Abstract
Background:
Fenoldopam mesylate is a selective agonist of DA-1 receptors. It is currently used for the in-hospital treatment of severe hypertension. DA-1 receptors have high density in renal pa-renchyma and for this reason, a possible reno-protective role of Fenoldopam mesylate was investigated.
Methods:
We examined all studies regarding the role of Fenoldopam mesylate in Acute Kidney Injury (AKI); particularly, those involving post-surgical patients, intensive care unit patients and contrast-induced nephropathy.
Results:
Fenoldopam mesylate was found to be effective in reducing the onset of postoperative AKI, when used before the development of the kidney damage. Positive results were also obtained in the management of intensive care unit patients with AKI, although the clinical studies investigated were few and conducted on small samples.
Conclusion:
Conflicting results were achieved in contrast-induced nephropathy.
Keywords: Fenoldopam mesylate, post-operative acute kidney injury, contrast-induced nephropathy, Intensive care unit-acute kidney injury, DA-1 receptor, dopamine, chronic kidney disease
1. INTRODUCTION
Clinical and pharmaceutical research is in continuous evolution. The synthesis of new drugs is sometimes faster than their clinical trials, with the risk of overlooking certain molecules that instead may have important therapeutic potential.
Fenoldopam mesylate (FM) is currently used for in-hospital, short-term (up to 48 hours) management of severe hypertension [1], but may potentially be used for different therapeutic and prophylactic purposes.
The following review analyzes the available literature on the possible role of FM in the prophylaxis of Acute Kidney Injury (AKI) in intensive care, in the surgical field, and in contrast-induced nephropathy (CIN). With these objectives, we reviewed all the existing literature on PUBMED and COCHRANE LIBRARY using the key-word “FENOLDOPAM”, obtaining 749 and 132 references, respectively. We then examined all the studies evaluating FM’s pharmacodynamic and pharmacokinetic properties in humans, focusing on the clinical studies demonstrating the possible kidney-protective effects of FM. Furthermore, we analyzed any eventual limits and bias in the clinical trials that studied the prophylactic and therapeutic effects of FM in AKI, as an attempt to explain the eventual discordance among the obtained results (Table 1).
Table 1. Major clinical studies on the effects of FM in three different subtypes of AKI.
| A) Fenoldopam Mesylate and PO-AKI | ||||||||
|---|---|---|---|---|---|---|---|---|
| References | Year | No. Patients | Type of Study | FM Dose | Type of Patients |
Effects of
FM |
Primary Outcome |
P Value for
Primary End-point |
| O’Hara JF [27] | 2013 | 90 | Double-blind RCT | 0.1 μg/Kg/min for 24-h period |
Nephrectomy in patients with solitary kidney | - | Change in GFR | P=0.15 |
| Ranucci M [28] | 2010 | 80 | Double-blind RCT | 0.1 μg/Kg/min started at the onset of CPB for the first 12 postoperative hours | Cardiac (coronary and valve) surgery patients | + | Adequacy of perfusion established by serial measurements of blood lactate concentration and oxygen delivery | P=0.048 |
| Biancofiore G [29] | 2004 | 140 | RCT | 0.1 μg/Kg/min from the time of anaesthesia induction to 96 hours post-operatively | Patients undergoing orthotopic liver transplantation | + | FM’s effect in preserving renal function after liver transplantation | P ˂ 0.001 (vs. placebo) P ˂ 0.05 (vs. dopamine) |
| Barr LF [30] | 2008 | 83 | Double-blind RCT | 0.1 μg/Kg/min was started intravenously at surgical induction and continued for 48 hours |
Cardiac surgery patients with preoperative clearance ≤ 40 mL/min but with creatinine ≤ 1.1 mg/dL | + | The difference between the preoperative and postoperative day 3 creatinine clearances | P=0.0286 |
| Cogliati AA [31] | 2007 | 193 | Double-blind RCT | Continuous infusion of FM, 0.1 μg/Kg/min for 24-h period | Cardiac surgery patients with at least one of the following risk factors: pre-operative serum creatinine >1.5 mg/dL, age >70 years, diabetes mellitus, or prior cardiac surgery | + | Patients who underwent elective cardiac surgery with CPB and received prophylactic FM for 24 hours had a lower incidence of AKI | P=0.02 |
| Caimmi PP [32] | 2003 | 160 | RCT | Continuous intravenous administration of low-dose FM (0.1-0.3 μg/Kg/min) during CPB and in the early postoperative period | Patients with serum creatinine >1.5 g/dL who underwent uncomplicated moderate hypothermic CPB for cardiac surgery | + | Improvement of postoperative renal parameters | P ˂0.001 |
| Bove T [33] | 2005 | 80 | Double-blind RCT | 0.05 μg/Kg/min after the induction of anaesthesia for a 24-h period | Cardiac surgical patients at high risk of perioperative renal dysfunction | Null | 25% creatinine increase from baseline levels after cardiac surgery | P=0.9 |
| Halpenny M [34] | 2002 | 28 | Double-blind RCT | 0.1 μg/Kg/min prior to surgical skin incision until release of the aortic clamp | ASA II-III patients undergoing elective aortic surgery requiring infrarenal aortic cross-clamping | + | Preservation of renal function in patients undergoing elective infrarenal aortic cross-clamping | P ˂ 0.01 (CrCl) P ˂ 0.01 (Cr concentration) |
| Halpenny M [35] | 2001 | 31 | Double-blind RCT | Continuous infusion of 0.1 µg/Kg/min for a 24-h period | Patients undergoing elective CPB | + | Renal and splanchnic effects of FM in patients undergoing coronary artery bypass grafting | P<0.01 |
| Oliver WC Jr [36] | 2006 | 60 | Double-blind RCT | 0.05 μg/Kg/min | Patients undergoing abdominal aortic surgery | Null | Renal protective ability of FM (by assessing 3-24-72h CrCl) | P=0.675 (3h CrCl) P=0.86 (24h CrCl) P=0.175(72h CrCl) |
| Della Rocca G [37] | 2004 | 43 | RCT non blinded, FM vs. Dopamine (2 µg/Kg/min) | 0.1 μg/Kg/min | Patients undergoing liver transplantation | + | Incidence of intraoperative and postoperative renal failure and/or dysfunction in patients undergoing liver transplantation comparing FM to small-dose dopamine. | P=0.004 (median Cr preop. vs. postop.) FM vs. dopamine; BUN P=0.0001 (median BUN preop. vs. postop.) FM patients fewer required furosemide compared with dopamine patients p=0.003 |
| McCune TR [38] | 2005 | 20 | Prospective RCT vs. Placebo | 0.1 µg/Kg/min for 48-h postoperatively |
Recipient over the age of 18 who would be receiving a deceased donor allograft with at least 12 h of cold ischemia time | Null | Evaluation of the effect FM on the renal function of patients receiving an allograft with at least 12 h of cold ischemia time. | P= 0.96 (sCr 7 days) P= 0.21 (sCr 14 days) P= 0.12 (sCr 30 days) |
| Ranucci M [40] | 2004 | 108 | Multicenter CT | 0.08 μg/Kg/min | Patients at high risk of AKI undergoing CPB | + | Incidence of RRT-AKI | P=0.028 |
| Roasio A [39] | 2008 | 92 | Case-matched study | Continuous 48-h infusion of FM, 0.1 μg/Kg/min | Patients with acute renal injury after cardiac surgery | + | Reduction of the need for RRT in high risk patients undergoing cardiac surgery | P=0.037 |
| Bove T [43] | 2014 | 667 | RCT, multicenter, double-blind | 0.1 μg/Kg/min (range, 0.025-0.3 μg/Kg/min) for days | Patients with AKI (cardiac-surgery patients) | Null/ increased risk for hypotension |
Rate of RRT | P=0.47 |
| B) Fenoldopam Mesylate and CI-AKI | ||||||||
| Kini AS [46] | 2002 | 260 | Retrospective study | 0.1 μg/Kg/min 15-20 min before and 6 h after the procedure |
Patients with CKD [baseline serum creatinine >1.5mg/ dL] undergoing PCI |
+ | Evaluation of incidence and predictors of CIN after PCI with use of FM in high-risk patients. | p=NS for creatinine increased after PCI procedure |
| Kini AS [45] | 2002 | 150 | Retrospective case-control study | 0.1 μg/Kg/min 15-20 min before and 6 h after the procedure |
Patients with CKD [baseline serum creatinine >1.5mg/ dL] undergoing PCI |
+ | Role of FM in the prevention of CIN during PCI (especially in diabetics) | P ˂ 0.001 |
| Madyoon H [47] | 2001 | 46 | Retrospective case-control study | 0.1 μg/Kg/min 2h before and ≥4 h after the procedure | Patients with CKD [serum creatinine ≥ 1.5mg/dL if diabetic and ≥1.7 mg/dL if non diabetic] | + | Evaluation of FM as a prophylactic strategy for CIN | CIN in 13% of patients treated with FM respect to 38% (expected value) |
| Briguori C [52] | 2004 | 192 | RCT not blinded | 0.1 μg/Kg/min 1h before and 12 h after the procedure | Patients with CKD | Null | Incidence of CIN | P = 0.019 |
| Allaqaband S [49] | 2002 | 123 | RCT | 0.1 μg/Kg/min 4h before and 4 h after the procedure | CKD patients, scheduled for cardiovascular procedures | Null | Incidence of CIN | P = 0.919 |
| Ng MK [51] | 2006 | 84 | RCT | 0.1 μg/Kg/min 2h before and 6 h after the procedure | Stable CKD | Null | Mean change in serum Cr level after 72h | P = 0.4 |
| Stone GW [50] | 2003 | 315 | Double-blind multicenter RCT | 0.05 to 0.1 μg/Kg/min 1h before and 12 h after the procedure | Patients with CKD | Null | Incidence of CIN | P = 0.66 |
| Tumlin JA [48] | 2002 | 45 | Double-blind RCT | 0.1 μg/Kg/min 1h before and 4 h after the procedure | CKD patients undergoing contrast angiography | + | Change in renal plasma flow 1h after contrast infusion | P ˂ 0.05 |
| C) Fenoldopam Mesylate and ICU-AKI | ||||||||
| Cobas M [57] | 2011 | 17 | RCT, double-blind | 0.05 µg/Kg/min and after 20 min increased to an infusion rate of 0.1 µg/Kg/min | Critically ill patients with impaired renal function | + | Low-dose FM for 24 h increases creatinine clearance in critically ill patients with renal insufficiency vs. placebo. | P = 0.045 |
| Brienza N [54] | 2005 | 100 | RCT, multicenter | 0.1µg/Kg/min FM continuous infusion over a 4 days period | Critically ill patients with early renal dysfunction | + | Comparison between FM and low-dose dopamine in early renal dysfunction | P< 0.05 |
| Morelli A [55] | 2005 | 300 | RCT, multicenter, double-blind | 0.09 µg/Kg/min, continuous infusion | Septic patients with baseline serum creatinine <150 µmol/L. | Null/+ | Prophylactic FM for renal protection in sepsis | P=0.005 |
| Tumlin JA [56] | 2005 | 155 | Double-blind RCT | 0.05 μg/Kg/min to 0.2 μg/Kg/min | Intensive care unit | Null | Incidence of need of dialysis therapy or all-cause mortality at 21 days in patients | P=0.163 (incidence of RRT) P=0.068 (21 days mortality) |
RCT: randomized clinical trial; GFR: glomerular filtration rate; CPB: cardio-pulmonary bypass; ASA: American society of anesthesiology classifications; FM: Fenoldopam mesylate; CrCl: creatinine clearance; RRT: renal replacement therapy; CKD: chronic kidney disease; BUN: blood urea nitrogen; PCI: percutaneous coronary intervention; CIN: contrast induced nephropathy.
FM, first named SK&F 82526, was synthesized in 1980 by the chemist Joseph Weinstock and his team at the Smith Kline & French laboratories in Philadelphia [2]. FM is a highly selective agonist of the Dopamine-1 like (DA-1) receptors. In the kidney, DA-1 receptors are localized on the middle layer of blood vessels, the proximal convoluted tubules, and the cortical collecting ducts. Their stimulation leads to renal vasodilation, urinary sodium excretion, and diuresis [3, 4]. DA-1 receptors are also localized on the middle layer of blood vessels of other organs, with different receptor density: the intravenous administration of FM induces peripheral arterial vasodilation in the splanchnic, muscular, and coronary vessels. Nevertheless, DA-1 receptors are present with greater density in renal and splanchnic arteries than in coronary and cerebral vessels [5].
All dopamine receptors belong to the family of G-protein coupled receptors [6]. FM does not exert any effects on the dopaminergic receptors of the DA-2 family, nor on α1 and β adrenergic receptors [7]. Moreover, FM does not cross the blood-brain barrier and thus does not exert any actions on the central nervous system.
The different therapeutic effects of FM and Dopamine are related to their receptor selectivity and density. The first one is selective for DA-1 receptors, and the second one acts on α1 and β adrenergic receptors, DA-1 and DA-2 receptors (Fig. 1). Moreover, dopamine has diverse effects according to its dosage. In the low-dose range (2-5 μg/Kg/min), so-called “renal dose”, dopamine mainly activates DA-1 and DA-2 receptors. At intermediate dosage (5-10 μg/Kg/min), it activates β-1 adrenergic receptors, while at high dosage (10-50 μg/Kg/min), α-adrenergic receptors are also activated 1. Furthermore, in the past, low-dose dopamine (LDD) (2 μg /Kg/min) was thought to have a kidney-protective role. Successive clinical studies failed to demonstrate that LDD protects the kidney of critically ill patients from AKI [8-11]. Therefore, FM currently remains the only drug with a “renal dose” that could induce vasodilation with a potential kidney-protective action, without systemic effects.
Fig. (1).
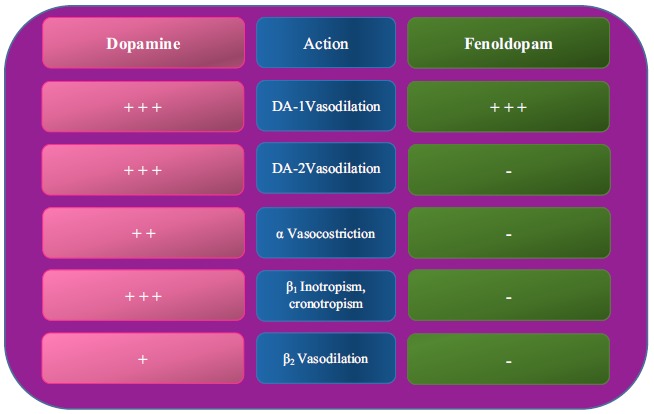
Actions of dopamine agonists (Dopamine and FM).
+++ = strong action; ++ = moderate action; + = minimal action; - = no action.
2. MECHANISM OF ACTION
Although FM can be administered orally or intravenously, the preferred route of administration is the latter, since the bioavailability of the drug after oral administration is reduced and influenced by food intake [12]. The drug can be infused intravenously at a dosage between 0.01-1.6 μg/Kg/min, reaching a steady state concentration proportional to the infusion rate. The half-life of FM is approximately 5 minutes and the steady-state is reached after a time equal to four half-lives of the drug (about 20 minutes) [13]. The volume of distribution (VD) of FM at steady-state varies according to its dosage (for dosages of 0.025 μg/Kg/min and 0.5 μg/Kg/min, VD increases from 0.23 L/Kg to 0.67 L/Kg respectively), as well as based on the enantiomer considered. In fact, the R-enantiomer has a steady-state distribution volume of 0.7 L/Kg compared to the S-enantiomer, which is inactive.
Once in the bloodstream, 88% of FM is bound to plasma proteins [12] and is metabolized in the liver by phase II reactions, without the intervention of cytochrome P-450 enzymes. Lastly, 10% of the drug is eliminated in the feces and 90% in the urine, of which 4% is excreted unchanged [13].
The mode of action of FM is that it interacts with peripheral DA-1-like dopaminergic receptors, resulting in the activation of adenylyl cyclase, which leads to a consequent increase in intracellular cyclic adenosine monophosphate (c-AMP) that in turn induces smooth muscle relaxation [14]. Within the kidney, the increase in c-AMP causes a modulation of the ionic channels with consequent diuretic and natriuretic effects. Activation of DA-1 receptors localized in renal tubular cells decreases sodium transport by c-AMP-dependent and independent mechanisms. The increase of c-AMP inhibits the sodium-hydrogen exchanger and the Na+/K+-ATPase pump [15-17]. Natriuresis is caused by an increase in hydrostatic pressure in the peritubular capillaries and a reduction in oncotic pressure, thereby inducing a decreased reabsorption of sodium by the proximal tubular cells [18].
Further actions of FM include:
Vasodilatation of splanchnic and coronary vasculature;
A slight increase in cardiac ejection fraction in severely hypertensive patients, associated with a modest increase in heart rate;
Reduction in the amplitude of T waves, up to an inversion of these same waves, observable on electrocardiographic recordings;
A slight increase in renin secretion and possible enhancement of norepinephrine levels.
Regarding its most important adverse effects, FM may lead to the possible onset of severe hypotension, tachycardia, flushing, headache, and nausea. FM is contraindicated in patients with sodium bisulfite hypersensitivity because the commercial formulations of the drug contain sodium bisulfite as an excipient, which may trigger potentially life-threatening anaphylactoid reactions. It is also contraindicated in patients receiving monoamine oxidase (MAO) inhibitors, and in patients with ocular hypertension or glaucoma since FM is able to increase intra-ocular pressure by decreasing aqueous humor drainage [19].
The current therapeutic indications are: a) the presence of a hypertensive emergency requiring a rapid control of blood pressure in adult or pediatric patients, and b) management of perioperative hypertension.
In adult patients, the initial dosage of FM is between 0.03-0.1 μg/Kg/min (in order to reduce the possible appearance of reflex tachycardia) which can be increased by 0.1 μg/Kg/min up to a dose of 1.6 μg/Kg/min (maximum dosage administered in clinical trials). In pediatric patients, the recommended starting dose is 0.2 μg/Kg/min, with a maximum effect obtained at a dose of 0.8 μg/Kg/min.
Higher doses do not seem to result in a further reduction of blood pressure values in the face of an increase in heart rate.
3. FENOLDOPAM MESYLATE AND THE KIDNEY
AKI is a term that is currently used to define an abrupt decrease in kidney function (within hours), characterized by both kidney injury (structural damage) and impairment (loss of function), which has substituted the previous term of acute renal failure (ARF) [20].
AKI is a clinical syndrome that can vary in severity and etiology, which can be divided into three main forms: pre-renal, intrinsic, and post-renal. Pre-renal AKI is linked to a renal perfusion deficit responsible for a reversible ARF (associated with functional damage), which - if left untreated - may evolve into an intrinsic renal injury (involving structural damage that can affect all the structures of the kidney) that is no longer immediately reversible. Post-renal AKI is less frequent and is linked to an obstruction along the renal excretory pathways that hinders normal urine outflow.
Among the types of AKI that present pre-renal and intrinsic damage, we can consider three sub-types for which today there is still no universally accepted therapeutic strategy and for which FM has been considered a prophylactic and therapeutic agent. These three sub-types are: post-operative acute kidney injury (PO-AKI), contrast-induced acute kidney injury (CI-AKI) and intensive care unit acute kidney injury (ICU-AKI). The rationale for the use of FM in these three forms of AKI resides in the drug’s renal vasodilator actions in both non-nephropathic and chronic kidney disease (CKD) patients [21].
All three of these AKI sub-types are characterized by different degrees of renal blood perfusion deficits. In PO-AKI, the pathogenesis of the renal damage is related to the reduction in mean arterial pressure that occurs during surgical procedures. The mechanisms of renal auto-regulation cannot counterbalance a severe drop in blood pressure below a certain threshold, which leads to the development of ischemic-hypoxic renal injury. The types of surgical interventions most closely associated with PO-AKI are major surgical interventions such as cardiac surgery, vascular surgery, and thoracic surgery [22, 23]. In CI-AKI, a double kidney damage mechanism is observed: firstly, iodinated contrast media (CM) can lead to the formation of reactive oxygen species (ROS), which cause direct toxic renal damage to tubular epithelial cells and secondly, release of ROS results in vessel vasoconstriction leading to hypoxic-ischemic renal damage [24]. Lastly, ICU-AKI has a multifactorial pathogenic mechanism, which in the majority of cases is due to the use of nephrotoxic substances and above all due to a condition of renal ischemia linked to general anesthesia, the use of CM, and the presence of heart failure and sepsis (the last of which being the main causes of ICU-AKI) [25].
4. POSSIBLE USE OF FENOLDOPAM MESYLATE AS A PROPHYLACTIC DRUG FOR PO-AKI
Many studies have examined FM as a possible therapeutic agent capable of preventing the onset of PO-AKI, the first dating back to 2001 [26-40]. The two most recent meta-analyses include the ones conducted by Zangrillo et al. (2012) [41] and Gilles et al. (2015) [42]. While Zangrillo et al. only examined patients undergoing cardiac surgery; Gilles et al. examined patients undergoing a greater variety of surgeries. Both meta-analyses evaluated only randomized placebo-controlled clinical trials. Overall, the resulting data is concordant: FM causes a decrease in the incidence of PO-AKI without however reducing the need for renal replacement therapy (RRT) or mortality.
The meta-analysis conducted by Gilles et al. contains six studies, in which total of 507 patients were examined. Among the studies analyzed, three studies involved patients undergoing cardiac surgery and the remaining three related to patients undergoing aortic surgery, transplantation, and partial nephrectomy. In these studies, the development of PO-AKI was examined in only 471 patients; hence, only five of the six studies evaluated were taken into consideration by the meta-analysis. The results were as follows: AKI occurred in 18.9% of patients treated with FM versus 26.6% of patients given the placebo (p = 0.004, OR 0.46, 95% CI: 0.27-0.79). On the other hand, concerning the risk of RRT, the result comparing treated versus untreated patients (p = 0.11, OR 0.27, 95% CI: 0.06-1.19) was not statistically significant. Likewise, concerning in-hospital mortality, no significant differences were found between the two groups (p = 0.60, OR 1.0, 95% CI: 0.14-7.37).
Zangrillo et al. reported similar results. In their meta-analysis, they considered six randomized placebo-controlled clinical trials conducted on patients undergoing cardiac surgery. The results of this meta-analysis were comparable to those obtained by Gilles and co-workers; there was a reduction in the risk of developing AKI in patients who were given FM. In particular, AKI was observed in 9.4% (19/202) of patients treated with FM versus 19.8% (41/207) of patients in the placebo group (OR = 0.41, 95% CI: 0.23-0.74). As observed in the previous study, a reduction in the risk of RRT was not observed: 3.8% (7/183) in the group treated with FM versus 6.9% (13/188) in the placebo group (OR = 0.67, 95% CI: 0.10-4.48). Moreover, the authors did not demonstrate a significant reduction of intra-hospital mortality: 5.4% (11/204) in the group treated with FM versus 4.8% (10/207) in the placebo group (OR = 1.12, 95% CI: 0.46-2.72).
Collectively, these two studies suggest that preventive administration of FM in the pre-operative period is able to reduce the incidence of AKI, but not the risk of RRT or intra-hospital death.
Although these results are promising, potential biases in these meta-analyses must be noted. In the studies examined, there were neither unequivocal criteria establishing when to initiate RRT, nor was a unique definition of AKI used. The latter is a source of bias not only affecting the PO-AKI studies, but also those on CI-AKI and ICU-AKI, since the first universally accepted definitions of AKI date back to 2004 (AKIN) and 2007 (RIFLE), with the most updated definition accepted by the international scientific community in 2012 (K-DIGO-AKI). Furthermore, the studies analyzed included only three types of surgeries, and it is not clear whether these results can be extended to other branches of surgery. Lastly, all the studies were underpowered, making conclusive interpretations on the use of FM in AKI, RRT or intra-hospital death challenging.
Further studies should also investigate in detail the dose of the drug to be administered and the duration of its administration. Although according to a meta-regression performed by Zangrillo and collaborators, neither the dose of the drug (p for regression coefficient = 0.7) nor the duration of its administration (p for regression coefficient = 0.4) are related to PO-AKI, on the contrary it is a common opinion among experts that the optimal dose and duration of therapy must still be precisely defined.
Recently, a multicenter randomized clinical trial was conducted by Bove et al. [43] on the largest post-surgical population treated with FM. The aim of this study was to evaluate the efficacy of FM in reducing the risk of RRT and intra-hospital mortality in 667 individuals with PO-AKI developed following cardiac surgery. However, the results obtained were not positive: FM did not lead to a reduction in the risk of RRT or intra-hospital mortality, but only to an increased risk of arterial hypotension compared to placebo, which forced the study to end prematurely. This study also presented several limitations potentially able to influence the results. Firstly, only 667 patients out of 1000 completed the study, decreasing its statistical power; secondly, the enrolled patients presented severely compromised general conditions (> 50% had New York Heart Association class III-IV heart failure); lastly, definite criteria to initiate RRT had not been previously established. Nevertheless, it should be noted that these results are not in conflict with those derived from the aforementioned meta-analyses. In fact, Bove’s study did not investigate the possible prophylactic role of FM in the development of PO-AKI but only the hypothetical role played by FM in reducing the risk of RRT and intra-hospital mortality once a condition of PO-AKI has already been manifested. This only reinforces the hypothesis that FM is an effective drug when used preventively (before hemodynamic damage to the kidney occurs) but is ineffective when administered for therapeutic purposes (after the damage has taken place).
In conclusion, FM is still today one of the most promising drugs in the prevention of PO-AKI and further randomized controlled clinical trials are necessary to confirm the positive data deduced from the meta-analyses conducted by Gilles and Zangrillo.
5. FENOLDOPAM MESYLATE IN THE PROPHYLAXIS OF CI-AKI
FM was first studied in 1999 as a possible agent to prevent contrast-induced nephropathy (CIN) by Professor George Bakris at the University of Chicago, who tried to corroborate the hypothesis that stimulation of DA-1 receptors was able to prevent the decrease in renal blood flow (RBF) and glomerular filtration rate (GFR), which are observed following the administration of iodinated CM [44]. This hypothesis was tested in six laboratory dogs, to which iodinated CM was administered simultaneously with the infusion of FM at a dose of 0.1 μg/Kg/min. The results obtained confirmed the hypothesis that FM infusion is able to prevent the fall of RBF and GFR. This pilot study aroused the interest of the international scientific community in the clinical testing of FM as a prophylactic agent for CIN. The first human studies were published between 2001 and 2002 [45-48] and consisted of non-randomized retrospective pilot clinical studies. The data obtained was decidedly positive, since the reduction in the risk of CIN in the examined patients turned out to be statistically significant. All patients enrolled in these studies were nephropathic, with serum creatinine values >1.5 mg/dL. In the following years up to 2006, randomized clinical trials were conducted, to consolidate these preliminary results [49-52]. However, these results disavowed those previously obtained, since FM did not prove to be effective in preventing CIN.
Currently, there are three meta-analyses [53-55] that agree on the inefficacy of FM. However, some considerations must be made. Firstly, various authors used different definitions of CI-AKI (all different from that of Kidney Disease: Improving Global Outcomes-KDIGO-AKI); secondly, in Stone’s study the development of hypotension (even at dose of FM’s 0.1 μg/Kg/min) caused drop out in 13% of the patients enrolled and made it necessary to reduce the dose by 50% (0.05 µg/Kg/min) in another 13% of patients [50]. Furthermore, the development of hypotension may have certainly contributed to worsening renal hemodynamics, increasing the number of cases of CIN in the group treated with FM. This side effect can, however, be mitigated by increasing patient blood volume with adequate preprocedural hydration.
Furthermore, it would be beneficial to examine the relationship between N-Acetyl Cysteine (NAC) and FM. In many studies, the two drugs have been compared, but they actually act with synergistic mechanisms. The rationale behind their combined use may be better understood by focusing on the pathogenetic mechanisms of CIN. The pathogenesis of CIN is multi-factorial. Firstly, it is correlated to the formation of free radicals, which reduce the synthesis of nitric oxide in the vasa recta and decrease the local levels of prostaglandins, resulting in vasoconstriction. Secondly, CIN is due to the direct cellular damage on renal tubular and endothelial cells, which is caused by altered mitochondrial membrane potential and the formation of ROS at the mitochondrial level, with consequent mitophagy [24]. The first pathogenetic mechanism induced by iodinated contrast media (CM) can be counteracted by NAC, and to a greater extent by FM, since both pharmacological agents have a vasodilatory action. The vasodilatory action of FM has been demonstrated in both healthy individuals and patients with nephropathy [21, 56]. The second damaging effect of CM is antagonized by NAC, via scavenging of ROS that is formed at tubular and endothelial levels [57]. Hence, FM and NAC could be used in combination versus placebo, in subsequent randomized clinical trials.
6. FENOLDOPAM MESYLATE AS PROPHYLAXIS OF ICU-AKI
FM has also been studied as a therapy for ICU-AKI. In total, four clinical trials were published from 2005 to 2008, studying the use of FM to prevent the progression or development of AKI in ICU patients. The results obtained showed efficacy of FM in increasing creatinine clearance and in reducing the serum creatinine peak, compared to placebo and dopamine administered at renal dose. However, the same positive results were not obtained concerning other renal outcomes, such as the need for dialytic therapy and 21-day mortality.
Brienza et al. [58] studied a population of 100 patients admitted to ICUs. The aim of their study was to investigate if FM can give greater benefit compared to dopamine infused at a renal dose in early renal dysfunction of critically ill patients. The authors observed a significant decrease in mean serum creatinine values in patients treated with FM, compared to patients receiving LDD. These authors concluded that a continuous infusion of FM (0.1 µg/Kg/min) improves renal function and does not induce a significant hemodynamic alteration with respect to LDD. This study indicated that it is important to administer FM promptly when the acute renal injury is early and severe renal failure is not established.
Morelli et al. [59] studied the effect of FM versus placebo on 300 patients admitted to the ICU for sepsis. The selected population consisted of non-nephrophatic patients, but unlike the other studies, FM was given as prophylaxis before AKI was developed. The results obtained were a reduction of incidence of AKI, a small increase in serum creatinine values and a small decrease in GFR compared to placebo. However, there was no direct effect on mortality in ICU patients treated with FM.
Tumlin et al. [60] evaluated if FM infusion compared to placebo can cause a reduction in the need for dialysis therapy and/or 21-day mortality in 155 patients with acute tubular necrosis (ATN) admitted to the ICU. Patients were eligible if serum creatinine value was 50% enhanced than the levels at admission within 24 hours and mean blood pressure was greater than 70 mm Hg. The authors observed a non-statistically significant reduction in serum creatinine values in the FM treated group and the need for dialysis therapy and 21-day mortality was not found significant. The interesting data from this study was the post-hoc analysis performed on non-diabetic patients not subjected to cardiac surgery: in this specific subgroup of patients, FM had shown to reduce significantly the need of dialysis therapy and the incidence of 21-day mortality.
A randomized controlled trial (RCT) versus crossover placebo performed by Cobas et al. [59] showed that infusion of FM was associated with an increase of creatinine clearance in 17 patients with impaired renal function admitted in trauma ICU. These authors confirmed that FM is a useful drug in ICU patients with early renal dysfunction.
The final analysis, in almost all clinical studies performed on FM, showed to induce a decrease in serum creatinine compared to placebo or LDD, with consequent improvement in renal function. FM is a drug that should be used for prophylactic and non-therapeutic purposes for ICU-AKI. In fact, as for the other forms of AKI, the damage must be prevented because once triggered, FM may no longer be sufficient to stop its progression.
Although most studies lead to concordant conclusions, they present divergent definitions of AKI, since the current definition of KDIGO-AKI became only subsequently available, creating a potential source of bias.
Furthermore, the meta-analysis performed by Landoni et al. [61] examined patients with or at risk of ARF, who were critically ill or underwent major surgery. The authors demonstrated that FM significantly reduced the risk for AKI, need for RRT, and in-hospital death.
Based on the results obtained, a RCT should be carried out with the following considerations:
The ability of FM to reduce the decrease in GFR if used as prophylaxis before the development of kidney injury;
The reduction in risk of death and/or need of renal replacement therapy at 21-days, highlighting the use of FM in post hoc analysis of patients without diabetes who underwent cardiothoracic surgery as reported in the study by Tumlin et al. [62].
The absence of a universally accepted definition of AKI in the previous studies (which today is present in the K-DIGO-AKI criteria).
CONCLUSION
In conclusion, based on the results reported in the major clinical trials that have studied FM, this drug has shown to reduce the risk of developing PO-AKI and ICU-AKI. The analysis of these studies suggested that FM would be effective in preventing certain forms of AKI. FM should be used as a prophylactic and non-therapeutic agent, before organ damage is established.
Regarding the role of FM in the prophylaxis of CI-AKI, it is important to underline the absence of studies that compare the efficacy of FM associated with NAC versus placebo. This association would lead to a synergistic effect of the two drugs, contrasting the formation of ROS (N-acetyl cysteine) and exerting positive effects on renal hemodynamics (FM). Furthermore, FM is very manageable when used at a renal dose, and in most of the clinical trials, it is neither associated with significant decreases in blood pressure nor whit significant increases in heart rate.
Moreover, these potential sources of bias should be considered:
The absence of unequivocal criteria for the beginning of the renal replacement therapy. In fact, in the clinical trial the need for RRT was established by clinician’s choice;
In ICU, the patients had unstable hemodynamic conditions regardless of FM infusion;
The absence of specific data about the effectiveness of FM according to the stage of chronic kidney disease. In fact, in the majority of the RCTs the patients were selected only for creatinine value >1.5 mg/dL.
A brief note must be made on the cost-benefit ratio of the drug: undoubtedly, FM is a relatively expensive drug but is lower in cost than prolonged hospitalization in a hospital unit or even more in an ICU, once AKI has been manifested.
ACKNOWLEDGEMENTS
We would like to thank Professor Simone Manca di Villahermosa for the language revision of the manuscript. We gratefully acknowledge Prof. Maurizio Casasco for his help in revising the manuscript.
LIST OF ABBREVIATIONS
- AKI
Acute Kidney Injury
- ARF
Acute Renal Failure
- ATN
Acute Tubular Necrosis
- c-AMP
Cyclic Adenosine Monophosphate
- CI-AKI
Contrast-Induced Acute Kidney Injury
- CIN
Contrast-Induced Nephropathy
- CKD
Chronic Kidney Disease
- DA-1
Dopamine 1 Like
- DA-2
Dopamine 2 Like
- FM
Fenoldopam Mesylate
- GFR
Glomerular Filtration Rate
- ICU-AKI
Intensive-Care Unit Acute Kidney Injury
- KDIGO-AKI
Kidney Disease Improving Global Outcomes-Acute Kidney Injury
- LDD
Low Dose Dopamine
- MAO
Monoamine Oxidase
- NAC
N-Acetyl Cysteine
- NYHA
New York Heart Association
- PO-AKI
Post-Operative Acute Kidney Injury
- RBF
Renal Blood Flow
- RCT
Randomized Controlled Trial
- ROS
Reactive Oxygen Species
- RRT
Renal Replacement Therapy
- VD
Volume of Distribution
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
We also thank Federazione Medico Sportiva Italiana for financial support. Liberal research contribution by the Italian Sports Medical Federation to our Department of Systems Medicine.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Murphy M.B., Murray C., Shorten G.D. Fenoldopam: A selective peripheral dopamine-receptor agonist for the treatment of severe hypertension. N. Engl. J. Med. 2001;345(21):1548–1557. doi: 10.1056/NEJMra010253. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock J., Wilson J.W., Ladd D.L., Brush C.K., Pfeiffer F.R., Kuo G.Y., Holden K.G., Yim N.C., Hahn R.A., Wardell J.R., Jr, Tobia A.J., Setler P.E., Sarau H.M., Ridley P.T. Separation of potent central and renal dopamine agonist activity in substituted 6-chloro-2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepines. J. Med. Chem. 1980;23(9):973–975. doi: 10.1021/jm00183a001. [DOI] [PubMed] [Google Scholar]
- 3.Carey R.M., Siragy H.M., Ragsdale N.V., Howell N.L., Felder R.A., Peach M.J., Chevalier R.L. Dopamine-1 and dopamine-2 mechanisms in the control of renal function. Am. J. Hypertens. 1990;3(6 Pt 2):59S–63S. doi: 10.1093/ajh/3.6.59s. [DOI] [PubMed] [Google Scholar]
- 4.Hughes J.M., Beck T.R., Rose C.E., Jr, Carey R.M. The effect of selective dopamine-1 receptor stimulation on renal and adrenal function in man. J. Clin. Endocrinol. Metab. 1988;66(3):518–525. doi: 10.1210/jcem-66-3-518. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg L.I. Dopamine receptors and hypertension. Physiologic and pharmacologic implications. Am. J. Med. 1984;77(4A):37–44. doi: 10.1016/s0002-9343(84)80036-4. [DOI] [PubMed] [Google Scholar]
- 6.Dohlman H.G., Thorner J., Caron M.G., Lefkowitz R.J. Model systems for the study of seven-transmembrane-segment receptors. Annu. Rev. Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 7.Hahn R.A., Wardell J.R., Jr, Sarau H.M., Ridley P.T. Characterization of the peripheral and central effects of SK&F 82526, a novel dopamine receptor agonist. J. Pharmacol. Exp. Ther. 1982;223(2):305–313. [PubMed] [Google Scholar]
- 8.Marik P.E. Low-dose dopamine: A systematic review. Intensive Care Med. 2002;28(7):877–883. doi: 10.1007/s00134-002-1346-y. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R., Chapman M., Finfer S., Hickling K., Myburgh J. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356(9248):2139–2143. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- 10.Jones D., Bellomo R. Renal-dose dopamine: From hypothesis to paradigm to dogma to myth and, finally, superstition? J. Intensive Care Med. 2005;20(4):199–211. doi: 10.1177/0885066605276963. [DOI] [PubMed] [Google Scholar]
- 11.Kellum J.A., Decker M.J. Use of dopamine in acute renal failure: a meta-analysis. Crit. Care Med. 2001;29(8):1526–1531. doi: 10.1097/00003246-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Brogden R.N., Markham A. Fenoldopam: A review of its pharmacodynamic and pharmacokinetic properties and intravenous clinical potential in the management of hypertensive urgencies and emergencies. Drugs. 1997;54(4):634–650. doi: 10.2165/00003495-199754040-00008. [DOI] [PubMed] [Google Scholar]
- 13. www.accessdata.fda.gov/drugsatfda_docs/label/2004/19922se5-005_colopam_lbl.pdf
- 14.Lokhandwala M.F., Barrett R.J. Cardiovascular dopamine receptors: physiological, pharmacological and therapeutic implications. J. Auton. Pharmacol. 1982;2(3):189–215. doi: 10.1111/j.1474-8673.1982.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 15.Felder C.C., Campbell T., Albrecht F., Jose P.A. Dopamine inhibits Na(+)-H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am. J. Physiol. 1990;259(2 Pt 2):F297–F303. doi: 10.1152/ajprenal.1990.259.2.F297. [DOI] [PubMed] [Google Scholar]
- 16.Mills K.T., Xu Y., Zhang W., Bundy J.D., Chen C.S., Kelly T.N., Chen J., He J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88(5):950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aperia A., Fryckstedt J., Svensson L., Hemmings H.C., Jr, Nairn A.C., Greengard P. Phosphorylated Mr 32,000 dopamine- and cAMP-regulated phosphoprotein inhibits Na+,K(+)-ATPase activity in renal tubule cells. Proc. Natl. Acad. Sci. USA. 1991;88(7):2798–27801. doi: 10.1073/pnas.88.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner B.M., Troy J.L., Daugharty T.M., MacInnes R.M. Quantitative importance of changes in postglomerular colloid osmotic pressure in mediating glomerulotubular balance in the rat. J. Clin. Invest. 1973;52(1):190–197. doi: 10.1172/JCI107164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piltz J.R., Stone R.A., Boike S., Everitt D.E., Shusterman N.H., Audet P., Zariffa N., Jorkasky D.K. Fenoldopam, a selective dopamine-1 receptor agonist, raises intraocular pressure in males with normal intraocular pressure. J. Ocul. Pharmacol. Ther. 1998;14(3):203–216. doi: 10.1089/jop.1998.14.203. [DOI] [PubMed] [Google Scholar]
- 20.Makris K., Spanou L. Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin. Biochem. Rev. 2016;37(2):85–98. [PMC free article] [PubMed] [Google Scholar]
- 21.Rovella V., Ferrannini M., Tesauro M., Marrone G., Busca A., Sorge R., Manca di Villahermosa S., Casasco M., Di Daniele N., Noce A. Effects of fenoldopam on renal blood flow in hypertensive chronic kidney disease. J. Nephrol. 2019;32(1):75–81. doi: 10.1007/s40620-018-0496-0. [DOI] [PubMed] [Google Scholar]
- 22.Romagnoli S., Ricci Z. Postoperative acute kidney injury. Minerva Anestesiol. 2015;81(6):684–696. [PubMed] [Google Scholar]
- 23.Park J.T. Postoperative acute kidney injury. Korean J. Anesthesiol. 2017;70(3):258–266. doi: 10.4097/kjae.2017.70.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pistolesi V., Regolisti G., Morabito S., Gandolfini I., Corrado S., Piotti G., Fiaccadori E. Contrast medium induced acute kidney injury: a narrative review. J. Nephrol. 2018;31(6):797–812. doi: 10.1007/s40620-018-0498-y. [DOI] [PubMed] [Google Scholar]
- 25.Huber W., Schneider J., Lahmer T., Kuchle C., Jungwirth B., Schmid R.M., Schmid S. Validation of RIFLE, AKIN, and a modified AKIN definition ("backward classification") of acute kidney injury in a general ICU: Analysis of a 1-year period. Medicine, (Baltimore) 2018;97(38):e12465. doi: 10.1097/MD.0000000000012465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert T.B., Hasnain J.U., Flinn W.R., Lilly M.P., Benjamin M.E. Fenoldopam infusion associated with preserving renal function after aortic cross-clamping for aneurysm repair. J. Cardiovasc. Pharmacol. Ther. 2001;6(1):31–36. doi: 10.1177/107424840100600104. [DOI] [PubMed] [Google Scholar]
- 27.O’Hara J.F., Jr, Mahboobi R., Novak S.M., Bonilla A.M., Mascha E.J., Fergany A.F., Campbell S.C., Kaouk J.H., Kaple K.M., Gill I.S., Ziegman S.A., Sessler D.I. Fenoldopam and renal function after partial nephrectomy in a solitary kidney: A randomized, blinded trial. Urology. 2013;81(2):340–345. doi: 10.1016/j.urology.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Ranucci M., De Benedetti D., Bianchini C., Castelvecchio S., Ballotta A., Frigiola A., Menicanti L. Effects of fenoldopam infusion in complex cardiac surgical operations: A prospective, randomized, double-blind, placebo-controlled study. Minerva Anestesiol. 2010;76(4):249–259. [PubMed] [Google Scholar]
- 29.Biancofiore G., Della Rocca G., Bindi L., Romanelli A., Esposito M., Meacci L., Urbani L., Filipponi F., Mosca F. Use of fenoldopam to control renal dysfunction early after liver transplantation. Liver Transpl. 2004;10(8):986–992. doi: 10.1002/lt.20145. [DOI] [PubMed] [Google Scholar]
- 30.Barr L.F., Kolodner K. N-acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Crit. Care Med. 2008;36(5):1427–1435. doi: 10.1097/CCM.0b013e31816f48ba. [DOI] [PubMed] [Google Scholar]
- 31.Cogliati A.A., Vellutini R., Nardini A., Urovi S., Hamdan M., Landoni G., Guelfi P. Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: A randomized clinical study. J. Cardiothorac. Vasc. Anesth. 2007;21(6):847–850. doi: 10.1053/j.jvca.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Caimmi P.P., Pagani L., Micalizzi E., Fiume C., Guani S., Bernardi M., Parodi F., Cordero G., Fregonara M., Kapetanakis E., Panella M., Degasperis C. Fenoldopam for renal protection in patients undergoing cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2003;17(4):491–494. doi: 10.1016/s1053-0770(03)00155-1. [DOI] [PubMed] [Google Scholar]
- 33.Bove T., Landoni G., Calabro M.G., Aletti G., Marino G., Cerchierini E., Crescenzi G., Zangrillo A. Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery: A prospective, double-blind, randomized clinical trial. Circulation. 2005;111(24):3230–3235. doi: 10.1161/CIRCULATIONAHA.104.509141. [DOI] [PubMed] [Google Scholar]
- 34.Halpenny M., Rushe C., Breen P., Cunningham A.J., Boucher-Hayes D., Shorten G.D. The effects of fenoldopam on renal function in patients undergoing elective aortic surgery. Eur. J. Anaesthesiol. 2002;19(1):32–39. doi: 10.1017/s0265021502000054. [DOI] [PubMed] [Google Scholar]
- 35.Halpenny M., Lakshmi S., O’Donnell A., O’Callaghan-Enright S., Shorten G.D. Fenoldopam: Renal and splanchnic effects in patients undergoing coronary artery bypass grafting. Anaesthesia. 2001;56(10):953–960. doi: 10.1046/j.1365-2044.2001.02220.x. [DOI] [PubMed] [Google Scholar]
- 36.Oliver W.C., Jr, Nuttall G.A., Cherry K.J., Decker P.A., Bower T., Ereth M.H. A comparison of fenoldopam with dopamine and sodium nitroprusside in patients undergoing cross-clamping of the abdominal aorta. Anesth. Analg. 2006;103(4):833–840. doi: 10.1213/01.ane.0000237273.79553.9e. [DOI] [PubMed] [Google Scholar]
- 37.Della Rocca G., Pompei L., Costa M.G., Coccia C., Scudeller L., Di Marco P., Monaco S., Pietropaoli P. Fenoldopam mesylate and renal function in patients undergoing liver transplantation: a randomized, controlled pilot trial. Anesth. Analg. 2004;99(6):1604–1609. doi: 10.1213/01.ANE.0000136420.01393.81. [DOI] [PubMed] [Google Scholar]
- 38.McCune T.R., Wombolt D.G., Whelan T.V., Thacker L.R., Colonna J.O. Vasodilatation vs. immunotherapy to prevent delayed graft function: Delayed graft function as an indication of immune activation. Int. Immunopharmacol. 2005;5(1):85–92. doi: 10.1016/j.intimp.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Roasio A., Lobreglio R., Santin A., Landoni G., Verdecchia C. Fenoldopam reduces the incidence of renal replacement therapy after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2008;22(1):23–26. doi: 10.1053/j.jvca.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Ranucci M., Soro G., Barzaghi N., Locatelli A., Giordano G., Vavassori A., Manzato A., Melchiorri C., Bove T., Juliano G., Uslenghi M.F. Fenoldopam prophylaxis of postoperative acute renal failure in high-risk cardiac surgery patients. Ann. Thorac. Surg. 2004;78(4):1332–1337. doi: 10.1016/j.athoracsur.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 41.Zangrillo A., Biondi-Zoccai G.G., Frati E., Covello R.D., Cabrini L., Guarracino F., Ruggeri L., Bove T., Bignami E., Landoni G. Fenoldopam and acute renal failure in cardiac surgery: a meta-analysis of randomized placebo-controlled trials. J. Cardiothorac. Vasc. Anesth. 2012;26(3):407–413. doi: 10.1053/j.jvca.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 42.Gillies M.A., Kakar V., Parker R.J., Honore P.M., Ostermann M. Fenoldopam to prevent acute kidney injury after major surgery-a systematic review and meta-analysis. Crit. Care. 2015;19:449. doi: 10.1186/s13054-015-1166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bove T., Zangrillo A., Guarracino F., Alvaro G., Persi B., Maglioni E., Galdieri N., Comis M., Caramelli F., Pasero D.C., Pala G., Renzini M., Conte M., Paternoster G., Martinez B., Pinelli F., Frontini M., Zucchetti M.C., Pappalardo F., Amantea B., Camata A., Pisano A., Verdecchia C., Dal Checco E., Cariello C., Faita L., Baldassarri R., Scandroglio A.M., Saleh O., Lembo R., Calabro M.G., Bellomo R., Landoni G. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: A randomized clinical trial. JAMA. 2014;312(21):2244–2253. doi: 10.1001/jama.2014.13573. [DOI] [PubMed] [Google Scholar]
- 44.Bakris G.L., Lass N.A., Glock D. Renal hemodynamics in radiocontrast medium-induced renal dysfunction: A role for dopamine-1 receptors. Kidney Int. 1999;56(1):206–210. doi: 10.1046/j.1523-1755.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 45.Kini A.S., Mitre C.A., Kim M., Kamran M., Reich D., Sharma S.K. A protocol for prevention of radiographic contrast nephropathy during percutaneous coronary intervention: Effect of selective dopamine receptor agonist fenoldopam. Catheter. Cardiovasc. Interv. 2002;55(2):169–173. doi: 10.1002/ccd.10038. [DOI] [PubMed] [Google Scholar]
- 46.Kini A.S., Mitre C.A., Kamran M., Suleman J., Kim M., Duffy M.E., Marmur J.D., Sharma S.K. Changing trends in incidence and predictors of radiographic contrast nephropathy after percutaneous coronary intervention with use of fenoldopam. Am. J. Cardiol. 2002;89(8):999–1002. doi: 10.1016/s0002-9149(02)02259-2. [DOI] [PubMed] [Google Scholar]
- 47.Madyoon H., Croushore L., Weaver D., Mathur V. Use of fenoldopam to prevent radiocontrast nephropathy in high-risk patients. Catheter. Cardiovasc. Interv. 2001;53(3):341–345. doi: 10.1002/ccd.1178. [DOI] [PubMed] [Google Scholar]
- 48.Tumlin J.A., Wang A., Murray P.T., Mathur V.S. Fenoldopam mesylate blocks reductions in renal plasma flow after radiocontrast dye infusion: A pilot trial in the prevention of contrast nephropathy. Am. Heart J. 2002;143(5):894–903. doi: 10.1067/mhj.2002.122118. [DOI] [PubMed] [Google Scholar]
- 49.Allaqaband S., Tumuluri R., Malik A.M., Gupta A., Volkert P., Shalev Y., Bajwa T.K. Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter. Cardiovasc. Interv. 2002;57(3):279–283. doi: 10.1002/ccd.10323. [DOI] [PubMed] [Google Scholar]
- 50.Stone G.W., McCullough P.A., Tumlin J.A., Lepor N.E., Madyoon H., Murray P., Wang A., Chu A.A., Schaer G.L., Stevens M., Wilensky R.L., O’Neill W.W., Investigators C. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: A randomized controlled trial. JAMA. 2003;290(17):2284–2291. doi: 10.1001/jama.290.17.2284. [DOI] [PubMed] [Google Scholar]
- 51.Ng T.M., Shurmur S.W., Silver M., Nissen L.R., O’Leary E.L., Rigmaiden R.S., Cieciorka M., Porter L.L., Ineck B.A., Kline M.E., Puumala S.E. Comparison of N-acetylcysteine and fenoldopam for preventing contrast-induced nephropathy (CAFCIN). Int. J. Cardiol. 2006;109(3):322–328. doi: 10.1016/j.ijcard.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Briguori C., Colombo A., Airoldi F., Violante A., Castelli A., Balestrieri P., Paolo Elia P., Golia B., Lepore S., Riviezzo G., Scarpato P., Librera M., Focaccio A., Ricciardelli B. N-Acetylcysteine versus fenoldopam mesylate to prevent contrast agent-associated nephrotoxicity. J. Am. Coll. Cardiol. 2004;44(4):762–765. doi: 10.1016/j.jacc.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 53.Kelly A.M., Dwamena B., Cronin P., Bernstein S.J., Carlos R.C. Meta-analysis: Effectiveness of drugs for preventing contrast-induced nephropathy. Ann. Intern. Med. 2008;148(4):284–294. doi: 10.7326/0003-4819-148-4-200802190-00007. [DOI] [PubMed] [Google Scholar]
- 54.Naeem M., McEnteggart G.E., Murphy T.P., Prince E., Ahn S., Soares G. Fenoldopam for the prevention of contrast-induced nephropathy (CIN)-do we need more trials? A meta-analysis. Clin. Imaging. 2015;39(5):759–764. doi: 10.1016/j.clinimag.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Su X., Xie X., Liu L., Lv J., Song F., Perkovic V., Zhang H. Comparative effectiveness of 12 treatment strategies for preventing contrast-induced acute kidney injury: A systematic review and bayesian network meta-analysis. Am. J. Kidney Dis. 2017;69(1):69–77. doi: 10.1053/j.ajkd.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 56.Mathur V.S., Swan S.K., Lambrecht L.J., Anjum S., Fellmann J., McGuire D., Epstein M., Luther R.R. The effects of fenoldopam, a selective dopamine receptor agonist, on systemic and renal hemodynamics in normotensive subjects. Crit. Care Med. 1999;27(9):1832–1837. doi: 10.1097/00003246-199909000-00021. [DOI] [PubMed] [Google Scholar]
- 57.Drager L.F., Andrade L., Barros de Toledo J.F., Laurindo F.R., Machado Cesar L.A., Seguro A.C. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol. Dial. Transplant. 2004;19(7):1803–1807. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 58.Brienza N., Malcangi V., Dalfino L., Trerotoli P., Guagliardi C., Bortone D., Faconda G., Ribezzi M., Ancona G., Bruno F., Fiore T. A comparison between fenoldopam and low-dose dopamine in early renal dysfunction of critically ill patients. Crit. Care Med. 2006;34(3):707–714. doi: 10.1097/01.CCM.0000201884.08872.A2. [DOI] [PubMed] [Google Scholar]
- 59.Morelli A., Ricci Z., Bellomo R., Ronco C., Rocco M., Conti G., De Gaetano A., Picchini U., Orecchioni A., Portieri M., Coluzzi F., Porzi P., Serio P., Bruno A., Pietropaoli P. Prophylactic fenoldopam for renal protection in sepsis: A randomized, double-blind, placebo-controlled pilot trial. Crit. Care Med. 2005;33(11):2451–2456. doi: 10.1097/01.ccm.0000186413.04875.ef. [DOI] [PubMed] [Google Scholar]
- 60.Tumlin J.A., Finkel K.W., Murray P.T., Samuels J., Cotsonis G., Shaw A.D. Fenoldopam mesylate in early acute tubular necrosis: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Kidney Dis. 2005;46(1):26–34. doi: 10.1053/j.ajkd.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Cobas M., Paparcuri G., De La Pena M., Cudemus G., Barquist E., Varon A. Fenoldopam in critically ill patients with early renal dysfunction. A crossover study. Cardiovasc. Ther. 2011;29(4):280–284. doi: 10.1111/j.1755-5922.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- 62.Landoni G., Biondi-Zoccai G.G., Tumlin J.A., Bove T., De Luca M., Calabro M.G., Ranucci M., Zangrillo A. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: A meta-analysis of randomized clinical trials. Am. J. Kidney Dis. 2007;49(1):56–68. doi: 10.1053/j.ajkd.2006.10.013. [DOI] [PubMed] [Google Scholar]


