Abstract
Significance: Oxidative stress in the cell is characterized by excessive generation of reactive oxygen species (ROS). Superoxide (O2−) and hydrogen peroxide (H2O2) are the main ROS involved in the regulation of cellular metabolism. As our fundamental understanding of the underlying causes of lung disease has increased it has become evident that oxidative stress plays a critical role.
Recent Advances: A number of cells in the lung both produce, and respond to, ROS. These include vascular endothelial and smooth muscle cells, fibroblasts, and epithelial cells as well as the cells involved in the inflammatory response, including macrophages, neutrophils, eosinophils. The redox system is involved in multiple aspects of cell metabolism and cell homeostasis.
Critical Issues: Dysregulation of the cellular redox system has consequential effects on cell signaling pathways that are intimately involved in disease progression. The lung is exposed to biomechanical forces (fluid shear stress, cyclic stretch, and pressure) due to the passage of blood through the pulmonary vessels and the distension of the lungs during the breathing cycle. Cells within the lung respond to these forces by activating signal transduction pathways that alter their redox state with both physiologic and pathologic consequences.
Future Directions: Here, we will discuss the intimate relationship between biomechanical forces and redox signaling and its role in the development of pulmonary disease. An understanding of the molecular mechanisms induced by biomechanical forces in the pulmonary vasculature is necessary for the development of new therapeutic strategies.
Keywords: biomechanical forces, shear stress, cyclic stretch, mitochondria, redox regulation, pulmonary disease
Introduction
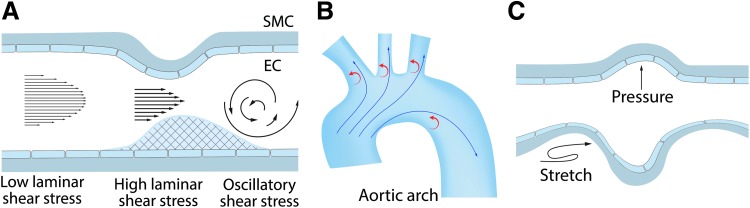
The entire pulmonary vasculature is exposed to biomechanical forces that can have profound physiological and pathological effects. In the vasculature, biomechanical forces are realized via two types of hemodynamic loads: tensile wall shear stress (WSS) caused by blood flow on the vessel and compressive circumferential stress caused by pressure loading. Flowing blood constantly exerts hemodynamic loads on the endothelium lining the blood vessels once the heart begins to produce a fetal circulation (75). As blood flow passes over the vessel luminal surface, it produces a frictional force known as shear stress (SS) or WSS, which acts tangentially to the vessel (75) (Fig. 1A).
FIG. 1.
Effect of biomechanical forces on blood vessels. Blood vessels are constantly exposed to the biomechanical forces associated with blood pressure and blood flow producing endothelial wall shear stress and circumferential wall stress, respectively. Physiological stresses and strains (stretch) exert vasoprotective roles via NO that generates antioxidant athero-protective signaling in the vessel wall (A). However, vessel geometry, such as that found in the aorta, can also create both athero-protective (high, laminar) and athero-prone (low, turbulent) areas of shear stress (B). Blood flow (shear stress) predominantly affects the endothelium, whereas changes in blood pressure cause mechanical distension (stretch) of the vessels affecting both the endothelium and the subjacent smooth muscle layer (C). EC, endothelial cell; NO, nitric oxide; SMC, smooth muscle cell. Color images are available online.
In vitro, many effects of physiological WSS can be reproduced by laminar shear stress (LSS), induced by steady laminar flow, and pulsatile shear stress, induced by periodic flow with a positive mean flow rate, stimulating a physiological response that maintains normal endothelial functions (Fig. 1A). LSS causes the alignment of the endothelial cells (ECs) in the direction of the flow (231). LSS globally affects EC homeostasis via multiple cell signaling cascades, the activation of specific transcription factors, and mechanosensitive gene expression. Blood vessels also contain athero-prone sites where wall geometry, afterload, and distal conditions combine to create areas of nonuniform flow such as turbulent or oscillatory flow as well as areas with modulated physiological SS (Fig. 1A, B). These increases or decreases in LSS (low and high SS) can have pathological consequences.
While SS acts tangentially to the vessel luminal surface (75) (Fig. 1A), the concomitant blood pressure exerts a load that acts perpendicularly to the cell surface, creating a compressive stress on the pulmonary vessel (75). As the blood pressure within the pulmonary system rises and falls depending on the cardiac cycle, this results in a circumferential stress and this is transmitted circumferentially to cells in the lung through contacts with the extracellular matrix (75) (Fig. 1C). The alveolar-capillary unit present in the lung is also exposed to mechanical forces as a result of the respiratory cycle (20), resulting in lung capillary strain (20).
Under certain conditions (such as high tidal volume lung mechanical ventilation or high blood pressure), excessive circumferential or compressive loading can induce pathological changes in the challenged cells. In vitro, an excessive circumferential loading can be reproduced by special devices designed to apply physiological (5% elongation) or excessive (15%–20% elongation) cyclic stretch (CS) to the cell monolayers. The following sections discuss our most up-to-date understanding of the effects of biomechanical forces on the lung and the role played by redox pathways in transducing these signals into both physiological and pathological cellular responses.
EC Surface Proteins as Mechanosensors
Integrins
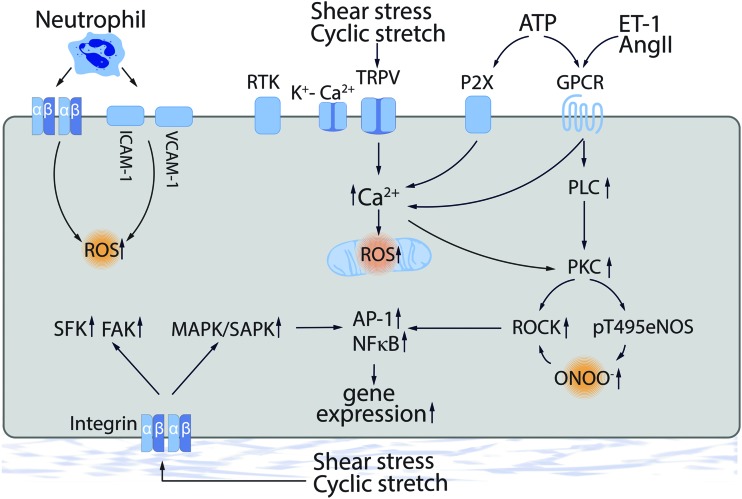
Integrins are heterodimeric transmembrane adhesion receptors responsible for cell focal adhesions (FAs) that function by linking cytoskeletal structures to the extracellular matrix (130). Integrins are also involved in cell signaling events via scaffolding specific signaling macromolecules (128). Integrins can also serve as mechanosensors, providing “outside-in” signaling in response to increased blood pressure, SS, or circumferential tensile stress (242) (Fig. 2). Low SS signaling via integrins has been linked to the activation of multiple proinflammatory pathways (60–62), whereas an excessive CS-dependent in vitro stimulation of β3-subunit expression has been shown to be protective for CS-challenged cells through cellular reorientation (257).
FIG. 2.
Mechanotransduction in the vessel wall. Direct mechanosensing occurs via multiple pathways including integrin complexes, caveolae-associated PECAM-1, VEGFR, and VE-cadherin, and ion channels such as TRPV4 and KCa. In indirect mechanosensing, shear stress-released agonists such as Ang II, ET-1, and ATP can stimulate specific receptors. Multiple of these downstream events can trigger ROS generation. Ang II, angiotensin II; ET-1, endothelin-1; GPCR, G-protein-coupled receptor; PKC, protein kinase C; ROCK, Rho kinase; ROS, reactive oxygen species; VCAM-1, vascular cell adhesion molecule 1. Color images are available online.
Immunofluorescence microscopy has identified a rapid reorganization of FA contacts and the activation of focal adhesion kinase, and the depletion of paxillin, an FA protein scaffold, delays the cell orientation changes indicating the importance of integrin-mediated signaling (127). Exposing smooth muscle cells (SMCs) to an excessive CS also induces both αv- and β3-integrin expression, Src activity, talin degradation, and binding and processing of prothrombin (173).
The integrin β4 has been shown to be involved in the anti-inflammatory response in EC (56) and in mouse models of acute lung injury (ALI) (57). Interestingly, the tyrosine phosphorylation in the C-terminal intracellular domain of integrin β4 is activated by CS-mediated mechanical stress, leading to the loss of its anti-inflammatory property in ECs (55). Mechanical forces appear to regulate integrin(s) via phosphorylation and this has been shown to be critical for proinflammatory cytokine expression (IL-6, IL-8, MCP-1, and RANTES) (55). Oscillatory SS- or high-pressure-dependent release of angiotensin II (Ang II), endothelin-1 (ET-1), vascular endothelial growth factor (VEGF), and other vasoactive factors can, in turn, activate integrin functions (204, 271).
Thus, integrins are implicated in downstream cell signaling events stimulated by other receptors including mechanosensors. Integrin signaling is also important in regulating reactive oxygen species (ROS) generation and oxidative stress. For example, superoxide release is induced in mouse neutrophils by α4-integrin-dependent adhesion on vascular cell adhesion molecule 1 (VCAM-1) (211), whereas tumor necrosis factor α (TNFα) has been shown to cause the redistribution of β2-integrins and NADPH oxidase (NOX) subunits (gp91phox, p22phox, p47phox, and p67phox) to a Triton X-100-insoluble fraction human neutrophils (299), suggesting an integrin-dependent activation of NOX. Ligation of β1-integrins has also been linked to p47phox membrane redistribution and hydrogen peroxide (H2O2) generation in human neutrophils (272). Thus, integrin-dependent signaling is intimately involved in the cellular response to biomechanical forces and the vascular damage induced by excessive ROS production.
Endothelial receptors and ion channels
The membrane microdomain, caveolae, is critically involved in mechanotransduction in EC. Caveolae serve as a platform that allows the assembly of cell signaling complexes, including receptors and ion channels, and components of cell–cell, and cell–matrix, contacts. Thus, caveolae can integrate “outside-in” signaling by functionally linking various mechanoreceptors with their downstream effectors. Caveolae microdomains are also important in assembling endothelial junctions and FAs into mechanosensitive signaling units. Therefore, perturbing the caveolae structure can produce an abnormal response to biomechanical forces applied to the endothelium.
Depleting the major structural protein of caveolae, caveolin-1 (cav-1) decreases the sensitivity to WSS in cav-1−/− mice that includes an attenuated increase in [Ca2+]i (45). Caveolae also support the ion channels involved in the EC hyperpolarization and Ca2+-dependent cell signaling that occurs in response to WSS. Studies in cav-1 knockout (KO) mice revealed that the impaired Ca2+-dependent signaling is linked to a decreased activity of the TRPV4 Ca2+ channel that normally colocalizes with cav-1 on the plasma membrane (226).
The TRPV4-dependent [Ca2+]i increase is essential for Ca2+-activated K+ channels (KCa), which induce endothelium-dependent hyperpolarization (EDH) and regulate vascular tone (109, 165). In EC, TRPV4 and KCa receptors are colocalized in caveolae (109). In human lung microvascular EC, under static conditions, TRPV4 colocalizes with small conductance KCa2.3 channel in caveolae, whereas SS stimulation also recruited intermediate conductance KCa3.1 channel to the complexes in caveolae (109), suggesting an importance of these channel complexes for vascular cell hyperpolarization (Fig. 2). Thus, mechanosensitive ion channels localized in caveolae are important players in the fine regulation of vascular tone and blood pressure.
The secretion of vasoactive factors (ET-1, Ang II, VEGF, PDGF, TNFα, etc.) is also regulated by biomechanical forces and these can be indirectly involved in mechanosensing via their respective endothelial or SMC receptors (Fig. 2). For example, mechanosensitive release of ATP (27, 278) can further stimulate P2X and P2Y purinoceptors, such as P2X4 (an ATP-dependent Ca2+ channel) (232, 297, 298) and P2Y1/P2Y2 (G-protein-coupled receptors [GPCRs]) (37, 38), followed by activation of respective cell signaling pathways (Fig. 2). All have been linked to ROS generation (Fig. 2).
Regulation of Vasoactive Molecules by Biomechanical Forces
Vasodilators
Nitric oxide (NO) is a vasorelaxant produced by NO synthase isoforms converting l-arginine to citrulline.
In blood vessels, NO is synthesized in ECs and diffuses to the adjacent SMCs, where it activates soluble guanylate cyclases (sGCs) (67). This leads to activation of cGMP-dependent PKG (cGMP-dependent protein kinase) and other effector proteins, including ion channels, ion pumps, and phosphodiesterases (PDEs) (43). In addition, NO in SMCs promotes the activation of cAMP-dependent protein kinase/protein kinase A (PKA), inhibiting SMC proliferation (136). NO is also involved in preventing platelet and leukocyte activation and adhesion to the vessel wall (147).
SS increases NO production via endothelial nitric oxide synthase (eNOS) phosphorylation and by stimulating EC receptors that increase intracellular Ca2+ (269). Exposing ECs to LSS can also suppress ROS levels (190, 289). In contrast, exposing EC to SS using an irregular flow pattern leads to higher levels of ROS and less available NO (166). NO generation attenuates insulin-like growth factor 1 (IGF-1) and the insulin-induced elevation in H2O2 levels via a cGMP-dependent event in SMC (313). As eNOS promoter activity and protein levels in ECs are suppressed by SMC-derived H2O2, this suggests that a feedback mechanism exists that may contribute to the NO signaling (287).
Derived from arachidonic acid via the action of cyclooxygenase-2 (COX-2) and prostaglandin I synthase (PGIS), prostacyclin (PGI2) is another vasodilator with a broad range of effects in the vasculature (Fig. 3).
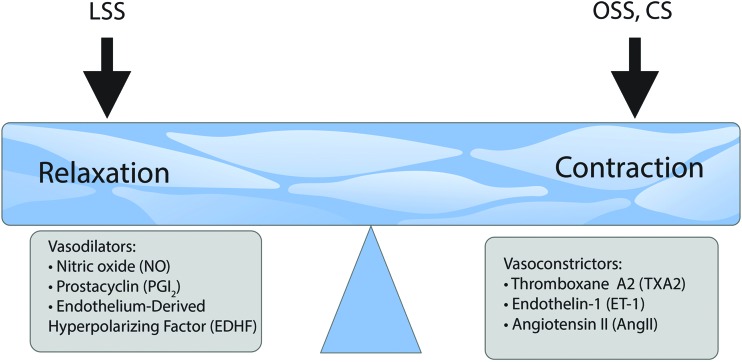
FIG. 3.
Biomechanical forces regulate vessel tone. Vascular tone is regulated by the opposing effects of vasodilators and vasoconstrictors that are predominantly produced by the vascular endothelium. These bioactive factors are heavily regulated by biomechanical forces such that LSS stimulates factors that enhance vasodilation, whereas OSS and excessive circumferential CS enhance vasoconstriction. CS, cyclic stretch; LSS, laminar shear stress; OSS, oscillatory shear stress. Color images are available online.
PGI2 binds to the PGI2 receptors (IP) (66) located on both platelets and SMCs (199), inhibiting platelet aggregation (63). Acting via Gs GPCR prostaglandin receptors, PGI2 induces cAMP synthesis and the well-described PKA-dependent pathway of the cytoskeletal reorganization and relaxation (263). The effects of PGI2 are tightly related to NO effects since PGI2 potentiates NO release and, in turn, NO potentiates the effect of PGI2 on SMCs (248).
PGI2 also exerts protective effects in the vasculature by inhibiting SMCs hypertrophy, migration, and proliferation (187). In patients with hypertension, production of vasoactive prostanoids is selectively impaired and this may contribute to the increased systemic vascular resistance and increased incidence of thrombosis (188). PGI2 exerts protective cardiovascular effects that counterbalance the harmful effects of thromboxane A2 (TxA2) (187). Disturbance of the balance between PGI2 and TxA2 has been associated with vascular disorders such as pulmonary hypertension (PH). ROS can activate COX-2 expression, enabling the production of both PGI2 and TxA2 (182). PGI2 has been shown to inhibit the activity of NOX, whereas peroxynitrite induces tyrosine nitration in PGIS, inactivating the enzyme (12, 314) and increasing the levels of TxA2 (2).
Endothelium-derived hyperpolarizing factor (EDHF) produced by the EC is a vasodilator of unknown nature that is shown to be important for vascular tone in smaller arteries, although a number of publications established its compensatory role for some pathological states, leading to an impairment of eNOS activity (300) (Fig. 3).
Vasorelaxation can also occur after endothelial stimulation through a non-NO nonprostanoid pathway originally ascribed to the actions of endothelium-derived hyperpolarizing factor (265) (Fig. 3). EDHF involves hyperpolarization, generated in the endothelium, which spreads via myoendothelial gap junctions to the SMCs, and it is this hyperpolarization that results in relaxation of SMCs (65, 85, 92, 228). Flow-induced vasodilation that is independent of endothelium-derived NO (EDNO) and PGI2 is typically due to EDH of the underlying SMCs (86).
EDHF initiates SMC hyperpolarization directly after its release from the endothelium (40, 84). The endothelial hyperpolarization is initiated by the activation of KCa channels (92). H2O2 is believed to be an EDHF that acts primarily on the prearterioles and arterioles where EDH-mediated relaxation becomes more important than EDNO (181, 243, 244). SS can induce the release of H2O2 from ECs that acts as an EDHF that contributes to flow-induced vasodilation in coronary arterioles (189). H2O2 can induce this hyperpolarization by several mechanisms, including cGMP or cAMP-meditated pathway, activation of PKA/PLA2, or the direct activation of various K+ channels (245).
Vasoconstrictors
The opposite effect on vascular tone and blood pressure occurs via vasoconstrictors (Fig. 3). Another arachidonic acid derivative, TxA2, secreted by platelets, acts via Gq GPCR thromboxane receptors (TP), inducing platelet aggregation and blood clot formation and reducing blood flow. TxA2 is a functional antagonist of PGI2 and their balance supports vascular homeostasis. TxA2 promotes platelet aggregation and expresses adhesive cofactors for platelets such as von Willebrand factor, fibronectin and thrombospondin, and procoagulant factors (262).
TxA2 exerts its biological activity through its cognate TP GPCR receptor (194). TxA2 receptor also promotes cell migration and proliferation of SMCs (133, 205, 301). TxA2 is a functional antagonist of PGI2 and their balance supports vascular homeostasis. ROS have been shown to induce the release of TxA2 in different tissues (1, 113, 114). ROS can enhance arteriolar tone by diminishing endothelium-derived NO responses, generate a COX-2-dependent endothelial-derived contracting factor (EDCF) that activates TP, and enhance vascular SMCs reactivity (182). In the vasculature, O2•− elicits constriction through activation of TP-dependent mechanisms (141, 266). Thus, ROS through the release of TxA2, a vasoconstrictor prostanoid, can also mediate vascular contraction.
ET-1 is a potent peptide vasoconstrictor produced by the EC. The product of the EDN1 gene, preproendothelin-1 (ppET-1), is proteolytically processed to an active 21-amino acid peptide ET-1 secreted from the EC into the circulation (72) (Fig. 3). ET-1 is a GPCR agonist inducing Ca2+ elevation in affected cells. In the vasculature, ET-1 has pleiotropic effects producing SMC constriction via ETA receptors and inducing relaxation via endothelial ETB receptors (72). ETA and ETB receptors promote the proliferation of pulmonary artery SMCs (PASMCs) (74). Increased ROS production caused by ET-1 promotes vasoconstriction and vascular remodeling via the suppression of NO activity (77). ET-1 messenger RNA (mRNA) and peptide expression are significantly upregulated in both PH models and patients (107, 274).
ET-1 receptor A and B antagonists have been used as pulmonary arterial hypertension (PAH) drugs with potent antiproliferative, anti-inflammatory, and endothelium-protective properties (48). Physiological levels of SS have a negative effect on the expression of ppET-1 and ET-1-converting enzyme (ECE-1) in the EC (178, 191). This downregulation of the ET-1 system depends on eNOS activation and oxidative stress (179, 191). ET-1 promotes a vascular and interstitial remodeling, stimulates the proliferation of SMCs, fibroblast activation, and proliferation (241) via increases in NOX-derived ROS (287). SS and NO are potent inhibitors of ET-1 gene expression (217, 222, 253, 255). Recently, it has been shown that mitochondria-targeted antioxidant, mitoTEMPO, can inhibit ET-1-induced constriction of rat mesenteric arteries (50), confirming a link between ET-1 and mitochondria-derived ROS that had been shown in EC (255).
Ang II is produced from angiotensin I in the lung by angiotensin-converting enzyme (ACE). Ang II is a potent vasoconstrictor acting via GPCR Ang II type 1 and type 2 receptors (AT1R and AT2R) (Fig. 3). LSS (10 dyn/cm2, 24 h) upregulates ACE expression in SMCs (111) and Ang II promotes SMC remodeling, cell growth, fibrosis, collagen deposition, and contractility (268, 313). AT1R is likely a redox-coupled mechanosensor that regulates oxidative stress as studies have demonstrated AT1R is closely associated with ROS production (25, 163, 282) via Nox-4-dependent oxidative stress pathways (312). LSS can also induce ROS levels by an AT1R-mediated downregulation of eNOS expression mediated via Akt1 and Erk activity (49). Ang II is also a proinflammatory mediator that stimulates the production of inflammatory cytokines and causes oxidative stress via AT1Rs to promote hypertension (18, 137, 308).
Regulation of ROS Generation by Biomechanical Forces
NADPH oxidase
The NOX family consists of seven isoforms (NOX-1–5 and DUOX-1 and DUOX-2) that act as transmembrane catalytic subunits and require additional proteins to assemble large functionally active complexes. NOX complexes produce ROS (superoxide anion and H2O2) using NADPH and molecular oxygen as substrates (152).
The regulation of NOX isoforms is diverse, including rather simple Ca2+-dependent activation of NOX-5 (267) and complex modulation of NOX-1/NOX-2 activities via association with various effector proteins such as Rac-1/2, NoxA1, p47phox, and p67phox that, in turn, can be regulated by a number of cell signaling pathways. In addition, NOX-4 is constitutively active and is mainly regulated by gene expression (152). NOX isoforms function in normal physiological processes and in the development/progression of vascular pathologies (261).
Owing to their complex regulation, NOX isoforms can be stimulated by biomechanical forces. In cell culture models, long-term LSS (30 dyn/cm2, 24 h) downregulates mRNA and protein expression of NOX-2 and p47phox in an eNOS-dependent manner (82). LSS also downregulates the expression of NOX-4 via antioxidant response element (ARE), Oct-1-binding site, and NF-E2-related factor 2 (Nrf2) (110), whereas oscillatory SS can stimulate expression/activity of NOX isoforms, p47phox-dependent superoxide generation, and monocyte adhesion (129).
More detailed studies of LSS and oscillatory SS have identified different roles of activated NOXs with LSS activating an NOX-2–p47phox complex that stimulates eNOS phosphorylation and NO production, and oscillatory SS leading to eNOS uncoupling via an NOX-1–NOXO1 complex (247). Disturbed flow (low and oscillatory SS) studied in vivo using a model of partial ligation of the mouse carotid artery identified a p47phox-dependent endothelial dysfunction, leucocyte recruitment, and infiltration (185), leading to the development of atherosclerosis (196. 197). In EC, NOX-4-derived superoxide has also been shown to interfere with PGI2 bioactivity (193).
Xanthine oxidase
Xanthine oxidoreductase is the enzyme that catalyzes the oxidation of hypoxanthine to xanthine and uric acid during purine metabolism (250, 286). The enzyme exists in two forms: xanthine dehydrogenase and xanthine oxidase (XO). XO is one of the major sources of ROS in the vasculature (183) producing superoxide and H2O2 and can be induced by TNFα (99). XO activity and superoxide generation are stimulated by oscillatory SS (183). A number of studies have identified a role for XO in the pathogenesis of ventilator-induced lung injury (VILI) via a p38-dependent mechanism (81), and p38/XO inhibition attenuates VILI pathogenesis (153, 258). Increased XO activity also impairs shear-dependent and endothelium-dependent vasodilation (80, 151).
Endothelial NO synthetase
A number of studies have established a regulatory role of post-translational modifications (PTMs) of eNOS. Multiple phosphorylation sites implicate several protein kinases in the modulation of eNOS activity.
Tyrosine phosphorylation of eNOS induced by H2O2 in EC increases the association of eNOS with caveolin-1 (104). Phosphorylation by Akt1 at Ser1177 increases NO synthesis (79), and LSS or pulsatile SS induces this PI3K/Akt1-dependent phosphorylation in a Ca2+-independent manner (94, 161).
Several reports describe the phosphorylation of the same site, Ser1177, by protein kinases A and G (PKA and PKG), AMP-dependent protein kinase (AMPK), and Ca2+-calmodulin-dependent protein kinase II (CaMKII); PKA also phosphorylates Ser633 and Ser615 [reviewed in Boo and Jo (31)]. LSS (15 dyn/cm2) induces PKA-dependent phosphorylation of eNOS at Ser633, which positively regulates its activity (30, 32).
eNOS-mediated NO signaling can also be inhibited by asymmetric dimethylarginine (ADMA), a product of cellular protein degradation (29). Increased levels of ADMA have been shown to be associated with PH (91, 229). ADMA levels have been shown to be stimulated by increased pulmonary blood flow (PBF) and pressure in vivo (254) and this leads to the uncoupling of eNOS and the peroxynitrite-mediated nitration and activation of Akt1 (216). This, in turn, induces the mitochondrial redistribution of eNOS that causes mitochondrial dysfunction and increases mitochondrial ROS generation and further increase in cellular oxidative stress (256). The ADMA degrading enzymes, dimethylaminohydrolases (DDAH), are now considered key regulators of eNOS-produced NO (93, 162).
In ALI models, the ADMA/DDAH balance is critical for the endothelial barrier disruption and disease progression, and DDAH II overexpression reduces lipopolysaccharide (LPS)-mediated increases in oxidative/nitrosative stress in vivo (3). DDAH II is inhibited via an Src-dependent phosphorylation (149, 238). As Src activity is stimulated by biomechanical forces (39, 76, 173), this could be a common mechanism for increasing cellular ADMA levels.
eNOS is also susceptible to a protein kinase C (PKC)-dependent phosphorylation at Thr495 (96, 186), this correlated with increases in NOS-derived superoxide and decreased NO levels (51). A similar Ang II-mediated increase in eNOS uncoupling was also recently identified in LPS-challenged EC that is mediated via a NOX-2-induced glutathionylation of eNOS (103, 292). Regulation of both eNOS gene expression and eNOS mRNA stability is also sensitive to various biomechanical stimuli, including LSS and oscillatory SS, LPS, and oxidative stress. The literature data regarding the regulation of eNOS gene expression have been extensively summarized by Searles (235).
Mitochondrial function, biogenesis, and network dynamics
Mitochondrial generation of ATP requires the activity of the electron transport complexes (ETCs) I–IV acting in concert with ATP synthase (Fig. 4A).
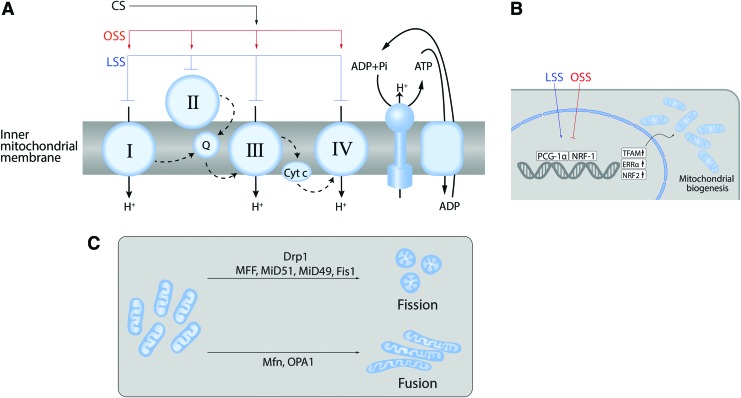
FIG. 4.
Effects of biomechanical forces on mitochondria. Biomechanical forces exert effects on the mitochondria at multiple levels. All of the ETCs can be regulated by biomechanical forces altering both mitochondrial function and ROS generation (A). Mitochondrial biogenesis (B) is regulated by PGC-1α via the transcription factors NRF1 and TFAM. Mitochondrial network dynamics (C) are regulated by the opposing effects of fission and fusion. Fission mediated by Drp1 guided by MFF, Fis1, MiD49, and MiD51. Fusion is mediated by mitofusins in the outer membrane and Opa1 in the inner membrane. Drp1, dynamin-related protein 1; ETCs, electron transport complexes; NRF, nuclear respiratory factor; PGC-1α, peroxisome proliferation and the activated receptor gamma coactivator-1α; TFAM, transcription factor A mitochondrial. Color images are available online.
Biomechanical forces have been shown to modulate ETC activity (Fig. 4A). For example, LSS-induced NO production mediates a sustained suppression of ETC I, II/III, and IV (116). Mitochondrial ROS generation is also regulated by SS due to the eNOS-derived NO and reactive nitrogen species (RNS)-mediated inhibition of mitochondrial electron transport (116). Increased PBF and pressure also attenuate mitochondrial function via the nitration-mediated inhibition of carnitine acetyl transferase (CrAT) and the reduction in CrAT, carnitine palmitoyltransferase type 1 (CPT1), and carnitine palmitoyltransferase type 2 (CPT2) expression (239, 256). The resulting disruption of β-oxidation leads to increased mitochondrial ROS generation.
The reduction in CrAT, CPT1, and CPT2 expression appears to be caused by a loss of peroxisome proliferator-activated receptor γ (PPARγ) signaling via increased WSS and/or increased pressure (240). PPARγ antagonists also induce mitochondrial ROS in the lung (237). Oscillatory shear stress also increases mitochondrial superoxide production via an NOX-c-Jun N-terminal kinase signaling pathway (260). At present the effect of CS on mitochondrial-mediated ROS in vascular cells is limited. One study in SMCs has shown that CS (15% elongation, 24 h) stimulates NOX-4 activity via a mechanism that requires CIII activity (288). How CS modulates mitochondrial-mediated ROS in EC is unresolved.
Mitochondrial biogenesis is a complex process involving the replication of mitochondrial DNA (mtDNA) that contains 37 genes encoding 13 subunits of electron transport chain complexes I, III, IV, and V (139). Again, biomechanical forces have been shown to regulate this process (Fig. 4B).
LSS has been shown to activate the AMPK pathway in EC (83, 200). As a result, AMPK stimulates DNMT1, RBBP7, and HAT1 signaling pathways (175) and stimulates mitochondrial biogenesis via peroxisome proliferation and the activated receptor gamma coactivator-1α (PGC-1α), which, in turn, activates nuclear respiratory factor (NRF)-1, NRF-2, transcription factor A mitochondrial, and transcription factor B mitochondrial (41, 277) (Fig. 4B). LSS can also stimulate mitochondrial biogenesis through Sirtuin 1, an NAD+-dependent deacetylase (59, 143). This laminar flow-enhanced mitochondrial biogenesis may also protect ECs against oxidative stress by stimulating PGC-1α-induced ROS-detoxifying enzymes (59). Thus, mitochondrial biogenesis is also involved in controlling the redox state of the endothelium.
A common misconception is that the mitochondria are present as static individual organelles, within the cell. In reality, mitochondria are dynamic: constantly forming elongated tubes, through the process of fusion and, through fission, splitting into small less connected mitochondria (44, 52, 131, 195) (Fig. 4C). This process has been termed “mitochondrial network remodeling.”
The correct balance of fission and fusion is critical for mitochondrial homeostasis. Mitochondrial fragmentation (fission) has been linked to increased apoptotic cell death (36, 158). However, the seminal work of Archer's group has shown that in PH, the increase in fission is associated with a hyperproliferative antiapoptotic SMC phenotype (10, 54, 223, 224).
Fusion permits the mixing of the contents between mitochondria and may be a pathway that protects the mitochondria (115) (Fig. 4C). Three mitochondrial guanosine triphosphatases (GTPases) regulate mitochondrial fusion: the mitofusins (Mfn)-1 and -2 and the optic atrophy 1 protein (OPA-1). Fusion is also an underappreciated regulator of cell proliferation as the initial term for Mfn-2 was “hyperplasia suppressor gene” due to its antiproliferative effect when overexpressed (46, 52).
Fission is mediated through the GTPase activity of dynamin-related protein 1 (Drp1) (Fig. 4C). Drp1 is present in the cytosol and translocates to the mitochondria when activated. On the mitochondrion, it assembles into oligomeric structures that mechanically constrict and fragment the mitochondria (170).
Drp1 is regulated by a complex array of PTMs, including S-nitrosylation, ubiquitination, sumoylation, O-GlcNAcylation, and phosphorylation (115, 203). The best studied PTM with respect to Drp1 is its phosphorylation that occurs at Ser616 and Ser637. Phosphorylation at Ser616 activates Drp1 to promote mitochondrial fission (135, 230). Cyclin-dependent kinase 1 (Cdk1) phosphorylates Drp1 at Ser616. Phosphorylation at Ser637 has been shown to occur through PKA, Cam kinase, and Pim1 (115). Phosphorylation at Ser637 inhibits Drp1 oligomerization, sequesters Drp1 in the cytosol, and can, therefore, suppress mitochondrial fission (135, 230). Ser637 can be dephosphorylated by calcineurin that enhances Drp1 mitochondrial translocation and so stimulates fission. Rho kinase (ROCK) has also been shown to phosphorylate Drp1 (34).
ROCK exists as two isoforms 1 and 2 and is known to be a major player in the pulmonary vascular disease (PVD) through its ability to reorganize the actin cytoskeleton. One of the major upstream activators of ROCK is RhoA (Ras homologous GTP-binding protein A) (275, 290). The canonical activation of RhoA GTPase involves the activation of GPCRs and/or tyrosine kinases, resulting in the activation of guanine nucleotide exchange factors (GEFs) that enhance the exchange of GDP for GTP and translocation of GTP-RhoA to the plasma membrane. Upon translocation to the plasma membrane, GTP-RhoA is able to activate ROCK.
A new mechanism of RhoA activation has been recently identified in which post-translational (PTM) nitration events can directly stimulate RhoA nucleotide exchange, independent of GEF activation (215). Thus, there could be a link between nitrosative stress and mitochondrial fission, although this has not been explored.
The effects of biomechanical forces on mitochondrial network remodeling are also still far from resolved as the limited published data are conflicting. For example, LSS has been shown to both increase mitochondrial fission and apoptosis (234) and increase mitochondrial fusion (293). As already described, the transient receptor potential cation channels are important players in mechanotransduction pathways (148). Increased calcium uptake is associated with LSS and is essential for the initiation of mitochondrial fission (35).
Nrf2 and Krüppel-like factor 2
Biomechanical forces can also regulate the removal of ROS. For example, LSS-dependent activation of Erk5 induces the activity of Nrf2 (144). Nrf2 acts via the ARE and stimulates expression of a number of antioxidant enzymes, including NAD(P)H:quinone oxidoreductase 1, glutathione reductase, glutathione peroxidase (GPx), and catalase (138). Nrf2-dependent upregulation of these enzymes has been shown to protect cardiac fibroblasts, macrophages, and cardiomyocytes against oxidative/nitrosative stress (42, 310, 311).
HO-1, the downstream target gene of Nrf2, is also capable of suppressing atherosclerotic lesion formation by reducing the oxLDL-induced transmigration of monocytes (132) and protecting against oxidative stress and inflammation, two of the predominant mechanisms in atherosclerosis.
The activation of transcription factor, Krüppel-like factor 2 (KLF2), is also stimulated by LSS (12 dyn/cm2, 16–24 h) (144). VCAM-1 mRNA levels are decreased in ECs exposed to LSS (209), whereas KLF2 inhibits the expression of vascular cell adhesion protein 1 (VCAM-1) as well as E-selectin (78, 281). This suggests a link between LSS-mediated increase in KLF-2 and a decrease in monocyte attachment to the endothelium. In human umbilical vein endothelial cell (HUVEC), KLF2 activity is also associated with SS-induced extracellular ATP release followed by P2X4 Ca2+-channel activation (232), suggesting a functional link between calcium-mediated signaling, antioxidant and antiatherogenic gene expression, and vasorelaxation.
KLF-2 also suppresses inflammatory gene expression via the inhibition of NFκB and AP-1 (33). KLF2 also improves the nuclear localization of Nrf2, and the combined actions of these two factors are thought to constitute the majority of the LSS-induced endothelial gene expression (95).
Mechanosensitive MicroRNAs and Cell Homeostasis
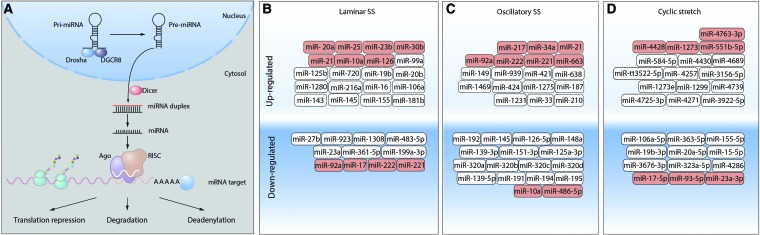
MicroRNAs (miRNAs) are single-stranded noncoding small RNAs that play an important role in the regulation of gene expression via binding targeted mRNA and suppressing their translation or inducing their degradation (157) (Fig. 5A).
FIG. 5.
MiRNA biogenesis and regulation by biomechanical forces. (A) miRNAs are transcribed by RNA polymerases II and III into pri-miRNAs that undergo a series of cleavage events to produce mature miRNA. The nuclear ribonuclease III Drosha binds to DGCR8 to form the microprocessor complex that cleaves the double-stranded pri-mRNA freeing a pre-miRNA (∼65 nt) that contains a characteristic stem-loop structure. The Pre-miRNA hairpin is then exported from the nucleus to the cytoplasm via the protein, Exportin-5. In the cytoplasm, the pre-miRNA hairpin is cleaved by the RNase III enzyme Dicer into an miRNA duplex (18–22 nt). Only one strand is usually incorporated into the RNA-induced silencing complex (RISC). When RISC binds to target mRNAs, a high degree of miRNA–mRNA degradation facilitates the Ago-catalyzed degradation of the target mRNA by cleavage. Mature miRNAs can modulate protein levels by enhancing mRNA degradation by inhibiting mRNA translation, or by enhancing mRNA deadenylation. (B-D) miRNAs have been identified that are sensitive to regulation by biomechanical forces: (B) laminar SS, (C) oscillatory SS, or (D) cyclic stretch. Those that can also affect cellular redox state are shown in red. miRNA, microRNA; mRNA, messenger RNA. Color images are available online.
In ECs, miRNA profiling has revealed 21 miRs that are differentially expressed (8 up- and 13 downregulated) after 24 h pulsatile SS (283) (Fig. 5B). Multiple miRNAs have been shown to be regulated by biomechanical forces (Fig. 5B). Fluid SS, like other physiological stimuli, can both induce and suppress gene expression, including expression of miRNA genes. Thus, miRNA expression patterns depend on specific transcription factors activated by different types of SS.
Pulsatile SS activates miR-10a expression via a retinoic acid receptor RARα/KLF2-dependent mechanism, and this miRNA downregulates VCAM-1 expression. In contrast, oscillatory SS induces histone deacetylase-dependent suppression of miR-10a expression (154). RARα/RXRα agonists rescue miR-10a expression in oscillatory SS regions in vivo. Moreover, induction of miR-10a by RARα/RXRα agonists protects ApoE−/− mice against atherosclerosis, inhibiting VCAM-1 expression and inflammatory cell infiltration (156). In addition, miR-23b, induced by pulsatile SS via KLF2-dependent transcription, possesses antiproliferative properties, repressing cyclin H and cell cycle progression. Oscillatory SS has no effect on miR-23b, however, and, therefore, does not induce cell cycle arrest (14). In HUVECs, induction of miR-19a by laminar flow leads to cyclin D1 downregulation and cell cycle arrest (214), whereas under the same conditions, miR-101 has an antiproliferative effect, suppressing mTOR expression (53).
Biomechanical forces affect the expression of several miRNAs that are either directly or indirectly involved in cellular redox balance [reviewed in Marin et al. (174)]. Among the mechanosensitive miRNAs, directly targeting ROS-regulating enzymes are miR-221/222 (252) and miR-92a (90, 294), which inhibit eNOS and miR-17* [inhibits superoxide dismutase (SOD) 2] (296) that are all downregulated by LSS. Conversely, miR-21 (252), miR-25 (100), and miR-23b (283) are upregulated by LSS and have been shown to inhibit NOX-4. miR-30b is also upregulated by LSS and inhibits catalase expression (117). Oscillatory SS upregulates miR-221/222 (252), miR-92a (90, 294), and miR-663, all of which inhibit eNOS expression. Oscillatory SS also upregulates miR-21 (304), which inhibits SOD3 expression.
Mechanosensitive miRNAs can also indirectly regulate oxidative stress by affecting ICAM-1/VCAM-1 expression and, therefore, adhesion and activation of neutrophils on the endothelium and subsequent ROS generation. LSS upregulates miR-10a (154) and miR-126 (118, 119), both of which inhibit VCAM-1 expression, whereas oscillatory SS downregulates miR-10a (154), which increases VCAM-1 expression. Several other miRs upregulated by oscillatory SS [miR-21 (309), miR-34a (89), and miR-663 (198)] have also been described as ICAM-1/VCAM-1-inhibitory miRs, suggesting a complex interplay in the regulation of these adhesion receptors by mechanical forces.
miR-486-5p, which is downregulated by oscillatory SS, may also be another indirect regulator of cellular redox balance as it can inhibit the expression of the phosphatase, PTEN, leading to increased Akt1 activity (125, 279). As Akt1 can phosphorylate eNOS, this could regulate NO levels.
Sirtuin-1 expression, a positive regulator of mitochondrial biogenesis, is inhibited by miR-92a (90, 164) and miR-217 (184), both of which are upregulated by oscillatory SS. LSS also upregulates miR-20a that inhibits VEGF expression (283).
Expression of PPARγ, a nuclear hormone receptor, is negatively regulated by miR-21 under oscillatory SS conditions (309). Detailed studies of PPARγ functions have identified its role in maintaining endothelial function and its loss has been shown to enhance the development of atherosclerosis, hypertension, and PH (6, 17, 126, 192, 237, 240). Experiments with pulmonary artery endothelial cells (PAECs) obtained from PH patients and mouse model of endothelial PPARγ loss-of-function showed that high levels of ET-1 correlated with the downregulation of PPARγ and miR-98 (140). Another miRNA family, miR-130/301, also negatively regulates the expression of PPARγ under conditions of excessive blood flow and pressure (15). miR-130/301-driven downregulation of PPARγ induces two downstream pathways that results in the decreased expression of miR-424/503 (in PAEC) and miR-204 (in PASMC); both pathways stimulate cell proliferation and are critical for PH promotion (16).
The exposure of ECs to CS (15% elongation, 24 h) also induces a dramatic change in the miRNA expression profile. Intriguingly, several miRs that inhibit NOX-4 expression are regulated with miR-4428, miR-1273 being downregulated and miR-17-5p, miR-93-5p, miR-23a-3p being upregulated. This divergent regulation is suggestive of a complex regulatory mechanism of NOX-4 expression via miRNAs. CS also downregulates miR-4763-3p that is a negative regulator of eNOS expression and miR-551b-5p that inhibits ICAM-1 expression (307). Taken together, these data demonstrate complex multileveled regulation of pathological pathways in vasculature by miRNAs that are responsive to biomechanical forces to either enhance vasoprotective effects or support excessive ROS generation.
Vascular Diseases/Pathologies Resulting from Altered Biomechanical Forces
Pulmonary hypertension
PH is biomechanically characterized as an increase in the resistive and reactive components of pulmonary vascular impedance (201, 285). In severe forms of PH, a progressive increase in the pulmonary vascular resistance leads to right heart pressure overload and right heart failure (102). Thus, changes in biomechanical forces are likely important in PH development. Increased levels of oxidative stress markers have been detected in PH patients (303) underpinned by multiple molecular, genetic, and epigenetic abnormalities, which cause endothelial dysfunction, pathological vascular remodeling, and mitochondrial metabolic abnormalities (4). WSS-dependent endothelial alterations within the complex pathobiology of PH play a very important role in blood clotting, inflammation, vascular tone, metabolism, angiogenesis, and repair. WSS is required for the development and maintenance of severe occlusive vascular lesions after Sugen-induced pulmonary vascular injury (280).
As was shown in healthy volunteers, a relationship between vascular WSS and flow-dependent vascular dilation can be directly accessed by phase contrast magnetic resonance imaging (MRI) (246), and in vivo data collected using MRI demonstrated site-specific WSS magnitudes in arterial system (206). Furthermore, using MRI, an occurrence of disturbed blood flow in pulmonary artery was directly demonstrated in PH patients. Vortex blood flow patterns and early systolic retrograde flow in main pulmonary artery were detected in all PH patients studied and were absent in healthy individuals; PA flow velocities and WSS were lower than those in control group (13, 202). Vortical blood flow duration in main pulmonary artery correlates with PH progression (220).
Disturbed blood flow is considered to be a critical trigger of PH development, since it stimulates numerous signaling pathways leading to oxidative stress, endothelial dysfunction, and expression of atherogenic factors. Experimental models of PH demonstrate dysregulation of oxidative signaling, with elevated ROS/RNS, reduced SOD, GPx, and catalase (4).
In the chronic hypoxia model of PH, pulmonary vascular remodeling is primarily mediated by NOX-2- and NOX-4-dependent ROS production (14, 134, 159). In PASMC, transforming growth factor (TGF)-β1 treatment stimulates increased NOX-4 levels, resulting in increased ROS that drives cellular proliferation, suggesting that NOX-4 mediates TGF-β1-dependent pulmonary vascular remodeling (4, 180, 251). NOX-4 also mediates the effects and hypoxia-induced factor-1α (HIF-1α) (28, 97, 227), which is critical to the pathogenesis of PAH.
Mitochondrial metabolic abnormalities are emerging as key players in the pathobiology of PAH (224, 239). Activation of HIF-1α causes the switch to a glycolytic phenotype, thereby suppressing oxidative phosphorylation, with multiple downstream consequences including mitochondrial depolarization (7). The underlying mechanism has been studied in animal models and is usually considered to be multifactorial through changes in eNOS production and uncoupling (256, 306), alteration in l-arginine metabolism (26), and increased NO consumption (295).
Evidence of mitochondrial fragmentation has also been identified due to a decrease in the expression of MFN2, and MFN2 overexpression attenuates the severity of PH (225). Several miRNAs are dysregulated in PH patients (19), including miR-204, which in healthy pulmonary artery SMCs (PASMCs) inhibits the STAT3/HIF-1α pathway (68).
Recent studies demonstrated that endothelial-to-mesenchymal transition (EndMT) could contribute to PH development and complexity. In PH models, various insults (such as hemodynamic stress and hypoxia) applied to the endothelium induce a loss of cell–cell and cell–matrix contacts, decrease of endothelial marker expression (VE-cadherin, PECAM-1, and von Willebrand factor), and increase of SMC- and fibroblast-specific proteins (α-SM-actin, fibronectin, SM-myosin, and calponin) (8, 98). TGF-β1-activated signaling was shown to contribute to EndMT (9, 98). Hypoxia-induced EndMT occurs via HIF-1α-mediated transcription followed by Twist1 activation (302), which, in turn, may lead to upregulation of TGF-β receptor 2 and Smad2 phosphorylation (172) linking hypoxia and TGF-β signaling.
Recently, HIF-2α-mediated transcription network was also demonstrated as critical for EndMT and PH development: siRNA-directed depletion of HIF-2α downregulated expression of Snai1/2 and EndMT in lung EC from idiopathic PAH patients (264). Also, HIF-2α-mediated upregulation of endothelial arginase II may contribute to an impairment of NO signaling in hypoxia-challenged EC and development of PH, since arginase II and eNOS utilize the same substrate, l-arginine (69, 146).
The diverse and complex mechanisms underlying the pathogenesis of PH offer the potential for new therapies. Specific therapies that have been developed for PH patients include the endothelin receptor antagonists, phosphodiesterase 5 (PDE5) inhibitors, prostanoids, sGC stimulators, and calcium channel blockers. New therapeutic targets have arisen since the emergence of the recent data that mitochondrial abnormalities and the presence of a hypoxic state are key to PH pathogenesis
Targeting various pathways (e.g., STAT3, mTORC, Akt1, PI3K, FoxO, and NFκB) in addition to dysregulated metabolic and mitochondrial signaling networks may help to reverse disease. Drugs aimed at blocking apoptosis might prevent the development of vascular remodeling in PAH, whereas promoting apoptosis in end-stage PAH might improve it (259). The treatment of PH could also benefit from advancements in precision medicine, by applying treatments that already exist in other areas. Combining two or more therapeutic approaches may be a strategy for the treatment of PH (101).
Congenital heart disease
In the United States, congenital heart disease (CHD) occurs in at least 8 of every 1000 live births and accounts for >24% of birth defect-related infant deaths (108). All congenital heart defects, in which a large intra- or extracardiac communication allows unrestricted pressure and volume overload of the pulmonary circulation, can lead to the development of PH (105, 221). The resulting shunt increases the pressure in the pulmonary arteries, leading to increased SS, circumferential wall stretching, and endothelial dysfunction. However, the classification of the PVD associated with CHD belies the complexity and varying physiology of predisposing cardiac lesions—from the classic example of unrestrictive ventricular septal defect to complex single ventricle lesions (Table 1).
Table 1.
Risk of Pulmonary Vascular Disease in Differing Lesions Associated with Congenital Heart Disease and Increased Pulmonary Blood Flow
| Defect | Risk of PVD (%) | Age of occurence (years) |
|---|---|---|
| CHD with increased pulmonary blood flow and/or pressure | ||
| Truncus arteriosus | ∼100 | <2 |
| A-V septal defect | ∼100 | ∼2 |
| Transportation of great arteries+VSD | ∼70 to 100 | 1–2 |
| Patent ductus arteriosus | ∼15 to 20 | >2 |
| Ventricular septal defect | ∼15 to 20 | >2 |
| ASD | ∼20 | >20 |
The natural history of PVD associated with systemic-to-pulmonary shunt reveals the differential, or perhaps incremental, effects of increased PBF and increased pulmonary arterial pressure. In patients with increased blood flow alone—pretricuspid valve lesions such as atrial septal defects—the development of PVD is uncommon and presents late, among 5%–15% of patients by the fourth decade of life (249). In stark contrast, in patients with increased blood flow and a direct pressure stimulus from the systemic ventricle—post-tricuspid lesions—the development of PVD is common, and develops early in life. Thus, the progression of PVD in these lesions reflects the severity of the hemodynamic insults to the pulmonary vasculature with lesions that exert only increased shear forces, from increases in blood flow alone, having a slower progression than those that have both flow and direct pressure stimuli (1, 120, 150).
Altered expression of vasoactive mediators, such as ET-1, PGI2, and NO, in CHD results in vasoconstriction, whereas aberrant expression of VEGF and fibroblast growth factor promotes vascular remodeling (1). These changes contribute to a progressive increase in pressures in the right ventricle (1). Compared with patients with other PH etiologies, the increases in pulmonary pressure seen in patients with PH–CHD occur early (during infancy rather than during adulthood), and this seems to provide PH–CHD patients with a prognostic advantage. More than 50% of patients with large unrestrictive ventricular septal defect will develop PH and cyanosis due to a reversal of left-to-right shunting, known as Eisenmenger syndrome (142).
Treatment of PH–CHD has evolved in recent years with options for either late repair in some patients (surgical) or PH disease-targeting therapy (87, 121). The use of PH-specific therapies in CHD significantly lowers the rate of cumulative mortality when compared with no therapy. Clinical studies evaluating oxidative stress and antioxidant status in children with CHD have revealed significant elevation of the oxidative stress biomarkers, malondialdehyde (MDA) and protein carbonyl, in patient plasma samples, as well as proinflammatory cytokines, such as IL-6 and TNFα (212). Superoxide and H2O2 levels have also been shown to increase (24). A comparison of total oxidant system (TOS) and total antioxidant system (TAS) in plasma collected from cyanotic acyanotic CHD patients and age-matched control individuals revealed a significant TOS increase and TAS decrease in cyanotic CHD patients (88).
Acute lung injury
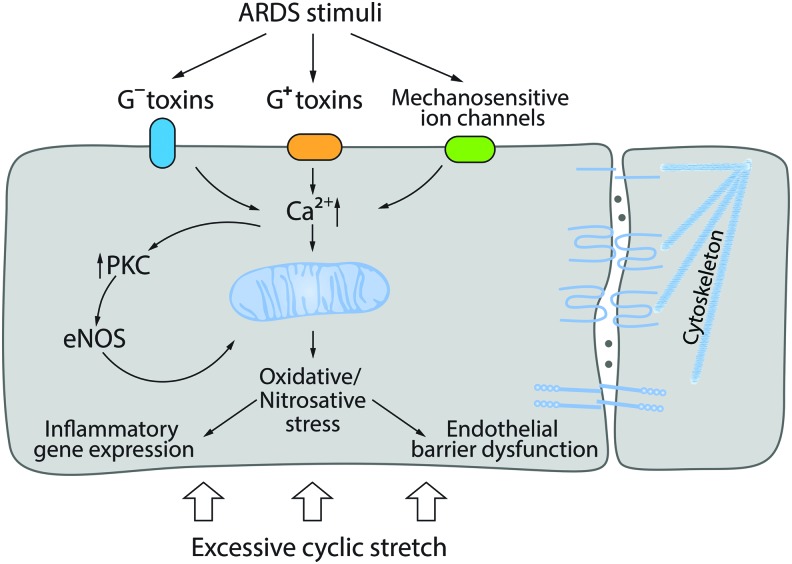
ALI and its more severe form, acute respiratory distress syndrome (ARDS), are pathological states of lung dysfunction of various etiology such as Gram-positive or Gram-negative respiratory infection, sepsis, trauma, acid aspiration, or toxic gas inhalation (219, 270). Although ALI/ARDS is not necessarily a pathology induced by biomechanical forces, it can be associated with VILI in patients. Therefore, studies of so-called two-hit models (bacterial toxin challenge or any other ALI-related stimuli plus in vitro CS or in vivo lung mechanical ventilation) are considered to be more clinically relevant (21–23) (Fig. 6). Published data demonstrate that the toxin pretreatment dramatically potentiates effects of excessive CS (18% elongation) inducing cell signaling pathways that lead to the barrier-disruptive cytoskeleton remodeling, cell–cell contact loss, expression of proinflammatory cytokines, and adhesive molecules (21–23, 291).
FIG. 6.
Effect of biomechanical forces on endothelial barrier function. In “two-hit” models, ALI/ARDS stimuli potentiate pathological effect of excessive CS/mechanical ventilation. Signaling pathways induced by bacterial toxins activate Ca2+-dependent PKC, eNOS uncoupling, and ROS generation; their effects are aggravated by excessive CS via mechanoreceptor signaling. ALI, acute lung injury; ARDS, acute respiratory distress syndrome; eNOS, endothelial NO synthetase. Color images are available online.
The most studied models of ALI are cultured pulmonary cell monolayers or animals challenged with either Gram-negative (LPS) or Gram-positive (pneumolysin, PLY; listeriolysin, LLO) bacterial toxins. In these models, the pivotal role of oxidative/nitrosative stress in the endothelial dysfunction is well documented. On molecular level, ALI is characterized by an excessive ROS generation and mitochondrial dysfunction. The major sources of ROS generation are NOX isoforms, which are activated by LPS in neutrophils (NOX-1) or EC (NOX-2) (171, 233). In ECs and SMCs, LPS induces oxidative stress and AP-1-/NFκB-mediated proinflammatory responses via NOX-4 activation via TLR4 (207, 208, 210). Uncoupled eNOS is another critical source of ROS in ALI/ARDS models (112, 238) that leads to peroxynitrite generation followed by protein tyrosine nitration and that plays an important role in ALI/ARDS pathogenesis (e.g., nitrated RhoA activation) (112, 149, 215).
Gram-positive bacterial toxins are pore-forming proteins that may rapidly induce oxidative stress via perturbance of [Ca2+]i homeostasis and mitochondrial dysfunction. PLY increases mito-ROS and decreases mitochondrial O2 consumption (160). In vitro, PLY or LLO shows dose-dependent negative effects on the endothelial barrier and, due to robust [Ca2+]i elevation, the stimulation of PKC and CaMKII activities (71). The activation of PKCα has been shown to trigger pulmonary endothelial dysfunction (168). PKC-dependent phosphorylation of eNOS at T495 leads to eNOS uncoupling (51, 255).
Actin stress fiber formation and contraction in PLY-challenged EC are the result of RhoA activation, Rac1 inhibition, myosin light chain (MLC), and filamin A phosphorylation causing the barrier disruption (70). Oxidative stress-mediated activation of protein tyrosine kinases followed by VE-cadherin tyrosine phosphorylation can also impair adherens junctions (167). In animal models, Gram-positive toxins induce endothelial dysfunction causing vascular leakage and pulmonary edema (71, 168).
Ventilator-induced lung injury
Mechanical ventilation of lungs is one of few clinical approaches effective for ARDS patients. However, excessive mechanical stress induced by ventilation may also cause lung tissue damage. This mechanical force, which is difficult to control in individual patients, may result in abnormal cyclic strain of the lung tissue (11). Such prolonged abnormal strain affects the lung vasculature inducing endothelial dysfunction and an inflammatory response [reviewed in Wang et al. (284)].
Experimental data obtained in EC subjected to excessive CS in specially designed devices or in animals subjected to mechanical ventilation demonstrate dramatic changes in cell signaling and cell metabolism affecting virtually all levels of EC and SMC homeostasis, including cytoskeletal structures and cell–cell contacts (adherens and tight junctions), protein modifications, gene expression, and cytokine/chemokine secretion.
Numerous studies have implicated ROS-modulating enzymes (NOX, NOS, and XO) as well as mitochondrial-derived ROS in VILI pathology (176, 177, 218, 276). Uncoupling of eNOS due to a functional BH4 shortage also exists in VILI (276). Exposing EC to various levels of CS has highlighted the critical role of the RhoA/ROCK signaling pathway in the development of endothelial dysfunction (21). Activation of ROCK appears to be due to a specific Rho-GEF, GEF-H1 (21, 47). GEF-H1 activation has been linked to microtubule disassembly (145) that occurs under excessive mechanical force (106). Importantly, GEF-H1 inhibition results in a decrease of proinflammatory factor levels in excessive CS-challenged EC. Therefore, a link between RhoA/ROCK pathway and NFκB-dependent proinflammatory response has been demonstrated (145).
Protective Approaches
Since the generation of excessive ROS/RNS and the resulting oxidative/nitrosative stress in the lung play a central role in the development and progression of a number of lung pathologies, antioxidant therapies have been tested as a general approach to protect the lung against the effects produced by abnormal biomechanical forces. However, such a general antioxidant approach has demonstrated either very modest or no therapeutic effect in humans due to inability to distinguish between harmful effects of excessive ROS and physiological ROS-mediated processes (213, 273). These failures have led to new ideas regarding antioxidant therapies that are designed to specifically target individual ROS/RNS-generating enzyme(s) or specific intracellular sites of ROS generation (e.g., dysfunctional mitochondria), which have been defined as critical for a particular disease.
Studies have focused on the specific inhibition of distinct NOX isoforms. Small-molecule inhibitors of NOX were tested in atherosclerosis mouse model (streptozotocin-treated ApoE−/− mice) and NOX-1/NOX-4 inhibitors (such as GKT137831) application showed decreased lesions and macrophage infiltration [reviewed in Altenhöfer et al. (5)]. NOX-inhibitory peptides have had success in inhibiting p22phox function, p47phox phosphorylation, and translocation and superoxide generation [reviewed in Cifuentes-Pagano et al. (64)].
Another protective approach being tested is the use of modulators of the downstream effectors regulated by excessive biomechanical forces and oxidative stress. For example, a number of publications have shown that ROCK inhibitors can be efficient in animal models of PH (58, 305). A more precise approach by preventing RhoA activation by Y34 tyrosine nitration using a specific RhoA-shielding peptide significantly protects the pulmonary vasculature against LPS-mediated damage in vivo (215). This type of precise targeting of ROS/RNS-dependent activation or inhibition of key regulators based on a fundamental understanding of a disease pathology could lead to more targeted and effective antioxidant therapies.
Another interesting therapeutic direction is the development of disintegrins, peptides originated from snake venoms that specifically interact and inhibit particular integrins [reviewed in Daavid et al. (73)]. This approach is aimed at preventing thrombocyte aggregation and leukocyte adhesion and activation and, therefore, has the potential to exert antioxidative and anti-inflammatory effects in the lung.
Recent preclinical and/or clinical studies in other complex pathologies (such as cancer) have demonstrated an increased therapeutic efficiency using drug combinations. Such combined approaches may be successful in the therapy of pulmonary diseases. In this regard, l-carnitine supplementation was successfully used in our laboratory to prevent eNOS uncoupling and nitration of mitochondrial proteins to improve eNOS function and protect/restore mitochondrial bioenergetics in a lamb model of CHD (236, 239, 256). Such supplementation can be tested in combination with other drugs in models of cardiovascular pathologies, wherein uncoupled eNOS-dependent mitochondrial dysfunction is observed.
Concluding Remarks
The exposure of the pulmonary vasculature to biomechanical forces affects the lung in a number of important ways, allowing cells in the vasculature to respond to a changing external environment via alterations in the production/secretion of vasoactive factors, gene expression changes, ROS generation, and mitochondrial bioenergetics/biogenesis/network dynamics. Lung injury and disease can alter the normal patterns of these forces, resulting in pathological signaling events that are intimately involved in disease progression. However, despite substantial investigations, there are still many unresolved issues surrounding how vascular cells respond to mechanical stress. This limitation is based on both the different types of forces to which the lung is exposed and the complexity of the lung itself.
Indeed, most of our data come from single cell types exposed to a single mechanical force. Thus, more sophisticated experimental systems that will allow the analysis of multiple cell types exposed to both SS and pressure/stretch will be necessary to more accurately determine how the pulmonary vasculature responds to a changing mechanical environment and how this is subverted in pathological conditions to drive the disease progression.
Acknowledgment
This research was supported, in part, by HL60190 (S.M.B.), HL137282 (S.M.B.), HL134610 (S.M.B.), HL142212 (S.M.B. and E.A.Z.), HL136603 (A.A.D.), HD072455 (E.M.), HL115014 (J.X.-J.Y.), and HL061284 (J.R.F.), all from the National Institutes of Health.
Abbreviations Used
- ACE
angiotensin-converting enzyme
- ADMA
asymmetric dimethylarginine
- ALI
acute lung injury
- AMPK
AMP-dependent protein kinase
- Ang II
angiotensin II
- ARDS
acute respiratory distress syndrome
- ARE
antioxidant response element
- AT1R
Ang II type 1 receptor
- CaMKII
Ca2+-calmodulin-dependent protein kinase II
- cav-1
caveolin-1
- CHD
congenital heart disease
- COX-2
cyclooxygenase-2
- CPT1
carnitine palmitoyltransferase type 1
- CPT2
carnitine palmitoyltransferase type 2
- CrAT
carnitine acetyl transferase
- CS
cyclic stretch
- DDAH
dimethylaminohydrolase
- Drp1
dynamin-related protein 1
- ECs
endothelial cells
- EDH
endothelium-dependent hyperpolarization
- EDHF
endothelium-derived hyperpolarizing factor
- EDNO
endothelium-derived NO
- EndMT
endothelial-to-mesenchymal transition
- eNOS
endothelial nitric oxide synthetase
- ET-1
endothelin-1
- ETC
electron transport complex
- FA
focal adhesion
- GEF
guanine nucleotide exchange factor
- GPCR
G-protein-coupled receptor
- GPx
glutathione peroxidase
- GTPase
guanosine triphosphatase
- H2O2
hydrogen peroxide
- HIF-1α
hypoxia-induced factor-1α
- HUVEC
human umbilical vein endothelial cell
- KLF2
Krüppel-like factor 2
- LLO
listeriolysin
- LPS
lipopolysaccharide
- LSS
laminar shear stress
- Mfn
mitofusin
- miRNA
microRNA
- MRI
magnetic resonance imaging
- mRNA
messenger RNA
- NO
nitric oxide
- NOX
NADPH oxidase
- NRF
nuclear respiratory factor
- Nrf2
NF-E2-related factor 2
- PAEC
pulmonary artery endothelial cell
- PAH
pulmonary arterial hypertension
- PASMC
pulmonary artery smooth muscle cell
- PBF
pulmonary blood flow
- PDE
phosphodiesterase
- PGC-1α
peroxisome proliferation and the activated receptor gamma coactivator-1α
- PGI2
prostacyclin
- PGIS
prostaglandin I synthase
- PH
pulmonary hypertension
- PKA
cAMP-dependent protein kinase/protein kinase A
- PKC
protein kinase C
- PKG
cGMP-dependent protein kinase/protein kinase G
- PLY
pneumolysin
- PPARγ
peroxisome proliferator-activated receptor γ
- ppET-1
preproendothelin-1
- PTM
post-translational modification
- PVD
pulmonary vascular disease
- RhoA
Ras homologous GTP-binding protein A
- RNS
reactive nitrogen species
- ROCK
Rho kinase
- ROS
reactive oxygen species
- sGC
soluble guanylate cyclase
- SMCs
smooth muscle cells
- SOD
superoxide dismutase
- SS
shear stress
- TAS
total antioxidant system
- TGF-β1
transforming growth factor β1
- TNFα
tumor necrosis factor α
- TOS
total oxidant system
- TP
thromboxane receptors
- TxA2
thromboxane A2
- VCAM-1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- VILI
ventilator-induced lung injury
- WSS
wall shear stress
- XO
xanthine oxidase
References
- 1. Adatia I, Kothari SS, and Feinstein JA. Pulmonary hypertension associated with congenital heart disease: pulmonary vascular disease: the global perspective. Chest 137: 52S–61S, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Agbani EO, Coats P, Mills A, and Wadsworth RM. Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: involvement of ERK and PKC. Pulm Pharmacol Ther 24: 100–109, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Aggarwal S, Gross CM, Kumar S, Dimitropoulou C, Sharma S, Gorshkov BA, Sridhar S, Lu Q, Bogatcheva NV, Jezierska-Drutel AJ, Lucas R, Verin AD, Catravas JD, and Black SM. Dimethylarginine dimethylaminohydrolase II overexpression attenuates LPS-mediated lung leak in acute lung injury. Am J Respir Cell Mol Biol 50: 614–625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aggarwal S, Gross CM, Sharma S, Fineman JR, and Black SM. Reactive oxygen species in pulmonary vascular remodeling. Compr Physiol 3: 1011–1034, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altenhöfer S, Radermacher KA, Kleikers PW, Wingler K, and Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal 23: 406–427, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, and Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 92: 1162–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Archer SL, Weir EK, and Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 121: 2045–2066, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arciniegas E, Frid MG, Douglas IS, and Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 293: L1–L8, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Arciniegas E, Sutton AB, Allen TD, and Schor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci 103: 521–529, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Atkins K, Dasgupta A, Chen K-H, Mewburn J, and Archer SL. The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease. Clin Sci 130: 1861–1874, 2016 [DOI] [PubMed] [Google Scholar]
- 11. ATS. International consensus conferences in intensive care medicine: ventilator-associated Lung Injury in ARDS. This official conference report was cosponsored by the American Thoracic Society, The European Society of Intensive Care Medicine, and The Societe de Reanimation de Langue Francaise, and was approved by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 160: 2118–2124, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Barbieri SS, Amadio P, Gianellini S, Zacchi E, Weksler BB, and Tremoli E. Tobacco smoke regulates the expression and activity of microsomal prostaglandin E synthase-1: role of prostacyclin and NADPH-oxidase. FASEB J 25: 3731–3740, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Barker AJ, Roldán-Alzate A, Entezari P, Shah SJ, Chesler NC, Wieben O, Markl M, and François CJ. Four-dimensional flow assessment of pulmonary artery flow and wall shear stress in adult pulmonary arterial hypertension: results from two institutions. Magn Reson Med 73: 1904–1913, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, and Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertero T, Cottrill K, Krauszman A, Lu Y, Annis S, Hale A, Bhat B, Waxman AB, Chau BN, Kuebler WM, and Chan SY. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem 290: 2069–2085, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, Saggar R, Wallace WD, Ross DJ, Vargas SO, Graham BB, Kumar R, Black SM, Fratz S, Fineman JR, West JD, Haley KJ, Waxman AB, Chau BN, Cottrill KA, and Chan SY. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest 124: 3514–3528, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beyer AM. de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, and Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res 103: 654–661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biancardi VC, Bomfim GF, Reis WL, Al-Gassimi S, and Nunes KP. The interplay between angiotensin II, TLR4 and hypertension. Pharmacol Res 120: 88–96, 2017 [DOI] [PubMed] [Google Scholar]
- 19. Bienertova-Vasku J, Novak J, and Vasku A. MicroRNAs in pulmonary arterial hypertension: pathogenesis, diagnosis and treatment. J Am Soc Hypertens 9: 221–234, 2015 [DOI] [PubMed] [Google Scholar]
- 20. Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal 11: 1651–1667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Birukova AA, Fu P, Xing J, Cokic I, and Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res 155: 44–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birukova AA, Moldobaeva N, Xing J, and Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Birukova AA, Tian Y, Meliton A, Leff A, Wu T, and Birukov KG. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. Am J Physiol Lung Cell Mol Physiol 1: L965–L975, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Black SM, Field-Ridley A, Sharma S, Kumar S, Keller RL, Kameny R, Maltepe E, Datar SA, and Fineman JR. Altered carnitine homeostasis in children with increased pulmonary blood flow due to ventricular septal defects. Pediatr Crit Care Med 18: 931–934, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blakely PK, Huber AK, and Irani DN. Type-1 angiotensin receptor signaling in central nervous system myeloid cells is pathogenic during fatal alphavirus encephalitis in mice. J Neuroinflamm 13: 196, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Block ER, Herrera H, and Couch M. Hypoxia inhibits L-arginine uptake by pulmonary artery endothelial cells. Am J Physiol 269: L574–L580, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Bodin P. and Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol 2001: 900–908, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Boehme J, Sun X, Tormos KV, Gong W, Kellner M, Datar SA, Kameny RJ, Yuan JX, Raff GW, Fineman JR, Black SM, and Maltepe E. Pulmonary artery smooth muscle cell hyperproliferation and metabolic shift triggered by pulmonary overcirculation. Am J Physiol Heart Circ Physiol 311: H944–H957, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Böger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 134: 2842S–2847S; discussion 2853S, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, and Jo H. Shear stress stimulates phosphorylation of eNOS at Ser635 by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 283: H1819–H1828, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Boo YC. and Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol 285: C499–C508, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Boo YC, Sorescu G, Bauer PM, Fulton D, Kemp BE, Harrison DG, Sessa WC, and Jo H. Phosphorylation of eNOS at Ser635 stimulates NO production in a Ca2+-independent manner. Free Radic Biol Med 35: 729–741, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Boon RA. and Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie 29: 39–40, 41–43, 2009 [PubMed] [Google Scholar]
- 34. Brand CS, Tan VP, Brown JH, and Miyamoto S. RhoA regulates Drp1 mediated mitochondrial fission through ROCK to protect cardiomyocytes. Cell Signal 50: 48–57, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bretón-Romero R, Acín-Perez R, Rodríguez-Pascual F, Martínez-Molledo M, Brandes RP, Rial E, Enríquez JA, and Lamas S. Laminar shear stress regulates mitochondrial dynamics, bioenergetics responses and PRX3 activation in endothelial cells. Biochim Biophys Acta 1843: 2403–2413, 2014 [DOI] [PubMed] [Google Scholar]
- 36. Brooks C, Cho SG, Wang CY, Yang T, and Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol 300: C447–C455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buvinic S, Briones R, and Huidobro-Toro JP. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol 136: 847–856, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buvinic S, Poblete MI, Donoso MV, Delpiano AM, Briones R, Miranda R, and Huidobro-Toro JP. P2Y1 and P2Y2 receptor distribution varies along the human placental vascular tree: role of nucleotides in vascular tone regulation. J Physiol 573: 427–443, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cai H, McNally JS, Weber M, and Harrison DG. Oscillatory shear stress upregulation of endothelial nitric oxide synthase requires intracellular hydrogen peroxide and CaMKII. J Mol Cell Cardiol 37: 121–125, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Campbell WB, Gebremedhin D, Pratt PF, and Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Cantó C. and Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cao Z, Zhu H, Zhang L, Zhao X, Zweier JL, and Li Y. Antioxidants and phase 2 enzymes in cardiomyocytes: chemical inducibility and chemoprotection against oxidant and simulated ischemia-reperfusion injury. Exp Biol Med (Maywood) 231: 1353–1364, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Carvajal JA, Germain AM, Huidobro-Toro JP, and Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol 184: 409–420, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Cerveny KL, Tamura Y, Zhang Z, Jensen RE, and Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol 17: 563–569, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Chai Q, Wang XL, Zeldin DC, and Lee HC. Role of caveolae in shear stress-mediated endothelium-dependent dilation in coronary arteries. Cardiovasc Res 100: 151–159, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22: 79–99, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Chang YC, Nalbant P, Birkenfeld J, Chang ZF, and Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell 19: 2147–2153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Channick RN, Sitbon O, Barst RJ, Manes A, and Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol 43: 62S–67S, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Chao Y, Ye P, Zhu L, Kong X, Qu X, Zhang J, Luo J, Yang H, and Chen S. Low shear stress induces endothelial reactive oxygen species via the AT1R/eNOS/NO pathway. J Cell Physiol 233: 1384–1395, 2018 [DOI] [PubMed] [Google Scholar]
- 50. Chen C, Gao JL, Liu MY, Li SL, Xuan XC, Zhang XZ, Zhang XY, Wei YY, Zhen CL, Jin J, Shen X, and Dong DL. Mitochondrial fission inhibitors suppress endothelin-1-induced artery constriction. Cell Physiol Biochem 42: 1802–1811, 2017 [DOI] [PubMed] [Google Scholar]
- 51. Chen F, Kumar S, Yu Y, Aggarwal S, Gross C, Wang Y, Chakraborty T, Verin AD, Catravas JD, Lucas R, Black SM, and Fulton DJ. PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G+-toxins. PLoS One 9: e99823, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen H. and Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14(Spec No. 2): R283–R289, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Chen K, Fan W, Wang X, Ke X, Wu G, and Hu C. MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells. Biochem Biophys Res Commun 427: 138–142, 2012 [DOI] [PubMed] [Google Scholar]
- 54. Chen KH, Dasgupta A, Lin J, Potus F, Bonnet S, Iremonger J, Fu J, Mewburn J, Wu D, Dunham-Snary K, Theilmann AL, Jing ZC, Hindmarch C, Ormiston ML, Lawrie A, and Archer SL. Epigenetic dysregulation of the dynamin-related protein binding partners MiD49 and MiD51 increases mitotic mitochondrial fission and promotes pulmonary arterial hypertension: mechanistic and therapeutic implications. Circulation 138: 287–304, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen W, Epshtein Y, Ni X, Dull RO, Cress AE, Garcia JGN, and Jacobson JR. Role of Integrin β4 in lung endothelial cell inflammatory responses to mechanical stress. Sci Rep 5: 16529, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen W, Garcia JG, and Jacobson JR. Integrin beta4 attenuates SHP-2 and MAPK signaling and reduces human lung endothelial inflammatory responses. J Cell Biochem 1: 718–724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen W, Sammani S, Mitra S, Ma SF, Garcia JG, and Jacobson JR. Critical role for integrin-β4 in the attenuation of murine acute lung injury by simvastatin. Am J Physiol Lung Cell Mol Physiol 303: L279–L285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen XY, Dun JN, Miao QF, and Zhang YJ. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses 5-hydroxytryptamine-induced pulmonary artery smooth muscle cell proliferation via JNK and ERK1/2 pathway. Pharmacology 83: 67–79, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Chen Z, Peng IC, Cui X, Li YS, Chien S, and Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A 107: 10268–10273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheng M, Liu X, Li Y, Tang R, Zhang W, Wu J, Li L, Liu X, Gang Y, and Chen H. IL-8 gene induction by low shear stress: pharmacological evaluation of the role of signaling molecules. Biorheology 44: 349–360, 2007 [PubMed] [Google Scholar]
- 61. Cheng M, Wu J, Li Y, Nie Y, and Chen H. Activation of MAPK participates in low shear stress-induced IL-8 gene expression in endothelial cells. Clin Biomech Bristol Avon 23(Suppl. 1): S96–S103, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Cheng M, Wu J, Liu X, Li Y, Nie Y, Li L, and Chen H. Low shear stress-induced interleukin-8 mRNA expression in endothelial cells is mechanotransduced by integrins and the cytoskeleton. Endothelium 14: 265–273, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Chow KB, Jones RL, and Wise H. Protein kinase A-dependent coupling of mouse prostacyclin receptors to Gi is cell-type dependent. Eur J Pharmacol 474: 7–13, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Cifuentes-Pagano E, Csanyi G, and Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci 69: 2315–2325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coleman HA, Tare M, and Parkington HC. Myoendothelial electrical coupling in arteries and arterioles and its implications for endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol 29: 630–637, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Coleman RA, Smith WL, and Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46: 205–229, 1994 [PubMed] [Google Scholar]
- 67. Cornwell TL, Arnold E, Boerth NJ, and Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol 267: C1405–C1413, 1994 [DOI] [PubMed] [Google Scholar]
- 68. Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Côté J, Simard MJ, and Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 208: 535–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cowburn AS, Crosby A, Macias D, Branco C, Colaço RD, Southwood M, Toshner M, Crotty Alexander LE, Morrell NW, Chilvers ER, and Johnson RS. HIF2alpha-arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci U S A 113: 8801–8806, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Czikora I, Alli AA, Sridhar S, Matthay MA, Pillich H, Hudel M, Berisha B, Gorshkov B, Romero MJ, Gonzales J, Wu G, Huo Y, Su Y, Verin AD, Fulton D, Chakraborty T, Eaton DC, and Lucas R. Epithelial sodium channel-α mediates the protective effect of the TNF-derived TIP peptide in pneumolysin-induced endothelial barrier dysfunction. Front Immunol 8: 842, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Czikora I, Sridhar S, Gorshkov B, Alieva IB, Kasa A, Gonzales J, Potapenko O, Umapathy NS, Pillich H, Rick FG, Block NL, Verin AD, Chakraborty T, Matthay MA, Schally AV, and Lucas R. Protective effect of growth hormone-releasing hormone agonist in bacterial toxin-induced pulmonary barrier dysfunction. Front Physiol 5: 259, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Davenport AP. and Maguire JJ. Endothelin. Handb Exp Pharmacol (176 Pt 1): 295–329, 2006 [DOI] [PubMed] [Google Scholar]
- 73. David V, Succar B, de Moraes JA, Saldanha-Gama RFG, Barja-Fidalgo C, and Zingali RB. Recombinant and chimeric disintegrins in preclinical research. Toxins (Basel) 10: pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Davie N, Haleen SJ, Upton PD, Polak JM, Yacoub MH, Morrell NW, and Wharton J. ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am J Respir Crit Care Med 165: 398–405, 2002 [DOI] [PubMed] [Google Scholar]
- 75. Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Davis ME, Cai H, Drummond GR, and Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res 89: 1073–1080, 2001 [DOI] [PubMed] [Google Scholar]
- 77. De Mey JG. and Vanhoutte PM. End o' the line revisited: moving on from nitric oxide to CGRP. Life Sci 118: 120–128, 2014 [DOI] [PubMed] [Google Scholar]
- 78. Deng Y, Lei T, Li H, Mo X, Wang Z, and Ou H. ERK5/KLF2 activation is involved in the reducing effects of puerarin on monocyte adhesion to endothelial cells and atherosclerotic lesion in apolipoprotein E-deficient mice. Biochim Biophys Acta 1864: 2590–2599, 2018 [DOI] [PubMed] [Google Scholar]
- 79. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, and Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 80. Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, and Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation 105: 2619–2624, 2002 [DOI] [PubMed] [Google Scholar]
- 81. Dolinay T, Wu W, Kaminski N, Ifedigbo E, Kaynar AM, Szilasi M, Watkins SC, Ryter SW, Hoetzel A, and Choi AM. Mitogen-activated protein kinases regulate susceptibility to ventilator-induced lung injury. PLoS One 3: e1601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Duerrschmidt N, Stielow C, Muller G, Pagano PJ, and Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J Physiol 576: 557–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, and Sharma K. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123: 4888–4899, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Edwards G, Dora KA, Gardener MJ, Garland CJ, and Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998 [DOI] [PubMed] [Google Scholar]
- 85. Edwards G, Félétou M, and Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch 459: 863–879, 2010 [DOI] [PubMed] [Google Scholar]
- 86. Ellinsworth DC, Earley S, Murphy TV, and Sandow SL. Endothelial control of vasodilation: integration of myoendothelial microdomain signalling and modulation by epoxyeicosatrienoic acids. Pflugers Arch 466: 389–405, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Engelfriet PM, Duffels MG, Möller T, Boersma E, Tijssen JG, Thaulow E, Gatzoulis MA, and Mulder BJ. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart 93: 682–687, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ercan S, Cakmak A, Kösecik M, and Erel O. The oxidative state of children with cyanotic and acyanotic congenital heart disease. Anadolu Kardiyol Derg 9: 486–490, 2009 [PubMed] [Google Scholar]
- 89. Fan W, Fang R, Wu X, Liu J, Feng M, Dai G, Chen G, and Wu G. Shear-sensitive microRNA-34a modulates flow-dependent regulation of endothelial inflammation. J Cell Sci 128: 70–80, 2015 [DOI] [PubMed] [Google Scholar]
- 90. Fang Y. and Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol 32: 979–987, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fang ZF, Huang YY, Tang L, Hu XQ, Shen XQ, Tang JJ, and Zhou SH. Asymmetric dimethyl-l-arginine is a biomarker for disease stage and follow-up of pulmonary hypertension associated with congenital heart disease. Pediatr Cardiol 36: 1062–1069, 2015 [DOI] [PubMed] [Google Scholar]
- 92. Félétou M. and Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 117: 139–155, 2009 [DOI] [PubMed] [Google Scholar]
- 93. Feng M, Liu L, Guo Z, and Xiong Y. Gene transfer of dimethylarginine dimethylaminohydrolase-2 improves the impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells induced by lysophosphatidylcholine. Eur J Pharmacol 584: 49–56, 2008 [DOI] [PubMed] [Google Scholar]
- 94. Fisslthaler B, Dimmeler S, Hermann C, Busse R, and Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol 168: 81–88, 2000 [DOI] [PubMed] [Google Scholar]
- 95. Fledderus JO, Boon RA, Volger OL, Hurttila H, Ylä-Herttuala S, Pannekoek H, Levonen AL, and Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol 28: 1339–1346, 2008 [DOI] [PubMed] [Google Scholar]
- 96. Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, and Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001 [DOI] [PubMed] [Google Scholar]
- 97. Frazziano G, Al Ghouleh I, Baust J, Shiva S, Champion HC, and Pagano PJ. Nox-derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4. Am J Physiol Heart Circ Physiol 306: H197–H205, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Frid MG, Kale VA, and Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res 90: 1189–1196, 2002 [DOI] [PubMed] [Google Scholar]
- 99. Friedl HP, Till GO, Ryan US, and Ward PA. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J 3: 2512–2518, 1989 [DOI] [PubMed] [Google Scholar]
- 100. Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, and Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am J Nephrol 32: 581–589, 2010 [DOI] [PubMed] [Google Scholar]
- 101. Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, and Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 62: D60–D72, 2013 [DOI] [PubMed] [Google Scholar]
- 102. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, and Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed) 69: 177, 2016 [DOI] [PubMed] [Google Scholar]
- 103. Galougahi KK, Liu CC, Gentile C, Kok C, Nunez A, Garcia A, Fry NA, Davies MJ, Hawkins CL, Rasmussen HH, and Figtree GA. Glutathionylation mediates angiotensin II-induced eNOS uncoupling, amplifying NADPH oxidase-dependent endothelial dysfunction. J Am Heart Assoc 3: e000731, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. García-Cardeña G, Fan R, Stern DF, Liu J, and Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem 271: 27237–27240, 1996 [DOI] [PubMed] [Google Scholar]
- 105. Gatzoulis MA, Alonso-Gonzalez R, and Beghetti M. Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur Respir Rev 18: 154–161, 2009 [DOI] [PubMed] [Google Scholar]
- 106. Gawlak G, Tian Y, O'Donnell JJ, 3rd, Tian X, Birukova AA, and Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. FASEB J 28: 3249–3260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, and Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328: 1732–1739, 1993 [DOI] [PubMed] [Google Scholar]
- 108. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, and Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127: e6–e245, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Goedicke-Fritz S, Kaistha A, Kacik M, Markert S, Hofmeister A, Busch C, Bänfer S, Jacob R, Grgic I, and Hoyer J. Evidence for functional and dynamic microcompartmentation of Cav-1/TRPV4/K(Ca) in caveolae of endothelial cells. Eur J Cell Biol 94: 391–400, 2015 [DOI] [PubMed] [Google Scholar]
- 110. Goettsch C, Goettsch W, Brux M, Haschke C, Brunssen C, Muller G, Bornstein SR, Duerrschmidt N, Wagner AH, and Morawietz H. Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res Cardiol 106: 551–561, 2011 [DOI] [PubMed] [Google Scholar]
- 111. Gosgnach W, Challah M, Coulet F, Michel JB, and Battle T. Shear stress induces angiotensin converting enzyme expression in cultured smooth muscle cells: possible involvement of bFGF. Cardiovasc Res 45: 486–492, 2000 [DOI] [PubMed] [Google Scholar]
- 112. Gross CM, Rafikov R, Kumar S, Aggarwal S, Ham PB, 3rd, Meadows ML, Cherian-Shaw M, Kangath A, Sridhar S, Lucas R, and Black SM. Endothelial nitric oxide synthase deficient mice are protected from lipopolysaccharide induced acute lung injury. PLoS One 10: e0119918, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gupte SA, Okada T, and Ochi R. Superoxide and nitroglycerin stimulate release of PGF2 alpha and TxA2 in isolated rat heart. Am J Physiol 271: H2447–H2453, 1996 [DOI] [PubMed] [Google Scholar]
- 114. Gupte SA, Okada T, Tateyama M, and Ochi R. Activation of TxA2/PGH2 receptors and protein kinase C contribute to coronary dysfunction in superoxide treated rat hearts. J Mol Cell Cardiol 32: 937–946, 2000 [DOI] [PubMed] [Google Scholar]
- 115. Hall AR, Burke N, Dongworth RK, and Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol 171: 1890–1906, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Han Z, Chen YR, Jones CI, 3rd, Meenakshisundaram G, Zweier JL, and Alevriadou BR. Shear-induced reactive nitrogen species inhibit mitochondrial respiratory complex activities in cultured vascular endothelial cells. Am J Physiol Cell Physiol 292: C1103–C1112, 2007 [DOI] [PubMed] [Google Scholar]
- 117. Haque R, Chun E, Howell JC, Sengupta T, Chen D, and Kim H. MicroRNA-30b-mediated regulation of catalase expression in human ARPE-19 cells. PLoS One 7: e42542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Harris TA, Yamakuchi M, Ferlito M, Mendell JT, and Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A 105: 1516–1521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Harris TA, Yamakuchi M, Kondo M, Oettgen P, and Lowenstein CJ. Ets-1 and Ets-2 regulate the expression of microRNA-126 in endothelial cells. Arterioscler Thromb Vasc Biol 30: 1990–1997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Haworth SG. Pulmonary vascular disease in different types of congenital heart disease. Implications for interpretation of lung biopsy findings in early childhood. Br Heart J 52: 557–571, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hjortshøj CMS, Kempny A, Jensen AS, Sørensen K, Nagy E, Dellborg M, Johansson B, Rudiene V, Hong G, Opotowsky AR, Budts W, Mulder BJ, Tomkiewicz-Pajak L, D'Alto M, Prokšelj K, Diller GP, Dimopoulos K, Estensen ME, Holmstrøm H, Turanlahti M, Thilén U, Gatzoulis MA, and Søndergaard L. Past and current cause-specific mortality in Eisenmenger syndrome. Eur Heart J 38: 2060–2067, 2017 [DOI] [PubMed] [Google Scholar]
- 122. Hoffman JI. and Rudolph AM. The natural history of ventricular septal defects in infancy. Am J Cardiol 16: 634–653, 1965 [DOI] [PubMed] [Google Scholar]
- 123. Hoffman JI. and Rudolph AM. The natural history of isolated ventricular septal defect with special reference to selection of patients for surgery. Adv Pediatr 17: 57–79, 1970 [PubMed] [Google Scholar]
- 124. Hoffman JI, Rudolph AM, and Danilowicz D. Left to right atrial shunts in infants. Am J Cardiol 30: 868–875, 1972 [DOI] [PubMed] [Google Scholar]
- 125. Holliday CJ, Ankeny RF, Jo H, and Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol 301: H856–H867, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hu C, Lu KT, Mukohda M, Davis DR, Faraci FM, and Sigmund CD. Interference with PPARγ in endothelium accelerates angiotensin II-induced endothelial dysfunction. Physiol Genomics 48: 124–134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Huang W, Sakamoto N, Miyazawa R, and Sato M. Role of paxillin in the early phase of orientation of the vascular endothelial cells exposed to cyclic stretching. Biochem Biophys Res Commun 418: 708–713, 2012 [DOI] [PubMed] [Google Scholar]
- 128. Humphries MJ, Travis MA, Clark K, and Mould AP. Mechanisms of integration of cells and extracellular matrices by integrins. Biochem Soc Trans 32: 822–825, 2004 [DOI] [PubMed] [Google Scholar]
- 129. Hwang SA, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, and Jo H. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003 [DOI] [PubMed] [Google Scholar]
- 130. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002 [DOI] [PubMed] [Google Scholar]
- 131. Ikeda Y, Shirakabe A, Brady C, Zablocki D, Ohishi M, and Sadoshima J. Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system. J Mol Cell Cardiol 78: 116–122, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ishikawa K, Navab M, Leitinger N, Fogelman AM, and Lusis AJ. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest 100: 1209–1216, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ishimitsu T, Uehara Y, Ishii M, Ikeda T, Matsuoka H, and Sugimoto T. Thromboxane and vascular smooth muscle cell growth in genetically hypertensive rats. Hypertension 12: 46–51, 1988 [DOI] [PubMed] [Google Scholar]
- 134. Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, and Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jahani-Asl A. and Slack RS. The phosphorylation state of Drp1 determines cell fate. EMBO Rep 8: 912–913, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jeremy JY, Rowe D, Emsley AM, and Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res 43: 580–594, 1999 [DOI] [PubMed] [Google Scholar]
- 137. Ji Y, Wang Z, Li Z, Zhang A, Jin Y, Chen H, and Le X. Angiotensin II enhances proliferation and inflammation through AT1/PKC/NF-kappaB signaling pathway in hepatocellular carcinoma cells. Cell Physiol Biochem 39: 13–32, 2016 [DOI] [PubMed] [Google Scholar]
- 138. Jones CI 3rd, Zhu H, Martin SF, Han Z, Li Y, and Alevriadou BR. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann Biomed Eng 35: 683–693, 2007 [DOI] [PubMed] [Google Scholar]
- 139. Jornayvaz FR. and Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem 47: 69–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kang BY, Park KK, Kleinhenz JM, Murphy TC, Green DE, Bijli KM, Yeligar SM, Carthan KA, Searles CD, Sutliff RL, and Hart CM. Peroxisome proliferator-activated receptor γ and microRNA 98 in hypoxia-induced endothelin-1 signaling. Am J Respir Cell Mol Biol 54: 136–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Katusić ZS, Schugel J, Cosentino F, and Vanhoutte PM. Endothelium-dependent contractions to oxygen-derived free radicals in the canine basilar artery. Am J Physiol 264: H859–H864, 1993 [DOI] [PubMed] [Google Scholar]
- 142. Kidd L, Driscoll DJ, Gersony WM, Hayes CJ, Keane JF, O'Fallon WM, Pieroni DR, Wolfe RR, and Weidman WH. Second natural history study of congenital heart defects. Results of treatment of patients with ventricular septal defects. Circulation 87: I38–I51, 1993 [PubMed] [Google Scholar]
- 143. Kim JS, Kim B, Lee H, Thakkar S, Babbitt DM, Eguchi S, Brown MD, and Park JY. Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. Am J Physiol Heart Circ Physiol 309: H425–H433, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kim M, Kim S, Lim JH, Lee C, Choi HC, and Woo CH. Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J Biol Chem 287: 40722–40731, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kratzer E, Tian Y, Sarich N, Wu T, Meliton A, Leff A, and Birukova AA. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol 47: 688–697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Krotova K, Patel JM, Block ER, and Zharikov S. Hypoxic upregulation of arginase II in human lung endothelial cells. Am J Physiol Cell Physiol 299: C1541–C1548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kubes P, Suzuki M, and Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 88: 4651–4655, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kuipers AJ, Middelbeek J, and van Leeuwen FN. Mechanoregulation of cytoskeletal dynamics by TRP channels. Eur J Cell Biol 91: 834–846, 2012 [DOI] [PubMed] [Google Scholar]
- 149. Kumar S, Sun X, Noonepalle SK, Lu Q, Zemskov E, Wang T, Aggarwal S, Gross C, Sharma S, Desai AA, Hou Y, Dasarathy S, Qu N, Reddy V, Lee SG, Cherian-Shaw M, Yuan JX, Catravas JD, Rafikov R, Garcia JGN, and Black SM. Hyper-activation of pp60Src limits nitric oxide signaling by increasing asymmetric dimethylarginine levels during acute lung injury. Free Radic Biol Med 102: 217–228, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lakier JB, Stanger P, Heymann MA, HoffmaN JI, and Rudolph AM. Early onset of pulmonary vascular obstruction in patients with aortopulmonary transposition and intact ventricular septum. Circulation 51: 875–880, 1975 [DOI] [PubMed] [Google Scholar]
- 151. Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, and Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106: 3073–3078, 2002 [DOI] [PubMed] [Google Scholar]
- 152. Lassègue B. San Martín A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Le A, Damico R, Damarla M, Boueiz A, Pae HH, Skirball J, Hasan E, Peng X, Chesley A, Crow MT, Reddy SP, Tuder RM, and Hassoun PM. Alveolar cell apoptosis is dependent on p38 MAP kinase-mediated activation of xanthine oxidoreductase in ventilator-induced lung injury. J Appl Physiol (1985) 105: 1282–1290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lee DY, Lin TE, Lee CI, Zhou J, Huang YH, Lee PL, Shih YT, Chien S, and Chiu JJ. MicroRNA-10a is crucial for endothelial response to different flow patterns via interaction of retinoid acid receptors and histone deacetylases. Proc Natl Acad Sci U S A 114: 2072–2077, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.This reference has been deleted
- 156. Lee DY, Yang TL, Huang YH, Lee CI, Chen LJ, Shih YT, Wei SY, Wang WL, Wu CC, and Chiu JJ. Induction of microRNA-10a using retinoic acid receptor-α and retinoid x receptor-α agonists inhibits atherosclerotic lesion formation. Atherosclerosis 271: 36–44, 2018 [DOI] [PubMed] [Google Scholar]
- 157. Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, and Burge CB. Prediction of mammalian microRNA targets. Cell 115: 787–798, 2003 [DOI] [PubMed] [Google Scholar]
- 158. Li MX. and Dewson G. Mitochondria and apoptosis: emerging concepts. F1000 Prime Rep 7: 42, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, and Hänze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 10: 1687–1698, 2008 [DOI] [PubMed] [Google Scholar]
- 160. Li X, Yu Y, Gorshkov B, Haigh S, Bordan Z, Weintraub D, Rudic RD, Chakraborty T, Barman SA, Verin AD, Su Y, Lucas R, Stepp DW, Chen F, and Fulton DJR. Hsp70 suppresses mitochondrial reactive oxygen species and preserves pulmonary microvascular barrier integrity following exposure to bacterial toxins. Front Immunol 9: 1309, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Li Y, Zheng J, Bird IM, and Magness RR. Mechanisms of shear stress-induced endothelial nitric-oxide synthase phosphorylation and expression in ovine fetoplacental artery endothelial cells. Biol Reprod 70: 785–796, 2004 [DOI] [PubMed] [Google Scholar]
- 162. Liu LH, Guo Z, Feng M, Wu ZZ, He ZM, and Xiong Y. Protection of DDAH2 overexpression against homocysteine-induced impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells. Cell Physiol Biochem 30: 1413–1422, 2012 [DOI] [PubMed] [Google Scholar]
- 163. Liu SY, Duan XC, Jin S, Teng X, Xiao L, Xue HM, and Wu YM. Hydrogen sulfide improves myocardial remodeling via downregulated angiotensin II/AT1R pathway in renovascular hypertensive rats. Am J Hypertens 30: 67–74, 2017 [DOI] [PubMed] [Google Scholar]
- 164. Loyer X, Potteaux S, Vion AC, Guérin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia P, Maccario J, Boulanger CM, Mallat Z, and Tedgui A. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res 114: 434–443, 2014 [DOI] [PubMed] [Google Scholar]
- 165. Lu T, Wang XL, Chai Q, Sun X, Sieck GC, Katusic ZS, and Lee HC. Role of the endothelial caveolae microdomain in shear stress-mediated coronary vasorelaxation. J Biol Chem 292: 19013–19023, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lu X. and Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol 561: 575–582, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lucas R, Sridhar S, Rick FG, Gorshkov B, Umapathy NS, Yang G, Oseghale A, Verin AD, Chakraborty T, Matthay MA, Zemskov EA, White R, Block NL, and Schally AV. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc Natl Acad Sci U S A 109: 2084–2089, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lucas R, Yang G, Gorshkov BA, Zemskov EA, Sridhar S, Umapathy NS, Jezierska-Drutel A, Alieva IB, Leustik M, Hossain H, Fischer B, Catravas JD, Verin AD, Pittet JF, Caldwell RB, Mitchell TJ, Cederbaum SD, Fulton DJ, Matthay MA, Caldwell RW, Romero MJ, and Chakraborty T. Protein kinase C-alpha and arginase I mediate pneumolysin-induced pulmonary endothelial hyperpermeability. Am J Respir Cell Mol Biol 47: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.This reference has been deleted
- 170. Macdonald PJ, Stepanyants N, Mehrotra N, Mears JA, Qi X, Sesaki H, and Ramachandran R. A dimeric equilibrium intermediate nucleates Drp1 reassembly on mitochondrial membranes for fission. Mol Biol Cell 25: 1905–1915, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Maitra U, Singh N, Gan L, Ringwood L, and Li L. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J Biol Chem 284: 35403–35411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Mammoto T, Muyleart M, Konduri GG, and Mammoto A. Twist1 in hypoxia-induced pulmonary hypertension through transforming growth factor-beta-Smad signaling. Am J Respir Cell Mol Biol 58: 194–207, 2018 [DOI] [PubMed] [Google Scholar]
- 173. Mao X, Said R, Louis H, Max JP, Bourhim M, Challande P, Wahl D, Li Z, Regnault V, and Lacolley P. Cyclic stretch-induced thrombin generation by rat vascular smooth muscle cells is mediated by the integrin alphavbeta3 pathway. Cardiovasc Res 96: 513–523, 2012 [DOI] [PubMed] [Google Scholar]
- 174. Marin T, Gongol B, Chen Z, Woo B, Subramaniam S, Chien S, and Shyy JY. Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free Radic Biol Med 64: 61–68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, Wang Y, Subramaniam S, Chien S, and Shyy JY. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal 10: pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Martínez-Caro L, Lorente JA, Marín-Corral J, Sánchez-Rodríguez C, Sánchez-Ferrer A, Nin N, Ferruelo A, de Paula M, Fernández-Segoviano P, Barreiro E, and Esteban A. Role of free radicals in vascular dysfunction induced by high tidal volume ventilation. Intensive Care Med 35: 1110–1119, 2009 [DOI] [PubMed] [Google Scholar]
- 177. Martínez-Caro L, Nin N, Sánchez-Rodríguez C, Ferruelo A, El Assar M, de Paula M, Fernández-Segoviano P, Esteban A, and Lorente JA. Inhibition of nitro-oxidative stress attenuates pulmonary and systemic injury induced by high-tidal volume mechanical ventilation. Shock 44: 36–43, 2015 [DOI] [PubMed] [Google Scholar]
- 178. Masatsugu K, Itoh H, Chun TH, Ogawa Y, Tamura N, Yamashita J, Doi K, Inoue M, Fukunaga Y, Sawada N, Saito T, Korenaga R, Ando J, and Nakao K. Physiologic shear stress suppresses endothelin-converting enzyme-1 expression in vascular endothelial cells. J Cardiovasc Pharmacol 31(Suppl. 1): S42–S45, 1998 [DOI] [PubMed] [Google Scholar]
- 179. Masatsugu K, Itoh H, Chun TH, Saito T, Yamashita J, Doi K, Inoue M, Sawada N, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, Yurugi T, and Nakao K. Shear stress attenuates endothelin and endothelin-converting enzyme expression through oxidative stress. Regul Pept 111: 13–19, 2003 [DOI] [PubMed] [Google Scholar]
- 180. Mata-Greenwood E, Meyrick B, Steinhorn RH, Fineman JR, and Black SM. Alterations in TGF-beta1 expression in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 285: L209–L221, 2003 [DOI] [PubMed] [Google Scholar]
- 181. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, and Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Matrougui K, Maclouf J, Lévy BI, and Henrion D. Impaired nitric oxide- and prostaglandin-mediated responses to flow in resistance arteries of hypertensive rats. Hypertension 30: 942–947, 1997 [DOI] [PubMed] [Google Scholar]
- 183. McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, and Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 184. Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, and Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 120: 1524–1532, 2009 [DOI] [PubMed] [Google Scholar]
- 185. Merino H, Parthasarathy S, and Singla DK. Partial ligation-induced carotid artery occlusion induces leukocyte recruitment and lipid accumulation—a shear stress model of atherosclerosis. Mol Cell Biochem 372: 267–273, 2013 [DOI] [PubMed] [Google Scholar]
- 186. Michell BJ, Chen ZP, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, and Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 276: 17625–17628, 2001 [DOI] [PubMed] [Google Scholar]
- 187. Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum 36: 37–49, 2006 [DOI] [PubMed] [Google Scholar]
- 188. Minuz P, Barrow SE, Cockcroft JR, and Ritter JM. Prostacyclin and thromboxane biosynthesis in mild essential hypertension. Hypertension 15: 469–474, 1990 [DOI] [PubMed] [Google Scholar]
- 189. Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, and Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003 [DOI] [PubMed] [Google Scholar]
- 190. Mohan S, Koyoma K, Thangasamy A, Nakano H, Glickman RD, and Mohan N. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappaB in endothelial cells. Am J Physiol Cell Physiol 292: C362–C371, 2007 [DOI] [PubMed] [Google Scholar]
- 191. Morawietz H, Talanow R, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, and Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J Physiol 525(Pt 3): 761–770, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Mukohda M, Stump M, Ketsawatsomkron P, Hu C, Quelle FW, and Sigmund CD. Endothelial PPAR-gamma provides vascular protection from IL-1beta-induced oxidative stress. Am J Physiol Heart Circ Physiol 310: H39–H48, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Muzaffar S, Jeremy JY, Angelini GD, and Shukla N. NADPH oxidase 4 mediates upregulation of type 4 phosphodiesterases in human endothelial cells. J Cell Physiol 227: 1941–1950, 2012 [DOI] [PubMed] [Google Scholar]
- 194. Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther 118: 18–35, 2008 [DOI] [PubMed] [Google Scholar]
- 195. Nakamura T. and Lipton SA. Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer's and Parkinson's diseases. Apoptosis 15: 1354–1363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, and Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297: H1535–H1543, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison DG, Giddens DP, and Jo H. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J Vis Exp 40: 1861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ni CW, Qiu H, and Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol 300: H1762–H1769, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Nicosia S, Oliva D, Noè MA, Corsini A, Folco GC, and Fumagalli R. PGI2 receptors in vasculature and platelets: 5Z-carbacyclin discriminates between them. Adv Prostaglandin Thromboxane Leukot Res 17A: 474–478, 1987 [PubMed] [Google Scholar]
- 200. O'Neill HM, Maarbjerg SJ, Crane JD, Jeppesen J, Jørgensen SB, Schertzer JD, Shyroka O, Kiens B, van Denderen BJ, Tarnopolsky MA, Kemp BE, Richter EA, and Steinberg GR. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci U S A 108: 16092–16097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. O'Rourke MF. Vascular impedance in studies of arterial and cardiac function. Physiol Rev 62: 570–623, 1982 [DOI] [PubMed] [Google Scholar]
- 202. Odagiri K, Inui N, Hakamata A, Inoue Y, Suda T, Takehara Y, Sakahara H, Sugiyama M, Alley MT, Wakayama T, and Watanabe H. Non-invasive evaluation of pulmonary arterial blood flow and wall shear stress in pulmonary arterial hypertension with 3D phase contrast magnetic resonance imaging. Springerplus 5: 1071, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Otera H, Ishihara N, and Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta 1833: 1256–1268, 2013 [DOI] [PubMed] [Google Scholar]
- 204. Otto S, Deussen A, Zatschler B, Müller B, Neisser A, Barth K, Morawietz H, and Kopaliani I. A novel role of endothelium in activation of latent pro-membrane type 1 MMP and pro-MMP-2 in rat aorta. Cardiovasc Res 109: 409–418, 2016 [DOI] [PubMed] [Google Scholar]
- 205. Pakala R, Willerson JT, and Benedict CR. Effect of serotonin, thromboxane A2, and specific receptor antagonists on vascular smooth muscle cell proliferation. Circulation 96: 2280–2286, 1997 [DOI] [PubMed] [Google Scholar]
- 206. Pantos I, Patatoukas G, Efstathopoulos EP, and Katritsis D. In vivo wall shear stress measurements using phase-contrast MRI. Expert Rev Cardiovasc Ther 5: 927–938, 2007 [DOI] [PubMed] [Google Scholar]
- 207. Park HS, Chun JN, Jung HY, Choi C, and Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 72: 447–455, 2006 [DOI] [PubMed] [Google Scholar]
- 208. Park HS, Jung HY, Park EY, Kim J, Lee WJ, and Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004 [DOI] [PubMed] [Google Scholar]
- 209. Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, and Evans PC. Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB J 21: 3553–3561, 2007 [DOI] [PubMed] [Google Scholar]
- 210. Patel DN, Bailey SR, Gresham JK, Schuchman DB, Shelhamer JH, Goldstein BJ, Foxwell BM, Stemerman MB, Maranchie JK, Valente AJ, Mummidi S, and Chandrasekar B. TLR4-NOX4-AP-1 signaling mediates lipopolysaccharide-induced CXCR6 expression in human aortic smooth muscle cells. Biochem Biophys Res Commun 347: 1113–1120, 2006 [DOI] [PubMed] [Google Scholar]
- 211. Pereira S, Zhou M, Mócsai A, and Lowell C. Resting murine neutrophils express functional alpha 4 integrins that signal through Src family kinases. J Immunol 166: 4115–4123, 2001 [DOI] [PubMed] [Google Scholar]
- 212. Pirinccioglu AG, Alyan O, Kizil G, Kangin M, and Beyazit N. Evaluation of oxidative stress in children with congenital heart defects. Pediatr Int 54: 94–98, 2012 [DOI] [PubMed] [Google Scholar]
- 213. Poljsak B, Šuput D, and Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013: 956792, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S, and Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A 107: 3240–3244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Rafikov R, Dimitropoulou C, Aggarwal S, Kangath A, Gross C, Pardo D, Sharma S, Jezierska-Drutel A, Patel V, Snead C, Lucas R, Verin A, Fulton D, Catravas JD, and Black SM. Lipopolysaccharide-induced lung injury involves the nitration-mediated activation of RhoA. J Biol Chem 289: 4710–4722, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Rafikov R, Rafikova O, Aggarwal S, Gross C, Sun X, Desai J, Fulton D, and Black SM. Asymmetric dimethylarginine induces endothelial nitric-oxide synthase mitochondrial redistribution through the nitration-mediated activation of Akt1. J Biol Chem 288: 6212–6226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Rapoport RM. Acute nitric oxide synthase inhibition and endothelin-1-dependent arterial pressure elevation. Front Pharmacol 5: 57, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Reddy SP, Hassoun PM, and Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal 9: 2003–2012, 2007 [DOI] [PubMed] [Google Scholar]
- 219. Reiss LK, Uhlig U, and Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol 91: 590–601, 2012 [DOI] [PubMed] [Google Scholar]
- 220. Reiter G, Reiter U, Kovacs G, Olschewski H, and Fuchsjäger M. Blood flow vortices along the main pulmonary artery measured with MR imaging for diagnosis of pulmonary hypertension. Radiology 275: 71–79, 2015 [DOI] [PubMed] [Google Scholar]
- 221. Rose ML, Strange G, King I, Arnup S, Vidmar S, O'Donnell C, Kermeen F, Grigg L, Weintraub RG, and Celermajer DS. Congenital heart disease-associated pulmonary arterial hypertension: preliminary results from a novel registry. Intern Med J 42: 874–879, 2012 [DOI] [PubMed] [Google Scholar]
- 222. Rossi NF, Black SM, Telemaque-Potts S, and Chen H. Neuronal nitric oxide synthase activity in the paraventricular nucleus buffers central endothelin-1- induced pressor response and vasopressin secretion. J Cardiovasc Pharmacol 44(Suppl. 1): S283–S288, 2004 [DOI] [PubMed] [Google Scholar]
- 223. Ryan J, Dasgupta A, Huston J, Chen KH, and Archer SL. Mitochondrial dynamics in pulmonary arterial hypertension. J Mol Med (Berl) 93: 229–242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Ryan JJ. and Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation 131: 1691–1702, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Ryan JJ, Marsboom G, Fang YH, Toth PT, Morrow E, Luo N, Piao L, Hong Z, Ericson K, Zhang HJ, Han M, Haney CR, Chen CT, Sharp WW, and Archer SL. PGC1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am J Respir Crit Care Med 187: 865–878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, and Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008 [DOI] [PubMed] [Google Scholar]
- 227. Sanders KA. and Hoidal JR. The NOX on pulmonary hypertension. Circ Res 101: 224–226, 2007 [DOI] [PubMed] [Google Scholar]
- 228. Sandow SL. and Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci 28: 61–67, 2007 [DOI] [PubMed] [Google Scholar]
- 229. Sandqvist A, Schneede J, Kylhammar D, Henrohn D, Lundgren J, Hedeland M, Bondesson U, Rådegran G, and Wikström G. Plasma l-arginine levels distinguish pulmonary arterial hypertension from left ventricular systolic dysfunction. Heart Vessels 33: 255–263, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Santel A. and Frank S. Shaping mitochondria: the complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life 60: 448–455, 2008 [DOI] [PubMed] [Google Scholar]
- 231. Sathanoori R, Bryl-Gorecka P, Müller CE, Erb L, Weisman GA, Olde B, and Erlinge D. P2Y2 receptor modulates shear stress-induced cell alignment and actin stress fibers in human umbilical vein endothelial cells. Cell Mol Life Sci 74: 731–746, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sathanoori R, Rosi F, Gu BJ, Wiley JS, Müller CE, Olde B, and Erlinge D. Shear stress modulates endothelial KLF2 through activation of P2X4. Purinergic Signal 11: 139–153, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, and Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J 16: 1713–1720, 2002 [DOI] [PubMed] [Google Scholar]
- 234. Scheitlin CG, Nair DM, Crestanello JA, Zweier JL, and Alevriadou BR. Fluid mechanical forces and endothelial mitochondria: a bioengineering perspective. Cell Mol Bioeng 7: 483–496, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Searles CD. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am J Physiol Cell Physiol 291: C803–C816, 2006 [DOI] [PubMed] [Google Scholar]
- 236. Sharma S, Aramburo A, Rafikov R, Sun X, Kumar S, Oishi PE, Datar SA, Raff G, Xoinis K, Kalkan G, Fratz S, Fineman JR, and Black SM. L-carnitine preserves endothelial function in a lamb model of increased pulmonary blood flow. Pediatr Res 74: 39–47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Sharma S, Barton J, Rafikov R, Aggarwal S, Kuo HC, Oishi PE, Datar SA, Fineman JR, and Black SM. Chronic inhibition of PPAR-gamma signaling induces endothelial dysfunction in the juvenile lamb. Pulm Pharmacol Ther 26: 271–280, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Sharma S, Smith A, Kumar S, Aggarwal S, Rehmani I, Snead C, Harmon C, Fineman J, Fulton D, Catravas JD, and Black SM. Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced acute lung injury: role of asymmetric dimethylarginine. Vascul Pharmacol 52: 182–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi P, Fineman JR, and Black SM. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 294: L46–L56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Sharma S, Sun X, Rafikov R, Kumar S, Hou Y, Oishi PE, Datar SA, Raff G, Fineman JR, and Black SM. PPAR-gamma regulates carnitine homeostasis and mitochondrial function in a lamb model of increased pulmonary blood flow. PLoS One 7: e41555, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, and Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell 15: 2707–2719, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, and Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res 304: 40–49, 2005 [DOI] [PubMed] [Google Scholar]
- 243. Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch 459: 915–922, 2010 [DOI] [PubMed] [Google Scholar]
- 244. Shimokawa H. and Godo S. Diverse functions of endothelial NO synthases system: NO and EDH. J Cardiovasc Pharmacol 67: 361–366, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Shimokawa H. and Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol 39: 725–732, 2005 [DOI] [PubMed] [Google Scholar]
- 246. Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, and Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J Am Coll Cardiol 38: 1859–1865, 2001 [DOI] [PubMed] [Google Scholar]
- 247. Siu KL, Gao L, and Cai H. Differential roles of protein complexes NOX1-NOXO1 and NOX2-p47phox in mediating endothelial redox responses to oscillatory and unidirectional laminar shear stress. J Biol Chem 291: 8653–8662, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Stankevicius E, Kevelaitis E, Vainorius E, and Simonsen U. Role of nitric oxide and other endothelium-derived factors [in Lithuanian]. Medicina (Kaunas) 39: 333–341, 2003 [PubMed] [Google Scholar]
- 249. Steele PM, Fuster V, Cohen M, Ritter DG, and McGoon DC. Isolated atrial septal defect with pulmonary vascular obstructive disease—long-term follow-up and prediction of outcome after surgical correction. Circulation 76: 1037–1042, 1987 [DOI] [PubMed] [Google Scholar]
- 250. Stirpe F. and Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem 244: 3855–3863, 1969 [PubMed] [Google Scholar]
- 251. Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, and Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 252. Suárez Y, Fernández-Hernando C, Pober JS, and Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res 100: 1164–1173, 2007 [DOI] [PubMed] [Google Scholar]
- 253. Sud N. and Black SM. Endothelin-1 impairs nitric oxide signaling in endothelial cells through a protein kinase Cdelta-dependent activation of STAT3 and decreased endothelial nitric oxide synthase expression. DNA Cell Biol 28: 543–553, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Sun X, Fratz S, Sharma S, Hou Y, Rafikov R, Kumar S, Rehmani I, Tian J, Smith A, Schreiber C, Reiser J, Naumann S, Haag S, Hess J, Catravas JD, Patterson C, Fineman JR, and Black SM. C-terminus of heat shock protein 70-interacting protein-dependent GTP cyclohydrolase I degradation in lambs with increased pulmonary blood flow. Am J Respir Cell Mol Biol 45: 163–171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Sun X, Kumar S, Sharma S, Aggarwal S, Lu Q, Gross C, Rafikova O, Lee SG, Dasarathy S, Hou Y, Meadows ML, Han W, Su Y, Fineman JR, and Black SM. Endothelin-1 induces a glycolytic switch in pulmonary arterial endothelial cells via the mitochondrial translocation of endothelial nitric oxide synthase. Am J Respir Cell Mol Biol 50: 1084–1095, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Sun X, Sharma S, Fratz S, Kumar S, Rafikov R, Aggarwal S, Rafikova O, Lu Q, Burns T, Dasarathy S, Wright J, Schreiber C, Radman M, Fineman JR, and Black SM. Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow. Antioxid Redox Signal 18: 1739–1752, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Suzuki M, Naruse K, Asano Y, Okamoto T, Nishikimi N, Sakurai T, Nimura Y, and Sokabe M. Up-regulation of integrin beta 3 expression by cyclic stretch in human umbilical endothelial cells. Biochem Biophys Res Commun 239: 372–376, 1997 [DOI] [PubMed] [Google Scholar]
- 258. Syrkina O, Jafari B, Hales CA, and Quinn DA. Oxidant stress mediates inflammation and apoptosis in ventilator-induced lung injury. Respirology 13: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 259. Szulcek R, Happé CM, Rol N, Fontijn RD, Dickhoff C, Hartemink KJ, Grünberg K, Tu L, Timens W, Nossent GD, Paul MA, Leyen TA, Horrevoets AJ, de Man FS, Guignabert C, Yu PB, Vonk-Noordegraaf A, van Nieuw Amerongen GP, and Bogaard HJ. Delayed microvascular shear adaptation in pulmonary arterial hypertension. Role of platelet endothelial cell adhesion molecule-1 cleavage. Am J Respir Crit Care Med 193: 1410–1420, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, Dharbandi F, Khalsa B, Bressler S, Barr ML, Li R, and Hsiai TK. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal 15: 1379–1388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Takac I, Schröder K, and Brandes RP. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep 14: 70–78, 2012 [DOI] [PubMed] [Google Scholar]
- 262. Tanaka KA, Key NS, and Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg 108: 1433–1446, 2009 [DOI] [PubMed] [Google Scholar]
- 263. Tanaka Y, Yamaki F, Koike K, and Toro L. New insights into the intracellular mechanisms by which PGI2 analogues elicit vascular relaxation: cyclic AMP-independent, Gs-protein mediated-activation of MaxiK channel. Curr Med Chem Cardiovasc Hematol Agents 2: 257–265, 2004 [DOI] [PubMed] [Google Scholar]
- 264. Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, Wang Z, Gupta A, Zhou T, Sun X, Dash S, Wang Z, Balistrieri A, Zheng Q, Cordery AG, Desai AA, Rischard F, Khalpey Z, Wang J, Black SM, Garcia JGN, Makino A, and Yuan JX. Endothelial HIF-2alpha contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol 314: L256–L275, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Taylor SG. and Weston AH. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci 9: 272–274, 1988 [DOI] [PubMed] [Google Scholar]
- 266. Tesfamariam B. Free radicals in diabetic endothelial cell dysfunction. Free Radic Biol Med 16: 383–391, 1994 [DOI] [PubMed] [Google Scholar]
- 267. Tirone F, Radu L, Craescu CT, and Cox JA. Identification of the binding site for the regulatory calcium-binding domain in the catalytic domain of NOX5. Biochemistry 49: 761–771, 2010 [DOI] [PubMed] [Google Scholar]
- 268. Touyz RM, Yao G, Quinn MT, Pagano PJ, and Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol 25: 512–518, 2005 [DOI] [PubMed] [Google Scholar]
- 269. Tran QK, Ohashi K, and Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res 48: 13–22, 2000 [DOI] [PubMed] [Google Scholar]
- 270. Tsushima K, King LS, Aggarwal NR, De Gorordo A, D'Alessio FR, and Kubo K. Acute lung injury review. Intern Med 48: 621–630, 2009 [DOI] [PubMed] [Google Scholar]
- 271. Tugues S, Honjo S, König C, Padhan N, Kroon J, Gualandi L, Li X, Barkefors I, Thijssen VL, Griffioen AW, and Claesson-Welsh L. Tetraspanin CD63 promotes vascular endothelial growth factor receptor 2-beta1 integrin complex formation, thereby regulating activation and downstream signaling in endothelial cells in vitro and in vivo. J Biol Chem 288: 19060–19071, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Umanskiy K, Robinson C, Cave C, Williams MA, Lentsch AB, Cuschieri J, and Solomkin JS. NADPH oxidase activation in fibronectin adherent human neutrophils: a potential role for beta1 integrin ligation. Surgery 134: 378–383, 2003 [DOI] [PubMed] [Google Scholar]
- 273. van der Vliet A, Janssen-Heininger YMW, and Anathy V. Oxidative stress in chronic lung disease: from mitochondrial dysfunction to dysregulated redox signaling. Mol Aspects Med 63: 59–69, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Van Hung T, Emoto N, Vignon-Zellweger N, Nakayama K, Yagi K, Suzuki Y, and Hirata K. Inhibition of vascular endothelial growth factor receptor under hypoxia causes severe, human-like pulmonary arterial hypertension in mice: potential roles of interleukin-6 and endothelin. Life Sci 118: 313–328, 2014 [DOI] [PubMed] [Google Scholar]
- 275. Vandenbroucke E, Mehta D, Minshall R, and Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci 1123: 134–145, 2008 [DOI] [PubMed] [Google Scholar]
- 276. Vaporidi K, Francis RC, Bloch KD, and Zapol WM. Nitric oxide synthase 3 contributes to ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 299: L150–L159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Ventura-Clapier R, Garnier A, and Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res 79: 208–217, 2008 [DOI] [PubMed] [Google Scholar]
- 278. Verónica Donoso M, Hernández F, Villalón T, Acuña-Castillo C, and Pablo Huidobro-Toro J. Pharmacological dissection of the cellular mechanisms associated to the spontaneous and the mechanically stimulated ATP release by mesentery endothelial cells: roles of thrombin and TRPV. Purinergic Signal 14: 121–139, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Viñas JL, Burger D, Zimpelmann J, Haneef R, Knoll W, Campbell P, Gutsol A, Carter A, Allan DS, and Burns KD. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int 90: 1238–1250, 2016 [DOI] [PubMed] [Google Scholar]
- 280. Vitali SH, Hansmann G, Rose C, Fernandez-Gonzalez A, Scheid A, Mitsialis SA, and Kourembanas S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: long-term follow-up. Pulm Circ 4: 619–629, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Wallace CS. and Truskey GA. Direct-contact co-culture between smooth muscle and endothelial cells inhibits TNF-alpha-mediated endothelial cell activation. Am J Physiol Heart Circ Physiol 299: H338–H346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Wang HW, Huang BS, White RA, Chen A, Ahmad M, and Leenen FH. Mineralocorticoid and angiotensin II type 1 receptors in the subfornical organ mediate angiotensin II - induced hypothalamic reactive oxygen species and hypertension. Neuroscience 329: 112–121, 2016 [DOI] [PubMed] [Google Scholar]
- 283. Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS, and Chien S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci U S A 107: 3234–3239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Wang T, Gross C, Desai AA, Zemskov E, Wu X, Garcia AN, Jacobson JR, Yuan JX, Garcia JG, and Black SM. Endothelial cell signaling and ventilator-induced lung injury: molecular mechanisms, genomic analyses, and therapeutic targets. Am J Physiol Lung Cell Mol Physiol 312: L452–L476, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Wang Z. and Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 1: 212–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Waud WR. and Rajagopalan KV. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys 172: 354–364, 1976 [DOI] [PubMed] [Google Scholar]
- 287. Wedgwood S. and Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 288: L480–L487, 2005 [DOI] [PubMed] [Google Scholar]
- 288. Wedgwood S, Lakshminrusimha S, Schumacker PT, and Steinhorn RH. Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309: L196–L203, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. White SJ, Hayes EM, Lehoux S, Jeremy JY, Horrevoets AJ, and Newby AC. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol 226: 2841–2848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Williams DA, Zheng Y, and Cancelas JA. Rho GTPases and regulation of hematopoietic stem cell localization. Methods Enzymol 439: 365–393, 2008 [DOI] [PubMed] [Google Scholar]
- 291. Wolfson RK, Mapes B, and Garcia JGN. Excessive mechanical stress increases HMGB1 expression in human lung microvascular endothelial cells via STAT3. Microvasc Res 92: 50–55, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Wu F, Szczepaniak WS, Shiva S, Liu H, Wang Y, Wang L, Wang Y, Kelley EE, Chen AF, Gladwin MT, and McVerry BJ. Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L987–L997, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Wu LH, Chang HC, Ting PC, and Wang DL. Laminar shear stress promotes mitochondrial homeostasis in endothelial cells. J Cell Physiol 233: 5058–5069, 2018 [DOI] [PubMed] [Google Scholar]
- 294. Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr., Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, and Shyy JY. Flow-dependent regulation of Kruppel-like factor 2 is mediated by microRNA-92a. Circulation 124: 633–641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, and Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 296. Xu Y, Fang F, Zhang J, Josson S, St Clair WH, and St Clair DK. miR-17* suppresses tumorigenicity of prostate cancer by inhibiting mitochondrial antioxidant enzymes. PLoS One 5: e14356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Yamamoto K, Korenaga R, Kamiya A, and Ando J. Fluid shear stress activates Ca(2+) influx into human endothelial cells via P2X4 purinoceptors. Circ Res 87: 385–391, 2000 [DOI] [PubMed] [Google Scholar]
- 298. Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, and Ando J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol 285: H793–H803, 2003 [DOI] [PubMed] [Google Scholar]
- 299. Yan SR, Fumagalli L, Dusi S, and Berton G. Tumor necrosis factor triggers redistribution to a Triton X-100-insoluble, cytoskeletal fraction of beta 2 integrins, NADPH oxidase components, tyrosine phosphorylated proteins, and the protein tyrosine kinase p58fgr in human neutrophils adherent to fibrinogen. J Leukoc Biol 58: 595–606, 1995 [DOI] [PubMed] [Google Scholar]
- 300. Yang YM, Huang A, Kaley G, and Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Yokota T, Shiraishi R, Aida T, Iwai K, Liu NM, Yokoyama U, and Minamisawa S. Thromboxane A(2) receptor stimulation promotes closure of the rat ductus arteriosus through enhancing neointima formation. PLoS One 9: e94895, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Zhang B, Niu W, Dong HY, Liu ML, Luo Y, and Li ZC. Hypoxia induces endothelial-mesenchymal transition in pulmonary vascular remodeling. Int J Mol Med 42: 270–278, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Zhang S, Yang T, Xu X, Wang M, Zhong L, Yang Y, Zhai Z, Xiao F, and Wang C. Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: a case control study. BMC Pulm Med 15: 50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Zhang X, Ng WL, Wang P, Tian L, Werner E, Wang H, Doetsch P, and Wang Y. MicroRNA-21 modulates the levels of reactive oxygen species by targeting SOD3 and TNFalpha. Cancer Res 72: 4707–4713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 305. Zhang X, Zhang T, Gao F, Li Q, Shen C, Li Y, Li W, and Zhang X. Fasudil, a Rho-kinase inhibitor, prevents intimamedia thickening in a partially ligated carotid artery mouse model: effects of fasudil in flowinduced vascular remodeling. Mol Med Rep 12: 7317–7325, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, and Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 119: 2009–2018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Zheng J, Zhang K, Wang Y, Cao J, Zhang F, Zhou Q, and Dong R. Identification of a microRNA signature in endothelial cells with mechanical stretch stimulation. Mol Med Rep 12: 3525–3530, 2015 [DOI] [PubMed] [Google Scholar]
- 308. Zhou C, Huang J, Chen J, Lai J, Zhu F, Xu X, and Wang DW. CYP2J2-derived EETs attenuated angiotensin II-induced adventitial remodeling via reduced inflammatory response. Cell Physiol Biochem 39: 721–739, 2016 [DOI] [PubMed] [Google Scholar]
- 309. Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, and Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A 108: 10355–10360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Zhu H, Itoh K, Yamamoto M, Zweier JL, and Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579: 3029–3036, 2005 [DOI] [PubMed] [Google Scholar]
- 311. Zhu H, Jia Z, Zhang L, Yamamoto M, Misra HP, Trush MA, and Li Y. Antioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp Biol Med (Maywood) 233: 463–474, 2008 [DOI] [PubMed] [Google Scholar]
- 312. Zhu X, Gao Q, Tu Q, Zhong Y, Zhu D, Mao C, and Xu Z. Prenatal hypoxia enhanced angiotensin II-mediated vasoconstriction via increased oxidative signaling in fetal rats. Reprod Toxicol 60: 21–28, 2016 [DOI] [PubMed] [Google Scholar]
- 313. Zhuang D, Ceacareanu AC, Lin Y, Ceacareanu B, Dixit M, Chapman KE, Waters CM, Rao GN, and Hassid A. Nitric oxide attenuates insulin- or IGF-I-stimulated aortic smooth muscle cell motility by decreasing H2O2 levels: essential role of cGMP. Am J Physiol Heart Circ Physiol 286: H2103–H2112, 2004 [DOI] [PubMed] [Google Scholar]
- 314. Zou MH, Klein T, Pasquet JP, and Ullrich V. Interleukin 1beta decreases prostacyclin synthase activity in rat mesangial cells via endogenous peroxynitrite formation. Biochem J 336: 507–512, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]