Abstract
Seborrheic dermatitis (SD) is a chronic recurrent erythemato-squamous condition that affects seborrheic areas causing flaking, erythema, and pruritus. Etiology is multifactorial and the role of Malassezia sp.remains controversial. We present a series of 12 patients with trichoscopic and direct microscopic exams. We analyzed the presence of the already known SD trichoscopic signs and its correlation to the amount of Malassezia sp. in the scalp. We describe three novel signs: the “dandelion” vascular conglomerate, the “cherry blossom” vascular pattern, and the intrafollicular oily material; of which the “dandelion” vascular conglomerate was the only trichoscopic sign to correlate with Malassezia colonization. This study correlates trichoscopic signs in SD and the quantity of Malassezia sp. We describe three new signs that can be useful to determine indirectly the fungal colonization of the scalp in SD.
Keywords: Seborrheic dermatitis, Malassezia sp., Trichoscopy
Introduction
Seborrheic dermatitis (SD) or seborrheic eczema is a chronic recurrent erythemato-squamous condition that affects seborrheic areas (scalp, face, chest) causing flaking, erythema, and pruritus. The etiology is multifactorial, and it is closely related with the presence of Malassezia sp.SD is a cosmopolitan disease, affects children and young adults, and is more frequent in males than females. It is a common reason for medical consultation (adults 1–6% and newborns 12%) [1].
SD presents as erythemato-squamous patches with greasy-looking, yellow to white scales, generally symmetrically distributed on seborrheic areas such as scalp, face, chest, and back, with a seasonal pattern (more frequent during winter). In immune-suppressed patients, it is more extensive, severe, and refractory to usual treatment; it may be an early sign of AIDS.
Pityriasis capitis or dandruff is a variant of SD. It is a chronic disease restricted to the scalp, involving flaking skin without inflammation signs and pruritus [2].
SD is associated with sex hormones (androgens), neurological disorders, psychiatric disease, chronic alcoholic pancreatitis, hepatitis C virus, and Down syndrome.
Trichoscopic Signs
Multiple trichoscopic signs of SD have been described, the most important findings are: diffuse yellowish scales, single and clusters of scales on an erythematous background distributed among the follicular units, peripilar casts, thin arborizing vessels increased in number compared with healthy controls, multicomponent vascular pattern – constituted by multiple dotted, comma, linear, and small arborizing vessels – and simple reed loops [3, 4].
Material and Methods
We searched for cases of SD of the scalp at “Dr. Manuel Gea Gonzalez” General Hospital, mycology section database in Mexico City, Mexico. Only cases with trichoscopic images were included. Fotofinder Dermoscope® was used to take the trichoscopic images. Direct microscopic examination was performed in order to quantify Malassezia sp. Diagnostic and trichoscopic findings were corroborated by two dermatologists, experts in trichoscopy (Fig. 1).
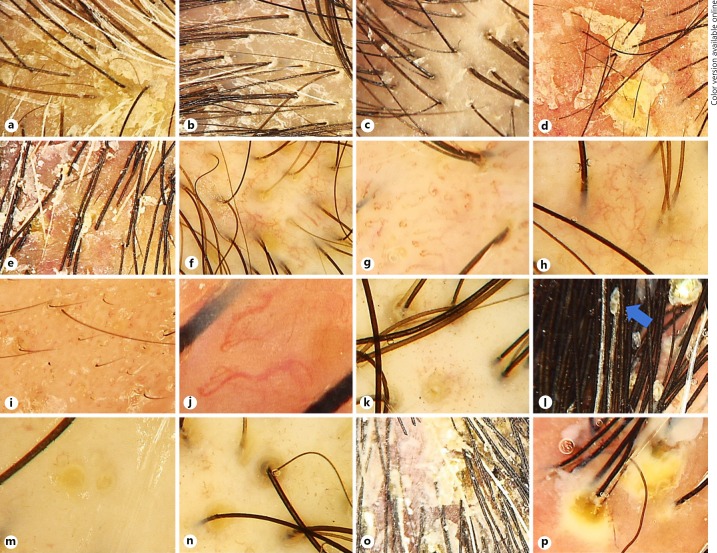
Fig. 1.
Trichoscopic signs found in our study. a Adherent scale. b Interfollicular white scale. c Peripilar white scale. d Interfollicular oily scale. e Peripilar oily scale. f Arborizing vessels. g Glomerular vessels. h “Cherry blossom” vascular pattern. i Comma vessels. j Serpentine vessels. k “Dandelion” vascular conglomerate. l Concretion (blue arrow). m Yellow dots. n Intrafollicular oily material. o Clusters of scales. p Interfollicular pustules.
Descriptive statistics were used for the analysis. Pearson correlation coefficient was used to analyze the trichoscopic signs and the amount of Malassezia sp. We used R version 3.4.2 for the statistical analysis.
Additionally, we set up a control group with patients diagnosed with androgenetic alopecia in order to search for SD trichoscopic signs and to compare Malassezia sp.colonization.
Results
We found 16 cases of patients diagnosed with SD with trichoscopic examination. The clinical variables analyzed were adherent scale, interfollicular white scale, interfollicular oily scale, peripilar white scale, peripilar oily scale, arborizing vessels, glomerular vessels, comma vessels, serpentine vessels, concretions, yellow dots, clusters of scales, interfollicular pustules, and the amount of Malassezia sp.
“Dandelion” vascular conglomerate, “cherry blossom” vascular pattern, and intrafollicular oily material were the three newly described signs (Fig. 2).
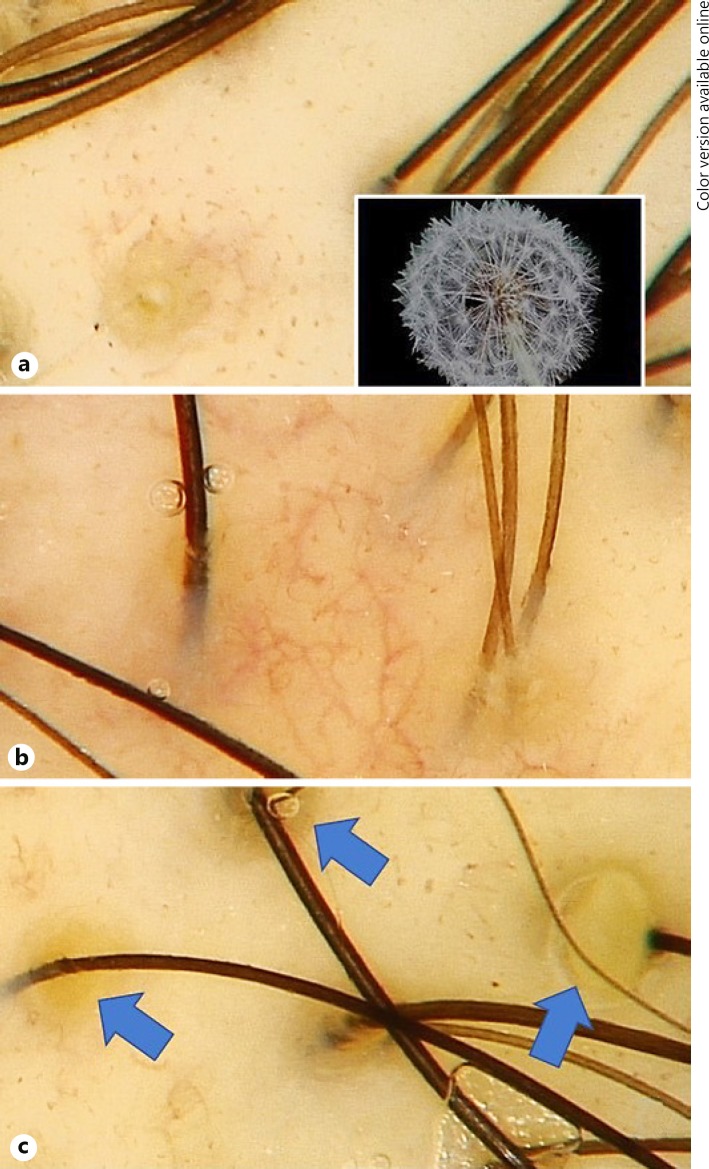
Fig. 2.
Novel trichoscopic signs. a “Dandelion” vascular conglomerate. b “Cherry blossom” vascular pattern. c Intrafollicular oily material (blue arrows).
We described “dandelion” vascular conglomerate as a yellow dot surrounded by glomerular and comma vessels (Fig. 2a). The “cherry blossom” vascular pattern is a conglomerate of arborizing vessels with multiple glomerular and comma vessels surrounding them (Fig. 2b). Intrafollicular oily material, as the term suggests, is a cumulus of sebum and keratin in the hair infundibulum (Fig. 2c).
Twelve cases were females and 4 males (3:1 ratio); mean age was 44.18 years. According to Fitzpatrick skin type, 11 cases were type 4 and 5 were type 3; 13 cases had a direct microscopical examination.
The clinical variables and their frequencies are presented in Table 1.
Table 1.
Trichoscopic signs presented in patients diagnosed witd seborrheic dermatitis
| Variable | Number of cases that presented it | Percentage |
|---|---|---|
| Adherent scale | 2 | 12.50 |
| Interfollicular white scale | 5 | 31.25 |
| Interfollicular oily scale | 6 | 37.50 |
| Peripilar white scale | 3 | 18.75 |
| Peripilar oily scale | 8 | 50.00 |
| Arborizing vessels | 11 | 68.75 |
| Glomerular vessels | 10 | 62.50 |
| “Cherry blossom” vascular pattern | 1 | 6.25 |
| Comma vessels | 1 | 6.25 |
| Serpentine vessels | 1 | 6.25 |
| “Dandelion” vascular conglomerate | 4 | 25.00 |
| Concretions | 2 | 12.25 |
| Yellow dots | 4 | 25.00 |
| Intrafollicular oily material | 5 | 31.25 |
| Clusters of scales | 4 | 25.00 |
| Interfollicular pustules | 1 | 6.25 |
Quantity of Malassezia sp.correlated only with one clinical sign, the “dandelion” vascular conglomerate (p = 0.05) (Fig. 2) (Table 2). Three patients were excluded from this correlation because of the lack of direct microscopical examination; comma vessels, serpentine vessels, and interfollicular pustules were spotted in those 3 patients, that is why they could not correlate with the quantity of Malassezia sp. (Table 2).
Table 2.
Correlation between trichoscopic signs and Malassezia sp. amount
| Variable | p value |
|---|---|
| Adherent scale | 0.45 |
| Interfollicular white scale | 0.27 |
| Interfollicular oily scale | 0.11 |
| Peripilar white scale | 0.67 |
| Peripilar oily scale | 0.47 |
| Arborizing vessels | 0.54 |
| Glomerular vessels | 0.65 |
| “Cherry blossom” vascular pattern | 0.21 |
| Comma vessels | NA |
| Serpentine vessels | NA |
| “Dandelion” vascular conglomerate | 0.05 |
| Concretions | 0.45 |
| Yellow dots | 0.67 |
| Intrafollicular oily material | 0.27 |
| Clusters of scales | 0.29 |
| Interfollicular pustules | NA |
NA, not available.
After comparing the three novel trichoscopic signs in SD with the control group, we found that only the “dandelion” vascular conglomerate was statistically significant (p = 0.045).
Discussion
Scalp SD has been associated with white-skinned people and high body mass index [2]; 5 of our patients were phototype 3, and 11 were phototype 4.
The link between SD and Malassezia sp.is supported by the favorable evolution of the symptoms with antifungal treatment and a decreasing count in Malasseziayeasts; nevertheless, the role of Malassezia stays controversial. Paulino [5] did not found any relation between Malasseziaand SD, neither on the species level nor on the subspecies level. She performed an in vivo analysis where she determined the amount of Malassezia cells before and after the treatment with topical ketoconazole; no decrease of fungal cell was seen, but there was symptomatic improvement, suggesting that this may be the consequence of an anti-inflammatory effect [5]. According to our analysis, there was no correlation between global clinical severity of the patient's SD and quantity of Malasseziacells, but individual analysis on each trichoscopic sign differed from this in one variable.
Fungal colonization, sebaceous gland activity, and factors conferring individual susceptibility have been previously analyzed [2, 6]. We only focused in the trichoscopic description and the severity of those signs related to Malassezia sp. quantity, and no association was found.
Previous trichoscopic signs associated with SD are yellow scaling (single and clusters), yellowish areas in the immersion trichoscopy, arborizing vessels, dotted, comma, linear, and small arborizing vessels, simple reed loops, peripilar casts, yellow dots, perifollicular pigmentation, multi-hair follicular unit, and hidden hair [3, 4, 7, 8].
Almost all of these signs were spotted in our cases, the most frequent being the arborizing vessels (68.75%), similar to the previous results published by Kibar et al. [8], where they found it in 82% of the cases.
We describe three new signs: the “dandelion” vascular conglomerate – the only trichoscopic sign corelated to high quantity of Malassezia sp. – present in 4 patients (25%), the “cherry blossom” vascular pattern, present in one case (6.25%), and the intrafollicular oily material, present in 5 patients (31.25%).
Conclusion
This is the first study that correlates the trichoscopic findings in SD with the quantity of Malassezia sp.“Dandelion” vascular conglomerate could be a good clinical indicator to estimate Malassezia colonization and could be the only trichoscopic sign that justifies the use of antifungal therapy intended to eradicate it.
Statement of Ethics
This study was approved by the Hospital ethics and investigation committee; all patients gave their approval to use the information and images taken during the protocol in an informed consent declaration.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Arenas R, Dermatitis Seborreica . In: Dermatología, atlas, diagnóstico y tratamiento. In: Arenas R, editor. Mc Graw Hill Interamericana Editores. 6ª edición. México: 2015. pp. pp. 65–70. [Google Scholar]
- 2.Borda LJ, Wikramanayake TC. Seborrheic Dermatitis and Dandruff: A Comprehensive Review. J Clin Investig Dermatol. 2015 Dec;3((2)):10. doi: 10.13188/2373-1044.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudnicka L, Sicinska J, Rakowska A, Warszawik-Hendzel O, Seborrheic Dermatitis . In: Atlas of Trichoscopy: Dermoscopy in Hair and Scalp Disease. In: Rudnicka L, Olszewska M, Rakowska A, editors. 1st edition. London, England: Springer-Verlag; 2012. pp. pp. 371–378. [Google Scholar]
- 4.Rossi A, Fortuna MC, Pranteda G, Garelli V, Di Nunno D, Mari E, et al. Clinical, Histological and Trichoscopic Correlations in Scalp Disorders. Dermatology. 2015;231((3)):201–8. doi: 10.1159/000430909. [DOI] [PubMed] [Google Scholar]
- 5.Paulino LC. New perspectives on dandruff and seborrheic dermatitis: lessons we learned from bacterial and fungal skin microbiota. Eur J Dermatol. 2017 Jun;27(S1):4–7. doi: 10.1684/ejd.2017.3038. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz JR, Messenger AG, Tosti A, Todd G, Hordinsky M, Hay RJ, et al. A comprehensive pathophysiology of dandruff and seborrheic dermatitis - towards a more precise definition of scalp health. Acta Derm Venereol. 2013 Mar;93((2)):131–7. doi: 10.2340/00015555-1382. [DOI] [PubMed] [Google Scholar]
- 7.Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012 Nov;67((5)):1040–8. doi: 10.1016/j.jaad.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Kibar M, Aktan Ş, Bilgin M. Dermoscopic findings in scalp psoriasis and seborrheic dermatitis; two new signs; signet ring vessel and hidden hair. Indian J Dermatol. 2015 Jan-Feb;60((1)):41–5. doi: 10.4103/0019-5154.147786. [DOI] [PMC free article] [PubMed] [Google Scholar]