Abstract
Aims
The development of novel and more effective medications for alcohol use disorder (AUD) is an important unmet medical need. Drug repositioning or repurposing is an appealing strategy to bring new therapies to the clinic because it greatly reduces the overall costs of drug development and expedites the availability of treatments to those who need them. Probenecid, p-(di-n-propylsulfamyl)-benzoic acid, is a drug used clinically to treat hyperuricemia and gout due to its activity as an inhibitor of the kidneys’ organic anion transporter that reclaims uric acid from urine. Probenecid also inhibits pannexin1 channels that are involved in purinergic neurotransmission and inflammation, which have been implicated in alcohol’s effects and motivation for alcohol. Therefore, we tested the effects of probenecid on alcohol intake in rodents.
Methods
We tested the effects of probenecid on operant oral alcohol self-administration in alcohol-dependent rats during acute withdrawal as well as in nondependent rats and in the drinking-in-the-dark (DID) paradigm of binge-like drinking in mice.
Results
Probenecid reduced alcohol intake in both dependent and nondependent rats and in the DID paradigm in mice without affecting water or saccharin intake, indicating that probenecid’s effect was selective for alcohol and not the result of a general reduction in reward.
Conclusions
These results raise the possibility that pannexin1 is a novel therapeutic target for the treatment of AUD. The clinical use of probenecid has been found to be generally safe, suggesting that it can be a candidate for drug repositioning for the treatment of AUD.
Probenecid, a drug currently used in the clinic to treat hyperuricemia, reduced alcohol intake in dependent and nondependent rats and binge-like drinking in mice. These results suggest that probenecid can be a candidate for drug repositioning for the treatment of alcohol use disorder (AUD).
INTRODUCTION
Alcohol is the most prevalent substance misused in the United States (SAMHSA, 2013). Approved medications for alcohol use disorder (AUD) have efficacy but are prescribed for fewer than 10% of US patients with AUD (Jonas et al., 2014). Thus, the development of novel and more efficacious medications for AUD is a pressing medical need (Litten et al., 2012).
Drug repositioning or repurposing is an appealing strategy to bring new therapies to the clinic because it greatly reduces the overall costs of drug development and expedites the availability of treatments to those who need them (Nosengo, 2016). We recently observed that the glycyrrhetinic acid derivative carbenoxolone (CBX; 3β-hydroxy-11-oxoolean-12-en-30-oic acid 3-hemisuccinate), a medication used to treat gastritis and peptic ulcer (Bertaccini and Coruzzi, 1985), reduced both dependent and nondependent alcohol intake in rodents (Sanna et al., 2016). CBX inhibits 11β-hydroxysteroid dehydrogenase (11β-HSD) isozymes, shaping cellular responses to glucocorticoids (Chapman et al., 2013), which are functionally involved in alcohol dependence in rats and AUD in humans (Vendruscolo et al., 2012, 2015; Repunte-Canonigo et al., 2015). In particular, 11β-HSD1 is broadly expressed in brain regions relevant to alcohol’s reinforcing properties, such as the amygdala (Pelletier et al., 2007). CBX also inhibits pannexin1 channels that contribute to adenosine triphosphate (ATP) release into the extracellular space (Dahl, 2015). Because probenecid was also shown to inhibit pannexin1 channels (Silverman et al., 2008), we tested its effect on alcohol intake, with the hypothesis that pannexin1 channel blockade may contribute to reducing alcohol drinking.
In the extracellular space, ATP is hydrolyzed to adenosine diphosphate (ADP) and adenosine, which contribute to the activation of purinergic receptors and neuroinflammation (Silverman et al., 2009; Velasquez and Eugenin, 2014). Alcohol has been shown to increase adenosine in the extracellular space, and adenosine neurotransmission contributes to alcohol intoxication and reinforcement (Ruby et al., 2010). ATP that is released into the extracellular space in turn inhibits pannexin1 channel opening (Dahl, 2015). Despite their controlled opening, pannexin1 channels have been shown to promote neuronal excitability after N-methyl-D-aspartate (NMDA) glutamate receptor activation (Thompson et al., 2008) and seizure activity (Dossi et al., 2018).
Probenecid is a medication used in the clinic primarily to increase uric acid excretion in the urine in hyperuricemic conditions, such as gout, through its activity as a competitive substrate for organic anion transporters (OATs) in the kidney (Pascale et al., 1952). We observed that, similarly to CBX, probenecid reduced alcohol intake both in dependent and nondependent rats and in the drinking-in-the-dark (DID) paradigm of binge-like drinking in mice (Rhodes et al., 2005). These results suggest that probenecid, a generally safe medication that has long been used in humans, is a candidate drug for repositioning for AUD and indicate that pannexin1 may be a potential therapeutic target for the treatment of AUD.
METHODS
Animals
Male Wistar rats (Charles River, Kingston, NY), weighing 225 to 275 g upon arrival, were group-housed (2 to 3 per cage) in standard plastic cages in a temperature- and humidity-controlled room and were maintained under a reverse 12 h/12 h light/dark cycle with food and water available ad libitum except during behavioral testing. Male C57BL/6 J mice, 7 weeks old upon arrival (The Jackson Laboratory, Bar Harbor, ME, USA), were individually housed in standard plastic cages and kept under a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water except during behavioral testing. The room was controlled for temperature and humidity.
All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition) and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute and the National Institute on Drug Abuse Intramural Research Program.
Drugs
Probenecid (Sigma Aldrich, St. Louis, MO) was administered in a Latin-square design at doses previously shown to be effective in studies targeting the central nervous system in mice (Dossi et al., 2018) and rats (Mousseau et al., 2018).
Operant alcohol self-administration and alcohol vapor exposure
Oral alcohol self-administration experiments were conducted as previously described using standard operant chambers (Med Associates, St. Albans, VT) fitted with 2 retractable levers and a dual-cup liquid receptacle (Vendruscolo and Roberts, 2014). After initial training to acquire operant responding for alcohol reinforcement, rats were split into 2 groups matched for alcohol consumption. In the next phase (vapor exposure) and for the remainder of these experiments, one group of rats (nondependent group) was exposed to air without alcohol, whereas the other group of rats (dependent group) was exposed to alcohol vapor in daily cycles designed to cause intoxication (14 h vapor ‘on’; 200 mg/dl target blood alcohol levels [BALs]) and withdrawal (10 h vapor ‘off’) to induce alcohol dependence (Vendruscolo and Roberts, 2014). During initial training and during the vapor exposure phase, rats in both groups were allowed 30-min operant sessions to lever press for alcohol (10%, w/v; 0.1 ml) and water (0.1 ml) on separate levers according to a concurrent fixed-ratio 1 (FR1) schedule of reinforcement (i.e. each lever press on each lever resulted in fluid delivery). Operant alcohol self-administration sessions were conducted 2–3 sessions per week during the 10 h ‘off’ period, 6–8 h into withdrawal. After each training session, the liquid receptacle and surrounding area were inspected to confirm the consumption of earned reinforcers. Probenecid was injected acutely intraperitoneally 60 min prior to behavioral testing.
Non-drug reinforcement: saccharin self-administration in rats
Rats were trained to self-administer saccharin (0.1% w/v) in 30 min sessions (FR1) until all rats responded over 100 times per session on two occasions. Next, all rats were given a baseline session of saccharin self-administration, followed by testing a range of probenecid doses administered in a Latin-square design on consecutive days.
Mouse drinking-in-the-dark paradigm
To evaluate the effects of probenecid on binge-like drinking, mice were tested in the DID paradigm (Rhodes et al., 2005). The DID paradigm utilizes a discrete time of access to alcohol starting 3 h into the dark phase to obtain pharmacologically significant alcohol drinking in a 4-day procedure. Blood alcohol levels in C57BL/6 J mice in the DID paradigm are reliably over 100 mg/dl (1 mg/ml) following the final drinking bout and produce behavioral intoxication (Rhodes et al., 2005). In the DID procedure, the water bottle is replaced with a tube containing 20% (v/v) ethanol for 2 h in the home cage 3 h after lights off. The design involves three daily drinking sessions of 2 h and a fourth 4 h session (Rhodes et al., 2005). The effects of probenecid were tested in the fourth 4 h session. Probenecid was administered acutely intraperitoneally 30 min before testing a range of doses of 0, 100, 150, and 200 mg/kg. Fluid weight was recorded to the nearest 0.01 g. On the last day (day 4), tubes were weighed before and after the first 2-h session and at the end of the second 2-h session. Blood samples were taken from the animals’ tail at the end of the 4-h session to assess BALs (mg%) using an Analox Alcohol Analyzer.
Non-drug reinforcement: saccharin drinking in mice
To assess the effect of probenecid on a non-drug reinforcer, mice were allowed to drink saccharin over 2-h sessions and were tested for the effect of probenecid on saccharin intake (0.066% w/v) over a 4-h session in a 2-day testing protocol.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 software (San Diego, CA, USA). The data are presented as mean ± S.E.M.
We examined the effect of probenecid on alcohol drinking in alcohol-dependent and nondependent rats using a two-way mixed ANOVA, followed by Holms–Sidak post-hoc tests. The same approach was used to assess probenecid’s effect on water drinking during this test. A one-way repeated-measures (RM) ANOVA was used to assess the effect of probenecid on saccharin self-administration in a separate cohort of rats.
We examined the effect of probenecid on alcohol drinking in the DID paradigm in mice using separate one-way RM ANOVAs for each variable: alcohol intake in the first 2 h of the 4-h drinking session, alcohol intake in the 4-h drinking session, and BALs, followed by the Bonferroni post hoc test. Two-tailed, paired t-tests were used to evaluate probenecid’s effect on saccharin intake in mice in the first 2 h of the 4-h drinking session and in the total 4-h drinking session.
RESULTS
We tested the effect of probenecid on alcohol drinking in rats. Rats were trained to self-administer alcohol as previously reported (Vendruscolo and Roberts, 2014; Sanna et al., 2016; Tunstall et al., 2019). Briefly, after acquisition of stable alcohol self-administration, some of the rats were made dependent by chronic intermittent exposure to alcohol vapor (Vendruscolo and Roberts, 2014; Sanna et al., 2016). With this model, dependent rats show physical and motivational (anxiety, dysphoria, and hypohedonia) signs of withdrawal, increased voluntary alcohol consumption, greater motivation for alcohol, reflected by an increase in progressive-ratio (PR) responding, increased persistence of alcohol consumption despite punishment (Vendruscolo and Roberts, 2014), and persistent electrophysiological changes in key components of reward and stress neurocircuitry (Francesconi et al., 2009; Roberto et al., 2010). The predictive validity of this model of alcohol dependence has been demonstrated by findings indicating that FDA-approved drugs and new experimental medications are effective in decreasing alcohol drinking in dependent and/or nondependent rats (Tunstall et al., 2017).
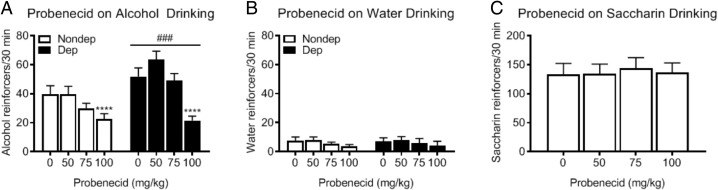
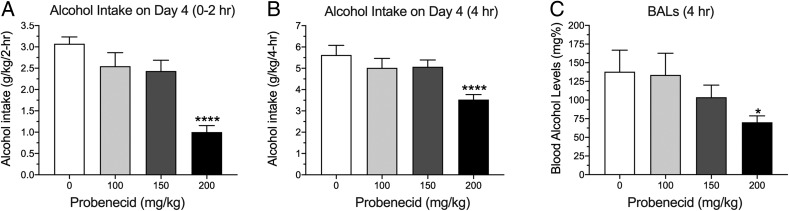
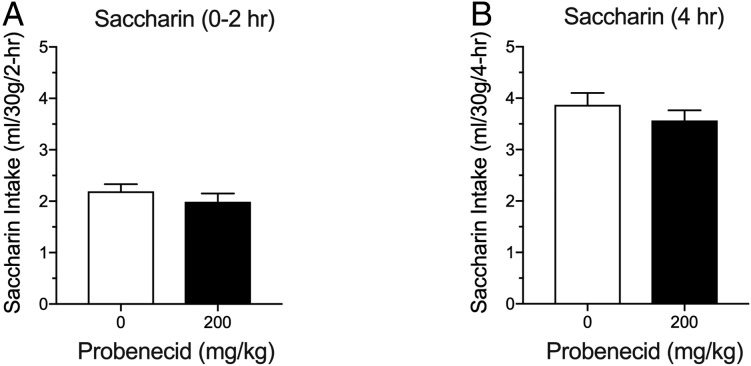
Alcohol-dependent rats consumed significantly more alcohol than nondependent rats during acute withdrawal compared to the nondependent rats (two-way RM ANOVA; Group: F1,22 = 14.88, P < 0.001; Fig. 1A). Probenecid decreased alcohol drinking in both groups (Dose: F3,66 = 13.54, P < 0.0001), with a significant effect at the 100 mg/kg dose compared to vehicle (P < 0.0001). Probenecid did not affect water or saccharin intake (Fig. 1B and C). The latter indicates that probenecid’s effect was selective for alcohol and not the result of a general reduction in reward. We next tested probenecid in the DID paradigm of binge-like drinking in mice (Rhodes et al., 2005). The DID paradigm is a limited-access drinking procedure, in which mice are tested during the period of the dark cycle when ingestive behaviors are heightened, resulting in high BALs without the need for any procedural manipulations, such as the inclusion of sweet compounds to motivate high levels of alcohol intake (Rhodes et al., 2005). As shown in Fig. 2A and B, probenecid significantly reduced alcohol drinking in the DID paradigm at a dose of 200 mg/kg (one-way RM ANOVA, F3,33 = 19.27, P < 0.0001, for the first 2 h of the session; one-way RM ANOVA, F3,33 = 10.07, P < 0.0001, for the entire 4-h session). As shown in Fig. 2C, probenecid also reduced BALs after the 4-h DID session (one-way RM ANOVA, F3,33 = 3.17, P < 0.05). At the dose used, probenecid did not reduce saccharin intake under similar experimental conditions as in DID (Fig. 3).
Fig. 1.
Probenecid reduces oral alcohol self-administration in rats. (A) Dependent rats self-administered greater amounts of alcohol than nondependent rats across testing (two-way RM ANOVA; Group: F1,22 = 14.88, P < 0.001). Probenecid dose-dependently decreased alcohol drinking in both groups (Dose: F3,66 = 13.54, P < 0.0001), with a significant effect at the 100 mg/kg dose compared to vehicle (P < 0.0001). (B) Dependent and nondependent rats did not differ in their water drinking during testing (two-way RM ANOVA; Group: F1,22 = 0.0036, P = 0.9528), nor did probenecid significantly alter water drinking (Dose: F3,66 = 0.9853, P = 0.4052; Group x Dose Interaction: F3,66 = 0.0128, P = 0.9980). (C) In rats trained to self-administer saccharin, probenecid treatment did not alter saccharin consumption (one-way RM ANOVA; Dose: F3,42 = 0.2238, P = 0.8793). Nondep = Nondependent, Dep = Dependent. ****P < 0.0001 compared to vehicle, ###P < 0.001 compared to Nondependent group.
Fig. 2.
Probenecid reduces alcohol drinking in the drinking-in-the-dark paradigm in mice. (A) Effects of probenecid on alcohol intake in the first 2 h of the 4-h drinking session (one-way RM ANOVA, F3,33 = 19.27; P < 0.0001, followed by Bonferroni post hoc). (B) Probenecid effect on alcohol intake in the 4-h drinking session (one-way RM ANOVA, F3,33 = 10.07, P < 0.0001, followed by Bonferroni post hoc). (C) Probenecid effect on blood alcohol levels after the 4-h drinking session (one-way RM ANOVA, F3,33 = 3.17, P = 0.05, followed by Bonferroni post hoc). ****P < 0.0001; *P < 0.05.
Fig. 3.
Probenecid does not reduce saccharin intake in a 2-day drinking-in-the-dark paradigm in mice. (A) Probenecid effect on saccharin intake in the first 2 h of the 4-h drinking session (two-tailed paired t-test, t = 1.510, df = 11; P = 0.1593). (B) Probenecid effect on saccharin intake in the 4-h drinking session (two-tailed paired t-test, t11 = 1.357, P = 0.2019).
DISCUSSION
Drug repositioning is an effective strategy to bring new medications to the clinic. Probenecid has been used clinically for over half a century and has proven to be generally safe, making it a potential candidate for repositioning for AUD.
Probenecid was synthesized in the 1940s and employed to extend the half-life of penicillin through interference with renal clearance (McKinney et al., 1951). Probenecid acts on the kidneys’ OATs, resulting in its hyperuricemia-reducing activity (Pascale et al., 1952). Probenecid also inhibits pannexin1 (Silverman et al., 2008). CBX, but not probenecid, inhibits connexins with lower potency (Dahl, 2015) and 11β-HSD isozymes that modulate glucocorticoid effects (Chapman et al., 2013), as well as pannexin1 (Dahl, 2015).
Here, we show that probenecid reduces alcohol drinking in both dependent and nondependent rats in the chronic intermittent alcohol vapor exposure model and reduces alcohol drinking in the DID paradigm in mice. These models have been developed to model escalated alcohol drinking and binge alcohol drinking patterns, respectively. Similar results, including reduced alcohol drinking in dependent and nondependent rats and in the DID procedure in mice, were previously demonstrated with CBX, which also inhibits pannexin1. Neither CBX (Sanna et al., 2016) nor probenecid (present study) affected saccharin intake, suggesting that their actions in decreasing alcohol drinking are independent of general reward/consummatory mechanisms. This may be beneficial for medication compliance and is a potentially important difference from the current FDA-approved medication naltrexone, which also affects natural reward (Tunstall et al., 2017).
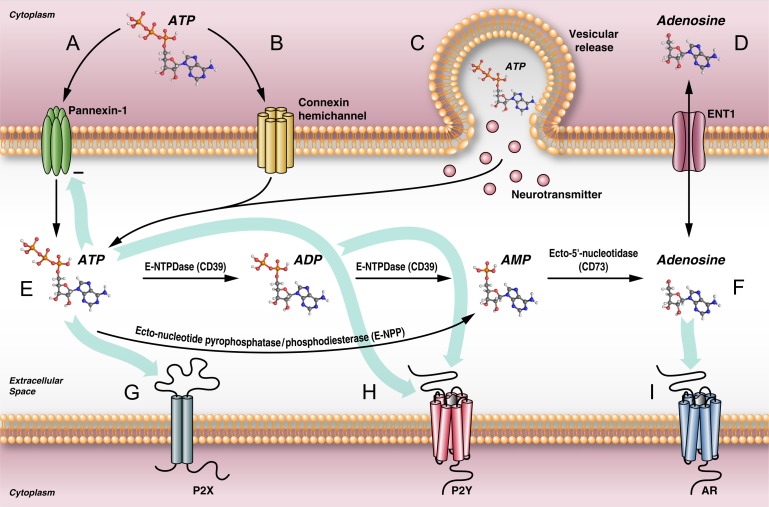
Pannexin1 is a source of extracellular ATP and, in turn, adenosine (Fig. 4). Adenosine signaling has been implicated in the regulation of alcohol drinking (Ruby et al., 2010). ATP is also released in vesicles from neuronal populations expressing the vesicular nucleotide transporter (VNUT)/SLC17A9 (Taruno, 2018). The equilibrative nucleoside transporters (ENTs) can transport adenosine bidirectionally according to the adenosine concentration gradient (Taruno, 2018). Alcohol is believed to inhibit ENT1, resulting in increased adenosine in the extracellular space (Ruby et al., 2010). In the extracellular space, ATP is hydrolyzed to ADP and adenosine that can bind purinoceptors.
Fig. 4.
Schematic representation of some of the mechanisms implicated in the release of ATP and purinergic neurotransmission in the brain. (A) Pannexin1 channels release ATP into the extracellular space, which in turn inhibits their opening (Dahl, 2015). (B) ATP can also be released from connexin hemichannels. Connexin hemichannel opening has been associated with cerebral insults and brain injury. (C) ATP is also released into the extracellular space by vesicular release along neurotransmitters, including acetylcholine, dopamine, serotonin, and norepinephrine. (D) The equilibrative nucleoside transporter type 1 (ENT1) allows adenosine to move in either direction, driven by the gradient. Alcohol is believed to inhibit ENT1 in vivo, resulting in increased adenosine in the extracellular space (Ruby et al., 2010). (E) Ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase1)/CD39 hydrolyzes ATP to ADP and ADP to AMP. Ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) can also hydrolyze ATP to AMP. Ecto-5’-nucleotidase/CD73 hydrolyzes adenosine monophosphate (AMP) to adenosine (Yegutkin, 2008) (F). Purinergic receptors include the ionotropic P2X (G) and metabotropic P2Y (H) receptors for ATP/ADP and (I) adenosine receptors (ARs) for adenosine.
Both probenecid and CBX exert pharmacological activities on more than one target. In the case of probenecid, the effective mechanism could be limited to its pannexin1 inhibitory activity. In the case of CBX, both known main activities, 11β-HSD1 and pannexin1 inhibition, are theoretically desirable in reducing alcohol drinking. It has been argued that multi-target molecules may actually be desirable as drugs with the ability to ameliorate complex conditions in the guise of a drug combination (e.g. statins and their dual anti-cholesterol and anti-inflammatory actions) because of their potential additive or synergistic pharmacological actions, more predictable pharmacokinetics, reduced likelihood of drug interactions, and better patient compliance (Talevi, 2015).
In conclusion, we showed that probenecid reduces both dependent and nondependent alcohol drinking in rats and binge-like drinking in mice. These results are similar to the effects of another drug, CBX, which shares the ability of probenecid to inhibit pannexin1 channels. Both probenecid and CBX are drugs that have been used extensively in the clinic and found to be generally safe, which makes them potential candidates for repositioning for AUD.
Funding
This work was supported by the National Institutes of Health (Intramural Research Program and grants AA021667, AA025012, DA041750, DA043268, DA046170, and DA046204).
Conflict of interest statement
PPS is an inventor of a patent related to this manuscript.
References
- Bertaccini G, Coruzzi G (1985) Pharmacology of the treatment of peptic ulcer disease. Dig Dis Sci 30:43S–51S. [DOI] [PubMed] [Google Scholar]
- Chapman K, Holmes M, Seckl J (2013) 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93:1139–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G. (2015) ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci 370:20140191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantis E, Kyriakos G, Quiles-Sanchez LV, et al. (2017) The anti-inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr Cardiol Rev 13:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossi E, Blauwblomme T, Moulard J, et al. (2018) Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci Transl Med 10:eaar3796. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Koob GF, et al. (2009) Intrinsic neuronal plasticity in the juxtacapsular nucleus of the bed nuclei of the stria terminalis (jcBNST). Prog Neuropsychopharmacol Biol Psychiatry 33:1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, et al. (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311:1889–900. [DOI] [PubMed] [Google Scholar]
- Kostrouchova M, Kostrouch Z, Kostrouchova M (2007) Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol (Praha) 53:37–49. [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, et al. (2012) Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol 17:513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Kinney S, Peck HM, Bochey JM, et al. (1951) Benemid, p-(DI-n-propylsulfamyl)-benzoic acid; toxicologic properties. J Pharmacol Exp Ther 102:208–14. [PubMed] [Google Scholar]
- Mousseau M, Burma NE, Lee KY, et al. (2018) Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci Adv 4:eaas9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosengo N. (2016) Can you teach old drugs new tricks? Nature 534:314–6. [DOI] [PubMed] [Google Scholar]
- Pascale LR, Dubin A, Hoffman WS (1952) Therapeutic value of probenecid (benemid) in gout. J Am Med Assoc 149:1188–94. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Luu-The V, Li S, et al. (2007) Localization and glucocorticoid regulation of 11beta-hydroxysteroid dehydrogenase type 1 mRNA in the male mouse forebrain. Neuroscience 145:110–5. [DOI] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Shin W, Vendruscolo LF, et al. (2015) Identifying candidate drivers of alcohol dependence-induced excessive drinking by assembly and interrogation of brain-specific regulatory networks. Genome Biol 16:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, et al. (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, et al. (2010) Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry 67:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Adams CA, Knight EJ, et al. (2010) An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev 3:163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (2013) Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. http://samhsagov/data/NSDUH/2012SummNatFindDetTables/Indexaspx.
- Sanna PP, Kawamura T, Chen J, et al. (2016) 11beta-hydroxysteroid dehydrogenase inhibition as a new potential therapeutic target for alcohol abuse. Transl Psychiatry 6:e760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, et al. (2009) The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284:18143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295:C761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talevi A. (2015) Multi-target pharmacology: possibilities and limitations of the ‘skeleton key approach’ from a medicinal chemist perspective. Front Pharmacol 6:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A. (2018) ATP release channels. Int J Mol Sci 19:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Jackson MF, Olah ME, et al. (2008) Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322:1555–9. [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Carmack SA, Koob GF, et al. (2017) Dysregulation of brain stress systems mediates compulsive alcohol drinking. Curr Opin Behav Sci 13:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Kirson D, Zallar LJ, et al. (2019) Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol 17:e2006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez S, Eugenin EA (2014) Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front Physiol 5:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, et al. (2012) Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32:7563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, et al. (2015) Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest 125:3193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ (2014) Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG. (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–94. [DOI] [PubMed] [Google Scholar]