Abstract
Catalpol is the main active ingredient of an extract from Radix rehmanniae, which in a previous study showed a protective effect against various types of tissue injury. However, a protective effect of catalpol on uterine inflammation has not been reported. In this study, to investigate the protective mechanism of catalpol on lipopolysaccharide (LPS)-induced bovine endometrial epithelial cells (bEECs) and mouse endometritis, in vitro and in vivo inflammation models were established. The Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling pathway and its downstream inflammatory factors were detected by enzyme-linked immunosorbent assay (ELISA), quantitative real-time polymerase chain reaction (qRT-PCR), western blot (WB), and immunofluorescence techniques. The results from ELISA and qRT-PCR showed that catalpol dose-dependently reduced the expression of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-1β, and IL-6, and chemokines such as C-X-C motif chemokine ligand 8 (CXCL8) and CXCL5, both in bEECs and in uterine tissue. From the experimental results of WB, qRT-PCR, and immunofluorescence, the expression of TLR4 and the phosphorylation of NF-κB p65 were markedly inhibited by catalpol compared with the LPS group. The inflammatory damage to the mouse uterus caused by LPS was greatly reduced and was accompanied by a decline in myeloperoxidase (MPO) activity. The results of this study suggest that catalpol can exert an anti-inflammatory impact on LPS-induced bEECs and mouse endometritis by inhibiting inflammation and activation of the TLR4/NF-κB signaling pathway.
Keywords: Catalpol, Endometritis, Inflammation, Toll-like receptor 4 (TLR4), Nuclear factor-κB (NF-κB)
1. Introduction
Bovine endometritis is a kind of mucinous or purulent inflammation of the uterus, which often occurs in perinatal dairy cows (Wagener et al., 2017). It can lead to infertility, delay the reproductive cycle, and reduce the production capacity of dairy cows, bringing huge economic losses to farm (Wu et al., 2016).
Clinically, bovine endometritis may be caused by mechanical injury of the uterus resulting from artificial insemination, calving, or midwifery during dystocia, or by infection caused by various pathogenic bacteria invading the uterus (van Schyndel and Pascottini, 2018). Clinical treatment of bovine endometritis relies mostly on antibiotics, but the use of antibiotics leads to increasing drug resistance and drug residues (Jiang et al., 2017), which has become a major hidden danger in the dairy cattle breeding industry. On the other hand, Chinese herbal medicine has become very popular in clinical medication because of its good safety, fewer toxic side effects, lack of residues, and convenient administration. In this context, the research and development of Chinese herbal medicine and its active ingredients have gradually become hot topics of interest.
Catalpol is an iridoid glycoside extracted from the rhizome of Rehmannia glutinosa. It has played an important pharmacological role in the study of many inflammatory diseases and exhibits a wide range of pharmacological functions. Catalpol can prevent D-galactose-induced mitochondrial dysfunction in mice by inhibiting nitric oxide synthase (NOS) activity and reactive oxygen species (ROS) production, and increasing respiratory complex activity and matrix metalloproteinase (MMP) levels (Zhang et al., 2010). It also increases hippocampal neuroplasticity and up-regulates protein kinase C (PKC) and brain-derived neurotrophic factor (BDNF) in aged rats (Liu et al., 2006). More and more studies have proved that catalpol plays an important role in protecting against Alzheimer’s disease (Zhang et al., 2009), and in neuroprotection, microcirculation improvement, and antioxidation (Huang et al., 2013). However, a protective effect of catalpol on uterine inflammation has not been reported. Whether it can protect against lipopolysaccharide (LPS)-challenged endometritis or alleviate endometrial inflammation remains unknown.
LPS, as an important stimulus leading to an inflammatory response in vitro and in vivo, can activate the intracellular signal transduction mechanism involving the nuclear factor-κB (NF-κB) in combination with the cell surface receptor, Toll-like receptor 4 (TLR4) (Zhao et al., 2019). This can then activate the production of many inflammatory factors downstream and aggravate the occurrence and development of inflammation. Upon stimulation, the subunit p65 of NF-κB transfers from the cytoplasm to the nucleus and is phosphorylated. This induces the production of many proinflammatory mediators such as interleukin (IL)-1β, IL-6, and tumor necrosis factor α (TNF-α) (Brown et al., 1993). These proinflammatory cytokines can induce the expression of other chemokines, which furthers the acute phase response to aggravate inflammatory damage (Israël, 2010). Therefore, the TLR4/NF-κB pathway is often used to study anti-inflammatory mechanisms in response to LPS-stimulated inflammatory diseases.
Therefore, the aim of this study was to establish LPS-stimulated bovine endometrial epithelial cell (bEEC) inflammation in vitro and LPS-induced mouse endometritis in vivo, and to investigate the anti-inflammatory mechanisms and protective effects of catalpol through the TLR4/NF-κB signaling pathway.
2. Materials and methods
2.1. Reagents
Catalpol (purity ≥98%; Fig. 1) was purchased from the Chengdu Mansite Bio-technology Co., Ltd. (Chengdu, China). Cell Counting Kit-8 (CCK-8) and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were obtained from the Beijing Neobioscience Biotechnology Co., Ltd. (Beijing, China). Myeloperoxidase (MPO) determination kits were obtained from the Jiancheng Bioengineering Institute of Nanjing (Nanjing, China).
Fig. 1.
Chemical structure of catalpol
β-Actin and its horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Santa Cruz Bio-technology, Inc. (Dallas, TX, USA). Primary antibodies for phospho-p65 (p-p65) were provided by Cell Signaling Technology (Beverly, MA, USA).
2.2. Cell culture
bEECs were purchased from the American Type Culture Collection (ATCC CRL-2398™; Manassas, USA), and cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 (HyClone, Logan, UT, USA) with 10% fetal bovine serum (FBS; PAN, Germany) for 24 h in a humidified incubator with 5% CO2 at 37 °C, then digested by trypsin for 3 min. The cells were mixed with DMEM/F-12 nutrient solution containing 10% FBS, transferred to six-well plates for another 24 h, and then pretreated with 1, 0.1, or 0.01 mmol/L catalpol for 1 h. LPS (1 μg/mL, from Escherichia coli O55:B5, Merck, Germany) alone or with different concentrations of catalpol was then added for a further 12 h. A dexamethasone (DXM; 1 μmol/L) group was used as a positive control. The supernatant and cells were collected for subsequent experiments.
2.3. CCK-8 assay
The effect of catalpol on the cell viability of bEECs was measured by CCK-8 assay (Chen Z et al., 2018). Briefly, bEECs were seeded in 96-well plates at 1×105 cells per well for 24 h, then treated with 100 μL of catalpol at different concentrations (1 mmol/L, 0.1 mmol/L, 0.01 mmol/L, 1 μmol/L, or 0.1 μmol/L) in an incubator for a further 12 h. Then 10 μL of water-soluble tetrazolium salt-8 (WST-8) was added to each well for another 2-h incubation. The optical density (OD) was measured using a microplate reader (Bio-Rad Instruments, Hercules, CA, USA) at 450 nm. The percentage cell viability was estimated as (ODtreatment–ODblank)/(ODcontrol–ODblank)×100%.
2.4. Immunofluorescence staining
The localization and expression of p-p65 in the cell nucleus were determined by cell immunofluorescence staining. The only difference from the culture protocol described above was that circular slides were added to each well before cells were transferred to six-well plates. After co-treatment with LPS and various concentrations of catalpol for 12 h, bEECs on the slides were washed three times with phosphate-buffered saline (PBS), and then fixed with 4% (0.04 g/mL) paraformaldehyde for 10 min, followed by incubation with primary antibodies and fluorescein isothiocyanate (FITC)-labeled secondary antibodies. Finally, after staining with 4',6-diamidino-2-phenylindole (DAPI) for 10 min, the localization and expression of p-p65 were observed using a fluorescence microscope (Olympus, Japan).
2.5. Cytokine assay by ELISA
The bEECs were treated according to the above method. The DXM group was treated as a positive control. The cell supernatants were collected. Cytokine levels (IL-1β, TNF-α, and IL-6) in cell supernatants were measured using ELISA kits according to the previous method (Chen BY et al., 2018).
2.6. qRT-PCR
Two-step quantitative real-time polymerase chain reaction (qRT-PCR) was used for detection (Guo et al., 2018). Firstly, the reverse transcription process was carried out using an HiScript® Q RT SuperMix for qRT-PCR (+gDNA wiper) kit (Vazyme Biotech Co., Ltd., Nanjing, China). Then the high-sensitivity qRT-PCR reaction was measured by AceQ® qPCR SYBR® Green Master Mix (Vazyme). The specific primers used in this study are listed in Table 1. The messenger RNA (mRNA) expression levels of IL-1β, TNF-α, IL-6, C-X-C motif chemokine ligand 5 (CXCL5), and CXCL8 were normalized with that of the control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and measured using the 2−ΔΔ C T comparative method (n=3).
Table 1.
Primers used for qRT-PCR
| Gene name | Primer sequence (5'→3') | Product size (bp) | GenBank accession number |
| IL-1β | F: AACCTTCATGCCCAGGTTTCTG R: GGTCATCAGCCTCAAATAACAGC | 113 | NM_174093.1 |
| IL-6 | F: TCTGGGTTCAATCAGGCGAT R: TGCGTTCTTTACCCACTCGT | 246 | NM_173923.2 |
| TNF-α | F: GCCCACGTTGTAGCCGA R: CCCTGAAGAGGACCTGTGAG | 157 | NM_173966.3 |
| GAPDH | F: ACTTATGACCACTGTCCACGC R: CACAACAGACACGTTGGGAGT | 211 | NM_001034034.2 |
| CXCL5 | F: AGCGCCGGTCCTGTCG R: GGTGGCTATCACTTCCACCTTG | 144 | NM_174300.2 |
| CXCL8 | F: TCTGTGTGAAGCTGCAGTTCTGT R: CTTGGGGTTTAGGCAGACCTCG | 190 | NM_173925.2 |
F: forward primer; R: reverse primer
2.7. Mice grouping and sample collection
Kunming mice weighing about 25 g on average were obtained from the Animal Experiment Center of Huazhong Agricultural University (Wuhan, China). The feeding environment of the mice was at room temperature (23 °C) and 60% relative humidity. The illumination time was 12 h per day, and the mice were free to eat and drink water. All experimental procedures involving animals and their care were approved by the Animal Welfare and Research Ethics Committee of Huazhong Agricultural University. The LPS-induced endometritis mouse model was developed as follows. Mice (n=5) were administered equal amounts of LPS (1 mg/kg) on each side of the uterus, under anesthesia. Twenty-four hours after LPS stimulation, catalpol at 1, 10, or 100 mg/kg, or DXM at 5 mg/kg, respectively, was injected intraperitoneally. Mice were then killed via CO2 inhalation 24 h later. Uterine tissues from each group were harvested and immersed in 4% (0.04 g/mL) paraformaldehyde for hematoxylin and eosin (H&E) staining. The remaining tissues were stored at −80 °C for subsequent study.
2.8. Histopathological analysis
Two sides of the uterus were collected and fixed in 4% (0.04 g/mL) paraformaldehyde solution for 24 h. Dehydration and paraffin embedding were carried out according to routine procedures. Sections of 4-µm thickness were stained using H&E and then examined under a microscope.
2.9. MPO activity assay
Uterine samples were homogenized in PBS and centrifuged at 12 000 r/min for 15 min. The supernatants were collected for further measurements according to the manufacturer’s instructions in the MPO activity kit (Jiancheng Biotechnology, Nanjing, China).
2.10. Western blot analysis
The methods for extracting tissue and cell proteins were as follows. Thirty milligrams of uterine tissues were ground with liquid nitrogen at low temperature. Then a protein lysate containing phenylmethylsulfonyl fluoride (PMSF) and protease inhibitors was added and fractured on ice for 30 min. The cell supernatants were collected from six-well plates. bEECs were washed three times with PBS, and then protein lysate was added. bEECs were then fractured in an ice shaker for 30 min. The protein concentration was measured by bicinchoninic acid (BCA) protein assay and adjusted to the same concentration using a loading buffer. The target protein was identified by 10% (0.1 g/mL) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to a nitrocellulose membrane to bind to the primary antibody at 4 °C overnight, and to the HRP-conjugated secondary antibody for 1.5 h at room temperature (Yang et al., 2018). β-Actin was used as the control. The protein levels of specific target genes expressed by electrophoresis were detected by a 3,3'-diaminobenzidine (DAB) substrate chromogenic assay (n=3).
2.11. Statistical analysis
Analysis was performed using GraphPad Prism 7.02 Software (GraphPad Software, Inc., USA). Differences between mean values of normally distributed data were analyzed using one-way analysis of variance (ANOVA) multiple comparisons. P<0.05 was considered statistically significant.
3. Results
3.1. Effect of catalpol on bEEC viability
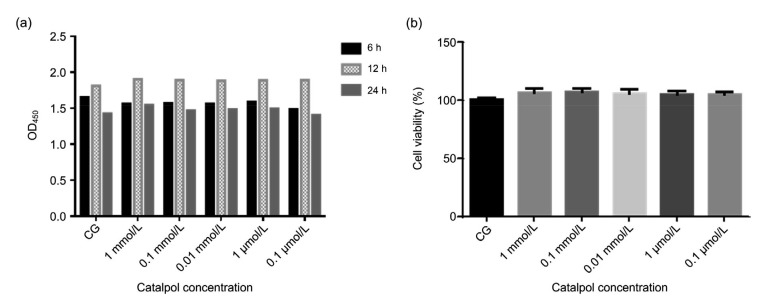
After 24 h of cell sticking cultivation, catalpol was added to bEECs for another 6, 12, or 24 h of incubation. When the cell culture time increased from 6 to 12 h, the cell proliferation rate increased, but by 24 h it declined (Fig. 2a). So we chose a cell culture time of 12 h as the basis for subsequent experiments. The effect of catalpol on the viability of bEECs was detected using the CCK-8 method. The results showed that catalpol had no effect on the viability of bEECs (Fig. 2b).
Fig. 2.
Effect of catalpol on the viability of bEECs
(a) Cells were cultured in 96-well cell plates at a density of 1×105 cells/mL for 24 h, and then catalpol was added at five concentrations (1 mmol/L, 0.1 mmol/L, 0.01 mmol/L, 1 μmol/L, or 0.1 μmol/L), followed by incubation for 6, 12, and 24 h. After CCK-8 was added for 2 h, the optical density was measured at a wavelength of 450 nm (OD450). (b) Catalpol at five concentrations was added to bEEC cultures for 12 h. CCK-8 was then added and after 2 h, OD450 was measured. The results were calculated using the following formula: cell viability (%)=(ODtreatment–ODblank)/(ODcontrol–ODblank)×100%. Data represent the mean±standard error of mean (SEM) of triplicate experiments. CG: control group; bEEC: bovine endometrial epithelial cell; CCK-8: Cell Counting Kit-8
3.2. Effect of catalpol on expression of inflammatory cytokines
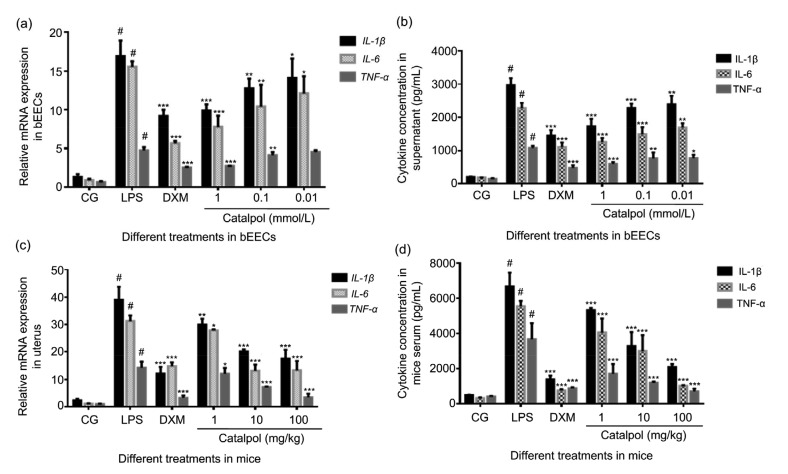
IL-1β, IL-6, and TNF-α are important inflammatory mediators involved in the inflammatory response. In this study, the expression of inflammatory factors in cell supernatants and mice serum was measured by ELISA, and cytokine gene expression in bEECs and uterine tissue was detected by qRT-PCR. LPS upregulated the cytokine expression of IL-1β, IL-6, and TNF-α in both in vitro and in vivo inflammation models, while catalpol inhibited the expression of inflammatory mediators in a concentration-dependent manner (Fig. 3).
Fig. 3.
Effect of catalpol on cytokine expression in bEECs and mice stimulated by LPS
(a, b) Cytokine expression in bEECs, stimulated by LPS (1 μg/mL) and co-treated with 1, 0.1, or 0.01 mmol/L catalpol in an incubator for 12 h, measured by qRT-PCR (a) and ELISA (b). DXM (1 μmol/L) was set as the positive control. (c, d) The mRNA expression and concentration of cytokines in mice uterine tissue and serum in the LPS (1 mg/kg) group, DXM (5 mg/kg) group, and three catalpol (1, 10, and 100 mg/kg) groups as above, were measured by qRT-PCR (c) and ELISA (d). Results are expressed as percentages relative to the untreated control after normalizing to GAPDH levels (n=3). Data represent the mean±standard error of mean (SEM) of triplicate experiments. # indicates P<0.05 vs. the control group. *, **, and *** indicate P<0.05, P<0.01, and P<0.001 vs. the LPS group, respectively. CG: control group; LPS: lipopolysaccharide; DXM: dexamethasone; bEEC: bovine endometrial epithelial cell
3.3. Effect of catalpol on chemokine gene expression in bEECs and mice uterus tissue stimulated by LPS
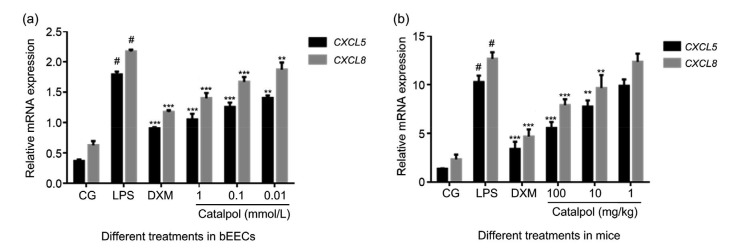
In addition to the dominant role of cytokines, chemokines play an important part in inflammatory response. Virtually every tissue and cell type tested to date can be induced to secrete chemokines (Chensue, 2001). By binding with its specific receptors, CXCL8 participates in and regulates reproductive physiological and pathological processes such as ovulation, endometrial proliferation and abortion (Kawamura et al., 2012). The pro-inflammatory chemokine CXCL5 is a member of the CXC-type chemokines. Research has revealed the roles of CXCL8 and CXCL5 in the physiological and pathological processes of uterine tissue (Begley et al., 2008). In this study, CXCL5 and CXCL8 were significantly expressed in both uterine tissue and endometrial epithelial cells stimulated by LPS compared with control groups, while chemokine gene expression in catalpol treatment groups was effectively reduced in a dose-dependent manner (Fig. 4).
Fig. 4.
CXCL5 and CXCL8 gene expression in bEECs and uterine tissue challenged by LPS and co-treated with catalpol
(a) CXCL5 and CXCL8 mRNA expression in bEECs treated by LPS alone and with catalpol at 1, 0.1, or 0.01 mmol/L for 12 h in cell culture, determined by qRT-PCR. DXM (1 μmol/L) was set as the positive control. (b) Mice were stimulated by LPS for 24 h, then injected intraperitoneally with 100, 10, or 1 mg/kg catalpol. Uterine tissues were collected 24 h later. DXM (5 mg/kg) was set as the positive control. CXCL5 and CXCL8 mRNA expression was determined by qRT-PCR. Results are expressed as percentages relative to the untreated control after normalizing to GAPDH levels (n=3). Data represent the mean±standard error of mean (SEM) of triplicate experiments. # indicates P<0.05 vs. CG. ** and *** indicate P<0.01 and P<0.001 vs. the LPS group, respectively. CXCL5: C-X-C motif chemokine ligand 5; CG: control group; LPS: lipopolysaccharide; DXM: dexamethasone; bEEC: bovine endometrial epithelial cell
3.4. Effect of catalpol on LPS-induced endometritis in mice
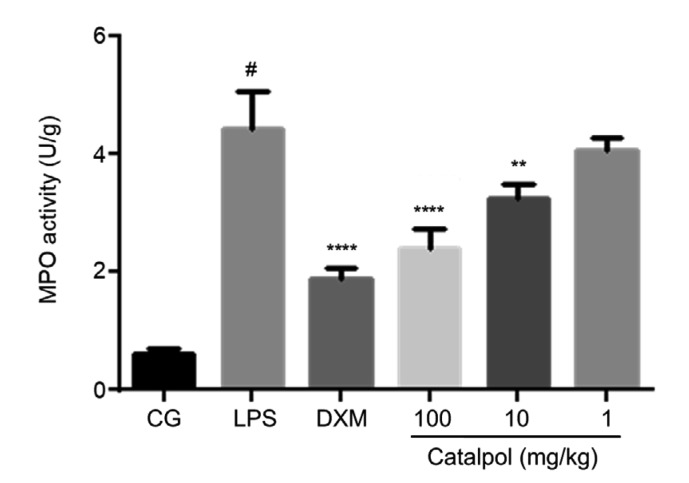
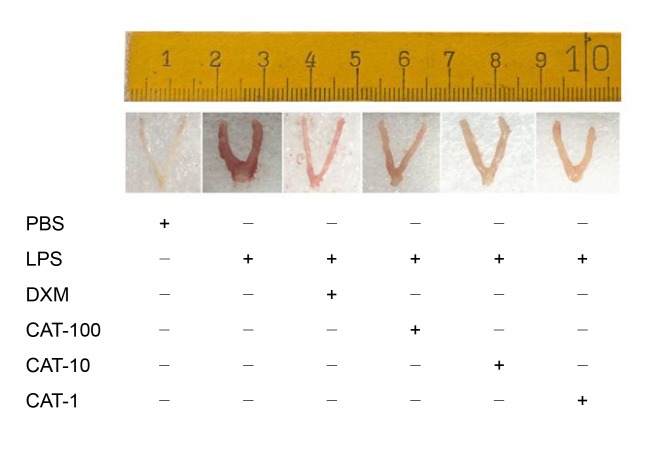
Intraperitoneal injection of catalpol at different concentrations in LPS-induced endometritis mice (Fig. 5) can decrease MPO activity (Fig. 6) and effectively reduce endometrial inflammation (Fig. 7), and alleviate pathological damage to the uterus (Fig. 8). MPO, known as a neutrophil granulocytes (PMN) marker enzyme, is used as a direct measure of the active state of neutrophil granulocytes in tissues (Zhao et al., 2019). The change in MPO level can reflect the degree of tissue damage. Fig. 7 shows that LPS can significantly increase the MPO level in uterine tissue, and lead to obvious damage (Fig. 6) compared with the control group. Catalpol can reduce MPO activity in the uterus (Fig. 7), thereby exerting a protective effect on the endometrium (Fig. 6). By observing histopathological sections of mouse uterine tissue stained with H&E in the control group, endometrial glands were seen to be well developed and glandular epithelial cells were columnar or square (Figs. 8a and 8b). In the LPS-treatment group (Figs. 8c and 8d), the endometrial layer was extensively exfoliated, endometrial stroma showed a large amount of vascular congestion, and inflammatory cells were infiltrating myometrial tissue. Co-treatment with catalpol exerted a good protective effect on LPS-induced endometritis in mice. In Figs. 8e and 8f, the structure of the uterine glands is obvious. Only in the superficial muscular layer did cells show light edema and a cubic shape. Inflammatory cell infiltration could be seen only in a small area.
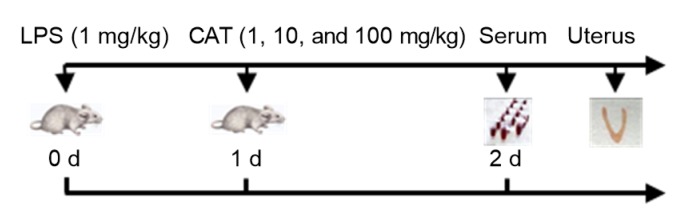
Fig. 5.
Time axis of experimental animal treatment
Catalpol was injected into mice at 1, 10, or 100 mg/kg, 1 d after intrauterine injection of 1 mg/kg LPS. The control group received an intrauterine injection of PBS. The serum and uterine tissues of mice were collected the next day. DXM (5 mg/kg) group was set as a positive control. LPS: lipopolysaccharide; CAT: catalpol; PBS: phosphate-buffered saline; DXM: dexamethasone
Fig. 6.
MPO activity in uterine tissue
Mice groups were set as above. Uterine tissues were collected, homogenized in PBS, and centrifuged at 12 000 r/min for 15 min. The supernatants were measured according to the manufacturer’s instruction in the MPO activity kit. Data represent the mean±standard error of mean (SEM) of triplicate experiments. # indicates P<0.05 vs. CG. ** and **** indicate P<0.01 and P<0.0001 vs. the LPS group, respectively. CG: control group; LPS: lipopolysaccharide; DXM: dexamethasone; MPO: myeloperoxidase; PBS: phosphate-buffered saline
Fig. 7.
Effect of catalpol on endometrial inflammation of LPS-induced endometritis in mice
Catalpol was injected into mice at 1, 10, or 100 mg/kg (CAT-1, CAT-10, or CAT-100) 1 d after intrauterine injection of 1 mg/kg LPS. The control group received an intrauterine injection of PBS. The DXM (5 mg/kg) group was set as a positive control. Uterine tissues of the mice were collected the next day, and immersed in 4% (0.04 g/mL) paraformaldehyde for H&E staining. PBS: phosphate-buffered saline; LPS: lipopolysaccharide; DXM: dexamethasone; CAT: catalpol; H&E: hematoxylin and eosin
Fig. 8.
H&E staining of uterine tissue in control, LPS-induced and catalpol (10 mg/kg) co-treated mice
(a, b) Control group. Histopathology of the endometrium from healthy mice. Black arrows point to the intact epithelium layer and red arrows to vacuoles. (c, d) LPS group. Histopathology of the endometrium from LPS-induced mice. Pathological changes are shown as hemorrhage in multiple areas (blue arrows), epithelial cell exfoliation (purple arrows), and a large amount of inflammatory cell aggregation (green arrows). The epithelial layer was severely damaged (yellow arrows). (e, f) LPS+catalpol group. Histopathology of the endometrium from LPS-induced mice co-treated with catalpol. Only a small part of the luminal epithelium was damaged (white arrows). The morphology of epithelial cells changed greatly, most becoming cubic in shape (brown arrows). A few inflammatory cells were seen in the epithelial layer (green arrows). LPS: lipopolysaccharide; H&E: hematoxylin and eosin
3.5. Effect of catalpol on the TLR4/NF-κB signal in bEECs and uterine tissue induced by LPS
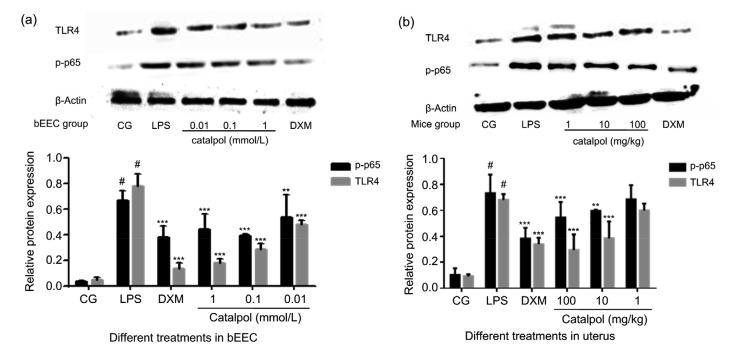
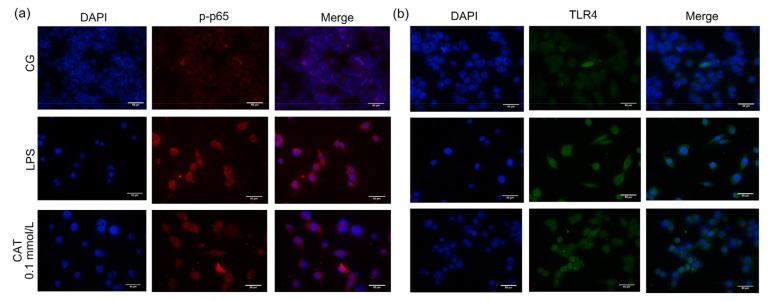
LPS binds to the cell surface receptor TLR4, transduces signals into the cell, and activates the downstream NF-κB pathway (Li and Verma, 2002; de Vries et al., 2014). Normally, NF-κB p65 and inhibitory protein inhibitor κBα (IκBα) are bound together in the cytoplasm (Lyu et al., 2017). Once stimulated, they dissociate rapidly. IκBα undertakes a phosphorylation reaction, while NF-κB p65 enters the nucleus, and then induces a large number of inflammatory factors (Nicholas et al., 2007). The protein expression of TLR4 and p-p65 increased markedly in both bEECs and uterine tissue after they were challenged by LPS, while catalpol had an inhibitory effect on the activation of TLR4/NF-κB in a dose-dependent manner (Fig. 9). Moreover, the detection by immunofluorescence of p-p65 and TLR4 in bEECs was consistent with the trend of above results (Fig. 10).
Fig. 9.
Effects of catalpol on the expression of TLR4 and p-p65 proteins in LPS-induced bEECs and mice uterine tissues
(a) bEECs were pretreated with catalpol at 1, 0.1, or 0.01 mmol/L for 1 h, and then LPS (1 µg/mL) was added, followed by incubation for a further 12 h. DXM (1 μmol/L) group was set as a positive control. (b) Catalpol was injected into mice at 1, 10, or 100 mg/kg, 1 d after intrauterine injection of 1 mg/kg LPS. The control group received an intrauterine injection of PBS. DXM (5 mg/kg) group was set as a positive control. Uterine tissues of mice were collected the next day. The phosphorylation levels of p65 and TLR4 were analyzed by western blot. Immunoblot signals were detected by an enhanced chemiluminescence detection system. Quantification of protein expression was normalized to β-actin using a densitometer (n=3). The data represent the mean±standard error of mean (SEM) of triplicate experiments. # indicates P<0.05 vs. CG. ** and *** indicate P<0.01 and P<0.001 vs. the LPS group, respectively. CG: control group; LPS: lipopolysaccharide; DXM: dexamethasone; PBS: phosphate-buffered saline; bEEC: bovine endometrial epithelial cell; TLR4: Toll-like receptor 4
Fig. 10.
Immunofluorescence detection of p-p65 and TLR4 in bEECs
The protein expression of p-p65 (a) and TLR4 (b) was detected by cell immunofluorescence assay in the control, LPS, and catalpol (0.1 mmol/L) groups. Bar=50 µm. CG: control group; LPS: lipopolysaccharide; CAT: catalpol; bEEC: bovine endometrial epithelial cell; DAPI: 4',6-diamidino-2-phenylindole; TLR4: Toll-like receptor 4
4. Discussion
Endometritis is an inflammatory change in endometrial structure resulting from various causes (Socha and Lada, 2018). Residual tissue fragments and a fluid environment in the uterus after parturition, weakness in uterine contraction after calving, and uterine injury caused by dystocia can easily to cause infection in the uterine lumen and endometritis (Sheldon et al., 2008). Endometritis in dairy cattle breeding can delay the regeneration of the endometrium, disrupt the resumption of cyclic ovarian function (Sicsic et al., 2018), and cause failure of artificial insemination and infertility, thereby causing huge economic losses (Machado Pfeifer et al., 2018). The endometrium is the first line of defense against reproductive tract infections after delivery (Shen et al., 2018). It plays an important role as a barrier (Koyama et al., 2018). Endometrial cells also play an important role in innate immune defense (Herath et al., 2009). Therefore, in this study, we first used LPS-induced bEECs to construct an in vitro anti-inflammatory model. We then used LPS-stimulated mouse uterine tissue to construct an endometritis model in vivo, to jointly observe the protective effect of catalpol on bEECs and mouse uterine tissue. Results showed that catalpol could reduce the inflammation and endometritis in LPS-induced bEECs and mice by inhibiting the expression of TLR4 and the downstream NF-κB pathway. In LPS-induced bEECs pretreated with catalpol, activation of the TLR4/NF-κB pathway was inhibited, and the phosphorylation level of p65 was significantly reduced. The expression and localization of p-p65 were observed by immunofluorescence in catalpol treatment groups, and the fluorescence intensity of translocation from the cytoplasm to the nucleus declined dramatically compared with the LPS group. Moreover, the reduction in inflammatory damage to the mouse uterus caused by LPS was accompanied by a decline in MPO activity.
The production of many pro-inflammatory cytokines, chemokines, and adhesion factors is known to be related to inflammation during the process of disease (Turner and Cronin, 2014; Ye et al., 2018). IL-1β, IL-6, and TNF-α play an important role in the development of endometritis during the early postpartum period (Brodzki et al., 2015). CXCL8 and CXCL5 are cytokines belonging to the chemokine family (Wang et al., 2018). The upregulation of pro-inflammatory mediators, including CXCL8, IL-1β, and IL-6, is involved in the pathogenesis of bacterial infection (Angrisano et al., 2010). Increased CXCL8 production can lead to the formation of pus in the uterine lumen (Haas et al., 2016). The upregulated chemokine CXCL5 in patients with uterine cervical cancer, in vivo and in vitro, contributes to the oncogenic potential of HeLa uterine cervical cancer cells (Feng et al., 2018). The elevated mRNA expression of chemokine CXCL5 plays a part in the pathogenesis of endometriosis in canines (Karlsson et al., 2015). In this study, the expression of both cytokines and chemokines increased significantly in both LPS-stimulated bEECs and LPS-induced endometritis in mice. To study the protective effect of catalpol on uterine tissue, we added different concentrations of catalpol to LPS-stimulated bEECs and LPS-induced endometritis mice. The results showed catalpol can effectively inhibit the secretion and expression of cytokines IL-1β, IL-6, and TNF-α and chemokines CXCL5 and CXCL8 in LPS-induced bEECs and endometritis in mice, exerting anti-inflammatory protective effects. This further confirmed the roles of CXCL8 and CXCL5 in the development of uterine inflammation. Today, drug residues are attracting increasing concern. As an extract based on traditional Chinese medicine, catalpol’s anti-inflammatory effects on LPS-induced endometritis in mice will provide a theoretical basis for the clinical treatment of bovine endometritis in the years to come.
Consequently, inhibiting activation of the TLR4/NF-κB pathway can partly control the development of endometritis in mice. The anti-inflammatory and protective effects of catalpol on LPS-induced endometritis may have potential therapeutic value for the treatment of bovine endometritis and other inflammatory diseases in the future.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31472254)
Contributors: Hua ZHANG and Zhi-min WU designed and performed this study. Ya-ping YANG, Jing YANG, and Ying-fang GUO assisted in carrying out the research. Aftab SHAUKAT assisted in editing language. Tao ZHANG, Xin-ying ZHU, and Jin-xia QIU assisted in analyzing the data. Gan-zhen DENG and Dong-mei SHI proofread the manuscript. All authors have approved the final manuscript. Therefore, all authors have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Hua ZHANG, Zhi-min WU, Ya-ping YANG, Aftab SHAUKAT, Jing YANG, Ying-fang GUO, Tao ZHANG, Xin-ying ZHU, Jin-xia QIU, Gan-zhen DENG, and Dong-mei SHI declare that they have no conflicts of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed. The animal experiments were carried out according to the guidelines of the Laboratory Animal Research Center of Hubei Province and approved by the Ethical Committee on Animal Research at Huazhong Agricultural University (HZAUMO-2015-12), Wuhan, China.
References
- 1.Angrisano T, Pero R, Peluso S, et al. LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol, 10:172. 2010 doi: 10.1186/1471-2180-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begley LA, Kasina S, Mehra R, et al. CXCL5 promotes prostate cancer progression. Neoplasia. 2008;10(3):244–254. doi: 10.1593/neo.07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodzki P, Kostro K, Brodzki A, et al. Inflammatory cytokines and acute-phase proteins concentrations in the peripheral blood and uterus of cows that developed endometritis during early postpartum. Theriogenology. 2015;84(1):11–18. doi: 10.1016/j.theriogenology.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Brown K, Park S, Kanno T, et al. Mutual regulation of the transcriptional activator NF-κB and its inhibitor, IκBα. Proc Natl Acad Sci USA. 1993;90(6):2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen BY, Jiang LX, Hao K, et al. Protection of plasma transfusion against lipopolysaccharide/D-galactosamine-induced fulminant hepatic failure through inhibiting apoptosis of hepatic cells in mice. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(6):436–444. doi: 10.1631/jzus.B1700277. [DOI] [Google Scholar]
- 6.Chen Z, Deng S, Yuan DC, et al. Novel nano-microspheres containing chitosan, hyaluronic acid, and chondroitin sulfate deliver growth and differentiation factor-5 plasmid for osteoarthritis gene therapy. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(12):910–923. doi: 10.1631/jzus.B1800095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chensue SW. Molecular machinations: chemokine signals in host-pathogen interactions. Clin Microbiol Rev. 2001;14(4):821–835. doi: 10.1128/CMR.14.4.821-835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries MA, Klop B, Eskes SA, et al. The postprandial situation as a pro-inflammatory condition. Clin Investig Arterioscler. 2014;26(4):184–192. doi: 10.1016/j.arteri.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Feng XN, Zhang DF, Li XY, et al. CXCL5, the upregulated chemokine in patients with uterine cervix cancer, in vivo and in vitro contributes to oncogenic potential of Hela uterine cervix cancer cells. Biomed Pharmacother. 2018;107:1496–1504. doi: 10.1016/j.biopha.2018.08.149. [DOI] [PubMed] [Google Scholar]
- 10.Guo S, Jiang KF, Wu HC, et al. Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Front Pharmacol, 9:982. 2018 doi: 10.3389/fphar.2018.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas M, Kaup FJ, Neumann S. Canine pyometra: a model for the analysis of serum CXCL8 in inflammation. J Vet Med Sci. 2016;78(3):375–381. doi: 10.1292/jvms.15-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herath S, Lilly ST, Santos NR, et al. Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility. Reprod Biol Endocrinol, 7:55. 2009 doi: 10.1186/1477-7827-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CL, Cui YL, Ji LL, et al. Catalpol decreases peroxynitrite formation and consequently exerts cardioprotective effects against ischemia/reperfusion insult. Pharm Biol. 2013;51(4):463–473. doi: 10.3109/13880209.2012.740052. [DOI] [PubMed] [Google Scholar]
- 14.Israël A. The IKK complex, a central regulator of NF-κB activation. Cold Spring Harb Perspect Biol. 2010;2(3):a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang KF, Chen XY, Zhao G, et al. IFN-τ plays an anti-inflammatory role in Staphylococcus aureus-induced endometritis in mice through the suppression of NF-κB pathway and MMP9 expression. J Interferon Cytokine Res. 2017;37(2):81–89. doi: 10.1089/jir.2016.0058. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson I, Hagman R, Guo YZ, et al. Pathogenic Escherichia coli and lipopolysaccharide enhance the expression of IL-8, CXCL5, and CXCL10 in canine endometrial stromal cells. Theriogenology. 2015;84(1):34–42. doi: 10.1016/j.theriogenology.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura M, Toiyama Y, Tanaka K, et al. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48(14):2244–2251. doi: 10.1016/j.ejca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Koyama T, Omori R, Koyama K, et al. Optimization of diagnostic methods and criteria of endometritis for various postpartum days to evaluate infertility in dairy cows. Theriogenology. 2018;119:225–232. doi: 10.1016/j.theriogenology.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Li QT, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, He QJ, Zou W, et al. Catalpol increases hippocampal neuroplasticity and up-regulates PKC and BDNF in the aged rats. Brain Res. 2006;1123(1):68–79. doi: 10.1016/j.brainres.2006.09.058. [DOI] [PubMed] [Google Scholar]
- 21.Lyu A, Chen JJ, Wang HC, et al. Punicalagin protects bovine endometrial epithelial cells against lipopolysaccharide-induced inflammatory injury. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(6):481–491. doi: 10.1631/jzus.B1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machado Pfeifer LF, de Souza Andrade J, Moreira EM, et al. Uterine inflammation and fertility of beef cows subjected to timed AI at different days postpartum. Anim Reprod Sci. 2018;197:268–277. doi: 10.1016/j.anireprosci.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Nicholas C, Batra S, Vargo MA, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J Immunol. 2007;179(10):7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 24.Sheldon IM, Williams EJ, Miller ANA, et al. Uterine diseases in cattle after parturition. Vet J. 2008;176(1):115–121. doi: 10.1016/j.tvjl.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Liu B, Mao W, et al. PGE2 downregulates LPS-induced inflammatory responses via the TLR4-NF-κB signaling pathway in bovine endometrial epithelial cells. Prostaglandins Leukot Essent Fatty Acids. 2018;129:25–31. doi: 10.1016/j.plefa.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Sicsic R, Goshen T, Dutta R, et al. Microbial communities and inflammatory response in the endometrium differ between normal and metritic dairy cows at 5–10 days post-partum. Vet Res, 49:77. 2018 doi: 10.1186/s13567-018-0570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Socha BM, Lada P, et al. The influence of experimentally induced endometritis on the PPAR expression profile in the bovine endometrium. Theriogenology. 2018;122:74–83. doi: 10.1016/j.theriogenology.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Turner ML, Cronin JG, et al. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology. 2014;155(4):1453–1465. doi: 10.1210/en.2013-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Schyndel SJ, Pascottini OB, et al. Comparison of cow-side diagnostic techniques for subclinical endometritis in dairy cows. Theriogenology. 2018;120:117–122. doi: 10.1016/j.theriogenology.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Wagener K, Gabler C, Drillich M. A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology. 2017;94:21–30. doi: 10.1016/j.theriogenology.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Yan XX, Nesengani LT, et al. LPS-induces IL-6 and IL-8 gene expression in bovine endometrial cells “through DNA methylation”. Gene. 2018;677:266–272. doi: 10.1016/j.gene.2018.07.074. [DOI] [PubMed] [Google Scholar]
- 32.Wu HC, Zhao G, Jiang KF, et al. IFN-τ alleviates lipopolysaccharide-induced inflammation by suppressing NF-κB and MAPKs pathway activation in mice. Inflammation. 2016;39(3):1141–1150. doi: 10.1007/s10753-016-0348-9. [DOI] [PubMed] [Google Scholar]
- 33.Yang C, Liu P, Wang S, et al. Shikonin exerts anti-inflammatory effects in LPS-induced mastitis by inhibiting NF-κB signaling pathway. Biochem Biophys Res Commun. 2018;505(1):1–6. doi: 10.1016/j.bbrc.2018.08.198. [DOI] [PubMed] [Google Scholar]
- 34.Ye GY, Wang KY, Gui QD, et al. Ureaplasma urealyticum-derived lipid-associated membrane proteins introduce IL-6, IL-8, and TNF-α cytokines into human amniotic epithelial cells via Toll-like receptor 2. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(8):654–661. doi: 10.1631/jzus.B1800005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang AH, Hao S, Bi J, et al. Effects of catalpol on mitochondrial function and working memory in mice after lipopolysaccharide-induced acute systemic inflammation. Exp Toxicol Pathol. 2009;61(5):461–469. doi: 10.1016/j.etp.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XL, Liu WD, Niu XH, et al. Systemic administration of catalpol prevents D-galactose induced mitochondrial dysfunction in mice. Neurosci Lett. 2010;473(3):224–228. doi: 10.1016/j.neulet.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 37.Zhao G, Zhang T, Wu HC, et al. MicroRNA let-7c improves LPS-induced outcomes of endometritis by suppressing NF-κB signaling. Inflammation. 2019;42(2):650–657. doi: 10.1007/s10753-018-0922-4. [DOI] [PubMed] [Google Scholar]