Abstract
We investigated the value of autoantibodies as biomarkers of chronic graft-versus-host disease (cGVHD) by analyzing the autoantibody profiles of 65 patients (34 cGVHD and 31 non-cGVHD) surviving longer than three months after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Autoantibodies to at least one antigen were detected in 45 patients (70.8%), with multiple autoantibodies detected in 30 patients (46.2%). Antinuclear antibodies (ANAs) were the most frequently detected autoantibodies, with a significantly higher prevalence in non-cGVHD patients and cGVHD patients than that in healthy controls (HCs). ANA-nucleolar (ANA-N) was the main immunofluorescence pattern of ANA-positivity in both the non-cGVHD and cGVHD groups. There was a higher prevalence of anti-Ro52-positivity in non-cGVHD and cGVHD patients than in HC. Liver cGVHD was significantly associated with anti-Ro52-positivity. However, cGVHD activity and severity were not associated with the presence of autoantibodies. Similarly, there were no significant differences in overall survival or relapse among the four groups of patients expressing autoantibodies. Our results suggest that autoantibodies have limited value in predicting cGVHD.
Keywords: Autoantibody, Chronic graft-versus-host disease (cGVHD), Anti-Ro52
1. Introduction
Chronic graft-versus-host disease (cGVHD) is the most common cause of late non-relapse morbidity and mortality among recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT). This complication occurs in 20% to 70% of patients surviving for more than 100 d (Anasetti et al., 2012; Fraser et al., 2006) and most commonly affects the skin, liver, gut, lung, eye, and mouth. The immune responses associated with cGVHD exhibit similarities with those in autoimmune disorders (ADs), such as Sjögren syndrome, polymyositis, myasthenia gravis, autoantibody-mediated hemolysis, and scleroderma (Sherer and Shoenfeld, 1998; Sanz et al., 2007; Assandri et al., 2017). However, the pathogenesis of cGVHD is not completely understood and the mechanisms by which these autoimmune responses develop remain to be clarified. cGVHD is initiated when the host immune system encounters the recipient tissues, but as with ADs, the clinical manifestations of cGVHD may take months, or even years, to manifest. There are increasing numbers of clinical reports of an association between transplantation and humoral autoimmunity, and although there can now be little doubt that transplantation may act as a trigger for the development of autoantibodies, their clinical significance and relationship with cGVHD remain to be elucidated (Quaranta et al., 1999; Patriarca et al., 2006; Ruck et al., 2008; Tyndall and Dazzi, 2008; Moon et al., 2009; Lepelletier et al., 2017).
In this study, we analyzed the autoantibody profiles of 65 patients surviving longer than three months after allo-HSCT for a diagnosis of malignant hematological disease with the aim of detecting a possible association between the occurrence of autoantibodies and development of cGVHD after allo-HSCT.
2. Patients and methods
2.1. Patients
Sixty-five consecutive patients who underwent allo-HSCT from Mar. 2010 to May 2017 were enrolled in this trial. Patients were subdivided in two groups, patients (n=34) with a history of cGVHD and a control group (n=31) of patients who did not have cGVHD. We also included a group of age-, sex-, and ethnicity-matched healthy volunteer blood donor controls (n=32) who had not undergone transplantation. Stem cells were obtained from the following sources: peripheral blood progenitor cells (56 patients; cGVHD (n=29) and non-cGVHD (n=27)) and bone marrow (9 patients; cGVHD (n=5) and non-cGVHD (n=4)). Following centrifugation (1200g, 10 min, 4 °C), serum samples were collected and stored at −80 °C prior to assay, usually within four weeks. Table 1 shows the demographic and clinical features of the patients included in this study.
Table 1.
Patient and transplantation characteristics
| Characteristics | Non-cGVHD (n=31) | cGVHD (n=34) | P-value |
| Age (year) | |||
| Median | 34 | 30 | 0.26 |
| Range | 18–66 | 15–61 | |
| Sex | |||
| Male | 20 (65) | 22 (65) | 0.99 |
| Female | 11 (35) | 12 (35) | |
| Diagnosis | |||
| AML | 15 (48) | 17 (50) | 0.53 |
| ALL | 7 (23) | 13 (38) | |
| CML | 5 (16) | 1 (3) | |
| MDS | 3 (10) | 1 (3) | |
| NHL | 1 (3) | 2 (6) | |
| Disease status at HSCT | |||
| Standard risk | 26 (84) | 28 (82) | 0.88 |
| High risk | 5 (16) | 6 (18) | |
| Conditioning regimens | |||
| Myeloablative | 27 (87) | 29 (85) | 0.84 |
| Reduced intensity conditionings | 4 (13) | 5 (15) | |
| Stem cell source | |||
| Bone marrow | 4 (13) | 5 (15) | 0.84 |
| Peripheral blood stem cells | 27 (87) | 29 (85) | |
| Stem cell donors | |||
| Related | 26 (84) | 26 (76) | 0.47 |
| Unrelated | 5 (16) | 8 (24) | |
| HLA-identical | 28 (90) | 27 (79) | 0.23 |
| HLA-mismatched | 3 (10) | 7 (21) | |
| aGVHD after HSCT | |||
| None | 18 (58) | 15 (44) | 0.27 |
| Grades ≥II | 13 (42) | 19 (56) | |
| GVHD involvement | |||
| Skin | 21 (62) | ||
| Eye | 1 (3) | ||
| Oral mucosa | 4 (12) | ||
| Liver | 4 (12) | ||
| Lung | 9 (26) | ||
| GI | 8 (24) | ||
| Genital | 1 (3) | ||
| Joint | 3 (8) | ||
| NIH global severity | |||
| Mild | 1 (3) | ||
| Moderate | 19 (56) | ||
| Severe | 14 (41) |
Data are expressed as number (percentage) except age. GVHD, graft-versus-host disease; cGVHD, chronic GVHD; aGVHD, acute GVHD; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin’s lymphoma; HSCT, hematopoietic stem cell transplantation; HLA, human leukocyte antigen; GI, gastrointestinal tract; NIH, National Institutes of Health
This study was approved by the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (Hangzhou, China). In accordance with the Declaration of Helsinki, all patients and control participants provided oral and written informed consent to the provision of blood samples.
2.2. GVHD prophylaxis and treatment
GVHD prophylaxis and treatment were performed according to the protocols described by Luo et al. (2009) with the following modifications. Main myeloablative conditioning regimen: busulfan/cyclophosphamide administration without total body irradiation. Reduced-intensity conditioning regimens: predominantly fludarabine-based combinations without total body irradiation. Prevention of acute GVHD (aGVHD): cyclosporin (administered at 2.5 mg/(kg·d) intravenously from Day −7, with a target blood level of 200–300 ng/mL and dosage tapered during the second month post-transplantation according to the chimeric status of the patient and evidence of GVHD); mycophenolate mofetil (started orally at 500 mg/d on Day +1, tapered from Day +28, and withdrawn on Day +100); and short-term methotrexate (administered at 10 mg/d on Days +1, +3, and +6). Primary treatment for GVHD: systemic corticosteroids.
2.3. GVHD assessment
All patients surviving for more than 7 d after transplantation were considered at risk of developing aGVHD, which was diagnosed and graded using established criteria (Przepiorka et al., 1995), whereas those surviving for 100 d or longer were monitored for cGVHD, which was diagnosed according to the definition of the National Institutes of Health (NIH) Consensus Group Criteria (Filipovich et al., 2005).
2.4. Autoantibody detection
Peripheral blood samples were analyzed for 3, 6, and 12 months and once a year for autoantibody profiling. Serum autoantibody levels in samples (diluted 1:100 (v/v) in phosphate-buffered saline (PBS) Tween-20 (pH 7.2) immediately before examination) were measured using indirect immunofluorescence assay (IIFA). HEp-2 cells, neutrophils, and monkey liver tissue (EUROIMMUN AG, Lübeck, Germany) were used as substrates to detect antinuclear antibodies (ANAs) and antineutrophil cytoplasmic antibodies (ANCAs). Serum titer of ≥1:100 was considered positive and samples were titered at 1:320, 1:640, and 1:1000 (v/v). ANA-positivity was subdivided into the following categories according to specific fluorescence patterns: nuclear homogeneous (ANA-H), nuclear speckled (ANA-S), centromere (ANA-C), nucleolar (ANA-N), cytoplasmic (ANA-CY), and other (ANA-O); the latter category included fluorescence patterns such as peripheral/rim or nuclear envelope, dense fine speckled (DFS), proliferating cell nuclear antigen (PCNA), and multiple/few nuclear dots. An IgG autoantibody panel of 27 specificities (Ro/SSA, La/SSB, Sm, small nuclear ribonucleoproteins (snRNPs), topoisomerase I (Scl-70), histidyl-tRNA synthetase (Jo-1), nucleosomes, histones, centromere protein A (CENP-A), CENP-B, polymyositis-scleroderma 100 (PM-Scl100), PM-Scl75, Th/To, fibrillarin, nucleolar organizing region 90 (NOR90), Ku, RNA Polymerase III subunits 11 (RP11), RP155, platelet-derived growth factor receptor (PDGFR), ribosomal P protein, anti-PCNA, anti-double-stranded DNA (anti-dsDNA), anti-mitochondrial antibody M2 (AMA-M2), anti-liver kidney microsomal type 1 antibody (anti-LKM-1), anti-liver cytosolic antigen type 1 (anti-LC-1), anti-soluble liver antigen/liver pancreas (anti-SLA/LP)) and anti-Ro52 antibodies was analyzed using the EUROLINE immunoassay system (EUROIMMUN AG) according to the manufacturer’s instructions and the results were graded from weak to strong (i.e., “+”, “++”, and “+++”). Serum samples were examined for anti-cyclic citrullinated peptide antibodies (anti-CCPs), anti-cardiolipin antibodies (ACLAs), anti-proteinase 3 (PR3), and anti-myeloperoxidase (MPO) (EUROIMMUN AG) by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions.
2.5. Statistical analysis
Differences between groups were compared using Student’s t-test or the Mann-Whitney U-test for continuous variables, and using χ 2 test or Fisher’s exact test for categorical variables. Overall survival (OS) was analyzed using the Kaplan-Meier test, and both groups were compared using a log-rank test or a Breslow test. Disease relapse and cGVHD were considered competing risks in this analysis. Time to cGVHD was measured from the date of transplantation (Zhang et al., 2017). Statistical analyses were performed with SPSS 19.0 software (IBM Company, Chicago, IL, USA). Two-sided P-values of <0.05 were considered to indicate statistical significance.
3. Results
3.1. GVHD development
We prospectively compared the clinical features and plasma autoantibody expression variables among patients with cGVHD (n=34) and without cGVHD (n=31) following HSCT and healthy controls (HCs) (n=32) to identify potential biomarkers of the occurrence and severity of cGVHD. In the cGVHD and non-GVHD groups, the median time interval between transplantation and enrollment in the study was 47 months (range, 7 to 108 months) and 36 months (range, 10 to 108 months), respectively. There were no significant differences in the incidences of grades ≥II aGVHD between the two groups of patients: 42% in the non-cGVHD group compared with 56% in the cGVHD group (P=0.27; Table 1). Furthermore, there were no significant differences in transplant characteristics among the patients in these two groups. Overall, the cGVHD occurred in 52% of the 65 patients. Among the 34 patients diagnosed as cGVHD, the most frequent organ manifestations were skin (62%), lung (26%), and gastrointestinal tract (GI) involvement (24%).
3.2. Prevalence of autoantibodies
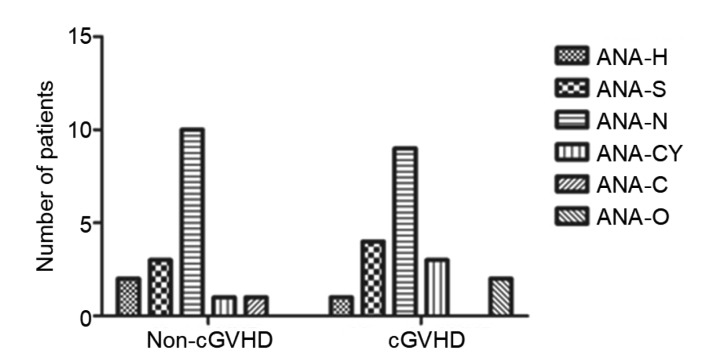
Among the 65 patients who underwent allog-HSCT, autoantibodies were detected in 45 patients (70.8%) and multiple autoantibodies were detected in 30 patients (46.2%). Furthermore, the median time to autoantibody detection was 23 months (range, 7 to 108 months) after transplantation. At least one autoantibody specificity was present in 21 non-cGVHD patients (67.7%). This prevalence was similar to that in 25 cGVHD patients (73.5%), but significantly higher than that for HC (67.7% vs. 9.4%, P<0.0003 and 73.5% vs. 9.4%, P<0.0001, respectively; Table 2). ANAs were the most frequently detected autoantibodies, with a significantly higher prevalence in non-cGVHD patients (17/31; 54.8%) and cGVHD patients (19/34; 55.9%) than in HC (54.8% vs. 6.2%, P<0.0001 and 55.9% vs. 6.2%, P<0.0001, respectively). ANA-N was the main immunofluorescence pattern (52.8%) of ANA-positivity in both the non-cGVHD and cGVHD groups (Fig. 1) followed by ANA-S (19.4%), ANA-CY (11.1%), ANA-H (8.3%), ANA-O (5.6%), and ANA-C (2.8%), although there was no significant difference between the two groups in terms of the proportions of the concomitant ANA staining patterns (Fig. 2). Furthermore, as expected, there was a higher prevalence of anti-Ro52 antibodies in non-cGVHD patients (6/31; 19.4%) and in cGVHD patients (6/34; 17.6%) than in HC (19.4% vs. 3.1%, P=0.047 and 17.6% vs. 3.1%, P=0.049, respectively). All the other antibodies assayed, including AMA-M2, anti-dsDNA, and anti-PDGFR, in patients were negative or the autoantibodies were detected at the same frequency in patients and HC.
Table 2.
Prevalence of the autoantibodies in the non-cGVHD, cGVHD, and healthy control (HC) groups
| Autoantibody | Positive*
|
P-value |
||||
| Non-cGVHD (n=31) | cGVHD (n=34) | HC (n=32) | Non-cGVHD vs. cGVHD | HC vs. non-cGVHD | HC vs. cGVHD | |
| Any autoantibody | 21 (67.7) | 25 (73.5) | 3 (9.4) | 0.506 | <0.0003a | <0.0001a |
| ANA (IFA) | 17 (54.8) | 19 (55.9) | 2 (6.2) | 0.978 | <0.0001a | <0.0001a |
| Anti-U1-nRNP | 0 (0.0) | 2 (5.9) | 0 (0.0) | 0.345 | 0.345 | |
| Anti-Sm | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-SS-A | 4 (12.9) | 1 (2.9) | 1 (3.1) | 0.148 | 0.148 | 0.966 |
| Anti-Ro/SS-A 52 kDa | 6 (19.4) | 6 (17.6) | 1 (3.1) | 0.772 | 0.047a | 0.049a |
| Anti-SS-B | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-Scl-70 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-Jo-1 | 1 (3.2) | 0 (0.0) | 0 (0.0) | 1.000 | 1.000 | |
| Anti-CENP-A | 1 (3.2) | 1 (2.9) | 0 (0.0) | 1.000 | 1.000 | 1.000 |
| Anti-CENP-B | 2 (6.5) | 1 (2.9) | 0 (0.0) | 0.345 | 0.500 | 1.000 |
| Anti-PM-Scl100 | 0 (0.0) | 2 (5.9) | 0 (0.0) | 0.500 | 0.500 | |
| Anti-PM-Scl75 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-PCNA | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1.000 | 1.000 | |
| Anti-dsDNA | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1.000 | 1.000 | |
| Anti-nucleosomes | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-histones | 0 (0.0) | 3 (8.8) | 0 (0.0) | 0.173 | 0.173 | |
| Anti-ribosomal P protein | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| AMA-M2 (LIA) | 1 (3.2) | 3 (8.8) | 0 (0.0) | 0.148 | 1.000 | 0.173 |
| Anti-RP11 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-RP155 | 1 (3.2) | 0 (0.0) | 0 (0.0) | 1.000 | 1.000 | |
| Anti-fibrillarin | 2 (6.5) | 0 (0.0) | 0 (0.0) | 0.345 | 0.345 | |
| Anti-NOR90 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-Th/To | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-Ku | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1.000 | 1.000 | |
| Anti-PDGFR | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| ACLA | 1 (3.2) | 0 (0.0) | 0 (0.0) | 1.000 | 1.000 | |
| Anti-CCP | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-PR3 (ELISA) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-MPO (ELISA) | 0 (0.0) | 3 (8.8) | 0 (0.0) | 0.159 | 0.107 | |
| Anti-SLA/LP | 1 (3.2) | 3 (8.8) | 0 (0.0) | 1.000 | 1.000 | 0.148 |
| Anti-LC-1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Anti-LKM-1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
Data are expressed as number (percentage).
Statistical significance between the groups (P<0.05). GVHD, graft-versus-host disease; cGVHD, chronic GVHD; ANA, anti-mitochondrial antibody; nRNP, nuclear ribonucleoprotein; Scl-70, topoisomerase I; Jo-1, histidyl-tRNA synthetase; CENP, centromere protein; PM, polymyositis; PCNA, proliferating cell nuclear antigen; RP, proteinase; NOR90, nucleolar organizing region 90; PDGFR, platelet-derived growth factor receptor; ACLA, anti-cardiolipin antibody; CCP, cyclic citrullinated peptide antibody; ELISA, enzyme-linked immunosorbent assay; MPO, myeloperoxidase; SLA/LP, soluble liver antigen/liver pancreas; LC-1, liver cytosolic antigen type 1; LKM-1, liver kidney microsomal type 1 antibody
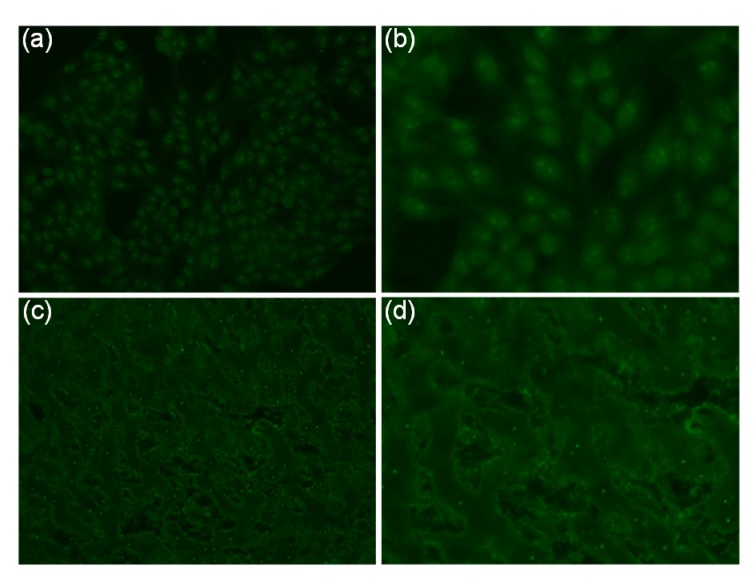
Fig. 1.
Example of nucleolar pattern of ANA in cGVHD sera detected by indirect immunofluorescence assay using HEp-2 cells and monkey liver tissue as substrates
(a) HEp-2 cells (magnification 20×). (b) HEp-2 cells (magnification 40×). (c) Monkey liver tissue (magnification 20×). (d) Monkey liver tissue (magnification 40×). ANA, anti-mitochondrial antibody; cGVHD, chronic graft-versus-host disease
Fig. 2.
Anti-nuclear antibody fluorescence patterns in the non-cGVHD and cGVHD groups
ANA, anti-nuclear antibody; ANA-H, ANA-homogeneous; ANA-S, ANA-speckled; ANA-N, ANA-nucleolar; ANA-CY, ANA-cytoplasmic patterns; ANA-C, ANA-centromere; ANA-O, ANA-other fluorescence patterns
3.3. Autoantibody expression according to cGVHD NIH global severity and activity
Correlations between the autoantibody profiles and cGVHD NIH global severity scores were analyzed as shown in Table 3. Twenty-five of 34 autoantibody-positive patients (73.5%) developed cGVHD (5 mild, 11 moderate, and 9 severe), whereas 9 of 34 autoantibody-negative patients (26.5%) developed cGVHD (4 moderate and 5 severe). However, there was no association between the overall incidence of autoantibodies and the cGVHD severity. Moreover, active cGVHD was present in 17 autoantibody-positive patients (68.0%) and in 6 autoantibody-negative patients (66.7%). Similarly, cGVHD activity was not correlated with the overall incidence of autoantibodies.
Table 3.
Autoantibody expression according to cGVHD NIH global severity and activity
| Characteristics | Autoantibody*
|
ANA*
|
||||||||
| Positive (n=25) | Negative (n=9) | OR | 95% CI | P | Positive (n=19) | Negative (n=15) | OR | 95% CI | P | |
| cGVHD severity at enrollment | ||||||||||
| Mild | 5 (20.0) | 0 (0.0) | 1.250 | 1.028–1.521 | 0.146 | 4 (21.1) | 1 (6.7) | 3.730 | 0.371–37.58 | 0.240 |
| Moderate | 11 (44.0) | 4 (44.4) | 0.982 | 0.212–4.553 | 0.982 | 9 (47.4) | 6 (40.0) | 1.350 | 0.343–5.315 | 0.667 |
| Severe | 9 (36.0) | 5 (55.6) | 0.450 | 0.096–2.120 | 0.307 | 6 (31.5) | 8 (53.3) | 0.404 | 0.099–1.640 | 0.201 |
| cGVHD activity | ||||||||||
| Active | 17 (68.0) | 6 (66.7) | 1.063 | 0.210–5.737 | 0.942 | 12 (63.2) | 11 (73.3) | 0.623 | 0.142–2.727 | 0.529 |
| Non-active | 8 (32.0) | 3 (33.3) | 7 (36.8) | 4 (26.7) | ||||||
|
| ||||||||||
|
| ||||||||||
| Characteristics | Anti-Ro/SS-A 52 kDa* | |||||||||
|
| ||||||||||
| Positive (n=6) | Negative (n=28) | OR | 95% CI | P | ||||||
|
| ||||||||||
| cGVHD severity at enrollment | ||||||||||
| Mild | 2 (33.3) | 3 (10.7) | 4.167 | 0.522–33.200 | 0.162 | |||||
| Moderate | 3 (50.0) | 11 (39.3) | 1.545 | 0.263–9.082 | 0.628 | |||||
| Severe | 1 (16.7) | 14 (50.0) | 0.200 | 0.021–1.930 | 0.136 | |||||
| cGVHD activity | ||||||||||
| Active | 3 (50.0) | 20 (71.4) | 0.400 | 0.066–2.415 | 0.309 | |||||
| Non-active | 3 (50.0) | 8 (28.6) | ||||||||
Data are expressed as number (percentage). cGVHD, chronic graft-versus-host disease; NIH, National Institutes of Health; ANA, anti-mitochondrial antibody; OR, odds ratio; CI, confidence interval
3.4. Circulating autoantibody expression and organ involvement with cGVHD
The frequencies of overall autoantibodies, ANA and anti-Ro52 antibodies were then evaluated for their association with specific organ involvement in cGVHD (Table 4). There was a lower frequency of overall antibodies in patients with skin and GI cGVHD (48.0% vs. 88.9%, P=0.033 and 12.0% vs. 44.4%, P=0.039, respectively). Similarly, a significantly lower frequency of ANA was detected in GI cGVHD patients (5.3% vs. 40.0%, P=0.013). However, a significantly higher frequency of anti-Ro52 antibodies was observed in liver cGVHD patients (66.7% vs. 4.1%, P=0.001). No significant association of the overall incidence of autoantibodies with oral mucosa, lung, joint, or fascia cGVHD was observed.
Table 4.
Autoantibody expression and organ involvement with cGVHD
| Organ | Autoantibody*
|
ANA*
|
Anti-Ro/SS-A-52 kDa*
|
||||||
| Positive (n=25) | Negative (n=9) | P | Positive (n=19) | Negative (n=15) | P | Positive (n=6) | Negative (n=28) | P | |
| Skin | 12 (48.0) | 8 (88.9) | 0.033a | 11 (57.9) | 9 (60.0) | 0.901 | 2 (33.3) | 18 (64.2) | 0.162 |
| Oral mucosa | 3 (12.0) | 0 (0.0) | 0.276 | 1 (5.3) | 2 (22.2) | 0.410 | 0 (0.0) | 3 (10.1) | 0.401 |
| Liver | 5 (20.0) | 0 (0.0) | 0.146 | 4 (21.1) | 1 (6.7) | 0.240 | 4 (66.7) | 1 (4.1) | 0.001d |
| Lung | 9 (36.0) | 2 (22.2) | 0.449 | 6 (31.6) | 3 (20.0) | 0.447 | 1 (16.7) | 8 (28.6) | 0.549 |
| GI | 3 (12.0) | 4 (44.4) | 0.039b | 1 (5.3) | 6 (40.0) | 0.013c | 0 (0.0) | 7 (25.0) | 0.169 |
| Joint and fascia | 6 (24.0) | 1 (11.1) | 0.412 | 4 (21.1) | 3 (20.0) | 0.940 | 0 (0.0) | 7 (25.0) | 0.169 |
Data are expressed as number (percentage). cGVHD, chronic graft-versus-host disease; GI, gastrointestinal tract; ANA, anti-mitochondrial antibody. Odds ratio (95% confidence interval):
0.115 (0.013–1.065);
0.17 (0.029–1.015);
0.083 (0.009–0.801);
54 (3.931–741.79)
3.5. Association between autoantibody expression and survival or relapse
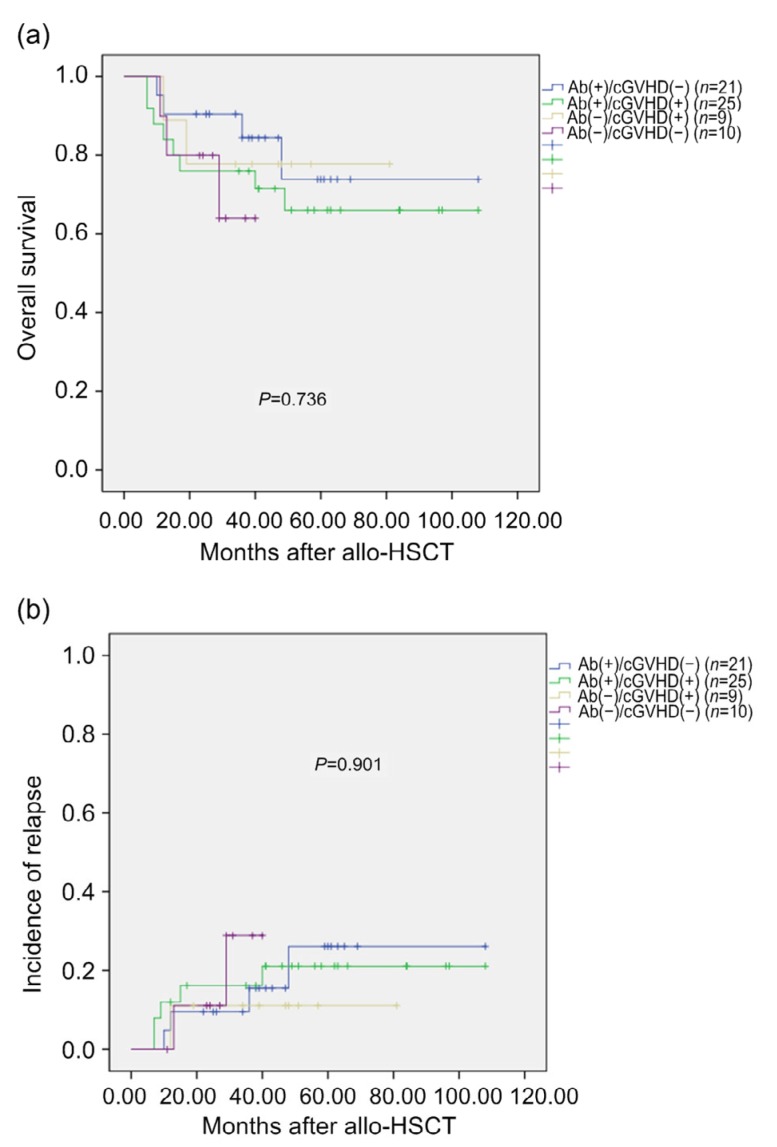
The value of autoantibody expression as a prognostic biomarker of survival and relapse of patients with cGVHD has been reported previously (Moon et al., 2009). Therefore, we analyzed autoantibody expression in patients who survived for more than 100 d post-transplantation. Patients were classified as autoantibody-positive/cGVHD-negative, autoantibody-positive/cGVHD-positive, autoantibody-negative/cGVHD-positive, and autoantibody-negative/cGVHD-negative groups. There were no significant differences in the 3-year OS rates between the groups (84.4%, 76.0%, 77.8%, and 64.0%, respectively; P=0.736). Similarly, there were no significant differences in the cumulative incidence of relapse between the groups (15.6%, 16.2%, 11.1%, and 28.9%, respectively; P=0.901).
Twelve (26.1%) of the 46 autoantibody-positive patients died, with the primary causes of death being relapse in nine cases (75.0%), cGVHD in two cases (16.7%), and infection in one case (8.3%). Five (26.3%) of the 19 autoantibody-negative patients died, with the primary causes of death being relapse in three cases (60.0%), cGVHD in one case (20%), and infection in one case (20%) (P=0.591). Patients in the autoantibody-positive group have relatively high relapse-related death, but there were no significant differences between the groups (Fig. 3).
Fig. 3.
Survival rates (a) and incidence of relapse (b)
Ab(+), autoantibody-positive; Ab(−), autoantibody-negative; cGVHD(+), cGVHD-positive; cGVHD(−), cGVHD-negative
4. Discussion
cGVHD is a serious complication of HSCT. The impairment is similar to that in some ADs (Tyndall and Dazzi, 2008; Pidala et al., 2011). High levels of serum autoantibodies detected in patients are important predictors of ADs (Sinico et al., 2005). In this study, we evaluated the potential value of autoantibodies as biomarkers of cGVHD after HSCT. An association between autoantibody expression and cGVHD has been reported previously (Patriarca et al., 2006; Moon et al., 2009). Although the prevalence of the autoantibodies detected in this study was higher in the cGVHD patients than in the non-cGVHD patients (73.5% vs. 67.7%), this difference was not statistically significant. Nevertheless, both rates were significantly higher than that among the HCs. Alloantibodies (predominantly to Y-chromosome-encoded histocompatibility (HY) antigen) have been described in cGVHD and correlate with disease activity (Miklos et al., 2005). In the present study, no significant association of autoantibodies with the severity or activity of cGVHD was found, which is consistent with the findings reported by Kuzmina et al. (2015). Thus, our findings suggest that patients receiving allo-HSCT have a higher incidence of autoantibodies. Indeed, B cells and pathogenic antibodies have been implicated in the pathogenesis of cGVHD (Panoskaltsis-Mortari et al., 2007; Srinivasan et al., 2012; Wang et al., 2017). In previous studies, elevated levels of soluble B cell activating factor (sBAFF) as well as increased numbers of T follicular helper (Tfh) cells, germinal center (GC) B cells, and antibodies that accumulate in target tissues have been reported in patients with cGVHD (Sarantopoulos et al., 2009; Flynn et al., 2014). These data suggest that, unlike healthy donors, B cells from HSCT patients are constantly exposed to allo-and auto-antigens. This leads to the activation and maturation of B cells that produce high-affinity class-switched antibodies in the case of cGVHD. In line with previous studies on HSCT and cGVHD (Patriarca et al., 2006; Lee et al., 2011), we observed that ANA was the most common autoantibody, being detected in more than half of the patients, usually at a low titer. The fluorescent ANA patterns were predominantly nucleolar types, which are often found in patients with systemic sclerosis (SSc) (Maddison et al., 1986). Scl-70 and anti-centromere antibodies are strongly associated with SSc (Catoggio et al., 1983). Although, similar to the results of Bell et al. (1996), we did not detect significant expression of anti-Scl-70 or anti-centromere antibodies related to cGVHD. Previous studies have shown that platelet-derived growth factor (PDGF) receptor-specific autoantibodies play a role in sclerodermatous cGVHD (Svegliati et al., 2007); however, this finding is controversial (Spies-Weisshart et al., 2013). In our study, comparisons of the cGVHD and the non-cGVHD groups showed no significant difference in the frequency of patients with anti-PDGFR antibodies. Thus, we hypothesize that the cGVHD does not affect the presence or functionality of PDGFR-specific autoantibodies. Evaluation of the SSc profiles showed a high proportion (90%) of SSc-positive sera with a nucleolar pattern (Ho and Reveille, 2003; Admou et al., 2009). In contrast, only 21.1% of sera with a nucleolar pattern from the cGVHD and non-cGVHD patients were found to be positive for SSc antigens, including CENP-A-B and the nucleolar (fibrillarin, RNA polymerases, Th/To, PM/Scl). However, most of the nucleolar staining patterns obtained using commercially available second level immunoblot tests did not reveal any nucleolar auto-antigens. Wesierska-Gadek et al. (1992) found that nucleolar proteins B23 and C23 were the major nucleolar autoantigens in the nucleolar staining pattern of cGVHD patients. However, they were not able to demonstrate the presence of antibodies to nucleolin in patients with scleroderma. All these autoantibodies can engage with the dense fibrillar component of the nucleolus, while having sequence homology with the binding sites of other different autoantigens. Previous studies have shown that ANAs with the anti-dense fine speckled 70 (anti-DFS70) staining pattern are commonly expressed in healthy subjects (Watanabe et al., 2004), whereas other studies demonstrated that the isolated anti-DFS70 antibodies were extremely rare in patients with systemic autoimmune rheumatic diseases (Muro et al., 2008; Sperotto et al., 2017). Thus, it can be speculated that the SSc profile defines a boundary between the two clinical conditions, and that these different autoantibody patterns can be used to distinguish between SSc and cGVHD. These conditions share a common potential autoantigen source required to initiate and maintain a B cell response.
In the present study, we detected a positive correlation between the specific organ involvement of cGVHD and autoantibody expression (Table 4). Anti-Ro52 antibodies were the primary autoantibodies detected in patients with liver cGVHD. In a previous study, antibodies to SSA antigen (Ro52/Ro60) were identified as a biomarker of Sjögren syndrome and systemic lupus erythematosus (Schulte-Pelkum et al., 2009). However, more recently, Ro52 and Ro60 were identified as two different protein components of the SSA antigen, representing two distinct autoantibody systems with different clinical associations (Defendenti et al., 2011). Patients with autoimmune liver diseases, such as primary biliary cirrhosis (PBC), are commonly anti-Ro52 antibody-positive, while anti-Ro52 antibodies, alone or in association with anti-SLA/LP antibodies, are associated with poorer prognosis in patients with autoimmune hepatitis type-1 (AIH-1) (Montano-Loza et al., 2012). Furthermore, unlike anti-SSA60 and SSB antibodies, anti-Ro52 antibodies target many transcription factors that dysregulate the production of proinflammatory IL23-Th17 pathway cytokines linked to tissue-specific inflammation and systemic autoimmunity (Espinosa et al., 2009). Our findings suggest that a biomarker, such as anti-Ro52 antibodies, that is specific for a target organ (liver) will provide important diagnostic and prognostic information.
In our study, analysis of autoantibody titers in patients with cGVHD did not reveal any significant associations with severity, activity, or clinical characteristics of cGVHD. Taken together with previous reports that autoantibody titers did not correlate with the severity of cGVHD or development of overt autoimmune disease (Moon et al., 2009; Kuzmina et al., 2015), these findings indicate that autoantibodies are not suitable biomarkers of the onset or severity of cGVHD. This is consistent with reports of the presence of autoantibodies in individuals without a history of chronic inflammatory or autoimmune disease and the absence of a correlation with disease activity (Langford, 2004; Finkielman et al., 2007). Moreover, a direct link between specific autoantibodies and tissue damage remains to be established in the majority of human chronic inflammatory and autoimmune diseases.
Previous studies have shown improved survival and reduced relapse rates in autoantibody-positive patients (Moon et al., 2009). In contrast, in our study, there were no significant differences in OS or relapse rates among the four groups of patients expressing autoantibodies (Fig. 3). The tyrosine kinase inhibitor (TKI), imatinib mesylate, which blocks the anti-PDGFR antibody pathway, has been used in steroid-refractory cGVHD. In a study of 15 cGVHD patients treated with imatinib, clinical outcomes were not correlated with the presence of anti-PDGFR antibodies (Chen et al., 2011). Imatinib is a promising therapy, although its clinical effect is not as strong as expected, which can be explained by the variation in antibody spectra in different cGVHD target organs (Olivieri et al., 2009). On the other hand, in patients with SSc, imatinib treatment led to a decreased modified Rodnan skin score (mRSS), which is a measure of skin fibrosis (P<0.001), and a trend toward improved lung functions (Khanna et al., 2011). Similar observations were made in other studies (Spiera et al., 2011; Fraticelli et al., 2014). These observations suggest that the multiple pathways involved in the development of cGVHD include autoantibody expression, although this condition is unlike conventional autoimmune diseases where autoantibodies do not play any beneficial role.
Some limitations of this study should be noted. The presence of autoantibodies prior to the development of autoimmune diseases has been reported (Arbuckle et al., 2003). Therefore, the presence of autoantibodies in the patients and donors prior to transplantation may influence the autoantibody status of recipients after transplantation.
In conclusion, we have demonstrated that autoantibodies are commonly present in patients after HSCT, and that anti-Ro52 antibodies may correlate with liver cGVHD. However, autoantibody profiles showed no relationship with the activity or severity of cGVHD and had no significant association with differences in survival rates. Our results suggest that autoantibodies have limited value in predicting cGVHD and that further studies are needed to clarify the diagnostic and prognostic value of autoantibodies in cGVHD.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY15H080002)
Contributors: Bing HAO and Song GAO performed the experimental research and data analysis, and wrote and edited the manuscript. Yi-wen SANG and Lin WANG performed the data analysis. Xue-qin MENG and Jing-ya YOU contributed to the ELISA assay. All authors have read and approved the final manuscript. Therefore, all authors have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Bing HAO, Song GAO, Yi-wen SANG, Lin WANG, Xue-qin MENG, and Jing-ya YOU declare that they have no conflicts of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for whom identifying information is included in this article.
References
- 1.Admou B, Essaadouni L, Amal S, et al. Autoantibodies in systemic sclerosis: clinical interest and diagnosis approach. Ann Biol Clin (Paris) 2009;67(3):273–281. doi: 10.1684/abc.2009.0332. [DOI] [PubMed] [Google Scholar]
- 2.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 4.Assandri R, Serana F, Montanelli A. Development of PBC/SSc overlap syndrome in chronic GVHD patient: immunological implications in the presence of mitochondrial, nucleolar and spindle midzone autoantigens. Gastroenterol Hepatol Bed Bench. 2017;10(4):323–331. [PMC free article] [PubMed] [Google Scholar]
- 5.Bell SA, Faust H, Mittermüller J, et al. Specificity of antinuclear antibodies in scleroderma-like chronic graft-versus-host disease: clinical correlation and histocompatibility locus antigen association. Br J Dermatol. 1996;134(5):848–854. doi: 10.1046/j.1365-2133.1996.116851.x. [DOI] [PubMed] [Google Scholar]
- 6.Catoggio LJ, Bernstein RM, Black CM, et al. Serological markers in progressive systemic sclerosis: clinical correlations. Ann Rheum Dis. 1983;42(1):23–27. doi: 10.1136/ard.42.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen GL, Arai S, Flowers MED, et al. A phase 1 study of imatinib for corticosteroid-dependent/refractory chronic graft-versus-host disease: response does not correlate with anti-PDGFRA antibodies. Blood. 2011;118(15):4070–4078. doi: 10.1182/blood-2011-03-341693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defendenti C, Atzeni F, Spina MF, et al. Clinical and laboratory aspects of Ro/SSA-52 autoantibodies. Autoimmun Rev. 2011;10(3):150–154. doi: 10.1016/j.autrev.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa A, Dardalhon V, Brauner S, et al. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med. 2009;206(8):1661–1671. doi: 10.1084/jem.20090585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147(9):611–619. doi: 10.7326/0003-4819-147-9-200711060-00005. [DOI] [PubMed] [Google Scholar]
- 12.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123(25):3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the bone marrow transplant survivor study. Blood. 2006;108(8):2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraticelli P, Gabrielli B, Pomponio G, et al. Low-dose oral imatinib in the treatment of systemic sclerosis interstitial lung disease unresponsive to cyclophosphamide: a phase II pilot study. Arthritis Res Ther. 2014;16(4):R144. doi: 10.1186/ar4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho KT, Reveille JD. The clinical relevance of autoantibodies in scleroderma. Arthritis Res Ther. 2003;5(2):80–93. doi: 10.1186/ar628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna D, Saggar R, Mayes MD, et al. A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum. 2011;63(11):3540–3546. doi: 10.1002/art.30548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuzmina Z, Gounden V, Curtis L, et al. Clinical significance of autoantibodies in a large cohort of patients with chronic graft-versus-host disease defined by NIH criteria. Am J Hematol. 2015;90(2):114–119. doi: 10.1002/ajh.23885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langford CA. Antineutrophil cytoplasmic antibodies should not be used to guide treatment in Wegener’s granulomatosis. Clin Exp Rheumatol. 2004;22(6 Suppl 36):S3–S6. [PubMed] [Google Scholar]
- 19.Lee DH, Huh SJ, Yoon HH, et al. Clinical significance of anti-mitochondrial antibodies in a patient with chronic graft-versus-host disease following hematopoietic stem cell transplantation. Korean J Hematol. 2011;46(3):200–202. doi: 10.5045/kjh.2011.46.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepelletier C, Bengoufa D, Lyes Z, et al. Dermatopulmonary syndrome associated with anti-MDA5 antibodies after allogeneic hematopoietic stem cell transplantation. JAMA Dermatol. 2017;153(2):184–188. doi: 10.1001/jamadermatol.2016.3976. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y, Lai XY, Tan YM, et al. Reduced-intensity allogeneic transplantation combined with imatinib mesylate for chronic myeloid leukemia in first chronic phase. Leukemia. 2009;23(6):1171–1174. doi: 10.1038/leu.2008.401. [DOI] [PubMed] [Google Scholar]
- 22.Maddison PJ, Skinner RP, Pereira RS, et al. Antinuclear antibodies in the relatives and spouses of patients with systemic sclerosis. Ann Rheum Dis. 1986;45(10):793–799. doi: 10.1136/ard.45.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montano-Loza AJ, Shums Z, Norman GL, et al. Prognostic implications of antibodies to Ro/SSA and soluble liver antigen in type 1 autoimmune hepatitis. Liver Int. 2012;32(1):85–92. doi: 10.1111/j.1478-3231.2011.02502.x. [DOI] [PubMed] [Google Scholar]
- 25.Moon JH, Lee SJ, Kim JG, et al. Clinical significance of autoantibody expression in allogeneic stem-cell recipients. Transplantation. 2009;88(2):242–250. doi: 10.1097/TP.0b013e3181ac6885. [DOI] [PubMed] [Google Scholar]
- 26.Muro Y, Sugiura K, Morita Y, et al. High concomitance of disease marker autoantibodies in anti-DFS70/LEDGF autoantibody-positive patients with autoimmune rheumatic disease. Lupus. 2008;17(3):171–176. doi: 10.1177/0961203307086311. [DOI] [PubMed] [Google Scholar]
- 27.Olivieri A, Locatelli F, Zecca M, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114(3):709–718. doi: 10.1182/blood-2009-02-204156. [DOI] [PubMed] [Google Scholar]
- 28.Panoskaltsis-Mortari A, Tram KV, Price AP, et al. A new murine model for bronchiolitis obliterans post-bone marrow transplant. Am J Respir Crit Care Med. 2007;176(7):713–723. doi: 10.1164/rccm.200702-335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34(3):389–396. doi: 10.1016/j.exphem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Pidala J, Kurland B, Chai XY, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 32.Quaranta S, Shulman H, Ahmed A, et al. Autoantibodies in human chronic graft-versus-host disease after hematopoietic cell transplantation. Clin Immunol. 1999;91(1):106–116. doi: 10.1006/clim.1998.4666. [DOI] [PubMed] [Google Scholar]
- 33.Ruck S, Hilgendorf I, Müller-Hilke B, et al. Autoantibody-mediated agranulocytosis in association with chronic GVHD. Bone Marrow Transplant. 2008;42(5):359–360. doi: 10.1038/bmt.2008.173. [DOI] [PubMed] [Google Scholar]
- 34.Sanz J, Arriaga F, Montesinos P, et al. Autoimmune hemolytic anemia following allogeneic hematopoietic stem cell transplantation in adult patients. Bone Marrow Transplant. 2007;39(9):555–561. doi: 10.1038/sj.bmt.1705641. [DOI] [PubMed] [Google Scholar]
- 35.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulte-Pelkum J, Fritzler M, Mahler M, et al. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8(7):632–637. doi: 10.1016/j.autrev.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Sherer Y, Shoenfeld Y. Autoimmune diseases and autoimmunity post-bone marrow transplantation. Bone Marrow Transplant. 1998;22(9):873–881. doi: 10.1038/sj.bmt.1701437. [DOI] [PubMed] [Google Scholar]
- 38.Sinico RA, Radice A, Corace C, et al. Value of a new automated fluorescence immunoassay (EliA) for PR3 and MPO-ANCA in monitoring disease activity in ANCA-associated systemic vasculitis. Ann N Y Acad Sci. 2005;1050(1):185–192. doi: 10.1196/annals.1313.019. [DOI] [PubMed] [Google Scholar]
- 39.Sperotto F, Seguso M, Gallo N, et al. Anti-DFS70 antibodies in healthy schoolchildren: a follow-up analysis. Autoimmun Rev. 2017;16(2):210–211. doi: 10.1016/j.autrev.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Spiera RF, Gordon JK, Mersten JN, et al. Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 1-year, phase IIa, single-arm, open-label clinical trial. Ann Rheum Dis. 2011;70(6):1003–1009. doi: 10.1136/ard.2010.143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spies-Weisshart B, Schilling K, Böhmer F, et al. Lack of association of platelet-derived growth factor (PDGF) receptor autoantibodies and severity of chronic graft-versus-host disease (GVHD) J Cancer Res Clin Oncol. 2013;139(8):1397–1404. doi: 10.1007/s00432-013-1451-z. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110(1):237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 44.Tyndall A, Dazzi F. Chronic GVHD as an autoimmune disease. Best Pract Res Clin Haematol. 2008;21(2):281–289. doi: 10.1016/j.beha.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang KS, Kim HT, Nikiforow S, et al. Antibodies targeting surface membrane antigens in patients with chronic graft-versus-host disease. Blood. 2017;130(26):2889–2899. doi: 10.1182/blood-2017-08-801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe A, Kodera M, Sugiura K, et al. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum. 2004;50(3):892–900. doi: 10.1002/art.20096. [DOI] [PubMed] [Google Scholar]
- 47.Wesierska-Gadek J, Penner E, Hitchman E, et al. Nucleolar proteins B23 and C23 as target antigens in chronic graft-versus-host disease. Blood. 1992;79(4):1081–1086. [PubMed] [Google Scholar]
- 48.Zhang AB, Wang Y, Hu C, et al. Laparoscopic versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a single-center experience. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(6):532–538. doi: 10.1631/jzus.B1600541. [DOI] [PMC free article] [PubMed] [Google Scholar]