<0.01 vs control group; ##p<0.01 vs glucose oxidase group.
P66Shc silencing abrogates glucose oxidase-induced oxidative stress in MCs
To determine the role of p66Shc in oxidative stress, the MCs were transduced with adenovirus expressing specific siRNA against p66Shc, which significantly decreased the protein levels (p<0.05, SNK-q test) (Figure 3). P66Shc knockdown significantly decreased the fluorescence intensity of DCFH-DA (Figure 4), as well as the percentage of apoptotic cell (Figure 5) in the glucose oxidase-treated cells compared to the un-transduced cells, to levels similar to that of the healthy control cells. This clearly indicates that p66Shc mediates the glucose oxidase-induced increase in ROS levels and apoptosis in MCs, and silencing its expression has a protective role against oxidative stress.
Ultrastructural effects of oxidative stress on MCs
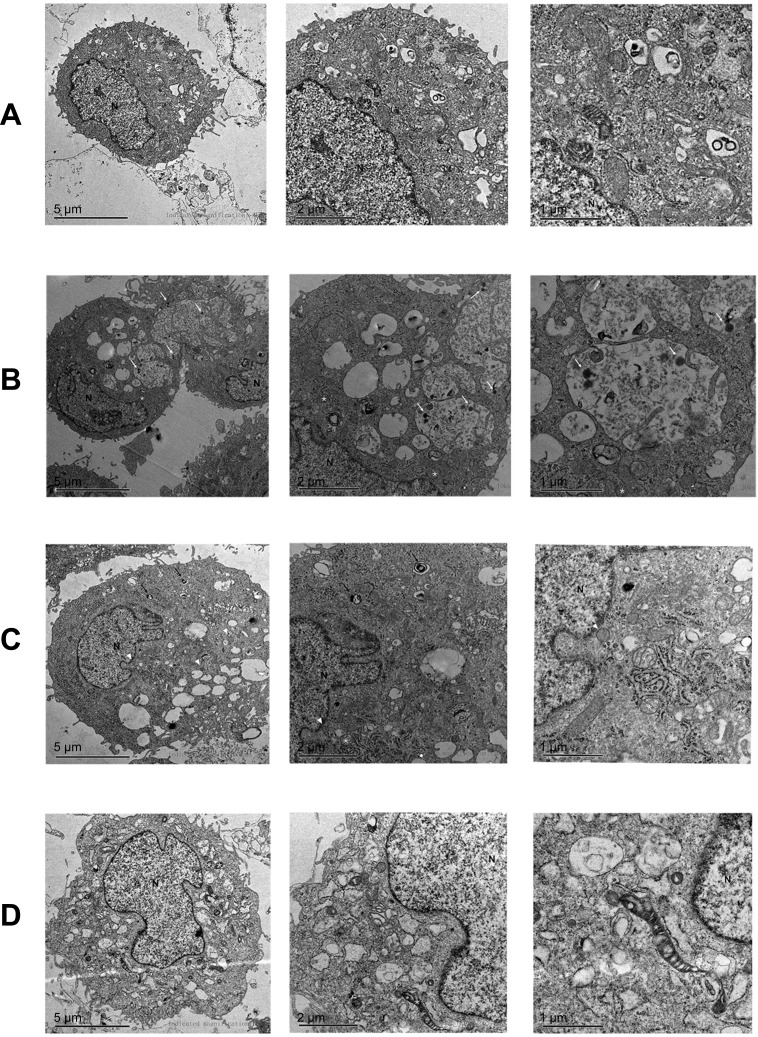
The ultrastructure of the differentially-treated MCs was observed by TEM (Figure 6). Viral particles were observed in the cytoplasmic inclusion bodies of the adenovirus-transduced cells. While the untreated controls and p66Shc-knockdown MCs showed complete nuclei, continuous cell membranes and healthy mitochondria, the glucose oxidase-treated cells had clear signs of oxidative stress, such as swollen mitochondria and endoplasmic reticulum, and numerous peroxisomes.
Figure 6.
TEM images showing ultrastructure of MCs treated with (A) adenovirus-siRNA and glucose oxidase, (B) adenovirus-siRNA, (C) glucose oxidase, and (D) untreated control. *mitochondria, long white arrow: virus, short white arrow: microbody, long black arrow: autophagic cells.
Abbreviation: N, nucleus.
Discussion
Glucose oxidase catalyzes the oxidation of glucose to produce persistently low levels of hydrogen peroxide, which causes oxidative stress. It is preferred over hydrogen peroxide and D-galactose since it can induce oxidative stress in a relatively short period of time. Rat MCs treated with glucose oxidase showed a significant decline in viability, along with high ROS levels, apoptosis and a large number peroxisomes, swollen mitochondria. all hallmarks of oxidative stress. Although the role of p66Shc in oxidative stress has been established, there are no reports so far regarding its involvement in the oxidative stress injury of the inner ear, which is associated with deafness. We observed a significant upregulation in p66Shc levels in the glucose oxidase-treated MCs, indicating that it is triggered by oxidative stress. In addition, knocking down p66Shc in the stressed cells alleviated the increased ROS levels and apoptosis. Taken together, p66Shc likely mediates the pathological effects of oxidative stress in the inner ear MCs, and its downregulation can protect the cells against oxidative stress injury.
Studies show that p66Shc increases intracellular ROS levels by promoting oxidase activity, decreasing antioxidant activity and increasing mitochondrial respiratory chain leakage.8,18,19 Oxidative stress factors (eg, H2O2 and UV light) activate protein kinase (PK)Cβ, which phosphorylates the serine 36 in the CH2 region of cytoplasmic p66Shc. The phosphorylated p66Shc activates GTPase Ras-related C3 botulinum toxin substrate 1 (RAC1) by substituting nucleotide exchange factor SOS in the growth factor receptor-bound protein 2 (GRB2) complex. The activated RAC1 then enables the formation of membrane-bound NADPH oxidase complex which triggers ROS production.20 The activated p66Shc also downregulates antioxidant enzymes and regulatory factors such as glutathione peroxidase-1 via the fork-head transcription factors (such as FOXO3A). In the resting cells, p66Shc is bound to the mitochondrial TOM/TIM complex. Under oxidative stress, p66Shc separates from the TOM/TIM complex and interacts with cytochrome C (cytC) of the mitochondrial respiratory chain, which promotes the former’s oxidation and ROS production. We found that p66Shc knockdown significantly decreased the number of peroxisomes in the glucose oxidase-treated cells. Peroxisome are secondary ROS-generating organelles21 that are involved in lipid metabolism.22 Any disruption in the peroxisomal oxidases and antioxidant enzymes can trigger cellular senescence.23 Our findings therefore indicate that p66Shc can also reduce oxidative stress by decreasing the number of peroxisomes, which has not been reported before.
Inhibition the expression of p66Shc can reduce the level of oxidative stress in Mcs, reducing the damage of oxidative stress to marginal cells, which plays an important role in maintaining the stability of the cochlear environment. Therefore, we speculate that inhibiting the expression of p66Shc may play a role in some kinds of deafness related to oxidative stress, such as noise-induced deafness, senile deafness, ischemia-hypoxia-induced deafness, and aminoglycoside-induced deafness,24–26 providing a possible treatment for this kind of deafness.
Taken together, oxidative stress increases p66Shc levels in the marginal cells of the inner ear, which aggravates ROS production and cellular injury. Downregulation of p66Shc expression can effectively reduce oxidative stress and protect the MCs. Our findings offer a new possibility for treating oxidative stress-induced hearing loss, such as noise-induced, sudden and senile deafness.
Conclusion
Downregulation of p66Shc can reduce oxidative stress and apoptosis in oxidative stress model of marginal cells of stria vascularis in SD rats, offering a new possibility for treating oxidative stress-induced hearing loss.
Acknowledgment
This study was supported by grants from the National Nature Science Foundation of China (No. 81400468 and 81401227).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gul F, Muderris T, Yalciner G, et al. A comprehensive study of oxidative stress in sudden hearing loss. Eur Arch Otorhinolaryngol. 2017;274(3):1301–1308. doi: 10.1007/s00405-016-4301-1 [DOI] [PubMed] [Google Scholar]
- 2.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- 3.Yuan H, Wang X, Hill K, et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal. 2015;22(15):1308–1324. doi: 10.1089/ars.2014.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheth S, Mukherjea D, Rybak LP, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front Cell Neurosci. 2017;11:338. doi: 10.3389/fncel.2017.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. [DOI] [PubMed] [Google Scholar]
- 6.Singh I. Mammalian peroxisomes: metabolism of oxygen and reactive oxygen species. Ann N Y Acad Sci. 1996;804:612–627. doi: 10.1111/j.1749-6632.1996.tb18648.x [DOI] [PubMed] [Google Scholar]
- 7.Wills MK, Jones N. Teaching an old dogma new tricks: twenty years of Shc adaptor signalling. Biochem J. 2012;447(1):1–16. doi: 10.1042/BJ20120769 [DOI] [PubMed] [Google Scholar]
- 8.Yang SK, Xiao L, Li J, Liu F, Sun L. Oxidative stress, a common molecular pathway for kidney disease: role of the redox enzyme p66Shc. Ren Fail. 2014;36(2):313–320. doi: 10.3109/0886022X.2013.846867 [DOI] [PubMed] [Google Scholar]
- 9.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402(6759):309–313. doi: 10.1038/46311 [DOI] [PubMed] [Google Scholar]
- 10.Kong X, Guan J, Li J, Wei J, Wang R. P66(Shc)-SIRT1 regulation of oxidative stress protects against cardio-cerebral vascular disease. Mol Neurobiol. 2017;54(7):5277–5285. doi: 10.1007/s12035-016-0073-2 [DOI] [PubMed] [Google Scholar]
- 11.Wangemann P. K(+) cycling and its regulation in the cochlea and the vestibular labyrinth. Audiol Neurootol. 2002;7(4):199–205. doi: 10.1159/000063736 [DOI] [PubMed] [Google Scholar]
- 12.Han C, Linser P, Park HJ, et al. Sirt1 deficiency protects cochlear cells and delays the early onset of age-related hearing loss in C57BL/6 mice. Neurobiol Aging. 2016;43:58–71. doi: 10.1016/j.neurobiolaging.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fetoni AR, Eramo SL, Paciello F, et al. The redox protein p66(shc) mediates cochlear vascular dysfunction and transient noise-induced hearing loss. Sci Rep. 2016;6:25450. doi: 10.1038/srep25450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Vega R, Garrido F, Partearroyo T, et al. Folic acid deficiency induces premature hearing loss through mechanisms involving cochlear oxidative stress and impairment of homocysteine metabolism. Faseb J. 2015;29(2):418–432. doi: 10.1096/fj.14-259283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, Sun Y, Hu YJ, et al. Increased p66Shc in the inner ear of D-galactose-induced aging mice with accumulation of mitochondrial DNA 3873-bp deletion: p66Shc and mtDNA damage in the inner ear during aging. PLoS One. 2012;7(11):e50483. doi: 10.1371/journal.pone.0050483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Someya S, Xu J, Kondo K, et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106(46):19432–19437. doi: 10.1073/pnas.0908786106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haller M, Khalid S, Kremser L, et al. Novel insights into the PKCbeta-dependent regulation of the oxidoreductase p66Shc. J Biol Chem. 2016;291(45):23557–23568. doi: 10.1074/jbc.M116.752766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arany I, Faisal A, Clark JS, Vera T, Baliga R, Nagamine Y. p66SHC-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol Renal Physiol. 2010;298(5):F1214–F1221. doi: 10.1152/ajprenal.00639.2009 [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto C, Yamasoba T. Oxidative stresses and mitochondrial dysfunction in age-related hearing loss. Oxidative Medicine and Cellular Longevity. 2014;2014:582849. doi: 10.1155/2014/582849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrader M, Costello J, Godinho LF, Islinger M. Peroxisome-mitochondria interplay and disease. J Inherit Metab Dis. 2015;38(4):681–702. doi: 10.1007/s10545-015-9819-7 [DOI] [PubMed] [Google Scholar]
- 22.Cipolla CM, Lodhi IJ. Peroxisomal dysfunction in age-related diseases. Trends Endocrinol Metab. 2017;28(4):297–308. doi: 10.1016/j.tem.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fransen M, Nordgren M, Wang B, Apanasets O, Van Veldhoven PP. Aging, age-related diseases and peroxisomes. Subcell Biochem. 2013;69:45–65. doi: 10.1007/978-94-007-6889-5_3 [DOI] [PubMed] [Google Scholar]
- 24.Shi X, Nuttall AL. Upregulated iNOS and oxidative damage to the cochlear stria vascularis due to noise stress. Brain Research. 2003;967(1–2):1–10. doi: 10.1016/s0006-8993(02)04090-8 [DOI] [PubMed] [Google Scholar]
- 25.Liu XZ, Yan D. Ageing and hearing loss. The Journal of Pathology. 2007;211(2):188–197. doi: 10.1002/path.2102 [DOI] [PubMed] [Google Scholar]
- 26.Keithley EM. Pathology and mechanisms of cochlear aging. Journal of Neuroscience Research. 2019. doi: 10.1002/jnr.24439 [DOI] [PMC free article] [PubMed] [Google Scholar]