Abstract
We report a rare case of hypertrophic pachymeningitis (HP) and cerebral venous thrombosis associated with proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA)-positive granulomatosis with polyangiitis (GPA). A 58-year-old male developed left headache after exudative otitis media. The laboratory data were positive for PR3-ANCA. Brain magnetic resonance imaging revealed bilateral paranasal sinusitis, left frontal lobe edema, and a thick dura mater with abnormal enhancement in the frontotemporal lobe. Magnetic resonance venography detected stenosis of the superior sagittal sinus. The patient was successfully treated with glucocorticoid, cyclophosphamide, and apixaban. Contrast neuroimaging should be performed for patients who present with unexplained headache, especially with middle ear and paranasal inflammation. These symptoms should be considered as GPA-related HP and cerebral venous thrombosis.
Keywords: Hypertrophic pachymeningitis, Cerebral venous thrombosis, Granulomatosis with polyangiitis, Antineutrophil cytoplasmic antibody, Central nervous system
Introduction
Granulomatosis with polyangiitis (GPA), formerly known as Wegener's granulomatosis, is characterized by necrotizing granulomatous inflammation, usually involving the upper and lower respiratory tracts with nodules, pulmonary capillaritis with alveolar hemorrhage, and necrotizing glomerulonephritis [1, 2, 3]. Central nervous system involvement was reported in 7–11% of patients with GPA [1, 2, 3]. Hypertrophic pachymeningitis (HP) presents various neurological symptoms due to focal or diffuse thickening of the dura mater. HP is an important neurologic complication of GPA [4, 5, 6, 7]. Recent reports revealed an association between HP and cerebral venous thrombosis [8, 9, 10]. However, HP and cerebral venous thrombosis associated with proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA)-positive GPA has not been previously reported. Here, we report a case of HP and cerebral venous thrombosis associated with PR3-ANCA-positive GPA.
Case Report
A 58-year-old male had developed exudative otitis media 2 months prior to his admission. He had had pain in the left maxillary third molar, which had been gradually progressing over 1 month. The symptoms had developed on the left side of the head 1 week prior to his admission. On admission, his temperature was 36.9°C. Physical examination showed normal findings. Neurological examination revealed paresthesia and pain in his left face and head. His motor function was intact. All deep tendon reflexes were normal. Bilateral Babinski reflexes were absent.
Laboratory tests showed a white blood cell count of 6,100/μL and a C-reactive protein level of 9.45 mg/dL, with an erythrocyte sedimentation rate of 134 mm. The serum procalcitonin and β-D-glucan levels were normal (procalcitonin: 0.04 ng/mL; β-D-glucan: 8.5 pg/mL). The serum PR3-ANCA level was elevated to 84.8 U/mL. Other autoimmune markers, such as antinuclear antibody, anti-DNA antibody, rheumatoid factor, myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA), and anti-cyclic citrullinated peptide, were unremarkable. Urine examination showed no proteinuria. Cerebrospinal fluid analysis showed normocytosis with an increased protein concentration of 82 mg/dL. A cerebrospinal fluid culture was negative.
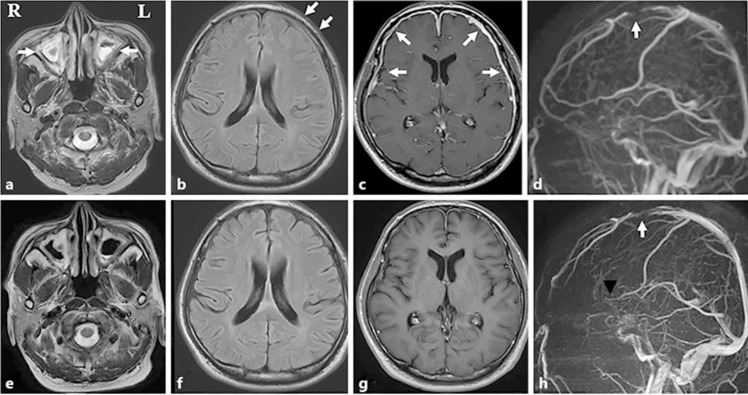
Brain magnetic resonance imaging revealed bilateral paranasal sinusitis on the T2-weighted image, left frontal lobe edema on fluid-attenuated inversion recovery (FLAIR) imaging, and a thick dura mater with abnormal enhancement in the frontotemporal lobe (Fig. 1a–c, arrows). Magnetic resonance venography detected stenosis of the superior sagittal sinus (Fig. 1d, arrow).
Fig. 1.
a Axial T2-weighted image showing bilateral paranasal sinusitis (arrows). b Axial fluid-attenuated inversion recovery (FLAIR) image showing left frontal lobe edema (arrows). c Axial T1-weighted contrast-enhanced image showing diffuse enhancement of the dura mater in the frontotemporal lobe (arrows). d Magnetic resonance venogram showing stenosis of the superior sagittal sinus (arrow). e–h The bilateral paranasal sinusitis on T2-weighted imaging (e), left frontal lobe edema on FLAIR imaging (f), diffuse enhancement of the dura matter in the frontotemporal lobe (g), and stenosis of the superior sagittal sinus (h, arrow) had improved by 30 days after admission.
The patient was diagnosed as having GPA associated with HP and cerebral venous thrombosis. He was treated with 3 courses of intravenous methylprednisolone (1,000 mg/day for 3 days) and 10 mg/day apixaban. He was followed by daily prednisolone (30 mg) and cyclophosphamide (50 mg). His C-reactive protein level had normalized by 25 days after admission. The bilateral paranasal sinusitis on T2-weighted imaging, left frontal lobe edema on fluid-attenuated inversion recovery imaging, diffuse enhancement of the dura matter in the frontotemporal lobe, and stenosis of the superior sagittal sinus had improved by 30 days after admission (Fig. 1e–h, arrow). His symptoms had disappeared by 33 days after admission. Upon discharge, 34 days after admission, the dose of prednisolone was tapered.
Discussion
We have reported a case of GPA associated with HP and cerebral venous thrombosis. Among patients with GPA-related HP, linear thickening of the falx and tentorium is the most common finding [4, 7]. Our case interestingly showed convexity of the frontotemporal lobe. Among HP cases, the most common cause is ANCA-related HP (34%), of which 69% are positive for MPO-ANCA and 31% are positive for PR3-ANCA [11]. Recent reports have revealed that HP associated with idiopathy, MPO-ANCA, cancer, or inflammatory bowel disease results in cerebral venous thrombosis [8, 9, 10, 12]. The fibrosing inflammation within the dura matter encased the sinuses, resulting in their progressive occlusion. HP and cerebral venous thrombosis associated with PR3-ANCA-positive GPA has not been previously reported.
In patients with GPA with central nervous system involvement, early use of immunosuppressants in combination with glucocorticoid is more effective than glucocorticoid monotherapy [8]. The approach to treatment of cerebral venous sinus thrombosis includes anticoagulation (intravenous heparin or subcutaneous low-molecular-weight heparin). In our patient, the use of direct oral anticoagulants to treat venous thrombosis associated with HP is novel.
Patients with GPA and HP have a lower incidence of systemic manifestations such as pulmonary and renal involvement [7]. This leads to a significant delay in diagnosis. Headache is a major symptom of GPA-related HP [7]. Our patient had left headache with exudative otitis media and paranasal sinusitis. However, it is difficult to distinguish GPA-related HP from other primary or secondary headaches. Contrast magnetic resonance imaging is useful in differential diagnosis of GPA-related HP. Our case indicates that contrast neuroimaging should be performed for patients who present with unexplained headache, especially in cases with middle ear and paranasal inflammation.
Statement of Ethics
The patient gave informed consent for the publication of this case report.
Disclosure Statement
The authors state that they have no conflicts of interest.
Funding Sources
The authors have nothing to disclose.
References
- 1.Di Comite G, Bozzolo EP, Praderio L, Tresoldi M, Sabbadini MG. Meningeal involvement in Wegener's granulomatosis is associated with localized disease. Clin Exp Rheumatol. 2006 Mar-Apr;24((2 Suppl 41)):S60–4. [PubMed] [Google Scholar]
- 2.Seror R, Mahr A, Ramanoelina J, Pagnoux C, Cohen P, Guillevin L. Central nervous system involvement in Wegener granulomatosis. Medicine (Baltimore) 2006 Jan;85((1)):54–65. doi: 10.1097/01.md.0000200166.90373.41. [DOI] [PubMed] [Google Scholar]
- 3.Fragoulis GE, Lionaki S, Venetsanopoulou A, Vlachoyiannopoulos PG, Moutsopoulos HM, Tzioufas AG. Central nervous system involvement in patients with granulomatosis with polyangiitis: a single-center retrospective study. Clin Rheumatol. 2018 Mar;37((3)):737–47. doi: 10.1007/s10067-017-3835-y. [DOI] [PubMed] [Google Scholar]
- 4.Lee YC, Chueng YC, Hsu SW, Lui CC. Idiopathic hypertrophic cranial pachymeningitis: case report with 7 years of imaging follow-up. AJNR Am J Neuroradiol. 2003 Jan;24((1)):119–23. [PMC free article] [PubMed] [Google Scholar]
- 5.Kupersmith MJ, Martin V, Heller G, Shah A, Mitnick HJ. Idiopathic hypertrophic pachymeningitis. Neurology. 2004 Mar;62((5)):686–94. doi: 10.1212/01.wnl.0000113748.53023.b7. [DOI] [PubMed] [Google Scholar]
- 6.Yonekawa T, Murai H, Utsuki S, Matsushita T, Masaki K, Isobe N, et al. A nationwide survey of hypertrophic pachymeningitis in Japan. J Neurol Neurosurg Psychiatry. 2014 Jul;85((7)):732–9. doi: 10.1136/jnnp-2013-306410. [DOI] [PubMed] [Google Scholar]
- 7.Choi HA, Lee MJ, Chung CS. Characteristics of hypertrophic pachymeningitis in patients with granulomatosis with polyangiitis. J Neurol. 2017 Apr;264((4)):724–32. doi: 10.1007/s00415-017-8416-0. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia R, Tripathi M, Srivastava A, Garg A, Singh MB, Nanda A, et al. Idiopathic hypertrophic cranial pachymeningitis and dural sinus occlusion: two patients with long-term follow up. J Clin Neurosci. 2009 Jul;16((7)):937–42. doi: 10.1016/j.jocn.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Xia Z, Chen-Plotkin A, Schmahmann JD. Hypertrophic pachymeningitis and cerebral venous sinus thrombosis in inflammatory bowel disease. J Clin Neurosci. 2010 Nov;17((11)):1454–6. doi: 10.1016/j.jocn.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang K, Xu Q, Ma Y, Zhan R, Shen J, Pan J. Cerebral venous sinus thrombosis secondary to idiopathic hypertrophic cranial pachymeningitis: case report and review of literature. World Neurosurg. 2017 Oct;106:1052.e13–21. doi: 10.1016/j.wneu.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Yokoseki A, Saji E, Arakawa M, Kosaka T, Hokari M, Toyoshima Y, et al. Hypertrophic pachymeningitis: significance of myeloperoxidase anti-neutrophil cytoplasmic antibody. Brain. 2014 Feb;137((Pt 2)):520–36. doi: 10.1093/brain/awt314. [DOI] [PubMed] [Google Scholar]
- 12.Di Stefano V, Dono F, De Angelis MV, Onofrj M. Hypertrophic pachymeningitis and cerebral venous thrombosis in myeloperoxidase-ANCA associated vasculitis. BMJ Case Rep. 2019 Jan;12((1)):e226780. doi: 10.1136/bcr-2018-226780. [DOI] [PMC free article] [PubMed] [Google Scholar]