Abstract
Background and Aims
Nocebo effects, adverse outcomes occurring in patients receiving inert therapy, contribute to adverse event [AE] reporting in randomized controlled trials [RCTs]. High placebo AE rates may result in inaccurate estimation of treatment-related AEs. We estimate the pooled rate of AEs in patients randomized to placebo compared to active therapy in inflammatory bowel disease [IBD] RCTs.
Methods
MEDLINE, EMBASE and CENTRAL were searched to March 1, 2017 for RCTs of conventional medical therapies for Crohn’s disease [CD] or ulcerative colitis [UC]. Rates of AEs, serious AEs [SAEs], AE-related trial withdrawal, infections and worsening IBD were pooled using a random-effects model.
Results
We included 124 CD [n = 26 042] and 71 UC RCTs [n = 16 798]. The pooled placebo AE rate was 70.6% (95% confidence interval [CI]: 65.3%, 75.4%) and 54.5% [47.8%, 61.1%] in CD and UC RCTs, respectively. There was no significant risk difference [RD] in AE, SAE or AE-related withdrawal rates between CD patients receiving placebo or active drug. A 1.6% [95% CI: 0.1%, 3.1%] increase in AE rates was observed among UC patients randomized to active therapy. Patients receiving active therapy had a higher risk of infection (RD 1.0% [95% CI: 0.4%, 1.7%] for CD, 2.9% [95% CI: 1.4%, 4.4%] for UC) although a lower risk of worsening CD (RD −3.2% [95% CI: −4.8%, −1.5%]) or UC (RD –3.7% [95% CI: –5.7%, –1.8%]).
Conclusions
AEs are commonly reported by patients randomized to either placebo or active treatment in IBD RCTs. Clinically relevant differences in AE, SAE and AE-related withdrawal were not observed.
Keywords: Adverse event, nocebo, inflammatory bowel disease
1. Introduction
Over the past two decades, therapy for Crohn’s disease [CD] and ulcerative colitis [UC] has expanded substantially to include aminosalicylates, corticosteroids, immunosuppressants, multiple classes of biologics and novel oral small molecules.1 The goal of medical therapy is to induce and maintain clinical and endoscopic remission, with the long-term aim of preventing bowel damage, averting surgery and optimizing quality of life.2 However, many patients experience adverse events [AEs] or serious adverse events [SAEs] that can negatively influence treatment adherence, reduce confidence in the efficacy of subsequent treatments and compromise treatment persistence.3
In clinical trials, patients randomized to either placebo or active comparator may develop adverse outcomes. Historically, AEs occurring in patients receiving inert therapy have been attributed to worsening of the underlying condition or the ‘nocebo’ effect, defined as negative consequences arising from the treatment context and patient expectations rather than from physiological actions of the drug itself.4 A notable example is the occurrence of myalgias in patients treated with HMG-CoA reductase inhibitors. In post-hoc analysis of the Anglo-Scandinavian Cardiac Outcomes Trial, when both patients and physicians were blinded to treatment assignment, muscle-related AEs occurred with similar frequency in patients receiving atorvastatin or placebo (hazard ratio [HR] 1.03, 95% confidence interval [CI]: 0.88–1.21, p = 0.72). However, during the open-label extension phase when treatment assignment was unblinded, muscle-related AEs were reported at a significantly higher rate by patients receiving atorvastatin (HR 1.41 [95% CI: 1.10–1.79], p = 0.006], an effect which was hypothesized to relate to highly publicized reports of potential statin-related AEs.5 The mechanisms underpinning the nocebo effect are complex and include patient-related, neurobiological, psychosomatic and psychosocial factors.6 Negative expectations for treatment are reinforced by patient perceptions of personal sensitivity to medication,7,8 social transmission and learning,9 and conditioned responses from past experiences10 that may heighten negative affectivity and lead to symptom misattribution or augmentation.11,12
Nocebo effects have important implications for drug development and randomized controlled trial [RCT] design. Large nocebo effects may result in inaccurate estimation of treatment-related AEs, either by increasing the proportion of AEs in the placebo group or by increasing the proportion of treatment-unrelated AEs in patients receiving active therapy.13 For example, in an analysis of 31 trials of 3271 patients who were switched from originator infliximab, adalimumab, etanercept or bevacizumab to the corresponding biosimilar, Odinet et al. demonstrated that the median rate of drug discontinuation for AEs was twice as high in patients unblinded to their switch status [5.60% vs 2.85%].14 Conversely, nocebo effects may also bias estimates of treatment efficacy by increasing study withdrawal or reducing medication compliance. These issues are particularly pertinent in inflammatory bowel disease [IBD] as nocebo effects are informed by the cumulative disease experience and are expected to be highest in chronic conditions such as CD or UC, especially when patients have required multiple therapies to control disease.15 Additionally, patients with IBD consistently describe the fear of side effects as an important consideration in choosing to start or continue medication.16
Although understanding and minimizing the nocebo effect is important for clinical trial design, it has not been well studied in IBD. Therefore, we conducted a systematic review and meta-analysis of placebo-controlled RCTs for CD and UC evaluating conventional medical therapies to: [1] estimate the risk of developing AEs, SAEs, system- and organ-specific AEs, and AE-related trial withdrawal among patients randomized to placebo; [2] determine if there is a difference in the proportion of AEs reported between patients randomized to placebo vs active comparator; and [3] evaluate trial-related factors that may influence AE rates.
2. Materials and methods
2.1 Search strategy
We identified eligible RCTs from three previously published systematic reviews evaluating conventional medical therapies for luminal CD,17 fistulizing CD18 and UC.19 MEDLINE [1948–2017], EMBASE [1947–2017] and the Cochrane CENTRAL Register of Controlled Trials [1994–2017] were searched from inception to March 1, 2017 without language restriction. Abstracts from Digestive Disease Week and United European Gastroenterology Week [2012–2017], and bibliographies of relevant studies and review articles were also screened to supplement the search. The search strategy is summarized in Supplementary File 1 and includes terms to capture IBD, randomization, placebo and blinding. All citations were screened, and potentially relevant studies underwent full text evaluation.
2.2 Study selection
Studies were eligible if they fulfilled the following inclusion criteria: [1] placebo-controlled induction and/or maintenance trial of adult patients with luminal or fistulizing CD or UC; [2] evaluation of conventional medical therapy for IBD, defined as an aminosalicylate, corticosteroid, immunosuppressant, biological agent or small molecule; [3] use of the Crohn’s Disease Activity Index [CDAI] or Harvey Bradshaw Index [HBI] in luminal CD trials or the Mayo Clinic Score [MCS] or UC Disease Activity Index [UCDAI] in UC trials for enrolment or outcome assessment; and [4] reporting of the proportion of patients experiencing AEs according to treatment assignment [placebo vs active comparator]. Trials of complementary therapies, antibiotics and probiotics were excluded as these are not currently recommended for induction or maintenance monotherapy. The inclusion criteria were limited to trials using modern disease activity indices to optimize relevance to current drug development.
2.3 Outcome assessment and data extraction
To identify eligible studies, articles were independently assessed by pairs of investigators using the predefined eligibility criteria. Data extraction for safety outcomes and baseline study features was independently performed by two reviewers [NP and TMN, and CM and IMH, respectively]. Discrepancies were resolved by consensus and with a third reviewer [VJ].
The primary outcome of interest was the risk difference [RD] in AE outcomes between patients treated with active comparator and placebo. Secondary outcomes were the proportion of patients randomized to placebo or active comparator experiencing AEs, SAEs, study withdrawal due to AEs, infectious AEs and worsening IBD. The number of patients who died or developed a malignancy during the trial was also extracted. Outcomes were collected by treatment assignment [placebo vs active comparator]. AE outcomes were defined according to the original study authors. Other trial features that were extracted included: [1] trial design features [induction vs maintenance, route of administration, trial phase and setting, number of trial centres, total number of patients and follow-up duration]; and [2] participant characteristics (patient age, disease duration, disease activity at trial entry, disease extent, and proportion of patients with concurrent and previous treatment exposure [biologic agents, corticosteroids and immunosuppressants]). For integrated studies with both induction and maintenance components, outcomes for each trial phase were reported separately. For trials with multiple active comparator arms, summary baseline characteristics were calculated using sample-size-weighted means and the proportion of patients with each outcome were pooled.
The Cochrane Collaboration Risk of Bias tool20 was used to assess the methodological quality of each of the included studies. The risk of bias assessment was published with the initial reviews and therefore are not reproduced here.
2.4 Data synthesis and statistical methods
The proportion of patients randomized to placebo or active comparator experiencing an AE was pooled separately for CD and UC trials and stratified by treatment class, using a restricted maximum likelihood random-effects model to account for between- and within-study variability,21 with associated 95% CI. A priori, we also decided to pool the proportion of patients experiencing an SAE, infectious AE, worsening IBD or trial withdrawal due to AEs. The RD in the proportion of patients experiencing an AE or SAE between active treatment and placebo arms was also calculated and pooled for CD and UC trials using a random-effects model. Corresponding odds ratios [ORs] for the AE outcomes were derived from fitting a meta-regression model adjusted for active comparator treatment class. Multivariable meta-regression was not possible due to an insufficient number of significant covariables in univariable analysis.
Statistical heterogeneity was quantified using the χ2 test and I2 statistic. I2 values of 25%, 50% and 75% were interpreted as representing small, moderate and high levels of relative heterogeneity. Univariable meta-regression was performed to assess the potential sources of heterogeneity and the impact of a priori-chosen study- and patient-related covariables on the AE rates in the placebo group. These included disease severity [remission, mild/moderate, moderate/severe], study phase, study design [induction vs maintenance], study setting [single centre, multicentre or multinational], publication year, active comparator treatment class (aminosalicylate, corticosteroid, immunosuppressant [azathioprine, 6-mercaptopurine or methotrexate], biologic, oral small molecule, or other), route of administration [oral, intravenous, subcutaneous or topical], duration of follow-up, time to primary outcome assessment, and concomitant immunosuppressant or corticosteroid use at trial entry.
Potential publication bias and small study effects for RDs were assessed using funnel plots and tested using Egger’s linear regression asymmetry test.22
All analyses were performed using the meta and metafor packages for R [version 3.5.1].23 The study is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] guidelines.24
3. Results
3.1 Search results and included studies
The final analysis included 124 CD RCTs and 71 UC RCTs [Supplementary Figure 1] [references provided in Supplementary File 2]. Characteristics of the included studies are summarized in Table 1 and individual study data are shown in Supplementary Table 1. A total of 120 induction trials [61.5%], 26 maintenance trials [13.3%] and 49 integrated induction/maintenance trials [25.1%] were included, enrolling a total of 26 042 CD patients and 16 798 UC patients. Amongst these patients, 8897 CD and 5563 UC patients were randomized to receive placebo. Most trials were either phase II [89/195, 45.6%] or phase III [96/195, 49.2%] studies. A total of 85 trials [43.6%] evaluated biologic agents. Any AEs were reported in 90 CD and 60 UC trials, SAEs were reported in 88 CD and 60 UC trials, AE-related withdrawal was reported in 100 CD and 50 UC trials, infectious AEs were reported in 72 CD and 35 UC trials, and IBD worsening was reported in 55 CD and 38 UC trials.
Table 1.
Summary characteristics of included trials
| Crohn’s disease | Ulcerative colitis | |
|---|---|---|
| [n = 124] | [n = 71] | |
| Trial design, n [%] | ||
| Induction | 70 [56.5] | 50 [70.4] |
| Maintenance | 22 [17.7] | 4 [5.6] |
| Integrated induction/maintenance | 32 [25.8] | 17 [23.9] |
| Trial phase, n [%] | ||
| Phase I | 5 [4.0] | 5 [7.0] |
| Phase II | 57 [46.0] | 32 [45.1] |
| Phase III | 62 [50.0] | 34 [47.9] |
| Trial setting, n [%] | ||
| Single centre | 9 [7.3] | 5 [7.0] |
| Multicentre, single nation | 29 [23.4] | 16 [22.5] |
| Multicentre, multinational | 86 [69.4] | 50 [70.4] |
| Active comparator, n [%] | ||
| Aminosalicylate | 10 [8.1] | 15 [21.1] |
| Corticosteroid | 8 [6.5] | 6 [8.5] |
| Immunosuppressant | 11 [8.9] | 2 [2.8] |
| Biologic | 57 [46.0] | 28 [39.4] |
| Oral small molecule | 13 [10.5] | 7 [9.9] |
| Other | 25 [20.2] | 13 [18.3] |
| Total patients, n | ||
| Total patients randomized | 26 042 | 16 798 |
| Patients randomized to active treatment | 15 680 | 10 934 |
| Patients randomized to placebo | 8987 | 5563 |
| Median follow-up duration [wk, range] | 17 [2–112] | 10 [4–96] |
SD standard deviation
3.2 Adverse event rates in Crohn’s disease
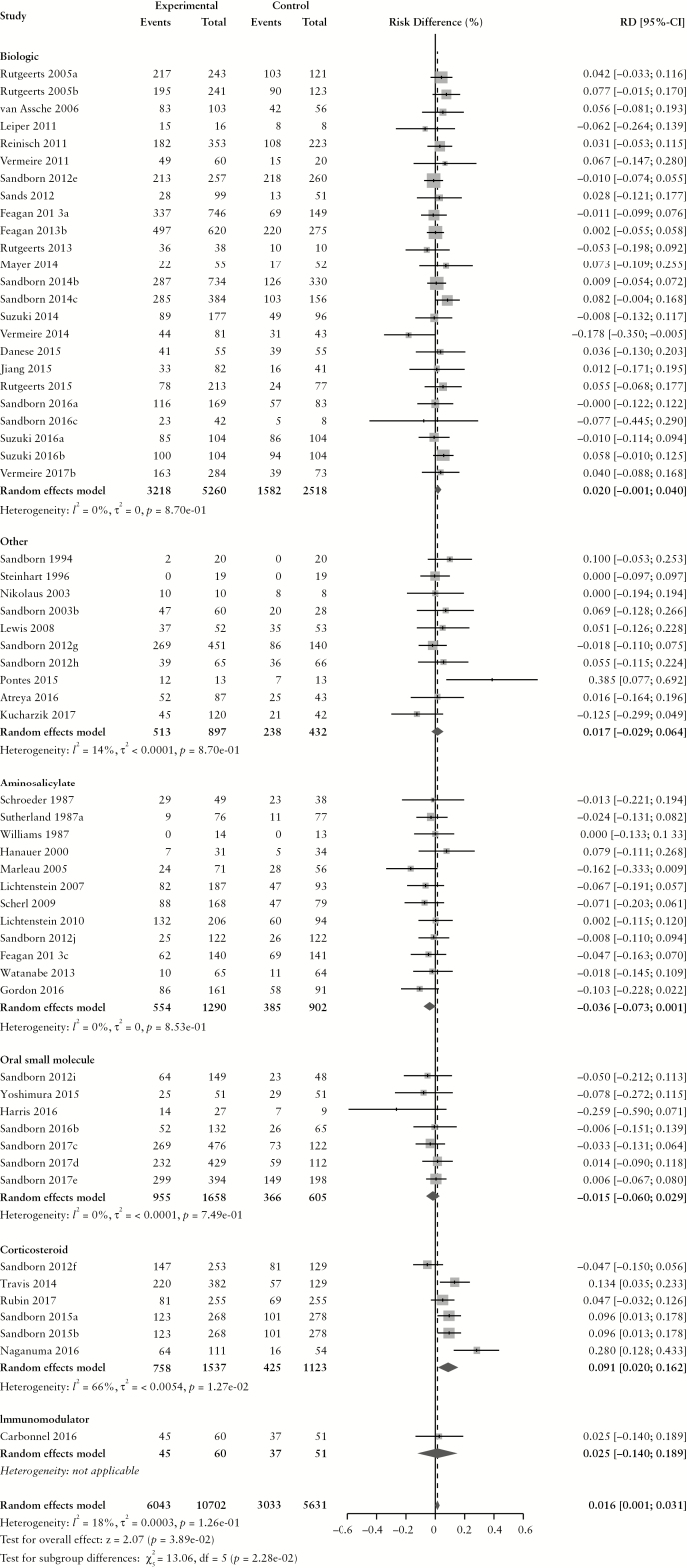
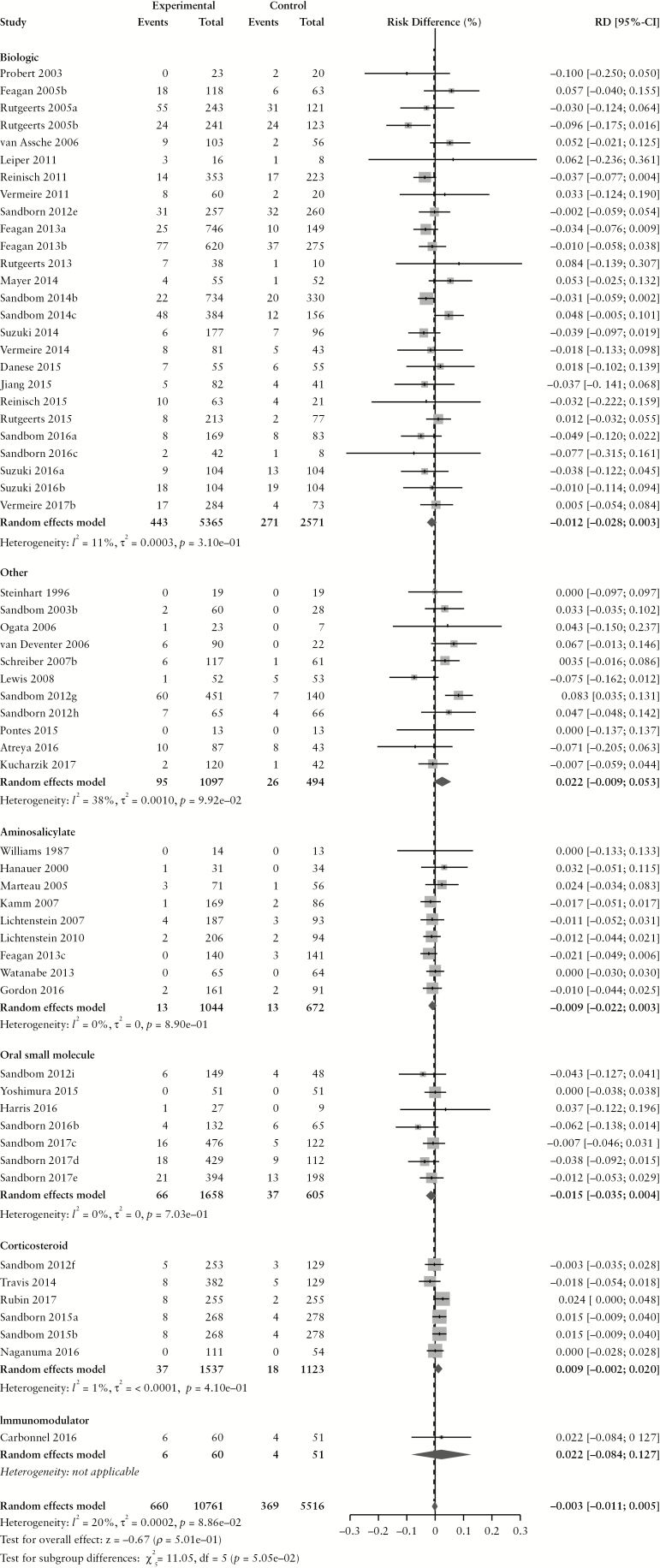
The overall pooled rate of AEs among CD patients randomized to placebo or active comparator is summarized in Table 2. Comparing patients receiving any active treatment to placebo, there was no difference in the pooled risk of the occurrence of any AE (RD −0.2% [95% CI: −1.5%, 1.2%]) with statistically significant homogeneity among RD estimates (χ2[89] = 130.56, p = 0.003; I2 = 32%). The pooled RD stratified by active comparator class is summarized in Figure 1. The pooled AE rate among CD patients randomized to placebo was 70.6% [95% CI: 65.8%, 74.9%] (χ2[89] = 808.35, p < 0.0001; I2 = 89%). The pooled AE rate among CD patients randomized to active comparator was 70.8% [95% CI: 65.7%, 75.3%] (χ2[89] = 1470.09, p < 0.0001; I2 = 94%). Pooled AE rates for CD patients randomized to placebo and active comparator are summarized in Supplementary Figures 2 and 3, respectively.
Table 2.
Pooled proportion of patients experiencing adverse events in the placebo and active treatment groups and pooled risk difference in adverse events in randomized controlled trials for Crohn’s disease and ulcerative colitis
| Outcome | Pooled proportion among patients randomized to placebo [%] | Pooled proportion among patients randomized to active treatment [%] | Pooled risk difference among active treatment compared to placebo [%]a |
|---|---|---|---|
| Crohn’s disease trials | |||
| Any adverse event | 70.6 [65.8, 74.9] | 70.8 [65.7, 75.3] | −0.2 [−1.5, 1.2] |
| Serious adverse events | 10.4 [9.1, 11.9] | 9.5 [8.3, 11.0] | −0.1 [−1.1, 0.8] |
| Treatment-related withdrawal | 7.7 [6.5, 9.2] | 8.2 [7.1, 9.4] | 1.2 [−0.1, 2.4] |
| Infections | 15.3 [11.9, 19.4] | 15.9 [12.6, 19.9] | 1.0 [0.4, 1.7]* |
| Worsening Crohn’s disease | 12.5 [10.0, 15.5] | 7.7 [6.0, 10.0] | −3.2 [−4.8, −1.5]* |
| Ulcerative colitis trials | |||
| Any adverse event | 54.5 [48.5, 60.4] | 56.5 [50.0, 62.9] | 1.6 [0.1, 3.1]* |
| Serious adverse events | 6.3 [5.1, 7.9] | 5.7 [4.6, 7.0] | −0.3 [−1.1, 0.1] |
| Treatment-related withdrawal | 7.6 [5.9, 9.8] | 5.3 [4.1, 6.8] | −1.1 [−2.2, 0.0] |
| Infections | 16.7 [13.0, 21.2] | 19.9 [15.5, 25.1] | 2.9 [1.4, 4.4]* |
| Worsening ulcerative colitis | 15.0 [10.8, 20.5] | 9.9 [7.0, 13.9] | −3.7 [−5.7, −1.8]* |
Proportions pooled random-effects model, with associated 95% confidence intervals.
aAn asterisk indicates risk difference is statistically different from zero at a 5% significance. level.
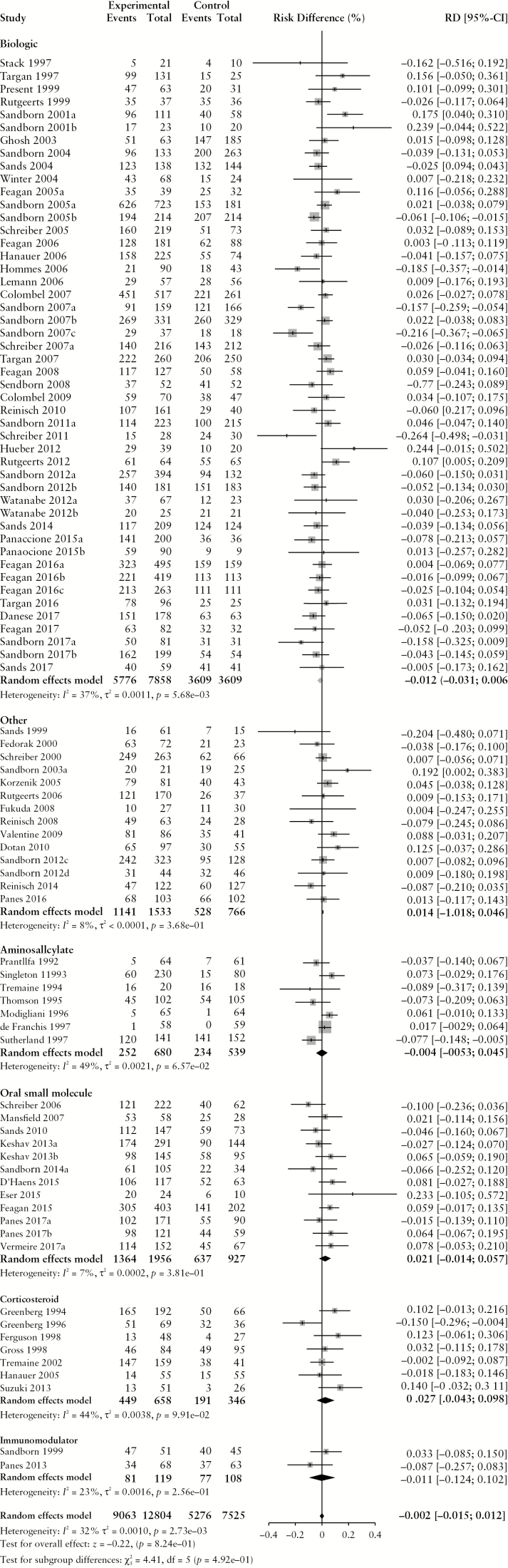
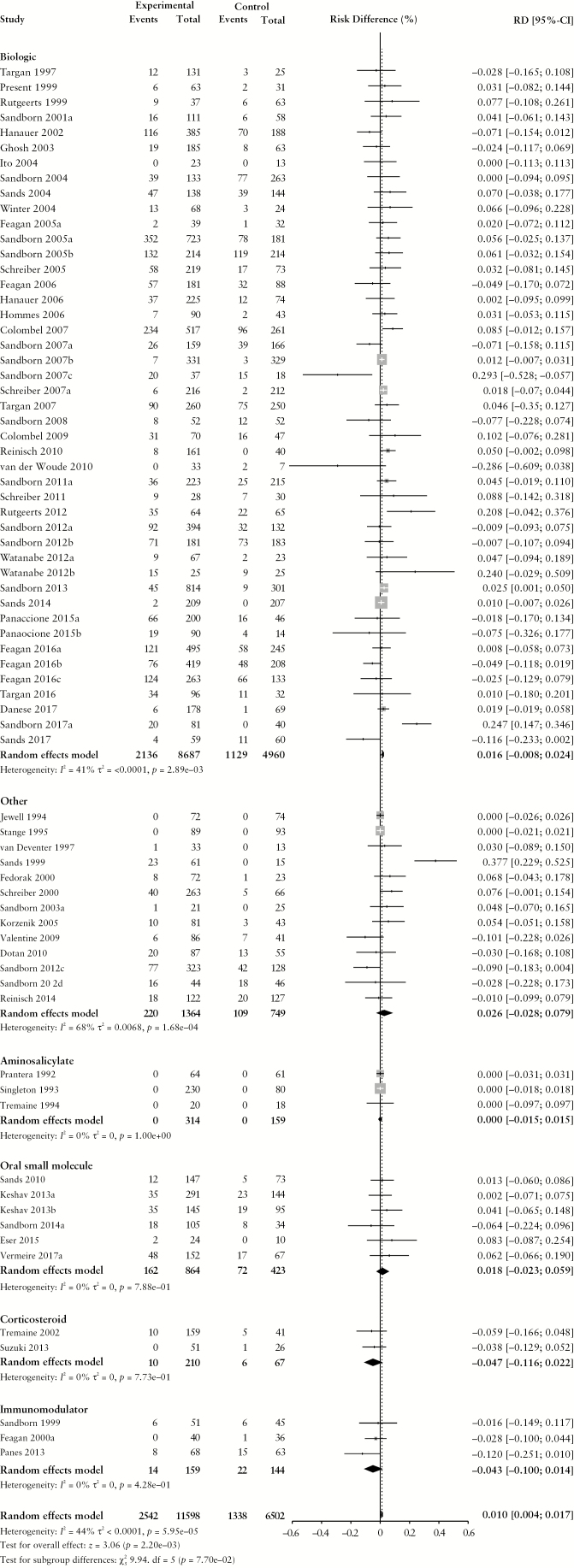
Figure 1.
Pooled risk difference of adverse event [A], serious adverse event [B], infectious adverse event [C] and worsening Crohn’s disease [D] rates, comparing patients treated with active comparator to placebo, stratified by active comparator class.
There were no differences in the pooled risk of SAEs (RD –0.1% [95% CI: –1.1%, 0.8%), or withdrawal due to AEs (RD 1.2% [95% CI: −0.1%, 2.4%) when comparing patients treated with active comparator to placebo. There was an increased risk of infections among patients treated with active comparator compared to placebo (RD 1.0% [95% CI: 0.4%, 1.7%]); the RD was significantly higher among patients receiving a biologic agent (RD 1.6% [95% CI: 0.8%, 2.4%]). The risk of CD worsening was significantly lower in the treatment group compared to placebo (RD –3.2% [95% CI: −4.8%, −1.5%]). By treatment class, the risk of worsening CD was significantly lower among patients treated with biologic agents (RD –4.4% [95% CI: –6.8%, –2.0%]) or corticosteroids (RD –16.2% [95% CI: –26.3%, –6.0%]). When the RD in AE and SAE rates was adjusted for active treatment class [Table 3], no statistically significant differences were found.
Table 3.
Risk difference, adjusted for active comparator class, associated with adverse events and serious adverse events in placebo-controlled randomized trials of patients with Crohn’s disease or ulcerative colitis
| Factor | Crohn’s disease | Ulcerative colitis |
|---|---|---|
| RD [%] [95% CI] | RD [%] [95% CI] | |
| Any adverse event [AE] | ||
| Active comparator | ||
| Biologic | −1.2 [−3.1, 0.6] | 2.0 [−0.1, 4.0] |
| Aminosalicylate | −0.2 [−2.5, 5.2] | −3.6 [−7.3, 0.1] |
| Oral small molecule | 2.0 [−2.0, 6.0] | −1.5 [−6.0, 2.9] |
| Corticosteroid | 2.6 [−3.1, 8.3] | 8.2 [4.4, 12.0] |
| Immunomodulator | −1.0 [−11.7, 9.8] | 2.5 [−14.0, 18.9] |
| Other | 1.4 [−2.5, 5.2] | 1.7 [−2.9, 6.4] |
| Any serious adverse event [SAE] | ||
| Active comparator | ||
| Biologic | −0.4 [−1.6, 0.9] | −1.4 [−2.7, −0.1] |
| Aminosalicylate | N/A | −0.1 [−2.2, 0.3] |
| Oral small molecule | 0.3 [−2.2, 2.8] | −1.5 [−3.5, 0.4] |
| Corticosteroid | −1.1 [−5.1, 2.9] | 0.9 [−0.1, 2.0] |
| Immunomodulator | −1.2 [−6.8, 4.3] | 2.2 [−8.4, 12.7] |
| Other | 1.0 [−1.6, 3.7] | 2.8 [0.6, 5.0] |
Abbreviations: CI, confidence interval; RD, risk difference.
Predictors of AEs in CD patients treated with placebo are summarized in Table 4. In univariable meta-regression, the risk of AEs among CD patients receiving placebo was higher in patients with moderate-to-severe disease activity at trial entry (OR 2.87 [95% CI: 1.49, 5.52] compared to remission) or intravenous [IV] dosing (OR 2.06 [95% CI: 1.25, 3.40] compared to oral) or subcutaneous [SC] [OR 2.34 [95% CI: 1.35, 4.03] compared to oral] Dosing. The rate of SAEs was higher in patients treated IV (OR 1.58 [95% CI: 1.09, 2.27] compared to oral) and lower among patients enrolled in RCTs where the active comparator was a corticosteroid (OR 0.33 [95% CI: 0.11, 0.96] compared to biologic therapy). No factors were statistically significantly associated with AE-related withdrawal in univariable meta-regression.
Table 4.
Univariable meta-regression of covariables associated with adverse events and serious adverse events in placebo-treated patients with Crohn’s disease or ulcerative colitis in placebo-controlled randomized trials.
| Factor | Crohn’s disease | Ulcerative colitis |
|---|---|---|
| OR [95% CI] | OR [95% CI] | |
| Any adverse event [AE] | ||
| Disease severity at trial entry | ||
| Remission | Reference | Reference |
| Mild-moderate | 0.66 [0.25, 1.74] | 1.04 [0.41, 2.63] |
| Moderate-severe | 2.87 [1.49, 5.52] | 2.57 [1.06, 6.22] |
| Study phase | ||
| Phase III | Reference | Reference |
| Phase II | 1.10 [0.70, 1.71] | 0.98 [0.58, 1.63] |
| Phase I | N/A | 2.66 [0.83, 8.55] |
| Study design | ||
| Induction | Reference | Reference |
| Maintenance | 0.65 [0.35, 1.22] | 0.66 [0.27, 1.61] |
| Induction and maintenance | 1.15 [0.68, 1.93] | 1.89 [1.15, 3.11] |
| Study setting | ||
| Multinational | Reference | Reference |
| Single nation, multicentre | 0.47 [0.28, 0.78] | 1.06 [0.55, 2.06] |
| Single centre | 1.80 [0.38, 8.51] | 0.51 [0.17, 1.57] |
| Publication year | ||
| Per 10-year increase | 1.26 [0.91, 1.73] | 1.51 [1.04, 2.18] |
| Active comparator | ||
| Biologic | Reference | Reference |
| Aminosalicylate | 0.19 [0.08, 0.45] | 0.36 [0.19, 0.65] |
| Oral small molecule | 0.80 [0.43, 1.50] | 0.76 [0.37, 1.58] |
| Corticosteroid | 0.39 [0.17, 0.88] | 0.35 [0.17, 0.74] |
| Immunomodulator | 1.05 [0.25, 4.36] | 1.46 [0.26, 8.11] |
| Other | 0.91 [0.49, 1.66] | 0.66 [0.33, 1.33] |
| Treatment route | ||
| Oral | Reference | Reference |
| Intravenous | 2.06 [1.25, 3.40] | 1.65 [0.99, 2.75] |
| Subcutaneous | 2.34 [1.35, 4.03] | 1.54 [0.84, 2.82] |
| Topical | 1.27 [0.19, 8.71] | 0.31 [0.16, 0.58] |
| Duration of follow-up | ||
| Per 1-week increase | 1.00 [0.99, 1.01] | 1.01 [0.99, 1.02] |
| Concomitant therapy | 1.09 [0.97, 1.23] | 1.22 [1.10, 1.35] |
| Per 10% increase in immunosuppressant use | 1.02 [0.93, 1.12] | 1.09 [1.01, 1.19] |
| Per 10% increase in corticosteroid use | ||
| Serious Adverse Events | ||
| Disease severity at trial entry | ||
| Remission | Reference | Reference |
| Mild-moderate | 0.46 [0.11, 1.85] | 1.38 [0.42, 4.48] |
| Moderate-severe | 1.61 [0.83, 3.16] | 4.89 [1.58, 15.14] |
| Study phase | ||
| Phase III | Reference | Reference |
| Phase II | 1.20 [0.89, 1.61] | 1.23 [0.75, 2.02] |
| Phase I | 2.36 [0.81, 6.82] | 1.37 [0.33, 5.65] |
| Study design | ||
| Induction | Reference | Reference |
| Maintenance | 1.31 [0.81, 2.09] | 0.39 [0.12, 1.32] |
| Induction and maintenance | 0.96 [0.70, 1.33] | 2.05 [1.33, 3.16] |
| Study setting | ||
| Multinational | Reference | Reference |
| Single nation, multicentre | 0.86 [0.51, 1.44] | 1.09 [0.57, 2.09] |
| Single centre | 1.42 [0.55, 3.67] | 1.48 [0.40, 5.56] |
| Publication year | ||
| Per 10-year increase | 0.84 [0.69, 1.03] | 1.02 [0.61, 1.71] |
| Active comparator | ||
| Biologic | Reference | Reference |
| Aminosalicylate | N/A | 0.19 [0.10, 0.37] |
| Oral small molecule | 0.88 [0.59, 1.33] | 0.60 [0.35, 1.02] |
| Corticosteroid | 0.33 [0.11, 0.96] | 0.16 [0.08, 0.29] |
| Immunomodulator | 1.63 [0.85, 3.11] | 0.72 [0.20, 2.68] |
| Other | 1.35 [0.91, 2.01] | 0.58 [0.34, 1.01] |
| Treatment route | ||
| Oral | Reference | Reference |
| Intravenous | 1.58 [1.09, 2.27] | 2.78 [1.70, 4.54] |
| Subcutaneous | 1.11 [0.75, 1.65] | 2.16 [1.23, 3.81] |
| Topical | 2.33 [0.76, 7.12] | 0.72 [0.34, 1.51] |
| Duration of follow-up | ||
| Per 1-week increase | 1.01 [1.00, 1.01] | 1.02 [1.01, 1.03] |
| Concomitant therapy | ||
| Per 10% increase in immunosuppressant use | 1.02 [0.92, 1.12] | 1.29 [1.17, 1.42] |
| Per 10% increase in corticosteroid use | 1.03 [0.96, 1.11] | 1.22 [1.14, 1.30] |
Abbreviations: CI, confidence interval; N/A, not applicable; OR, odds ratio.
3.3 Adverse event rates in ulcerative colitis
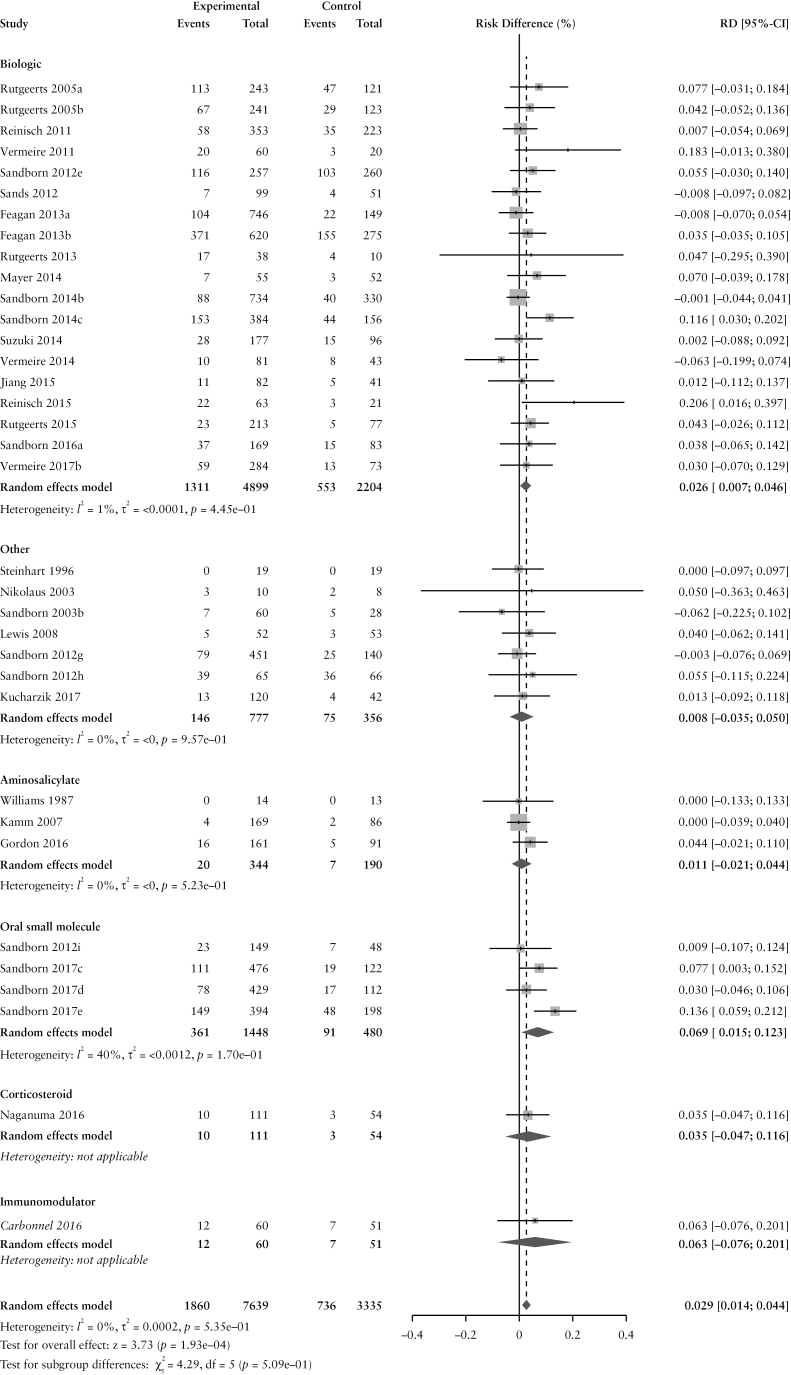
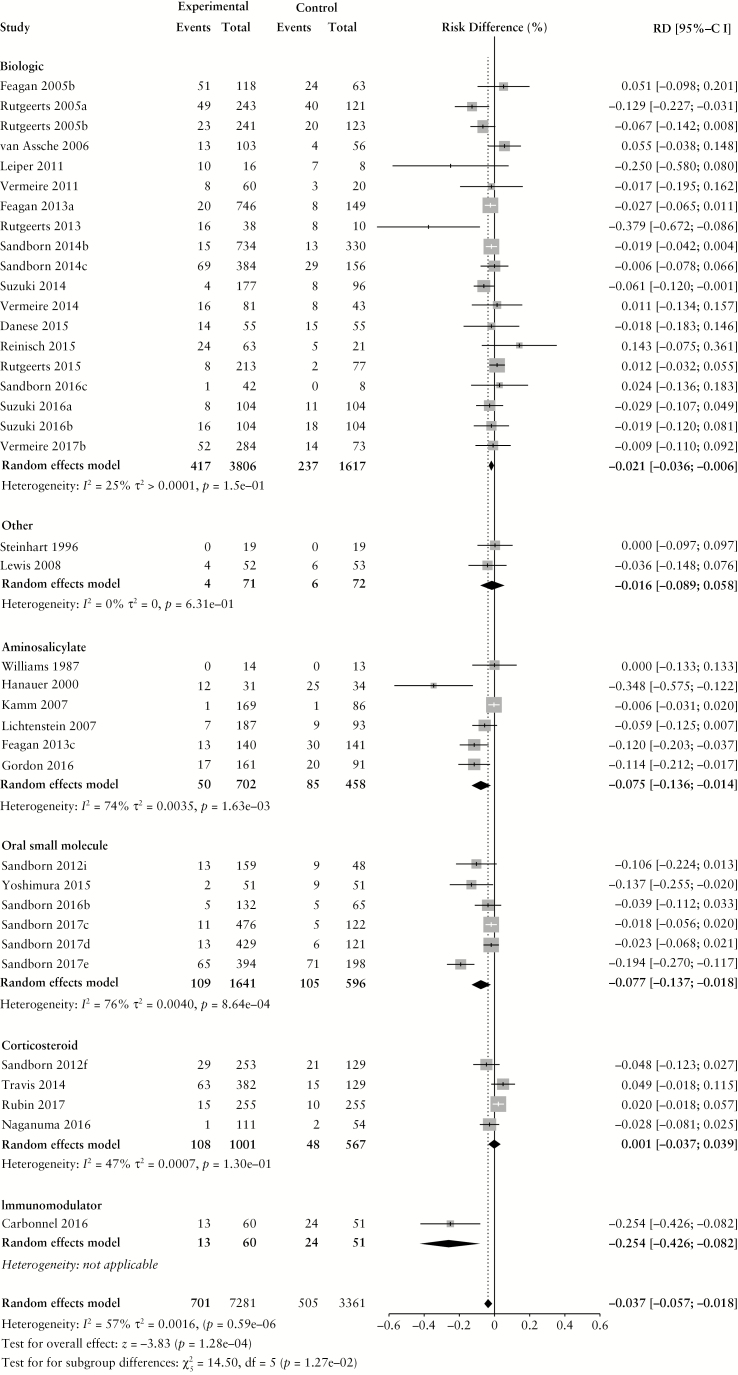
The overall pooled rate of AEs among UC patients randomized to placebo or active comparator is summarized in Table 2. Comparing patients receiving active treatment vs placebo, there was a higher risk of AEs with treatment (RD 1.6% [95% CI: 0.1%, 3.1%]) without statistically significant heterogeneity (χ2[59] = 71.58, p = 0.13; I2 = 18%) The pooled RD, stratified by active comparator class, is summarized in Figure 2. The pooled AE rate among UC patients randomized to placebo was 54.5% [95% CI: 48.5%, 60.4%] (χ2[59] = 766.62, p < 0.0001; I2 = 92%). The pooled AE rate among UC patients randomized to active comparator was 56.5% [95% CI: 50.0%, 62.9%] (χ2[59] = 1118.97, p < 0.0001; I2 = 95%). Pooled AE rates for UC patients randomized to placebo and active comparator are summarized in Supplementary Figures 4 and 5, respectively.
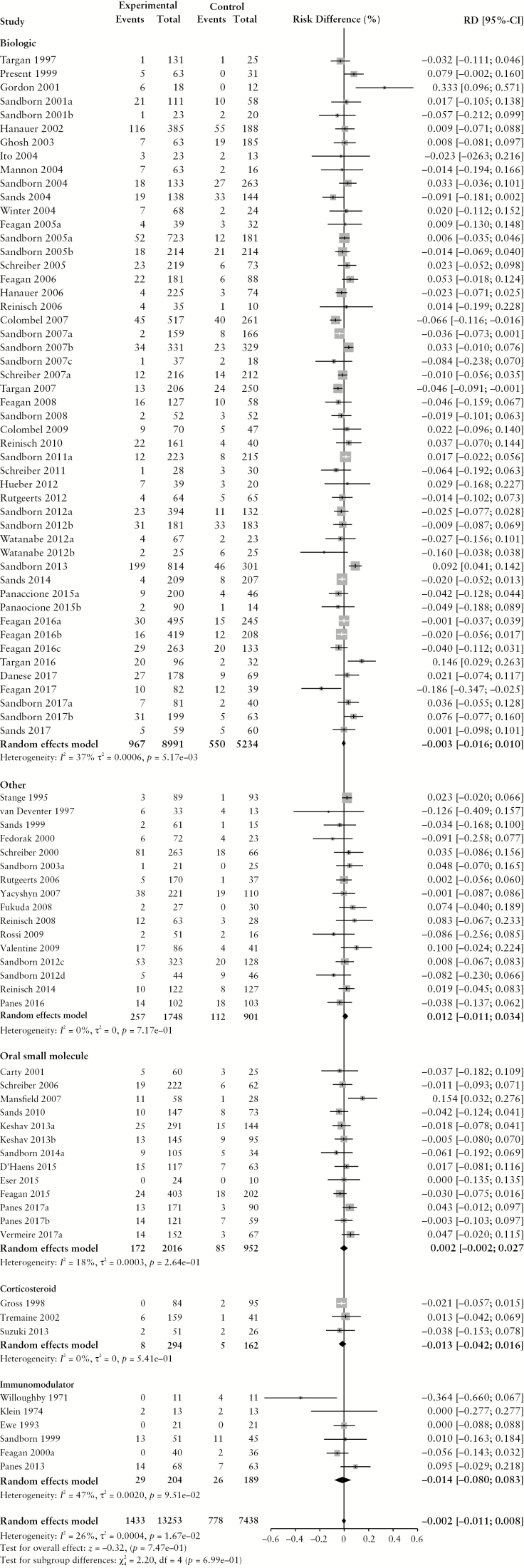
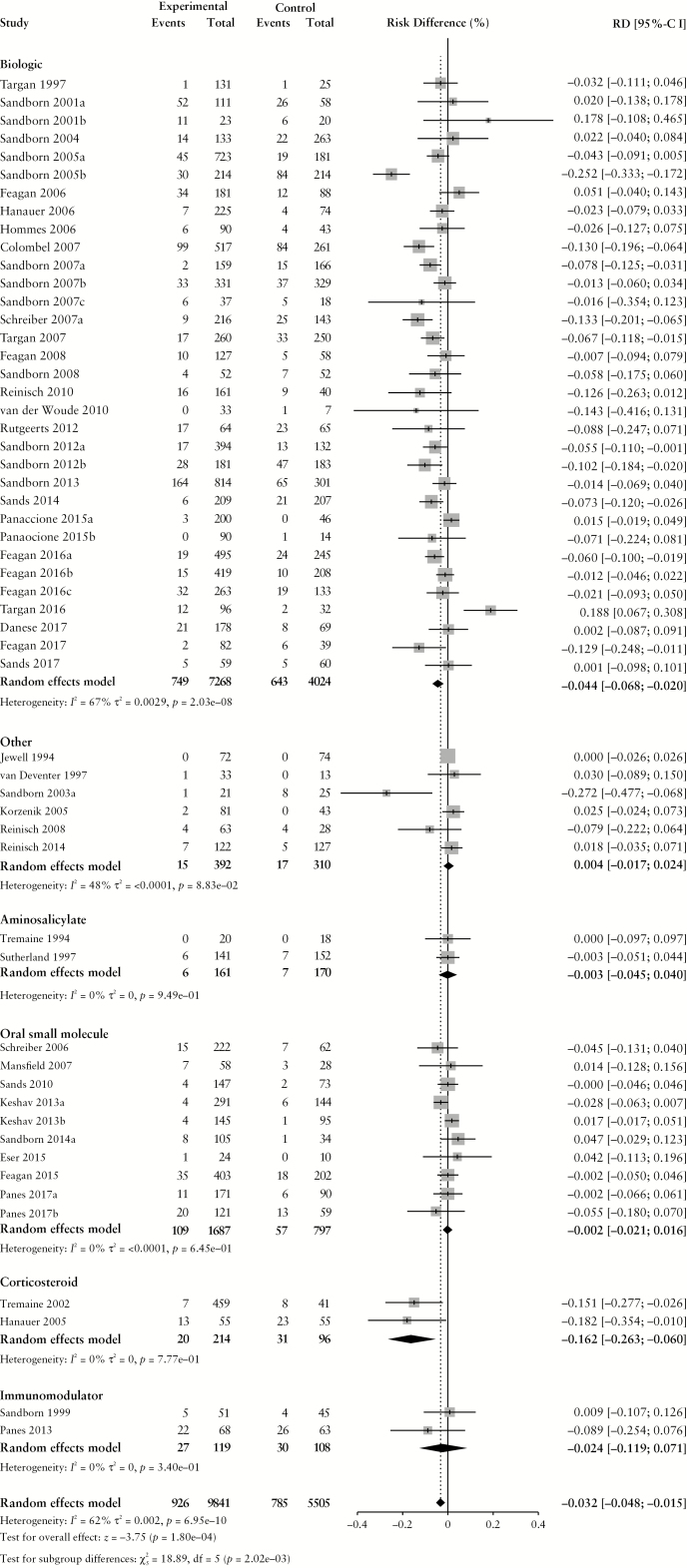
Figure 2.
Pooled risk difference of adverse event [A], serious adverse event [B], infectious adverse event [C] and worsening ulcerative colitis [D] rates, comparing patients treated with active comparator to placebo, stratified by active comparator class.
There were no differences in the pooled risk of SAEs (RD −0.3% [95% CI: −1.1%, 0.1%]), or withdrawal due to AEs (RD –1.1% [95% CI: –2.2%, 0.0%]) when comparing patients treated with active comparator vs placebo. There was an increased risk of infectious AEs among patients treated with active comparator compared to placebo (RD 2.9% [95% CI: 1.4%, 4.4%]); the RD was statistically significant among patients receiving a biologic agent (RD 2.7% [95% CI: 0.7%, 4.6%]) or an oral small molecule (RD 6.9% [95% CI: 1.5%, 12.3%]). The risk of UC worsening was significantly lower in the treatment group compared to placebo (RD –3.7% [95% CI: –5.7%, –1.8%]). Stratified by treatment class, the risk was significantly lower among patients treated with biologic agents, corticosteroids, aminosalicylates, oral small molecules and immunomodulators. After adjusting for active treatment class [Table 3], AE rates were greater in patients treated with corticosteroids compared to placebo [adjusted RD 8.2%, 95% CI: 4.4%, 12.0%]. SAE rates were lower in patients treated with biologics [adjusted RD -−1.4%, 95% CI: −2.7%, −0.1%] compared to placebo.
Predictors of AEs in UC patients treated with placebo are summarized in Table 4. In univariable meta-regression, moderate-to-severe disease activity at trial entry (OR 2.57 [95% CI: 1.06, 6.22] compared to remission), integrated induction and maintenance trial design (OR 1.89 [95% CI: 1.15, 3.11] compared to induction only), later date of publication (OR 1.51 [95% CI: 1.04, 2.18] per 10-year increment), concomitant immunosuppressant use (OR 1.22 [95% CI: 1.10, 1.35] per 10% increase), and concomitant corticosteroid use (OR 1.09 [95% CI: 1.01, 1.19]) increased the risk of AEs in UC patients randomized to placebo. SAEs were more likely among patients with moderate-to-severe disease (OR 4.89 [95% CI: 1.58, 15.14] compared to remission) at trial enrolment, enrolment in integrated induction/maintenance trials (OR 2.05 [95% CI: 1.33, 3.16] compared to stand-alone induction trials), IV treatment (OR 2.78 [95% CI: 1.70, 4.54]) or SC treatment (OR 2.16 [95% CI: 1.23, 3.81] compared to oral) and when concomitant immunosuppressants (OR 1.29 [95% CI: 1.17, 1.42] per 10% increase) or concomitant corticosteroids (OR 1.22 [95% CI: 1.14, 1.30] per 10% increase) were used.
3.4 Other safety outcomes
From all trials, a total of 37 deaths [0.09%] were reported. The time of death was not available in most trials so precise estimation of exposure time is unclear; however, based on the number of randomized patients and planned study follow-up duration, 30 deaths occurred in approximately 2.5 million patient-years of follow-up amongst patients randomized to active comparator and seven deaths occurred in 1.4 million patient-years of follow-up amongst patients randomized to placebo. The most commonly reported causes of death were cardiac events [n = 8] and sepsis/infection-related complications [n = 10]. A total of 76 malignancies [0.18%] were reported [28 in the placebo group, 48 in the active comparator groups]. The most common malignancies were dermatological [n = 19], primarily basal cell or squamous cell carcinomas. Ten cases of colorectal cancer and three cases of lymphoma were reported.
3.5 Publication bias
There was no evidence of publication bias for most outcomes [Supplementary Figure 4]. There was possible publication bias for the outcome of UC worsening [funnel plot regression test p = 0.012], probably due to selective reporting of this outcome.
4. Discussion
In addition to evaluating efficacy, clinical trials play an important role in identifying potential treatment-related AEs and safety signals. However, the nocebo effect plays an important role in the reporting of AEs and, consequently, influences RCT design and interpretation.13 This phenomenon has been well studied in trials of analgesics, statins and anti-depressants where negative perceptions of drug safety result in increased reports of subjective AEs.25 However, the influence of the nocebo effect has not been well evaluated in IBD, despite patients with CD and UC being prone to subjective gastrointestinal symptoms that are influenced by patient expectations, including nausea, food intolerance and abdominal pain.15 In this meta-analysis of AEs reported in all adult RCTs of conventional medical therapies for CD and UC, we found that the pooled rate of AEs among patients randomized to placebo was higher for CD compared to UC [~70% and ~50% respectively], with 1:10 CD patients and 1:15 UC patients developing a SAE over the course of the study duration, despite not receiving active treatment. These SAEs may in part be attributable to worsening of the underlying disease state and/or the use of concomitant medications. However, when AE, SAE and AE-related withdrawal rates between active treatment and placebo arms were compared, clinically relevant differences were not observed, suggesting that while RCTs are the most robust study design for assessing treatment efficacy, they have limitations for distinguishing differences in adverse outcomes.
The findings from this meta-analysis have important implications for trial design and interpretation. First, we identified a substantial 20% difference in absolute AE rates between CD and UC trials. We postulate that this may relate to the higher burden of non-specific symptoms experienced by patients with CD, encompassing both disease-related and disease-unrelated, as well as physical and psychological symptoms.26 The high background rate of AEs limits the statistical power for detecting true treatment-related differences between placebo and active comparator in RCTs. Designing an RCT to detect small differences in AE rates, which might feasibly be important in the setting of comparative effectiveness trials, may require infeasibly large sample sizes. In contrast, some AEs have enough specificity [e.g. infections] that they are less related to nocebo effects. Second, we demonstrated a significant, albeit small difference in AE rates between patients randomized to placebo and active comparator in patients with UC. Therefore, a possible ceiling to the nocebo effect may exist. To maximize trial efficiency, identifying the factors that may mitigate the nocebo response is critical. Generally, trial duration, study phase, study setting, publication year, follow-up duration and concomitant therapy were not consistently associated with the nocebo response. This highlights the need to assess individual patient data to identify potential patient-related predictors of the nocebo response and, more generally, to increase the ability to detect safety signals across multiple trials.
Treatment context is a crucial determinant of the nocebo response. The RCT setting itself may lead to the development of negative treatment expectations. During the informed consent process, presenting patients with an exhaustive list of potential AEs may facilitate future symptom misattribution. For example, in a trial of patients with unstable angina, Myers et al. identified that the listing of possible gastrointestinal side effects during informed consent resulted in a six-fold increase in withdrawals for gastrointestinal symptoms compared to when these risks were not explicitly disclosed.27 In our meta-regression, we identified parenteral placebo administration as being associated with AEs and SAEs. Interestingly, parenteral administration has also been previously associated with higher rates of positive placebo response,28,29 suggesting that the effect of IV or SC dosing may be mediated by modulating patient expectations of both benefit and harm.
Although minimizing the nocebo effect would be beneficial, strategies to do so have been poorly studied in the clinical trial environment, and those that may be effective in daily practice may not translate to RCTs. For example, providing less information about rare or irrelevant side effects in a ‘contextualized’ informed consent process has been proposed to reduce nocebo effects.30 However, withholding such information has ethical implications for patient autonomy that are magnified when patients are enrolling in a clinical trial.31 Some authors have proposed optimizing treatment expectations by using positive framing to focus on the higher proportion of patients who do not experience adverse outcomes.32,33 In clinical trials, this strategy may be difficult to adopt given the high proportion of patients [>50% in this meta-analysis] who will report AEs and the state of clinical equipoise with respect to treatment efficacy.34 However, counselling patients regarding the risk of worsening disease-related symptoms may reduce negative expectations of experiencing drug-related AEs. Third, Crichton and Petrie have proposed educating patients regarding the nocebo effect as part of the informed consent process.35 The scope and impact of this intervention require further investigation.
In addition to the nocebo effect, there are several other potential explanations for the high rates of AEs that we observed in this meta-analysis. First, both CD and UC are chronic, progressive diseases that accumulate irreversible bowel damage.36,37 Some reported AEs, particularly gastrointestinal symptoms, may reflect the natural history of untreated inflammation rather than the nocebo effect. This is empirically supported by the observation that RDs for worsening IBD are significantly higher for both CD and UC in patients treated with placebo compared to active drug. Furthermore, most trials included in this meta-analysis evaluated patients with moderate-to-severe disease who had already failed other therapies. These patients are at high risk of disease progression without novel treatment options, and in meta-regression more severe disease activity at trial entry was identified as a risk factor for higher placebo AE rates in both CD and UC. Second, AEs experienced in the placebo group may be attributable to concomitant therapies, particularly corticosteroids and immunosuppressants such as thiopurines and methotrexate. Side effects of corticosteroids are well documented and patients with IBD are at high risk of corticosteroid-related AEs due to repeated, high-dose and chronic exposure.38 However, we found small and inconsistent differences in AE rates after adjusting for concomitant corticosteroids or immunomodulator use.
A considerable degree of heterogeneity in the estimates of AE rates for both CD and UC were observed and were not completely explained in meta-regression. Some of this heterogeneity is a statistical artefact of the effect measure scale. When assessing risk between placebo and active treatment groups on an absolute [RD] or relative [relative risk] scale, the degree of heterogeneity is relatively low. Differences in AE reporting are also likely to contribute to this heterogeneity, a covariable that is difficult to capture based on published data alone without individual trial protocols. Furthermore, there is heterogeneity in whether individual trial authors considered worsening IBD as an AE. Systematic collection of AEs in clinical trials is likely to result in higher AE capture rates compared to spontaneous patient reporting.39 Thus, standardized outcome assessment is required as implicit in the Medical Dictionary for Regulatory Activities [MedDRA] and National Cancer Institute Common Terminology Criteria for Adverse Events [CTCAE].40,41 This is particularly important for mitigating potential nocebo effects because subjective symptoms such as myalgias or fatigue may be inconsistently described.
Importantly, our meta-analysis demonstrates a lack of clinically significant RDs in AE, SAE and AE-related withdrawal rates between active treatment and placebo groups. This emphasizes two important concepts. First, while it has been previously posited that placebo groups are essential for determining the relative safety of treatment,42 our findings underline that RCTs are not necessarily the ideal study design for evaluating adverse outcomes. Rare and serious AEs occur in only a minority of patients and RCTs are underpowered to detect these outcomes based on their limited sample size and follow-up duration. Furthermore, clinical trial populations are highly restricted and designed to enroll younger, healthier patients without comorbidities, who are inherently less likely to experience AEs compared to the general population who would be subsequently treated with the drug in a real world situation.43 Therefore, long-term prospective post-marketing registries are essential to adequately characterize the safety profile of novel therapies.44 Second, the high rate of AEs in the active treatment arm and lack of an RD compared to placebo suggests that AE reporting among patients receiving active therapy may also be subject to nocebo effects. This may be differentiated in a multiple treatment allocation trial where patients are randomized to active comparator, placebo or no treatment25; however, this design is unlikely to be ethically acceptable.
Our study has limitations. First, we were unable to capture differences in AE recording methodology, which is a potential source of heterogeneity. While we used AE definitions as reported by the original study authors, these may feasibly vary by publication year, by investigator vs sponsor-initiated trials, and by monitoring plan. Second, assessing the predictors of nocebo response among patients randomized to placebo would best be accomplished using individual rather than trial-level data, which would permit controlling for potential confounders such as disease duration, disease activity and previously failed therapies. Furthermore, individual patient data are required to adjust risk estimates for exposure time, but this is not possible with trial-level data alone. Third, in pooled analysis from trial-level data, we were able to determine the proportion of patients experiencing AEs although it is plausible that there are some patients who will experience multiple AEs and this patient subset has not been well characterized. Fourth, we did not include trials of complementary therapies, probiotics or antibiotics. The rationale for this decision was two-fold. First, we based our meta-analysis on previously published systematic reviews that excluded complementary therapies to focus on conventional IBD treatments. Second, differentiating nocebo effects in the placebo group from the active treatment arm may be biased in studies of complementary therapy given that the true treatment effect of most complementary therapies is unclear.
Finally, we recognize that not all AEs occurring in the placebo group are related to the nocebo effect, nor are all AEs occurring in the active comparator group treatment-related. Rather, both nocebo- and non-nocebo-related factors contribute to AE reporting, regardless of treatment assignment. Some AEs occurring in the placebo group may be due to disease progression and, conversely, the nocebo effect may contribute to AE reporting in patients receiving active therapy, particularly given the close medical contacts that occur throughout the course of an RCT. The precise attributable risk in each arm is difficult to distinguish although this meta-analysis offers a detailed evaluation of the AE RD between placebo and active comparator across multiple therapies.
In conclusion, we conducted a comprehensive systematic review and meta-analysis demonstrating that patients randomized to placebo in IBD RCTs have a high risk of reporting AEs, related to both nocebo and non-nocebo factors. When active treatment and placebo groups were compared, there were no clinically significant differences in safety outcomes, highlighting the importance of non-RCT study designs for accurately documenting treatment-related AEs. Further investigations are required to determine patient-level predictors of the nocebo response in IBD.
Funding
C.M. is supported by a Clinician Fellowship from the Canadian Institutes of Health Research and the Canadian Association of Gastroenterology. N.V.C. is supported by a Research Scholar Award from the American Gastroenterological Association. S.S. is supported by the American College of Gastroenterology Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award.
Conflict of Interest
C.M., N.P. and I.H. have no conflicts of interest to declare. T.N., L.G. and C.P. are employees of Robarts Clinical Trials, Inc. N.V.C. has received consulting fees from MSD, Janssen, Pfizer, UCB, and Takeda; and speaker’s bureau fees from Abbvie. R.K. has received scientific advisory board fees from AbbVie, Janssen, Pfizer, Takeda; consulting fees from AbbVie, Janssen, Takeda, Robarts Clinical Trials; payments for lectures/speakers’ bureau fees from AbbVie, Janssen, Shire and Takeda. P.D. has received research support, honorarium and travel support from Takeda; research support from Pfizer; and serves on the advisory board for Janssen. S.S. has received research support from Pfizer and AbbVie; and consulting fees from AbbVie. B.F. has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma Inc., and Sigmoid Pharma; and speaker’s fees from UCB, AbbVie, and J&J/Janssen. V.J. has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert and Celltrion; and speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie and Pfizer.
Author Contributions
C.M. contributed to study concept and design, data acquisition, data interpretation, manuscript drafting and editing. N.P., T.M.N., C.E.P. and I.M.H. contributed to data collection and manuscript editing. L.G. contributed to data analysis and interpretation and manuscript editing. N.V.C., R.K., P.S.D., S.S. and B.G.F. contributed to manuscript editing. V.J. contributed to study concept and design, data interpretation, manuscript drafting and editing, and study supervision. All authors approved the final version of the manuscript. V.J. is guarantor of the manuscript.
Supplementary Material
References
- 1. Hemperly A, Sandborn WJ, Vande Casteele N. Clinical pharmacology in adult and pediatric inflammatory bowel disease. Inflamm Bowel Dis 2018;24:2527–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levesque BG, Sandborn WJ, Ruel J, Feagan BG, Sands BE, Colombel JF. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.e1. [DOI] [PubMed] [Google Scholar]
- 3. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol 2013;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benedetti F, Shaibani A. Nocebo effects: more investigation is needed. Expert Opin Drug Saf 2018;17:541–3. [DOI] [PubMed] [Google Scholar]
- 5. Gupta A, Thompson D, Whitehouse A, et al.; ASCOT Investigators Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017;389:2473–81. [DOI] [PubMed] [Google Scholar]
- 6. Kleine-Borgmann J, Bingel U. Nocebo effects: neurobiological mechanisms and strategies for prevention and optimizing treatment. Int Rev Neurobiol 2018;138:271–83. [DOI] [PubMed] [Google Scholar]
- 7. Faasse K, Grey A, Horne R, Petrie KJ. High perceived sensitivity to medicines is associated with higher medical care utilisation, increased symptom reporting and greater information-seeking about medication. Pharmacoepidemiol Drug Saf 2015;24:592–9. [DOI] [PubMed] [Google Scholar]
- 8. Horne R, Faasse K, Cooper V, et al.. The perceived sensitivity to medicines (PSM) scale: an evaluation of validity and reliability. Br J Health Psychol 2013;18:18–30. [DOI] [PubMed] [Google Scholar]
- 9. Lorber W, Mazzoni G, Kirsch I. Illness by suggestion: expectancy, modeling, and gender in the production of psychosomatic symptoms. Ann Behav Med 2007;33:112–6. [DOI] [PubMed] [Google Scholar]
- 10. Rheker J, Winkler A, Doering BK, Rief W. Learning to experience side effects after antidepressant intake - results from a randomized, controlled, double-blind study. Psychopharmacology [Berl] 2017;234:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA 2002;287:622–7. [DOI] [PubMed] [Google Scholar]
- 12. Benedetti F, Durando J, Vighetti S. Nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway. Pain 2014;155:921–8. [DOI] [PubMed] [Google Scholar]
- 13. Carlino E, Vase L. Can knowledge of placebo and nocebo mechanisms help improve randomized clinical trials? Int Rev Neurobiol 2018;138:329–57. [DOI] [PubMed] [Google Scholar]
- 14. Odinet JS, Day CE, Cruz JL, Heindel GA. The biosimilar nocebo effect? A systematic review of double-blinded versus open-label studies. J Manag Care Spec Pharm 2018;24:952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 2015;12:472–85. [DOI] [PubMed] [Google Scholar]
- 16. Irvine EJ. Review article: patients’ fears and unmet needs in inflammatory bowel disease. Aliment Pharmacol Ther 2004;20[Suppl 4]:54–9. [DOI] [PubMed] [Google Scholar]
- 17. Ma C, Hussein IM, Al-Abbar YJ, et al.. Heterogeneity in definitions of efficacy and safety endpoints for clinical trials of Crohn’s disease: a systematic review. Clin Gastroenterol Hepatol 2018;16:1407–1419.e22. [DOI] [PubMed] [Google Scholar]
- 18. Lee MJ, Parker CE, Taylor SR, et al.. Efficacy of medical therapies for fistulizing Crohn’s disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16:1879–92. [DOI] [PubMed] [Google Scholar]
- 19. Ma C, Panaccione R, Fedorak RN, et al.. Heterogeneity in definitions of endpoints for clinical trials of ulcerative colitis: a systematic review for development of a core outcome set. Clin Gastroenterol Hepatol 2018;16:637–647.e13. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gøtzsche PC, et al.; Cochrane Bias Methods Group; Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010;29:3046–67. [DOI] [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software 2010;36:48. [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howick J, Webster R, Kirby N, Hood K. Rapid overview of systematic reviews of nocebo effects reported by patients taking placebos in clinical trials. Trials 2018;19:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farrell D, McCarthy G, Savage E. Self-reported symptom burden in individuals with inflammatory bowel disease. J Crohns Colitis 2016;10:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myers MG, Cairns JA, Singer J. The consent form as a possible cause of side effects. Clin Pharmacol Ther 1987;42:250–3. [DOI] [PubMed] [Google Scholar]
- 28. Jairath V, Zou G, Parker CE, et al.. Systematic review with meta-analysis: placebo rates in induction and maintenance trials of Crohn’s disease. Aliment Pharmacol Ther 2017;45:1021–42. [DOI] [PubMed] [Google Scholar]
- 29. Ma C, Guizzetti L, Panaccione R, et al.. Systematic review with meta-analysis: endoscopic and histologic placebo rates in induction and maintenance trials of ulcerative colitis. Aliment Pharmacol Ther 2018;47:1578–96. [DOI] [PubMed] [Google Scholar]
- 30. Wells RE, Kaptchuk TJ. To tell the truth, the whole truth, may do patients harm: the problem of the nocebo effect for informed consent. Am J Bioeth 2012;12:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller FG. Clarifying the nocebo effect and its ethical implications. Am J Bioeth 2012;12:30–1. [DOI] [PubMed] [Google Scholar]
- 32. Bingel U; Placebo Competence Team Avoiding nocebo effects to optimize treatment outcome. JAMA 2014;312:693–4. [DOI] [PubMed] [Google Scholar]
- 33. Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov 2013;12:191–204. [DOI] [PubMed] [Google Scholar]
- 34. Petrie KJ, Rief W. Psychobiological mechanisms of placebo and nocebo effects: pathways to improve treatments and reduce side effects. Annu Rev Psychol 2019;70:599–625. [DOI] [PubMed] [Google Scholar]
- 35. Crichton F, Petrie KJ. Health complaints and wind turbines: The efficacy of explaining the nocebo response to reduce symptom reporting. Environ Res 2015;140:449–55. [DOI] [PubMed] [Google Scholar]
- 36. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 37. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018;16:343–356.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waljee AK, Wiitala WL, Govani S, et al.. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One 2016;11:e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rief W, Nestoriuc Y, von Lilienfeld-Toal A, et al.. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta-analysis. Drug Saf 2009;32:1041–56. [DOI] [PubMed] [Google Scholar]
- 40. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999;20:109–17. [DOI] [PubMed] [Google Scholar]
- 41. Trotti A, Colevas AD, Setser A, et al.. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81. [DOI] [PubMed] [Google Scholar]
- 42. Danese S, Schabel E, Masure J, Plevy S, Schreiber S. Are we ready to abandon placebo in randomised clinical trials for inflammatory bowel disease? Pros and cons. J Crohns Colitis 2016;10[Suppl 2]:S548–52. [DOI] [PubMed] [Google Scholar]
- 43. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012;10:1002–7; quiz e78. [DOI] [PubMed] [Google Scholar]
- 44. Lichtenstein GR, Feagan BG, Cohen RD, et al.. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol 2006;4:621–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.