Abstract
Background:
Myocardial infarction (MI) is the most severe ischemic heart disease and di-rectly leads to heart failure till death. Target molecules have been identified in the event of MI including increasing angiogenesis, promoting cardiomyocyte survival, improving heart function and restraining inflammation and myocyte activation and subsequent fibrosis. All of which are substantial in cardiomy-ocyte protection and preservation of cardiac function.
Methodology:
To modulate target molecule expression, virus and non-virus-mediated gene transfer have been investigated. Despite successful in animal models of MI, virus-mediated gene transfer is hampered by poor targeting efficiency, low packaging capacity for large DNA sequences, immunogenicity induced by virus and random integration into the human genome.
Discussion:
Nanoparticles could be synthesized and equipped on purpose for large-scale production. They are relatively small in size and do not incorporate into the genome. They could carry DNA and drug within the same transfer. All of these properties make them an alternative strategy for gene transfer. In the review, we first introduce the pathological progression of MI. After concise discussion on the current status of virus-mediated gene therapy in treating MI, we overview the history and development of nanoparticle-based gene delivery system. We point out the limitations and future perspective in the field of nanoparticle vehicle.
Conclusion:
Ultimately, we hope that this review could help to better understand how far we are with nanoparticle-facilitated gene transfer strategy and what obstacles we need to solve for utilization of na-nomedicine in the treatment of MI.
Keywords: Myocardial infarction, cardiomyocytes, angiogenesis, inflammation, gene transfer, nanoparticles
1. INTRODUCTION
Ischemic heart disease (IHD) is the leading cause of morbidity and mortality for decades [1]. Among IHD, myocardial infarction is the most serious one and directly leads to heart failure. Atherosclerosis is the fundamental pathological basis of IHD, resulting from endothelial injury [2, 3], lipoprotein sub-endothelium retention [3, 4], inflammatory infiltration and accumulation [5-7] and collagen deposition [8]. As atherosclerotic plaque grows, it narrows coronary arteries and limits blood supply to cardiomyocytes. Myocardial infarction happens when severe coronary stenosis blocks blood flow and deprives oxygen and nutrient supply. Reperfusion exposes cardiomyocytes under oxidative stress which further accelerates cell death. In response to cell apoptosis, innate immune cells, and subsequently, T lymphocytes and B lymphocytes infiltrate into the infarcted area for further cardiomyocyte destruction. For instance, following myocardial infarction, chronic β-adrenergic activation is a potent stimulator for the production of TNF-α, IL-1β, IL-6 and IL-18, all of which contribute significantly to myocardial damage and accelerate fibrotic progression [9]. Adult mammalian cardiomyocytes carry low proliferative potential and could not replace the dead cardiomyocytes [10]. Therefore, insufficient healing results in scar formation and heart failure. By far, accumulated studies have demonstrated how cardiomyocytes are subjected to apoptosis and how inflammation stimulates fibrosis, the key questions/strategies focus on improving angiogenesis to protect ischemic cardiomyocytes and preserve heart function, regenerating cardiomyocytes and restraining inflammation and ventricular remodeling.
1.1. Angiogenesis for Cardiomyocytes Survival and Function
Early thrombolysis and stenting reopen stenosed coronary vessel and recapitulate the supply of oxygen and nutrients to promote cardiomyocyte survival in the acute phase. Nevertheless, some patients miss the best chance for thrombolysis and catheter intervention. When these patients are in a chronic stage, how to minimize cardiomyocyte damage, maintain cell function and inhibit the progression of fibrosis are under investigation.
Manipulating key angiogenic factors not only protect coronary endothelial cells but also assist new vessel formation. In the mice received coronary ligation and lectin and hypoxyprobe injection, Kobayashi et al. demonstrated that new vessels developed from the endocardium on day 3 in the ischemic area and became mature on day 14. These primitive vessels are independent from coronary circulation but could perfuse ischemic area with oxygen supply. They further showed that VEGF-VEGFR2 signaling pathway was crucial in the formation of primitive vessels [11].
VEGF is a very potent factor to stimulate angiogenesis. Among these family members, VEGF-B is the most abundantly expressed in cardiomyocytes [12]. Huusko et al. injected adenoviral vector containing VEGF-A, or VEGF-B or VEGF-E into the anterior wall of the left ventricle in C57BL/6 mice. By ultrasound and perfusion analyses, they found that VEGF-B- and VEGF-E-induced angiogenesis was more physical than that of VEGF-A. Although neither injection altered left ventricular function, VEGF-A had more side effects than VEGF-B and VEGF-E [13]. In agreement with this report, when rats underwent I/R injury and then VEGF-B injection, it increased Akt phosphorylation and Bcl-2 expression, reduced p38MAPK phosphorylation, all of which contributed to the inhibition of autophagy for cell survival [14]. Topical expression of VEGF-B by adeno- or AAV-9-mediated gene transfer could increase the density of the capillary area and cardiomyocyte proliferation and enhance cardiac function in mice model with myocardial infarction [15, 16]. Unlike VEGF-B, the role of VEGF-C in cardiomyocytes is uncertain. On one hand, in a rat I/R model with pretreatment of VEGF-C in the left ventricle myocardium, VEGF-C/VEGFR2 activates Akt phosphorylation and inhibits Bax expression, leading to increased cardiomyocyte survival and function [17]. On the other hand, binding to its receptor VEGF-R3 on myofibroblasts, VEGF-C could activate TGF-β1 and ERK phosphorylation and participate fibrosis [18].
1.2. Improving Cardiac Function
Except angiogenesis that could promote cardiomyocyte survival with function, calcium stimulates cardiomyocyte contraction, and thus, is an important mediator for cardiac function. Cardiac action potential consists of two cycles, a rest phase and an active phase. Ca2+ influx into cytoplasmic compartment depolarizes cardiomyocyte contraction. Immediately after that, Ca2+ is removed from cytosol for Ca2+ homeostasis. The Ca2+ efflux is controlled by Sarco/Endoplasmic reticulum Ca2-ATPase (SERCA-2a), a calcium ATPase in the sarcoplasmic reticulum in cardiomyocytes. As the Ca2+ transporter, it facilitates Ca2+ transportation from cytosolic compartment to the Sarcoplasmic Reticulum. In cardiomyocyte-specific SERCA-2-/- mice, Ca2+ transient amplitude was reduced which was accompanied with O2 consumption dysfunction [19]. In the patients with heart failure, calcium cycling was impaired partially due to decreased SERCA-2 activity [20]. By contrast, direct [21] and indirect [22, 23] increase of SERCA-2 expression improved energy utilization and cardiac contractility. Apart from that, connexin 43 has been identified as the major mediator of intracellular Ca2+ propagation between cardiomyocytes [24]. Down-regulation of connexin 43 could enhance cardiomyocyte proliferation under myocardial infarction [24].
1.3. Restraining Inflammation and Myofibroblast Activation
Inflammation is the main drive for cardiomyocyte fibrosis and cardiac remodeling. In the presence of MI, endothelial cells become activated and express a series of adhesion molecules to attract neutrophils, macrophages, monocytes and lymphocytes for infiltrating into injured site [25, 26]. These inflammatory cells release inflammatory cytokines such as IL-1β, TNF-a and IL-17A that strengthen cardiomyocyte apoptosis [27-29], MMPs for matrix degradation [30, 31] and myofibroblast activation [32, 33].
Beside inflammatory cells, β1-adrenergic receptor (β1-AR) and mineralocorticoid receptor (MR) pathways are activated in cardiomyocytes, both of which stimulate inflammatory cytokine production to exaggerate inflammation cascade. From the mechanism view, stress activates β1-adrenergic receptor (β1-AR) on cardiomyocytes for reactive oxygen species production, which, in turn, increases inflammasome component NLRP3 production for caspase-1 activation. Activated caspase-1 cleaves pro-IL-18 into active IL-18 to further reinforce inflammation. In contrast, blockade of IL-18 by neutralizing antibody reverted cardiac inflammation and fibrosis [9].
The mineralocorticoid aldosterone is produced and secreted from adrenal gland to regulate water and electrolyte homeostasis. By cell-type-specific gene targeting, MR is detected in extra-renal cells including endothelial cells, vascular smooth cells, macrophages and cardiomyocytes in mice [34]. MR pathways are involved in inflammation and fibrosis in cardiomyocyte infarction by the following evidence: (1) activation of MR pathways in endothelial cells could stimulate adhesion molecule expressions such as vascular adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) [35, 36]; (2) NGAL (neutrophil gelatinase-associated lipocalin) promotes cardiac damage and remodeling which is a downstream target of MR activation [37]; (3) deletion of MR in VSMCs improves left ventricular dysfunction in mice MI model [38]; and (4) binding of aldosterone to MR induces a panel of fibrotic molecule expression in cardiomyocytes including activation of extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK) and p38MAPK, transforming growth factor (TGF)-β1 pathways and increased production of collagen and α-smooth muscle actin (SMA). Inhibition of MR expression abolishes the above fibrotic marker protein expression [39].
2. VIRUS-MEDIATED GENE TRANSFER IN THE TREATMENT OF MI
2.1. Virus as Vectors for Modulating Candidate Protein Expression
As we described above, MI is a complicated process, among which, key factors are critically involved in cardiomyocyte death, calcium handling, inflammation and scar formation. Selective overexpression or deletion of the key factor could promote cardiomyocyte survival and attenuate inflammation and fibrosis.
By far, virus-mediated gene transfer remains the most potent means to modulate certain protein expression in animal models. Both systemic and topical gene transfers by different types of viral vectors have shown beneficial effects in various animal MI models. Adenoviruses and adenovirus-associated viruses are more frequently used than retroviruses and lentiviruses, because these vectors could induce sustained gene expression for months and they do not integrate into the host genome [40-43]. Table 1 lists the effects of virus-mediated gene transfer in animal models of MI. As shown in Table 1, they can be generalized as promotion of angiogenesis, [44-62] regulation of SERCA2a expression, [22, 63-67] and enhancement of cardiac functions partially via modifying β2-adrenergic signaling pathways [68-71] by virus-mediated gene transfer.
Table 1. Summary of the effects of virus-mediated gene transfer in animal models of myocardial infarction.
| Molecular Target | Vector Type | Animal MI Models | Results |
|---|---|---|---|
| Angiogenesis | |||
| CD151 [44] | rAAV, local | Rat | Increased VEGF expression and improved left ventricular function |
| VEGF [45-49] | Adenovirus, local | Rat, sheep | Improved ejection fraction; reduced myocardial fibrosis |
| β-adrenoceptor [50] | Ad | rats | Activation of VEGF pathway for increased angiogenesis and global contractility |
| Fibroblast growth factor 9 [51, 52] | Ad | mice | Conditional expression of FGF9 promotes myocardial vascularization and hypertrophy with enhanced systolic function and reduced heart failure mortality after MI. |
| Endothelial nitric oxide synthase [53-56] | Ad | rat | eNOS provided cardiac protection after myocardial infarction injury through inhibition of cardiac apoptosis and collagen deposition, and suppression of TGF-β1 |
| Stromal cell-derived factor or CXCR4 [57, 58] | Ad | Rat | SDF-1 alpha could improve cardiac structure and function after Myocardial infarction through angiogenic and anti-fibrotic actions. |
| Hepatic growth factor [59-61] | Ad | Rabbit, rat, canine | improved left ventricular ejection fraction and fractional shortening, reduced the fibrotic area, and increased the capillary density in the risk area. |
| apoA-I [62] | Ad | mice | Increased endothelial progenitor number and function and the peak rate of isovolumetric relaxation by AdapoA-I |
| SERCA2a expression | |||
| SERCA2a [63, 64] | AAV1; Lentivirus | Sheep, rat | Improved contractility, reduced myocyte apoptosis and myocyte hypertrophy |
| antisense phospholamban (asPLB) [22] | AAV | rat | enhanced myocardium SERCA activity; prevented the progression of heart failure |
| Urocortin-2 [65] | AAV8 | mice | increased LV systolic and diastolic function |
| Small ubiquitin-like modifier 1 (SUMO-1) [66] | AAV1 | swine | improved cardiac function and stabilized LV volumes |
| S100A1 [67] | AAV9, | porcine | Prevented heart failure and reduced scar side |
| Cardiomyocyte preservation and regeneration | |||
| Stem cell factor [68] | Adenovirus | Swine | Recruitment of cKit+ cells, improved cardiac function |
| Connexin43 [69] | Ad | pig | Targeted manipulation of Cx43 levels improved conduction velocity and reduced ventricular tachycardia susceptibility. |
| G protein-coupled receptor kinase 2 (GRK2) [70, 71] | scAAV | Sheep, pig | preservation of regional and global systolic function |
2.2. Virus-mediated Gene Transfer in Reprogramming Stem Cells for Cardiac Repair
Following the appearance of induced pluripotent stem cells (iPSCs), reprogramming of cardiac fibroblasts into induced cardiomyocytes holds great promise for regenerating functional cardiomyocytes after MI.
When fibroblasts are induced with Gata4, Mef2c and Tbx5 expression, these factors are sufficient to drive fibroblasts transdifferentiating into cardiomyocytes [72-74]. As adenovirus and lentivirus vectors are often used in reprogramming, Mathison et al. compared their potency and efficacy in a rat model of MI. Three weeks after coronary ligation, rats were injected with adenovirus or Lentivirus, encoding the cocktail of Gata4, Mef2c and Tbx5. By immunohistochemistry, cardiomyocyte marker troponin T expression was comparable and injection fraction was increased in a similar extent in both Ad and lentivirus gene transfer groups shown by echocardiography. These data imply that Adeno and lentiviral vectors are equally effective in inducing fibroblast transdifferentiating into induced cardiomyocyte-like cells with function [72]. Despite promising, the main obstacles in cell reprogramming are low transfection efficiency, time-consumption and genome integration.
Recently, Miyamoto et al. demonstrated that Sendai vectors encoding Gata4, Mef2c and Tbf5 could rapidly reprogram fibroblasts into induced cardiomyocyte-like cells without any sign of integration. In mouse fibroblasts, Sendai virus system generated 100-fold more beating induced cardiomyocyte-like cells than retroviral-GMT and the duration to induce beating cells was shortened from 30 to 10 days. Accordingly, by in vivo lineage tracing, injection of Sentai virus encoding the factors above was more potent to induce cardiomyocyte-like cell expression with improved cardiac function and reduced fibrosis when compared with retroviral-Gata4/Mef2c/Tbf5 group [74].
2.3. Genetic Modified MSC for MI Treatment
Except reprogramming, mesenchymal stem cells (MSCs) are multipotent stem cells derived from the mesoderm of early-phase embryos. They are self-renewable and capable of differentiating into a variety of cell types, such as osteoblasts, cartilage, skeletal muscle, tendon, fat, endothelial cells and nerves [75]. In addition, MSCs could produce a variety of proteins and RNAs, so-called secretome and exome, to attenuate inflammation and enhance angiogenesis [76, 77]. Delivery of MSCs or subpopulation of MSCs to animal models of acute myocardial infarction improves left ventricular function ejection fraction and increases blood vessel density in the ischemic area and reduces collagen deposition [78, 79].
To gain better homing and angiogenesis efficiency, MSCs are genetically equipped with certain character prior to injection in MI animals. For instance, when MSCs were transfected with VEGF cDNA or hepatic growth factor cDNA and then injected into the border of infarcted cardiomyocytes individually, MSC-HGF and MSC-VEGF injection showed the most advantageous effect than other groups [46]. In line with this, using the facially amphipathic bile acid-modified polyethyleneimine (BA-PEI) conjugates, transfection efficiency was further improved, resulting in higher VEGF expression in MSCs and increased angiogenesis in infarcted cardiomyocytes [80].
3. NANOPARTICLES AS VEHICLES FOR GENE DELIVERY
Although virus-mediated gene transfer is successful in promoting cardiomyocyte survival, improving cardiac function and mitigating fibrosis in mice, the safety issues are the most concerned that hinder its application moving from animal studies to clinical practice. The safety issues mainly comprise poor targeting efficiency, immunogenicity and unpredicted insertion site of the human genome. As an alternative approach, nanoparticles provide another option for gene delivery.
3.1. General Introduction
As how it is named, nanoparticles are small particles between 1 and 100 nm in size. The interfacial layer typically consists of ions, inorganic and organic molecules, which affects the properties of nanoparticles. They do not belong to modern science. In fact, the history is traced back to the fourth century as a component for dichroic glass by artisans in Roma. But till 1857, its scientific terms were first described as the optical properties of nanometer-scale metals. Nanoparticles elicited biologists’ interest because they could be linked to biological molecules such as tags that direct nanoparticles to specific sites within living cells for tracing [81-83]. Nowadays, the applications of nanoparticles have been extended for imaging, drug and gene delivery system.
3.2. Types of Nanoparticles
Nanoparticles used in medical research consist of micelles or liposomes, polymers, dendrimers, carbon nanotubes and metallic nanoparticles. Micelles are hydrophilic. When micelles are incorporated with hydrophobic therapeutic agents, the solubility problems are solved [84]. Liposomes are compatible with the cell membrane and thus the most popular carriers for cell endocytosis [85, 86]. Polymers could incorporate both hydrophilic and hydrophobic agents to increase solubility [87, 88]. Among polymers, hydrogels have been used in patients for wound healing, antibacterial infection and hemostasis [88-91]. Dendrimers are organic nanoparticles, synthesized step by step to finely tune their properties and formed in the three-dimensional structure
[92]. Carbon nanotubes are graphite sheets rolled up into a tubular form in which drugs are filled [93]. Different from others, metallic nanoparticles are functional themselves. The conjugation of metal nanoparticles with biomolecules could be used in biosensing, bioimaging and tissue engineering [94]. When nanoparticles circulate in the peripheral blood, it could be taken by white blood cells, leading to reduced homing. To reduce phagocytosis and obtain better targeting efficiency, nanoparticles are pegylated on the surface to escape the recognition by circulating and resident phagocytes [95].
3.3. Nanomedicine in Imaging
Magnetic resonance imaging (MRI) allows us to visualize the structure and characteristic of atherosclerotic plaques, coronary stenosis and the extent of infarcted cardiomyocyte area. Three MRI techniques are T1, T2 and off-resonance. In off-resonance, pulse sequences excite and refocus off-resonance water to give the positive contrast. Paramagnetic contrast agents including gadolinium chelates and nanoparticles enhance T1 contrast to give bright contrast in MR image. In addition, iron oxide nanoparticles enhance predominantly T2 contrast and give dark contrast. Some nanoparticles contain several contrast agents such as 18F-cross-linked iron oxide which is formed by superparamagnetic iron oxide core and functionalized with the radionuclide 18F. When iron oxide nanoparticles are injected to animals with MI, macrophages take up these nanoparticles and thus become labeled by MRI. From the detected signals on MRI, we could know how severe the inflammation is, present in the infarcted area and plaque and whether the plaques are stable or not [96]. Another substantial application of nanomedicine in imaging is cell tracing. When stem cells are labeled with certain nanoparticles before injection, they could be followed up by MRI for weeks to assess homing efficiency and fate of the injected stem cells [97].
3.4. Drug Delivery System Using Nanoparticles
The golden criteria of drug delivery system include specific targeting, controlled drug release, pharmaceutical efficacy and degradable delivery material for safety. Receptors and intracellular molecules are equally important mediators for cell function. Using antibodies immune therapies, antibodies could recognize receptors on the cell surface and a limited number of extracellular targets [98, 99]. Nevertheless, how to control intracellular targets is more demanding. These issues could not be reached by conventional drug assembling system. The development of nanotechnology has brought a solution to solve these issues.
Accumulated studies have consistently delineated the therapeutic effects in the treatment of cardiovascular diseases using nanoparticle-based drug delivery system (nano-DDS). Using microchip technology and 3D dynamic contrast-enhanced MRI, Kim et al., found that nanoparticles could translocate over endothelium with controllable permeability in rabbits [100]. These data imply that nanoparticles delivery systems could target molecules inside the cells.
Recently, two polymers, polylactide (PLA) and poly(lactide-co-glycolide) (PLGA) were approved by the FDA for nanoparticle synthesis. PLGA polymers could incorporate hydrophilic and hydrophilic agents and become biodegradable. In a mouse model of atherosclerotic plaque rupture, a single injection of PLGA nanoparticles containing pioglitazone significantly reduced the number of Ly6chigh monocytes. After weekly intravenous injection for 4 weeks, the fibrous cap in atherosclerotic plaque became thickened and stabilized [101]. Similarly, when mice were subjected with coronary ligation and injected with pitavastatin alone or with pitavastatin-incorporating nanoparticles for consecutive 3 to 5 days, administration of pitavastatin-incorporating nanoparticles decreased the number of monocytes/macro- phages in the infarcted heart and inhibited left ventricular remodeling whereas pitavastatin alone did not [102]. These data indicate that nanoparticles are more efficient than traditional drug delivery system.
3.5. Gene Delivery System Using Nanoparticles
Besides for imaging and drug delivery, nanoparticles hold some advantages for gene transfer. First, they are small molecules that easily and efficiently penetrate to target cells; second, nanoparticles could be covalently linked to specific tags in a controlled number per nanoparticle and thus they could be taken by target cells; third, multivalent nanoparticles could cluster receptor to activate signaling pathways; and fourth, the synthesis of nanoparticles would be faster than the virus packaging system [103, 104].
There are two types of nanoparticle systems carrying DNA or RNA: an entrapping system which is a reservoir type nanosphere system and surface binding system which supports an ionic interaction between the cationic polymer and the anionic nucleic acid. The entrapping system could protect DNA or RNA from degradation. How to synthesize and modify nanoparticles for higher transfection efficiency with the least toxicity is under investigation. A substantial progress has been made on how to modify nanoparticles for better gene delivery, which is summarized below:
3.5.1. PEGylation
The complex of PEG-polycation block copolymers and DNA are water soluble, colloidally stable, non-toxic and effective in transfection. Based on this, conjugation of polylysine to PEG further condenses plasma DNA into DNA nanoparticles. When transfected, the transgene expression was 10- fold higher than controls [105]. In parallel, Dasari et al. linked PEG on the N-terminal cysteine of a peptide for ocular delivery. This construct enables gene transfer in the retina. Using the reducible PEG-POD/DNA nanoparticles, FLT1 cDNA was transfected intro retina cells in vitro and FLT1 expression was induced without any change of LDH activity. When tested in vivo, the reducible PEG-POD/DNA induced 21- fold increase in transgene expression which resulted in 50% reduction in choroidal neovascularization in a murine model of age-related macular degeneration [106]. Because it is quickly degraded by the extracellular environment, this reducible PEG-SS-POD/DNA nanoparticle is a powerful and safe gene delivery system.
3.5.2. Chitosan
Chitosan is a cationic polysaccharide derived from partial deacetylation of chitin. It is an ideal carrier for drug, DNA and siRNA delivery because of good incorporation and long-term release [107-109]. The strategies of making better chitosan/DNA nanoparticles have been revolutionized in the aspects of size, concentration and the stoichiometry of polymer for better efficiency and safety. Chitosan nanoparticle systems have been applied in vaccines and intranasal delivery of chitosan-DNA complex against Coxsackievirus B and hepatitis B infections [110-112]. Intriguingly, Mannosylated chitosan nanoparticles are preferentially taken by macrophages [113]. Thus, whether Mannosylated chitosan nanoparticles could be used for suppressing inflammation and attenuation of myocardial infarction awaits for future exploration.
3.5.3. Polyethyleneimine (PEI)
Polyethyleneimine (PEI) is one of the most widely studied cationic polymeric vectors. An advanced strategy was reported in which low molecular weight PEI was linked with succinic acid which improved the hydrophilic and hydrophobic balance within the polymer and in the meantime, minimized the toxicity. The modified PEI could condense plasmid DNA and the formed complex was approximately 130 nm in size. When tested using the CD200 gene as the reporter, the transgene expression was increased 1.5-fold than controls in vitro and the expression pattern was distributed in a variety of organs and could even penetrate the blood-brain barrier [114].
3.5.4. Solid Lipid Nanoparticles
Due to shared compatibility to the cell membrane, liposomes have been used extensively for gene transfer for research. As alternatives, Solid Lipid Nanoparticles (SLNs) and Nanostructure Lipid Carriers (NLCs) have been developed since the 1990s. Because they could be equipped on purpose, they could protect DNA/RNA from degradation during delivery, reach specific target cells, and pass through all barriers, resulting in better transgene expression as desired. SLNs have a solid lipid core with a surfactant layer in an aqueous dispersion whereas NLCs are mixtures of solid and liquid lipids.
SLNs and NLCs carry several properties superior to liposome in gene transfer: (1) Depending on the charges of nucleic acids to be transferred, SLNs and NLCs could either be cationic or anionic to obtain stable binding in an electrostatic manner which helps DNA/RNA condensation and protects them from being degraded by enzymes in the environment [115]; (2) After injection, they bind to serum proteins which serve as carriers and deliver them to cells. When they reach the cells, the positively charged SLCs and NLCs interact with the negatively charged cell membrane to mediate endocytosis. Once they are equipped with the target ligands that recognize the receptors on cell and/or nuclear membrane, the transfection efficiency would increase by receptor and ligand interaction on top of endocytosis [116, 117]; and (3) in addition to gene transfer, SLNs and possibly NLCs are also carriers for drug delivery. Currently, SLNs and NLCs have been widely tested in the field of cancer, infectious disease and ocular disease [118-120]. Nevertheless, limited studies have been performed using SLCs and NCLs for treating ischemic heart. Notably, modification of SLCs and NLCs makes them feasible for penetration of cell and nuclear membranes. Different pathways are involved in different types of cells. The endocytosis pathways have to be clarified before SLCs and NCLs are designed for drug and gene dual transfer into cardiomyocytes.
3.5.5. Magnetic Nanoparticles
As introduced earlier, magnetic nanoparticles such as iron oxide and Fe3O4 could accumulate to favored cells for imaging-based cell tracing. When they are coated by different polymers, they become stabilized and activated by pH, temperature and microwave [121]. Coating strategies are the main issues in preparing these nanoparticles. Recently, a type of mesoporous silica-coated magnetic nanoparticles was reported. Their magnetic targeting abilities, magnetic hyperthermia performance and MRI properties have made them a superior candidate for suicide gene therapy in cancer treatment [122]. Considering the different nature and mechanisms between cancer and infarcted cardiomyocytes, whether magnetic nanoparticles could be used for gene therapy is not yet known in myocardial infarction.
All types of nanoparticles that could be applied for the treatment of cardiovascular diseases are summarized in Table 2.
Table 2. Summary of the nanoparticles that could be applied for the treatment of cardiovascular disease.
| Type | Structure and Compositions | Size | Modification for Improved Efficiency |
|---|---|---|---|
| Micelle [128] | Lipids and synthetic amphiphilic polymeric molecules dispersed in a liquid colloid | 10-100 nanometers | Micelle-like nanoparticles: half micelle and half polymeric. |
| Liposome [129] | Phospholipid bilayers and an aqueous core | Up to thousands of nanometers | polyethylene glycol (PEG) |
| Polymeric nanosphere [129, 130] | Polymeric materials | An average of 700 nanometers | Poly(lactide-co-glycolide) (PLGA) or polylactide (PLA)-based biodegradable nanoparticles. Could carry both hydrophobic and hydraulic drugs |
| Dendrimer [131, 132] | A monodisperse assembly with complex structure | Starting from several nanometers | GPIb to PLGA; probucol loaded PLGA |
| Metallic nanoparticle [133, 134] | Could be gold, iron oxide to superparamagnetic iron oxide | Varied | Dextran or poly(ethyleneglycol); suitable for magnetic resonance imaging |
| Nanogel [135, 136] | A nanosized spherical hydrogel | Varied | Thermally responsive manipulation for better delivery |
3.5.6. Limitations and Future Perspective
In virus-mediated gene transfer, there were potential limitations in nanoparticle-facilitated gene therapy as well. First, although nanoparticles-based gene delivery could induce faster and higher transgene expression than controls, the duration of transgene expression is relatively short and lasts for days. Second, the nucleic acids that nanoparticles carry do not integrate into human genome which makes it safer than virus vectors. However, the cellular toxicity is still present and could not be ignored. The toxic effects of engineered nanoparticles on germ cells, embryos and reproductive systems have been noted [123]. And third, detailed understanding of how nanoparticles are distributed, metabolized and eliminated is demanding to enhance targeting efficiency and prolonged efficacy with least of toxicity. Huge amount of work is anticipated for improving nanoparticle-mediated gene therapy for better targeting and least side effects.
Nonetheless, nanoparticles are potent in gene therapy. The dual properties in drug and gene transfer by nanoparticles could not be replaced by any other delivery systems. Here is an example, demonstrating elaborately how to utilize nanomedicine for better treatment. Tang et al., set up a combinatorial library of 15 high-density lipoprotein-inspired nanoparticles, a PEGylated micellar and a long-circulating liposomal nanoparticle. All of which had distinct physiochemical properties such as size and chemical composition. They screened the injected nanoparticles by half-life and accumulation in the organs by near-infrared fluorescence imaging and assessed their cholesterol efflux capacity in vitro. They further evaluated the effects of nanoparticles on inflammatory cells in aorta, spleen and blood by flow cytometry. Thus, the best candidate was screened which had high cholesterol efflux capacity, relatively long half-life, predominantly accumulated in aorta and liver and a high relative aortic-to-splenic macrophage association ratio. They formulated the candidate with a Liver X receptor agonist GW3965 (Rx-HDL) and confirmed its effects on atherosclerosis in apoE-/- mice. As expected, this nanoparticle processed all the characters above and inhibited atherosclerosis with the least liver toxicity [124]. This study gives a direction to utilize nanoparticles to deliver a gene of interest and drug of interest to the targeted cells for specificity, accuracy, efficacy and safety.
Based on their unique characters, hybrid nanoparticles could inherit both advantages to achieve better gene delivery efficiency. For instance, polymer-lipid hybrid nanoparticles hold the property of polymeric materials and also a lipid formulation. When they were incorporated with PEG-distearoyphosphatidylethanolamine, gene transfer efficiency was 3-fold higher than conventional transfection method [125]. Another elaborate example is the incorporation of gold nanoparticles (Au NPs) into the liposome. Once administered under near-infrared irradiation, liposomes are fragmented and gold nanoparticles are released and penetrated into tumor tissues to proceed photothermal treatment, leading to superior inhibition of tumor cell growth in vitro and in vivo [126]. Therefore, how to make the best use of hybrid nanoparticles for gene delivery is under investigation. When polyethyleneimine particles are loaded with modified cholesterol, they could bind DNA with more affinity and protect DNA from degradation [127]. Therefore, these “lipopolyplexes” would be essential tools for more efficient gene delivery to target cells.
CONCLUSION AND FUTURE OUTLOOK
In conclusion, nanoparticles possess several advantages while other vectors do not. They could be synthesized in a controlled manner and produced on a large scale. They are biocompatible and could be designed to carry certain properties to protect DNA/RNA content from enzyme-induced degradation, recognize target of interest more specifically, and efficiently penetrate cell and nuclear membrane (Fig. 1). They could facilitate dual transfer-gene and drug at once. The biggest challenge that nanoparticles need to overcome is coating. With the development of technologies and knowledge on cell biology and pathology, nanoparticles would be desirable for clinical purpose. Different from cancer treatment, application of nanoparticle-mediated gene transfer in cardiomyocyte infarction is relatively rare which definitely opens the space for future exploration.
Fig. (1).
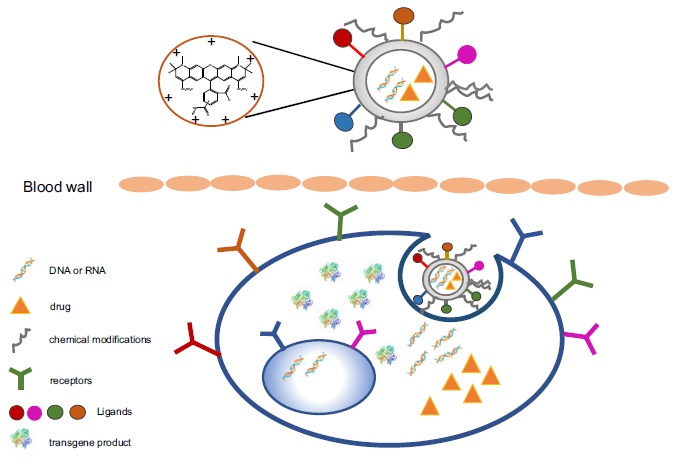
Proposed nanoparticle-based gene transfer system. Nanoparticle-based delivery system could carry transferred genes and drug at the same time whereas other systems could not. The basic structure of nanoparticles could be manipulated by charges that regulate interaction between nanoparticles and DNA to be transferred and by chemical modifications on the surface, leading to DNA stability, resistance to enzyme destruction in the environment and improved cellular uptake. To further enhance uptake, ligands could be wrapped on the surface that could specific recognize the receptors on the target cells.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was partly supported by National Science Funding in China (#81470566, #81670765 and # 81772943).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Roth G.A., Johnson C., Abajobir A., et al. Global, Regional, and National Burden of cardiovascular diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017;70(1):1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonard A., Rahman A., Fazal F. Importins alpha and beta signaling mediates endothelial cell inflammation and barrier disruption. Cell. Signal. 2018;44:103–117. doi: 10.1016/j.cellsig.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin F., Pei L., Zhang Q., et al. Ox-LDL induces endothelial cell apoptosis and macrophage migration by regulating caveolin-1 phosphorylation. J. Cell. Physiol. 2018;233(10):6683–6692. doi: 10.1002/jcp.26468. [DOI] [PubMed] [Google Scholar]
- 4.Karki P., Birukov K.G. Lipid mediators in the regulation of endothelial barriers. Tissue Barriers. 2018;6(1):e1385573. doi: 10.1080/21688370.2017.1385573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin M1 Ginsenoside F1 ameliorates endothelial cell inflammatory injury and prevents atherosclerosis in mice through A20-Mediated suppression of NF-kB signaling. Front. Pharmacol. 2017;8:953. doi: 10.3389/fphar.2017.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pankratz F., Hohnloser C., Bemtgen X., et al. MicroRNA-100 Suppresses chronic vascular inflammation by stimulation of endothelial autophagy. Circ. Res. 2018;122(3):417–432. doi: 10.1161/CIRCRESAHA.117.311428. [DOI] [PubMed] [Google Scholar]
- 7.Ooi B.K., Goh B.H., Yap W.H. Oxidative stress in cardiovascular diseases: Involvement of Nrf2 Antioxidant Redox Signaling in macrophage foam cells formation. Int. J. Mol. Sci. 2017;18(11):e2336. doi: 10.3390/ijms18112336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halper J. Basic components of vascular connective tissue and extracellular matrix. Adv. Pharmacol. 2018;81:95–127. doi: 10.1016/bs.apha.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Xiao H., Li H., Wang J.J., et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon beta-adrenergic insult. Eur. Heart J. 2018;39(1):60–69. doi: 10.1093/eurheartj/ehx261. [DOI] [PubMed] [Google Scholar]
- 10.Rühle K.H., Domanski U., Franke K.J., Nilius G. Studies of leakage measurements of automatic CPAP-devices. Pneumologie. 2007;61(4):213–218. doi: 10.1055/s-2007-959155. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi K., Maeda K., Takefuji M., et al. Dynamics of angiogenesis in ischemic areas of the infarcted heart. Sci. Rep. 2017;7(1):7156. doi: 10.1038/s41598-017-07524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash A.D., Baca M., Wright C., Scotney P.D. The biology of vascular endothelial growth factor-B (VEGF-B). Pulm. Pharmacol. Ther. 2006;19(1):61–69. doi: 10.1016/j.pupt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Huusko J., Merentie M., Dijkstra M.H., et al. The effects of VEGF-R1 and VEGF-R2 ligands on angiogenic responses and left ventricular function in mice. Cardiovasc. Res. 2010;86(1):122–130. doi: 10.1093/cvr/cvp382. [DOI] [PubMed] [Google Scholar]
- 14.Li G-H., Luo B., Yan-xia L.V., et al. Dual effects of VEGF-B on activating cardiomyocytes and cardiac stem cells to protect the heart against short- and long-term ischemia-reperfusion injury. J. Transl. Med. 2016;14(1):116. doi: 10.1186/s12967-016-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zentilin L., Puligadda U., Lionetti V., et al. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J. 2010;24(5):1467–1478. doi: 10.1096/fj.09-143180. [DOI] [PubMed] [Google Scholar]
- 16.Huusko J., Lottonen L., Merentie M., et al. AAV9-mediated VEGF-B gene transfer improves systolic function in progressive left ventricular hypertrophy. Mol. Ther. 2012;20(12):2212–2221. doi: 10.1038/mt.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X.G., Lv Y.X., Zhao D., et al. Vascular endothelial growth factor-C protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Mol. Cell. Biochem. 2016;413(1-2):9–23. doi: 10.1007/s11010-015-2622-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhao T., Zhao W., Meng W., Liu C., Chen Y., Sun Y. Vascular endothelial growth factor-C: Its unrevealed role in fibrogenesis. Am. J. Physiol. Heart Circ. Physiol. 2014;306(6):H789–H796. doi: 10.1152/ajpheart.00559.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boardman N.T., Aronsen J.M., Louch W.E., et al. Impaired left ventricular mechanical and energetic function in mice after cardiomyocyte-specific excision of Serca2. Am. J. Physiol. Heart Circ. Physiol. 2014;306(7):H1018–H1024. doi: 10.1152/ajpheart.00741.2013. [DOI] [PubMed] [Google Scholar]
- 20.Kawase Y., Ly H.Q., Prunier F., et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 2008;51(11):1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Isman F1 Serum hyaluronidase levels in patients with aneurysmal subarachnoid haemorrhage. Singapore Med. J. 2008;49(5):405–409. [PubMed] [Google Scholar]
- 22.Zhao X.Y., Hu S.J., Li J., et al. rAAV-asPLB transfer attenuates abnormal sarcoplasmic reticulum Ca2+ -ATPase activity and cardiac dysfunction in rats with myocardial infarction. Eur. J. Heart Fail. 2008;10(1):47–54. doi: 10.1016/j.ejheart.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Kho C., Lee A., Jeong D., et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477(7366):601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W.E., Li L., Xia X., et al. Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation. 2017;136(9):834–848. doi: 10.1161/CIRCULATIONAHA.116.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younce C.W., Niu J., Ayala J., et al. Exendin-4 improves cardiac function in mice overexpressing monocyte chemoattractant protein-1 in cardiomyocytes. J. Mol. Cell. Cardiol. 2014;76:172–176. doi: 10.1016/j.yjmcc.2014.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao T., Lu W., Zhu J., et al. Role of CD11b+Gr-1+ myeloid cells in AGEs-induced myocardial injury in a mice model of acute myocardial infarction. Int. J. Clin. Exp. Pathol. 2015;8(3):3238–3249. [PMC free article] [PubMed] [Google Scholar]
- 27.Veltman D., Laeremans T., Passante E., Huber H.J. Signal transduction analysis of the NLRP3-inflammasome pathway after cellular damage and its paracrine regulation. J. Theor. Biol. 2017;415:125–136. doi: 10.1016/j.jtbi.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Su S.A., Yang D., Zhu W., et al. Interleukin-17A mediates cardiomyocyte apoptosis through Stat3-iNOS pathway. Biochim. Biophys. Acta. 2016;1863(11):2784–2794. doi: 10.1016/j.bbamcr.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Jarrah A.A., Schwarskopf M., Wang E.R., et al. SDF-1 induces TNF-mediated apoptosis in cardiac myocytes. Apoptosis. 2018;23(1):79–91. doi: 10.1007/s10495-017-1438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opstad T.B., Seljeflot I., Bøhmer E., Arnesen H., Halvorsen S. MMP-9 and its regulators TIMP-1 and EMMPRIN in patients with acute ST-Elevation Myocardial Infarction: A NORDISTEMI Substudy. Cardiology. 2018;139(1):17–24. doi: 10.1159/000481684. [DOI] [PubMed] [Google Scholar]
- 31.Mongue-Din H., Patel A.S., Looi Y.H., et al. NADPH Oxidase-4 driven cardiac macrophage polarization protects against myocardial infarction-induced remodeling. JACC Basic Transl. Sci. 2017;2(6):688–698. doi: 10.1016/j.jacbts.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Maggio S., Milano G., De Marchis F., et al. Non-oxidizable HMGB1 induces cardiac fibroblasts migration via CXCR4 in a CXCL12-independent manner and worsens tissue remodeling after myocardial infarction. Biochim. Biophys. Acta. 2017;1863(11):2693–2704. doi: 10.1016/j.bbadis.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Su S.A., Yang D., Wu Y., et al. EphrinB2 regulates cardiac fibrosis through modulating the interaction of Stat3 and TGF-beta/Smad3 signaling. Circ. Res. 2017;121(6):617–627. doi: 10.1161/CIRCRESAHA.117.311045. [DOI] [PubMed] [Google Scholar]
- 34.Lother A., Moser M., Bode C., Feldman R.D., Hein L. Mineralocorticoids in the heart and vasculature: New insights for old hormones. Annu. Rev. Pharmacol. Toxicol. 2015;55:289–312. doi: 10.1146/annurev-pharmtox-010814-124302. [DOI] [PubMed] [Google Scholar]
- 35.Caprio M., Newfell B.G., la Sala A., et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ. Res. 2008;102(11):1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung J., Stewart J.E., Barbeau H. The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal cord injured subjects. J. Neurol. Sci. 1990;100(1-2):85–93. doi: 10.1016/0022-510x(90)90017-h. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-Martínez E., Buonafine M., Boukhalfa I., et al. Aldosterone Target NGAL (Neutrophil Gelatinase-Associated Lipocalin) is involved in cardiac remodeling after myocardial infarction through nfkappab pathway. Hypertension. 2017;70(6):1148–1156. doi: 10.1161/HYPERTENSIONAHA.117.09791. [DOI] [PubMed] [Google Scholar]
- 38.Gueret A., Harouki N., Favre J., et al. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension. 2016;67(4):717–723. doi: 10.1161/HYPERTENSIONAHA.115.06709. [DOI] [PubMed] [Google Scholar]
- 39.Tsai C.F., Yang S.F., Chu H.J., Ueng K.C. Cross-talk between mineralocorticoid receptor/angiotensin II type 1 receptor and mitogen-activated protein kinase pathways underlies aldosterone-induced atrial fibrotic responses in HL-1 cardiomyocytes. Int. J. Cardiol. 2013;169(1):17–28. doi: 10.1016/j.ijcard.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J., Lever A.M. Lentivirus-mediated gene expression. Methods Mol. Biol. 2007;366:343–355. doi: 10.1007/978-1-59745-030-0_20. [DOI] [PubMed] [Google Scholar]
- 41.Hammoudi N., Ishikawa K., Hajjar R.J. Adeno-associated virus-mediated gene therapy in cardiovascular disease. Curr. Opin. Cardiol. 2015;30(3):228–234. doi: 10.1097/HCO.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castañeda-Lopez M.E., Garza-Veloz I., Lopez-Hernandez Y., Barbosa-Cisneros O.Y., Martinez-Fierro M.L. Anti-inflammatory effects of modified adenoviral vectors for gene therapy: A view through animal models tested. Immunol. Invest. 2016;45(5):450–470. doi: 10.3109/08820139.2016.1168831. [DOI] [PubMed] [Google Scholar]
- 43.Poletti V., Mavilio F. Interactions between retroviruses and the Host Cell Genome. Mol. Ther. Methods Clin. Dev. 2018;8:31–41. doi: 10.1016/j.omtm.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu H., Tan J., Yin Q. Effects of recombinant adeno-associated virus-mediated CD151 gene transfer on the expression of rat vascular endothelial growth factor in ischemic myocardium. Exp. Ther. Med. 2015;9(1):187–190. doi: 10.3892/etm.2014.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerrero M., Athota K., Moy J., et al. Vascular endothelial growth factor-165 gene therapy promotes cardiomyogenesis in reperfused myocardial infarction. J. Interv. Cardiol. 2008;21(3):242–251. doi: 10.1111/j.1540-8183.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 46.Deuse T., Peter C., Fedak P.W., et al. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120(11) Suppl.:S247–S254. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- 47.Olea F.D., De Lorenzi A., Cortés C., et al. Combined VEGF gene transfer and erythropoietin in ovine reperfused myocardial infarction. Int. J. Cardiol. 2013;165(2):291–298. doi: 10.1016/j.ijcard.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 48.Vera Janavel G.L., De Lorenzi A., Cortés C., et al. Effect of vascular endothelial growth factor gene transfer on infarct size, left ventricular function and myocardial perfusion in sheep after 2 months of coronary artery occlusion. J. Gene Med. 2012;14(4):279–287. doi: 10.1002/jgm.1608. [DOI] [PubMed] [Google Scholar]
- 49.Mathison M., Gersch R.P., Nasser A., et al. In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor. J. Am. Heart Assoc. 2012;1(6):e005652. doi: 10.1161/JAHA.112.005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rengo G., Zincarelli C., Femminella G.D., et al. Myocardial beta(2)-adrenoceptor gene delivery promotes coordinated cardiac adaptive remodelling and angiogenesis in heart failure. Br. J. Pharmacol. 2012;166(8):2348–2361. doi: 10.1111/j.1476-5381.2012.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao X., Månsson-Broberg A., Gustafsson T., et al. Angiogenic effects of dual gene transfer of bFGF and PDGF-BB after myocardial infarction. Biochem. Biophys. Res. Commun. 2004;315(4):1058–1063. doi: 10.1016/j.bbrc.2004.01.165. [DOI] [PubMed] [Google Scholar]
- 52.Korf-Klingebiel M., Kempf T., Schlüter K.D., et al. Conditional transgenic expression of fibroblast growth factor 9 in the adult mouse heart reduces heart failure mortality after myocardial infarction. Circulation. 2011;123(5):504–514. doi: 10.1161/CIRCULATIONAHA.110.989665. [DOI] [PubMed] [Google Scholar]
- 53.Smith R.S., Agata J., Xia C.F., Chao L., Chao J. Human endothelial nitric oxide synthase gene delivery protects against cardiac remodeling and reduces oxidative stress after myocardial infarction. Life Sci. 2005;76(21):2457–2471. doi: 10.1016/j.lfs.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 54.Chen L.L., Yin H., Huang J. Inhibition of TGF-beta1 signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through angiogenesis and reduction of apoptosis. Cardiovasc. Pathol. 2007;16(4):221–230. doi: 10.1016/j.carpath.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Chen LL1 Inhibition of MAPK signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through reduction of inflammation. Mol. Biol. Rep. 2010;37(7):3067–3072. doi: 10.1007/s11033-009-9879-6. [DOI] [PubMed] [Google Scholar]
- 56.Qin W., Chen X., Liu P. Inhibition of TGF-beta1 by eNOS gene transfer provides cardiac protection after myocardial infarction. J. Biomed. Res. 2010;24(2):145–152. doi: 10.1016/S1674-8301(10)60023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang J., Wang J., Yang J., Kong X. Adenovirus-mediated stromal cell-derived- factor-1alpha gene transfer induces cardiac preservation after infarction via angiogenesis of CD133+ stem cells and anti-apoptosis. Interact. Cardiovasc. Thorac. Surg. 2008;7(5):767–770. doi: 10.1510/icvts.2007.169896. [DOI] [PubMed] [Google Scholar]
- 58.Tang J., Wang J., Song H., et al. Adenovirus-mediated stromal cell-derived factor-1 alpha gene transfer improves cardiac structure and function after experimental myocardial infarction through angiogenic and antifibrotic actions. Mol. Biol. Rep. 2010;37(4):1957–1969. doi: 10.1007/s11033-009-9642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmet I., Sawa Y., Yamaguchi T., Matsuda H. Gene transfer of hepatocyte growth factor improves angiogenesis and function of chronic ischemic myocardium in canine heart. Ann. Thorac. Surg. 2003;75(4):1283–1287. doi: 10.1016/s0003-4975(02)04677-5. [DOI] [PubMed] [Google Scholar]
- 60.Jayasankar V., Woo Y.J., Bish L.T., et al. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation. 2003;108(Suppl. 1):II230–II236. doi: 10.1161/01.cir.0000087444.53354.66. [DOI] [PubMed] [Google Scholar]
- 61.Chen X.H., Minatoguchi S., Kosai K., et al. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia-reperfusion injury through its multiple actions. J. Card. Fail. 2007;13(10):874–883. doi: 10.1016/j.cardfail.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Gordts S.C., Van Craeyveld E., Muthuramu I., Singh N., Jacobs F., De Geest B. Lipid lowering and HDL raising gene transfer increase endothelial progenitor cells, enhance myocardial vascularity, and improve diastolic function. PLoS One. 2012;7(10):e46849. doi: 10.1371/journal.pone.0046849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niwano K., Arai M., Koitabashi N., et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol. Ther. 2008;16(6):1026–1032. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 64.Fargnoli A.S., Katz M.G., Yarnall C., et al. Cardiac surgical delivery of the sarcoplasmic reticulum calcium ATPase rescues myocytes in ischemic heart failure. Ann. Thorac. Surg. 2013;96(2):586–595. doi: 10.1016/j.athoracsur.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lai N.C., Gao M.H., Giamouridis D., et al. Intravenous AAV8 Encoding Urocortin-2 increases function of the failing heart in mice. Hum. Gene Ther. 2015;26(6):347–356. doi: 10.1089/hum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tilemann L., Lee A., Ishikawa K., et al. SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Sci. Transl. Med. 2013;5(211):211ra159. doi: 10.1126/scitranslmed.3006487. [DOI] [PubMed] [Google Scholar]
- 67.Fish K.M., Ladage D., Kawase Y., et al. AAV9.I-1c delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodeling. Circ Heart Fail. 2013;6(2):310–317. doi: 10.1161/CIRCHEARTFAILURE.112.971325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishikawa K., Fish K., Aguero J., et al. Stem cell factor gene transfer improves cardiac function after myocardial infarction in swine. Circ Heart Fail. 2015;8(1):167–174. doi: 10.1161/CIRCHEARTFAILURE.114.001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greener I.D., Sasano T., Wan X., et al. Connexin43 gene transfer reduces ventricular tachycardia susceptibility after myocardial infarction. J. Am. Coll. Cardiol. 2012;60(12):1103–1110. doi: 10.1016/j.jacc.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raake P.W., Schlegel P., Ksienzyk J., et al. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur. Heart J. 2013;34(19):1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swain J.D., Fargnoli A.S., Katz M.G., et al. MCARD-mediated gene transfer of GRK2 inhibitor in ovine model of acute myocardial infarction. J. Cardiovasc. Transl. Res. 2013;6(2):253–262. doi: 10.1007/s12265-012-9418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathison M, Singh VP, Chiuchiolo MJ, et al. In situ reprogramming to transdifferentiate fibroblasts into cardiomyocytes using adenoviral vectors: Implications for clinical myocardial regeneration. J Thorac Cardiovasc Surg. 2017;132(2):329-39. doi: 10.1016/j.jtcvs.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma H., Wang L., Liu J., Qian L. Direct Cardiac Reprogramming as a novel therapeutic strategy for treatment of myocardial infarction. Methods Mol. Biol. 2017;1521:69–88. doi: 10.1007/978-1-4939-6588-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyamoto K, Akiyama M, Tamura F, et al. Direct in vivo reprogramming with sendai virus vectors improves cardiac function after myocardial infarction. Cell Stem Cell. 2018;22(1):91-103. doi: 10.1016/j.stem.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Knuth C.A., Kiernan C.H., Palomares C.V., et al. Isolating paediatric mesenchymal stem cells with enhanced expansion and differentiation capabilities. Tissue Eng. Part C Methods. 2018;24(6):313–321. doi: 10.1089/ten.TEC.2018.0031. [DOI] [PubMed] [Google Scholar]
- 76.Keshtkar S., Azarpira N., Ghahremani M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018;9(1):63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Q., Jia L., Li X., et al. Long noncoding RNAs: New players in the osteogenic differentiation of bone marrow- and adipose-derived mesenchymal stem cells. Stem Cell Rev. 2018;14(3):297–308. doi: 10.1007/s12015-018-9801-5. [DOI] [PubMed] [Google Scholar]
- 78.Luger D., Lipinski M.J., Westman P.C., et al. Intravenously delivered mesenchymal stem cells: Systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ. Res. 2017;120(10):1598–1613. doi: 10.1161/CIRCRESAHA.117.310599. [DOI] [PubMed] [Google Scholar]
- 79.Houtgraaf J.H., de Jong R., Kazemi K., et al. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ. Res. 2013;113(2):153–166. doi: 10.1161/CIRCRESAHA.112.300730. [DOI] [PubMed] [Google Scholar]
- 80.Moon H.H., Joo M.K., Mok H., et al. MSC-based VEGF gene therapy in rat myocardial infarction model using facial amphipathic bile acid-conjugated polyethyleneimine. Biomaterials. 2014;35(5):1744–1754. doi: 10.1016/j.biomaterials.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 81.Hoshino A., Fujioka K., Oku T., et al. Quantum dots targeted to the assigned organelle in living cells. Microbiol. Immunol. 2004;48(12):985–994. doi: 10.1111/j.1348-0421.2004.tb03621.x. [DOI] [PubMed] [Google Scholar]
- 82.Wang G.D., Tan Y.Z., Wang H.J., Zhou P. Autophagy promotes degradation of polyethyleneimine-alginate nanoparticles in endothelial progenitor cells. Int. J. Nanomedicine. 2017;12:6661–6675. doi: 10.2147/IJN.S141592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhuo H., Zheng B., Liu J., et al. Efficient targeted tumor imaging and secreted endostatin gene delivery by anti-CD105 immunoliposomes. J. Exp. Clin. Cancer Res. 2018;37(1):42. doi: 10.1186/s13046-018-0712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hashemi M.M., Holden B.S., Taylor M.F., et al. Antibacterial and antifungal activities of poloxamer micelles containing ceragenin CSA-131 on ciliated tissues. Molecules. 2018;23(3):E596. doi: 10.3390/molecules23030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang J., Yang C., Pan S., et al. Eph A10-modified pH-sensitive liposomes loaded with novel triphenylphosphine-docetaxel conjugate possess hierarchical targetability and sufficient antitumor effect both in vitro and in vivo. Drug Deliv. 2018;25(1):723–737. doi: 10.1080/10717544.2018.1446475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang L., Chen F., Zheng J., Wang H., Qin X., Pan W. Chitosan-based liposomal thermogels for the controlled delivery of pingyangmycin: Design, optimization and in vitro and in vivo studies. Drug Deliv. 2018;25(1):690–702. doi: 10.1080/10717544.2018.1444684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li H., Teng Y., Xu X., Liu J. Enhanced rapamycin delivery to hemangiomas by lipid polymer nanoparticles coupled with anti-VEGFR antibody. Int. J. Mol. Med. 2018;41(6):3586–3596. doi: 10.3892/ijmm.2018.3518. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y., Sui Y., Liu C., et al. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018;188:27–36. doi: 10.1016/j.carbpol.2018.01.093. [DOI] [PubMed] [Google Scholar]
- 89.Ginot F., Decaux J.F., Cognet M., et al. Transfection of hepatic genes into adult rat hepatocytes in primary culture and their tissue-specific expression. Eur. J. Biochem. 1989;180(2):289–294. doi: 10.1111/j.1432-1033.1989.tb14646.x. [DOI] [PubMed] [Google Scholar]
- 90.Lu M., Liu Y., Huang Y.C., Huang C.J., Tsai W.B. Fabrication of photo-crosslinkable glycol chitosan hydrogel as a tissue adhesive. Carbohydr. Polym. 2018;181:668–674. doi: 10.1016/j.carbpol.2017.11.097. [DOI] [PubMed] [Google Scholar]
- 91.Wang Z., Lee S.J., Cheng H.J., Yoo J.J., Atala A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018;70:48–56. doi: 10.1016/j.actbio.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caminade A.M., Majoral J.P. Which dendrimer to attain the desired properties? focus on phosphorhydrazone dendrimers. Molecules. 2018;23(3):E622. doi: 10.3390/molecules23030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boussema F., Gross A.J., Hmida F., et al. Dawson-type polyoxometalate nanoclusters confined in a carbon nanotube matrix as efficient redox mediators for enzymatic glucose biofuel cell anodes and glucose biosensors. Biosens. Bioelectron. 2018;109:20–26. doi: 10.1016/j.bios.2018.02.060. [DOI] [PubMed] [Google Scholar]
- 94.Miao Z., Gao Z., Chen R., Yu X., Su Z., Wei G. Surface-bioengineered gold nanoparticles for biomedical applications. Curr. Med. Chem. 2018;25(16):1920–1944. doi: 10.2174/0929867325666180117111404. [DOI] [PubMed] [Google Scholar]
- 95.Niu Z., Samaridou E., Jaumain E., et al. PEG-PGA enveloped octaarginine-peptide nanocomplexes: An oral peptide delivery strategy. J. Control. Release. 2018;276:125–139. doi: 10.1016/j.jconrel.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z., Mascheri N., Dharmakumar R., Li D. Cellular magnetic resonance imaging: Potential for use in assessing aspects of cardiovascular disease. Cytotherapy. 2008;10(6):575–586. doi: 10.1080/14653240802165699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ta H.T., Prabhu S., Leitner E., et al. Enzymatic single-chain antibody tagging: A universal approach to targeted molecular imaging and cell homing in cardiovascular disease. Circ. Res. 2011;109(4):365–373. doi: 10.1161/CIRCRESAHA.111.249375. [DOI] [PubMed] [Google Scholar]
- 98.Chames P., Regenmortel M.V., Weiss E., Baty D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009;157(2):220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robinson J.G., Farnier M., Krempf M., et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015;372(16):1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 100.Kim Y., Lobatto M.E., Kawahara T., et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc. Natl. Acad. Sci. USA. 2014;111(3):1078–1083. doi: 10.1073/pnas.1322725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matoba T., Koga J.I., Nakano K., Egashira K., Tsutsui H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol. 2017;70(3):206–211. doi: 10.1016/j.jjcc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 102.Mao Y., Koga J.I., Tokutome M., et al. Nanoparticle-Mediated delivery of pitavastatin to monocytes/macrophages inhibits left ventricular remodeling after acute myocardial infarction by inhibiting monocyte-mediated inflammation. Int. Heart J. 2017;58(4):615–623. doi: 10.1536/ihj.16-457. [DOI] [PubMed] [Google Scholar]
- 103.Sung K.M., Mosley D.W., Peelle B.R., Zhang S., Jacobson J.M. Synthesis of monofunctionalized gold nanoparticles by fmoc solid-phase reactions. J. Am. Chem. Soc. 2004;126(16):5064–5065. doi: 10.1021/ja049578p. [DOI] [PubMed] [Google Scholar]
- 104.Fu A., Micheel C.M., Cha J., Chang H., Yang H., Alivisatos A.P. Discrete nanostructures of quantum dots/Au with DNA. J. Am. Chem. Soc. 2004;126(35):10832–10833. doi: 10.1021/ja046747x. [DOI] [PubMed] [Google Scholar]
- 105.Sun W., Davis P.B. Reducible DNA nanoparticles enhance in vitro gene transfer via an extracellular mechanism. J. Control. Release. 2010;146(1):118–127. doi: 10.1016/j.jconrel.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dasari B.C., Cashman S.M., Kumar-Singh R. Reducible PEG-POD/DNA nanoparticles for gene transfer in vitro and in vivo: Application in a mouse model of age-related macular degeneration. Mol. Ther. Nucleic Acids. 2017;8:77–89. doi: 10.1016/j.omtn.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Picola I.P., Shi Q., Fernandes J.C., et al. Chitosan derivatives for gene transfer: effect of phosphorylcholine and diethylaminoethyl grafts on the in vitro transfection efficiency. J. Biomater. Sci. Polym. Ed. 2016;27(16):1611–1630. doi: 10.1080/09205063.2016.1225333. [DOI] [PubMed] [Google Scholar]
- 108.Kong F., Liu G., Zhou S., Guo J., Chen S., Wang Z. Superior transfection efficiency of phagocytic astrocytes by large chitosan/DNA nanoparticles. Int. J. Biol. Macromol. 2017;105(Pt 2):1473–1481. doi: 10.1016/j.ijbiomac.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 109.Ma P.L., Lavertu M., Winnik F.M., Buschmann M.D. Stability and binding affinity of DNA/chitosan complexes by polyanion competition. Carbohydr. Polym. 2017;176:167–176. doi: 10.1016/j.carbpol.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 110.Tang R., Zhai Y., Dong L. Immunization with dendritic cell-based DNA vaccine pRSC-NLDC145.gD-IL21 protects mice against herpes simplex virus keratitis. Immunotherapy. 2018;10(3):189–200. doi: 10.2217/imt-2017-0060. [DOI] [PubMed] [Google Scholar]
- 111.Huang T., Song X., Jing J., et al. Chitosan-DNA nanoparticles enhanced the immunogenicity of multivalent DNA vaccination on mice against Trueperella pyogenes infection. J. Nanobiotechnology. 2018;16(1):8. doi: 10.1186/s12951-018-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khademi F., Derakhshan M., Yousefi-Avarvand A., Tafaghodi M. Potential of polymeric particles as future vaccine delivery systems/adjuvants for parenteral and non-parenteral immunization against tuberculosis: A systematic review. Iran. J. Basic Med. Sci. 2018;21(2):116–123. doi: 10.22038/IJBMS.2017.22059.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Asthana G.S., Asthana A., Kohli D.V., Vyas S.P. Mannosylated chitosan nanoparticles for delivery of antisense oligonucleotides for macrophage targeting. BioMed Res. Int. 2014;2014:526391. doi: 10.1155/2014/526391. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Nouri F., Sadeghpour H., Heidari R., Dehshahri A. Preparation, characterization, and transfection efficiency of low molecular weight polyethylenimine-based nanoparticles for delivery of the plasmid encoding CD200 gene. Int. J. Nanomedicine. 2017;12:5557–5569. doi: 10.2147/IJN.S140734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McClements D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018;253:1–22. doi: 10.1016/j.cis.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 116.Han Y., Zhang Y., Li D., et al. Transferrin-modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int. J. Nanomedicine. 2014;9:4107–4116. doi: 10.2147/IJN.S67770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abd-Rabou A.A., Bharali D.J., Mousa S.A. Taribavirin and 5-Fluorouracil-Loaded Pegylated-Lipid nanoparticle synthesis, p38 docking, and antiproliferative effects on MCF-7 breast cancer. Pharm. Res. 2018;35(4):76. doi: 10.1007/s11095-017-2283-3. [DOI] [PubMed] [Google Scholar]
- 118.Del Pozo-Rodriguez A., Solinis M.A., Rodriguez-Gascon A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016;109:184–193. doi: 10.1016/j.ejpb.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 119.Sanchez-Lopez E., Espina M., Doktorovova S., Souto E.B., Garcia M. L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye - Part II - Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017;110:58–69. doi: 10.1016/j.ejpb.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 120.Buyukkoroglu G., Senel B., Basaran E., Yenilmez E., Yazan Y. Preparation and in vitro evaluation of vaginal formulations including siRNA and paclitaxel-loaded SLNs for cervical cancer. Eur. J. Pharm. Biopharm. 2016;109:174–183. doi: 10.1016/j.ejpb.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 121.Shen L., Li B., Qiao Y. Fe(3)O(4) Nanoparticles in targeted drug/gene delivery systems. Materials (Basel) 2018;11(2):324. doi: 10.3390/ma11020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Z., Chang Z., Lu M., et al. Shape-controlled magnetic mesoporous silica nanoparticles for magnetically-mediated suicide gene therapy of hepatocellular carcinoma. Biomaterials. 2018;154:147–157. doi: 10.1016/j.biomaterials.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 123.Das J., Choi Y.J., Song H., Kim J.H. Potential toxicity of engineered nanoparticles in mammalian germ cells and developing embryos: Treatment strategies and anticipated applications of nanoparticles in gene delivery. Hum. Reprod. Update. 2016;22(5):588–619. doi: 10.1093/humupd/dmw020. [DOI] [PubMed] [Google Scholar]
- 124.Tang J., Baxter S., Menon A., et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl. Acad. Sci. USA. 2016;113(44):E6731–E6740. doi: 10.1073/pnas.1609629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li J., He Y.Z., Li W., Shen Y.Z., Li Y.R., Wang Y.F., et al. A novel polymer-lipid hybrid nanoparticle for efficient nonviral gene delivery. Acta Pharmacol. Sin. 2010;31(4):509–514. doi: 10.1038/aps.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xing S., Zhang X., Luo L., et al. Doxorubicin/gold nanoparticles coated with liposomes for chemo-photothermal synergetic antitumor therapy. Nanotechnology. 2018;29(40):405101. doi: 10.1088/1361-6528/aad358. [DOI] [PubMed] [Google Scholar]
- 127.Song H., Wang G., He B., et al. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int. J. Nanomedicine. 2012;7:4637–4648. doi: 10.2147/IJN.S33923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Navarro G., Pan J., Torchilin V.P. Micelle-like nanoparticles as carriers for DNA and siRNA. Mol. Pharm. 2015;12(2):301–313. doi: 10.1021/mp5007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chistiakov D.A., Melnichenko A.A., Orekhov A.N., Bobryshev Y.V. Engineered nanoparticles: Their properties and putative applications for therapeutic approaches utilizing stem cells for the repair of atherosclerotic disease. Curr. Drug Targets. 2017;19(14):1639–1648. doi: 10.2174/1389450118666171027111528. [DOI] [PubMed] [Google Scholar]
- 130.Matoba T., Koga J.I., Nakano K., Egashira K., Tsutsui H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol. 2017;70(3):206–211. doi: 10.1016/j.jjcc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 131.Nafee N., Gouda N. Nucleic acids-based nanotherapeutics crossing the blood brain barrier. Curr. Gene Ther. 2017;17(2):154–169. doi: 10.2174/1566523217666170510155803. [DOI] [PubMed] [Google Scholar]
- 132.Leiro V., Santos S.D., Pego A.P. Delivering siRNA with dendrimers: In vivo applications. Curr. Gene Ther. 2017;17(2):105–119. doi: 10.2174/1566523217666170510160527. [DOI] [PubMed] [Google Scholar]
- 133.Prabhu P., Patravale V. The upcoming field of theranostic nanomedicine: An overview. J. Biomed. Nanotechnol. 2012;8(6):859–882. doi: 10.1166/jbn.2012.1459. [DOI] [PubMed] [Google Scholar]
- 134.Vosen S., Rieck S., Heidsieck A., et al. Improvement of vascular function by magnetic nanoparticle-assisted circumferential gene transfer into the native endothelium. J. Control. Release. 2016;241:164–173. doi: 10.1016/j.jconrel.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 135.Swendeman D., Fehrenbacher A.E., Ali S., et al. “Whatever I have, I have made by coming into this profession”: The intersection of resources, agency, and achievements in pathways to sex work in Kolkata, India. Arch. Sex. Behav. 2015;44(4):1011–1023. doi: 10.1007/s10508-014-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y., Xu H., Ma L. Recent advances of thermally responsive nanogels for cancer therapy. Ther. Deliv. 2015;6(10):1157–1169. doi: 10.4155/tde.15.63. [DOI] [PubMed] [Google Scholar]


