Abstract
Background:
Different genetic and environmental factors can explain the heterogeneity of obesity-induced metabolic alterations between individuals. In this study, we aimed to screen factors that predict metabolically healthy (MHP) and unhealthy (MUP) phenotypes using genetic and lifestyle data in overweight/obese participants.
Methods:
In this cross-sectional study we enrolled 298 overweight/obese Spanish adults. The Adult Treatment Panel III criteria for metabolic syndrome were used to categorize MHP (at most, one trait) and MUP (more than one feature). Blood lipid and inflammatory profiles were measured by standardized methods. Body composition was determined by dual-energy X-ray absorptiometry. A total of 95 obesity-predisposing single-nucleotide polymorphisms (SNPs) were genotyped by a predesigned next-generation sequencing system. SNPs associated with a MUP were used to compute a weighted genetic-risk score (wGRS). Information concerning lifestyle (dietary intake and physical activity level) was collected using validated questionnaires.
Results:
The prevalence of MHP and MUP was 44.3% and 55.7%, respectively, in this sample. Overall, 12 obesity-related genetic variants were associated with the MUP. Multiple logistic regression analyses revealed that wGRS (OR = 4.133, p < 0.001), total dietary fat [odds ratio (OR) = 1.105, p = 0.002], age (OR = 1.064, p = 0.001), and BMI (OR = 1.408, p < 0.001) positively explained the MUP, whereas female sex (OR = 0.330, p = 0.009) produced a protective effect. The area under the receiver operating characteristic curve using the multivariable model was high (0.8820). Interestingly, the wGRS was the greatest contributor to the MUP (squared partial correlation = 0.3816, p < 0.001).
Conclusions:
The genetic background is an important factor explaining MHP and MUP related to obesity, in addition to lifestyle variables. This information could be useful to metabolically categorize individuals, as well as for the design/implementation of personalized nutrition interventions aimed at promoting metabolic health and nutritional wellbeing.
Keywords: diet, genetic-risk score, lifestyle, metabolically healthy phenotype, metabolically unhealthy phenotype, obesity, personalized nutrition
Introduction
The global prevalence of obesity has been increasing at an alarming rate in recent decades, being considered as a major public health concern; not only in developed countries, but also in many transitionary countries.1 Approximately, 1.9 billion adults are estimated as overweight worldwide, and at least 650 million are classified as obese according to body mass index (BMI) cut-offs according to World Health Organization (WHO) criteria.2
Obesity is recognized as an important risk factor in the onset and development of metabolic complications and chronic diseases such as insulin resistance, dyslipidemia, hypertension, inflammation, cardiovascular diseases, and type 2 diabetes mellitus.3 However, the presence of metabolically healthy (MHP) or metabolically unhealthy (MUP) phenotypes varies widely among obese participants regardless of the degree of excessive adiposity.4 In this context, apparently a subset of patients with obesity may display a metabolically healthy phenotype (MHP) characterized by insulin sensitivity similar to normal-weight individuals, as well as normal blood pressure and safe lipid and inflammatory profiles.5
Although the mechanisms underlying the heterogeneity of obesity-induced metabolic interindividual alterations are still not clearly understood, evidence suggests that genetic predisposition and lifestyle factors can influence such phenotypes.6 Accordingly, genetic variants mapped to lipid regulatory genes7 and adiponectin8,9 have been associated with MUP in obese individuals. Moreover, dietary fat and vegetable intakes, as well as physical activity and sleep patterns have been identified as independent predictors of MHP in some populations.10,11 Nonetheless, these factors could differ between populations, which makes it important to undertake more studies that allow the timely categorization of obesity phenotypes and improvement of therapeutic decision making. The aim of this study was to identify factors that explain a MUP using genetic and lifestyle data in participants with excessive adiposity for personalized precision interventions to facilitate diagnosis, characterization, and therapeutic prescriptions.
Materials and methods
Participants
In this cross-sectional study we enrolled 298 Spanish adults with overweight (BMI: 25–29.9 kg/m2) or obesity (BMI: 30–40 kg/m2), who were recruited at the Center for Nutrition Research of the University of Navarra, Pamplona, Spain. Major exclusion criteria included a clinical history of cardiovascular disease, type 1 diabetes, or type 2 diabetes treated with insulin; pregnant or lactating women; unstable dose of medication for hyperlipidemia or hypertension; and weight change > 3 kg within 3 months before the study. This investigation was approved by the Research Ethics Committee of the University of Navarra (reference number 132/2015) and followed the ethical principles for medical research in humans from the 2013 Helsinki Declaration.12 In addition, participants signed consent to participate in the study [ClinicalTrials.gov identifier: NCT02737267].
Anthropometry and blood pressure
Habitual anthropometric measurements such as height, body weight, and waist circumference (WC) were collected by trained nutritionists following standardized procedures.13 BMI was calculated as the ratio between body weight and squared height (kg/m2). Total body fat (TFAT) and visceral fat (VFAT) were quantified by dual-energy X-ray absorptiometry (DXA) following the supplier’s instructions (Lunar Prodigy, software version 6.0, Madison, WI, USA). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an automated sphygmomanometer according to accepted WHO/International Society of Hypertension criteria.14
Biochemical measurements
Overnight fasting blood samples (10 ml) were drawn by venipuncture and serum was obtained by centrifugation for further processing. Appropriate commercial kits were used to determine glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), and triglycerides in the chemistry analyzer Pentra C-200 (HORIBA ABX, Madrid, Spain). Low-density lipoprotein cholesterol (LDL-c) was calculated following the Friedewald equation:15 LDL-c = TC – (triglycerides/5)–HDL-c. Also, plasma concentrations of adiponectin, insulin, leptin, tumor necrosis factor alpha (TNF-α), and high-sensitive C-reactive protein (CRP) were measured using specific enzyme-linked immunosorbent assays and read in a fully automated analyzer system (Triturus, Grifols, Barcelona, Spain). The homeostatic model assessment–insulin resistance (HOMA-IR) index was calculated according to the Matthews formula:16 fasting insulin (µU/l) × fasting glucose (nmol/l)/22.5. Furthermore, the triglyceride–glucose (TyG) index was estimated as previously described:17 {ln[fasting triglycerides (mg/dl) × fasting plasma glucose (mg/dl)/2]}.
Definition of metabolically healthy and unhealthy phenotypes
Metabolic health status was evaluated using the criteria for diagnosis of metabolic syndrome according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines: WC > 102 cm for men or >88 cm for women, triglycerides ⩾ 150 mg/dl, HDL-c < 40 mg/dl for men or <50 mg/dl for women, blood pressure ⩾ 130/85 mmHg, and fasting glucose ⩾ 100 mg/dl.18 Participants with MHP had none or one of these altered cut-off points. Instead, MUP was based on the presence of more than one NCEP–ATP III criteria.
Lifestyle factors
A food frequency questionnaire validated for the Spanish population was used to assess the habitual dietary intake.19,20 Information concerning frequency consumption of 137 foods (daily, weekly, monthly, or never) during the previous year was collected and then converted into energy and nutrient intakes with appropriate software based on the available equivalences from standard Spanish food composition tables.21
The level of physical activity was evaluated using a validated questionnaire (Spanish version) that included 17 items.22 Metabolic equivalents (METs) were used to express the intensities of each of the physical activities relative to the resting metabolic rates, as previously described.23
The variable ‘duration of overweight/obesity’ was obtained from medical records under the section ‘weight evolution’ and referred to the time elapsed since the volunteer presented with overweight or obesity (<5 years or ⩾5 years).
SNP selection and genotyping
A total of 95 obesity-predisposing genetic variants were analyzed. Details regarding the selection procedure and genomic information (including chromosome location and minor allele frequencies) have been recently reported.24 Genomic deoxyribonucleic acid (DNA) was isolated from buccal cells using the Maxwell® 16 Buccal Swab LEV DNA Purification Kit (Promega Corp., Madison, WI, USA) according to the manufacturer’s protocol. Genotyping was performed with the Ion Torrent™ Next-Generation Sequencing system (Thermo Fisher Scientific Inc., Waltham, MA, USA), as described elsewhere.25,26 Raw data were processed with the Ion Torrent Sequencing platform and R software.
GRS calculation
First, the frequency of a MUP was compared between genotypes to select the single-nucleotide polymorphisms (SNPs) with a p value ⩽ 0.20, with BMI and WC as covariates, to adjust random baseline phenotypical differences. Subsequently, genotypes with similar effects (p > 0.05) were clustered and coded as risk and nonrisk groups. A risk genotype was defined as that associated with a higher frequency of MUP. Then, SNPs whose risk genotypes presented at least a marginal statistical trend (p < 0.10) among MHP and MUP were finally selected. SNPs with low sample (<10%) or collinearity were excluded. Afterwards, a weighted genetic-risk score (wGRS) was constructed under the assumption that all selected SNPs had independent effects and contributed in an additive manner to a MUP, as described elsewhere.27 Briefly, the wGRS was computed by multiplying the number of high-risk genotypes at each locus for the corresponding effect sizes (β coefficients), and then summing the products.28 This wGRS was used as a continuous variable in the multiple logistic regression models, as described elsewhere.24
Statistical analyses
Quantitative variables were expressed as means ± standard deviations, whereas categorical variables were presented as numbers and percentages. Statistical differences in adiposity, biochemical, and dietary intake markers according to metabolically healthy and unhealthy phenotype status were estimated using student t tests. Instead, chi-square tests were used to determine variations in the frequencies/proportions of risk genotypes between MHP and MUP groups. Mean values of wGRS according to the number of metabolic alterations were compared using analysis of variance and post hoc tests.
Multiple logistic regression models were performed to explain the MUP. The screening of genetic and lifestyle data to introduce into the models was carried out through the least-angle regression (LARS) test, as previously described.29 Collinearity was evaluated using the variance inflation factor. Area under the receiver operating characteristic (ROC) curves were built to evaluate the predictive values of the LARS-selected variables. Moreover, squared partial correlations (PC2) were used to estimate the individual contribution of each significant variable to the MUP. Statistical analyses were computed in the statistical program Stata 12 (StataCorp LLC, College Station, TX, USA; www.stata.com). Statistical significance was set at p < 0.05. Figure plots were designed using the GraphPad Prism® software, version 6.0C (La Jolla, CA, USA) and Stata 12.
Results
Anthropometrics, clinical, and biochemical characteristics of the study population categorized by metabolically healthy and unhealthy status are reported in Table 1. The prevalence of MHP and MUP were 44.3% and 55.7%, respectively. Participants with MUP were older, had greater TFAT and VFAT, as well as higher blood glucose levels, worse lipid profiles, insulin levels, HOMA-IR, and inflammatory markers than those showing a MHP.
Table 1.
Anthropometric, clinical, and biochemical characteristics of the study population categorized by metabolically healthy and unhealthy status: MHP (n = 132); MUP (n = 166).
| Variable | MHP | MUP | p value |
|---|---|---|---|
| Age (year) | 42.4 ± 10.3 | 48.0 ± 9.9 | <0.001 |
| Sex (female/male) | 102/30 | 106/60 | 0.012 |
| Anthropometrics and clinical data | |||
| Weight (kg) | 83.1 ± 11.3 | 91.5 ± 13.0 | <0.001 |
| BMI (kg/m2) | 30.1 ± 3.0 | 32.9 ± 3.3 | <0.001 |
| WC (cm) | 96.7 ± 9.4 | 106.5 ± 9.4 | <0.001 |
| TFAT (kg) | 34.8 ± 7.0 | 38.7 ± 7.6 | <0.001 |
| VFAT (kg) | 1.01 ± 0.62 | 1.84 ± 0.91 | <0.001 |
| SBP (mmHg) | 120 ± 11 | 135 ± 18 | <0.001 |
| DBP (mmHg) | 74 ± 8 | 84 ± 10 | <0.001 |
| Biochemical profile | |||
| Glucose (mg/dl) | 90.9 ± 6.4 | 101.0 ± 16.9 | <0.001 |
| Total cholesterol (mg/dl) | 211.1 ± 35.3 | 219.1 ± 40.2 | 0.073 |
| LDL-c (mg/dl) | 134.5 ± 32.5 | 143.9 ± 33.9 | 0.017 |
| HDL-c (mg/dl) | 61.4 ± 11.2 | 50.5 ± 12.3 | <0.001 |
| Triglycerides (mg/dl) | 76.0 ± 23.7 | 123.5 ± 60.8 | <0.001 |
| Adiponectin (µg/ml) | 12.9 ± 5.2 | 10.4 ± 4.4 | <0.001 |
| Insulin (mU/l) | 5.7 ± 2.8 | 9.6 ± 5.2 | <0.001 |
| Leptin (ng/ml) | 35.8 ± 27.4 | 37.9 ± 28.6 | 0.585 |
| CRP (µg/ml) | 2.19 ± 2.61 | 3.56 ± 3.73 | 0.002 |
| TNFα (pg/ml) | 0.78 ± 0.29 | 0.98 ± 0.37 | <0.001 |
| HOMA-IR (ratio) | 1.29 ± 0.68 | 2.40 ± 1.46 | <0.001 |
| TyG index (ratio) | 8.01 ± 0.34 | 8.63 ± 0.49 | <0.001 |
Variables are expressed as means ± standard deviations.
Bold numbers indicate p < 0.05. MHP, no more than one metabolic syndrome criteria; MUP, more than one metabolic syndrome criteria.
BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment–insulin resistance index; LDL-c, low-density lipoprotein cholesterol; MHP, Metabolically healthy phenotype; MUP, metabolically unhealthy phenotype; SBP, systolic blood pressure; TFAT, total body fat; TNFα, tumoral necrosis factor alpha; TyG index, triglyceride–glucose index; VFAT, visceral fat; WC, waist circumference.
Interestingly, MUP individuals consumed significantly higher portions of total and white cereals, and low amounts of dairy products and olive oil compared with MHP participants (Table 2). Regarding the nutritional profile, higher intakes of energy, total fat, saturated fatty acids and cholesterol were found among people exhibiting a MUP than their metabolic counterparts.
Table 2.
Nutritional profile and physical activity patterns of the study participants according to metabolically healthy (MHP) and unhealthy (MUP) status.
| Variable | MHP | MUP | p value |
|---|---|---|---|
| Energy (calories/d) | 2812 ± 756 | 3087 ± 1030 | 0.011 |
| Foods | |||
| Vegetables (g/d) | 418 ± 240 | 427 ± 166 | 0.705 |
| Fruits (g/d) | 280 ± 189 | 272 ± 209 | 0.717 |
| Legumes (g/d) | 17 ± 10 | 17 ± 9 | 0.825 |
| Total cereals (g/d) | 241 ± 127 | 295 ± 158 | 0.002 |
| Whole grains (g/d) | 45 ± 68 | 43 ± 77 | 0.816 |
| White cereals (g/d) | 116 ± 95 | 162 ± 134 | 0.001 |
| Dairy products (g/d) | 447 ± 230 | 390 ± 219 | 0.031 |
| Yogurt (g/d) | 112 ± 116 | 95 ± 99 | 0.183 |
| Meat (g/d) | 207 ± 79 | 223 ± 98 | 0.148 |
| Processed sausages (g/d) | 9 ± 13 | 14 ± 27 | 0.052 |
| Olive oil (g/d) | 43 ± 18 | 36 ± 19 | 0.002 |
| Fish | 99 ± 47 | 101 ± 55 | 0.756 |
| Macronutrients | |||
| Carbohydrates (% energy/d) | 41.1 ± 6.2 | 40.3 ± 7.3 | 0.322 |
| Protein (% energy/d) | 17.3 ± 2.7 | 16.7 ± 3.1 | 0.062 |
| Total fat (% energy/d) | 39.6 ± 5.7 | 41.1 ± 6.0 | 0.035 |
| SFA (% energy/d) | 17.9 ± 3.7 | 18.9 ± 3.3 | 0.010 |
| MUFA (% energy/d) | 5.7 ± 1.7 | 5.9 ± 1.6 | 0.195 |
| PUFA (% energy/d) | 11.1 ± 2.1 | 11.4 ± 2.6 | 0.321 |
| Cholesterol (mg/d) | 508 ± 161 | 578 ± 257 | 0.007 |
| Fiber (g/d) | 28.1 ± 10.5 | 28.1 ± 10.6 | 0.958 |
| Lifestyle | |||
| Physical activity (METs/d) | 25.6 ± 20.5 | 22.3 ± 19.5 | 0.166 |
Variables are expressed as means ± standard deviations.
Bold numbers indicate p < 0.05. MHP, no more than one metabolic syndrome criteria; MUP, more than one metabolic syndrome criteria.
METs, metabolic equivalents; MHP, metabolically healthy phenotype; MUFA, monounsaturated fatty acids; MUP, metabolically unhealthy phenotype; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
A total of 12 obesity-predisposing genetic variants were marginally or statistically associated with a MUP. The characteristics and coding of each of these SNPs are reported in Table 3. Stronger associations were detected for rs7799039 (LEP), rs4731426 (LEP), rs1801260, (CLOCK), rs3123554 (CNR2), rs569805 (ABCB11), rs6265 (BDNF), and rs1685325 (UCP3). Nine polymorphisms were excluded by low sample or collinearity: rs1055144 (NFE2L3), rs2867125 (TMEM18), rs4846567 (LYPLAL1), rs494874 (ABCB11), rs13021737 (TMEM18), rs10938397 (GNPDA2), rs1800629 (TNFA), rs206936 (NUDT3), and rs17066866 (MC4R). A wGRS was calculated using these 12 genetic variants (range 1.17–5.86).
Table 3.
Genomic and statistical characteristics of SNPs significantly associated with a MUP in participants with excessive body weight.
| No. | SNP (gene) | Alleles | Risk genotype | Risk genotype in MHP, n (%) | Risk genotype in MUP, n (%) | p value |
|---|---|---|---|---|---|---|
| 1 | rs7799039 (LEP) | G/A | GA | 56 (39.2) | 87 (60.8) | 0.006 |
| 2 | rs4731426 (LEP) | G/C | GC | 49 (36.8) | 84 (63.2) | 0.009 |
| 3 | rs1801260 (CLOCK) | A/G | AA+AG | 118 (42.8) | 158 (57.2) | 0.011 |
| 4 | rs3123554 (CNR2) | A/G | AG+GG | 91 (40.6) | 133 (59.4) | 0.015 |
| 5 | rs569805 (ABCB11) | A/T | TT | 44 (37.6) | 73 (62.4) | 0.028 |
| 6 | rs6265 (BDNF) | C/T | CC+TT | 79 (40.7) | 115 (59.3) | 0.028 |
| 7 | rs1685325 (UCP3) | T/C | TC+CC | 89 (40.3) | 132 (59.7) | 0.039 |
| 8 | rs1052700 (PLIN1) | A/T | AA | 12 (36.4) | 21 (63.6) | 0.061 |
| 9 | rs8192678 (PPARGC1A) | C/T | CC+TT | 69 (39.4) | 106 (60.6) | 0.073 |
| 10 | rs6123837 (GNAS) | G/A | AA | 15 (29.4) | 36 (70.6) | 0.086 |
| 11 | rs1800497 (ANKK1) | G/A | GG+AA | 84 (40.6) | 123 (59.4) | 0.087 |
| 12 | rs2860323 (TMEM18) | A/G | AG | 33 (35.5) | 60 (64.5) | 0.093 |
Data are expressed as number (percentage) and sorted in decreasing level of significance. wGRS calculated by multiplying the number of risk genotypes for the corresponding effect sizes, and then summing the products.
Bold numbers indicate p < 0.05.
MHP, metabolically healthy phenotype; MUP, metabolically unhealthy phenotype; SNPs, single-nucleotide polymorphisms; wGRS, weighted genetic-risk score.
Multiple logistic regression models explaining a MUP using LARS-selected variables are also reported (Table 4). Of note, wGRS [odds ratio (OR) = 4.133, p < 0.001], total dietary fat (OR = 1.105, p = 0.002), age (OR = 1.064, p = 0.001), and BMI (OR = 1.408, p < 0.001) contributed to explain a MUP, whereas female sex (OR = 0.330, p = 0.009) produced a protective effect. Remarkably, the wGRS remained statistically significant when analyzed individually or when combined with other variables (model 1 versus model 2; Table 4). In addition, partial-correlation analyses revealed that wGRS was the greatest predictor of the MUP, with about a 38% contribution (PC2 = 0.3816). No statistically significant gene–environment interactions concerning the MUP were found.
Table 4.
Odds ratios concerning genetic and nongenetic variables explaining a MUP in participants with excessive body weight.
| Variable | OR (CI 95%) | p value |
|---|---|---|
| Model 1 | ||
| wGRS (∑ weighted-risk genotypes) | 2.718 (1.960, 3.770) | <0.001 |
| χ 2 | 44.39 | <0.001 |
| R 2 | 0.1100 | – |
| Model 2 | ||
| wGRS (∑ weighted-risk genotypes) | 4.133 (2.490, 6.858) | <0.001 |
| Energy (100 calories/d) | 1.000 (0.999, 1.000) | 0.265 |
| Total dietary fat (%) | 1.105 (1.037, 1.176) | 0.002 |
| Physical activity (METs/d) | 0.983 (0.964, 1.001) | 0.067 |
| Age (years) | 1.064 (1.025, 1.103) | 0.001 |
| Sex (female) | 0.330 (0.144, 0.757) | 0.009 |
| BMI (kg/m2) | 1.408 (1.249, 1.588) | <0.001 |
| Duration of overweight/obesity (⩾5 years) | 2.051 (0.668, 6.297) | 0.209 |
| χ 2 | 127.96 | <0.001 |
| R 2 | 0.3755 |
Predictive variables are expressed as OR values.
Bold numbers indicate p < 0.05.
BMI, body mass index; CI, confidence interval; METs, metabolic equivalents; MUP, metabolically unhealthy phenotype; OR, odds ratio; wGRS, weighted genetic-risk score.
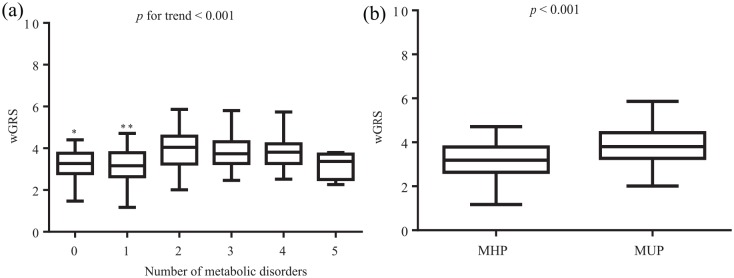
Comparisons of wGRS mean values according to metabolic status are plotted in Figure 1. The number of cases of each metabolic category were: none (n = 37), one (n = 95), two (n = 97), three (n = 38), four (n = 27), and five (n = 4). Of note, lower levels of wGRS were found among no and one metabolic alterations than two, three, and four, but not five types of disorder [Figure 1(a), p for trend < 0.001]. By contrast, the MUP was characterized by higher wGRS rates compared with MHP [Figure 1(b), p < 0.001].
Figure 1.
Comparisons of wGRS mean values.
Comparisons of wGRS mean values according to the number of metabolic alterations (a) or to the presence of a MUP (b).
*No versus two, three, and four metabolic disorders, p < 0.05.
**One versus two, three, and four metabolic disorders, p < 0.05.
MHP, metabolically healthy phenotype; MUP, metabolically unhealthy phenotype; wGRS, weighted genetic-risk score.
Figure 2 shows ROC curves of only genetic and the combination of both genetic and nongenetic factors explaining a MUP. Interestingly, an important predictive value (area under ROC curve = 0.7279) was obtained when the wGRS was separately analyzed [Figure 2, curve (a)]. However, the strongest performance was obtained when the wGRS was combined with the rest of variables including energy intake, total dietary fat, age, BMI, sex, physical activity and years of being overweight/obese [area under ROC curve = 0.8820, Figure 2, curve (b)]. A statistically significant difference (p < 0.0001) was found when both ROC curves were compared.
Figure 2.
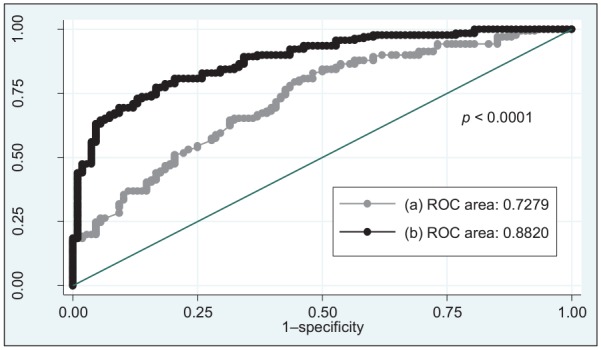
ROC curves of only genetic and the combination of both genetic and nongenetic factors explaining a MUP.
(a) wGRS; (b) wGRS, energy intake, total dietary fat, age, BMI, female sex, physical activity, and duration of overweight/obesity.
BMI, body mass index; MUP, metabolically unhealthy phenotype; ROC, area under the receiver operating characteristic; wGRS, weighted genetic-risk score.
Discussion
The prevalence of MHP and MUP in obese and normal-weight participants varies substantially across studies, mainly due to the lack of a uniform definition concerning different adiposity/metabolic features and thresholds as well as ethnicity/cultural reasons.30 In this investigation, 56% of the overweight/obese individuals presented with a MUP, which is within the range reported (55–85%) in the global literature.31 Similar results were found in a large Spanish obese population, where the prevalence of MUP was about 60% according to consensus criteria.32 Meanwhile, a higher frequency (near 70%) was observed in obese Spanish participants from the ENRICA cohort;33 however, unlike our study, they also included the homeostasis model assessment of insulin resistance value and C-reactive protein as cardiometabolic abnormalities. Conversely, only 30% of Spanish workers with overweight and obesity had a MUP using modified NCEP–ATP III cutoffs.34 Together, these findings highlight the heterogeneity of the obesity disorder and highlight the importance of establishing more universal criteria to define this phenotype.
Increasing evidence in humans and animal models suggests that the genetic background predisposes the susceptibility of developing obesity-related complications by affecting diverse essential metabolic pathways.35 Until now, most available studies have focused on analyzing the association of genetic variants with individual cardiometabolic traits; whereas the identification of polymorphisms specifically related to MUP or MHP remains less explored. In this research, 12 obesity-predisposing genetic variants were associated with a MUP, which regulate physiological processes such as food intake (LEP), circadian cycle (CLOCK), neuronal synapse signaling (CNR2, BDNF), bile secretion (ABCB11), energy expenditure (UCP3), lipid metabolism (PLIN1, PPARGC1A), hormone production (GNAS), food rewarding (ANKK1), and insulin signaling (TMEM18), according to human gene databases (www.genecards.org).
Furthermore, in this study, a wGRS constructed of the aforementioned obesity-risk alleles was strongly associated with a MUP and was the major contributor to this phenotype in the multivariate regression models, with the highest predictive value. Consistent with our results, polygenic risk scores for waist-to-hip ratio, a measure of genetic predisposition to abdominal adiposity, were associated with higher blood pressure and triglyceride levels, as well as higher risk of diabetes and coronary heart disease in different population-based cohorts.36,37 Moreover, individuals carrying genetically determined favorable adiposity phenotypes were at lower risk of diabetes, heart disease, and hypertension in two cohorts.38,39 These results demonstrate a link between the genetic susceptibility to excessive adiposity and cardiometabolic disease risks.
In addition to innate biological mechanisms, demographic factors and lifestyle have been shown to explain, at least in part, the difference between MHP and MUP in obese individuals.31 Thus, MUP participants are generally older, male, have higher adiposity indices, tend to consume a nutritionally poorer diet and often are less physically active than their obese counterparts.31 Herein, age was positively associated with a MUP, whereas female sex had a protective effect, in line with previous research.31 Meanwhile, greater visceral fat accumulation (measured by DXA) was phenotypically related to a MUP. Moreover, the diet of MUP participants was characterized by a high intake of fat (mainly saturated fat and cholesterol) and a low consumption of dairy products. Regarding this latter point, a systematic review and meta-analysis of published observational studies suggested an inverse dose–response relationship between dairy consumption and risk of metabolic syndrome.40 The differences in cereals/carbohydrate consumption may also explain the incidence of MUP.41 Moreover, the higher consumption of olive oil in the MHP group could be exerting a protective metabolic effect in these patients, which is attributed not only to the content of monounsaturated fatty acids, but also to other minor components such as phenolic compounds (e.g. hydroxytyrosol and oleuropein), with antioxidant, anti-inflammatory and other beneficial properties.42,43
Current precision nutrition/medicine approaches are adding knowledge into disease-risk prediction and individualized nutritional prescriptions by taking into account a number of clinical, phenotypical, environmental and biological factors to personalize the prevention and management of obesity and related complications.44 In this context, as a proof of concept, we constructed an integrative model covering conventional (age, sex), phenotypic (body composition), genetic (multiple SNPs), and lifestyle data (diet and physical activity) to explain MHP and MUP in overweight/obese individuals. Also, the predictive model was adjusted by the duration of overweight and obesity, which is a potential confounding factor associated with the presence of metabolic alterations. Additionally, this study enrolled a genetically homogeneous White/European population, thus minimizing the possible effect of population stratification in our results. On the other hand, we consider a drawback of this study was the relatively small sample analyzed, which could explain the lack of statistical differences in the levels of wGRS between the group with five risk factors and the other metabolic groups. Also, the findings of this research may not be extended to other ethnic groups exposed to different gene–environmental interactions. Moreover, given that this study has a cross-sectional design, no temporal/causal relationships between exposures and outcomes can be established. Furthermore, the role of other emerging factors affecting metabolic homeostasis, such as microbiota composition, epigenetic phenomena, sleep patterns, and metabolomic profiles, also need to be explored.45–47
In conclusion, the genetic background is an important factor explaining MHP and MUP in participants with overweight/obesity, in addition to lifestyle variables, such as dietary fat and physical activity, as well as age, female sex, and BMI. This information could be useful to metabolically categorize individuals for disease-risk prediction purposes, as well as for the design/implementation of personalized nutritional interventions aimed at promoting metabolic health and nutritional wellbeing, using precise criteria.
Acknowledgments
Omar Ramos-Lopez and Jose Riezu-Boj contributed equally to this research. The authors thank Laura Olazaran and Iosune Zubieta for nutritional assistance; Ana Lorente for laboratory support; and Blanca Martinez de Morentin (physician). The tractor role of CINFA with regards the genetic tools is also gratefully acknowledged, regarding a grant from the Gobierno de Navarra.
Footnotes
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: this work was funded by grants from the Gobierno de Navarra (grant number PT024); Centro de Investigación Biomédica en Red: Fisiopatología de la Obesidad y Nutrición (grant number CB12/03/30002), and Nutrición personalizada y biomarcadores nutrigenómicos de la inflamación asociada a la dieta y la obesidad (grant number AGL2013–45554-R).
Conflict of interest statement: The authors declare that they have no conflicts of interest concerning this investigation.
ORCID iD: J. Alfredo Martinez  https://orcid.org/0000-0001-5218-6941
https://orcid.org/0000-0001-5218-6941
Contributor Information
Omar Ramos-Lopez, Department of Nutrition, Food Science and Physiology, and Center for Nutrition Research, University of Navarra, Pamplona, Spain; Medicine and Psychology School, Autonomous University of Baja California, Tijuana, Baja California, Mexico.
Jose I. Riezu-Boj, Department of Nutrition, Food Science and Physiology, and Center for Nutrition Research, University of Navarra, Pamplona, Spain Navarra Institute for Health Research (IdiSNA), Pamplona, Spain.
Fermin I. Milagro, Department of Nutrition, Food Science and Physiology, and Center for Nutrition Research, University of Navarra, Pamplona, Spain Navarra Institute for Health Research (IdiSNA), Pamplona, Spain; CIBERobn, Fisiopatología de la Obesidad y la Nutrición; Carlos III Health Institute, Madrid, Spain.
Marta Cuervo, Department of Nutrition, Food Science and Physiology, and Center for Nutrition Research, University of Navarra, Pamplona, Spain; Navarra Institute for Health Research (IdiSNA), Pamplona, Spain; CIBERobn, Fisiopatología de la Obesidad y la Nutrición; Carlos III Health Institute, Madrid, Spain.
Leticia Goni, Department of Nutrition, Food Science and Physiology, and Center for Nutrition Research, University of Navarra, Pamplona, Spain.
J. Alfredo Martinez, Department of Nutrition, Food Science and Physiology, and Center for Nutrition Research, University of Navarra, 1 Irunlarrea Street, Pamplona, 31008, Spain; Navarra Institute for Health Research (IdiSNA), Pamplona, Spain; CIBERobn, Fisiopatología de la Obesidad y la Nutrición; Carlos III Health Institute, Madrid, Spain; Madrid Institute of Advanced Studies (IMDEA Food), Madrid, Spain.
References
- 1. Friedrich MJ. Global obesity epidemic worsening. JAMA 2017; 318: 603. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Obesity and overweight, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2018, accessed 11 April 2019).
- 3. Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: a review. World J Diabetes 2010; 1: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011; 35: 971–981. [DOI] [PubMed] [Google Scholar]
- 5. Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol 2017; 960: 1–17. [DOI] [PubMed] [Google Scholar]
- 6. Navarro E, Funtikova AN, Fíto M, et al. Can metabolically healthy obesity be explained by diet, genetics, and inflammation? Mol Nutr Food Res 2015; 59: 75–93. [DOI] [PubMed] [Google Scholar]
- 7. Hovsepian S, Javanmard SH, Mansourian M, et al. Lipid regulatory genes polymorphism in children with and without obesity and cardiometabolic risk factors: the CASPIAN-III study. J Res Med Sci 2018; 23: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang CS, Lu YJ, Chang HH, et al. Role of adiponectin gene variants, adipokines and hydrometry-based percent body fat in metabolically healthy and abnormal obesity. Obes Res Clin Pract 2018; 12: 49–61. [DOI] [PubMed] [Google Scholar]
- 9. Berezina A, Belyaeva O, Berkovich O, et al. Prevalence, risk factors, and genetic traits in metabolically healthy and unhealthy obese individuals. Biomed Res Int 2015; 2015: 548734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prince RL, Kuk JL, Ambler KA, et al. Predictors of metabolically healthy obesity in children. Diabetes Care 2014; 37: 1462–1468. [DOI] [PubMed] [Google Scholar]
- 11. Nasreddine L, Tamim H, Mailhac A, et al. Prevalence and predictors of metabolically healthy obesity in adolescents: findings from the national “Jeeluna” study in Saudi-Arabia. BMC Pediatr 2018; 18: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 13. Lopez-Legarrea P, De la Iglesia R, Abete I, et al. Short-term role of the dietary total antioxidant capacity in two hypocaloric regimes on obese with metabolic syndrome symptoms: the RESMENA randomized controlled trial. Nutr Metab (Lond) 2013; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitworth JA, Chalmers J. World Health Organisation-International Society of Hypertension (WHO/ISH) hypertension guidelines. Clin Exp Hypertens 2004; 26: 747–752. [DOI] [PubMed] [Google Scholar]
- 15. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- 16. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 17. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, et al. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev Med 2016; 86: 99–105. [DOI] [PubMed] [Google Scholar]
- 18. Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004; 109: 433–438. [DOI] [PubMed] [Google Scholar]
- 19. Martin-Moreno JM, Boyle P, Gorgojo L, et al. Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol 1993; 22: 512–519. [DOI] [PubMed] [Google Scholar]
- 20. De la Fuente-Arrillaga C, Ruiz ZV, Bes-Rastrollo M, et al. Reproducibility of an FFQ validated in Spain. Public Health Nutr 2010; 13: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 21. Moreiras O, Carbajal Á, Cabrera L, et al. Tablas de Composición de Alimentos. 16th ed. Madrid: Piramide, 2013; 1–416. [Google Scholar]
- 22. Martínez-González MA, López-Fontana C, Varo JJ, et al. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ follow-up study. Public Health Nutr 2005; 8: 920–927. [DOI] [PubMed] [Google Scholar]
- 23. Basterra-Gortari FJ, Bes-Rastrollo M, Pardo-Fernández M, et al. Changes in weight and physical activity over two years in Spanish alumni. Med Sci Sports Exerc 2009; 41: 516–522. [DOI] [PubMed] [Google Scholar]
- 24. Ramos-Lopez O, Riezu-Boj JI, Milagro FI, et al. Prediction of blood lipid phenotypes using obesity-related genetic polymorphisms and lifestyle data in subjects with excessive body weight. Int J Genomics 2018; 2018: 4283078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramos-Lopez O, Riezu-Boj JI, Milagro FI, et al. Association of the Gly482Ser PPARGC1A gene variant with different cholesterol outcomes in response to two energy-restricted diets in subjects with excessive weight. Nutrition 2018; 47: 83–89. [DOI] [PubMed] [Google Scholar]
- 26. Ramos-Lopez O, Riezu-Boj JI, Milagro FI, et al. Differential lipid metabolism outcomes associated with ADRB2 gene polymorphisms in response to two dietary interventions in overweight/obese subjects. Nutr Metab Cardiovasc Dis 2018; 28: 165–172. [DOI] [PubMed] [Google Scholar]
- 27. Hung CF, Breen G, Czamara D, et al. A genetic risk score combining 32 SNPs is associated with body mass index and improves obesity prediction in people with major depressive disorder. BMC Med 2015; 13: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin X, Song K, Lim N, et al. Risk prediction of prevalent diabetes in a Swiss population using a weighted genetic score–the CoLaus Study. Diabetologia 2009; 52: 600–608. [DOI] [PubMed] [Google Scholar]
- 29. Efron B, Hastie T, Johnstone I, et al. Least angle regression. Ann Statist 2004; 32: 407–499. [Google Scholar]
- 30. Mathew H, Farr OM, Mantzoros CS. Metabolic health and weight: understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism 2016; 65: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loos RJF, Kilpeläinen TO. Genes that make you fat, but keep you healthy. J Intern Med 2018; 284: 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martínez-Larrad MT, Corbatón Anchuelo A, Del Prado N, et al. Profile of individuals who are metabolically healthy obese using different definition criteria. A population-based analysis in the Spanish population. PLoS One 2014; 9: e106641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lopez-Garcia E, Guallar-Castillon P, Leon-Muñoz L, et al. Prevalence and determinants of metabolically healthy obesity in Spain. Atherosclerosis 2013; 231: 152–157. [DOI] [PubMed] [Google Scholar]
- 34. Goday A, Calvo E, Vázquez LA, et al. Prevalence and clinical characteristics of metabolically healthy obese individuals and other obese/non-obese metabolic phenotypes in a working population: results from the Icaria study. BMC Public Health 2016; 16: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang LO, Loos RJF, Kilpeläinen TO. Evidence of genetic predisposition for metabolically healthy obesity and metabolically obese normal weight. Physiol Genomics 2018; 50: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Emdin CA, Khera AV, Natarajan P, et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 2017; 317: 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lotta LA, Wittemans LBL, Zuber V, et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA 2018; 320: 2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yaghootkar H, Lotta LA, Tyrrell J, et al. Genetic evidence for a link between favorable adiposity and lower risk of type 2 diabetes, hypertension, and heart disease. Diabetes 2016; 65: 2448–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yiorkas AM, Frau F, Mook-Kanamori D, et al. Genome-wide and abdominal MRI data provide evidence that a genetically determined favorable adiposity phenotype is characterized by lower ectopic liver fat and lower risk of type 2 diabetes, heart disease, and hypertension. Diabetes 2019; 68: 207–219. [DOI] [PubMed] [Google Scholar]
- 40. Chen GC, Szeto IM, Chen LH, et al. Dairy products consumption and metabolic syndrome in adults: systematic review and meta-analysis of observational studies. Sci Rep 2015; 5: 14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Santiago S, Zazpe I, Bes-Rastrollo M, et al. Carbohydrate quality, weight change and incident obesity in a Mediterranean cohort: the SUN Project. Eur J Clin Nutr 2015; 69: 297–302. [DOI] [PubMed] [Google Scholar]
- 42. Yubero-Serrano EM, Lopez-Moreno J, Gomez-Delgado F, et al. Extra virgin olive oil: more than a healthy fat. Eur J Clin Nutr 2019; 72: 8–17. [DOI] [PubMed] [Google Scholar]
- 43. Tsartsou E, Proutsos N, Castanas E, et al. Network meta-analysis of metabolic effects of olive-oil in humans shows the importance of olive oil consumption with moderate polyphenol levels as part of the Mediterranean diet. Front Nutr 2019; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramos-Lopez O, Milagro FI, Allayee H, et al. Guide for current nutrigenetic, nutrigenomic, and nutriepigenetic approaches for precision nutrition involving the prevention and management of chronic diseases associated with obesity. J Nutrigenet Nutrigenomics 2017; 10: 43–62. [DOI] [PubMed] [Google Scholar]
- 45. Goni L, Cuervo M, Milagro FI, et al. Future perspectives of personalized weight loss interventions based on nutrigenetic, epigenetic, and metagenomic data. J Nutr 2016; 146: 905S–912S. [DOI] [PubMed] [Google Scholar]
- 46. Ramos-Lopez O, Samblas M, Milagro FI, et al. Circadian gene methylation profiles are associated with obesity, metabolic disturbances and carbohydrate intake. Chronobiol Int 2018; 35: 969–981. [DOI] [PubMed] [Google Scholar]
- 47. Ramos-Lopez O, Riezu-Boj JI, Milagro FI, et al. DNA methylation signatures at endoplasmic reticulum stress genes are associated with adiposity and insulin resistance. Mol Genet Metab 2018; 123: 50–58. [DOI] [PubMed] [Google Scholar]