Abstract
Oxidative stress is well recognized to contribute to the pathogenesis of diverse diseases, including the devastating disease of the lung's blood vessels, pulmonary arterial hypertension (PAH), however, antioxidant-based therapies have been overall disappointing. With the evolution of the field of redox biology, it is now becoming clear that redox reactions are highly selective and targeted, allowing for precise control of redox-regulated signaling in health and disease. This special Forum of the journal describes the current state of knowledge on the regulation of redox-regulated signaling during the development of pulmonary vascular disease, focusing on distinct compartmentalized mechanisms outside and within the cell, including regulation of extracellular and intracellular membrane receptors and channels; responses to changes in biomechanical forces; intracellular thiol redox control; regulation of the nuclear transcription factor, peroxisome proliferator-activated receptor-γ; and regulation of mitochondrial metabolism. Collectively, they exemplify the complex, precise, and localized signaling pathways that drive PAH pathogenesis. This group of authors suggests ways that our increased understanding of these events may pave the way to improved therapeutic approaches for the treatment of this lethal disease.
Keywords: redox-regulated signaling, pulmonary hypertension, pulmonary vasoconstriction, pulmonary vascular remodeling
Pulmonary arterial hypertension (PAH) is a devastating disease of the lung's blood vessels. One of the hallmarks of PAH is elevated vascular resistance due to enhanced vasoconstriction and remodeling of the normally low-pressure pulmonary vasculature leading to vascular obstruction, right ventricular hypertrophy, right ventricular failure, and eventually death. Although a strong genetic link to mutations in BMPR2 signaling has been identified in familial PAH, this accounts for only ∼20% of all cases. The remaining individuals, those with idiopathic PAH as well as other forms of pulmonary hypertension, develop PAH due to an incompletely understood, but complex, etiology.
While there have been advances in the understanding of certain elements of PAH pathobiology such as defining the importance of alterations in endothelin-1 (ET-1) and NO signaling (4), a deep understanding of the inducing and regulatory pathways involved in the disease process remains poorly understood. This is important as current therapies are confined to augmenting the NO-cGMP or prostacyclin cascades and/or blocking the ET-1 cascade. However, less than 1/3 of PAH patients respond to an acute vasodilator challenge with a substantial fall in PA pressure, indicating that factors other than increased tone contribute to the progression of PAH. In fact, other than Flolan, vasodilator therapies have not been proven to prolong survival. Thus, current treatment decisions are based on disease severity as opposed to disease pathobiology.
To advance the field, our understanding of the specific mechanisms of disease must be increased. Diverse redox-sensitive mechanisms are proposed to work together to orchestrate this process. These series of review articles summarize the current perspective on a number of these different compartmentalized mechanisms, including regulation of extracellular and intracellular membrane receptors and channels; responses to changes in biomechanical forces; intracellular thiol redox control; regulation of the nuclear transcription factor, peroxisome proliferator-activated receptor-γ (PPARγ); and regulation of mitochondrial metabolism. Collectively, they exemplify the complex compartmentalized signaling pathways regulated by redox signaling in the pulmonary circulation.
Redox-sensitive mechanisms transduce signals from the extracellular environment to change cellular signaling. Two examples of this are the regulation of cell membrane receptors and channels responsible for transmembrane ion flux, and the endothelial cell (EC) membrane response to mechanical forces. Redox stress contributes to pathologic vasoconstriction by altering the regulation and activity of membrane receptors, K+ channels, and intracellular Ca2+ homeostasis.
Weise-Cross et al. describe our latest understanding of the role of reactive oxygen species on K+ conductance and membrane potential, and both receptor-operated and store-operated Ca2+ entry (8). Emerging data on how specific redox changes that result in post-translational modifications modulate membrane receptor and ion channel activity in PAH are described and related to context-specific molecular mechanisms of channel regulation.
Zemskov et al. discuss how mechanical forces modulate signaling molecules within the vessel wall to modulate vascular health and disease (9). ECs that line the blood vessels are constantly under the influence of hemodynamic forces, including (i) shear stress, the tangential friction force acting on the vessel wall due to blood flow; (ii) hydrostatic pressure, the perpendicular force acting on the vascular wall; and (iii) cyclic strain, the circumferential stretch of the vessel wall. Mechanosensors on EC detect these forces and transduce them into biochemical signals that trigger vascular responses (2). These mechanical forces are intimately associated with the development of pulmonary vascular disease associated with congenital heart defects, and the progression of disease in these lesions reflects the differing hemodynamic insults to the pulmonary vasculature. Furthermore, regardless of the underlying etiology of advanced pulmonary vascular disease (i.e., flow, hypoxia, hypoplasia, toxin, infection, vasculitis, and thrombus), once vascular remodeling occurs, abnormal flow patterns disrupt the mechanical force patterns to which the lung is exposed. Thus, disruption of blood flow that exists in children born with complex congenital heart defects likely participate in the progression of adults with WHO Group I PAH.
Within the cell, protein thiols are critical regulators of protein function, and the thiol state of proteins is regulated by a coordinated series of thiol regulatory proteins. Ofman and Tipple discuss the emerging role the thiol redox system plays in the normal development of the lung and how loss of this protective system is involved in hyperoxic lung injury in neonates treated with high levels of oxygen in the intensive care unit (5). The redox regulation of proteins through S-nitrosylation and S-glutathionylation is also discussed in relation to the development of hyperoxic lung injury and PAH.
Insufficient PPARγ signaling contributes to PAH pathogenesis, and therapeutic restoration of PPARγ activity attenuates PAH in a number of preclinical models. Indeed, one of the mechanisms underlying the loss of mitochondrial function in PAH is due to the disruption of β-oxidation due to the loss of PPARγ signaling. However, it is clear that PPARγ exerts significantly greater effects on pulmonary vascular health than just its effect on cellular metabolism and Tseng et al. discuss the pleotropic effects of PPARγ on cellular antioxidant defense systems, inflammation, control of cell cycle and proliferation, and regulation of vascular tone through interactions with NO and endogenous vasoactive molecules (7). Furthermore, they assess the scope of PPARγ interconnected pathways that exert a broad impact on cell signaling through the ability to interact with an extensive regulatory network of transcription factors and micro-RNAs, leading to a broad impact on cell signaling.
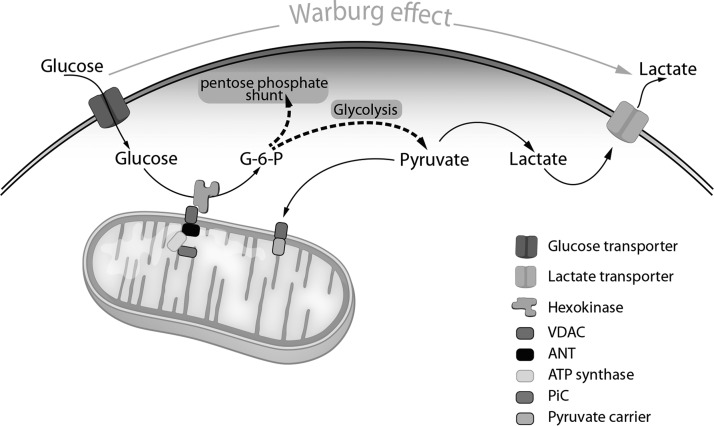
Finally, redox-regulated signaling is central to mitochondrial respiration and mitochondrial metabolism, and quality control is increasingly recognized as a key redox mechanism contributing to pulmonary hypertension (PH). A central feature of PAH is the hyperproliferative nature of pulmonary artery smooth muscle cells and pulmonary artery endothelial cells in end-stage disease. Suliman and Nozik-Grayck discuss the evidence that, like cancer, the development of pulmonary vascular disease relies on a metabolic reprogramming to favor glycolysis (6) (Fig. 1). Furthermore, Sulliman and Nozik-Grayck describe the causal link between this metabolic reprogramming and the development of the hyperproliferative endothelial phenotype that exists in PAH (6). This link is, in part, because proliferating cells have different metabolic needs than nonproliferating ones; in addition to generating adenosine triphosphate (ATP), proliferating cells must also produce biosynthetic precursors such as nucleic acids and lipids required for cell division. Transformed cells therefore frequently exhibit altered bioenergetic flux patterns. An example of this is aerobic glycolysis, in which glucose is converted into lactate even under normoxic conditions. In addition to generating ATP, glycolysis is embedded in a complex network of metabolic reactions involved in the biosynthesis of anabolic precursors required for cell growth and proliferation. For instance, glucose supplies the carbon backbone for the nonessential amino acids cysteine, glycine, serine, and alanine. Furthermore, by generating 5-phosphoribosyl-a-pyrophosphate through the pentose phosphate pathway (PPP), glycolysis actively supports the de novo synthesis of purines and pyrimidines required for DNA biosynthesis (Fig. 1). Thanks to branching of the oxidative arm of the PPP, glycolysis also contributes to the regeneration of NADPH, an important player in maintaining redox homeostasis and lipid biosynthesis. Importantly, aerobic glycolysis has been observed in highly proliferative but nontransformed cells as well, including activated lymphocytes, embryonic stem cells, and smooth muscle and ECs in the setting of PH. The emerging role of mitochondrial network remodeling, that is, the process of mitochondrial fusion and through fission, splitting into small, less connected mitochondria in the pathology of PAH, is also described.
FIG. 1.
Metabolic reprogramming in PAH favors glycolysis. Glucose brought across the plasma membrane by glucose transporters is phosphorylated by HK-2 bound to VDAC on the OMM. VDAC channels ATP generated by the ATP synthesizing machinery on the inner mitochondrial membrane, facilitating direct access of ATP to VDAC-bound HK-2. In this manner, HK-2 can convert glucose to G-6-P. To maintain this high rate of glycolytic metabolism and to stimulate proliferation, G-6-P rapidly distributes across two key metabolic routes: entry of G-6-P into the PPP for biosynthetic reactions; and conversion of G-6-P via the glycolytic pathway to lactic acid, which is transported out of the cell to regenerate NAD+ and maintain glycolysis. Some pyruvate is directed to the mitochondria to provide substrates for the TCA cycle for energy generation, as well as lipid and amino acid biosynthesis (not shown). ANT, adenine nucleotide translocator; ATP, adenosine triphosphate; HK-2, hexokinase-2; OMM, outer mitochondrial membrane; PAH, pulmonary arterial hypertension; PiC, inorganic phosphate carrier; PPP, pentose phosphate pathway; VDAC, voltage dependent anion channel.
Overall, these series of Reviews highlight the importance of redox-sensitive signaling in the pathogenesis of pulmonary vascular diseases, and the highly regulated and compartmentalized reactions involved in disease progression. This knowledge provides new opportunities to design rational therapeutic strategies to improve the health outcomes of these serious disease conditions.
Abbreviations Used
- ATP
adenosine triphosphate
- EC
endothelial cells
- ET-1
endothelin-1
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- PPARγ
peroxisome proliferator-activated receptor-γ
- PPP
pentose phosphate pathway
References
- 1. Archer SL. Pyruvate kinase and Warburg metabolism in pulmonary arterial hypertension: uncoupled glycolysis and the cancer-like phenotype of pulmonary arterial hypertension. Circulation 136: 2486–2490, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chatterjee S, Fujiwara K, Perez NG, Ushio-Fukai M, and Fisher AB. Mechanosignaling in the vasculature: emerging concepts in sensing, transduction and physiological responses. Am J Physiol Heart Circ Physiol 308: H1451–H1462, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hansen T, Galougahi KK, Celermajer D, Rasko N, Tang O, Bubb KJ, and Figtree G. Oxidative and nitrosative signalling in pulmonary arterial hypertension—implications for development of novel therapies. Pharmacol Ther 165: 50–62, 2016 [DOI] [PubMed] [Google Scholar]
- 4. Kim D. and George MP. Pulmonary hypertension. Med Clin North Am 103: 413–423, 2019 [DOI] [PubMed] [Google Scholar]
- 5. Ofman G. and Tipple TE. Thiol-redox regulation in lung development and vascular remodeling. Antioxid Redox Signal 2019. DOI: 10.1089/ars.2018.7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suliman HB. and Nozik-Grayck E. Mitochondrial dysfunction: metabolic drivers of pulmonary hypertension. Antioxid Redox Signal 2019. DOI: 10.1089/ars.2018.7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tseng V, Sutliff RL, and Hart CM. Redox biology of peroxisome proliferator-activated receptor-gamma in pulmonary hypertension. Antioxid Redox Signal 2019. DOI: 10.1089/ars.2018.7695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weise-Cross L, Resta TC, and Jernigan NL. Redox regulation of ion channels and receptors in pulmonary hypertension. Antioxid Redox Signal 2019. DOI: 10.1089/ars.2018.7699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zemskov EA, Lu Q, Ornatowski W, Klinger CN, Desai AA, Maltepe E, Yuan JX, Wang T, Fineman JR, and Black SM. Biomechanical forces and oxidative stress: implications for pulmonary vascular disease. Antioxid Redox Signal 2019. DOI: 10.1089/ars.2018.7720 [DOI] [PMC free article] [PubMed] [Google Scholar]