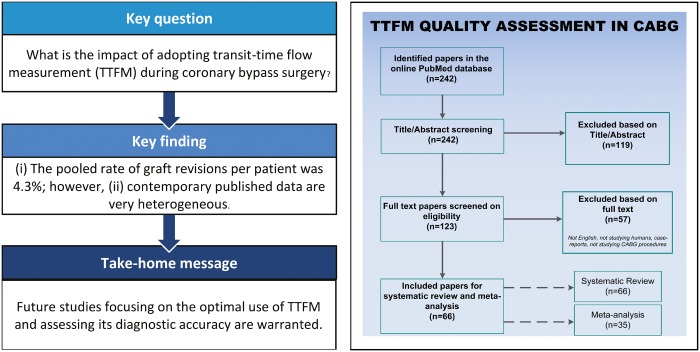
Summary
Despite there being numerous studies of intraoperative graft flow assessment by transit-time flow measurement (TTFM) on outcomes after coronary artery bypass grafting (CABG), the adoption of contemporary TTFM is low. Therefore, on 31 January 2018, a systematic literature search was performed to identify articles that reported (i) the amount of grafts classified as abnormal or which were revised or (ii) an association between TTFM and outcomes during follow-up. Random-effects models were used to create pooled estimates with 95% confidence intervals (CI) of (i) the rate of graft revision per patient, (ii) the rate of graft revision per graft and (iii) the rate of graft revision among grafts deemed abnormal based on TTFM parameters. The search yielded 242 articles, and 66 original articles were included in the systematic review. Of those articles, 35 studies reported on abnormal grafts or graft revisions (8943 patients, 15 673 grafts) and were included in the meta-analysis. In 4.3% of patients (95% CI 3.3–5.7%, I2 = 73.9) a revision was required and 2.0% of grafts (95% CI 1.5–2.5%; I2 = 66.0) were revised. The pooled rate of graft revisions among abnormal grafts was 25.1% (95% CI 15.5–37.9%; I2 = 80.2). Studies reported sensitivity ranging from 0.250 to 0.457 and the specificity from 0.939 to 0.984. Reported negative predictive values ranged from 0.719 to 0.980 and reported positive predictive values ranged from 0.100 to 0.840. This systematic review and meta-analysis showed that TTFM could improve CABG procedures. However, due to heterogeneous data, drawing uniform conclusions appeared challenging. Future studies should focus on determining the optimal use of TTFM and assessing its diagnostic accuracy.
Keywords: Coronary artery bypass, Intraoperative quality control, Transit time, Transit-time flow measurement, Intraoperative graft flow assessment, Coronary artery bypass grafting
INTRODUCTION
Outcomes of coronary artery bypass grafting (CABG) have significantly improved over the first 50 years since the introduction of the modern CABG procedure [1]. Despite increasing use of percutaneous coronary intervention (PCI), CABG remains the treatment of choice for patients with complex multivessel disease [2]. While outcomes of PCI are continuously improving with new advancements, many new techniques to optimize short- and long-term outcomes of CABG have not been adopted widely [3].
One of such techniques to improve CABG outcomes and graft patency is intraoperative graft flow assessment. Early graft failure can occur due to limited outflow, graft kinking upon chest closure, thrombosis, yet also due to anastomotic problems. A meta-analysis reported a graft failure rate of ∼5% and 25% at 3 and 12 months, respectively [4]. Fabricius et al. [5] reported that 23 of 2052 patients (1.1%) who underwent CABG had severely compromised haemodynamics due to postoperative myocardial infarction (MI). In 21.7% of patients, the cause of this adverse event was found to be an incorrect anastomosis. Intraoperative graft assessment has therefore been introduced to identify anastomotic problems and limited outflow before chest closure.
Multiple techniques for intraoperative graft assessment have been proposed: coronary angiography (CAG), transit-time flow measurement (TTFM), high-resolution-epicardial ultrasonography (HR-ECUS) and intraoperative fluorescence imaging (IFI) [6]. Although angiography is thought to be the best and most reliable method for assessing flow, the infrastructure required for CAG is rarely available in standard operating theatres. Therefore, the most adopted strategy to assess graft functioning is TTFM. Several studies reported associations between TTFM parameters and the necessity for graft revisions as well as with short- and medium-term outcomes after CABG; however, results vary considerably [7, 8]. A summary of the evidence could provide more incentive to adopt TTFM, especially as TTFM has been criticized [9].
We performed a systematic review and meta-analysis to evaluate the value of TTFM during CABG by determining (i) the rate of abnormal grafts and graft revisions required when using TTFM and (ii) the impact of TTFM parameters on angiographic and clinical outcomes.
METHODS
Search strategy
On 31 January 2018, a systematic literature search in the PubMed database was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline (Supplementary Materials) to identify full-length, English-language articles with the following search term: ‘(transit time OR transit-time) AND coronary artery bypass’. Studies with the following criteria were included: (i) graft assessment was performed using TTFM; (ii) subjects were adult patients undergoing CABG; and (iii) it was reported how many grafts had abnormal TTFM parameters or were revised, or an association between TTFM parameters and outcomes during follow-up was investigated.
Titles and abstracts were then screened for inclusion. When eligible, full-text articles were reviewed (D.J.F.M.T. and S.J.H.). Only original articles, articles in English, studying humans and studying TTFM in CABG procedures were considered for inclusion. If multiple articles described the same patient population, only the article with the largest number of patients or the most relevant information was included.
Data extraction
The following study characteristics were extracted: prospective versus retrospective study, the year of publication, the authors and the number of patients. Furthermore, the following data on surgical characteristics and TTFM parameters were obtained from each study: surgical procedure (on-pump or off-pump), which grafts were used (e.g. internal thoracic artery, great saphenous vein, radial artery and gastroepiploic artery), the number of grafts assessed with TTFM, the number of grafts deemed abnormal based on intraoperative TTFM parameters, the number of grafts that were revised based on intraoperative TTFM parameters, the reasons why grafts were deemed ‘abnormal’ or ‘required revision’ based on intraoperative TTFM parameters [mean graft flow (MGF), pulsatility index (PI), diastolic filling % (DF%) and percentage of backflow (%BF) (e.g. insufficiency percentage)] and fast Fourier transformation (FFT). Sensitivity, specificity, negative predictive values (NPV) and positive predictive values (PPV) were extracted to assess the diagnostic accuracy of TTFM. Postoperative short-term outcomes (e.g. till 30 days) and outcomes during follow-up (e.g. beyond 30 days) that were extracted consisted of major adverse cardiac and cerebrovascular events (MACCE), mortality, MI, postoperative cardiac enzyme release, stroke, requirement for intra-aortic balloon pump placement, angina and graft failure.
Statistical analyses
The quality of the included studies used in the meta-analysis was assessed according to the Newcastle-Ottawa-Scale (NOS) [10]. Random-effects models were generated to estimate pooled study outcomes with 95% confidence intervals (95% CI) of (i) the proportion of revised grafts compared to the total number of patients studied by TTFM, (ii) the proportion of revised grafts compared to the total number of grafts on which TTFM was applied and (iii) the proportion of revised grafts compared to the total number of abnormal grafts found by TTFM. For studies that reported zero events (e.g. no abnormal or revised grafts), 0.1 events were used to calculate an estimated event rate with 95% CI. The I2 statistic was used to describe the proportion of variation across studies based on heterogeneity, where low values relate to homogeneity (range 0–100). Statistical analyses were executed with Comprehensive Meta-Analysis software Version 3.3 (Biostat, Englewood, NJ, USA).
RESULTS
Study selection
This systematic review included 66 studies (Fig. 1). In total, 35 unique studies reported on abnormal grafts or graft revisions with 8943 CABG patients and 15 673 grafts (Table 1) and were included in the meta-analysis. An overview of the NOS quality assessment is presented in the Supplementary Material, Table S1. Eight studies reported on the diagnostic accuracy of TTFM (Supplementary Material, Table S2). Forty-two studies reported graft patency and clinical outcomes related to TTFM assessments (Supplementary Material, Tables S3 and S4).
Figure 1:
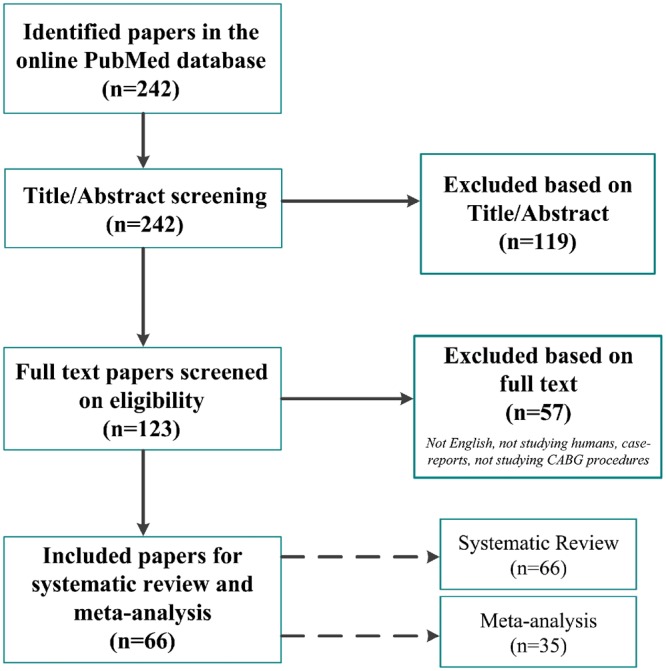
Flow chart of the systematic review process. Studies not written in English, not studying humans, reporting on the same patient population, reporting on transit-time flow measurement in other procedures besides CABG and case reports or reviews were excluded. In total, 66 studies were included, of which 35 studies were incorporated in the meta-analysis. CABG: coronary artery bypass grafting.
Table 1:
Studies reporting rates of abnormal grafts and/or revised grafts assessed by TTFM
| Study | Year | Design | Number of grafts/patients | Procedure specificsa | Graft outcome | Reasons for abnormal or revised grafts | Results |
|---|---|---|---|---|---|---|---|
| Hashim et al. [11] | 2017 | Prospective | 86/60 | TTFM on ITA | Abnormal | PI >1.0 with an MGF <20 ml/min in an arrested heart | Not specified |
| Revision | Not specified | 3.5% (n = 3 grafts) | |||||
| Hiraoka et al. [12] | 2017 | Prospective | 104/63 | TTFM on ITA, RA and SVG | Abnormal | PI >5.0 and an MGF <20 ml/min in ITA-graft or <40 ml/min in SVG | 8.7% (n = 9 grafts) |
| Leon et al. [13] | 2017 | Retrospective | 543/177 | TTFM on ITA and SVG | Revision | PI ≥5.0 | 0.9% (n = 5 grafts) |
| Handa et al. [14] | 2016 | Retrospective | 196/68 | OPCAB with TTFM on ITA and SVG | Abnormal | Abnormal TTFM parameters: MGF <15 ml/min, DF <50% and PI >5.0 | 40% (n = 46 grafts) of which 54% appeared patent on postoperative CAG |
| Revision | MGF <5 ml/min or DF <50% or PI >5.0 | 3.0% (n = 6 grafts) | |||||
| Oshima et al. [15] | 2016 | Retrospective | 214/196 | TTFM on ITA and SVG | Abnormal | Lower mean flow (21.3 ± 16.2 ml/min) and higher PI (5.5 ± 4.7) | 7.0% (n = 15 grafts) |
| Honda et al. [16] | 2015 | Retrospective | 72/72 | TTFM on in situ ITA | Abnormal | MGF <20 ml/min and PI = 2.0 | 1.4% (n = 1 graft) |
| Di Giammarco et al. [17] | 2014 | Prospective | 717/333 | TTFM on ITA and SVG | Abnormal | Grafts with MGF ≤15 ml/min and PI ≥3.0 were defined as failing | 5.4% (n = 39 grafts) |
| Revision | Failing grafts based on TTFM and surgical inspection | 0.3% (n = 2 grafts) | |||||
| Quin et al. [18] | 2014 | Retrospective | 2738/1067 | TTFM on ITA, SVG and RA | Abnormal | MGF <20 ml/min | 20.7% (n = 568 grafts) |
| Revision | MGF <20 ml/min and abnormal PI <3.0 (0.7%), 3.0–5.0 (2.9%) and >5.0 (5.8%) | 2.0% (n = 54 grafts) | |||||
| Harahsheh [19] | 2012 | Prospective | 1394/436 | Not specifiedb | Abnormal | MGF <20 ml/min, PI >5.0 and DF <50% | 7.2% (n = 100 grafts) |
| Revision | Not specified | 1.0% (n = 14 grafts) | |||||
| 1.1% (n = 5 patients) | |||||||
| Kuroyanagi et al. [20] | 2012 | Retrospective | 435/159 | OPCAB with TTFM on ITA and SVG | Revision | Cut-off values not specified | 2.0% (n = 9 grafts) |
| Kieser et al. [8] | 2010 | Prospective | 1015/336 | TTFM on ITA, SVG and RA | Abnormal | PI >5.0 | 7% (n = 74 grafts) |
| Revision | PI >5, MGF ≤15 ml/min and DF ≤25 with surgical signs of graft malfunctioning | 18% (n = 59 patients) | |||||
| 2.0% (n = 20 grafts) | |||||||
| 4.2% (n = 14 patients) | |||||||
| Handa et al. [21] | 2009 | Retrospective | 116/39 | OPCAB with TTFM on ITA and SVG | Abnormal | MGF <10 ml/min, PI >5.0 or DF <50% | 2.6% (n = 3 grafts) |
| Revision | MGF = 0 ml/min | 1.7% (n = 2 grafts) | |||||
| Nordgaard et al. [22] | 2009 | Retrospective | 1390/581 | TTFM on ITA and SVG | Revision | Low MGF and high PI | 0.4% (n = 5 grafts) |
| Santarpino et al. [23] | 2009 | Prospective | 238/238 | TTFM on LITA + RA versus LITA + SVG | Revision | TTFM systolic waveform and PI >4.0 based on thrombosis (n = 2) and torsion of the graft (n = 1) | 1.3% (n = 3 grafts) |
| 1.3% (n = 3 patients) | |||||||
| Waseda et al. [24] | 2009 | Retrospective | 289/116 | TTFM on ITA, SVG, RA and GEA | Abnormal | MGF ≤5 ml/min and PI >5 | 7.3% (n = 21 grafts) |
| Revision | Failing grafts on IFI, yet acceptable TTFM (MGF >5 ml/min and PI ≤5) results | 2.1% (n = 6 grafts) | |||||
| Herman et al. [25] | 2008 | Prospective | …/985 | TTFM on ITA and SVG | Abnormal | PI >5 | 18.7% (n = 184 patients) |
| Revision | Anastomotic (n = 9), conduit (n = 8), subclavian stenosis (n = 1) and unidentified (n = 2) | 2.0% (n = 20 patients) | |||||
| Onorati et al. [26] | 2008 | Retrospective | …/433 | TTFM on ITA and RA | Abnormal | PI >5 and low MGF (not specified) | 0.2% (n = 1 patients) |
| Revision | MGF ≤3 ml/min and PI ≥5 | 0.7% (n = 3 patients) | |||||
| Becit et al. [27] | 2007 | Retrospective | 303/200 | TTFM versus without TTFM on ITA, SVG or RA | Revision | Unsatisfactory TTFM parameters due to kinked/twisted grafts (n = 4) or stenosis in proximal LITA (n = 2) or poor native coronary vessel (n = 3) | 3.0% (n = 9 grafts) |
| 9.0% (n = 9 patients) | |||||||
| Mujanovic et al. [28] | 2007 | Prospective | 2872/1000 | Not specifiedb | Revision | Cut-off values not specified | 2.2% (n = 64 grafts) |
| 6.3% (n = 63 patients) | |||||||
| Onorati et al. [29] | 2007 | RCT | 90/90 | TTFM on single-SVG versus sequential-SVG | Abnormal | PI >5 and low MGF (not specified) | 5.6% (n = 5 grafts) |
| Revision | ‘Systolic’ pattern of the curve with low MGF (4 ml/min) and high PI (7.8)” | 5.6% (n = 5 patients) | |||||
| 1.1% (n = 1 graft) | |||||||
| 1.1% (n = 1 patient) | |||||||
| Desai et al. [30] | 2006 | RCT | 139/106 | TTFM and IFI on ITA, SVG and RA | Abnormal | DF <50%, PI >5.0 and MGF <10 ml/min | 2.6% (n = 3 grafts) |
| Revision | MGF = 0 ml/min | 1.4% (n = 2 grafts) | |||||
| Poston et al. [31] | 2006 | Prospective | 410/410 | TTFM on SVG | Revision | MGF <10 ml/min | 0.5% (n = 2 grafts) |
| Balacumaraswami et al. [32] | 2005 | Prospective | 266/100 | TTFM on ITA and RA | Abnormal | Not specified | 9.4% (n = 25 grafts) |
| Revision | Persistent poor MGF with TTFM and IFI under adequate MAP (>80 mmHg) | 25.0% (n = 25 patients) | |||||
| 3.0% (n = 8 grafts) | |||||||
| 8.0% (n = 8 patients) | |||||||
| Kim et al. [33] | 2005 | Retrospective | 117/58 | OPCAB with TTFM on ITA, RA and GEA | Abnormal | Low MGF <3 ml/min or high PI (>20.0) | 12.0% (n = 14 grafts) |
| Leong et al. [34] | 2005 | Prospective | 322/116 | TTFM on ITA and SVG | Revision | Low MGF, high PI and unsatisfactory flow curve (values not specified) due to occluded, stretched, kinked/twisted grafts or anastomotic stenosis | 2.2% (n = 7 grafts) |
| 5.2% (n = 6 patients) | |||||||
| Onorati et al. [35] | 2005 | Prospective | …/297 | TTFM on ITA and RA | Abnormal | Low MGF and high PI, without systolic peak pattern on the flow curves | 2.4% (n = 7 patients) |
| Revision | Systolic wave-pattern, low MGF (9 ml/min) and high PI | 0.3% (n = 1 patient) | |||||
| Bergsland et al. [36] | 2004 | Prospective | 113/46 | OPCAB with TTFM on ITA and SVG | Revision | Abnormal MGF in 5 grafts due to distal anastomosis problems (n = 3), long grafts (n = 1) and vein graft stenosis (n = 1) | 4.4% (n = 5 grafts) |
| Gwozdziewicz [37] | 2004 | Prospective | …/50 | OPCAB with TTFM on ITA and SVG | Revision | Grafts with low MGF and high PI (>5) | 0.0% (n = 0 grafts) |
| 0.0% (n = 0 patients) | |||||||
| Guden et al. [38] | 2003 | RCT | …/300 | TTFM on ITA | Revision | MGF close to 0 ml/min and PI >5.0, due to intimal flaps and localized dissections at anastomosis site | 1.3% (n = 4 grafts) |
| Sanisoglu et al. [39] | 2003 | Prospective | 49/20 | OPCAB with TTFM on ITA and SVG | Revision | Graft failure based on low MGF (5.2 ml/min) and high PI (11.9) | 5.0% (n = 1 grafts) |
| 2.0% (n = 1 patients) | |||||||
| Groom et al. [40] | 2001 | Prospective | 298/125 | TTFM in ITA and SVG | Revision | Low MGF and/or high PI (not specified) | 3.0% (n = 9 grafts) |
| 7.2% (n = 9 patients) | |||||||
| D’Ancona et al. [41] | 2000 | Prospective | 1145/409 | OPCAB with TTFM on ITA and SVG | Revision | Abnormal systolic flow patterns, PI >5.0 and low MGF due to (i) kinking, (ii) coronary dissection or (iii) thrombosis/stenosis at the anastomosis site | 3.5% (n = 41 grafts) |
| 7.9% (n = 33 patients) | |||||||
| Jakobsen and Kjaergard [42] | 1999 | Prospective | …/280 | TTFM on ITA and SVG | Abnormal | MGF <10 ml/min due to kinking, rotation or occlusion | 1.8% (n = 5 grafts) |
| Walpoth et al. [43] | 1998 | Prospective | 46/46 | TTFM on ITA | Abnormal | Low-flow through ITA-graft (<0.5 ± 0.7 ml/min), high PI (147 ± 96) and elevated vascular resistance | 6.5% (n = 3 grafts) 6.5% (n = 3 patients) |
| Revision | 1 distal ITA dissection, 1 ITA intramural haematoma and 1 abnormal ECG and poor LV-anterior wall contractility | 6.5% (n = 3 grafts) 6.5% (n = 3 patients) | |||||
| Canver and Dame [44] | 1994 | Prospective | …/63 | TTFM on ITA | Abnormal | Absence of ITA flow due to twisting at the anastomosis site | 3.2% (n = 2 patients) |
Results are presented as percentages with the number of grafts and (if available) by the number of patients.
On-pump unless specified.
No specification on which grafts were assessed by TTFM.
CAG: coronary angiography; DF: diastolic filling; ECG: electrocardiogram; GEA: gastroepiploic artery; HR-ECUS: high-resolution-epicardial ultrasonography; IFI: intraoperative fluorescence imaging; ITA: internal thoracic artery; LITA: left internal thoracic artery; LV: left ventricular; MAP: mean arterial pressure; MGF: mean graft flow; OPCAB: off-pump coronary artery bypass; PI: pulsatility index; RA: radial artery; RCT: randomized controlled trial; SVG: saphenous vein graft; TTFM: transit-time flow measurement.
Meta-analysis: abnormal grafts and graft revisions
Individual studies classified grafts as abnormal based either on low MGF (arterial grafts: <15 ml/min and venous grafts: <20 ml/min), an increased PI ≥5 for both venous and arterial grafts, or decreased diastolic filling % (<50%). Overall reasons to revise abnormal grafts were due to kinking or twisting of a graft, graft or coronary dissection or anastomotic stenosis/thrombosis.
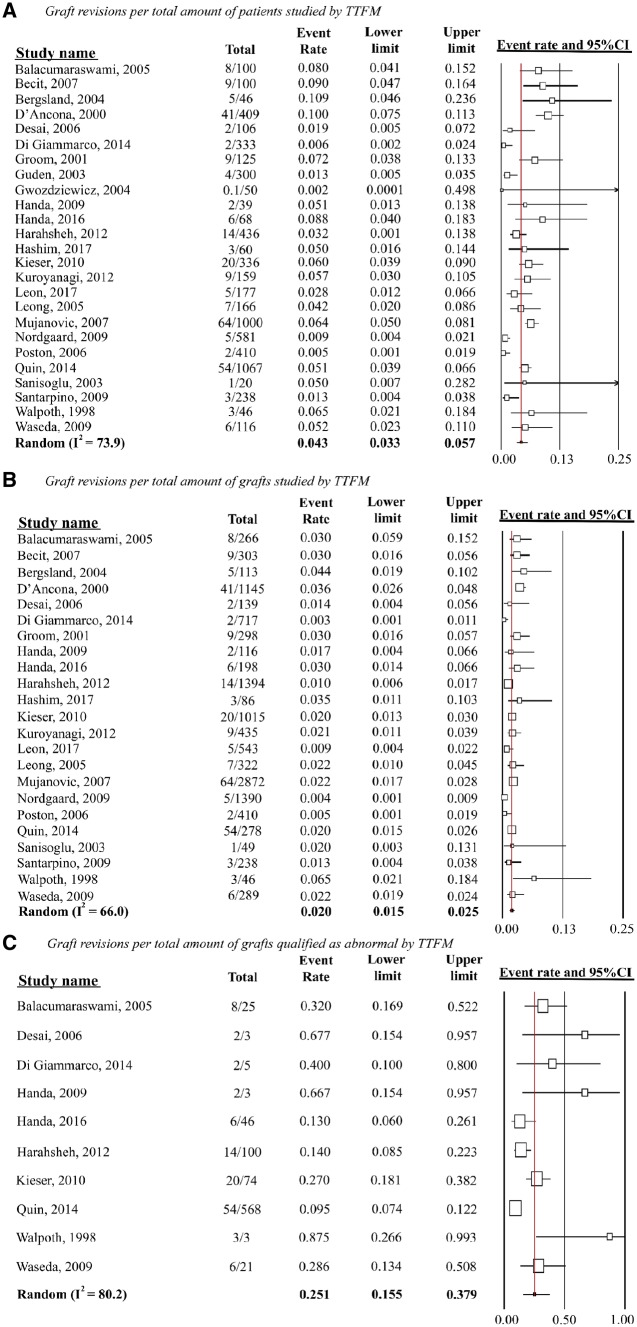
In 25 studies (n = 6488), the pooled rate of graft revisions was 4.3% when estimated per patient (95% CI 3.3–5.7%; I2 = 73.9), and 2.0% (95% CI 1.5–2.5%; I2 = 66.0) in 23 studies with 12 662 grafts when estimated per graft (Fig. 2). The pooled rate of graft revision among abnormal classified grafts was 25.1% (95% CI 15.5–37.9%; I2 = 80.2) among 10 studies that reported both the number of abnormal grafts and graft revisions. Main reasons for not revising abnormal classified grafts were (i) no suspicion on graft failure after careful surgical inspection or (ii) no better alternative due to bad quality of the native coronary arteries.
Figure 2:
Random-effects models on pooled TTFM study outcomes. (A) Graft revision per total amount of patients studied by TTFM, (B) graft revisions per total amount of grafts studied by TTFM and (C) graft revisions per total amount of grafts qualified as abnormal by TTFM. I2 indicates heterogeneity (range 0–100; 0 being entirely homogenous). CI: confidence intervals; TTFM: transit-time flow measurement.
Diagnostic accuracy
Sensitivity rates, describing the accuracy of TTFM, ranged from 0.250 to 0.457 (Supplementary Material, Table S2). Specificity varied between 0.941 and 0.984 [14, 17, 30]. The probability of having adequate TTFM values with an open graft (e.g. NPV) ranged from 0.719 to 0.980. The probability of having abnormal TTFM values with failing graft (e.g. PPV) varied from 0.100 to 0.840 [14, 17, 30]. These NPV and PPV values were based on the outcomes of angiography performed intraoperatively or on the 4th postoperative day.
Graft patency outcomes
Thirty-two studies evaluated graft patency according to TTFM values, summarized in Supplementary Material, Table S3 [7, 12, 14–17, 30, 38, 44–68]. Currently, only one small, randomized clinical trial assigned patients to undergo isolated CABG with or without TTFM and/or IFI [69]. The Graft Imaging to Improve Patency (GRIIP) study randomized 156 patients and performed a follow-up CAG at 1 year. No differences were found in the rate of graft occlusion on CAG (30.9% vs 28.9%, imaging vs control, respectively, P = 0.82) [69]. Several observational studies reported that abnormal TTFM parameters predicted graft failure at 6 months to 1 year [7, 54, 70]. One study reported no predictive correlation between TTFM parameters and angiographic graft stenosis at 3 and 12 months [65].
Studies reporting on TTFM cut-off values which predict graft patency found that predictors of early graft failure were a PI >5.85 and MGF <20 ml/min for venous grafts, and an MGF of <11.5 ml/min for arterial grafts [15, 46]. Lehnert et al. [45] found internal thoracic artery graft patency at 1 year to be significantly worse when MGF <20 ml/min and worsening with 4% failure with every 1 ml/min decrease in MGF [odds ratio (OR) 0.96, 95% CI 0.93–0.99; P = 0.005]. Une et al. [71] reported that a higher MGF was an independent predictor of great saphenous vein failure at 1-year follow-up (OR 0.95, 95% CI 0.91–0.99), with an optimal cut-off value of 31 ml/min. The NPV on intermediate-term graft patency (156 days) in relation to abnormal TTFM values was 0.890 (Supplementary Material, Table S2) [59]. Follow-up on venous graft patency at 3 years showed that MGF was significantly higher among patent grafts versus failing grafts (41.3 ± 22.9 ml/min vs 29.6 ± 18.7 ml/min, respectively; P = 0.01) [55].
Short-term clinical outcomes
Twelve studies evaluated short-term outcomes in relation with TTFM parameters (Supplementary Material, Table S4) [8, 13, 25, 27, 54, 62, 69, 71–75]. Bauer et al. [75] compared CABG with TTFM versus without TTFM and found an increased rate of intraoperative redo-anastomoses, which coincided with significantly lower incidences of ventricular fibrillation, perioperative MI and postoperative mortality when TTFM was performed. Furthermore, another study found that CABG with TTFM resulted in lower rates of postoperative mortality (0% vs 4%), MI (0% vs 5%), intra-aortic balloon pump placement (1% vs 7%) and overall morbidity (6% vs 16%, all P < 0.05) [27]. Jokinen et al. [54] did not confirm the predictive capability of TTFM for these outcomes.
Studies that assessed TTFM cut-off values demonstrated that a PI >5, in arterial and venous grafts, was an independent predictor of early (30 days) major adverse cardiac events with an OR ranging from 1.8 (95% CI 1.1–2.7, P = 0.0097) [25] to 4.23 (95% CI 1.69–10.59, P = 0.002) [8]. Yet, these abnormal TTFM values did not predict mid-term mortality or hospital readmission [25].
A study which evaluated off-pump versus on-pump CABG found lower overall TTFM values to be associated with an increased incidence of low cardiac output syndrome (P = 0.049). Off-pump surgery was not associated with higher PI or lower diastolic filling %, and no differences were found in 30-day mortality and MI between patients who underwent off-pump versus on-pump CABG [72]. One study reported higher MGF and lower PI in off-pump procedures [62], while another study showed no differences in TTFM parameters between on-pump and off-pump [52].
Clinical outcomes during follow-up
The GRIPP trial found no differences in the composite of death, MI and repeat revascularization at 1 year in patients who underwent CABG with intraoperative graft flow assessment versus those without intraoperative graft flow assessment (7.7% vs 7.7%, respectively) [69]. However, observational studies showed the positive predictive capability of TTFM on intermediate-term clinical outcomes, such as major adverse cardiac events, mortality, intra-aortic balloon pump placement or cardiac enzyme release [8, 75]. Other studies reported data that showed no correlation between TTFM parameters and mid-term hospital readmission (during 1.8-year follow-up) [25], survival after 3.8 years [74] or even long-term survival (7.8 ± 0.2 years, Supplementary Material, Table S4) [13].
DISCUSSION
This systematic review and meta-analysis provides an overview of the literature on intraoperative graft flow assessment by TTFM. We found that 4.3% of patients undergoing CABG required graft revisions based on TTFM parameters. However, of all grafts that were classified as abnormal, only 25% of grafts were revised. The surgeons’ clinical interpretation of the graft and the quality of the anastomosis in respect to the quality of the native coronary system was the main reason for not revising all these grafts. Indeed, the reported sensitivity of TTFM was fairly low, ranging from 0.250 to 0.457 with TTFM reported specificity varying from 0.941 to 0.984. Nevertheless, abnormal TTFM parameters were associated with postoperative mortality and MI, and showed to be of particular importance in predicting graft patency during follow-up.
Intraoperative graft flow assessment is currently most frequently performed by TTFM, as it performs well compared to other methods of intraoperative graft quality assessment, such as thermal CAG, IFI or CAG. Although IFI is associated with higher sensitivity and specificity compared to TTFM [30], major limitations of IFI consist of not being able to visualize the entire graft at once and the need to reposition the heart for the laser camera to capture the immunofluorescent flow, possibly compromising natural blood flow. While intraoperative CAG would be ideal to assess graft patency and anastomotic quality, this strategy requires a ‘hybrid’ operating theatre that is not common in all institutions.
In this meta-analysis, we found that graft revisions were required in 2.0% of grafts and in 4.3% of patients undergoing CABG. Compared to other intraoperative complication rates, such as stroke at 1.1%, this provides the ability to significantly improve short-term outcomes, considering that TTFM usage led to graft revision and may have prevented a perioperative MI or increased cardiac enzyme release which is associated with impaired long-term outcomes [76].
So far, no randomized controlled trial focusing exclusively on CABG with TTFM versus without TTFM has been published. Only one small randomized controlled trial primarily studied IFI in combination with TTFM (n = 78) versus without intraoperative graft assessment (n = 78) [69]. No differences existed in intraoperative graft revisions, perioperative adverse events, 1-year graft patency or clinical outcomes. However, only 1.7% of the grafts were studied with TTFM exclusively, and thus, the study provides limited information on the actual impact of TTFM. Larger trials evaluating the benefit of routinely performing TTFM on early and late clinical outcomes are warranted.
Observational study data on the impact of TTFM are essential before randomized data will be available. So far, numerous studies reported improved outcomes in patients undergoing CABG with TTFM and only a few reporting no association between TTFM and postoperative outcomes. However, a great diversity in different TTFM cut-off values and methods exists. Studies used in our meta-analysis applied different methods of performing TTFM, including varying surgical and clinical scenarios, such as on-pump or off-pump procedures, varying haemodynamics, venous versus arterial grafts, different locations and number of coronary stenosis or using single versus sequential anastomoses. All of these factors have a major influence on coronary flow and thereby on TTFM parameters. Furthermore, reasons for surgeons not to revise a graft despite abnormal TTFM parameters were that after inspecting the anastomosis, no suspicion of an anastomotic problem or graft failure was raised, or that there was no better surgical alternative due to poor native coronary arteries. Lacking standardized methods of performing and interpreting TTFM parameters, in combination with non-existent standardized TTFM cut-off values, still introduces a subjective aspect to TTFM and whether a graft should be revised. The heterogeneity of study methods and study outcomes could have contributed to the varying results on the diagnostic accuracy of TTFM (Supplementary Material, Table S2). This may also have contributed to the statistical heterogeneity (I2 ≥ 66).
The strength of TTFM is that it is able to detect truly failing and truly patent grafts (true positives and true negatives, respectively). False positives (e.g. patent graft with high PI) rarely occur; however, difficulties exist in detecting poor grafts with a low PI or high MGF (false negatives), which could lead to unnecessary graft revisions [14]. Therefore, it remains challenging to interpret TTFM results and translate it into decision-making during each CABG procedure. Di Giammarco et al. [77] showed that the diagnostic accuracy of TTFM increased to 100% NPV and 100% PPV when it was combined with HR-ECUS. Adding HR-ECUS to TTFM thereby overcomes the relatively modest diagnostic accuracy of TTFM alone. By including HR-ECUS to the surgeon’s appraisal of graft and anastomotic quality, in relation to native coronary targets and run-off, the best surgical and clinical outcomes for patients undergoing CABG could be ensured. Besides, HR-ECUS with TTFM could provide beneficial insights for young surgeons to further improve their surgical techniques.
Standardization on how to perform TTFM, what TTFM values to expect for specific grafts and anastomoses and which cut-offs to use for graft revision are essential to increase the use of TTFM amongst surgeons. Moreover, the studies included in current meta-analysis are of moderate quality, according to the NOS criteria. Surgeons may not be therefore convinced to use TTFM. The prospective, multicentre REQUEST registry collected information on standardized TTFM and ultrasound assessments in patients undergoing CABG (n = 1046, ClincalTrials.gov: NCT02385344). This registry could provide crucial information on how to incorporate TTFM in daily clinical practice by providing insights into whether TTFM is effective and improves outcomes in patients undergoing CABG. Furthermore, it could quantify potential benefits of combining HR-ECUS with TTFM on graft and anastomosis quality assessment.
Finally, a clinical issue that remains is that long-term graft failure may still occur as a result of other mechanisms than those controlled by TTFM. This could be one of the reasons why surgeons doubt its clinical impact and consequently why routine use of TTFM has been limited. Other factors that potentially influence the adoption rate of intraoperative quality assessment are (i) adequately interpreting and acting upon TTFM determinants come with a learning curve, (ii) the time of the procedure might increase (e.g. depending on the need to revise a graft) and (iii) concerns might remain of needlessly revising a patent graft (e.g. due to limited diagnostic accuracy of TTFM alone) [17, 78]. Furthermore, no high-quality data on the impact of TTFM on surgical and clinical outcomes after CABG exist that could influence the adoption rate of TTFM by individual surgeons. Nevertheless, this systematic review does show that TTFM provides valuable intraoperative data on graft and anastomotic quality, which could contribute to improved surgical and clinical outcomes. Despite potential shortcomings, the 2018 ESC/EACTS Guidelines on myocardial revascularization gave TTFM for intraoperative graft assessment a class-IIa recommendation [79].
Limitations
As with any meta-analysis of observational studies, limitations related to the observational nature of studies cannot be overcome. One important challenge is that, currently, no consensus exists on uniform TTFM cut-off values to classify grafts as ‘abnormal’ or requiring revision. This could have caused the relatively increased heterogeneity of the results. To allow a conservative estimate, we have analysed the data using random-effects models, as recommended for meta-analyses on observational studies [80]. However, considering the heterogeneity of study definitions and end points in papers reporting the association between TTFM and graft patency and clinical outcomes, no meta-analysis was performed on these outcomes, as it was considered to be inappropriate to pool heterogeneous results.
CONCLUSION
TTFM has potential to further improve the quality of CABG procedures and could improve clinical outcomes for patients. In 4.3% of patients undergoing CABG, there was a need to revise grafts after TTFM assessment. However, only 25% of grafts, classified as abnormal based on TTFM values, were revised, suggesting that the use of TTFM can be further optimized. Indeed, reaching consensus on TTFM remains difficult due to the substantial heterogeneity in published TTFM data, which could be related to varying haemodynamics during assessment, the location of the TTFM probe (e.g. proximal or distal on the graft), varying cut-off values for revision and the use of different graft types (e.g. internal thoracic artery, saphenous vein or radial artery) on unique coronary arteries with varying degrees of stenosis. Future studies should focus on determining the optimal use of TTFM and thereby further guiding surgeons to improve outcomes after CABG. A multicentre study with standardized TTFM use and definitions on graft revision may provide more insights into the optimal use of this technique.
Conflict of interest: Daniel J.F.M. Thuijs, Margreet W.A. Bekker, David P. Taggart, A. Pieter Kappetein, Teresa M. Kieser, Daniel Wendt, Gabriele Di Giammarco, John D. Puskas and Stuart J. Head received travelling support and/or speaking fees from Medistim ASA, Oslo, Norway. A. Pieter Kappetein and Stuart J. Head report to work as employees of Medtronic, outside the submitted work. Gregory D. Trachiotis declares no conflict of interest.
Supplementary Material
REFERENCES
- 1. Head SJ, Kieser TM, Falk V, Huysmans HA, Kappetein AP.. Coronary artery bypass grafting: part 1—the evolution over the first 50 years. Eur Heart J 2013;34:2862–72. [DOI] [PubMed] [Google Scholar]
- 2. Head SJ, Milojevic M, Daemen J, Ahn JM, Boersma E, Christiansen EH.. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939–48. [DOI] [PubMed] [Google Scholar]
- 3. Head SJ, Borgermann J, Osnabrugge RL, Kieser TM, Falk V, Taggart DP. et al. Coronary artery bypass grafting: part 2—optimizing outcomes and future prospects. Eur Heart J 2013;34:2873–86. [DOI] [PubMed] [Google Scholar]
- 4. Balacumaraswami L, Taggart DP.. Intraoperative imaging techniques to assess coronary artery bypass graft patency. Ann Thorac Surg 2007;83:2251–7. [DOI] [PubMed] [Google Scholar]
- 5. Fabricius AM, Gerber W, Hanke M, Garbade J, Autschbach R, Mohr FW.. Early angiographic control of perioperative ischemia after coronary artery bypass grafting. Eur J Cardiothorac Surg 2001;19:853–8. [DOI] [PubMed] [Google Scholar]
- 6. Mack MJ. Intraoperative coronary graft assessment. Curr Opin Cardiol 2008;23:568–72. [DOI] [PubMed] [Google Scholar]
- 7. Kitamura H, Okabayashi H, Hanyu M, Soga Y, Nomoto T, Johno H. et al. Early and midterm patency of the proximal anastomoses of saphenous vein grafts made with a symmetry aortic connector system. J Thorac Cardiovasc Surg 2005;130:1028–31. [DOI] [PubMed] [Google Scholar]
- 8. Kieser TM, Rose S, Kowalewski R, Belenkie I.. Transit-time flow predicts outcomes in coronary artery bypass graft patients: a series of 1000 consecutive arterial grafts. Eur J Cardiothorac Surg 2010;38:155–62. [DOI] [PubMed] [Google Scholar]
- 9. Niclauss L. Techniques and standards in intraoperative graft verification by transit time flow measurement after coronary artery bypass graft surgery: a critical review. Eur J Cardiothorac Surg 2017;51:26–33. [DOI] [PubMed] [Google Scholar]
- 10. Wells GSB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (16 January 2019, date last accessed).
- 11. Hashim SA, Amin MA, Nair A, Raja Mokhtar RA, Krishnasamy S, Cheng K.. A flowmeter technique to exclude internal mammary artery anastomosis error in an arrested heart. Heart Lung Circ 2018;27:e59–63. [DOI] [PubMed] [Google Scholar]
- 12. Hiraoka A, Fukushima S, Miyagawa S, Yoshikawa Y, Saito S, Domae K. et al. Quantity and quality of graft flow in coronary artery bypass grafting is associated with cardiac computed tomography study-based anatomical and functional parameters. Eur J Cardiothorac Surg 2017;52:909–16. [DOI] [PubMed] [Google Scholar]
- 13. Leon M, Stanham R, Soca G, Dayan V.. Do flow and pulsatility index within the accepted ranges predict long-term outcomes after coronary artery bypass grafting? Thorac Cardiovasc Surg 2017; doi: 10.1055/s-0037-1600116 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Handa T, Orihashi K, Nishimori H, Yamamoto M.. Maximal blood flow acceleration analysis in the early diastolic phase for aortocoronary artery bypass grafts: a new transit-time flow measurement predictor of graft failure following coronary artery bypass grafting. Surg Today 2016;46:1325–33. [DOI] [PubMed] [Google Scholar]
- 15. Oshima H, Tokuda Y, Araki Y, Ishii H, Murohara T, Ozaki Y. et al. Predictors of early graft failure after coronary artery bypass grafting for chronic total occlusion. Interact CardioVasc Thorac Surg 2016;23:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honda K, Okamura Y, Nishimura Y, Uchita S, Yuzaki M, Kaneko M. et al. Graft flow assessment using a transit time flow meter in fractional flow reserve-guided coronary artery bypass surgery. J Thorac Cardiovasc Surg 2015;149:1622–8. [DOI] [PubMed] [Google Scholar]
- 17. Di Giammarco G, Canosa C, Foschi M, Rabozzi R, Marinelli D, Masuyama S. et al. Intraoperative graft verification in coronary surgery: increased diagnostic accuracy adding high-resolution epicardial ultrasonography to transit-time flow measurement. Eur J Cardiothorac Surg 2014;45:e41–5. [DOI] [PubMed] [Google Scholar]
- 18. Quin J, Lucke J, Hattler B, Gupta S, Baltz J, Bishawi M. et al. Surgeon judgment and utility of transit time flow probes in coronary artery bypass grafting surgery. JAMA Surg 2014;149:1182–7. [DOI] [PubMed] [Google Scholar]
- 19. Harahsheh B. Transit time flowmetry in coronary artery bypass grafting-experience at Queen Alia Heart Institute, Jordan. Oman Med J 2012;27:475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuroyanagi S, Asai T, Suzuki T.. Intraoperative fluorescence imaging after transit-time flow measurement during coronary artery bypass grafting. Innovations 2012;7:435–40. [DOI] [PubMed] [Google Scholar]
- 21. Handa T, Katare RG, Sasaguri S, Sato T.. Preliminary experience for the evaluation of the intraoperative graft patency with real color charge-coupled device camera system: an advanced device for simultaneous capturing of color and near-infrared images during coronary artery bypass graft. Interact CardioVasc Thorac Surg 2009;9:150–4. [DOI] [PubMed] [Google Scholar]
- 22. Nordgaard H, Vitale N, Haaverstad R.. Transit-time blood flow measurements in sequential saphenous coronary artery bypass grafts. Ann Thorac Surg 2009;87:1409–15. [DOI] [PubMed] [Google Scholar]
- 23. Santarpino G, Onorati F, Scalas C, De Gori M, Cristodoro L, Zofrea S. et al. Radial artery achieves better flowmetric results than saphenous vein in the elderly. Heart Vessels 2009;24:108–15. [DOI] [PubMed] [Google Scholar]
- 24. Waseda K, Ako J, Hasegawa T, Shimada Y, Ikeno F, Ishikawa T. et al. Intraoperative fluorescence imaging system for on-site assessment of off-pump coronary artery bypass graft. JACC Cardiovasc Imaging 2009;2:604–12. [DOI] [PubMed] [Google Scholar]
- 25. Herman C, Sullivan JA, Buth K, Legare JF.. Intraoperative graft flow measurements during coronary artery bypass surgery predict in-hospital outcomes. Interact CardioVasc Thorac Surg 2008;7:582–5. [DOI] [PubMed] [Google Scholar]
- 26. Onorati F, Santarpino G, Lerose MA, Impiombato B, Mastroroberto P, Renzulli A.. Intraoperative behavior of arterial grafts in the elderly and the young: a flowmetric systematic analysis. Heart Vessels 2008;23:316–24. [DOI] [PubMed] [Google Scholar]
- 27. Becit N, Erkut B, Ceviz M, Unlu Y, Colak A, Kocak H.. The impact of intraoperative transit time flow measurement on the results of on-pump coronary surgery. Eur J Cardiothorac Surg 2007;32:313–18. [DOI] [PubMed] [Google Scholar]
- 28. Mujanovic E, Kabil E, Bergsland J.. Transit time flowmetry in coronary surgery—an important tool in graft verification. Bosn J Basic Med Sci 2007;7:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onorati F, Pezzo F, Esposito A, Impiombato B, Comi MC, Polistina M. et al. Single versus sequential saphenous vein grafting of the circumflex system: a flowmetric study. Scand Cardiovasc J 2007;41:265–71. [DOI] [PubMed] [Google Scholar]
- 30. Desai ND, Miwa S, Kodama D, Koyama T, Cohen G, Pelletier MP. et al. A randomized comparison of intraoperative indocyanine green angiography and transit-time flow measurement to detect technical errors in coronary bypass grafts. J Thorac Cardiovasc Surg 2006;132:585–94. [DOI] [PubMed] [Google Scholar]
- 31. Poston RS, Gu J, Brown JM, Gammie JS, White C, Nie L. et al. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2006;131:122–30. [DOI] [PubMed] [Google Scholar]
- 32. Balacumaraswami L, Abu-Omar Y, Choudhary B, Pigott D, Taggart DP.. A comparison of transit-time flowmetry and intraoperative fluorescence imaging for assessing coronary artery bypass graft patency. J Thorac Cardiovasc Surg 2005;130:315–20. [DOI] [PubMed] [Google Scholar]
- 33. Kim KB, Kang CH, Lim C.. Prediction of graft flow impairment by intraoperative transit time flow measurement in off-pump coronary artery bypass using arterial grafts. Ann Thorac Surg 2005;80:594–8. [DOI] [PubMed] [Google Scholar]
- 34. Leong DK, Ashok V, Nishkantha A, Shan YH, Sim EK.. Transit-time flow measurement is essential in coronary artery bypass grafting. Ann Thorac Surg 2005;79:854.. [DOI] [PubMed] [Google Scholar]
- 35. Onorati F, Olivito S, Mastroroberto P, di Virgilio A, Esposito A, Perrotti A. et al. Perioperative patency of coronary artery bypass grafting is not influenced by off-pump technique. Ann Thorac Surg 2005;80:2132–40. [DOI] [PubMed] [Google Scholar]
- 36. Bergsland J, Hol PK, Lingas PS, Lundblad R, Rein KA, Andersen R. et al. Intraoperative and intermediate-term angiographic results of coronary artery bypass surgery with symmetry proximal anastomotic device. J Thorac Cardiovasc Surg 2004;128:718–23. [DOI] [PubMed] [Google Scholar]
- 37. Gwozdziewicz M. Cardiomed coronary flow meter for prevention of early occlusion in aortocoronary bypass grafting. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2004;148:59–61. [DOI] [PubMed] [Google Scholar]
- 38. Guden M, Sanisoglu I, Sagbas E, Ergenoglu MU, Ozbek U, Akpinar B.. Hemodilution during off-pump coronary artery bypass grafting: can we improve flow and reduce hypercoagulability? Heart Surg Forum 2003;6:399–402. [PubMed] [Google Scholar]
- 39. Sanisoglu I, Guden M, Balci C, Sagbas E, Duran C, Akpinar B.. Comparison of intraoperative transit-time flow measurement with early postoperative magnetic resonance flow mapping in off-pump coronary artery surgery. Tex Heart Inst J 2003;30:31–7. [PMC free article] [PubMed] [Google Scholar]
- 40. Groom R, Tryzelaar J, Forest R, Niimi K, Cecere G, Donegan D. et al. Intra-operative quality assessment of coronary artery bypass grafts. Perfusion 2001;16:511–18. [DOI] [PubMed] [Google Scholar]
- 41. D’Ancona G, Karamanoukian HL, Ricci M, Schmid S, Bergsland J, Salerno TA.. Graft revision after transit time flow measurement in off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg 2000;17:287–93. [DOI] [PubMed] [Google Scholar]
- 42. Jakobsen HL, Kjaergard HK.. Severe impairment of graft flow without electrocardiographic changes during coronary artery bypass grafting. Scand Cardiovasc J 1999;33:157–9. [DOI] [PubMed] [Google Scholar]
- 43. Walpoth BH, Bosshard A, Genyk I, Kipfer B, Berdat PA, Hess OM. et al. Transit-time flow measurement for detection of early graft failure during myocardial revascularization. Ann Thorac Surg 1998;66:1097–100. [DOI] [PubMed] [Google Scholar]
- 44. Canver CC, Dame NA.. Ultrasonic assessment of internal thoracic artery graft flow in the revascularized heart. Ann Thorac Surg 1994;58:135–8. [DOI] [PubMed] [Google Scholar]
- 45. Lehnert P, Moller CH, Damgaard S, Gerds TA, Steinbruchel DA.. Transit-time flow measurement as a predictor of coronary bypass graft failure at one year angiographic follow-up. J Card Surg 2015;30:47–52. [DOI] [PubMed] [Google Scholar]
- 46. Takazawa A, Nakajima H, Iguchi A, Tabata M, Morita K, Koike H. et al. Impacts of intraoperative flow on graft patency of sequential and individual saphenous vein grafts. Innovations (Phila) 2015;10:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uehara M, Muraki S, Takagi N, Yanase Y, Tabuchi M, Tachibana K. et al. Evaluation of gastroepiploic arterial grafts to right coronary artery using transit-time flow measurement. Eur J Cardiothorac Surg 2015;47:459–63. [DOI] [PubMed] [Google Scholar]
- 48. Jelenc M, Jelenc B, Klokocovnik T, Lakic N, Gersak B, Knezevic I.. Understanding coronary artery bypass transit time flow curves: role of bypass graft compliance. Interact CardioVasc Thorac Surg 2014;18:164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Milani R, Moraes D, Sanches A, Jardim R, Lumikoski T, Miotto G. et al. Analysis of transit time flow of the right internal thoracic artery anastomosed to the left anterior descending artery compared to the left internal thoracic artery. Rev Bras Cir Cardiovasc 2014;29:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Genoni M, Odavic D, Loblein H, Dzemali O.. Use of the eSVS Mesh: external vein support does not negatively impact early graft patency. Innovations (Phila) 2013;8:211–14. [DOI] [PubMed] [Google Scholar]
- 51. Walker PF, Daniel WT, Moss E, Thourani VH, Kilgo P, Liberman HA. et al. The accuracy of transit time flow measurement in predicting graft patency after coronary artery bypass grafting. Innovations (Phila) 2013;8:416–19. [DOI] [PubMed] [Google Scholar]
- 52. Cerqueira Neto FM, Guedes MA, Soares LE, Almeida GS, Guimaraes AR, Barreto MA. et al. Flowmetry of left internal thoracic artery graft to left anterior descending artery: comparison between on-pump and off-pump surgery. Rev Bras Cir Cardiovasc 2012;27:283–9. [DOI] [PubMed] [Google Scholar]
- 53. Balkhy HH, Wann LS, Krienbring D, Arnsdorf SE.. Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg 2011;92:821–7. [DOI] [PubMed] [Google Scholar]
- 54. Jokinen JJ, Werkkala K, Vainikka T, Perakyla T, Simpanen J, Ihlberg L.. Clinical value of intra-operative transit-time flow measurement for coronary artery bypass grafting: a prospective angiography-controlled study. Eur J Cardiothorac Surg 2011;39:918–23. [DOI] [PubMed] [Google Scholar]
- 55. Kim HJ, Lee TY, Kim JB, Cho WC, Jung SH, Chung CH. et al. The impact of sequential versus single anastomoses on flow characteristics and mid-term patency of saphenous vein grafts in coronary bypass grafting. J Thorac Cardiovasc Surg 2011;141:750–4. [DOI] [PubMed] [Google Scholar]
- 56. Takami Y, Tajima K, Terazawa S, Okada N, Fujii K, Sakai Y.. Transit-time flow characteristics of in situ right gastroepiploic arterial grafts in coronary artery bypass grafting. J Thorac Cardiovasc Surg 2009;138:669–73. [DOI] [PubMed] [Google Scholar]
- 57. Tokuda Y, Song MH, Oshima H, Usui A, Ueda Y.. Predicting midterm coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg 2008;86:532–6. [DOI] [PubMed] [Google Scholar]
- 58. Hatada A, Yoshimasu T, Kaneko M, Kawago M, Yuzaki M, Honda K. et al. Relation of waveform of transit-time flow measurement and graft patency in coronary artery bypass grafting. J Thorac Cardiovasc Surg 2007;134:789–91. [DOI] [PubMed] [Google Scholar]
- 59. Hol PK, Andersen K, Skulstad H, Halvorsen PS, Lingaas PS, Andersen R. et al. Epicardial ultrasonography: a potential method for intraoperative quality assessment of coronary bypass anastomoses? Ann Thorac Surg 2007;84:801–7. [DOI] [PubMed] [Google Scholar]
- 60. Tokuda Y, Song MH, Ueda Y, Usui A, Akita T.. Predicting early coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg 2007;84:1928–33. [DOI] [PubMed] [Google Scholar]
- 61. Di Giammarco G, Pano M, Cirmeni S, Pelini P, Vitolla G, Di Mauro M.. Predictive value of intraoperative transit-time flow measurement for short-term graft patency in coronary surgery. J Thorac Cardiovasc Sur 2006;132:468–74. [DOI] [PubMed] [Google Scholar]
- 62. Hassanein W, Albert AA, Arnrich B, Walter J, Ennker IC, Rosendahl U. et al. Intraoperative transit time flow measurement: off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2005;80:2155–61. [DOI] [PubMed] [Google Scholar]
- 63. Manchio JV, Gu J, Romar L, Brown J, Gammie J, Pierson RN 3rd. et al. Disruption of graft endothelium correlates with early failure after off-pump coronary artery bypass surgery. Ann Thorac Surg 2005;79:1991–8. [DOI] [PubMed] [Google Scholar]
- 64. Hirotani T, Kameda T, Shirota S, Nakao Y.. An evaluation of the intraoperative transit time measurements of coronary bypass flow. Eur J Cardiothorac Surg 2001;19:848–52. [DOI] [PubMed] [Google Scholar]
- 65. Hol PK, Fosse E, Mork BE, Lundblad R, Rein KA, Lingaas PS. et al. Graft control by transit time flow measurement and intraoperative angiography in coronary artery bypass surgery. Heart Surg Forum 2001;4:254–7; discussion 57–8. [PubMed] [Google Scholar]
- 66. Takami Y, Ina H.. Relation of intraoperative flow measurement with postoperative quantitative angiographic assessment of coronary artery bypass grafting. Ann Thorac Surg 2001;72:1270–4. [DOI] [PubMed] [Google Scholar]
- 67. Takami Y, Ina H.. A simple method to determine anastomotic quality of coronary artery bypass grafting in the operating room. Cardiovasc Surg 2001;9:499–503. [DOI] [PubMed] [Google Scholar]
- 68. Handa T, Orihashi K, Nishimori H, Fukutomi T, Yamamoto M, Kondo N. et al. Maximal blood flow acceleration analysis in the early diastolic phase for in situ internal thoracic artery bypass grafts: a new transit-time flow measurement predictor of graft failure following coronary artery bypass grafting. Interact CardioVasc Thorac Surg 2015;20:449–57. [DOI] [PubMed] [Google Scholar]
- 69. Singh SK, Desai ND, Chikazawa G, Tsuneyoshi H, Vincent J, Zagorski BM. et al. The Graft Imaging to Improve Patency (GRIIP) clinical trial results. J Thorac Cardiovasc Surg 2010;139:294–301.e1. [DOI] [PubMed] [Google Scholar]
- 70. Di Giammarco G, Pano M, Cirmeni S, Pelini P, Vitolla G, Di Mauro M.. Predictive value of intraoperative transit-time flow measurement for short-term graft patency in coronary surgery. J Thorac Cardiovasc Surg 2006;132:468–74. [DOI] [PubMed] [Google Scholar]
- 71. Une D, Deb S, Chikazawa G, Kommaraju K, Tsuneyoshi H, Karkhanis R. et al. Cut-off values for transit time flowmetry: are the revision criteria appropriate? J Card Surg 2013;28:3–7. [DOI] [PubMed] [Google Scholar]
- 72. Forcillo J, Noiseux N, Dubois MJ, Mansour S, Prieto I, Basile F. et al. Intra-operative graft blood flow measurements for composite and sequential coronary artery bypass grafting. Int J Artif Organs 2014;37:382–91. [DOI] [PubMed] [Google Scholar]
- 73. Song MH. Reappraisal of importance of the left internal mammary artery to the left anterior descending artery in improving mid-term outcome in patients with severe left ventricular dysfunction. Nagoya J Med Sci 2013;75:113–19. [PMC free article] [PubMed] [Google Scholar]
- 74. Beran E, Kapitan M, Machler H, Salaymeh L, Anelli-Monti M, Oberwalder P. et al. Accurate preoperative echocardiography has more impact on prediction of long-term mortality than intra-operatively measured flow in coronary bypass grafts. Eur J Cardiothorac Surg 2011;40:245–8. [DOI] [PubMed] [Google Scholar]
- 75. Bauer SF, Bauer K, Ennker IC, Rosendahl U, Ennker J.. Intraoperative bypass flow measurement reduces the incidence of postoperative ventricular fibrillation and myocardial markers after coronary revascularisation. Thorac Cardiovasc Surg 2005;53:217–22. [DOI] [PubMed] [Google Scholar]
- 76. Domanski MJ, Mahaffey K, Hasselblad V, Brener SJ, Smith PK, Hillis G. et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011;305:585–91. [DOI] [PubMed] [Google Scholar]
- 77. Di Giammarco G, Canosa C, Foschi M, Rabozzi R, Marinelli D, Masuyama S. et al. Intraoperative graft verification in coronary surgery: increased diagnostic accuracy adding high-resolution epicardial ultrasonography to transit-time flow measurement. Eur J Cardiothorac Surg 2013;45:e41–5. [DOI] [PubMed] [Google Scholar]
- 78. Kieser TM, Taggart DP.. The use of intraoperative graft assessment in guiding graft revision. Ann Cardiothorac Surg 2018;7:652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sousa-Uva M, Neumann F-J, Ahlsson A, Alfonso F, Banning AP, Benedetto U. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4–90. [DOI] [PubMed] [Google Scholar]
- 80. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.