Summary
This systematic review and meta-analysis aims to determine outcomes following aortic occlusion with the transthoracic clamp (TTC) versus endoaortic balloon occlusion (EABO) in patients undergoing minimally invasive mitral valve surgery. A subgroup analysis compares TTC to EABO with femoral cannulation separately from EABO with aortic cannulation. We searched Medline and Embase up to December 2018. Two people independently and in duplicate screened title and abstracts, full-text reports, extracted data and assessed the risk of bias using the Cochrane risk-of-bias tool for non-randomized studies. We identified 1564 reports from which 11 observational studies with 4181 participants met the inclusion criteria. We found no evidence of difference in the risk of postoperative death or cerebrovascular accident (CVA) between the 2 techniques. Evidence for a reduction in aortic dissection with TTC was found: 4 of 1590 for the TTC group vs 19 of 2492 for the EABO group [risk ratio 0.33, 95% confidence interval (CI) 0.12–0.93; P = 0.04]. There was no difference in aortic cross-clamp (AoX) time between TTC and EABO [mean difference (MD) −5.17 min, 95% CI −12.40 to 2.06; P = 0.16]. TTC was associated with a shorter AoX time compared to EABO with femoral cannulation (MD −9.26 min, 95% CI −17.00 to −1.52; P = 0.02). EABO with aortic cannulation was associated with a shorter AoX time compared to TTC (MD 7.77 min, 95% CI 3.29–12.26; P < 0.001). There was no difference in cardiopulmonary bypass (CPB) time between TTC and EABO with aortic cannulation (MD −4.98 min, 95% CI −14.41 to 4.45; P = 0.3). TTC was associated with a shorter CPB time compared to EABO with femoral cannulation (MD −10.08 min, 95% CI −19.93 to −0.22; P = 0.05). Despite a higher risk of aortic dissection with EABO, the rates of survival and cerebrovascular accident across the 2 techniques are similar in minimally invasive mitral valve surgery.
Keywords: Cardiac surgery, Minimally invasive surgery, Mitral valve surgery, Endoaortic balloon occlusion, Chitwood clamp, Transthoracic clamp, Systematic review, Meta-analysis
INTRODUCTION
Minimally invasive mitral valve surgery (MIMVS) is recognized as a safe surgical approach with patients reporting less pain, shorter hospital stay and better cosmetic results compared to other more invasive approaches [1]. To perform this, cardiopulmonary bypass (CPB) is needed and aortic occlusion is a critical step in its setup. This is achieved currently by 2 techniques available to surgeons: transthoracic clamp (TTC) and endoaortic balloon occlusion (EABO). The TTC technique is simpler and involves inserting a clamp through the intercostal spaces to clamp the ascending aorta [2]. The EABO technique is associated with a longer learning curve as the procedure requires more monitoring and experience [3]. It involves accessing the aorta through a catheter inserted either in the femoral artery or directly through the ascending aorta with an inflatable balloon at its tip. This is guided by transoesophageal echocardiography, the balloon is inflated and the aorta occluded.
A previous meta-analysis of observational studies and abstracts reports that there was no significant difference in the occurrence of cerebrovascular accidents (CVAs) and mortality when comparing TTC and EABO [4]. However, it found that EABO was associated with significantly higher risk of iatrogenic aortic dissection and a trend towards increased CPB and aortic cross-clamp (AoX) times. In this review, we perform a subgroup analysis separating EABO with femoral cannulation to EABO with aortic cannulation to see whether the cannulation approach in EABO has an impact on these clinical outcomes. In addition, we only include research published in full-text reports aiming to deliver a more comprehensive assessment of risk of bias (ROB).
METHODS
To perform this research, we followed the review process as outlined by the Cochrane Handbook for Systematic Reviews of Interventions [5].
Criteria for considering studies for this review
Types of studies.
We searched for both observational and randomized studies that compared TTC and EABO in patients undergoing MIMVS. Studies included, reported as full text and excluded those published as abstract only. No unpublished data were examined.
Types of participants.
We included patients of any age, sex or ethnicity with mitral valve pathology and required MIMVS. Patients undergoing concomitant surgery alongside mitral valve surgery were not excluded.
Types of interventions.
We included studies comparing TTC (also called external aortic clamp or external transthoracic aortic clamp) with EABO [also called endoaortic clamp occlusion (EACO)] in MIMVS.
Types of outcome measures.
-
Primary outcomes
a. Primary outcomes
b. All-cause mortality, within 3 months of MIMVS.
c. CVA <30 days following MIMVS.
d. Aortic dissection.
-
Secondary outcomes
a. AoX time (min).
b. CPB time (min).
Search methods for identification of studies
Electronic search.
We searched Medline and Embase on Ovid for published observational and randomized studies comparing TTC to EABO in MIMVS between January 2000 and December 2018. We decided to use the year 2000 as a cut-off to target the latest reports only. The following keywords were used: minimally invasive surgical procedures, mitral valve, mini-MVS, mini-thoracotomy, ministernotomy, hemisternotomy, endoclamp, endo-aortic, endoluminal, endo-balloon, Chitwood clamp, Intraclude clamp, Heartport clamp, ESTECH and flex clamp (Supplementary Material, Appendix S1).
Data collection and analysis
Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery [6] were consulted.
Selection of studies.
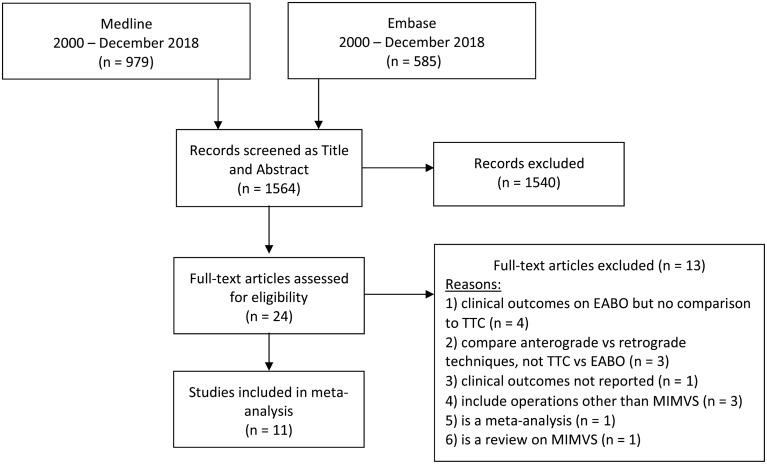
Two reviewers (P.M.R. and Z.D.T.) independently screened titles and abstracts for relevance. Any disagreements were resolved by conversation and a consensus was reached with the supervision of a third reviewer (H.H.). We retrieved the full-text reports and 1 reviewer (P.M.R.) screened these for inclusion or exclusion. We recorded the selection process and completed a PRISMA [7] flow diagram (Fig. 1) and a ‘characteristics of excluded studies table’ (Supplementary Material, Appendix S2).
Figure 1:
Study flow diagram according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysed) statement. EABO: endoaortic balloon occlusion; MIMVS: minimally invasive mitral valve surgery; TTC: transthoracic clamp.
Data extraction and management.
Three independent reviewers (P.M.R., T.H.M.M. and A.M.) performed data collection, including assessment of bias, for each study and extracted information on the following:
1. Methods: date of study, country of origin and study design.
2. Patient characteristics: total number of patients; and number of patients, mean age, percentage of female patients and reported previous CVAs in each group.
3. Interventions: type of clamp used in the TTC technique, type of endoballoon used in the EABO technique and if the EABO approach was with femoral or aortic cannulation.
4. Outcomes: primary and secondary outcomes as reported in the earlier section.
The data were then transferred to the review manager (Version 5.3. Copenhagen: The Nordic Cochrane Centre) [8].
Assessment of risk of bias in included studies.
Three independent reviewers (P.M.R., T.H.M.M. and A.M.) used the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool to assess ROB in the included studies [9]. This tool assesses ROB by comparing each observational study with a hypothetical target randomized control trial (RCT) that addresses the same question as the observational study (Supplementary Material, Appendix S3). ROBINS-I assesses ROB in 7 domains:
Bias due to confounding.
Bias in selection of participants.
Bias in classification of interventions.
Bias due to departures from intended intervention.
Bias due to missing data.
Bias in measurement outcomes.
Bias in selection of the reported results.
Each domain was marked as having either a low; moderate; high; serious or critical ROB. ROBINS-I requires the prespecification of confounders. Numerous confounding factors were identified and could be divided into the 2 following categories:
Patient-based confounders: sex, diameter of femoral artery, age, New York Heart Association (NYHA) class, history of previous CVA, history of previous vascular disease (hypertension, diabetes), history of previous rheumatic heart disease and history of previous cardiac surgery.
Technical confounders: type of surgery, complexity of the surgery, additional interventions during the surgery such as maze procedure or tricuspid surgery, use of robotics and surgeon experience or the learning curve.
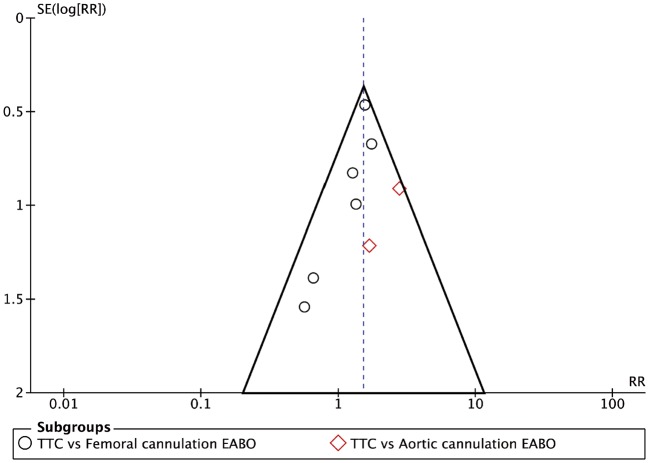
The surgeon learning curve was accounted for within the domain of ‘bias due to confounding’. Studies that both accounted for this confounding factor and looked balanced for the main baseline confounders were judged to be at moderate ROB for this domain. Finally, in an attempt to investigate the publication bias, we produced a funnel plot looking at the outcome of all-cause mortality (Fig. 2).
Figure 2:
Funnel plot analysis of all-cause mortality for TTC versus EABO. On the horizontal axis is RR, and on the vertical axis is SE. EABO: endoaortic balloon occlusion; RR: risk ratio; SE: standard error; TTC: transthoracic clamp.
Measures of the treatment effect.
Continuous data were analysed as the mean difference (MD) with 95% confidence intervals (CIs). We analysed dichotomous data as risk ratios (RRs) with 95% CIs. We chose RRs over odds ratios because they are considered easier to interpret [5]. Events are reported as a number per cohort and weighted using a random effects model.
Unit of analysis issues.
When collecting data for the CPB and AoX times, we converted any reports that were in ‘hours’ to ‘minutes’. In addition, for other outcomes, we converted any reports of ‘percentage of incidence’ to a ‘number of events per group’ by using the total number of patients in that group.
Assessment of heterogeneity.
χ 2 and I2 statistics were used to measure the presence and extent of heterogeneity between the groups in each analysis. P-values were considered statistically significant when ≤0.05.
Data analysis.
We used the Mantel–Haenszel method for all meta-analyses with dichotomous outcomes and the Inverse Variance random effects model for continuous outcomes according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions [5]. Because of the heterogeneity of the interventions and comparators, we used a random effects model in all instances. We performed all analyses using the Review Manager 5 (RevMan 5) software [8], following an intention-to-treat principle.
Subgroup analysis.
Where there were sufficient numbers of events for a specific outcome, we performed subgroup analyses investigating the effect of cannulation location for EABO.
‘Summary of findings’ tables.
We created ‘Summary of findings’ tables for each intervention type for primary and secondary outcomes. We used the 5 GRADE considerations to assess the quality of body of evidence as described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions [5], employing the GRADEpro GDT software [10].
RESULTS
Results of the search
We identified 11 papers with 4181 participants for inclusion in our review. We retrieved 1564 references from the electronic search of the literature. After removing studies that were clearly not eligible for inclusion we assessed 24 full-text articles. Of these, we excluded 13 studies and recorded reasons for exclusion (Supplementary Material, Appendix S2).
Included studies with patient characteristics
Included studies were performed in Germany [11, 12], Italy [13–16], Canada [17], the USA [18–20] and the Netherlands [21] (Table 1). All studies were observational with 3 studies being prospective and 8 studies being retrospective in design (Table 1). These represented a total of 4181 patients undergoing MIMVS with cohort sizes ranging from 36 to 1064 patients. The total number of patients undergoing MIMVS with the TTC technique was 1606 (38%) as opposed to 2575 (62%) with the EABO technique. Of those, 2056 (80%) had EABO with femoral cannulation and 519 (20%) had EABO with direct aortic cannulation. Eight studies used the femoral cannulation technique for EABO [11, 12, 15–19, 21]. Two studies used the direct aortic cannulation technique for EABO [14, 20]. One study, Barbero et al. [13], offered 2 separate EABO cohorts, 1 with femoral cannulation and 1 with aortic cannulation (Table 1).
Table 1:
Included studies with patient characteristics
| Study | Country | Study type | Type of TTC | Type of EABO with cannulation approach | Patient characteristics |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = total | n = TTC group | n = EABO group | Age TTC group (years), mean ± SD | Age EABO group (years), mean ± SD | Female TTC group (%) | Female EABO group (%) | Previous CVA in TTC group (%) | Previous CVA in EABO group (%) | |||||
| Aybek et al. [11] | Germany | Prospective | Chitwood Clamp (Scanlan International, Inc., St Paul, MN, USA) | Heartport Endoaortic Clamp (Heartport, Redwood City, CA, USA)—femoral cannulation | 58 | 35 | 23 | 56.3 ± 12.9 | 58.3 ± 16.4 | 45.7 | 52.2 | 2.9 | 0 |
| Reichenspurner et al. [12] | Germany | Retrospective | Chitwood Clamp (Scanlan International, Inc., St Paul, MN, USA) | EndoClamp (Cardiovation, Ethicon Inc., Somerville, NJ, USA)—femoral cannulation | 120 | 60 | 60 | 62.1 ± 10.5 | 70.8 | NA | NA | ||
| Maselli et al. [14] | Italy | Prospective | Chitwood Clamp (Scanlan International, Inc., St Paul, MN, USA) | Cardiovations EndoClamp Aortic Catheter (Edwards Lifesciences Corporation, Irvine, CA, USA)—aortic cannulation | 36 | 16 | 20 | 54.7 ± 5.4 | 56.5 ± 6.4 | 70 | 75 | NA | NA |
| Ius et al. [15] | Italy | Retrospective | Cygnet Flexible Clamp (Novare Surgical Systems Inc., Cupertino, CA, USA) | EndoClamp Aortic Catheter (Edwards Lifesciences Corporation, Irvine, CA, USA)—femoral cannulation | 127 | 95 | 32 | 62 ± 11 | 63 ± 9 | 50.5 | 40.6 | NA | NA |
| Modi et al. [18] | USA | Prospective | NA | NA—femoral cannulation | 1052 | 573 | 479 | 61.1 ± 13.9 | 51 | Not recorded | |||
| Glower and Desai [20] | USA | Retrospective | Cosgrove Flexible Clamp (Cardinal Health V, Edwards Lifesciences Corporation, Irvine, CA, USA) | EndoClamp Aortic Catheter (Edwards Lifesciences Corporation, Irvine, CA, USA)—aortic cannulation | 671 | 235 | 436 | 58 ± 14 | 59 ± 13 | 59.1 | 52.5 | NA | NA |
| Loforte et al. [16] | Italy | Retrospective | Cygnet Flexible Clamp (Novare Surgical Systems Inc., Cupertino, CA, USA) | EndoClamp Aortic Catheter (Edwards Lifesciences Corporation, Irvine, CA, USA)—femoral cannulation | 138 | 93 | 45 | 58.8 ± 7.8 | 58.1 ± 11.4 | 73.1 | 77.7 | NA | NA |
| Mazine et al. [17] | Canada | Retrospective | Chitwood Clamp (Scanlan International, Inc., St Paul, MN, USA) | Cardiovations EndoClamp Aortic Catheter (Edwards Lifesciences Corporation, Irvine, CA, USA)—femoral cannulation | 243 | 103 | 140 | 61.9 ± 11 | 55.4 ± 1.9 | 38.8 | 40 | 5.8 | 6.4 |
| Atluri et al. [19] | USA | Retrospective | Chitwood Clamp (Scanlan International, Inc., St Paul, MN, USA) | EndoClamp Aortic Catheter (Edwards Lifesciences Corporation, Irvine, CA, USA)—femoral cannulation | 1064 | 189 | 875 | 58.9 ± 15.9 | 59.4 ± 12.6 | 52.4 | 42.5 | NA | NA |
| Bentala et al. [21] | Netherlands | Retrospective | Chitwood Clamp (Scanlan International, Inc., St Paul, MN, USA) | EndoClamp Aortic Catheter, IntraClude Intra-Aortic Occlusion Device (Edwards Lifesciences Corporation, Irvine, CA, USA)—femoral cannulation | 221 | 57 | 164 | 62 | 66 | 43.9 | 43.9 | 7 | 1.9 |
| Barbero et al. [13] | Italy | Retrospective | Chitwood Clamp (Scanlan International, Inc., St Paul, MN, USA) | Endoreturn/ IntraClude Intra-Aortic Occlusion Device (Edwards Lifesciences Corporation, Irvine, CA, USA)—one group (1) with femoral cannulation, the other (2) with aortic cannulation | 451 | 150 | 238 (1) | 67.1 ± 12.2 | 61.3 ± 13.9 (1) | 42.7 | 51.7 (1) | 11.3 | 6.3 (1) |
| 63 (2) | 69.2 ± 9.4 (2) | 23.8 (2) | 9.5 (1) | ||||||||||
| Total |
|
|
|
4181 | 1606 | 2575 | |||||||
CVA: cerebrovascular accident; EABO: endoaortic balloon occlusion; TTC: transthoracic clamp; SD: standard deviation.
Risk of bias
ROB is summarized in Table 2. Out of the 11 included studies, 9 studies were deemed to be at an overall ‘serious’ ROB whereas the remaining 2 were deemed to be at an overall ‘moderate’ risk. The main source of bias was bias due to confounding.
Table 2:
Assessment of risk of bias using ROBINS-I
| Study | Bias due to confounding | Bias in selection of participants | Bias in classification of interventions | Bias due to departures from intended intervention | Bias due to missing data | Bias in measurement outcomes | Bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Aybek et al. [11] | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Reichenspurner et al. [12] | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Maselli et al. [14] | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Ius et al. [15] | Serious | Low | Low | Low | Moderate | Low | Moderate | Serious |
| Modi et al. [18] | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Glower and Desai [20] | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Loforte et al. [16] | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Mazine et al. [17] | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Atluri et al. [19] | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Bentala et al. [21] | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Barbero et al. [13] | Serious | Low | Low | Low | Moderate | Low | Moderate | Serious |
ROBINS-I: Risk Of Bias In Non-randomized Studies of Interventions.
Table 3:
Summary of findings table for primary outcomes
| Summary of findings | |||||||
|---|---|---|---|---|---|---|---|
| TTC compared to EABO for MIMVS | |||||||
| Patient or population: patients undergoing MIMVS | |||||||
| Setting: cardiac surgery | |||||||
| Intervention: TTC | |||||||
| Comparison: EABO | |||||||
| Outcomes | Anticipated absolute effectsa (95% CI) |
Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE)b | Comments | ||
| Risk with EABO | Risk with TTC | ||||||
| CVA [11–13, 15–21] | 19 per 1000 | 16 per 1000 (9–27) | RR 0.83 (0.48–1.44) | 4145 (11 observational studies) | ⊕◯◯◯ | I 2 = 6% | |
| Very low | |||||||
| All-cause mortality [11–21] | 11 per 1000 | 17 per 1000 (9–29) | RR 1.52 (0.86–2.66) | 4181 (12 observational studies) | ⊕◯◯◯ | I 2 = 0% | |
| Very low | |||||||
| Aortic dissection [11–13, 15–19, 21] | 7 per 1000 | 2 per 1000 (1–7) | RR 0.33 (0.12–0.93) | 3336 (9 observational studies) | ⊕◯◯◯ | I 2 = 0% | |
| Very low | |||||||
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bGRADE Working Group grades of evidence: high certainty: we are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: the true effect may be substantially different from the estimate of the effect; and very low certainty: the true effect is likely to be substantially different from the estimate of effect.
CI: confidence interval; CVA: cerebrovascular accident; EABO: endoaortic balloon occlusion; MIMVS: minimally invasive mitral valve surgery; RR: risk ratio; TTC: transthoracic clamp.
Bias due to confounding.
Nine included studies [11–13, 15–20] were rated as being at a ‘serious’ ROB due to confounding and 2 [14, 21] were rated at a ‘moderate’ risk. Nine studies provided the preoperative patient characteristics tables. Of these, most reported were the unbalanced patient characteristics between TTC and EABO cohorts. All included studies were non-randomized, and patient allocation to either TTC or EABO intervention group was based on the policy of the surgical centre at the time of operation, patient characteristics and surgeon preference. Many studies compared 2 time periods, 1 when EABO was the technique of choice and 1 when TTC was the preferred technique. This introduces the possibility of differences in the outcome due to differences in surgeon experience in MIMVS and due to changes in procedures over time, which were not controlled for.
Bias in selection of participants.
All 11 included studies were at a ‘low’ ROB due to selection of participants because patient selection was not related to the intervention (or the effect of the intervention) and the outcome. Patients who underwent elective ventricular fibrillation were, for most outcomes, removed as part of the study designs.
Bias in classification of interventions.
All 11 studies were at a ‘low’ ROB due to classification of interventions, with both TTC and EABO intervention groups clearly defined before the start of the operation.
Bias due to departures from intended intervention.
All 11 studies were at a ‘low’ ROB due to departures from intended intervention.
Bias due to missing data.
Eight studies were at a ‘low’ ROB due to the missing data. Three studies [13, 15, 20] were at a ‘moderate’ risk due to a very small number of patients having been allocated a group and then dropping out or being excluded from the intervention group due to conversion to full sternotomy or inadequate data reporting.
Bias in measurement of outcomes.
All 11 studies were at a ‘low’ ROB in measurement of outcomes as all outcomes were objective in nature.
Bias in selection of the reported results.
All 11 studies were at a ‘moderate’ ROB in selection of reported results because there was no protocol or a prespecified analysis plan.
Bias in publication.
A funnel plot was produced to investigate the publication bias, which showed that studies comparing TTC to EABO with aortic cannulation could be missing from the literature if they reported a higher risk of mortality than TTC. However, overall, there did not appear to be a publication bias when comparing TTC to EABO (Fig. 2).
Summary of bias
Overall, allocation bias seemed to favour TTC over EABO. Four studies [13, 17–19] favoured TTC over EABO by having more patients with previous cardiac surgery allocated to their EABO cohorts compared to their TTC ones. With only 2 studies [19, 21] specifically controlling for surgeon experience by stating that their surgeons had completed the learning curve for MIMVS, we can confidently say that they were competent in both TTC and EABO techniques. All other studies are at risk of having surgeons experienced in TTC and not EABO, which is a serious potential confounder.
Primary outcomes
Summary of findings table.
All-cause mortality.
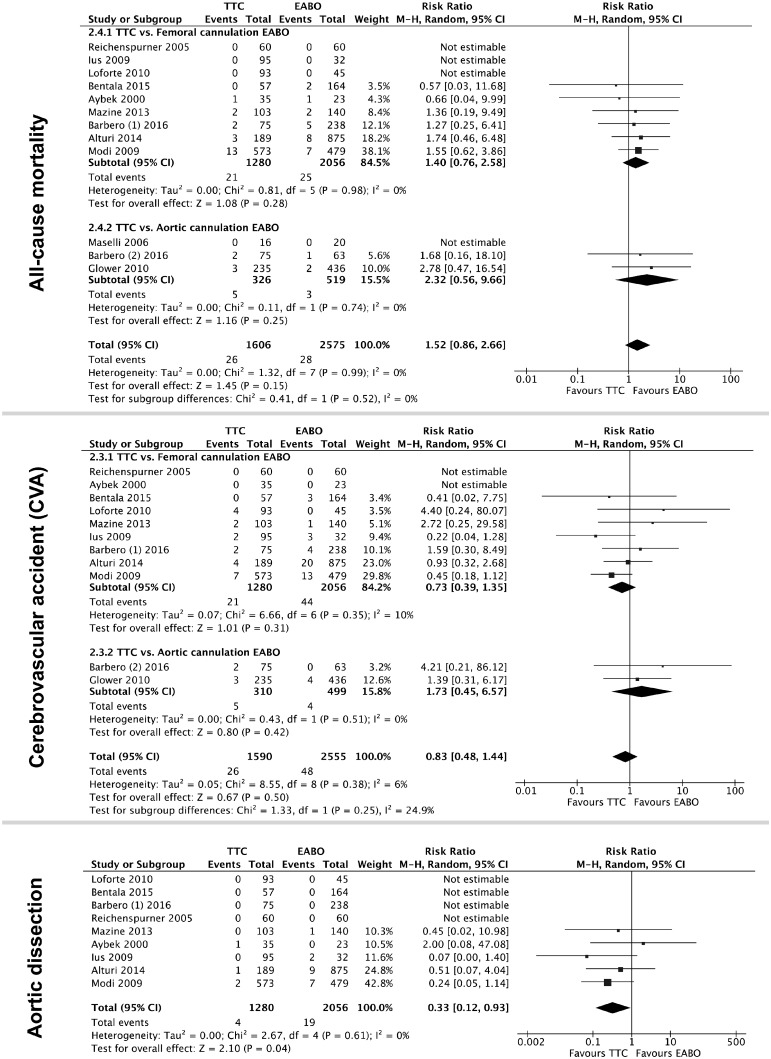
All studies reported all-cause mortality as postoperative mortality within 3 months following the surgery (Fig. 3). In a random effects model, there was no difference in the risk of death between the 2 techniques or within any of the subgroups (overall RR 1.52, 95% CI 0.86–2.66; P = 0.15, I2 = 0%).
Figure 3:
Forest plot of primary outcomes with pooled risk ratios (diamonds) and 95% CIs (horizontal lines). Sizes of the squares are proportional to the weight of each study. CIs: confidence intervals; CVA: cerebrovascular accident; EABO: endoaortic balloon occlusion; TTC: transthoracic clamp.
Cerebrovascular accident.
Ten studies reported CVA either as a stroke or a transient ischaemic attack within 30 days after surgery (Fig. 3). Maselli et al. [14] did not report this outcome and therefore was not included in the analysis. There was no difference in the risk of CVA between the 2 techniques in a random effects model (RR 0.83, 95% CI 0.48–1.44; P = 0.5, I2 = 6%).
Aortic dissection.
For studies comparing TTC to EABO with femoral cannulation, there was a significantly lower risk of aortic dissection with TTC as opposed to the EABO with femoral cannulation (RR 0.33, 95% CI 0.12–0.93; P = 0.04, I2 = 0%) (Fig. 3). None of the studies that compared TTC to EABO with aortic cannulation found any events of aortic dissection in either group, and therefore we did not include them in this analysis.
Secondary outcomes
Summary of findings table.
Aortic cross-clamp time.
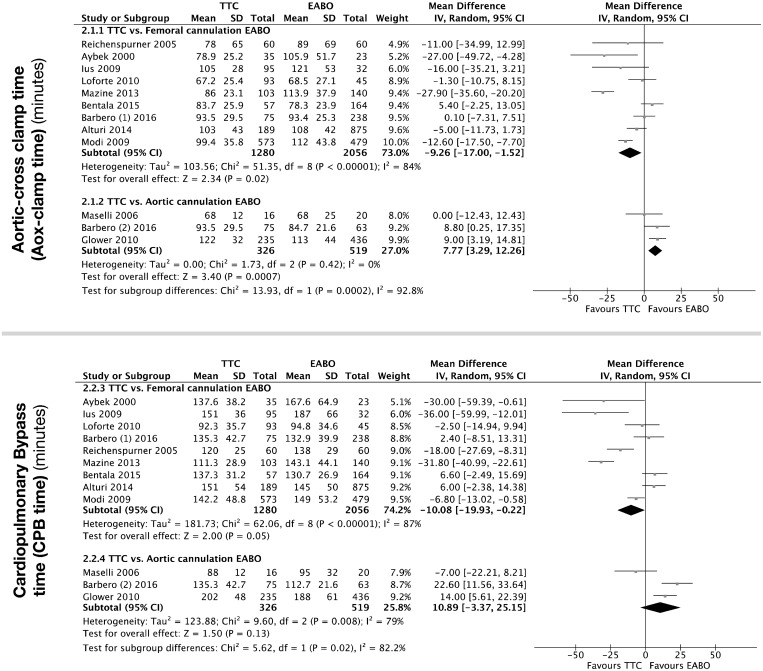
Evidence for AoX time in MIMVS was very low (Fig. 4 and Table 4). The summary estimates for subgroups were significantly different (test for heterogeneity P < 0.001, I2 = 92%); therefore, we focus on the subgroup summary estimates. The AoX time for MIMVS using TTC was on average 9 min shorter than EABO with femoral cannulation (MD −9.26 min, 95% CI −17.00 to −1.52 min; P = 0.02, I2 = 84%). In contrast, the AoX time for TTC was over 7 min longer than EABO with aortic cannulation (MD 7.77 min, 95% CI 3.29–12.26 min; P < 0.001, I2 = 0%).
Figure 4:
Forest plot for AoX-clamp and CPB times (min) with mean differences (diamonds) and 95% CIs (horizontal lines). Sizes of the squares are proportional to the weight of each study. AoX-clamp: aortic cross-clamp; CIs: confidence intervals; CPB: cardiopulmonary bypass; EABO: endoaortic balloon occlusion; SD: standard deviation; TTC: transthoracic clamp.
Table 4:
Summary of findings table for secondary outcomes
| Summary of findings | |||||||
|---|---|---|---|---|---|---|---|
| TTC compared to EABO (with technical subgroups) for MIMVS | |||||||
| Patient or population: patients undergoing MIMVS | |||||||
| Setting: cardiac surgery | |||||||
| Intervention: TTC | |||||||
| Comparison: EABO (with technical subgroups) | |||||||
| Outcomes | Anticipated absolute effectsa (95% CI) |
Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE)b | Comments | ||
| Risk with EABO (with technical subgroups) | Risk with TTC | ||||||
| Cross-clamp time (min) − TTC versus femoral cannulation EABO [11–13, 15–19, 21] | The mean cross-clamp time (min) − TTC versus femoral cannulation EABO was 0 min | The mean cross-clamp time (min) − TTC versus femoral cannulation EABO in the intervention group was 9.26 min faster (17–1.52 min faster) | - | 3336 (9 observational studies) | ⊕◯◯◯ | I 2 = 84% | |
| Very low | |||||||
| Cross-clamp time (min) − TTC versus Aortic cannulation EABO [13–14, 20] | The mean cross-clamp time (min) − TTC versus Aortic cannulation EABO was 0 min | The mean cross-clamp time (min) − TTC versus Aortic cannulation EABO in the intervention group was 7.77 min faster (3.29–12.26 min longer) | - | 845 (3 observational studies) | ⊕◯◯◯ | I 2 = 0% | |
| Very low | |||||||
| CPB time (min) − TTC versus femoral cannulation EABO [11–13, 15–19, 21] | The mean CPB time (min) − TTC versus femoral cannulation EABO was 0 min | The mean CPB time (min) − TTC versus femoral cannulation EABO in the intervention group was 10.08 min faster (19.93–0.22 min faster) | - | 3336 (9 observational studies) | ⊕◯◯◯ | I 2 = 87% | |
| Very low | |||||||
| CPB time (min) − TTC versus Aortic cannulation EABO [13–14, 20] | The mean CPB time (min) − TTC versus Aortic cannulation EABO was 0 min | The mean CPB time (min) − TTC versus Aortic cannulation EABO in the intervention group was 10.89 min longer (3.37 min faster to 25.15 min longer) | - | 845 (3 observational studies) | ⊕◯◯◯ | I 2 = 79% | |
| Very low | |||||||
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bGRADE Working Group grades of evidence: high certainty: we are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: the true effect may be substantially different from the estimate of the effect; and very low certainty: the true effect is likely to be substantially different from the estimate of effect.
CI: confidence interval; CVA: cerebrovascular accident; EABO: endoaortic balloon occlusion; MIMVS: minimally invasive mitral valve surgery; TTC: transthoracic clamp.
Cardiopulmonary bypass time.
Evidence for CPB time was very low (see Fig. 4 and Table 4). The summary estimates for the subgroups were significantly different (test for heterogeneity P = 0.02, I2 = 82%); therefore, we focus on the subgroup summary estimates. The CPB time for MIMVS using TTC was on average 10 min faster than CPB using EABO with femoral cannulation (MD −10.08 min, 95% CI −19.93 to −0.22 min; P = 0.05, I2 = 87%). The data were heterogeneous (χ2 test for heterogeneity <0.001; I2 = 87%). The CPB time for MIMVS using EABO with aortic cannulation was 10 min faster (MD 10.89 min, 95% CI −3.37 to 25.15 min; P = 0.13, I2 = 79%) The data were heterogeneous (χ2 test for heterogeneity P < 0.001; I2 = 79%), and the lower 95% CI indicates CPB could be 3 min longer than when using TTC.
DISCUSSION
General summary of results
All studies reported postoperative survival rates. The meta-analysis showed overall good survival rates with no difference between TTC and the 2 EABO techniques. However, there was weak evidence of no difference suggesting that both EABO techniques were associated with fewer postoperative deaths compared to TTC. The reasons for this were undetermined and most probably secondary to confounding. Several studies performed multivariable analyses to determine whether there were any predictors of mortality [13, 18]. They found that the aortic clamping technique was not a predictor of mortality but preoperative patient risk factors such as an NYHA class of III/IV, diabetes, renal failure, atrial fibrillation, age >70 years and prolonged CPB time were predictors of mortality. These findings suggest that patient comorbidities are more influential on survival rates than the aortic occlusion technique.
Ten studies reported postoperative CVA. The meta-analysis showed no difference in the risk of CVA with both techniques overall, and no difference when subgroup analyses were used to explore differences between TTC and EABO (femoral) and TTC and EABO (aortic). The mean summary estimates from the subgroup analysis indicate a higher risk in the subgroup analysis of CVA with EABO (femoral) when compared to TTC but an opposite effect of a lower risk of EABO (aortic) when compared with TTC. However, the 95% CI for both these means cross the null. More evidence from additional studies would be helpful in determining whether these trends persist and inform a hypothesis that EABO (aortic) is the occlusion approach with the least CVA risk. A previous meta-analysis [4] comparing TTC and EABO suggested several reasons for higher incidences of CVA with EABO (femoral): introduction of a guidewire along the aortic arch/ascending aorta, the balloon catheter being prone to migration during atrial wall retraction especially in patients with wide aortas and atheromatous disease and, finally, having to reposition the balloon intraoperatively with deflation and reinflation of the balloon, which can increase the risk of embolization from the aorta. These reasons can explain why we found that EABO (femoral) was associated with a higher risk of CVA compared to TTC. On the other hand, the question still remains regarding how EABO (aortic) has the lowest risk of CVA. Two papers (Glower and Desai [20] and Schmitz et al. [22]) have investigated this question and state that the advantages of the aortic cannulation approach over the femoral approach are a more direct and controlled placement of the balloon and the elimination of balloon migration because the balloon is pulled snuggly against the fixed aortic cannula and cannot move. In addition, we found that EABO (aortic) was associated with the shortest AoX time and CPB times, which suggests that shorter extracorporeal support times may be another reason for a low risk of CVA. Overall, both TTC and EABO are associated with similarly low risks of CVA; however, EABO (aortic) seems the least risk prone for this outcome.
Ten studies reported AoX-clamp and CPB times. The meta-analysis showed no difference in the length of AoX times and CPB times between TTC and the pooled EABO techniques. Overall, it showed weak evidence of no difference between the 2 techniques suggesting that TTC was associated with shorter AoX times and CPB times. However, upon the subgroup analysis, we found that TTC was associated with shorter AoX times and CPB times compared to EABO (femoral) (P = 0.02; P = 0.05) but not EABO (aortic). EABO (aortic) was associated with shorter AoX times and CPB times compared to TTC (P < 0.001; P = 0.13). These results show that, out of all 3 approaches, EABO (aortic) is associated with the shortest AoX times and CPB times. Reasons for this may include an easier and more straightforward cannulation manoeuvre compared to femoral cannulation and a more reliable occlusion of the aorta than with TTC. In addition, the surgeon learning curve is an important confounder to consider for this outcome. It is a difficult confounder to control for because in most cases the reports from operations performed at the early stages of the surgeon learning curve were not separated from those when surgeons had gained more experience. An example of bias caused by confounding due to changes over time is found by Mazine et al. [17]. In this study, the experience from 2006 to 2009, when EABO was available, was compared with the experience from 2009 to 2011, when TTC was available. With the same surgeons operating, the potential for confounding due to the surgeon learning curve suggests that these surgeons were less experienced both at MIMVS and EABO between 2006 and 2009 compared to when they started using TTC. We know that EABO is, by the nature of the technique, associated with a longer surgeon learning curve [3]. Surgeon experience has an impact not only on intraoperative times but also on clinical outcomes for patients. In Atluri et al. [19], for example, the authors noticed that the learning curve associated with EABO had a significant impact on the number of iatrogenic aortic dissections in their patients. They reported a 3% rate of aortic dissection with EABO in their first 100 cases as opposed to only a 0.6% rate in their last 500. These 2 examples show how surgeon experience plays an important role in influencing these outcomes of interest.
Ten studies reported on aortic dissection. The meta-analysis showed lower rates of aortic dissection with TTC compared to EABO: 4 of 1590 for the TTC group vs 19 of 2492 for the EABO group (RR 0.33, 95% CI 0.12–0.93; P = 0.04). This finding has been previously reported [4] and it is thought that EABO is associated with higher rates of aortic dissection because of the need to insert a guidewire in the femoral artery which can damage the lining of the vessel and the need for higher perfusion pressures with this technique [17]. We were unable to perform a subgroup analysis for this outcome because all studies using EABO (aortic) found no incidence of aortic dissection to compare to TTC.
Quality of evidence and limitations of review
The overall quality of the evidence was low. For AoX times and CPB times, we found substantial heterogeneity within the results (I2 > 80%). We suspect the heterogeneity to be secondary to variations in surgeon experience, variations in recording technique of extracorporeal support times across studies, variations in the types of clamps used in TTC (Chitwood Clamp versus Cygnet flexible clamp) and the balloons used in EABO [Heartport Endoaortic Clamp (Heartport) versus EndoClamp Aortic Catheter (Edwards Lifesciences)] across the studies. Such high levels of heterogeneity (I2 > 80%) suggest that it is very difficult to infer anything from these findings because the variation in the measurement technique is too influential. Based on the meta-analysis, the studies included did not report data on myocardial protection, acute right and/or left ventricular failure and need for postoperative extracorporeal membrane oxygenation to achieve a meaningful conclusion in this regard. The literature search, data collection and analysis have been performed in a transparent and reproducible form. This will have reduced any ROB in the review process.
CONCLUSIONS
This systematic review and meta-analysis report safe and similar rates of CVA and survival with both TTC and EABO in MIMVS. EABO was associated with a higher risk of aortic dissection. EABO with aortic cannulation offers the shortest extracorporeal support times.
Implications for research
To this day, all evidence for use of the 2 occlusion techniques are drawn from observational cohort studies. At present, there is little evidence to support the adoption of 1 technique over another other than personal choice. In such a position of true equipoise, we would therefore encourage the careful design of an RCT comparing TTC and EABO in specific participant subgroups. Adoption of an RCT design would remove the heavy bias of confounding factors. In our opinion, a threshold of 100 operations could be used to determine adequate surgeon experience in both techniques and would remove the confounding of the surgeon learning curve.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Sarah Dawson from the School of Social and Community Medicine (UOB) for her guidance on performing the literature search.
Funding
T.H.M.M. time was supported by NIHR CLAHRC West. This study was supported by the NIHR Biomedical Centre at the University Hospitals Bristol NHS Foundation Trust, the British Heart Foundation and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, the British Heart Foundation or the Department of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943–52. [DOI] [PubMed] [Google Scholar]
- 2. Chitwood WR Jr, Elbeery JR, Moran JF.. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann Thorac Surg 1997;63:1477–9. [DOI] [PubMed] [Google Scholar]
- 3. Marullo A, Irace F, Vitulli P, Peruzzi M, Rose D, D’Ascoli R.. Recent developments in minimally invasive cardiac surgery: evolution or revolution? Biomed Res Int 2015;2015:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kowalewski M, Malvindi P, Suwalski P, Raffa G, Pawliszak W, Perlinski D. et al. Clinical safety and effectiveness of endoaortic as compared to transthoracic clamp for small thoracotomy mitral valve surgery: meta-analysis of observational studies. Ann Thoracic Surg 2017;103:676–86. [DOI] [PubMed] [Google Scholar]
- 5. Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. http://handbook.cochrane.org (1 July 2017, date last accessed). [Google Scholar]
- 6. Hickey GL, Dunning J, Seifert B, Sodeck G, Carr MJ, Burger HU. et al. Statistical and data reporting guidelines for the European Journal of Cardiothoracic Surgery and the Interactive Cardiovascular and Thoracic Surgery. Eur J Cardiothorac Surg 2015;48:180–93. [DOI] [PubMed] [Google Scholar]
- 7. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 9. Sterne J, Hernán M, Reeves B, Savović J, Berkman N, Viswanathan M. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brozek J, Oxman A, Schünemann H.. GRADEpro GDT. Version Accessed Prior to 9 October 2017. Hamilton, ON: McMaster University (developed by Evidence Prime; ), 2015. [Google Scholar]
- 11. Aybek T, Dogan S, Wimmer-Greinecker G, Westphal K, Mortiz A.. The micro-mitral operation comparing the port-access technique and the transthoracic clamp technique. J Card Surg 2000;15:76–81. [DOI] [PubMed] [Google Scholar]
- 12. Reichenspurner H, Detter C, Deuse T, Boehm D, Treede H, Reichart B.. Video and robotic-assisted minimally invasive mitral valve surgery: a comparison of the port-access and transthoracic clamp techniques. Ann Thorac Surg 2005;79:485–90. [DOI] [PubMed] [Google Scholar]
- 13. Barbero C, Marchetto G, Ricci D, El Qarra S, Attisani M, Filippini C. et al. Right minithoracotomy for mitral valve surgery: impact of tailored strategies on early outcome. Ann Thorac Surg 2016;102:1989–95. [DOI] [PubMed] [Google Scholar]
- 14. Maselli D, Pizio R, Borelli G, Musumeci F.. Endovascular balloon versus transthoracic aortic clamping for minimally invasive mitral valve surgery: impact on cerebral microemboli. Interact CardioVasc Thorac Surg 2006;5:183–6. [DOI] [PubMed] [Google Scholar]
- 15. Ius F, Mazzaro E, Tursi V, Guzzi G, Spagna E, Vetrugno L. et al. Clinical results of minimally invasive mitral valve surgery: endoaortic clamp versus external aortic clamp techniques. Innovations 2009;4:311–18. [DOI] [PubMed] [Google Scholar]
- 16. Loforte A, Luzi G, Montalto A, Ranocchi F, Polizzi V, Sbaraglia F. et al. Video-assisted minimally invasive mitral valve surgery external aortic clamp versus endoclamp techniques. Innovations 2010;5:413–18. [DOI] [PubMed] [Google Scholar]
- 17. Mazine A, Pellerin M, Lebon J-S, Dionne P-O, Jeanmart H, Bouchard D.. Minimally invasive mitral valve surgery: influence of aortic clamping technique on early outcomes. Ann Thorac Surg 2013;96:2116–22. [DOI] [PubMed] [Google Scholar]
- 18. Modi P, Rodriguez E, Hargrove W, Hassan A, Szeto W, Chitwood W.. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg 2009;137:1481–7. [DOI] [PubMed] [Google Scholar]
- 19. Atluri P, Goldstone AB, Fox JY, Szeto W, Hargrove WC.. Port access cardiac operations can be safely performed with either endoaortic balloon or Chitwood clamp. Ann Thorac Surg 2014;98:1579–84. [DOI] [PubMed] [Google Scholar]
- 20. Glower D, Desai B.. Transaortic endoclamp for mitral valve operation through right minithoracotomy in 369 patients. Innovations 2010;5:394–9. [DOI] [PubMed] [Google Scholar]
- 21. Bentala M, Heuts S, Vos R, Maessen J, Scohy TV, Gerritse BM. et al. Comparing the endo-aortic balloon and the external aortic clamp in minimally invasive mitral valve surgery. Interact CardioVasc Thorac Surg 2015;21:359–65. [DOI] [PubMed] [Google Scholar]
- 22. Schmitz C, Ashraf O, Bimmel D, Welz A.. Direct aortic cannulation in minimally invasive mitral-valve operations. Heart Surg Forum 2002;5:370–2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.