Abstract
The indications of immune checkpoint inhibitors (ICPIs) for cancer treatment have rapidly expanded, and their use is increasing in clinical settings worldwide. Despite the considerable clinical benefits of ICPIs, frequent immune-related adverse events (irAEs) have become nonnegligible concerns. Among irAEs, ICPI-induced colitis/diarrhea is frequent and recognized not only by oncologists but also by gastroenterologists or endoscopists. The endoscopic findings show similarity to those of inflammatory bowel disease to a certain extent, particularly ulcerative colitis, but do not seem to be identical. The pathological findings of ICPI-induced colitis may vary among drug classes. They show acute or chronic inflammation, but it may depend on the time of colitis suggested by colonoscopy, including biopsy or treatment intervention. In the case of chronic inflammation determined by biopsy, the endoscopy findings may overlap with those of inflammatory bowel disease. Here, we provide a comprehensive review of ICPI-induced colitis based on clinical, endoscopic and pathologic findings.
Keywords: Immune checkpoint inhibitor, Colitis, Diarrhea, Endoscopic, Pathologic
Core tip: Immune checkpoint inhibitor (ICPI)-induced colitis/diarrhea is frequent and recognized not only by oncologists but also by gastroenterologists or endoscopists. The endoscopic findings resemble those of inflammatory bowel disease to a certain extent, particularly ulcerative colitis, but are not identical. The pathological findings of ICPI-induced colitis may vary among drug classes. The findings show acute or chronic phases but may depend on the diagnostic timing or treatment intervention. Colonoscopy with biopsy is necessary to confirm ICPI-induced colitis, and early evaluation may avoid exacerbating or prolonging colitis due to treatment resistance.
INTRODUCTION
In 1992, Ishida et al[1] identified a protein on activated T lymphocytes called programmed cell death protein 1 (PD-1), a key player in tumor immunology. In 1996, Leach et al[2] identified a protein called cytotoxic T-lymphocyte antigen-4 (CTLA-4), another major blocking pathway for the human immune system that was similar to PD-1. Since then, their discoveries have led to the development of immune checkpoint inhibitors (ICPIs) as anticancer drugs and have brought about a major revolution in cancer treatment strategy. Both CTLA-4 and PD-1 deliver negative signals to T-cell-mediated excessive immune activation, known as checkpoints, and ICPIs disrupt the signals mediated by CTLA-4 and PD-1 to prevent T cells from blocking pathways. By inhibiting immune checkpoints, activation of T cells is maintained, thereby helping cancer cells to induce cytotoxic T cell-mediated death. In 2018, Professor Honjo and Professor Allison won the Nobel prize in Physiology or Medicine for their work.
Presently, there are six ICPIs available and approved by the United States Food and Drug Administration for different cancers. Despite the significant clinical benefits of ICPIs, frequent immune-related adverse events (irAEs) in the skin, endocrine organs, gastrointestinal (GI) tract, liver, and lungs and in the musculoskeletal, renal, nervous, hematologic, cardiovascular, and ocular systems have become nonnegligible concerns. Most irAEs have a delayed onset and prolonged duration compared with those from chemotherapy[3]. The incidence of irAEs appears to be similar across tumor types[4]. Among irAEs, ICPI-induced colitis/diarrhea is frequent and recognized not only by oncologists but also by gastroenterologists or endoscopists. In this review, we provide a comprehensive review of ICPI-induced colitis based on clinical, endoscopic and pathologic findings.
ONSET TIMING OF ICPI-INDUCED DIARRHEA/COLITIS
ICPI-induced diarrhea occurs after an average of three infusions[5], although it can occur immediately after the first infusion. Recent reports suggest that the onset timing of ICPI-induced diarrhea/colitis may differ by ICPI type. ICPI-induced diarrhea/colitis induced by ipilimumab (anti-CTLA-4) usually occurs 6 to 7 wk after the initiation of ipilimumab[6]. The median time from last the ipilimumab treatment to diarrhea onset is 11-14 d (range 0-59 d)[7,8]. On the other hand, Wang et al[9] reported that 3.2% of patients (30/973) receiving anti-PD-1 developed ICPI-induced colitis at a median of 25.4 wk (range 0.6-120 wk). ICPI-induced diarrhea/colitis induced by anti-PD-1 seems to occur later than that induced by anti-CTLA-4. After the combined use of ipilimumab and nivolumab or pembrolizumab, 24.4% of patients (79/324) developed ICPI-induced diarrhea/colitis significantly earlier, at a median of 7.2 wk (range 0.7-51 wk)[9]. Because the ranges of its onset timing are widely distributed, it is difficult to predict the development of ICPI-induced diarrhea/colitis. In addition, it may be influenced by other drugs, including NSAIDs, antibiotics, or previous anticancer drugs. Moreover, it seems difficult to predict the development of colitis before patients have symptoms[10]. We should keep in mind that ICPI-related colitis can occur at any point, even after discontinuation of ICPIs.
LOCATION
Geukes Foppen et al[11] reported total colonoscopy in 62 of 92 patients (67%) suspected of ICPI-induced colitis. Of these patients, 68% showed pancolitis (> 3 affected segments), and the ascending colon had more severe colitis than the descending colon. In cases where a total colonoscopy was not performed, patients with colitis in the ascending colon can be underestimated by sigmoidoscopy alone. Abdominal computed tomography (CT) findings may be useful not only to evaluate perforation, obstruction, and toxic megacolon but also to evaluate inflamed lesions due to ICPIs. The common CT findings of 16 patients treated with ipilimumab showed that 75% of patients had diffuse colitis patterns, and 25% had segmental colitis[12]. CT was not sufficient to diagnose colitis when using endoscopic evaluation as the gold standard because it has a high false-negative rate and low sensitivity[13]. In contrast, Garcia-Neuer et al[14] reported that CT was useful for predicting ICPI-induced colitis with a positive predictive value of 96% and a negative likelihood ratio of 0.2 in 34 diarrhea patients who underwent both CT and colonoscopy with biopsy. Early sigmoidoscopy without bowel preparation has merit to assess ICPI-induced colitis because it can be performed more easily and earlier than total colonoscopy. Therefore, the combined use of sigmoidoscopy and CT may be useful to evaluate ICPI-induced colitis at an earlier stage.
ENDOSCOPIC EVALUATION AND FINDINGS
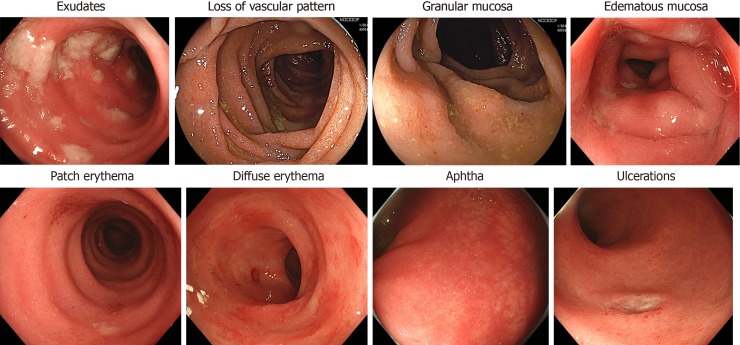
There are several reports about the endoscopic findings of ICPI-induced colitis. Wang et al[13] observed that endoscopic inflammatory findings were found in more than 80% of patients with ICPI-induced diarrhea/colitis. Common endoscopic inflammation findings are reported as exudates, loss of vascular pattern, granular or edematous mucosa, patch or diffuse erythema, aphtha and ulcerations (Figure 1)[15,16]. Most of the inflammatory changes, including pathological changes, are dominantly more diffuse than patchy[10], but patchy distribution was endoscopically observed in half of the patients with diarrhea[17]. These endoscopic findings resemble those of inflammatory bowel disease (IBD) to a certain extent, particularly with ulcerative colitis (UC)[16,18], but sometimes look different from a UC-like pattern (Table 1).
Figure 1.
Endoscopic findings caused by an immune checkpoint inhibitor.
Table 1.
Summary of endoscopic and pathological findings of immune-related diarrhea and colitis
| Endoscopic and pathological findings of immune-related diarrhea and colitis | |
| Endoscopic findings | |
| Endoscopic features | (1) Exudates; (2) loss of vascular pattern; (3) granular or edematous mucosa; (4) patch or diffuse erythema; (5) aphtha; (6) ulceration |
| Inflammatory distribution | (1) Diffuse; (2) patchy (dominantly more diffuse than patchy) |
| Risk factors for steroid-refractory colitis | (1) Extensively inflamed area (e.g., pancolitis); (2) deeper ulceration |
| Pathological findings | |
| Anti-CTLA-4 associated colitis | Like autoimmune colitis: (1) lamina propria expansion due to dense lymphoplasmacytic infiltrate; (2) increased intraepithelial lymphocytosis; (3) apoptosis in the crypts; (4) neutrophilic cryptitis and crypt abscess; (5) occasional prominent eosinophilia in the lamina propria; (6) the lack of findings of basal plasmacytosis, crypt distortion, or granulomas |
| Anti-PD1/anti-PDL1-associated colitis | (1) Expansion of lamina propria by lymphoplasmacytic infiltrate; (2) the increase in intraepithelial neutrophils and neutrophilic crypt abscess; (3) crypt distortion; (4) increased crypt cell apoptosis |
CTLA-4: Cytotoxic T-lymphocyte antigen-4; PD1: Programmed cell death protein 1; PDL1: Programmed cell death receptor ligand 1.
Wang et al[13] reported in 53 patients with diarrhea, clinical symptoms did not always correlate with other endoscopic findings except for the presence of ulceration, which had a strong relationship with higher colitis. Similarly, another retrospective study showed that there was no significant correlation between diarrhea/colitis symptoms and endoscopic findings in 92 patients who developed diarrhea.
They also reported that pancolitis and the presence of ulceration are indicators for steroid-refractory colitis[11]. Geukes Foppen et al[11] reported that the Mayo score was associated with the presence of ulceration. Abu-Sbeih et al[19] categorized endoscopic findings as low-risk and high-risk for steroid-responsiveness. High-risk findings included either ulcers deeper than 2 mm and/or larger than 1 cm in surface area or endoscopically extensive colitis from the proximal colon to the splenic flexure. These patients require frequent use of infliximab or vedolizumab and more frequent and longer hospital stays than non-high-risk patients[19]. They also reported that timely early colonoscopy decreased the duration of steroid treatment[19]. If the colonoscopy shows normal mucosal findings, we are not always able to exclude the presence of ICPI-induced colitis, as cases of isolated ileitis[20] or enteritis without colitis[21] can also occur. We can also rule out microscopic colitis or other infectious diseases such as Clostridioides difficile or cytomegalovirus[7]. Therefore, early colonoscopy with mucosal biopsy from colorectal and ileum-end mucosa is necessary not only to evaluate the severity and distribution of colitis[11] but also to ensure shorter and less intense treatment[19].
PATHOLOGY
The histologic features of ICPI-associated colitis may vary among drug classes, i.e., CTLA-4 inhibitors and PD-1/PDL1 inhibitors. Although they are nonspecific, some findings can be helpful clues to diagnose and speculate about the class of inhibitors. On the other hand, there is significant overlap between ICPI-associated colitis and other types of colitis, making the differential diagnosis difficult.
The histologic findings of CTLA-4-associated colitis are relatively consistent across most studies. The previously reported histologic features of CTLA-4 associated colitis are similar to those of autoimmune colitis[22]. They include lamina propria expansion due to dense lymphoplasmacytic infiltrate, increased intraepithelial lymphocytosis, and apoptosis in the crypts. Neutrophilic cryptitis and crypt abscess are also found. At times, there is prominent eosinophilia in the lamina propria. Although dense lymphoplasmacytic lamina propria expansion is reminiscent of other mimics, the lack of findings of basal plasmacytosis, crypt distortion, or granulomas can help the differentiation.
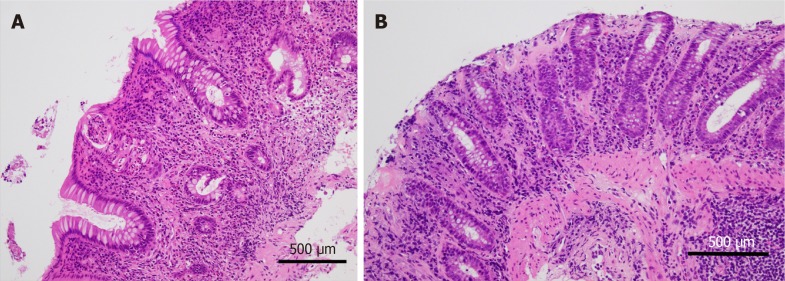
The most common findings of anti-PD1/anti-PDL1-associated colitis are the expansion of the lamina propria by lymphoplasmacytic infiltrate and features of active colitis[23-27]. The latter are characterized by an increase in intraepithelial neutrophils and neutrophilic crypt abscess (Figure 2A). Other findings include crypt distortion, increased crypt cell apoptosis, features of ischemic colitis, and collagenous colitis (Figure 2B). Although, in the study by Gonzalez et al[26], there were no cases with increased intraepithelial lymphocytosis commonly observed in CTLA-4-associated colitis, Chen et al[23] and Bavi et al[27] described features of lymphocytic colitis in a minority of their cases with anti-PD1/anti-PDL1. In the latter studies, a PD-1 inhibitor and CLTA-4 inhibitor were prescribed for their patient population either in combination or sequentially. Therefore, it is unlikely that this finding is related to PD-1 inhibition alone.
Figure 2.
Programmed cell death protein 1 inhibitor-associated colitis. A: This colon biopsy reveals lamina propria expansion by lymphoplasmacytic infiltrate. Crypt distortion, crypt abscess, and cryptitis are prominent in the mucosa. In the stroma, a significantly increased eosinophilic infiltrate is observed; B: In another case of immune checkpoint inhibitors-related colitic mucosa, a subluminal collagen band thickening is prominent as observed in collagenous colitis. (Hematoxylin and eosin original magnification × 20, a scale bar represents 500 µm).
As mentioned, the histologic features of ICPI-associated colitis are nonspecific and can mimic other type of colitis, including infectious colitis, IBD, graft versus host disease (GVHD), and other drug-induced colitis. Although infectious colitis typically shows features of active colitis, increased apoptosis and crypt atrophy/dropout are not typical features[28]. ICPI-associated colitis lacks the features of chronicity that characterize IBD[29]. The lamina propria expansion by lymphoplasmacytic infiltrate can discriminate from GVHD although increased crypt apoptosis is the sine qua none of the diagnosis of GVHD[30]. Despite the histopathological differential diagnostic points, clinical correlation and medical history are indispensable for discrimination between ICPI-associated colitis and mimics (Table 1).
MORBIDITY ASSOCIATED WITH ICPI-INDUCED DIARRHEA/COLITIS AND TREATMENT
IrAEs involving the GI tract range from mild to severe events[31] and are well reported for anti-CTLA4 but less well reported for anti-PD-1 and anti-PD-L1 and for combined anti-CTLA4 plus anti-PD-1. Most clinical trials distinguish diarrhea from colitis even though they overlap in most practical cases. Diarrhea is evaluated based on an increase in stool per day or ostomy output. Colitis is evaluated based on clinical symptoms (abdominal pain, mucus or blood in stool) or diagnostic observations based on radiographic and/or colonoscopy findings. The severity is usually classified based on the Common Terminology Criteria for Adverse Events[32] (Table 2).
Table 2.
Definition of diarrhea and colitis based on Common Terminology Criteria for Adverse Events v5.0[32]
| CTCAE Term | Definition | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | CTCAE v5.0 Change |
| Diarrhea | A disorder characterized by an increase in frequency and/or loose or watery bowel movements | Increase of < 4 stools per day over baseline; mild increase in ostomy output compared to baseline | Increase of 4-6 stools per day over baseline; moderate increase in ostomy output compared to baseline; limiting instrumental ADL | Increase of ≥ 7 stools per day over baseline; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated | Death | Clarification: Grade 2, 3, Definition |
| Colitis | A disorder characterized by inflammation of the colon | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Abdominal pain; mucus or blood in stool | Severe abdominal pain; peritoneal signs | Life-threatening consequences; urgent intervention indicated | Death | Addition: Navigational note; Clarification: Grade 3 |
ADL: Activities of daily living; CTCAE: Common Terminology Criteria for Adverse Events.
Moderate to severe ICPI-related colitis may lead to severe deterioration in organ function and quality of life and life-threatening events. Diarrhea and colitis occurred in 8% to 22% of patients treated with anti-CTLA4[15]. A recent systemic review reported that 613 fatal ICPI toxic events were fond from 2009 through January 2018 searched by Vigilyze, which included 135 anti–CTLA-4 deaths and 32 combination anti-CTLA-4 plus anti-PD-1 deaths from colitis (27%)[33]. Colonic perforation was reported to occur in 1-5% of melanoma patients treated with ipilimumab (anti-CTLA-4)[7,15,34], and 0.6% of patients treated with ipilimumab died due to ICPI-induced colitis[35].
Anti-CTLA4-related colitis is reportedly associated with mouth ulcers, anal lesions and extraintestinal irAEs[17]. A recent meta-analysis of 34 studies that included 8863 patients in clinical trials revealed that, for anti-CTLA4 alone (ipilimumab), all grades of colitis occurred in 9.1% (95% confidence interval (CI), 6.6%-12.5%) of participants, grade 3/4 colitis occurred in 6.8% (95%CI: 5.3%-8.6%) of participants, and grade 3/4 diarrhea occurred in 7.9% (95%CI: 5.5%-11.4%) of participants. Similarly, for anti-PD-1 alone (nivolumab or pembrolizumab), the rates were 1.4% (95%CI: 1.1%-1.8%), 0.9% (95%CI: 0.7%-1.3%), and 1.3% (95%CI: 1.0%-1.7%), respectively. For anti-PD-L1 alone (atezolizumab), the rates were 1.0% (95%CI: 0.4%-2.2%), 0.6% (95%CI: 0.2%-1.6%), and 0.3% (95%CI: 0.1%-1.1%), respectively[36]. For anti-CTLA4 (Ipilimumab) plus anti-PD-1 (nivolumab), the rates were 13.6% (95%CI: 7.7%-22.9%), 9.4% (95%CI: 4.8%-117.4%), and 9.2% (95%CI: 6.8%-12.3%), respectively. ICPI-induced diarrhea/colitis induced by anti-CTLA-4 can develop more often and more severely than ICPI-induced diarrhea/colitis induced by anti-PD-1. Combined anti-CTLA4 plus anti-PD-1 treatment is also more strongly associated with diarrhea/colitis than single-drug treatment[36]. Ipilimumab is commonly used at either 10 mg/kg or 3 mg/kg. There were similar rates of severe colitis at these doses, but severe diarrhea was more frequent at a dose of 10 mg/kg than at 3 mg/kg[36]. Recently, Marthey et al[17] showed that the use of nonsteroidal anti-inflammatory drugs (NSAIDs) was associated with an increased risk of ICPI-induced colitis induced by CTLA-4 (2/38, 5% vs 11/35, 31%, P = 0.003). Therefore, the use of NSAIDs may affect the incidence of ICPI-induced diarrhea/colitis. Table 3 shows a summary of the incidence of immune-related diarrhea or colitis based on representative clinical trials.
Table 3.
Summary of incidence of immune-related diarrhea and colitis
| ICPI | Target | Author | Year | Plus other drugs | n | Cancer type | Any grade diarrhea/colitis, n (%) | Grade 3-5 diarrhea/colitis, n (%) |
| Nivolumab | PD-1 | Topalian et al[41] | 2012 | None | 296 | Solid cancer | 33 (11)/ND | 3 (1)/ND |
| Weber et al[42] | 2013 | None ipilimumab-naive | 34 | Melanoma | 13 (38.2)/0 (0) | Not observed1 | ||
| Ipilimumab-refractory | 56 | 11 (19.6)/0 (0) | ||||||
| Weber et al[43] | 2015 | None | 268 | Melanoma2 | 30 (11.2)/ND | 1 (0.4)/ND | ||
| Larkin et al[44] | 2015 | None | 315 | Melanoma | 60 (19.2)/4 (1.3) | 7 (2.2)/2 (0.6) | ||
| Ferris et al[45] | 2016 | None | 236 | SCCHN | 16 (6.8)/0 (0) | 0 (0)/0 (0) | ||
| Kang et al[46] | 2017 | None | 330 | GC/GEJC | 23 (7)/2 (1) | 2 (1)/1 (< 1) | ||
| Pembrolizumab | PD-1 | Hamid et al[47] | 2013 | None | 135 | Melanoma | 27 (20) | 1(1) |
| Garon et al[48] | 2015 | None | 495 | NSCLC | 40 (8.1)/ND | 3 (0.6)/ND | ||
| Ribas et al[49] | 2015 | None | 361 | Melanoma2 | 32 (8.9)/5 (1.4) | 2 (0.6)/2 (0.6) | ||
| Herbst et al[50] | 2016 | None | 690 | NSCLC | 46 (6.7)/6 (0.9) | 2 (0.3)/4 (0.6) | ||
| Ribas et al[51] | 2016 | None | 655 | Melanoma | 115 (18)/11(2) | 6 (1)/7 (1.1) | ||
| Mok et al[52] | 2019 | None | 636 | NSCLC | 34 (5)/7 (1) | 5 (< 1)/4 (< 1) | ||
| Ipilimumab | CTLA-4 | Weber et al[53] | 2008 | None | 88 | Melanoma | ND | 5 (5.6)/4 (4.5) |
| Weber et al[54] | 2009 | None | 57 | Melanoma | 20 (35)/ND | 10 (18)/ND | ||
| budesonide | 58 | 19 (33)/ND | 8 (14)/ND | |||||
| Wolchok et al[55] | 2010 | None | 214 | Melanoma | 58 (27)/ND | 11(5.1)/ND | ||
| Hodi et al[56] | 2010 | None | 131 | Melanoma | 43 (32.8)/10 (7.6) | 7 (5.3)/7 (5.3) | ||
| gp100 | 380 | 146 (38.4)/20 (5.3)3 | 17 (4.5)/12(3.2)3 | |||||
| Robert et al[57] | 2011 | Dacarbazine | 247 | Melanoma | 81 (32.8)/11 (4.5) | 10 (4.0)/5 (2.0) | ||
| Margolin et al[58] | 2012 | None | 72 | Melanoma | 30 (42)/ND | 6 (8.3)/ND | ||
| Kwon et al[59] | 2014 | None | 399 | Prostate cancer | 199 (51)/27 (7) | 64 (16)/18 (5) | ||
| Larkin et al[44] | 2015 | None | 311 | Melanoma | 103 (33.1)/36 (11.6) | 19 (6.1)/27 (8.7) | ||
| Eggermont et al[35] | 2016 | None | 471 | Melanoma | 194 (41.2)/73 (15.5) | 46 (9.8)/39 (8.2) | ||
| Ipilimumab plus nivolumab | CTLA4 and PD1 | Wolchok et al[60] | 2013 | None | 53 | Melanoma | 18 (34.0)/5 (9) | 3 (6)/2 (4) |
| Larkin et al[44] | 2015 | None | 315 | Melanoma | 138 (44.1)/37 (11.8) | 29 (9.3)/24 (7.7) | ||
| Schadendorf et al[61] | 2017 | None | 407 | Melanoma | 30 (7.4)/40 (9.8) | 25 (6.1)/32 (7.9) | ||
| Wolchok et al[62] | 2017 | None | 313 | Melanoma | 142 (45)/40 (13) | 29 (9)/26 (8) | ||
| Hellmann et al[63] | 2017 | None | 77 | NSCLC | 16 (21)/4 (5.2) | 1 (1.3)/3 (3.9) | ||
| Motzer et al[64] | 2018 | None | 547 | Renal cell carcinoma | 145 (27)/ND | 21 (4)/ND | ||
| Durvalumab | PD-L1 | Antonia et al[65] | 2017 | None | 473 | NSCLC | 87 (18.3)/ND | 3 (0.6)/ND |
| Motzer et al[66] | 2018 | None | 475 | NSCLC | 88 (18.5)/ND | 3 (0.6)/ND | ||
| Loibl et al[67] | 2019 | None | 92 | Breast cancer | 26 (28.3)/ND | 3 (3.3)/ND | ||
| Atezolizumab | PD-L1 | Herbst et al[68] | 2014 | None | 277 | Solid tumors or hematological malignancies | 29 (10.5)/ND | 0 (0)/ND |
| Rosenberg et al[69] | 2016 | None | 311 | Urothelial carcinoma | 24 (8)/3 (1) | 1 (0.3)/2 (1) | ||
| Fehrenbacher et al[70] | 2016 | None | 142 | NSCLC | ND | ND/2 (1) | ||
| Socinski et al[71] | 2018 | ABCP | 393 | NSCLC | 70 (17.8) | 11 (2.8) | ||
| Avelumab | PD-L1 | Chung et al[72] | 2019 | None | 150 | GC/GEJC | ND/2 (1.3) | ND/1 (0.7)4 |
| Barlesi et al[73] | 2019 | None | 396 | NSCLC | 24 (6)/ND | 0 (0)/ND |
Dose-limiting colitis was not observed in this trial;
Progressed after ipilimumab;
Immune-related event;
No atezolizumab-related grade 4 but adverse events were reported, but only one patient showed Grade 5 cardiac failure. SCCHN: Squamous cell carcinoma of the head and neck; NSCLC: Non-small-cell lung cancer; ABCP: Atezolizumab plus bevacizumab plus carboplatin plus paclitaxel; GC/GEJC: Gastric/gastroesophageal cancer; ND: Not described.
In the case of grade 1 diarrhea/colitis, antidiarrheal drugs and/or oral hydration with electrolyte substitution can be initiated. In cases of persistent or grade 2 or higher diarrhea or rectal bleeding, it is necessary to confirm colitis or to rule out GI infection by testing for stool leukocytes, stool cultures, IBD, or tumor-related GI symptoms. In particular, Clostridioides difficile toxin and/or antigen test, cytomegalovirus DNA polymerase chain reaction, and tests for stool ova and parasites should be carried out in every patient with diarrhea treated with ICPIs. Sigmoidoscopy or colonoscopy combined with mucosal biopsy needs to be performed to evaluate the presence of colitis and to rule out GI metastasis because it is not uncommon in lung cancer or melanoma. If ICPI-induced colitis is diagnosed, an oral steroid is recommended. In the case of grade 3/4 diarrhea/colitis or persistent symptoms after oral steroids for several days, changing the treatment to intravenous steroids should be considered, and an infusion solution with electrolytes should be given. If patients respond to intravenous steroids within several days, they should be switched to oral steroids and tapered. However, if they fail to respond to steroid infusion, treatment with anti-TNF-α should be considered[15,37]. Recently, a case series reported that vedolizumab was a safer and more theoretic alternative than anti-TNF in patients with steroid-dependent or partially refractory ICPI-induced enterocolitis[38]. In the near future, vedolizumab may be effective and safe because it inhibits the migration of mucosal-associated T lymphocytes without inducing immune suppression and does not show an increased risk of serious infections in patients with UC or Crohn’s disease[39,40].
CONCLUSION
The combination of endoscopic and pathological findings may help diagnose ICPI-induced colitis as well as exclude infectious colitis, including Clostridioides difficile or cytomegalovirus, ischemic colitis, other drug-induced colitis, or segmental diverticular colitis. However, there are no specific findings because the endoscopic and pathological findings can depend on the time of colitis proven by biopsy or treatment intervention. In cases of persistent or grade 2 or higher diarrhea or rectal bleeding, colonoscopy evaluation is necessary to confirm ICPI-induced colitis and to rule out other diseases. Early evaluation and intervention may avoid exacerbating or prolonging colitis.
Footnotes
Conflict-of-interest statement: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Manuscript source: Invited manuscript
Peer-review started: May 9, 2019
First decision: June 6, 2019
Article in press: August 21, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abd Elhamid SM, Morini S, Yang ZH S-Editor: Yan JP L-Editor: A E-Editor: Li X
Contributor Information
Tsutomu Nishida, Department of Gastroenterology, Toyonaka Municipal Hospital, Osaka 560-8565, Japan, tnishida.gastro@gmail.com.
Hideki Iijima, Department of Gastroenterology and Hepatology, Osaka University Graduate School of Medicine, Osaka 565-0871, Japan.
Shiro Adachi, Department of Pathology, Toyonaka Municipal Hospital, Osaka 560-8565, Japan.
References
- 1.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 3.Matsubara T, Nishida T, Higaki Y, Tomita R, Shimakoshi H, Shimoda A, Osugi N, Sugimoto A, Takahashi K, Nakamatsu D, Mukai K, Yamamoto M, Fukui K, Adachi S, Inada M. Nivolumab Induces Sustained Liver Injury in a Patient with Malignant Melanoma. Intern Med. 2018;57:1789–1792. doi: 10.2169/internalmedicine.9851-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maughan BL, Bailey E, Gill DM, Agarwal N. Incidence of Immune-Related Adverse Events with Program Death Receptor-1- and Program Death Receptor-1 Ligand-Directed Therapies in Genitourinary Cancers. Front Oncol. 2017;7:56. doi: 10.3389/fonc.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 7.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lord JD, Hackman RC, Moklebust A, Thompson JA, Higano CS, Chielens D, Steinbach G, McDonald GB. Refractory colitis following anti-CTLA4 antibody therapy: Analysis of mucosal FOXP3+ T cells. Dig Dis Sci. 2010;55:1396–1405. doi: 10.1007/s10620-009-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang DY, Mooradian MJ, Kim D, Shah NJ, Fenton SE, Conry RM, Mehta R, Silk AW, Zhou A, Compton ML, Al-Rohil RN, Lee S, Voorhees AL, Ha L, McKee S, Norrell JT, Mehnert J, Puzanov I, Sosman JA, Chandra S, Gibney GT, Rapisuwon S, Eroglu Z, Sullivan R, Johnson DB. Clinical characterization of colitis arising from anti-PD-1 based therapy. Oncoimmunology. 2018;8:e1524695. doi: 10.1080/2162402X.2018.1524695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman D, Parker SM, Siegel J, Chasalow SD, Weber J, Galbraith S, Targan SR, Wang HL. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- 11.Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, van Leerdam ME, van den Heuvel M, Blank CU, van Dieren J, Haanen JBAG. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3:e000278. doi: 10.1136/esmoopen-2017-000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KW, Ramaiya NH, Krajewski KM, Shinagare AB, Howard SA, Jagannathan JP, Ibrahim N. Ipilimumab-associated colitis: CT findings. AJR Am J Roentgenol. 2013;200:W468–W474. doi: 10.2214/AJR.12.9751. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA, Zobniw C, Johnson DH, Samdani R, Lum P, Shuttlesworth G, Blechacz B, Bresalier R, Miller E, Thirumurthi S, Richards D, Raju G, Stroehlein J, Diab A. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm Bowel Dis. 2018;24:1695–1705. doi: 10.1093/ibd/izy104. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Neuer M, Marmarelis ME, Jangi SR, Luke JJ, Ibrahim N, Davis M, Weinberg J, Donahue H, Bailey N, Hodi FS, Buchbinder EL, Ott PA. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5:286–291. doi: 10.1158/2326-6066.CIR-16-0302. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, De Felice KM, Loftus EV, Jr, Khanna S. Systematic review: Colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42:406–417. doi: 10.1111/apt.13281. [DOI] [PubMed] [Google Scholar]
- 16.Kubo K, Kato M, Mabe K. Nivolumab-Associated Colitis Mimicking Ulcerative Colitis. Clin Gastroenterol Hepatol. 2017;15:A35–A36. doi: 10.1016/j.cgh.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, Zallot C, Peyrin-Biroulet L, Rahier JF, Bourdier de Beauregard M, Mortier L, Coutzac C, Soularue E, Lanoy E, Kapel N, Planchard D, Chaput N, Robert C, Carbonnel F. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:395–401. doi: 10.1093/ecco-jcc/jjv227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamauchi R, Araki T, Mitsuyama K, Tokito T, Ishii H, Yoshioka S, Kuwaki K, Mori A, Yoshimura T, Tsuruta O, Torimura T. The characteristics of nivolumab-induced colitis: An evaluation of three cases and a literature review. BMC Gastroenterol. 2018;18:135. doi: 10.1186/s12876-018-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6:95. doi: 10.1186/s40425-018-0411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venditti O, De Lisi D, Caricato M, Caputo D, Capolupo GT, Taffon C, Pagliara E, Battisi S, Frezza AM, Onetti Muda A, Tonini G, Santini D. Ipilimumab and immune-mediated adverse events: A case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87. doi: 10.1186/s12885-015-1074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messmer M, Upreti S, Tarabishy Y, Mazumder N, Chowdhury R, Yarchoan M, Holdhoff M. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9:705–713. doi: 10.1159/000452403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oble DA, Mino-Kenudson M, Goldsmith J, Hodi FS, Seliem RM, Dranoff G, Mihm M, Hasserjian R, Lauwers GY. Alpha-CTLA-4 mAb-associated panenteritis: A histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32:1130–1137. doi: 10.1097/PAS.0b013e31817150e3. [DOI] [PubMed] [Google Scholar]
- 23.Chen JH, Pezhouh MK, Lauwers GY, Masia R. Histopathologic Features of Colitis Due to Immunotherapy With Anti-PD-1 Antibodies. Am J Surg Pathol. 2017;41:643–654. doi: 10.1097/PAS.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 24.Baroudjian B, Lourenco N, Pagès C, Chami I, Maillet M, Bertheau P, Bagot M, Gornet JM, Lebbé C, Allez M. Anti-PD1-induced collagenous colitis in a melanoma patient. Melanoma Res. 2016;26:308–311. doi: 10.1097/CMR.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 25.Marginean EC. The Ever-Changing Landscape of Drug-Induced Injury of the Lower Gastrointestinal Tract. Arch Pathol Lab Med. 2016;140:748–758. doi: 10.5858/arpa.2015-0451-RA. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez RS, Salaria SN, Bohannon CD, Huber AR, Feely MM, Shi C. PD-1 inhibitor gastroenterocolitis: Case series and appraisal of 'immunomodulatory gastroenterocolitis'. Histopathology. 2017;70:558–567. doi: 10.1111/his.13118. [DOI] [PubMed] [Google Scholar]
- 27.Bavi P, Butler M, Serra S, Chetty R. Immune modulator-induced changes in the gastrointestinal tract. Histopathology. 2017;71:494–496. doi: 10.1111/his.13224. [DOI] [PubMed] [Google Scholar]
- 28.Turner K. Philadelphia: Wolters Kluwer; 2017. Fenoglio-Preiser’s Gastrointest Pathology. Infectious Colitis. 4th ed; pp. 754–796. [Google Scholar]
- 29.Gordon I. Philadelphia: Wolters Kluwer; 2017. Fenoglio-Preiser’s Gastrointest Pathology; pp. 814–816. [Google Scholar]
- 30.Turner K. Philadelphia: Wolters Kluwer; 2017. Fenoglio-Preiser’s Gastrointest Pathology. 4th ed; pp. 814–816. [Google Scholar]
- 31.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 32.NCI Common Terminology Criteria for Adverse Events (CTCAE) data files [cited 3 April 2019] Available from: https://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 33.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yervoy: Highlights of Presecribing Information [cited 27 March 2019]. 2015. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125377s073lbl.pdf.
- 35.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M, Weber JS, Maio M, Bastholt L, Mortier L, Thomas L, Tahir S, Hauschild A, Hassel JC, Hodi FS, Taitt C, de Pril V, de Schaetzen G, Suciu S, Testori A. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1344805. doi: 10.1080/2162402X.2017.1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 38.Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, Carneiro A, Marsal J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581–592. doi: 10.1007/s00262-017-1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 40.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 41.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, Kroeger J, Eysmans C, Sarnaik AA, Chen YA. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 44.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 47.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 49.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, Cranmer LD, Blank CU, O'Day SJ, Ascierto PA, Salama AK, Margolin KA, Loquai C, Eigentler TK, Gangadhar TC, Carlino MS, Agarwala SS, Moschos SJ, Sosman JA, Goldinger SM, Shapira-Frommer R, Gonzalez R, Kirkwood JM, Wolchok JD, Eggermont A, Li XN, Zhou W, Zernhelt AM, Lis J, Ebbinghaus S, Kang SP, Daud A. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 51.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, Gangadhar TC, Hersey P, Dronca R, Joseph RW, Zarour H, Chmielowski B, Lawrence DP, Algazi A, Rizvi NA, Hoffner B, Mateus C, Gergich K, Lindia JA, Giannotti M, Li XN, Ebbinghaus S, Kang SP, Robert C. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 52.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 53.Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, Snively J, Hersh E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 54.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, Siegel J, O'Day SJ. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 55.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O'Day SJ, Lebbé C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 56.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 58.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF, Richards J, Michener T, Balogh A, Heller KN, Hodi FS. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 59.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Chesney J, Robert C, Grossmann K, McDermott D, Walker D, Bhore R, Larkin J, Postow MA. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J Clin Oncol. 2017;35:3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, Shepherd FA, Laurie SA, Geese WJ, Agrawal S, Young TC, Li X, Antonia SJ. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B CheckMate 214 Investigators. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 66.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH, Jr, Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, Grischke EM, Furlanetto J, Tesch H, Hanusch C, Engels K, Rezai M, Jackisch C, Schmitt WD, von Minckwitz G, Thomalla J, Kümmel S, Rautenberg B, Fasching PA, Weber K, Rhiem K, Denkert C, Schneeweiss A. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple negative breast cancer - clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;pii:mdz158. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 68.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 71.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 72.Chung HC, Arkenau HT, Lee J, Rha SY, Oh DY, Wyrwicz L, Kang YK, Lee KW, Infante JR, Lee SS, Kemeny M, Keilholz U, Melichar B, Mita A, Plummer R, Smith D, Gelb AB, Xiong H, Hong J, Chand V, Safran H. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: Phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer. 2019;7:30. doi: 10.1186/s40425-019-0508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): An open-label, randomised, phase 3 study. Lancet Oncol. 2018;19:1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]