Introduction
For almost 20 years, the Breast Committee of the German Gynecological Oncology Group (Arbeitsgemeinschaft Gynäkologische Onkologie, AGO) has been preparing and updating evidence-based recommendations for the diagnosis and treatment of patients with early and metastatic breast cancer. The AGO Breast Committee consists of gynecological oncologists specialized in breast cancer and interdisciplinary members specialized in pathology, radiological diagnostics, medical oncology, and radiation oncology. This update has been performed according to a documented rule-fixed algorithm, by thoroughly reviewing and scoring chapter by chapter the recent publications for their scientific validity (Oxford level of evidence (LoE) [1] and clinical relevance (AGO grade of recommendation [GR]; Table 1). We present the 2019 update; the full version of the updated slide set is available online as a PDF file in both English and German [2].
Table 1.
German Gynecological Oncology Group grades of recommendation
| ++ | This investigation or therapeutic intervention is highly beneficial for patients, can be recommended without restriction, and should be performed |
| + | This investigation or therapeutic intervention is of limited benefit for patients and can be performed |
| +/– | This investigation or therapeutic intervention has not shown benefit for patients and may be performed only in individual cases; according to current knowledge, a general recommendation cannot be given |
| - | This investigation or therapeutic intervention can be of disadvantage for patients and might not be performed |
| –/– | This investigation or therapeutic intervention is of clear disadvantage for patients and should be avoided or omitted in any case |
Options for Primary Prevention: Principally Modifiable Lifestyle Factors
Individual risk factors can be classified into nonmodifiable and principally modifiable lifestyle factors. Currently, there is good evidence that changes in some modifiable risk factors could substantially decrease individual breast cancer risk. Relevant lifestyle factors such as obesity, alcohol consumption, physical inactivity, fiber-containing foods, smoking, and exposition to ionizing radiation are well known. A Mediterranean diet with consumption of extra virgin olive oil, nuts (>10 g/day), and reduced consumption of fat and red meat can decrease the incidence of breast cancer. For other factors, such as supplementation of vitamin D, vegetarian or vegan diet, vegetables, fruits, or phytoestrogens, we have insufficient or contradictory data for reduction of breast cancer incidence [3]. Physical exercise (metabolic equivalents to 3–5 h of moderate pace walking per week) is very efficient [4]. Avoidance of hormonal therapy (especially estrogen/progestin combination regimens) in postmenopausal women is a controllable factor that may reduce breast cancer risk [5]. Oral contraception does not increase the risk of mortality from breast cancer.
Breast Cancer Risk and Prevention
Gene analyses for high-risk breast and ovarian cancer patients have been performed since the middle of the nineties. Besides risk evaluation, intensified surveillance programs and prophylactic procedures have been developed, and genetic testing is currently being expanded to panel tests. A checklist facilitates the identification of persons with familial criteria for whom genetic counseling is an option and inclusion criteria for testing have been defined. In addition, testing should be performed in the context of a therapeutic option (e.g., PARP inhibitor) as a consequence of data on the use of PARP inhibitors in BRCA1/2 mutation carriers with advanced breast cancer (LoE 1b/GR B/AGO+) [6] (see also the chapter on treatment of metastatic breast cancer). The FDA has granted fast-track approval for both olaparib and talazoparib. Currently, the Committee for Medicinal Products for Human Use of the European Medicines Agency has adopted a positive opinion for a new indication of olaparib as monotherapy for the treatment of adult patients with germline BRCA1/2 mutations who have HER2-negative locally advanced or metastatic breast cancer.
Follow-up results of the German Consortium for Hereditary Breast and Ovarian Cancer [7] have recently been published for 4,573 patients based on a total of 14,142 screening rounds with MRI. A total of 185 invasive breast cancers and 36 ductal carcinomas in situ (DCIS) were observed, with the highest detection rates in BRCA1 mutation carriers, and underline the benefit of early intensive care. For high-risk patients (BRCA1/2-negatives), the surveillance program revealed a relatively lower positive predictive value of MRI as compared to the BRCA1/2 mutation carriers; however, also in this group the positive predictive value significantly increased above 40 years of age, leading to an identical or even higher MRI-only cancer rate compared with the BRCA1/2-positives. Program sensitivity was 89.6%. The data confirmed the importance of MRI in high-risk screening, as 30.8% of all screen-detected cancers were detected by MRI alone.
Women with BRCA1/2 mutations should be offered nondirective counseling for the uptake of primary preventive measures (e.g., bilateral risk-reducing salpingo-oophorectomy [RRSO] after completion of family planning, LoE 2c/GR B/AGO++; risk-reducing bilateral mastectomy [RRBM], LoE 2a/GR B/AGO+; or medical prevention with tamoxifen [LoE 1a/GR A/AGO+], raloxifene [LoE 1b/GR A/AGO+], or an aromatase inhibitor [AI] [LoE 1b/GR A/AGO+]) in addition to participation in an intensified surveillance program. RRBM after ovarian cancer is predominantly not indicated and could be thoroughly discussed depending on tumor stage, recurrence-free time (≥5 years), and age (<55 years) (LoE 4/GR C/AGO+/–). However, uni- or bilateral mastectomy is not indicated in the absence of clearly defined genetic risk factors (LoE 2a/GR B/AGO+). For RRSO, a current meta-analysis [8] demonstrated a significant reduction in the incidence of breast cancer, but publication year was a critical interaction factor, and most recent studies failed to find a significant reduction in breast cancer risk associated with RRSO (LoE 2a/GR B/AGO+/–). However, RRSO is associated with a significant reduction in the incidence of ovarian cancer and overall mortality (LoE 2a/GR B/AGO++).
Data regarding the clinical benefit of risk-reducing contralateral mastectomy in BRCA1/2 mutation carriers suggested a disease-free survival (DFS) and an overall survival (OS) benefit in specific subgroups only, particularly in patients aged <40 years with G1/2 tumors, no triple-negative breast cancer (TNBC), and no chemotherapy. Therefore, this intervention should be discussed with each individual patient (LoE 2b/GR B/AGO+/–). Breast-conserving therapy (BCT) is safe (LoE 2a/GR B/AGO+) and systemic therapy can be given according to recommendations for sporadic breast cancer (LoE 3a/GR B/AGO+). The addition of carboplatin represents not only an effective treatment option for metastatic cancer (LoE 2b/GR B/AGO+), but also in the neoadjuvant chemotherapy (NACT) setting (the last regardless of BRCA1 or BRCA2 mutation status) [9].
Breast Cancer Diagnostics
All available evidence confirms that mammography screening is capable of significantly reducing breast cancer mortality [10]. Based on a review by Oeffinger et al. [11], the number needed to screen to prevent one breast cancer death with a mortality reduction of 20% (40%) was estimated for women aged 40–49 years to be 1,770 (753), for women aged 50–59 years 1,087 (462), and for women aged 60–69 years 835 (355). Screening mammography for breast cancer is recommended for women aged 50–74 years (LoE 1a/GR A/AGO++) [12, 13, 14, 15, 16]. For women aged 40–49 years, individual shared decision-making is recommended as these women would have smaller beneficial health effects than women of older age groups, e.g., higher recall rates for additional imaging and biopsy rates (LoE 1a/GR B/AGO+) [17, 18]. There are no studies on women >75 years of age. However, in view of an aging population, screening can be offered to women in good health with a life expectancy of ≥10 years (LoE 4/GR C/AGO+).
Ultrasound as the sole modality for breast cancer screening cannot be recommended, neither using hand-held ultrasound nor automated whole-breast ultrasound (LoE 3a/GR C/AGO–). The reasons are lack of reproducibility, high false-positive rate, low positive predictive value for biopsy, and lack of quality assurance.
Glandular tissue density as a risk factor for breast cancer development as well as for breast cancer detectability is a well-known issue of mammographic imaging; hence, new technologies to supersede or complete mammographic limitations are highly welcome. However, harms of supplemental imaging in women with dense breasts included higher recall and biopsy rates for women who did not have breast cancer. With digital breast tomosynthesis, the recall and biopsy rates were low (LoE 2b/GR B/AGO+). The radiation dose, which is increased with dual acquisition of digital mammography and tomosynthesis, can be significantly reduced by synthetic 2D image reconstruction of the 3D data set [19, 20, 21, 22, 23, 24]. Digital breast tomosynthesis is particularly recommended if further mammographic diagnostic interventions such as digital spot compression view are requested [25, 26].
Mammographic breast composition is newly classified according to the detectability of masses (A–D), in mammography strongly affected by the percentage of glandular tissue (1–4) [27, 28]. The systematic review by Melnikow et al. [24] underlined interobserver variability for categorizing breast composition, reporting reclassification rates of 12.6–18.7%. As computer-aided density assessment and calculation is getting more widely available, this limitation will be overcome soon.
To assess breast symptoms or lesions, clinical examination (LoE 3b/GR B/AGO++), mammography (LoE 1b/GR A/AGO++), digital breast tomosynthesis where available (LoE 2b/GR B/AGO+), ultrasound (LoE 2b/GR B/AGO++), and minimally invasive biopsies (LoE 1c/GR A/AGO++) should be performed. Elastography (LoE 2b/GR B/AGO+) serves as an adjunct diagnostic modality and shows potential to decrease the false-positive biopsy rate.
MRI can be useful in high-risk patients and if clinical examination, mammography, ultrasound, and needle biopsy do not allow a definitive diagnosis (LoE 3b/GR B/AGO+). Second-look ultrasound is recommended in cases of lesions detected by MRI only.
MRI should not be used in general for preoperative staging purposes in the case of BCT. According to a meta-analysis, the re-excision rate was not reduced. Furthermore, the initial and total mastectomy rate increased if a preoperative breast MRI was performed compared with no preoperative breast MRI [29, 30]. Preoperative breast MRI neither reduced the rate of local recurrences nor local recurrence-free survival or distant metastasis-free survival [31]. For some patients, e.g., with a reduced lesion detectability in mammography and ultrasound (detectability C–D), nipple involvement, lobular invasive cancer, suspicion of multilocular disease, and/or high risk, MRI can be considered (LoE 1b/GR B/AGO+/–) [32, 33]. The feasibility of performing MRI-guided vacuum-assisted biopsies is mandatory if suspicious lesions are detected by MRI of the breast.
In the case of clinically and/or sonographically suspicious axillary lymph nodes, core needle biopsy is recommended to detect extensive axillary disease (LoE 2b/GR B/AGO++). If lymph node involvement is proven by needle biopsy in a patient undergoing neoadjuvant therapy who is a potential candidate for targeted axillary dissection, a marker should be inserted into the metastatic lymph node. The marker should have a proven detectability and stable location also after completion of therapy. The standard procedure in patients with clinically negative axillary lymph nodes is sentinel lymph node biopsy (SLNB).
Imaging for metastasis is recommended with CT scan of the thorax/abdomen and bone scan in patients with high metastatic potential (e.g., lymph node-positive) and/or symptoms, or in case of decision-making for chemotherapy and/or HER2 treatment (LoE 2b/GR B/AGO+) [34].
Pathology
Immunohistochemistry Scores for the Determination of Hormone Receptors
Hormone receptors are both of prognostic and therapeutic importance, and one of the most important parameters to be determined in breast cancer. Traditionally, the results of immunohistological determination of estrogen and progesterone receptors were reported using immunohistochemistry (IHC) scores, such as the Allred score, the Remmele score, or the H score. These scores are based on the determination of the percentage of positive tumor cells and staining intensities. When using IHC scores, the definitions of receptor positivity or negativity depends to a large extend on the staining intensity and on the type of scoring system. For example, estrogen receptor (ER) positivity is assumed for <1% ER-positive tumor cells with intermediate or strong staining when using the Allred score (score results 3 or 4). However, with the Remmele score, a negative result (score 2) will be calculated with up to 50% positive tumor cells when low staining intensity is present. Therefore, the use of IHC scores is discouraged, and this is in line with other national and international guidelines.
ER Low-Positive Tumors
The ER low-positive group is characterized molecularly by having features of TNBC in the majority of cases. This includes basal-like phenotype [35], high incidence of germline BRCA mutation [36], and high risk score by Oncotype DX® [37]. Also, distant disease-free survival is similar to TNBC in these cases [38]. Therefore, a low threshold of 1% for ER positivity may lead to the false categorization of biologically ER-negative tumors as ER-positive ones in the low ER receptor expression group. We recommend careful consideration in the low ER-positive situation to rule out clinically insignificant ER expression in a tumor dominated by features of TNBC otherwise.
Adoption of the Updated ASCO/CAP Guideline for HER2 Testing
The recently updated ASCO/CAP guideline for HER2 testing in breast cancer [39] contains newly developed recommendations and testing algorithms regarding less common scenarios when using a dual-probe in situ hybridization (ISH) assay. Five different scenarios for HER2 copy numbers and centromere 17 ratios were described, and groups 2–4 are rare, involving <5% of cases. The diagnostic approach for these cases includes more rigorous interpretation criteria for ISH and requires concomitant IHC review to allow for combined interpretation of the ISH and IHC assays.
Immunohistological Detection of PD-L1 on Immune Cells
The immunohistological expression of PD-L1 on immune cells was shown to be predictive for prolonged progression-free survival among patients with metastatic TNBC treated with atezolizumab plus nab-paclitaxel [40]. In this clinical trial, the IHC staining was performed using the Ventana PD-L1 (SP142) assay with positive control (tonsil), either on the primary cancer or on metastatic tumor biopsy. Cytoplasmic staining of at least 1% of the leukocyte stromal infiltrate (lymphocytes, macrophages, plasma cells, granulocytes outside of abscesses) was shown to be predictive. For quality assurance, obligatory participation in further education and training measures is strongly recommended. Reference pathology should be requested in case of not yet completed qualification.
Lesions of Uncertain Malignant Potential (B3)
The group of lesions of uncertain malignant potential (B3) are detected typically in core or vacuum-assisted biopsy in asymptomatic women. The risk associated with B3 lesions cannot be strictly categorized according to the type of lesion (atypical ductal hyperplasia [ADH], flat epithelial atypia [FEA], lobular intraepithelial neoplasia, papilloma, radial scar), but additional clinical and pathological factors must be taken into consideration. The aim of further excision of B3 lesions is to detect more severe lesions (DCIS), but also to minimize the risk of progression of a lesion of low malignant potential to an in situ or invasive carcinoma.
ADH is characterized by a low-grade, monomorphic proliferation of atypical ductal epithelial cells which either partially or completely involve terminal ductal spaces, but involve the ductal spaces to a total extent of <2 mm. There is a particularly high risk for breast cancer when combined with BIRADS IV/V and high breast volume. DCIS or carcinoma occur in 30 and 8%, respectively, within a mean follow-up time of 76 months [41]. The reason for the high upgrade rate on open biopsy after the diagnosis of ADH is believed to be that ADH on core biopsy not infrequently represents inadequately sampled DCIS.
The role of lobular neoplasia as a precursor lesion for invasive lobular carcinoma has been confirmed recently. However, because of the low-risk nature of classical lobular neoplasia, a consensus has been reached that open excision can be avoided after the diagnosis of classical lobular neoplasia has been established on core biopsy and no discordant imaging, especially no focal lesion, is present [42]. In contrast, high-risk variants of lobular neoplasia, which include pleomorphic and florid lobular carcinoma in situ, are recommended for open biopsy and preferably complete excision.
A similar approach of conservative management avoiding open biopsy in the typical case of FEA has been recommended, provided that no mass lesion is present and imaging findings are concordant with pathological findings [43].
The diagnosis of solitary or multiple papillomas on core biopsy carries a risk of up to 30% (with atypia) for an invasive carcinoma or DCIS. However upgrade rates are widely different in the literature and may be as low as 3.1% [44]. It is important to distinguish papillomas, which are associated with mass lesions from peripheral papillomas that are often smaller but are commonly associated with proliferative breast disease. Certain clinical and imaging risk factors are associated with increased risk (patient age, lesion multiplicity, and peripheral location). Conservative management is justified provided that the biopsy has been sufficiently representative and no discordance to imaging results was evident.
A radial scar may mimic carcinoma mammographically because of its stellate appearance. Radial sclerosing lesions are only rarely associated with atypia or DCIS. Therefore, radial scars most often are benign lesions and recent studies with careful radiological correlation have indicated that open biopsy may not be necessary for small lesions and complete removal of the imaging abnormality. When radial scar is associated with atypia (such as FEA, ADH, or classical lobular neoplasia), management can the same as recommended in cases of atypia alone [45].
In summary, there is accumulating evidence that open biopsy may be necessary only for a subset of patients with FEA, lobular intraepithelial neoplasia, papilloma, or radial scar lesions, provided that careful radiological-pathological correlation was performed, no clinical, imaging, or pathological high-risk factors are present, and the imaging abnormality was completely or at least sufficiently representatively removed. This can often be achieved with a diagnostic-therapeutic vacuum-assisted biopsy. ADH is an exception and should be excised in most instances.
Prognostic and Predictive Factors
Prognostic and predictive factors are an essential part of therapy concepts in early and advanced breast cancer. In 2019, the AGO guidelines for prognostic and predictive factors did not change substantially, since the data presented in 2018 altered the LoEs, but not the AGO recommendations.
Pathological complete response (pCR) predicts a survival benefit [46]. However, patients with a large tumor (cT3/4), nodal involvement before NACT, and an invasive lobular subtype have an increased recurrence risk, despite pCR (LoE 2a/GR B/AGO+/–) [47].
In hormone receptor-positive HER2-negative early breast cancer with 0–3 involved lymph nodes, gene expression assays may be used if established clinical pathological factors do not allow therapy decisions regarding the use of chemotherapy in addition to the standard of endocrine therapy. Patients with an estimated risk of recurrence of >10% at 10 years are generally considered candidates for upfront or adjuvant chemotherapy. AGO recommends four tests (AGO “+”) that have been thoroughly validated retrospectively (LoE 1b for Endopredict®, Prosigna®) and prospectively (LoE IA for MammaPrint®, Oncotype DX®) for use in clinical routine. Prospective 5-year outcome data for Oncotype DX® from the WSG PlanB Trial [48] and for MammaPrint® from the MINDACT trial [49] confirmed an excellent outcome in pN0–1 patients who had low-risk test results. Clinical outcome data from an analysis of a large prospectively designed registry investigating patients over a longer period confirm these results in real-life clinical practice [50]. However, in 2018 the 9-year data of the prospectively randomized TAILORx trial were published, lacking a chemotherapy benefit in the intermediate-risk group with a recurrence score of 11–25 for patients >50 years and with a 36% relative risk increase with endocrine therapy compared to chemo-endocrine therapy in patients <50 years. Patients with a recurrence score of 26–100 had a distant metastasis rate of 13% despite chemotherapy. The whole intermediate-risk study cohort (recurrence score 11–25) had a distant disease-free survival of 94.5% without and of 95% with chemotherapy combined with endocrine treatment [51].
In HER2-positive early breast cancer, a meta-analysis (n = 967) demonstrated that pCR after neoadjuvant therapy (chemotherapy + anti-HER2 therapy) is significantly lower in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA)-mutant versus wild-type tumors (16.2 vs. 29.6%; p < 0.001) (LoE 1b). This difference was mostly due to a substantial difference in hormone receptor-positive tumors with pCR rates of 7.6 versus 24.2% (p < 0.001); in hormone receptor-negative tumors, the numerical difference was not significant (27.2 vs. 36.4%; p = 0.125) [52]. Due to the lack of clinical consequences, there is no AGO recommendation for PIK3CA mutation analysis before NACT so far (AGO+/–).
In triple-negative early breast cancer, germline BRCA status is predictive in response to NACT. In the neoadjuvant GeparQuinto trial, in 74 (15.8%) out of 469 TNBC patients with available germline DNA, BRCA1 (n = 61) or BRCA2 (n = 13) mutations were detected. pCR (ypT0/ypN0) was observed in 50% (n = 37) of the mutation carriers, but in only 31.1% (n = 123) of patients without mutations (p = 0.002). In patients without BRCA mutations (HR = 0.20, 95% CI 0.11–0.34, p < 0.001) but not in mutation carriers (HR = 0.48, 95% CI 0.18–1.27, p = 0.129), pCR (ypT0/ypN0) was significantly correlated with DFS [53]. As there are evidence-based consequences for patient management beyond the neoadjuvant setting, gBRCA determination is recommended in TNBC (LoE II/GR B/AGO+). Yet, as shown earlier in the GeparSixto trial, the use of platinum should not depend on the gBRCA status.
In patients with NACT, detection of ≥1 circulating tumor cell is an independent prognostic factor for locoregional relapse-free survival (HR = 1.8, 95% CI 1.2–2.7, p = 0.001), distant disease-free survival (HR = 2.4, 95% CI 1.9–3.1, p < 0.0001), as well as OS (HR = 2.6, 95% CI 1.9–3.4, p < 0.0001). Circulating tumor cell positivity was not correlated with pCR in this meta-analysis comprising 2,156 patients from 21 studies [54]. Despite the high LoE (1a/GR B), the AGO does not recommend circulating tumor cell detection in clinical routine due to the lack of therapeutic consequences (AGO+/–). In metastatic breast cancer, the presence of disseminated tumor cells is correlated with prognosis. Despite a new meta-analysis [55], the AGO did not change their recommendation grade, only the LoE (1/GR A/AGO+/–). Also cell-free DNA has a prognostic value in the adjuvant and the metastatic setting, but data regarding its clinical utility are still limited and the detection techniques different (LoE 1/GR B/AGO+/–) [56, 57].
Ductal Carcinoma in Situ
The diagnosis of DCIS increased following the introduction of screening mammography and comprises >20% of all newly diagnosed breast cancers [58]. However, epidemiological studies demonstrate that the removal of DCIS lesions has not been accompanied by a reduction in the incidence of invasive breast cancer [59, 60]. The challenge of DCIS is to minimize the risk of overdiagnosis, to avoid under- or overtreatment, and potentially to prevent the development of invasive breast cancer. DCIS is commonly diagnosed by mammography, but up to 20% of DCIS remain mammographically occult due to lack of calcifications and/or small tumor dimensions. The use of additional imaging techniques may theoretically be helpful to detect more DCIS or to observe the extent of a DCIS lesion and define surgical treatment. However, the role of MRI in DCIS is not well defined. Breast MRI has a high sensitivity in the diagnosis of invasive breast cancer, varying from 90 to 100%. The sensitivity for the diagnosis of DCIS is lower (77–96%). However, MRI does not improve the results of surgical therapy of DCIS [61, 62].
The biological characteristics of DCIS often predict recurrence and the type of invasive cancer that may develop in the future (e.g., no receptor expression versus hormone receptor-positive tumors). Among patients with DCIS, the breast cancer-specific mortality was associated with age at diagnosis, ethnicity, grade, size, and ER status [59]. With appropriate risk prediction of subsequent development of invasive cancer, there is a better chance for individualized therapy. Breast cancer surgery aims at the complete removal of the DCIS and represents the most favorable treatment in a majority of patients. Negative margins of at least 2 mm are associated with a reduced risk of ipsilateral DCIS or ipsilateral breast tumor recurrence (IBTR) compared with positive margins defined as ink on DCIS. Negative margins <2 mm alone are not an indication for mastectomy, and factors known to impact the rates of IBTR should be considered in determining the need for re-excision [63].
The majority of trials reveal that postsurgical endocrine treatment as well as radiation therapy both reduce IBTR, but do not show any effect on mortality. Radiotherapy after breast cancer surgery has been shown to reduce both in situ and invasive recurrences in five phase III trials, two of which have also demonstrated that tamoxifen 20 mg/day reduces the risk of ipsilateral and contralateral events by approximately 30% at both 10 and 15 years (LoE 1a/GR A/AGO+) [64, 65]. Omitting radiotherapy implies a slightly elevated risk for local recurrence without an effect on OS. The omission of adjuvant radiotherapy in selected low-risk cases such as those with tumors <2.5 cm, low and intermediate nuclear grade, and mammographically detected DCIS might be discussed in individual patients according to retrospective data analysis; this of course lacks level 1 evidence. The number needed to irradiate for IBTR is 9 over all risk groups. Retrospective evaluation of the ER status showed that tamoxifen reduced any subsequent breast events by 42% in ER-positive DCIS [64, 66]. AIs offer an endocrine option for postmenopausal women with ER-positive DCIS, depending on the patient's medical (e.g., osteoporosis and venous thrombosis) and short-term tolerability. However, OS is not improved by endocrine therapy. The number needed to treat with tamoxifen or AI for any ipsilateral breast event is 15.
The optimal management in particular of adjuvant treatment and long-term risks must be discussed with the individual patient. Hence, potential side effects of radiation therapy and endocrine therapy, albeit small, must be weighed more carefully when making treatment decisions for patients with DCIS.
Breast Cancer Surgery under Oncological Aspects
The extent of breast surgery has been consistently reduced in recent years. No ink on tumor is the accepted standard for resection margins for patients who undergo primary breast-conserving surgery or surgery, provided that all suspicious lesions according to preoperative imaging are resected (LoE 2a/GR A/AGO++).
Choi et al. [67] published the first study investigating the impact of the margins after NACT in respect to DFS and OS. Due to these findings, the authors concluded that after NACT there was no reason to aim for extended resection boundaries compared to the primary surgery.
Preoperative axillary assessment consists of thorough clinical and ultrasound examination. SLNB is the standard of care staging procedure in cN0 patients with invasive disease (LoE 1b/GR A/AGO++). Suspicious lymph nodes should be assessed by core needle biopsy.
In contrast, the role of axillary surgery is still under debate in patients with 1–2 positive sentinel lymph nodes (SLNs) after primary surgery. This was mainly due to limitations of the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial [68] and the AMAROS trial [69] as well as to the lack of confirmable studies. In 2017 the ACOSOG Z0011 trial presented 10-year follow-up data [70], with no differences in locoregional recurrences, DFS, and OS.
Three studies concerning healthcare research have been published in the last year [71, 72, 73] investigating the development of axillary surgery in patients with 1–2 positive lymph nodes in clinical routine. Morrow et al.'s [71] findings stated that 51% of the surgeons advised against axillary lymph node dissection (ALND). Poodt et al. [72] studied the operative procedure performed on 37,520 women by means of the Netherlands Cancer Registry. The rate of ALND declined from 75% in the year 2011 to 17% in the year 2015. Hennigs et al. [73] identified 13,741 patients with a cT1/2 cN0 tumor undergoing breast-conserving surgery with 1–2 positive SLNs. The rate of ALND declined from 94.5% (2008) to 46.9% (2015). Considerable differences in standard of care have been shown. ALND rates varied between 80.1 and 3.45% in the different institutions! The decrease in ALND covered women meeting the ACOSOG criteria, but was also seen in women undergoing mastectomy (from 79% in 2011 to 26% in 2015!). Although confirming studies are still pending (POSNOC, SENOMAC, SINOBAR-1, INSEMA Random2), there remains the need for clarification in clinical routine. Therefore the AGO, allowing for these results, changed its 2019 guidelines, advising to forego ALND procedure (LoE 1b/GR B/AGO–).
In the neoadjuvant setting the role of SLNB is still a matter of intense debate. Axillary staging after NACT offers a number of benefits: reduction to one-step surgery, reduction of the ALND rate due to conversion from cN+ to ycN0, and determination of pCR as an important prognostic parameter. However, older data on the feasibility and reliability of SLNB in this setting were controversial. The French GANEA 2 study [74] is the first prospective multicenter cohort study which enrolled 418 clinically node-negative patients who underwent SLNB alone after NACT. The detection rate for the SLN was 97%. Only 1 (0.2%) axillary recurrence was observed after 3 years. In a retrospective single-center study, Galimberti et al. [75] published similar results. After 5 years of follow-up, only 1 (0.4%) axillary recurrence was observed in 249 clinically node-negative patients who underwent SLNB after NACT. The AGO favors SLNB after NACT in cN0 patients (LoE 2b/GR B/AGO+). SLNB before NACT remains an option if an impact on adjuvant treatment decisions is expected (LoE 2b/GR B/AGO+/–).
For patients who initially present with (histologically proven) positive axillary lymph nodes (pN+), the feasibility and accuracy of SLNB is lower than in the adjuvant setting [76, 77, 78]. It is unclear whether the unfavorable false-negative rates translate into higher recurrence rates. Suggestions have been provided to improve the false-negative rate for patients who convert from cN+ to ycN0 after NACT. An unplanned retrospective analysis of the ACOSOG Z1071 revealed that the false-negative rate could be improved when >2 SLNs were removed or when a dual tracer technique was applied. More than 2 SLNs were, however, identified in only 43.1% of the patients in the ACOSOG study and in 34% in the SENTINA trial. The improvement of the false-negative rate by use of a dual tracer technique was not confirmed in the multivariate analysis of the SENTINA trial, but patients had significantly more lymph nodes removed (3 vs. 2).
Targeted axillary dissection offers a new treatment option in biopsy-proven node-positive patients who convert to cN0. Targeted axillary dissection implies the combined removal of the SLN and the core biopsy-positive target lymph node, which was marked with a clip, coil, seeds, or tattoo at the time of diagnosis. Hereby the false-negative rate can be reduced significantly [79]. In the retrospective analysis of a prospective register, Caudle et al. [79] described a false-negative rate of 10.1% with SLNB alone, of 4.2% with dissection of the target lymph node alone, and of 1.4% in case of combined dissection of SLN and target lymph node (targeted axillary dissection). In 23% of the cases the target lymph node was no SLN, which underlines the combination of both methods.
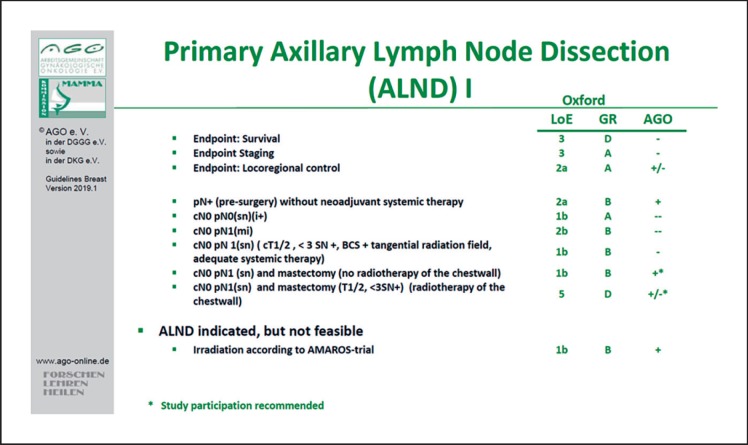
Despite the fact that many issues, including the surgical and the marker technique or the reproducibility of this retrospective single-center study, remain uncertain, routine ALND in patients who convert from pN+ to ycN0 appears to be overtreatment for many patients. Therefore, in the updated 2019 guideline the AGO recommends targeted axillary dissection as the procedure of choice (as opposed to ALND) for pN+/ycN0 patients (LoE 3b/GR C/AGO+) (Fig. 1). Due to open questions it seems advisable to gather patient data in a registry. Furthermore, the results of several studies (RISAS, SENTA, GANEA 3) are eagerly anticipated and will facilitate a binding clarification.
Fig. 1.
Indications for primary axillary lymph node dissection.
Oncoplastic and Reconstructive Surgery
Oncoplastic surgery today represents an essential component in the framework of an integrated treatment strategy for patients with breast carcinoma. It is defined as the use of plastic surgery techniques at the time of tumor removal, in order to achieve safe resection margins on the one hand, while on the other allowing an aesthetic breast shape [80]. Oncoplastic surgery focuses on favorable scar positioning, adequate soft tissue shaping, the choice of a suitable reconstruction procedure (particularly when radiotherapy is indicated), and reconstruction of the contralateral side in order to achieve symmetry. The basic principles of reconstructive surgery (AGO++) require planning in an interdisciplinary tumor board prior to the actual surgical procedure. In general, the least burdensome surgical technique with an aesthetic result that will be stable over the longer term should be selected. The patient must receive detailed information and advice about all surgical techniques and their advantages and disadvantages, and about the option of obtaining a second opinion. In case of an unfavorable tumor-breast ratio, neoadjuvant systemic therapy (NAST) might be considered, depending on the tumor biology. The preoperative counseling should include possible procedures for the contralateral breast if indicated. Contralateral procedures and subsequent operations in order to achieve symmetry should therefore be discussed with the patient even before the first operation. These operations are usually performed as secondary procedures after an interval of at least 3–6 months. The effects of radiotherapy on the affected side must be taken into account (e.g., volume reduction). Importantly, adjuvant therapy should not be delayed by breast reconstruction.
When mastectomy is necessary, skin-sparing techniques in suitably selected patients are associated with a similar recurrence rate and a better quality of life (LoE 2b/GR B/AGO++). Depending on the location of the tumor, the mammillary and areola complex can also be preserved. The same also applies to prophylactic mastectomy (LoE 2a/GR B/AGO+).
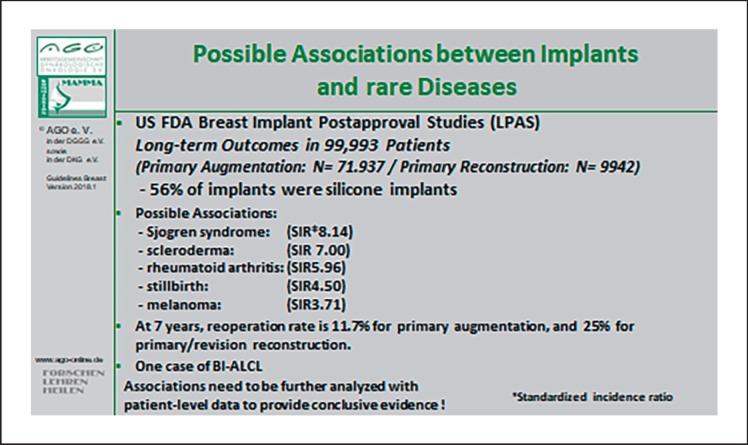
Following a mastectomy, reconstruction can be carried out using pedicled (LoE 3b/GR C/AGO+) or free tissue transfer (LoE 2a/GR B/AGO+), and also with the use of silicone gel-filled implants (LoE 2a/GR B/AGO+). A recent analysis including 55,279 patients reported equal oncological safety compared to saline implants [81]. Possible associations between implants and rare diseases are summarized in Figure 2 [82]. Reconstruction can be carried out either immediately or after an interval (LoE 3b/GR B/AGO++). The latter does not delay any necessary adjuvant therapies, but may result in shrinkage of the skin cover. When free tissue transfer is used, the deep inferior epigastric perforator or free transverse rectus abdominis muscle flaps are available (LoE 2a/GR B/AGO+). Both procedures are potentially muscle-sparing, but the deep inferior epigastric perforator is associated with a lower rate of herniation.
Fig. 2.
Possible associations between implants and rare diseases.
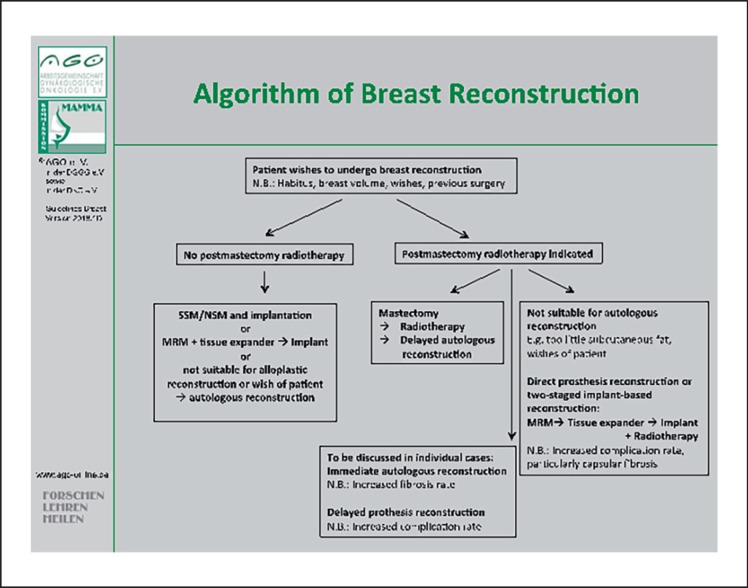
In case of heterologous reconstruction, immediate reconstruction with implants should be completed before starting radiotherapy to avoid a negative outcome (LoE 2a/GR B/AGO+) [83]. However, the patient must receive information about the high complication rate (e.g., capsule contraction, revision operations, failure of the reconstruction, impaired cosmetic appearance) and about the lower level of patient satisfaction in comparison with autologous reconstruction plus radiotherapy (LoE 2b/GR B) [84, 85](Fig. 3).
Fig. 3.
Algorithm for decision-making in cases with an indication for breast reconstruction.
In cases with indication for meshes, autologous tissue flaps (e.g., deepithelialized corium-fat flaps), acellular dermis, or synthetic mesh are available and of equivalent value (AGO+). Volume deficits and scars can be corrected using lipotransfer both after breast preservation and also after mastectomy with reconstruction (LoE 2a/GR B/AGO+). Numerous studies confirming the oncological safety of these approaches have been published in the meantime [86].
NACT: Subtype-Specific Therapies
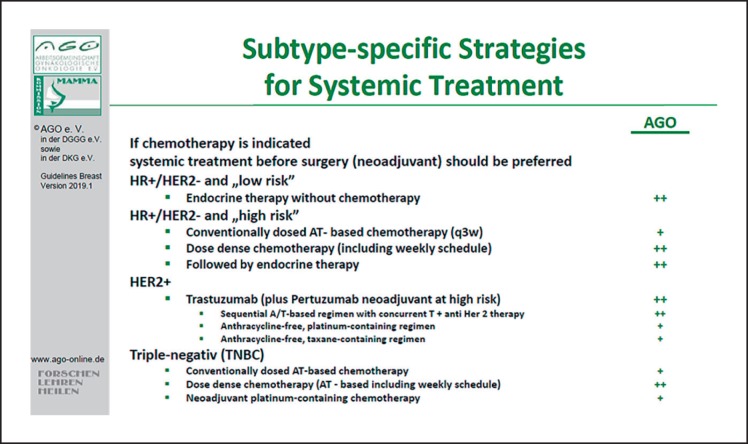
If there is an indication for systemic chemotherapy, the AGO recommends NAST. For patients with hormone receptor-positive, HER2-negative tumors at high risk, dose-dense chemotherapy, including weekly regimens, is preferred. For patients with HER2-positive tumors, neoadjuvant treatment should contain anthracyclines and taxanes plus trastuzumab (in high-risk patients plus pertuzumab), or an anthracycline-free regimen with carboplatin is recommended. For patients with TNBC, dose-dense chemotherapy including weekly regimens with anthracyclines and taxanes is recommended. For some patients the additions of a platinum analog is recommended (Fig. 4).
Fig. 4.
Subtype-specific strategies for systemic treatment indicating the recommendation to add pertuzumab only among cases with increased risk.
Survival rates are similar after primary systemic (“preoperative,” “neoadjuvant”) chemotherapy (NAST) and adjuvant therapy. pCR defined as ypT0 ypN0 or ypT0/is ypN0 is associated with improved survival [46, 87]. NAST is the preferred therapeutic option in patients who have an indication for adjuvant chemotherapy. A recent EBCTCG meta-analysis of NAST trials with a follow-up of 15 years detected an increased rate of breast conservation with equal DFS and OS (compared to adjuvant therapy). The increased rate of local recurrence cannot be attributed to NAST, but to different rates of recurrence after breast cancer surgery versus mastectomy [88]. Modern regimens are considered to be more efficient (higher pCR), and better surgical and radiotherapy approaches as well as pathology assessment have improved outcome after NAST. NAST allows response-guided therapies and novel post-neoadjuvant therapies in low- or nonresponding tumors. Dose-dense therapy regimens should be preferred (AGO++). In particular, in patient subgroups among whom a pCR is strongly associated with improved survival such as in TNBC, HER2-positive, and luminal B-like (hormone receptor-positive/HER2-negative/grade 3, high Ki-67) cancer, NACT (plus targeted therapy) should be the preferred therapeutic approach (AGO++). In patients with TNBC (regardless of gBRCA1/2 mutation status), a platinum-containing regimen may be considered (LoE 2b/GR B/AGO+) based on data from phase II randomized trials (e.g., GeparSixto, CALGB 40603) [89]. The addition of carboplatin was not only associated with an increased pCR rate in both neoadjuvant trials, but also resulted in a significant improvement in GeparSixto with a DFS rate of 85.8% (with carboplatin) versus 76.1% without carboplatin (HR = 0.56, p = 0.0350) and a clinically meaningful albeit statistically not significant improvement in DFS (absolute 5%) in the CALGB 40603 study. Furthermore, the results of the GeparSepto trial suggest particular benefit from using nab-paclitaxel 125 mg/m2 weekly instead of paclitaxel for patients with TNBC (LoE 1b/GR B/AGO+) [90, 91, 92].
For patients with HER2-positive tumors, HER2-directed therapy is standard as part of neoadjuvant and adjuvant therapy. Given the significant increase in pCR rates and the trend for improved progression-free survival observed in the neoadjuvant NeoSphere trial with the addition of pertuzumab to trastuzumab, dual blockade is highly recommended in patients at high risk for recurrence [93, 94, 95, 96] (LoE 2b/GR B/AGO++).
In patients with N+ or hormone receptor-negative/HER2-positive tumors, adjuvant therapy with trastuzumab and pertuzumab after surgery for a total of 52 weeks should be considered (LoE 2b/GR B/AGO+). With respect to endocrine neoadjuvant therapy, in exceptional situations, endocrine treatment with gonadotropin-releasing hormone analogs plus an AI may be considered for premenopausal women (LoE 1b/GR C/AGO+/–). Response-guided treatment has been shown to be beneficial within the GeparTrio trial. Consequently, in the case of response after two cycles of DAC (docetaxel, Adriamycin, cyclophosphamide) in hormone receptor-positive breast cancer, a total of eight instead of six cycles of DAC may be considered to be appropriate (LoE 2b/GR C/AGO+). In the case of no response after two cycles of DAC, continuation of NACT with a non-cross-resistant regimen (LoE 2b/GR B/AGO+) such as 4× vinorelbine/capecitabine (LoE 1b/GR B/AGO+) may be beneficial [97]. This can be an option in individual cases but cannot be considered as a routine approach. Post-neoadjuvant concepts are currently being investigated in clinical trials, and trial participation is recommended if possible, particularly in the case of no pCR. NAST is also recommended to allow a risk-adapted postoperative therapy (LoE 1b/GR A/AGO++).
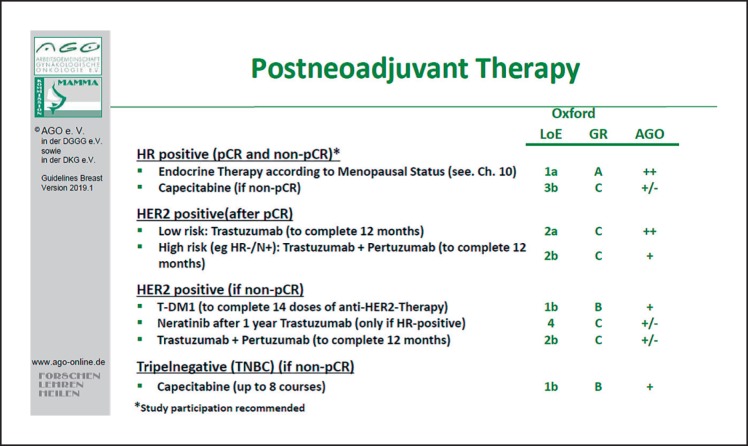
These are the AGO recommendations for post-neoadjuvant therapy: for patients with hormone receptor-positive tumors, endocrine therapy according to the menopausal status is recommended (Fig. 5). Given the positive results of the CREATE-X study, further chemotherapy with capecitabine in TNBC for no pCR can be considered (LoE 2b/GR B/AGO+) [98].
Fig. 5.
Residual disease after neoadjuvant systemic therapy – treatment recommendations.
For patients with HER2-positive tumors, adjuvant anti-HER2 therapy with either trastuzumab alone or plus pertuzumab (especially in high risk patients – node-positive or hormone receptor-negative) is recommended.
If residual disease is present after NAST (non-pCR), trastuzumab emtansine to complete 14 doses of anti-HER2 therapy is highly recommended [99], irrespective of the hormone receptor status, the volume of the residual disease, or other risk factors.
Novel predictive factors, such as tumor-infiltrating lymphocytes (LoE 1/GR B/AGO+) or PIK3CA mutation in the tumor (LoE 2/GR B/AGO+/–), are promising tools, but currently not recommended in the routine clinical setting [100, 101]. Patients with gBRCA mutations have a higher probability to achieve a pCR. The indications for mastectomy after NACT remain unchanged: positive margins after repeated excisions (LoE 3b/GR C/AGO++), lack of feasibility of radiotherapy (LoE 5/GR D/AGO++), and presence of inflammatory breast cancer (with no more than clinical complete response, LoE 2b/GR C/AGO+). In inflammatory breast cancer with pCR after NACT, BCT may be discussed with the patient. Furthermore, large tumors (cT4a–c) at first diagnosis represent only a relative indication for mastectomy after NACT (LoE 2b/GR B/AGO+/–). In the case of nodal involvement, biopsy and clip marking of the positive nodes is recommended if possible (LoE 5/GR D/AGO+). The SLN procedure is recommended preferably after neoadjuvant therapy in the case of cN0 (LoE 2b/GR B/AGO+) (see also the chapter on surgery) [77, 102, 103, 104]. Delayed initiation of NACT for thorough diagnosis (imaging and/or molecular pathology) is not correlated with a negative outcome (LoE 2b, GR B). Surgery after NACT should be planned 2–4 weeks after the last chemotherapy cycle, after patients have recovered from hematological toxicities (LoE 2b/GR B/AGO++) [105, 106].
Adjuvant Cytotoxic and Targeted Therapy
It is well known that adjuvant anthracycline- and taxane-based chemotherapy reduced breast cancer mortality by approximately one-third depending on the absolute risk without chemotherapy [107]. Furthermore, there was no significant difference in survival between adjuvant and NACT in early breast cancer despite higher local recurrence rates in tumors downsized with NACT [88]. In addition to downsizing tumors in the breast and axillary lymph nodes, NAST provides the unique opportunity to assess the in vivo efficacy of the therapy used and potentially to tailor post-neoadjuvant therapy appropriately. Hence, if chemotherapy is indicated, systemic treatment before surgery (neoadjuvant) is preferred.
Very recently, a patient-level meta-analysis of 37,298 women with early breast cancer in 26 randomized trials showed that increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling moderately improved the 10-year risk of recurrence and death from breast cancer without increasing mortality from other causes [108]. Because of this strong evidence, dose-dense chemotherapy (weekly regimen) (AGO++) instead of conventionally dosed anthracycline- and taxane-based chemotherapy (every 3 weeks) (AGO+) should be the preferred treatment option both for high-risk ER-positive breast cancer and TNBC. Especially in high-risk breast cancer with more than three involved axillary lymph nodes, dose-dense and dose-intensified chemotherapy showed superior survival compared with a conventional schedule [109]. Most strikingly, OS was improved with an absolute difference of 10% after 10 years of follow-up (AGO++).
Even though anthracycline- and taxane-containing chemotherapy is generally recommended, some patients might not be suitable for this due to preexisting comorbidities. In patients with reduced left ventricular ejection fraction, an anthracycline-free therapy with docetaxel/cyclophosphamide (AGO+) [110] might be an option, while other regimens such as weekly paclitaxel (AGO+/–) [111] or cyclophosphamide, methotrexate, and fluorouracil (AGO+/–) [107] may not be sufficiently effective. Patients suffering from sensory neuropathy might be treated taxane-free with fluorouracil, epirubicin, and cyclophosphamide (AGO+). In patients with HER2-positive early breast cancer, anti-HER2 therapy with trastuzumab is highly recommended (AGO++). Trastuzumab might be either combined with an anthracycline- and taxane-based chemotherapy (AGO++) or an anthracycline-free regimen like carboplatin/docetaxel (AGO+) [112]. In HER2-positive node-negative early breast cancer with a maximum diameter of 2 cm, trastuzumab might be combined with weekly paclitaxel (AGO+) [113]. In patients with HER2-positive early breast cancer with positive lymph nodes and/or ER negativity, pertuzumab in addition to trastuzumab and chemotherapy improved invasive disease-free survival (AGO+). In HER2-positive patients not achieving a pCR after NAST, the antibody-drug conjugate trastuzumab emtansine for the completion of 12 months anti-HER2 treatment considerably improved invasive disease-free survival (88.3 vs. 77.0%) (AGO++) [99]. In TNBC patients not achieving pCR after NACT, post-neoadjuvant therapy with capecitabine is an option (AGO+) [98].
Adjuvant Endocrine Therapy
Endocrine therapy is indicated in all patients with hormone receptor-positive breast cancer as well as those with low-positive cancer (≥1–9%; LoE 1/GR A/AGO++). In case of ER negativity/progesterone receptor (PR) positivity (≥10%), immunohistochemical reevaluation should be performed. This phenotype is extremely rare and therefore false positivity for PR should be excluded [114]. Treatment duration of 5 years remains to be the standard of care. Extended adjuvant treatment (EAT) might be indicated in patients with increased risk of relapse based on the individual risk-benefit ratio. There are still no validated biomarkers identifying patients at increased risk of late relapse.
Premenopausal Patients
No changes have been made. All statements from 2018 are still valid.
Postmenopausal Patients
The recommendations from 2018 have not been changed. However, for patients with lobular breast cancer, a nonsteroidal AI should be preferred over exemestane (steroidal) due to better OS as previously shown by the MA.27 trial [115] (LoE 2b/GR B/AGO+).
EAT in Premenopausal Women
In case of increased risk, tamoxifen therapy can be extended for up to 10 years (LoE 1a/GR A/AGO++). Based on the opinion of the AGO experts, EAT with 5 years of tamoxifen should also be offered to those patients with ovarian suppression and tamoxifen or AI for their initial treatment (LoE 5/GR D/AGO+). If the patient is confirmed as being postmenopausal within the first 5 years, endocrine therapy can be continued after 5 years of tamoxifen with 2.5–5 years of letrozole based on the results of the MA.17 trial (LoE 1b/GR B/AGO+).
EAT in Postmenopausal Women
After 5 years of tamoxifen, extended therapy with 5 years of tamoxifen is still an option in high-risk patients (LoE 1a/GR A/AGO+), but switching to an AI for 2–5 years should be preferred (LoE 1a/GR A/AGO++). If patients have received an AI-containing therapy (upfront or switch), only those patients who are at higher risk and have well tolerated AI therapy should be offered 2–5 additional years of AI (LoE 1b/GR B/AGO+). Factors including a clinical benefit from EAT are, e.g., initial treatment with 5 years of tamoxifen, positive lymph node status, T2/T3 tumors, high CTS5 score, or elevated risk based on immunohistochemical criteria and/or multigene expression assays [116]. EAT with AI can be safely interrupted for up to 3 months in selected patients who require a break based on the results of the SOLE trial [117] (LoE 1b/GR B/AGO+–).
Adjuvant Radiotherapy
As in previous years, the treatment recommendations for adjuvant radiotherapy were jointly developed by the AGO and the German Society of Radiation Oncology (Deutsche Gesellschaft für Radioonkologie, DEGRO). There is also consensus that hypofractionated radiation is the basis of adjuvant radiation therapy after BCT. Conventional radiation, on the other hand, has lost significance and is therefore only recommended with one (+). Complete renunciation of adjuvant radiation may be considered for patients with favorable prognostic factors and a life expectancy <10 years due to comorbidities or age. In this context, partial breast irradiation may be considered as a therapy option in patients aged >70 years (LoE 1a/GR A/AGO+). Boost irradiation of the tumor bed should be mandatory in premenopausal patients, whereas in the presence of a risk factor (>T1, G3, HER2-positive, triple-negative, EIC), boost irradiation should also be considered in postmenopausal patients.
In the case of mastectomy, postmastectomy irradiation should be mandatory in patients with more than three involved lymph nodes. In 1–3 involved lymph nodes and high-risk situation, postmastectomy irradiation should urgently be considered. At present, there are no subgroup analyses from the large studies on postmastectomy irradiation available that can clearly define specific low-risk cohorts. Boost irradiation during postmastectomy irradiation has no effect on breast cancer-specific OS [118] and should therefore be performed only in case of proven R1/R2 resection. For tumors close to or in touch with the pectoral fascia, but not growing beyond, a R0 resection should be assumed if the pectoral fascia has been resected.
In case of 1–2 involved SLNs and no consecutive ALND (according to the ACOSOG Z0011 criteria) and breast-conserving surgery, radiotherapy should encompass those parts of levels 1 and 2 located >5 mm below the axillary vein. This clarification is due to the fact that the application of intensity-modulated radiotherapy significantly reduces the effective radiation dose in levels 1 and 2 compared to the application of a 3D or high-tangent radiation field [119]. If the ACOSOG Z0011 criteria are not met, the axilla should be irradiated according to the criteria of the AMAROS study.
Irradiation of the infra- and supraclavicular lymph nodes should be performed in cases with >4 involved lymph nodes as well as in infestation of level 3 or proven infestation of the supra-/infraclavicular lymph nodes. In case of 1–3 involved lymph nodes, irradiation of infra- and supraclavicular lymph nodes should be considered in the presence of risk factors (premenopausal patient and G2–3 or ER/PR-negative as well as central or medial tumor seat). However, micrometastases are excluded from this risk category. This also applies to the irradiation of internal mammary lymph nodes. Irradiation should not be performed in the presence of cardiac risk factors and concomitant trastuzumab therapy.
According to current data, the increasing use of molecular risk profiles does not constitute a basis for deciding to forego radiation therapy in the case of a favorable risk profile. The increasing data with regard to post-neoadjuvant therapy concepts must be taken into account since molecular therapies or cytostatics are also used parallel to irradiation. Therapy with the combination of trastuzumab/pertuzumab [120] and trastuzumab emtansine [99], checkpoint inhibitors [121], or capecitabine [122] is possible with respect to toxicity.
Breast Cancer: Special Situations
Breast cancer in pregnancy should be treated as close as possible according to guidelines for nonpregnant patients [123, 124]. However, staging, systemic therapy and radiotherapy do have some restrictions due to potential fetal harm. Recent cohort studies indicate that whole-body MRI without a contrast agent provides valuable staging information and may be considered in individual high-risk cases (AGO+/–) [125, 126]. Surgery should be performed as in nonpregnant women and SLNB with technetium, but not with blue dye, is feasible [127]. Regarding systemic therapy, anthracyclines and taxanes seem to be safe for use in pregnancy after the first trimester [128]. Platinum salts may be considered (AGO+/–) based on data mostly from gynecological tumors [129, 130]. Radiotherapy, endocrine therapy, and monoclonal antibodies should be avoided during pregnancy [131, 132]. Early termination of pregnancy does not improve maternal outcome, and preterm delivery for maternal reasons should be avoided as well as delivery during chemotherapy-induced leukocyte nadir. The prognosis of patients with adequate treatment is not worse than that of nonpregnant women [122, 133, 134].
Male breast cancer should be treated mostly according to that in females, especially with chemotherapy and HER2-targeted treatment [135]. Tamoxifen is the standard for endocrine treatment. Some evidence also exists for AIs and fulvestrant [136, 137].
In inflammatory breast cancer, mastectomy is the standard of care (AGO+) [138]. Patients without distant metastases should receive chemotherapy and radiotherapy [139].
In axillary metastases of occult breast cancer (axillary carcinoma of unknown primary), radiotherapy of the ipsilateral breast seems to improve outcome based on retrospective data (AGO+): In a large case series from the National Cancer Database, axillary metastases with an occult breast cancer were very rare (0.09%). Treatment with radiotherapy and axillary dissection was an independent predictor of OS in multivariate analysis (HR = 0.509, 95% CI 0.321–0.808, p = 0.004) [140]. Breast imaging should include mammography, ultrasound, and MRI [141].
Metaplastic breast cancer is a rare subtype with an incidence of 0.2–5% of all breast cancers [142]. These tumors are characterized by an epithelial and mesenchymal differentiation and a high proliferation rate. These different components are the basis for the classification according to the World Health Organization criteria [143]. More than 90% of metaplastic breast cancers are negative for ER, PR, and HER2 but, in contrast, there is an overexpression of HER1 and cytokeratin 5/6 (stem cell and BRCA-like) [144] and the molecular profile is more basal-like [145]. The clinical features show often large tumors at diagnosis (>5 cm), frequent hematogenous metastases, and nodal involvement in about 20% of all cases. Imaging and gaining histology for diagnosis should be performed according to standard (AGO++). Due to the high frequency of hematogenous metastases, staging should encompass chest and abdominal CT (AGO++). Surgical treatment can be performed according to standard (including SLNB, AGO+), but mastectomy is more often necessary due to more advanced tumor stage and the goal of tumor-free resection margins [146, 147]. Adjuvant treatment consists of chemotherapy (even tumors are more chemoresistant) [148], endocrine therapy (only in hormone receptor-positive tumors) [149], and standard radiotherapy.
Breast implant-associated anaplastic large cell lymphoma, a rare peripheral T-cell lymphoma associated with microtextured implant surfaces, is increasing in incidence. It represents 3% of non-Hodgkin lymphomas and causes only 0.04–0.5% of all malignant breast diseases with an incidence of 0.6–1.2/100.00 women with breast implants [150]. The median interval to diagnosis of breast implant-associated anaplastic large cell lymphoma is about 8 years. The symptoms include swelling and late seroma (60%), solid tumor (17%), or seroma and solid tumor (20%). The histology is defined by a CD30-positive ALK T-cell lymphoma, which has to be reported as a serious adverse event to the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM). The 5-year OS is 89% [151]. All newly diagnosed seromas more than a year after breast implant placement should be clarified by ultrasound and cytology (AGO++). In addition, breast MRI for confirmation of diagnosis, and consecutively the nodal status as well as PET-CT and bone marrow biopsy, should be performed (AGO++). Therapy includes implant removal (documentation of the implant) and total capsulectomy plus removal of tumor and suspicious lymph nodes (AGO++). Chemotherapy might be indicated in case of extracapsular extension of the tumor and radiation in case of unresectable tumor or residual disease (AGO+/–). In case of planned implant-based reconstruction, a risk disclosure for breast implant-associated anaplastic large cell lymphoma is mandatory. Semiannual clinical examinations for the first 5 years and semiannual ultrasound examinations for the first 2 years are recommended. In case of late seroma and surgically resected implant capsule, histological exclusion of an anaplastic large cell lymphoma is indicated [152].
Complementary Therapy and Survivorship
The integration of complementary methods continues to be a challenge in the standard treatment of breast cancer. The two main reasons for this are (1) the still lacking general definition of complementary medicine and (2) the fact that only a few “conventional studies” exist that provide clear evidence of the efficacy of complementary approaches and risk-benefit ratios.
Since it is estimated that more than half of the patients make use of complementary medicine, this is an important topic for the medical consultation. Patients should be made aware of possible risks and interactions with standard breast cancer therapy. They should be informed that complementary medicine can support their evidence-based standard therapy, but that they should not consider it as a substitute.
In 2019, the AGO recommendations “Complementary Therapy and Survivorship” did not change substantially compared to 2018. It has been added that diagnostic procedures offered in complementary and alternative therapy concepts without evidence (e.g., iris diagnostics) should not be recommended.
Further recently published studies underline the effects of physical exercise on breast cancer-related secondary lymphedema (LoE 1a/GR A/AGO+). A recently published systematic review including eleven RCTs found evidence that exercise can improve subjective and objective parameters in patients with breast cancer-related lymphedema [153].
Evidence is growing that mind-body interventions, including cognitive therapies, behavioral therapies, relaxation techniques, and meditation, improve quality of life among breast cancer patients, and therefore clinical guidelines have begun to include recommendations. A systemic review and meta-analysis of 19 RCTs (n = 2,806) revealed evidence that mind-body interventions are efficacious for reducing fear of cancer recurrence, although further investigation are recommended to analyze the optimal integration of mind-body practices (LoE 1a/GR A/AGO+) [154].
Some small studies have shown an effect of yoga on perioperative shoulder and arm pain (LoE 2b/GR C/AGO+) and music therapy on pain in women undergoing mastectomy (LoE 2b/GR C/AGO+/–) [155, 156]. However, the quality of evidence was low and the results were inconsistent. First pilot trials have investigated the feasibility and effects on quality of life of short-term fasting during chemotherapy in breast and ovarian cancer patients (LoE 3b/GR C/AGO+/–) [157]. Larger studies are needed to investigate the effect of fasting during chemotherapy.
Gynecological Issues in Breast Cancer Patients and Contraception
Treatment of Menopausal Symptoms
Classical hormonal replacement therapy to alleviate menopausal symptoms is not indicated in breast cancer patients, particularly in ER-positive disease (LoE 1b/GR B/AGO–), but might be considered in individual cases and after failure of other nonhormonal treatments (LoE 3b/GR B/AGO+/–) considering individual patients' combination of low-dose estradiol with tamoxifen (LoE 2b/GR B/AGO+/–). Tibolone is contraindicated (LoE 1b/GR A/AGO–/–), while topical vaginal application of low-dose estriol may be used for urogenital symptoms (LoE 4/GR D/AGO+/–). Menopausal symptoms such as hot flushes, night sweats, or sleep disturbances may be treated with various nonhormonal remedies, e.g., serotonin reuptake inhibitors (i.e., venlafaxine [LoE 1a/GR A/AGO+]), which carry the potential to reduce hot flushes by about 60%. At SABCS 2018, a randomized double-blind placebo-controlled trial of oxybutynin for hot flushes (ACCRU study SC-1603) was presented. Oxybutynin significantly improves the frequency and severity of hot flushes and was associated with a positive impact on several quality of life metrics. Patients on 5 mg (two times a day) experienced more reduction in hot flushes and improvement in more quality of life measures (LoE 1b/GR A/AGO+/–) [158].
According to a randomized double-blind placebo-controlled trial, duloxetine (30 mg in one capsule daily for 1 week, followed by two capsules daily for 11 weeks, followed by tapering of the medication during which patients took one capsule daily for 1 week) in AI-treated patients was able to significantly reduce arthralgia (LoE 1b/GR B/AGO+) [159].
The majority of studies on the efficacy of herbal treatments for menopausal symptoms – mostly hot flushes – were not conducted in women with breast cancer. Neither flax seed, black cohosh (Cimicifuga racemosa), St. John's wort, ginseng root, nor soy could improve menopausal symptoms.
Physical exercise and cognitive behavioral therapy have positive effects on menopausal symptoms and, to a lesser degree, on the sexuality and physical functioning of patients with breast cancer experiencing treatment-induced menopause (LoE 1b/GR B/AGO+). Mind-body medicine (relaxation training, yoga, hypnosis) is reported to result in a moderate and even significant improvement in scores of hot flushes, joint pain, fatigue, sleep, mood, and relaxation (LoE 1b/GR B/AGO+/+). These effects are seen even after longer periods of application and some months after stopping mind-body medicine. There are contradictory data about the effect of acupuncture on hot flushes, but in a review comparing twelve randomized controlled trials, the authors concluded that acupuncture had no significant effect on reducing hot flushes (LoE 1a/GR A/AGO+/–) [160]. Acupuncture or electroacupuncture might be used to treat AI-induced joint pain (LoE 1b/GR B/AGO+) [161, 162].
Fertility Preservation
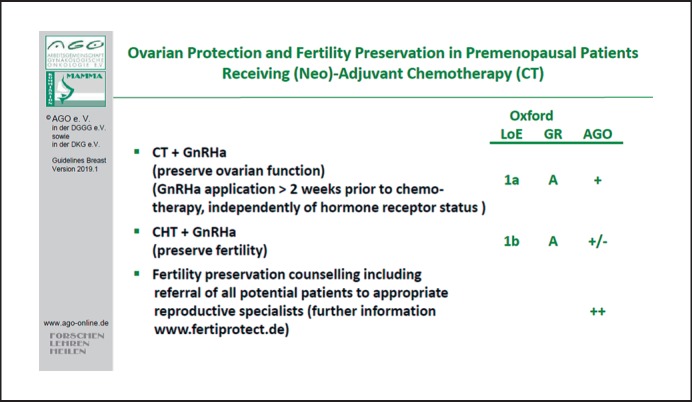
Counseling on fertility preservation is suggested in all patients who wish to retain their fertility (LoE 4/GR C/AGO+). Application of gonadotropin-releasing hormone analogs 2 weeks prior to chemotherapy has been shown to result in a higher rate of recovery of ovarian function after 2 years (LoE 1a/GR B/AGO+) and might have a moderate effect on fertility preservation (LoE 2a/GR B/AGO+/–) [163]. No negative effect with regard to prognosis could be observed independent of hormone receptor status of the primary tumor (Fig. 6).
Fig. 6.
Ovarian protection and fertility preservation in premenopausal patients receiving (neo)adjuvant chemotherapy.
Menstrual history is reliable only in women under 45 years of age. A more precise evaluation of the ovarian reserve (particularly in perimenopausal patients) may be obtained by the measurement of follicle-stimulating hormone and estradiol levels in the peripheral blood. Low anti-Müllerian hormone levels seem to be indicative of reduced ovarian reserve and chemotherapy-related amenorrhea in chemotherapy-treated breast cancer patients [164]. An antral follicle count, defined as the sum of the follicle diameters of all follicles of 10 mm in both ovaries, can be easily performed at little extra cost.
Contraception
All patients of childbearing potential must be counseled about adequate contraception prior to systemic therapy, since cytotoxic treatment, including endocrine therapy, by itself does not confer reliable protection against pregnancy. The majority of contraceptive measures have not been tested in women after breast cancer, and hormone-free methods are the first choice for patients with breast cancer.
Sexual Health
Sexual complaints are common in breast cancer patients and should be assessed. They include sexual desire disorder/decreased libido (23–64% of patients), arousal or lubrication concerns (20–48% of patients), and dyspareunia (35–38% of patients). Screening tools may help physicians to address sexual health issues (LoE 4/GR C/AGO+).
Nonhormonal lubricants and moisturizers are the primary treatment for vaginal dryness. Silicone-based products may last longer than water- or glycerin-based products (LoE 1b/GR B/AGO+). Microablative fractionated laser or vaginal YAG/erbium laser may be an option for some patients to alleviate genital atrophy (LoE 2b/GR B/AGO+/–) [165].
Follow-Up of Breast Cancer
The rationale in breast cancer follow-up is the early detection of curable breast cancer events, i.e., breast or locoregional recurrence (LoE 1a/GR B/AGO++) or early detection of contralateral cancer (LoE 1a/GR B/AGO++). Early detection of symptomatic metastases is desirable (LoE 3b/GR C/AGO+); however, with regard to the early detection of asymptomatic metastases (LoE 1a/GR A/AGO–), data are inconsistent and, most importantly, do not suggest a survival benefit.
Beyond improvement of survival, additional issues such as improvement of quality of life and physical performance and the reduction and early detection of treatment-related side effects are important concerns in this matter (LoE 2b/GR B/AGO+). In addition, reevaluation of current adjuvant therapies and the assessment or improvement of treatment adherence (especially to endocrine therapy) is an essential part of follow-up care (LoE 2b/GR B/AGO++). It should thus be pointed out that every patient has the right to obtain a second opinion (LoE 2c/GR B/AGO++); genetic counseling should be offered if indicated, as should hormone replacement therapy, prophylactic surgery, and breast reconstruction (LoE 2c/GR C/AGO+). Further issues, such as pregnancy, contraception, sexuality, quality of life, menopausal symptoms, and specific psychological aspects, should be addressed proactively (LoE 4/GR C/AGO+). Inclusion of related persons (partner, family, friends, caregivers) in counseling is highly recommended.
Lifestyle modifications (cessation of smoking, fat-reduced diet, reduced alcohol consumption, physical activity, nightly fasting, distress reduction) and interventions with regard to comorbidities (diabetes) are further important aspects of follow-up.
From the patient's perspective, examination of the breast, reassurance, guidance and answering questions, evaluation of treatment including side effects, and psychosocial support are also essential.
Most importantly, follow-up examinations in asymptomatic patients in routine situations should not consist of tumor marker measurements, liver ultrasound, bone scans, X-ray, CT or PET scans, and monitoring of circulating tumor cells. For the detection of curable events, self-examination and physical examination in combination with mammography and adjunctive ultrasound are recommended. Follow-up of male breast cancer should follow the same procedures as in female breast cancer (LoE 5/GR D/AGO+).
In case of increased risk, such as age <50 years, hormone receptor negativity, and decreased diagnostic accessibility C/D in mammography and ultrasound, MRI should be considered [166].
In this context, screening for secondary malignancies according to guidelines (e.g., colorectal cancer, endometrial cancer, ovarian cancer, cervical cancer, lymphoma) is meaningful. Patients and physicians should be aware of the increased risk of myelodysplastic syndrome and acute myeloid leukemia after chemotherapy and lung cancer after radiotherapy to the chest wall, especially in smokers. Further, a DXA scan at baseline and a repeat scan according to individual risk in women with premature ovarian failure or in women on AI therapy are recommended [167].
Disclosure Statement
The conflict of interest statements of all authors with conflicts of interest can be found in the supplementary material (see www.karger.com/doi/10.1159/000501000).
Supplementary Material
Supplementary data
References
- 1. www.cebm.net.
- 2.Empfehlungen Gynäkologische Onkologie Kommission Mamma 2019 www.ago-online. [Google Scholar]
- 3.Marc P. McRae. The Benefits of Dietary Fiber Intake on Reducing the Risk of Cancer: An Umbrella Review of Meta-analyses. Nutr J. 2018 Sep;17((1)):87. doi: 10.1016/j.jcm.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Boer MC, Wörner EA, Verlaan D, van Leeuwen PA. The Mechanisms and Effects of Physical Activity on Breast Cancer. Clin Breast Cancer. 2017 Jul;17((4)):272–8. doi: 10.1016/j.clbc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, et al. WHI Investigators Menopausal hormone therapy and long-term all-cause and cause-specific mortality, the women's health initiative randomized trials. JAMA. 2017 Sep;318((10)):927–38. doi: 10.1001/jama.2017.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017 Aug;377((6)):523–33. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 7.Bick U, Engel C, Krug B, Heindel W, Fallenberg EM, Rhiem K, et al. German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC) High-risk breast cancer surveillance with MRI: 10-year experience from the German consortium for hereditary breast and ovarian cancer. Breast Cancer Res Treat. 2019 Feb;175((1)):217–28. doi: 10.1007/s10549-019-05152-9. 10.1007/s10549-019-05152-98. [DOI] [PubMed] [Google Scholar]
- 8.Xiao YL, Wang K, Liu Q, Li J, Zhang X, Li HY. Risk Reduction and Survival Benefit of Risk-Reducing Salpingo-oophorectomy in Hereditary Breast Cancer: Meta-analysis and Systematic Review. Clin Breast Cancer. 2019 Feb;19((1)):e48–65. doi: 10.1016/j.clbc.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Loibl S, Weber KE, Timms KM, Elkin EP, Hahnen E, Fasching PA, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018 Dec;29((12)):2341–7. doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 10.Sankatsing VD, van Ravesteyn NT, Heijnsdijk EA, Looman CW, van Luijt PA, Fracheboud J, et al. The effect of population-based mammography screening in Dutch municipalities on breast cancer mortality: 20 years of follow-up. Int J Cancer. 2017 Aug;141((4)):671–7. doi: 10.1002/ijc.30754. [DOI] [PubMed] [Google Scholar]
- 11.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. American Cancer Society Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA. 2015 Oct;314((15)):1599–614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ECIBC. 2016. European Commission on Breast Cancer: Statements. Abruf Evidencereport update 24/11/2016, Zugriff 20122016: http://ecibc.jrc.ec.europa.eu/recommendations/list/3
- 13.Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. International Agency for Research on Cancer Handbook Working Group Breast-cancer screening—viewpoint of the IARC Working Group. N Engl J Med. 2015 Jun;372((24)):2353–8. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 14.Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016 Feb;164((4)):244–55. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 15.Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of Breast Cancer Screening: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016 Feb;164((4)):256–67. doi: 10.7326/M15-0970. [DOI] [PubMed] [Google Scholar]
- 16.USPSTF 2016: US Preventive Services Task Force Final Recommendation Statement for mammographyScreening (12.01.2016); IACR Handbook 2016: Website for the IARC publications: http://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Breast-Cancer-Screening-2016
- 17.Moss SM, Wale C, Smith R, Evans A, Cuckle H, Duffy SW. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age trial at 17 years' follow-up: a randomised controlled trial. Lancet Oncol. 2015 Sep;16((9)):1123–32. doi: 10.1016/S1470-2045(15)00128-X. [DOI] [PubMed] [Google Scholar]
- 18.ACS 2015: Systematic Review of Cancer Screening Literature for Updating American Cancer Society Breast Cancer Screening Guidelines Duke Evidence Synthesis Group. 2016. http://www.cancer.org/acs/groups/content/documents/document/acspc-046315.pdf.
- 19.Skaane P, Bandos AI, Niklason LT, Sebuødegård S, Østerås BH, Gullien R, et al. Digital Mammography versus Digital Mammography Plus Tomosynthesis in Breast Cancer Screening: The Oslo Tomosynthesis Screening Trial. Radiology. 2019 Apr;291((1)):23–30. doi: 10.1148/radiol.2019182394. [DOI] [PubMed] [Google Scholar]
- 20.Caumo F, Bernardi D, Ciatto S, Macaskill P, Pellegrini M, Brunelli S, et al. Incremental effect from integrating 3D-mammography (tomosynthesis) with 2D-mammography: increased breast cancer detection evident for screening centres in a population-based trial. Breast. 2014 Feb;23((1)):76–80. doi: 10.1016/j.breast.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014 Jun;311((24)):2499–507. doi: 10.1001/jama.2014.6095. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert FJ, Tucker L, Gillan MG, Willsher P, Cooke J, Duncan KA, et al. The TOMMY trial: a comparison of TOMosynthesis with digital MammographY in the UK NHS Breast Screening Programme—a multicentre retrospective reading study comparing the diagnostic performance of digital breast tomosynthesis and digital mammography with digital mammography alone. Health Technol Assess. 2015 Jan;19((4)):i–xxv. doi: 10.3310/hta19040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson R, Heywang-Köbrunner SH, Harvey SC, Edwards M, Shaikh J, Arber M, et al. Systematic review of 3D mammography for breast cancer screening. Breast. 2016 Jun;27:52–61. doi: 10.1016/j.breast.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental Screening for Breast Cancer in Women With Dense Breasts: A Systematic Review for the U.S. Preventive Service Task Force Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Jan. Report No.: 14-05201-EF-3. [PubMed] [Google Scholar]
- 25.Morel JC, Iqbal A, Wasan RK, Peacock C, Evans DR, Rahim R, et al. The accuracy of digital breast tomosynthesis compared with coned compression magnification mammography in the assessment of abnormalities found on mammography. Clin Radiol. 2014 Nov;69((11)):1112–6. doi: 10.1016/j.crad.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Cornford EJ, Turnbull AE, James JJ, Tsang R, Akram T, Burrell HC, et al. Accuracy of GE digital breast tomosynthesis vs supplementary mammographic views for diagnosis of screen-detected soft-tissue breast lesions. Br J Radiol. 2016;89((1058)):20150735. doi: 10.1259/bjr.20150735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Radiology (ACR) In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. D'Orsi CJ, Sickles EA, Mendelson EB, et al., editors. Reston, VA, USA: American College of Radiology; 2013. [Google Scholar]
- 28.Müller-Schimpfle M. BI-RADS die 5.–Eine Kurzmitteilung aus deutsch-/ österreichischer Sicht. Senologie - Zeitschrift für Mammadiagnostik und -therapie. 2016;13((03)):132–143. [Google Scholar]
- 29.El Sharouni MA, Postma EL, Menezes GL, van den Bosch MA, Pijnappel RM, Witkamp AJ, et al. High prevalence of MRI-detected contralateral and ipsilateral malignant findings in patients with invasive ductolobular breast cancer: impact on surgical management. Clin Breast Cancer. 2016 Aug;16((4)):269–75. doi: 10.1016/j.clbc.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013 Feb;257((2)):249–55. doi: 10.1097/SLA.0b013e31827a8d17. [DOI] [PubMed] [Google Scholar]
- 31.Houssami N, Turner RM, Morrow M. Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res Treat. 2017 Sep;165((2)):273–83. doi: 10.1007/s10549-017-4324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piato JR, de Andrade RD, Chala LF, de Barros N, Mano MS, Melitto AS, et al. MRI to Predict Nipple Involvement in Breast Cancer Patients. AJR Am J Roentgenol. 2016 May;206((5)):1124–30. doi: 10.2214/AJR.15.15187. [DOI] [PubMed] [Google Scholar]
- 33.Luparia A, Mariscotti G, Durando M, et al. Accuracy of tumour size assessment in the preoperative staging of breast cancer: comparison of digital mammography, tomosynthesis, ultrasound and MRI. Radiol Med. 2013 Oct;118((7)):1119–1136. doi: 10.1007/s11547-013-0941-z. [DOI] [PubMed] [Google Scholar]
- 34.deSouza NM, Liu Y, Chiti A, et al. Strategies and technical challenges for imaging oligometastatic disease: Recommendations from the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2018 Jan 10mar;91:153–163. doi: 10.1016/j.ejca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Deyarmin B, Kane JL, Valente AL, van Laar R, Gallagher C, Shriver CD, et al. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013 Jan;20((1)):87–93. doi: 10.1245/s10434-012-2588-8. [DOI] [PubMed] [Google Scholar]
- 36.Sanford RA, Song J, Gutierrez-Barrera AM, Profato J, Woodson A, Litton JK, et al. High incidence of germline BRCA mutation in patients with ER low-positive/PR low-positive/HER-2 neu negative tumors. Cancer. 2015 Oct;121((19)):3422–7. doi: 10.1002/cncr.29572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon JM, Cameron DA, Arthur LM, Axelrod DM, Renshaw L, Thomas JS, et al. Accurate Estrogen Receptor Quantification in Patients with Negative and Low-Positive Estrogen-Receptor-Expressing Breast Tumors: Sub-Analyses of Data from Two Clinical Studies. Adv Ther. 2019 Apr;36((4)):828–41. doi: 10.1007/s12325-019-0896-0. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Yi M, Huo L, Koenig KB, Mittendorf EA, Meric-Bernstam F, Kuerer HM, et al. Which threshold for ER positivity? a retrospective study based on 9639 patients. Ann Oncol. 2014 May;25((5)):1004–11. doi: 10.1093/annonc/mdu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018 Nov;142((11)):1364–82. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 40.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. IMpassion130 Trial Investigators Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018 Nov;379((22)):2108–21. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 41.Renshaw AA, Gould EW. Long term clinical follow-up of atypical ductal hyperplasia and lobular carcinoma in situ in breast core needle biopsies. Pathology. 2016 Jan;48((1)):25–9. doi: 10.1016/j.pathol.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Sinn P, Brucker SY, Budach W, et al. 2017. DCIS und Risikoläsionen; in Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms (Hrsg: Leitlinienprogramm Onkologie der AWMF, DKG und DKH) pp. pp 79–89. [Google Scholar]
- 43.Calhoun BC, Sobel A, White RL, Gromet M, Flippo T, Sarantou T, et al. Management of flat epithelial atypia on breast core biopsy may be individualized based on correlation with imaging studies. Mod Pathol. 2015 May;28((5)):670–6. doi: 10.1038/modpathol.2014.159. [DOI] [PubMed] [Google Scholar]
- 44.Khan S, Diaz A, Archer KJ, Lehman RR, Mullins T, Cardenosa G, et al. Papillary lesions of the breast: to excise or observe? Breast J. 2018 May;24((3)):350–5. doi: 10.1111/tbj.12907. [DOI] [PubMed] [Google Scholar]
- 45.Rageth CJ, O'Flynn EA, Pinker K, et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions) Review, Breast Cancer Res Treat, 2018 doi: 10.1007/s10549-018-05071-1. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 Jul;384((9938)):164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 47.Huober J, Schneeweiss A, Blohmer J-U, et al. Factors predicting relapse in early breast cancer patients with a pathological complete response after neoadjuvant therapy – Results of a pooled analysis based on the GBG meta-database. SABCS. 2018 P2-08-01. [Google Scholar]
- 48.Gluz O, Nitz UA, Christgen M, Kates RE, Shak S, Clemens M, et al. West German Study Group Phase III PlanB Trial: First Prospective Outcome Data for the 21-Gene Recurrence Score Assay and Concordance of Prognostic Markers by Central and Local Pathology Assessment. J Clin Oncol. 2016 Jul;34((20)):2341–9. doi: 10.1200/JCO.2015.63.5383. [DOI] [PubMed] [Google Scholar]
- 49.Cardoso F, van't Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. MINDACT Investigators 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016 Aug;375((8)):717–29. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 50.Stemmer SM, Steiner M, Rizel S, Soussan-Gutman L, Ben-Baruch N, Bareket-Samish A, et al. Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017 Sep;3((1)):33. doi: 10.1038/s41523-017-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018 Jul;379((2)):111–21. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loibl S, Majewski I, Guarneri V, Nekljudova V, Holmes E, Bria E, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol. 2016 Aug;27((8)):1519–25. doi: 10.1093/annonc/mdw197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fasching PA, Loibl S, Hu C, Hart SN, Shimelis H, Moore R, et al. BRCA1/2 Mutations and Bevacizumab in the Neoadjuvant Treatment of Breast Cancer: Response and Prognosis Results in Patients With Triple-Negative Breast Cancer From the GeparQuinto Study. J Clin Oncol. 2018 Aug;36((22)):2281–7. doi: 10.1200/JCO.2017.77.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A, et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J Natl Cancer Inst. 2018 Jun;110((6)):560–7. doi: 10.1093/jnci/djy018. [DOI] [PubMed] [Google Scholar]
- 55.Hartkopf AD, Brucker SY, Taran F-A, et al. International pooled analysis of the prognostic impact of disseminated tumor cells from the bone marrow in early breast cancer: Results from the PADDY study. SABCS. 2018 GS5-07. [Google Scholar]
- 56.Tan G, Chu C, Gui X, Li J, Chen Q. The prognostic value of circulating cell-free DNA in breast cancer: A meta-analysis. Medicine (Baltimore) 2018 Mar;97((13)):e0197. doi: 10.1097/MD.0000000000010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J, Cheng L, Zhang J, Chen L, Wang D, Guo X, et al. Predictive value of circulating cell-free DNA in the survival of breast cancer patients: A systemic review and meta-analysis. Medicine (Baltimore) 2018 Jul;97((28)):e11417. doi: 10.1097/MD.0000000000011417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.American Cancer Society . Breast Cancer Facts and Figures 2015-2016. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 59.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015 Oct;1((7)):888–96. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 60.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute. 2014 Nov; SEER data submission. http://seer.cancer.gov/csr/1975_2012/. Accessed September 23, 201569. [Google Scholar]
- 61.Fancellu A, Rosman AS, Sanna V, et al. Meta-analysis of the effect of preoperative breast MRI on the surgical management of ductal carcinoma in situ BJS. 2015;102:883–893. doi: 10.1002/bjs.9797. [DOI] [PubMed] [Google Scholar]
- 62.Ryan R, Tawfik O, Jensen RA, Anant S. Current Approaches to Diagnosis and Treatment of Ductal Carcinoma In Situ and Future Directions. Prog Mol Biol Transl Sci. 2017;151:33–80. doi: 10.1016/bs.pmbts.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Kuerer HM, Smith BD, Chavez-MacGregor M, Albarracin C, Barcenas CH, Santiago L, et al. DCIS Margins and Breast Conservation: MD Anderson Cancer Center Multidisciplinary Practice Guidelines and Outcomes. J Cancer. 2017 Aug;8((14)):2653–62. doi: 10.7150/jca.20871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014 Feb;348(feb11 9):g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solin LJ, Gray R, Hughes LL, Wood WC, Lowen MA, Badve SS, et al. Surgical Excision Without Radiation for Ductal Carcinoma in Situ of the Breast: 12-Year Results From the ECOG-ACRIN E5194 Study. J Clin Oncol. 2015 Nov;33((33)):3938–44. doi: 10.1200/JCO.2015.60.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCormick B, Winter K, Hudis C, Kuerer HM, Rakovitch E, Smith BL, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015 Mar;33((7)):709–15. doi: 10.1200/JCO.2014.57.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi J, Laws A, Hu J, Barry W, Golshan M, King T. Margins in Breast-Conserving Surgery After Neoadjuvant Therapy. Ann Surg Oncol. 2018 Nov;25((12)):3541–7. doi: 10.1245/s10434-018-6702-4. [DOI] [PubMed] [Google Scholar]
- 68.Giuliano AE, Morrow M, Duggal S, Julian TB. Should ACOSOG Z0011 change practice with respect to axillary lymph node dissection for a positive sentinel lymph node biopsy in breast cancer? Clin Exp Metastasis. 2012 Oct;29((7)):687–92. doi: 10.1007/s10585-012-9515-z. [DOI] [PubMed] [Google Scholar]
- 69.Straver ME, Meijnen P, van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010 Jul;17((7)):1854–61. doi: 10.1245/s10434-010-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017 Sep;318((10)):918–26. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrow M, Jagsi R, McLeod MC, Shumway D, Katz SJ. Surgeon Attitudes Toward the Omission of Axillary Dissection in Early Breast Cancer. JAMA Oncol. 2018 Nov;4((11)):1511–6. doi: 10.1001/jamaoncol.2018.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poodt IG, Spronk PE, Vugts G, van Dalen T, Peeters MT, Rots ML, et al. Trends on Axillary Surgery in Nondistant Metastatic Breast Cancer Patients Treated Between 2011 and 2015: A Dutch Population-based Study in the ACOSOG-Z0011 and AMAROS Era. Ann Surg. 2018 Dec;268((6)):1084–90. doi: 10.1097/SLA.0000000000002440. [DOI] [PubMed] [Google Scholar]
- 73.Hennigs A, Köpke M, Feißt M, Riedel F, Rezai M, Nitz U, et al. Which patients with sentinel node-positive breast cancer after breast conservation still receive completion axillary lymph node dissection in routine clinical practice? Breast Cancer Res Treat. 2019 Jan;173((2)):429–38. doi: 10.1007/s10549-018-5009-2. [DOI] [PubMed] [Google Scholar]
- 74.Classe JM, Loaec C, Gimbergues P, Alran S, de Lara CT, Dupre PF, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. 2019 Jan;173((2)):343–52. doi: 10.1007/s10549-018-5004-7. [DOI] [PubMed] [Google Scholar]
- 75.Galimberti V, Cole BF, Viale G, Veronesi P, Vicini E, Intra M, et al. International Breast Cancer Study Group Trial 23-01 Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018 Oct;19((10)):1385–93. doi: 10.1016/S1470-2045(18)30380-2. [DOI] [PubMed] [Google Scholar]
- 76.Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance) Ann Surg. 2016 Apr;263((4)):802–7. doi: 10.1097/SLA.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013 Jun;14((7)):609–18. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 78.Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015 Jan;33((3)):258–64. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 79.Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016 Apr;34((10)):1072–8. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertozzi N, Pesce M, Santi PL, Raposio E. Oncoplastic breast surgery: comprehensive review. Eur Rev Med Pharmacol Sci. 2017 Jun;21((11)):2572–85. [PubMed] [Google Scholar]
- 81.Singh N, Picha GJ, Hardas B, Schumacher A, Murphy DK. Five-Year Safety Data for More than 55,000 Subjects following Breast Implantation: Comparison of Rare Adverse Event Rates with Silicone Implants versus National Norms and Saline Implants. Plast Reconstr Surg. 2017 Oct;140((4)):666–79. doi: 10.1097/PRS.0000000000003711. [DOI] [PubMed] [Google Scholar]
- 82.Coroneos CJ, Selber JC, Offodile AC, 2nd, Butler CE, Clemens MW. Patients Reference US FDA Breast Implant Postapproval Studies: long-term Outcomes in 99,993. Ann Surg. 2019 Jan;269((1)):30–6. doi: 10.1097/SLA.0000000000002990. [DOI] [PubMed] [Google Scholar]
- 83.Magill LJ, Robertson FP, Jell G, Mosahebi A, Keshtgar M. Determining the outcomes of post-mastectomy radiation therapy delivered to the definitive implant in patients undergoing one- and two-stage implant-based breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2017 Oct;70((10)):1329–35. doi: 10.1016/j.bjps.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 84.Jagsi R, Momoh AO, Qi J, Hamill JB, Billig J, Kim HM, et al. Impact of Radiotherapy on Complications and Patient-Reported Outcomes After Breast Reconstruction. J Natl Cancer Inst. 2018 Feb;110((2)):157–65. doi: 10.1093/jnci/djx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuerer HM, Cordeiro PG, Mutter RW. Optimizing Breast Cancer Adjuvant Radiation and Integration of Breast and Reconstructive Surgery. Am Soc Clin Oncol Educ Book. 2017;37:93–105. doi: 10.1200/EDBK_175342. [DOI] [PubMed] [Google Scholar]
- 86.Cohen O, Lam G, Karp N, Choi M. Determining the Oncologic Safety of Autologous Fat Grafting as a Reconstructive Modality: An Institutional Review of Breast Cancer Recurrence Rates and Surgical Outcomes. Plast Reconstr Surg. 2017 Sep;140((3)):382e–92e. doi: 10.1097/PRS.0000000000003576. [DOI] [PubMed] [Google Scholar]
- 87.Berruti A, Amoroso V, Gallo F, Bertaglia V, Simoncini E, Pedersini R, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol. 2014 Dec;32((34)):3883–91. doi: 10.1200/JCO.2014.55.2836. [DOI] [PubMed] [Google Scholar]
- 88.EBCTCG Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol pii: S1470-2045(17)30777-5. 2017 doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Von Minckwitz G, Loibl S, Schneeweiss A, et al. “Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto)”. SABCS. 2015 Abstract S2-04. [Google Scholar]
- 90.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015 Jan;33((1)):13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, et al. German Breast Group (GBG) Arbeitsgemeinschaft Gynäkologische Onkologie—Breast (AGO-B) Investigators Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016 Mar;17((3)):345–56. doi: 10.1016/S1470-2045(15)00542-2. [DOI] [PubMed] [Google Scholar]
- 92.Gianni L, Mansutti M, Anton A, Calvo L, Bisagni G, Bermejo B, et al. Comparing Neoadjuvant Nab-paclitaxel vs Paclitaxel Both Followed by Anthracycline Regimens in Women With ERBB2/HER2-Negative Breast Cancer-The Evaluating Treatment With Neoadjuvant Abraxane (ETNA) Trial: A Randomized Phase 3 Clinical Trial. JAMA Oncol. 2018 Mar;4((3)):302–8. doi: 10.1001/jamaoncol.2017.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016 Jun;17((6)):791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 94.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Waldron-Lynch M, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018 Jan;89:27–35. doi: 10.1016/j.ejca.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 95.Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018 Jan;19((1)):115–26. doi: 10.1016/S1470-2045(17)30716-7. [DOI] [PubMed] [Google Scholar]
- 96.Swain SM, Ewer MS, Viale G, Delaloge S, Ferrero JM, Verrill M, et al. BERENICE Study Group Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018 Mar;29((3)):646–53. doi: 10.1093/annonc/mdx773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013 Oct;31((29)):3623–30. doi: 10.1200/JCO.2012.45.0940. [DOI] [PubMed] [Google Scholar]
- 98.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017 Jun;376((22)):2147–59. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 99.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. KATHERINE Investigators Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019 Feb;380((7)):617–28. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 100.Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, et al. Tumor-Infiltrating Lymphocytes: A Predictive and Prognostic Biomarker in Neoadjuvant-Treated HER2-Positive Breast Cancer. Clin Cancer Res. 2016 Dec;22((23)):5747–54. doi: 10.1158/1078-0432.CCR-15-2338. [DOI] [PubMed] [Google Scholar]
- 101.Loibl S, Darb-Esfahani S, Huober J, et al. Integrated Analysis of PTEN and p4EBP1 Protein Expression as Predictors for pCR in HER2-Positive Breast Cancer. Clin Cancer Res. 2016 Jan;22((11)):2675–2683. doi: 10.1158/1078-0432.CCR-15-0965. [DOI] [PubMed] [Google Scholar]
- 102.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Alliance for Clinical Trials in Oncology Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013 Oct;310((14)):1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Classe JM, Bordes V, Campion L, Mignotte H, Dravet F, Leveque J, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009 Feb;27((5)):726–32. doi: 10.1200/JCO.2008.18.3228. [DOI] [PubMed] [Google Scholar]
- 104.El Hage Chehade H, Headon H, El Tokhy O, Heeney J, Kasem A, Mokbel K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3,398 patients. Am J Surg. 2016 Nov;212((5)):969–81. doi: 10.1016/j.amjsurg.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 105.Loibl S, Werutsky G, Nekljudova V, et al. Impact in delay of start of chemotherapy and surgery on pCR and survival in breast cancer – a pooled analysis of individual patient data from six prospectively randomized neoadjuvant trials. ASCO. 2017 (abs 571) [Google Scholar]
- 106.Sanford RA, Lei X, Barcenas CH, Mittendorf EA, Caudle AS, Valero V, et al. Impact of Time from Completion of Neoadjuvant Chemotherapy to Surgery on Survival Outcomes in Breast Cancer Patients. Ann Surg Oncol. 2016 May;23((5)):1515–21. doi: 10.1245/s10434-015-5020-3. [DOI] [PubMed] [Google Scholar]
- 107.Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012 Feb;379((9814)):432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. doi: 10.1016/S0140-6736(18)33137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Möbus V, Jackisch C, Lück HJ, du Bois A, Thomssen C, Kuhn W, et al. AGO Breast Study Group (AGO-B) Ten-year results of intense dose-dense chemotherapy show superior survival compared with a conventional schedule in high-risk primary breast cancer: final results of AGO phase III iddEPC trial. Ann Oncol. 2018 Jan;29((1)):178–85. doi: 10.1093/annonc/mdx690. [DOI] [PubMed] [Google Scholar]
- 110.Nitz U, Gluz O, Clemens M, Malter W, Reimer T, Nuding B, et al. West German Study Group PlanB Investigators West German Study PlanB Trial: Adjuvant Four Cycles of Epirubicin and Cyclophosphamide Plus Docetaxel Versus Six Cycles of Docetaxel and Cyclophosphamide in HER2-Negative Early Breast Cancer. J Clin Oncol. 2019 Apr;37((10)):799–808. doi: 10.1200/JCO.18.00028. [DOI] [PubMed] [Google Scholar]
- 111.Shulman LN, Berry DA, Cirrincione CT, Becker HP, Perez EA, O'Regan R, et al. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance) J Clin Oncol. 2014 Aug;32((22)):2311–7. doi: 10.1200/JCO.2013.53.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Breast Cancer International Research Group Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011 Oct;365((14)):1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015 Jan;372((2)):134–41. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell'Orto P, Rasmussen BB, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007 Sep;25((25)):3846–52. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 115.Strasser-Weippl K, Sudan G, Ramjeesingh R, Shepherd LE, O'Shaughnessy J, Parulekar WR, et al. Outcomes in women with invasive ductal or invasive lobular early stage breast cancer treated with anastrozole or exemestane in CCTG (NCIC CTG) MA.27. Eur J Cancer. 2018 Feb;90:19–25. doi: 10.1016/j.ejca.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 116.Gray R, on behalf of EBCTCG Extended aromatase inhibitor treatment following 5 or more years of endocrine therapy: a metaanalysis of 22192 women in 11 randomised trials. SABCS. 2018 GS3-03. [Google Scholar]
- 117.Colleoni M, Luo W, Karlsson P, Chirgwin J, Aebi S, Jerusalem G, et al. SOLE Investigators Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018 Jan;19((1)):127–38. doi: 10.1016/S1470-2045(17)30715-5. [DOI] [PubMed] [Google Scholar]
- 118.Mayadev J, Fish K, Valicenti R, West D, Chen A, Martinez S, et al. Utilization and impact of a postmastectomy radiation boost for invasive breast cancer. Pract Radiat Oncol. 2014 Nov-Dec;4((6)):e269–78. doi: 10.1016/j.prro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 119.Jagsi R, Chadha M, Moni J, Ballman K, Laurie F, Buchholz TA, et al. Radiation field design in the ACOSOG Z0011 (Alliance) Trial. J Clin Oncol. 2014 Nov;32((32)):3600–6. doi: 10.1200/JCO.2014.56.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. APHINITY Steering Committee and Investigators Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017 Jul;377((2)):122–31. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu ZI, Ho AY, McArthur HL. Combined Radiation Therapy and Immune Checkpoint Blockade Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys. 2017 Sep;99((1)):153–64. doi: 10.1016/j.ijrobp.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 122.Woodward WA, Fang P, Arriaga L, Gao H, Cohen EN, Reuben JM, et al. A phase 2 study of capecitabine and concomitant radiation in women with advanced breast cancer. Int J Radiat Oncol Biol Phys. 2017 Nov;99((4)):777–83. doi: 10.1016/j.ijrobp.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loibl S, Schmidt A, Gentilini O, Kaufman B, Kuhl C, Denkert C, et al. Breast Cancer Diagnosed During Pregnancy: Adapting Recent Advances in Breast Cancer Care for Pregnant Patients. JAMA Oncol. 2015 Nov;1((8)):1145–53. doi: 10.1001/jamaoncol.2015.2413. [DOI] [PubMed] [Google Scholar]
- 124.Amant F, von Minckwitz G, Han SN, Bontenbal M, Ring AE, Giermek J, et al. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. 2013 Jul;31((20)):2532–9. doi: 10.1200/JCO.2012.45.6335. [DOI] [PubMed] [Google Scholar]
- 125.Han SN, Amant F, Michielsen K, De Keyzer F, Fieuws S, Van Calsteren K, et al. Feasibility of whole-body diffusion-weighted MRI for detection of primary tumour, nodal and distant metastases in women with cancer during pregnancy: a pilot study. Eur Radiol. 2018 May;28((5)):1862–74. doi: 10.1007/s00330-017-5126-z. [DOI] [PubMed] [Google Scholar]
- 126.Peccatori FA, Codacci-Pisanelli G, Del Grande M, Scarfone G, Zugni F, Petralia G. Whole body MRI for systemic staging of breast cancer in pregnant women. Breast. 2017 Oct;35:177–81. doi: 10.1016/j.breast.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 127.Han SN, Amant F, Cardonick EH, Loibl S, Peccatori FA, Gheysens O, et al. International Network on Cancer, Infertility and Pregnancy Axillary staging for breast cancer during pregnancy: feasibility and safety of sentinel lymph node biopsy. Breast Cancer Res Treat. 2018 Apr;168((2)):551–7. doi: 10.1007/s10549-017-4611-z. [DOI] [PubMed] [Google Scholar]
- 128.Mir O, Berveiller P, Ropert S, Goffinet F, Pons G, Treluyer JM, et al. Emerging therapeutic options for breast cancer chemotherapy during pregnancy. Ann Oncol. 2008 Apr;19((4)):607–13. doi: 10.1093/annonc/mdm460. [DOI] [PubMed] [Google Scholar]
- 129.Köhler C, Oppelt P, Favero G, Morgenstern B, Runnebaum I, Tsunoda A, et al. How much platinum passes the placental barrier? Analysis of platinum applications in 21 patients with cervical cancer during pregnancy. Am J Obstet Gynecol. 2015 Aug;213((2)):206.e1–5. doi: 10.1016/j.ajog.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 130.Zheng X, Zhu Y, Zhao Y, Feng S, Zheng C. Taxanes in combination with platinum derivatives for the treatment of ovarian cancer during pregnancy: A literature review. Int J Clin Pharmacol Ther. 2017 Sep;55((9)):753–60. doi: 10.5414/CP202995. [DOI] [PubMed] [Google Scholar]
- 131.Zagouri F, Sergentanis TN, Chrysikos D, Papadimitriou CA, Dimopoulos MA, Bartsch R. Trastuzumab administration during pregnancy: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013 Jan;137((2)):349–57. doi: 10.1007/s10549-012-2368-y. [DOI] [PubMed] [Google Scholar]
- 132.Cunha GR, Taguchi O, Namikawa R, Nishizuka Y, Robboy SJ. Teratogenic effects of clomiphene, tamoxifen, and diethylstilbestrol on the developing human female genital tract. Hum Pathol. 1987 Nov;18((11)):1132–43. doi: 10.1016/s0046-8177(87)80381-7. [DOI] [PubMed] [Google Scholar]
- 133.Gerstl B, Sullivan E, Ives A, Saunders C, Wand H, Anazodo A. Pregnancy Outcomes After a Breast Cancer Diagnosis: A Systematic Review and Meta-analysis. Clin Breast Cancer. 2018 Feb;18((1)):e79–88. doi: 10.1016/j.clbc.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 134.Lambertini M, Kroman N, Ameye L, Cordoba O, Pinto A, Benedetti G, et al. Long-term Safety of Pregnancy Following Breast Cancer According to Estrogen Receptor Status. J Natl Cancer Inst. 2018 Apr;110((4)):426–9. doi: 10.1093/jnci/djx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leon-Ferre RA, Giridhar KV, Hieken TJ, Mutter RW, Couch FJ, Jimenez RE, et al. A contemporary review of male breast cancer: current evidence and unanswered questions. Cancer Metastasis Rev. 2018 Dec;37((4)):599–614. doi: 10.1007/s10555-018-9761-x. [DOI] [PubMed] [Google Scholar]
- 136.Zagouri F, Sergentanis TN, Koutoulidis V, Sparber C, Steger GG, Dubsky P, et al. Aromatase inhibitors with or without gonadotropin-releasing hormone analogue in metastatic male breast cancer: a case series. Br J Cancer. 2013 Jun;108((11)):2259–63. doi: 10.1038/bjc.2013.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Agrawal A, Cheung KL, Robertson JF. Fulvestrant in advanced male breast cancer. Breast Cancer Res Treat. 2007 Jan;101((1)):123. doi: 10.1007/s10549-006-9266-0. [DOI] [PubMed] [Google Scholar]
- 138.Audisio RA. Inflammatory Breast Cancer: updates on diagnosis and treatment options. Eur J Surg Oncol. 2018 Aug;44((8)):1127. doi: 10.1016/j.ejso.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 139.Rueth NM, Lin HY, Bedrosian I, Shaitelman SF, Ueno NT, Shen Y, et al. Underuse of trimodality treatment affects survival for patients with inflammatory breast cancer: an analysis of treatment and survival trends from the National Cancer Database. J Clin Oncol. 2014 Jul;32((19)):2018–24. doi: 10.1200/JCO.2014.55.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hemminki K, Bevier M, Sundquist J, Hemminki A. Site-specific cancer deaths in cancer of unknown primary diagnosed with lymph node metastasis may reveal hidden primaries. Int J Cancer. 2013 Feb;132((4)):944–50. doi: 10.1002/ijc.27678. [DOI] [PubMed] [Google Scholar]
- 141.Fehm T, R. Axillary lymph node metastasis in CUP. Onkologe. 2013;19((1)):40–43. [Google Scholar]
- 142.Fehm T, Souchon R. Axillary lymph node metastasis in CUP. Onkologe. 2013;19((1)):40–3. [Google Scholar]
- 143.Lakhani SR, Ellis IO, Schnitt SJ, et al. World Health Organization classification of tumours. 4th ed. Lyon: IARC Press; 2012. WHO classification of tumors of the breast; pp. pp. 48–52. [Google Scholar]
- 144.Song Y, Liu X, Zhang G, Song H, Ren Y, He X, et al. Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. World J Surg Oncol. 2013 Jun;11((1)):129. doi: 10.1186/1477-7819-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Y, Lv F, Yang Y, Qian X, Lang R, Fan Y, et al. Clinicopathological Features and Prognosis of Metaplastic Breast Carcinoma: Experience of a Major Chinese Cancer Center. PLoS One. 2015 Jun;10((6)):e0131409. doi: 10.1371/journal.pone.0131409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007 Jan;14((1)):166–73. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 147.Beatty JD, Atwood M, Tickman R, Reiner M. Metaplastic breast cancer clinical significance. Am J Surg. 2006 May;191((5)):657–664. doi: 10.1016/j.amjsurg.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 148.Beatty JD, Atwood M, Tickman R, Reiner M. Metaplastic breast cancer: clinical significance. Am J Surg. 2006 May;191((5)):657–64. doi: 10.1016/j.amjsurg.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 149.Tzanninis IG, Kotteas EA, Ntanasis-Stathopoulos I, Kontogianni P, Fotopoulos G. Management and Outcomes in Metaplastic Breast Cancer. Clin Breast Cancer. 2016 Dec;16((6)):437–43. doi: 10.1016/j.clbc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 150.Leberfinger AN, Behar BJ, Williams NC, Rakszawski KL, Potochny JD, Mackay DR, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma: A Systematic Review. JAMA Surg. 2017 Dec;152((12)):1161–8. doi: 10.1001/jamasurg.2017.4026. [DOI] [PubMed] [Google Scholar]
- 151.Kricheldorff J, Fallenberg EM, Solbach C, Gerber-Schäfer C, Rancsó C, Fritschen UV. Breast implant-associated lymphoma. Dtsch Arztebl Int. 2018 Sep;115((38)):628–635. doi: 10.3238/arztebl.2018.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blohmer JU, Sinn HP. Zum möglichen Zusammenhang von Brustsilikonimplantaten und dem Auftreten von Lymphomen. 243rd Statement by the German Society of Gynecology and Obstetrics (DGGG) in Response to the call for Data on the Safety of PIP Silicone Breast Implants and the Possible Association between Breast Implants and ALCL by the Scientific Committee on Health Environmental and Emerging Risks (SCHEER) of the European Commission. Geburtshilfe Frauenheilkd. 2017;77((6)):617. [Google Scholar]
- 153.Kricheldorff J, Fallenberg EM, Solbach C, Gerber-Schäfer C, Rancsó C, Fritschen UV. Breast Implant-Associated Lymphoma. Dtsch Arztebl Int. 2018 Sep;115((38)):628–35. doi: 10.3238/arztebl.2018.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hall DL, Luberto CM, Philpotts LL, Song R, Park ER, Yeh GY. Mind-body interventions for fear of cancer recurrence: A systematic review and meta-analysis. Psychooncology. 2018 Nov;27((11)):2546–58. doi: 10.1002/pon.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eyigor S, Uslu R, Apaydın S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Complement Ther Clin Pract. 2018 Aug;32:40–5. doi: 10.1016/j.ctcp.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 156.Bradt J, Dileo C, Magill L, Teague A. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev. 2016 Aug;((8)):CD006911. doi: 10.1002/14651858.CD006911.pub3. [DOI] [PubMed] [Google Scholar]
- 157.Bauersfeld SP, Kessler CS, Wischnewsky M, Jaensch A, Steckhan N, Stange R, et al. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer a randomized cross-over pilot study. BMC Cancer. 2018 Apr;18((1)):476. doi: 10.1186/s12885-018-4353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bauersfeld SP, Kessler CS, Wischnewsky M, Jaensch A, Steckhan N, Stange R, et al. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. BMC Cancer. 2018 Apr;18((1)):476. doi: 10.1186/s12885-018-4353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leon-Ferre RA, Novotny PJ, Faubion SS, et al. A randomized, double-blind, placebo-controlled trial of oxybutynin for hot flashes: ACCRU study SC-1603. SABCS. 2018 doi: 10.1093/jncics/pkz088. GS6-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chien TJ, Hsu CH, Liu CY, Fang CJ. Effect of acupuncture on hot flush and menopause symptoms in breast cancer – a systematic review and meta-analysis. PLoS One. 2017 Aug;12((8)):e0180918. doi: 10.1371/journal.pone.0180918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chien TJ, Hsu CH, Liu CY, Fang CJ. Effect of acupuncture on hot flush and menopause symptoms in breast cancer- A systematic review and meta-analysis. PLoS One. 2017 Aug;12((8)):e0180918. doi: 10.1371/journal.pone.0180918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hershman DL, Unger JM, Greenlee H, Capodice JL, Lew DL, Darke AK, et al. Effect of Acupuncture vs Sham Acupuncture or Waitlist Control on Joint Pain Related to Aromatase Inhibitors Among Women With Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA. 2018 Jul;320((2)):167–76. doi: 10.1001/jama.2018.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leonard RC, Adamson DJ, Bertelli G, Mansi J, Yellowlees A, Dunlop J, et al. Anglo Celtic Collaborative Oncology Group and National Cancer Research Institute Trialists GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol. 2017 Aug;28((8)):1811–6. doi: 10.1093/annonc/mdx184. [DOI] [PubMed] [Google Scholar]
- 164.Fréour T, Barrière P, Masson D. Anti-müllerian hormone levels and evolution in women of reproductive age with breast cancer treated with chemotherapy. Eur J Cancer. 2017 Mar;74:1–8. doi: 10.1016/j.ejca.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 165.Gambacciani M, Levancini M. Vaginal erbium laser as second-generation thermotherapy for the genitourinary syndrome of menopause: a pilot study in breast cancer survivors. Menopause. 2017 Mar;24((3)):316–9. doi: 10.1097/GME.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 166.Shah C, Ahlawat S, Khan A, Tendulkar RD, Wazer DE, Shah SS, et al. The Role of MRI in the Follow-up of Women Undergoing Breast-conserving Therapy. Am J Clin Oncol. 2016 Jun;39((3)):314–319. doi: 10.1097/COC.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 167.Shah C, Ahlawat S, Khan A, Tendulkar RD, Wazer DE, Shah SS, et al. The Role of MRI in the Follow-up of Women Undergoing Breast-conserving Therapy. Am J Clin Oncol. 2016 Jun;39((3)):314–9. doi: 10.1097/COC.0000000000000290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data