Abstract
Objective: In this study, we investigated the changes in peripheral blood inflammatory factors and intestinal flora in acquired immune deficiency syndrome (AIDS) and human immunodeficiency virus (HIV)-positive individuals (AIDS/HIV patients), and explored the relationships among intestinal flora, peripheral blood inflammatory factors, and CD4+ T lymphocytes. Methods: Thirty blood and stool samples from an AIDS group and a control group were collected. The levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were determined by enzyme-linked immunosorbent assay (ELISA), and the number of CD4+ T lymphocytes by a FACSCount automated instrument. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to determine the messenger RNA (mRNA) levels of Bifidobacterium, Lactobacillus, Escherichia coli, Enterococcus faecalis, and Enterococcus faecium. Correlations among intestinal flora, inflammatory factor levels, and CD4+ T lymphocyte values were evaluated using the Spearman correlation coefficient. Results: The levels of TNF-α and IL-6 in the AIDS group were higher than those in the control group, while the number of CD4+ T lymphocytes was lower. The amounts of Bifidobacterium and Lactobacillus in the AIDS group were significantly lower than those in control group, while the amounts of E. coli, E. faecalis, and E. faecium were much higher. The amounts of Bifidobacterium and Lactobacillus were negatively correlated with the content of TNF-α and IL-6 and the CD4+ T lymphocyte count, while those correlations were reversed for E. coli, E. faecalis, and E. faecium. Conclusions: The intestinal microbiota of AIDS/HIV patients were disordered, and there was a correlation between the amount of intestinal flora and the number of CD4+ T lymphocytes and the levels of TNF-α and IL-6.
Keywords: Acquired immune deficiency syndrome (AIDS), Tumor necrosis factor-α(TNF-α), Interleukin-6 (IL-6), Intestinal flora, CD4+ T lymphocytes
1. Introduction
Acquired immune deficiency syndrome (AIDS) is an immune system disease caused by human immunodeficiency virus (HIV) infection and CD4+ T cell depletion. AIDS patients have several co-morbidities, including gastrointestinal disease, systemic immune activation, malignancy, liver disease, bone disease, and neurological complications (Capeau, 2011; Kolgiri and Patil, 2017), which have a clear impact on survival. Previous studies have reported that antiretroviral therapy (ART) successfully controls systemic HIV replication, but immune recovery is variable (Maartens et al., 2014; Monaco et al., 2016). One of the characteristics of AIDS is a rapid depletion of CD4+ T cells in the gut-associated lymphoid tissue (Klatt et al., 2008). In addition, AIDS can lead to gastrointestinal disease characterized by increased gastrointestinal inflammation, diarrhea, and malabsorption (Brenchley, 2013).
It has been reported that dysbiosis of the microbiota plays an important role in gut dysfunction and gastrointestinal inflammation (Klase et al., 2015). The human intestine is a complex ecosystem inhabited by the microbiome, including bacteria, viruses, archaea, and eukaryotic organisms. Previous studies have suggested that HIV infection may be related to obvious changes in enteric bacterial populations (Dillon et al., 2014; Dinh et al., 2015; Monaco et al., 2016). The bacterial microbiome includes Bifidobacterium, Lactobacillus, Escherichia coli, Enterococcus faecalis, and Enterococcus faecium. E. faecalis and E. faecium are commonly used in human intestinal colonization (Quiloan et al., 2012). Lactobacillus and Bifidobacterium can prevent gastrointestinal infections and restore an effective intestinal barrier. They also have a positive effect on the immune function of HIV/AIDS patients (Irvine et al., 2010). E. coli is the most common bacterium associated with diarrhea in HIV patients (Shah et al., 2016). Moreover, bacterial microbiome alteration has been reported to be linked to inflammatory bowel disease (IBD) and inflammation in HIV-1 infection (Bäckhed et al., 2012; Lyte, 2013; Márquez et al., 2016). However, a better understanding of the relationships established among inflammatory factors, CD4+ T lymphocyte counts, and the intestinal flora compartment in AIDS is urgently needed.
In this study, we investigated the levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and the amounts of CD4+ T lymphocytes, E. coli, Lactobacillus, Bifidobacterium, E. faecalis, and E. faecium, as well as the correlations among them. Our results revealed that the intestinal microbiota of AIDS/HIV patients were disordered. The results also confirmed that there was a correlation between the amount of intestinal flora and the number of CD4+ T lymphocytes and levels of TNF-α and IL-6. The findings of our study may provide a new theoretical foundation for the clinical treatment of AIDS/HIV patients.
2. Materials and methods
2.1. Patients and groups
Thirty AIDS/HIV patients (18 males and 12 females, aged 20–65 years) were included in the AIDS group. They were selected from the outpatient (n=21) and inpatient (n=9) departments of Qingdao Sixth People’s Hospital (Qingdao, China) from April 2016 to April 2018. The statistical characteristics of the patients are shown in Table 1. Subjects who had used antacids within the previous two months were excluded. Patients with an active opportunistic infection or inflammation were also excluded from our cohort. Simultaneously, 30 healthy volunteers (15 males and 15 females, aged 23–61 years), were set as the control group. None of the volunteers had a history of gastrointestinal diseases or hepatobiliary diseases, and none had used antibiotics or microecological preparations within two months before the experiment. This study was approved by the Ethics Committee of Qingdao Sixth People’s Hospital and informed consent was signed by the patients and healthy volunteers.
Table 1.
Clinical characteristics of AIDS/HIV patients
| Characteristics | Patients |
|
| Number | Percentage (%) | |
| Male | 18 | 60.0 |
| Female | 12 | 40.0 |
| Outpatient | 21 | 70.0 |
| Inpatient | 9 | 30.0 |
| Infection pathway | ||
| Drug abuse | 10 | 33.3 |
| Sexual transmission | 16 | 53.4 |
| Blood transmission | 4 | 13.3 |
| Antiretroviral therapy (ART) | ||
| Yes | 10 | 33.3 |
| No | 20 | 66.7 |
| Antibiotics | ||
| Yes | 25 | 83.3 |
| No | 5 | 16.7 |
| Acute illness | ||
| Yes | 3 | 10.0 |
| No | 27 | 90.0 |
| CD4+ cell count (cells/µL) | ||
| >500 | 5 | 16.7 |
| 200–500 | 15 | 50.0 |
| <200 | 10 | 33.3 |
|
| ||
| Median age (year) | 39.5 | |
| Median CD4+ cell count (cells/µL) | 304.5 | |
| HIV viral load* (copies/mL plasma) | ||
| ART treatment | 20 (0–50) | |
| No treatment | 95 672 (24 455–285 548) | |
The values for HIV viral load are expressed as median (range)
2.2. Detection of inflammatory factors by ELISA
Fasting venous blood samples (3–5 mL) were collected and stored at 4 °C, centrifuged at 2000 r/min for 10 min, and the plasma was dispensed into 2-mL Eppendorf (EP) tubes and stored at −80 °C. The levels of TNF-α and IL-6 were detected using an enzyme-linked immunosorbent assay (ELISA) kit (Cloud-Clone Corp., USA). The absorbance value was measured by an absorbance detector at 450 nm.
2.3. Detection of CD4+ T lymphocytes by flow cytometry
The number of CD4+ T lymphocytes was assessed using a FACSCalibur type flow cytometer and a tricolor fluorescent antibody labeling kit (BD, USA), as described by Yao et al. (2017) and Zhang SG et al. (2017). According to the reagent specification, each sample was processed in a TruCONT tube dedicated to flow cytometry. A total of 20 µL of monoclonal antibody (mAb) was firstly added to the TruCONT tube, and then 50 μL of whole blood containing ethylenediaminetetraacetic acid (EDTA) was added. After 15 min in the dark, 450 μL of 10% (0.1 g/mL) fluorescence activated cell sorter (FACS) hemolysin was added and the tube was placed in the dark for 15 min. Finally, the number of CD4+ T lymphocytes was determined using MultiSET software.
2.4. qRT-PCR
Fresh feces from the two groups were collected and placed on ice. A total of 220 mg of medium stool was weighed, and the fecal genomic DNA was extracted using a bacterial genomic DNA extraction kit (Tiangen Biochemical Technology Co., Ltd., Beijing, China). The DNA concentration of each sample was tested and adjusted to 20 µg/mL, and then the amounts of Bifidobacterium, Lactobacillus, E. coli, E. faecalis, and E. faecium in fecal samples were separately quantified by quantitative real-time polymerase chain reaction (qRT-PCR). Standards were set for simultaneous amplification in each reaction, and the DNA template was replaced with sterile ddH2O as a negative control. A total of 20 µL of reaction system was used for qRT-PCR, including 10 µL 2×SYBR mixture uracil-N-glycosylase (UNG; including reactive oxygen species (ROX)), 0.75 µL upstream and downstream primers, 2.0 µL DNA template, and 6.5 µL sterilized ddH2O. Then, the amplification was carried out according to the following procedures: UNG enzymes acted at 50 °C for 5 min, pre-denatured at 95 °C for 10 min followed by 35 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 30 s. The C T value and the standard curve were calculated as a quantitative result. The target gene sequence was searched from GenBank (https://www. ncbi.nlm.nih.gov), and primer design was carried out using Primer Premier 5.0 (Primer, Canada). Five primers were verified online by BLAST software (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and synthesized by Shanghai Biotech Engineering Services Co., Ltd., Shanghai, China. The primer sequences are shown in Table 2.
Table 2.
Primer sequences
| Name | Sequence (5'→3') |
| Bifidobacterium | F: TCGCCTCCGGGTGAGAGTGG |
| R: CGAAGCCATGGTGGGCCGTT | |
| Lactobacillus | F: TGGAAACAGGTGCTAATACCG |
| R: GTCCATTGTGGAAGATTCCC | |
| Escherichia coli | F: CTCGCTGGCATTTGCGTAC |
| R: ATCTTTTGCCGTCCGTTTTGG | |
| Enterococcus faecalis | F: TTGGCATTCCACAAGTACCA |
| R: AATTGCTCGGGCATCATAAC | |
| Enterococcus faecium | F: TCGGACGACTTTGCCTGGCG |
| R: TCGCACCGGCTCAATCCGC |
F: forward; R: reverse
2.5 Statistical analysis
The experimental measurements are expressed as mean±standard deviation (SD), and a sample t-test was performed using GraphPad Prism 5.0 software. The correlations between the amount of intestinal flora and the levels of TNF-α and IL-6 and the number of CD4+ T lymphocytes were analyzed by Spearman correlation. All experiments were carried out three times. If P<0.05, statistical significance was accepted.
3. Results
3.1. Study population characteristics
The median age of patients in the AIDS group was 39.5 years (range 20–65 years) and 60% were male. Drug abuse (33.3%), sexual transmission (53.4%), and blood transmission (13.3%) were the main routes of infection. Ten subjects (33.3%) in the AIDS group received ART treatment for six months, which consisted of tenofovir (20 mg/kg) and emtricitabine (30 mg/kg). In addition, 25 patients were treated with antibiotics consisting of vancomycin (15 mg/kg) and enrofloxacin (10 mg/kg) for one month. ART-treated participants had undetectable viral loads (<20 copies/mL plasma) and participants without ART treatment had high viral loads (95 672 copies/mL plasma). Three patients suffering acute illness were clinically diagnosed AIDS patients and the other 27 were HIV-positive patients, including patients not yet under treatment. Among the 30 patients, 10 (33.3%) had a CD4+ cell count of <200 cells/µL, 15 (50.0%) had a CD4+ cell count of 200–500 cells/µL, and 5 (16.7%) had a CD4+ cell count of >500 cells/µL. Three (10.0%) were suffering acute illness (Table 1).
3.2. Higher levels of TNF-α and IL-6 in the peripheral blood of AIDS/HIV patients
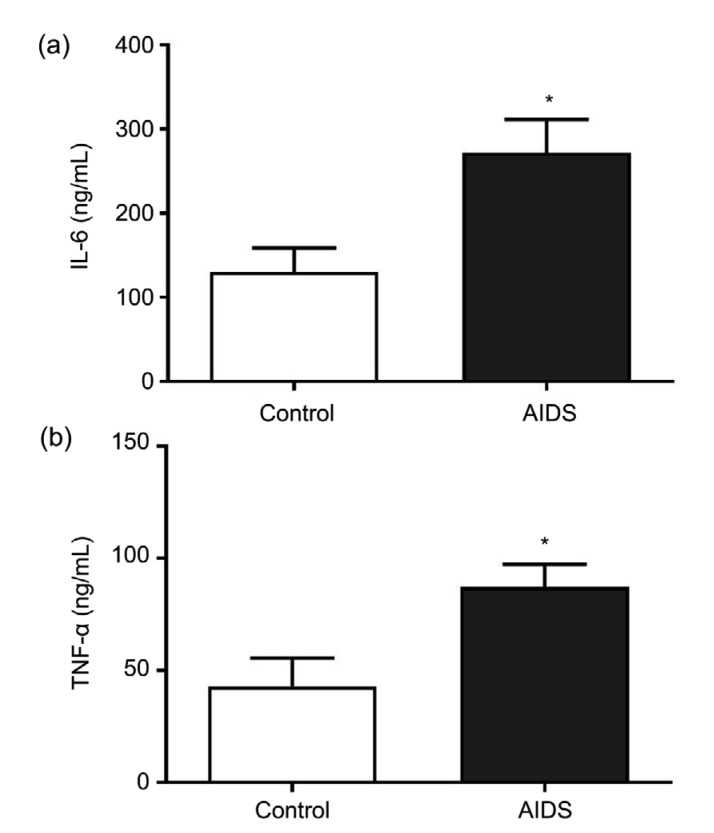
The measured levels of inflammatory factors in the peripheral blood of the AIDS group and the control group are shown in Fig. 1. TNF-α and IL-6 levels were markedly higher in subjects in the AIDS group than in the control group (P<0.05). The results illustrated that the expression of inflammatory factors in the peripheral blood of AIDS/HIV patients was abnormally high.
Fig. 1.
Expression levels of IL-6 and TNF-α in the AIDS and control groups
(a) The expression level of IL-6; (b) The expression level of TNF-α. Data from experiments carried out three times are presented as mean±SD. * P<0.05, vs. control
3.3. Lower number of CD4+ T lymphocytes in AIDS/HIV patients
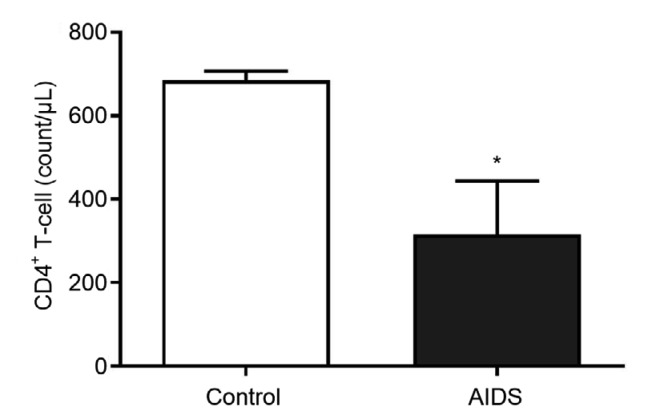
The numbers of CD4+ T lymphocytes in the AIDS and control groups are shown in Fig. 2. The number of CD4+ T lymphocytes in the AIDS group was much lower than that in the control group (P<0.05), suggesting that the number of CD4+ T lymphocytes was abnormally low in AIDS/HIV patients.
Fig. 2.
Numbers of CD4+ T lymphocytes in the AIDS and control groups
Data from experiments carried out three times are presented as mean±SD. * P<0.05, vs. control
3.4. Changes in the amounts of Bifidobacterium, Lactobacillus, E. coli, E. faecalis, and E. faecium
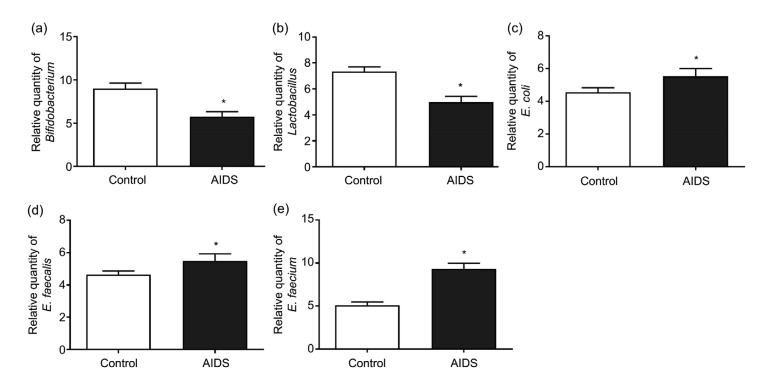
The relative amounts of Bifidobacterium and Lactobacillus in the AIDS group were markedly lower than those in the control group (P<0.05; Figs. 3a and 3b). However, the amounts of E. coli, E. faecalis, and E. faecium in the AIDS group were significantly higher than those in the control group (P<0.05; Figs. 3c–3e). These findings indicated that the amounts of intestinal flora changed abnormally and the intestinal microbiota were destroyed.
Fig. 3.
Amounts of different bacteria in the AIDS and control groups
(a) Bifidobacterium; (b) Lactobacillus; (c) Escherichia coli; (d) Enterococcus faecalis; (e) Enterococcus faecium. Data from experiments carried out three times are presented as mean±SD. * P<0.05, vs. control
3.5. Correlations of the amount of intestinal flora with the levels of TNF-α and IL-6 in peripheral blood
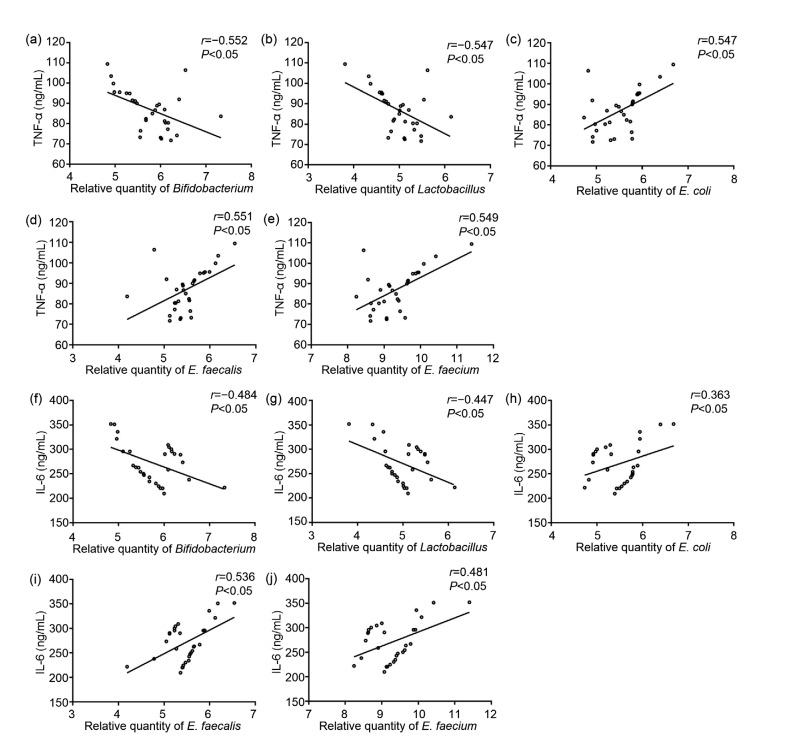
Spearman correlation analysis showed that the amounts of Bifidobacterium and Lactobacillus in the AIDS group were negatively correlated with the level of TNF-α (P<0.05; Figs. 4a and 4b), while the amounts of E. coli, E. faecalis, and E. faecium were positively correlated with the level of TNF-α (P<0.05; Figs. 4c–4e). Similarly, the amounts of Bifidobacterium and Lactobacillus in the AIDS group were negatively correlated with the level of IL-6 (P<0.05; Figs. 4f and 4g). In contrast, the amounts of E. coli, E. faecalis, and E. faecium were positively correlated with the level of IL-6 (P<0.05; Figs. 4h–4j).
Fig. 4.
Correlation analysis between the contents of intestinal flora and inflammatory factors
Correlations between the content of Bifidobacterium and the level of TNF-α (a), Lactobacillus and the level of TNF-α (b), Escherichia coli and the level of TNF-α (c), Enterococcus faecalis and the level of TNF-α (d), Enterococcus faecium and the level of TNF-α (e), Bifidobacterium and the level of IL-6 (f), Lactobacillus and the level of IL-6 (g), E. coli and the level of IL-6 (h), E. faecalis and the level of IL-6 (i), E. faecium and the level of IL-6 (j)
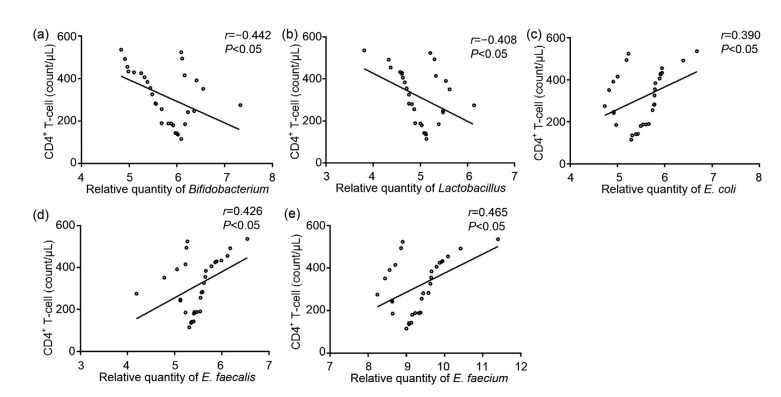
3.6. Correlations of the amount of intestinal flora with the number of CD4+ T lymphocytes in peripheral blood
The correlations between the intestinal flora and the number of CD4+ T lymphocytes were determined using Spearman correlation analysis. The results showed that the amounts of Bifidobacterium and Lactobacillus in the AIDS group were negatively correlated with the number of CD4+ T lymphocytes (P<0.05; Figs. 5a and 5b). In contrast, the amounts of E. coli, E. faecalis, and E. faecium in the AIDS group were positively correlated with the number of CD4+ T lymphocytes (P<0.05; Figs. 5c–5e).
Fig. 5.
Correlation analysis between the number of CD4+ T lymphocytes and the content of intestinal flora
Correlations between the number of CD4+ T lymphocytes and the content of Bifidobacterium (a), Lactobacillus (b), Escherichia coli (c), Enterococcus faecalis (d), Enterococcus faecium (e)
4. Discussion
Intestinal parasitic infections and HIV/AIDS are major public health problems in developing countries, and are the main causes of high mortality rates (Gedle et al., 2017). The progression of AIDS is a diverse process. Slow or rapid progression could present distinct behaviors of specific cell subpopulations and compartments, and components of the patient’s microbiota. HIV/AIDS is characterized by the targeting and destruction of CD4+ T lymphocytes in peripheral blood. Importantly, CD4+ T cells are the key to host defense and the primary driver of immune-mediated disease (O'Shea and Paul, 2010; Zhang J et al., 2017). In this study, the number of CD4+ T lymphocytes in peripheral blood was shown to be very low in AIDS/HIV patients. This was consistent with previous research which revealed that high levels of CD4+ T lymphocytes could improve the quality of life of AIDS patients (Taiwo et al., 2009; Ren, 2018).
Immune activation has long been recognized as an important consequence of untreated HIV infection and a predictor of AIDS progression (Utay and Hunt, 2016). In particular, IL-6, a pro-inflammatory cytokine, has a proinflammatory role in the regulation of immune responses (Hirano and Kishimoto, 1989). Elevated levels of IL-6 can indicate a systemic inflammatory status, which is used as a marker of the HIV infection process (de Medeiros et al., 2016). Sobti et al. (2010) reported that suppressing IL-6 might decrease viral replication in the host after infection. TNF-α is an important mediator of inflammation and immune response (Fragoso et al., 2014). The level of TNF-α is much higher in AIDS/HIV patients than in healthy people, and continuous changes of TNF-α levels might lead to tissue damage and contribute to the development of other diseases (de Pablo-Bernal et al., 2014). In this study, levels of TNF-α and IL-6 were higher in the AIDS group, strongly suggesting that the progression of AIDS might be related to inflammatory response.
Elevated IL-6 in HIV-infected patients was related to impeded recovery of low CD4 levels (Rosado-Sánchez et al., 2017). CD4 is a T cell receptor for major histocompatibility complex class II antigens, a key regulator of immune response, and also a receptor for HIV (Wang et al., 1994). Evidence suggests that poor CD4+ T lymphocyte recovery is associated with microbial translocation in HIV-infected patients (Ponte et al., 2016). The enrichment of gut bacteria has been reported to be associated with poor CD4+ T cell reconstruction (Lu et al., 2018). Previous studies have indicated that the composition of gut microbiota is related to cancer immunotherapy (Routy et al., 2018) as well as HIV infection (Vujkovic-Cvijin et al., 2013). The human gastrointestinal tract is a complex ecosystem inhabited by the microbiome, including bacteria, viruses, archaea, and eukaryotic organisms. Probiotics can improve intestinal barrier function and even enhance the immunity of HIV-infected individuals by reducing the duration of diarrhea (Monachese et al., 2011). When the intestinal flora of AIDS patients is dysfunctional, the levels of Bifidobacterium and Lactobacillus are significantly lower, and the contents of E. coli, E. faecalis and E. faecium are higher (Lei et al., 2012). Shah et al. (2016) reported that E. coli is the most common bacterium associated with diarrhea in HIV patients. In addition, bacterial microbiome alteration is linked to IBD and inflammation in HIV-1 infection (Bäckhed et al., 2012; Lyte, 2013; Márquez et al., 2016). In the current study, the amounts of Bifidobacterium and Lactobacillus were low and negatively correlated with TNF-α and IL-6, and the number of CD4+ T lymphocytes. The indications and correlations above in E. coli, E. faecalis, and E. faecium were exactly opposite to those of Bifidobacterium and Lactobacillus. Our results were consistent with those of previous studies. For example, He et al. (2014) pointed out that the amount of Enterococcus in AIDS/HIV patients increased markedly, and CD4+ T lymphocyte counts were strongly and negatively correlated with Enterococcus counts. Li and Zhong (2017) reported that the expression of inflammatory cytokines (IL-6 and TNF-α) in peripheral blood of patients with ulcerative colitis was higher than that of healthy people, and was negatively correlated with the contents of Bifidobacterium and Lactobacillus, but positively correlated with the contents of Enterobacter and Enterococcus.
There were some limitations in our study. Firstly, our study was based on a small number of Chinese subjects and did not include people from other countries. Next, the effects of ART and antibiotic dosages and treatment regimens on the microbiome of AIDS/HIV patients were not investigated. These limitations will be addressed in our future research.
5. Conclusions
In conclusion, the intestinal microbiota of AIDS/HIV patients were disordered, and the amount of intestinal flora was correlated with the number of CD4+ T lymphocytes and the levels of TNF-α and IL-6, providing strong data to support the clinical treatment of AIDS/HIV patients.
Footnotes
Contributors: Jing LU and Sai-sai MA: conception and design of this study, and analysis of data. Wei-ying ZHANG and Jian-ping DUAN: revising the article critically for important intellectual content. All authors have read and approved the final manuscript. Therefore, all authors have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Jing LU, Sai-sai MA, Wei-ying ZHANG, and Jian-ping DUAN declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Bäckhed F, Fraser CM, Ringel Y, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Brenchley JM. Mucosal immunity in human and simian immunodeficiency lentivirus infections. Mucosal Immunol. 2013;6(4):657–665. doi: 10.1038/mi.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capeau J. Premature aging and premature age-related comorbidities in HIV-infected patients: facts and hypotheses. Clin Infect Dis. 2011;53(11):1127–1129. doi: 10.1093/cid/cir628. [DOI] [PubMed] [Google Scholar]
- 4.de Medeiros RM, Valverde-Villegas JM, Junqueira DM, et al. Rapid and slow progressors show increased IL-6 and IL-10 levels in the pre-AIDS stage of HIV infection. PLoS ONE. 2016;11(5):e0156163. doi: 10.1371/journal.pone.0156163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Pablo-Bernal RS, Ruiz-Mateos E, Rosado I, et al. TNF-α levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J Antimicrob Chemother. 2014;69(11):3041–3046. doi: 10.1093/jac/dku263. [DOI] [PubMed] [Google Scholar]
- 6.Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7(4):983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinh DM, Volpe GE, Duffalo C, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211(1):19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fragoso JM, Vargas Alarcón G, Jiménez Morales S, et al. Tumor necrosis factor alpha (TNF-α) in autoimmune diseases (AIDs): molecular biology and genetics. Gac Méd Méx. 2014;150(4):334–344. (in Spanish) [PubMed] [Google Scholar]
- 9.Gedle D, Kumera G, Eshete T, et al. Intestinal parasitic infections and its association with undernutrition and CD4 T cell levels among HIV/AIDs patients on HAART in Butajira, Ethiopia. J Health Popul Nutr, 36:15. 2017 doi: 10.1186/s41043-017-0092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He YW, Cai WP, Cen YW, et al. A preliminary study on intestinal microflora of AIDS patients and its correlation with CD4+ T lymphocytes. Guangdong Med J. 2014;35(14):2172–2174. doi: 10.3969/j.issn.1001-9448.2014.14.012. (in Chinese) [DOI] [Google Scholar]
- 11.Hirano T, Kishimoto T. Interleukin-6: possible implications in human diseases. Res Clin Lab. 1989;19(1):1–10. doi: 10.1007/BF02871787. [DOI] [PubMed] [Google Scholar]
- 12.Irvine SL, Hummelen R, Hekmat S, et al. Probiotic yogurt consumption is associated with an increase of CD4 count among people living with HIV/AIDs. J Clin Gastroenterol. 2010;44(9):e201–e205. doi: 10.1097/MCG.0b013e3181d8fba8. [DOI] [PubMed] [Google Scholar]
- 13.Klase Z, Ortiz A, Deleage C, et al. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol. 2015;8(5):1009–1020. doi: 10.1038/mi.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klatt NR, Villinger F, Bostik P, et al. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest. 2008;118(6):2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolgiri V, Patil VW. Protein carbonyl content: a novel biomarker for aging in HIV/AIDs patients. Braz J Infect Dis. 2017;21(1):35–41. doi: 10.1016/j.bjid.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei Y, Wang KH, Gong KM, et al. Real-time fluorescence quantitative PCR analysis of intestinal flora in patients with acquired immune deficiency syndrome. J Pract Med. 2012;28(1):69–71. doi: 10.3969/j.issn.1006-5725.2012.01.030. (in Chinese) [DOI] [Google Scholar]
- 17.Li L, Zhong Q. Correlation of intestinal microflora with cytokines and Toll-like receptors expression in patients with ulcerative colitis. Infect Dis Inf. 2017;30(6):361–364. doi: 10.3969/j.issn.1007-8134.2017.06.012. (in Chinese) [DOI] [Google Scholar]
- 18.Lu W, Feng YQ, Jing FH, et al. Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front Microbiol, 9:1451. 2018 doi: 10.3389/fmicb.2018.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9(11):e1003726. doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384(9939):258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- 21.Márquez M, del Álamo CFG, Girón-González JA. Gut epithelial barrier dysfunction in human immunodeficiency virus-hepatitis C virus coinfected patients: influence on innate and acquired immunity. World J Gastroenterol. 2016;22(4):1433–1448. doi: 10.3748/wjg.v22.i4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monachese M, Cunningham-Rundles S, Diaz MA, et al. Probiotics and prebiotics to combat enteric infections and HIV in the developing world: a consensus report. Gut Microbes. 2011;2(3):198–207. doi: 10.4161/gmic.2.3.16106. [DOI] [PubMed] [Google Scholar]
- 23.Monaco CL, Gootenberg DB, Zhao GY, et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe. 2016;19(3):311–322. doi: 10.1016/j.chom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponte R, Mehraj V, Ghali P, et al. Reversing gut damage in HIV infection: using non-human primate models to instruct clinical research. EBioMedicine. 2016;4:40–49. doi: 10.1016/j.ebiom.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quiloan MLG, Vu J, Carvalho J. Enterococcus faecalis can be distinguished from Enterococcus faecium via differential susceptibility to antibiotics and growth and fermentation characteristics on mannitol salt agar. Front Biol. 2012;7(2):167–177. doi: 10.1007/s11515-012-1183-5. [DOI] [Google Scholar]
- 27.Ren FN. Analysis on the influence of traditional Chinese medicine comprehensive intervention on AIDS/HIV patients. Guangming J Chin Med. 2018;33(11):1585–1587. doi: 10.3969/j.issn.1003-8914.2018.11.026. (in Chinese) [DOI] [Google Scholar]
- 28.Rosado-Sánchez I, Jarrín I, Pozo-Balado MM, et al. Higher levels of IL-6, CD4 turnover and Treg frequency are already present before cART in HIV-infected subjects with later low CD4 recovery. Antiviral Res. 2017;142:76–82. doi: 10.1016/j.antiviral.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Routy B, le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 30.Shah S, Kongre V, Kumar V, et al. A study of parasitic and bacterial pathogens associated with diarrhea in HIV-positive patients. Cureus. 2016;8(9):e807. doi: 10.7759/cureus.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobti RC, Berhane N, Mahedi SA, et al. Polymorphisms of IL-6 174 G/C, IL-10-592 C/A and risk of HIV/AIDS among North Indian population. Mol Cell Biochem. 2010;337(1-2):145–152. doi: 10.1007/s11010-009-0293-0. [DOI] [PubMed] [Google Scholar]
- 32.Taiwo BO, Li X, Palella F, et al. Higher risk of AIDS or death in patients with lower CD4 cell counts after virally suppressive HAART. HIV Med. 2009;10(10):657–660. doi: 10.1111/j.1468-1293.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Utay NS, Hunt PW. Role of immune activation in progression to AIDS. Curr Opin HIV AIDS. 2016;11(2):131–137. doi: 10.1097/COH.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5(193):193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ZQ, Orlikowsky T, Dudhane A, et al. Deletion of T lymphocytes in human CD4 transgenic mice induced by HIV-gp120 and gp120-specific antibodies from AIDS patients. Eur J Immunol. 1994;24(7):1553–1557. doi: 10.1002/eji.1830240715. [DOI] [PubMed] [Google Scholar]
- 36.Yao F, Lu YQ, Jiang JK, et al. Immune recovery after fluid resuscitation in rats with severe hemorrhagic shock. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(5):402–409. doi: 10.1631/jzus.B1600370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Liu H, Wei B. Immune response of T cells during herpes simplex virus type 1 (HSV-1) infection. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(4):277–288. doi: 10.1631/jzus.B1600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang SG, Song YX, Shu XM, et al. A simple method for removing low-density granulocytes to purify T lymphocytes from peripheral blood mononuclear cells. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(7):605–614. doi: 10.1631/jzus.B1600064. [DOI] [PMC free article] [PubMed] [Google Scholar]