Abstract
The distribution and content of fibronectin is closely related to the occurrence and development of tumors. Fibronectin is widely involved in cell migration, adhesion, proliferation, hemostasis, and tissue repair. Fibronectin type III domain containing 1, as a primary component of the structural domain of fibronectin, is closely related to the occurrence of some cancers. However, the molecular mechanism of fibronectin type III domain containing 1 in gastric cancer has not been elaborated. In this study, we analyzed the expression and prognosis of fibronectin type III domain containing 1 by collecting data from Oncomine and GEPIA database. The expression of fibronectin type III domain containing 1 in gastric cancer cells was detected by quantitative real-time polymerase chain reaction in vitro. After knockdown of fibronectin type III domain containing 1 by small interfering RNA, the proliferation, invasion, and migration of AGS (human gastric adenocarcinoma cell line) cells and the function of epithelial–mesenchymal transition were measured by Cell Counting Kit-8, colony formation, transwell, and Western blot. The results showed that fibronectin type III domain containing 1 was highly expressed in gastric cancer tissues and its overexpression was significantly correlated with the prognosis of gastric cancer. In vitro, experiments revealed that knockdown of fibronectin type III domain containing 1 could inhibit the proliferation, migration, and invasion of gastric cancer cells, possibly by changing the epithelial–mesenchymal transition pathway. The findings elaborated the biological role of fibronectin type III domain containing 1 in gastric cancer and potential mechanism of action, possibly providing a new insight for future clinical diagnosis or even molecular therapy.
Keywords: gastric cancer, FNDC1, invasion, migration, EMT
Introduction
Gastric cancer (GC) is a common disease threatening human health. Several precipitating factors were reported to play important role in promoting GC occurrence, such as Helicobacter pylori infection, dietary habits, familial inheritance, and atrophic gastritis.1,2 The survival rate of GC is related to many factors, such as disease stage, tumor size, location of GC, histological type, pathological stage, and lymph node metastasis.3 For early detection and diagnosis of GC, the 5-year survival rate can exceed 90%, while for advanced GC, the figure is usually less than 50%.4 Gastric cancer is a complex, undetectable, multifactorial, metastatic disease, and the specific mechanism of occurrence and development has not been clarified.5 So to find the biological indicators, it is necessary to improve the cure rate of GC.
In recent years, the role of extracellular matrix macromolecular characteristics in the invasion and metastasis of tumor has been paid more and more attention.6 However, as one of the major macromolecular characteristics of extracellular matrix, fibronectin (FN) and its main structural domain fibronectin type III domain containing 1 (FNDC1) have rarely been reported in GC about its performance and function. It has been reported that FNDC1 is a pathogenic gene in children with acute otitis media.7 Some studies found that knockdown AGS8 (also known as FNDC1) had a limited effect on epidermal growth factor and basic fibroblast growth factor signaling, indicating the specificity of AGS8-mediated trafficking. Some researchers investigated that AGS8 is required for hypoxia-induced apoptosis of cardiomyocytes.8 There have also been some reports about FNDC1 in cancers. Some papers explored that FNDC1 can promote apoptosis in human salivary gland adenoid cystic carcinoma by hypermethylation.9 Some findings observed that in prostate cancer, increased expression of microRNA-1207-3p significantly limited migration and proliferation and induces apoptosis via direct molecular targeting of FNDC1.10 A recent study found the correlation between the expression pattern and clinical features of FNDC1 in GC,11 but its mechanism has not yet been clarified. Thus, the specific mechanism of FNDC1 in GC still needs to be elucidated.
In this study, we found that FNDC1 was highly expressed in GC tissues and cell lines, and knockdown of FNDC1 reduced AGS cells proliferation, invasion, and migration abilities, which were closely linked with epithelial–mesenchymal transition (EMT) repression. Patients with high FNDC1 expression had a short overall survival (OS). Thus, findings from this work indicate a significant role of FNDC1 and underlying mechanism in GC progression.
Materials and Methods
Data Acquisition
To get the expression pattern of the FNDC1 in GC, we used the data sets from Oncomine database (https://www.oncomine.org) and The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov/). Differential expression and prognostic analysis of FNDC1 were obtained from the TCGA database and analyzed by GEPIA (http://gepia.cancer-pku.cn/). Then, we downloaded 407 samples including 32 normal cases and 375 tumor cases for FNDC1 expression and prognosis analysis. Subsequently, patients with complete clinical data were selected for clinicopathological correlation analysis. Low and high expression of FNDC1 in the GC sas defined according to the median, and those above the median were defined as high expression and those under the median were defined as low expression. And the prognosis was analyzed by Kaplan-Meier plotter.
Cell Culture
Human GC cell lines AGS, MKN45, HGC-27, and MGC-803 and normal control cell line GES-1 were purchased from the Shanghai Cell Bank of the Chinese Academy of Medical Sciences (Shanghai, China). The cells were incubated at 37°C, 5% CO2, 10% the concentration of serum, 100 U/mL the concentration of penicillin, and 0.1 mg/mL the concentration of streptomycin (Gibco, Invitrogen, Carlsbad, California). Under the logarithmic growth stage, cells were washed by phosphate-buffered saline (PBS) 3 times and then digested with trypsin (Gibco, Invitrogen, Carlsbad, California). Added culture solution to stop digestion when the cells became round, blowed them into single-cell suspension, then put them into a 6-well plate for subsequent experiments.
Transfection
The sequences of small interfering RNA (siRNA) were synthesized byGenePharma (Shanghai, China). The siRNA was transfected into AGS cells using Lipofectamine2000 (Invitrogen, Carlsbad, California) for 6 hours when the cells grows logarithmically. After transfection, the cells were cultured for 24 hours and then detected transfection efficiency by Western blot and quantitative polymerase chain reaction with the following primers: FNDC1 si-RNA: 5′-CCGAAGGGAAGGCGTAGATAA-3′, si-con: 5′-TTCTCCGAACGTGTCACGT-3′.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from cells using RNA extraction kit (Invitrogen, Carlsbad, California). Five micrograms RNA was reverse-transcribed into complementary DNA and then amplified by polymerase chain reaction (PCR) with the following primers: FNDC1: forward: 5′-GGGGTTTTCTCCTGGGCTAC-3′, reverse: 5′-CAGCCCTGTAGACTGGTTGG-3′; Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH): forward: 5′-GGAGCGAGATCCCTCCAAAAT-3′, reverse: 5′-GGCTGTTGTCATACTTCTCATGG-3′.
The expression of FNDC1 was detected by quantitative real-time polymerase chain reaction (qRT-PCR). GAPDH was amplified for internal standardization. Quantitative real-time polymerase chain reaction was performed by 40 cycles consisting of 95°C for 5 minutes, 95°C for 30 seconds, and an extension step at 60°C for 45 seconds and 72°C for 30 minutes. Each group sample was extracted from 3 plates and the experiments were repeated 3 times independently. The relative expression data from the qRT-PCR experiments were statistically analyzed using the 2−ΔΔCT method.
Western Blot
We extracted the total protein from the cells transfected with siRNA for 48 hours, and the total protein concentration was measured by the bicinchoninic acid method. The same amount of protein (20 μg) from each sample was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene difluoride membranes. The membranes were incubated with 5% skim milk powder for 1 hour at room temperature and then incubated with the primary antibodies (1:1000; Sigma, St. Louis, MO, USA) at 4°C overnight. After washing, the membrane was incubated with the secondary antibodies (1:2000; Sigma, St. Louis, MO, USA) for 1 hour. The signal of the protein was visualized by enhanced chemiluminescence. The relative expression of protein bands was measured by Quantity-One software and took GAPDH as the internal control. All the experiments were repeated 3 times independently.
Cell Counting Kit-8 Analysis
The cells were digested after transfected siRNA for 24 hours and then cultured in 96-well plates at a density of 1 × 104 cells/well. Cells were cultured routinely in carbon dioxide incubator. The cells activity was measured by Cell Counting Kit-8 (CCK-8; BestBio, Shanghai, China) every other 24 hours, before each detection, cultured in the incubator for 1.5 hours after added the CCK-8 agent into the plate, and cultured in the incubator for 1.5 hours. The optical density was analyzed by enzyme-linked immunosorbent assay microplate reader at wavelength of 450 nm and the proliferation was fitted with a curve. All the experiments were repeated 3 times independently.
Transwell
The 100 μL of Matrigel melted overnight was added into the upper chamber of the transwell chamber of the 24-well plate, shaken evenly, and placed in a carbon dioxide incubator at 37°C for 4 to 6 hours to form a gel. Then, the culture medium was dried, and 500 μL of serum-free culture fluid was added into the lower chamber, and the substrate membrane was hydrated for half an hour. Cells transfected with siRNA were cultured for 24 hours using serum-free culture to prepare cell suspension; 100 μL cell suspension (1 × 105) was added into the upper chamber and 500 µL complete culture solution was added into the lower chamber. After overnight, the small chamber was removed, and the remaining cells in the upper chamber were wiped off with cotton swab. After PBS cleaning, 4% paraformaldehyde was fixed for 30 minutes; 0.1% crystal violet was stained for 20 minutes, PBS was cleaned, and 5 visual fields were randomly selected under the microscope for observation and counting.
The migration procedure is similar to the invasion experiment without precoated Matrigel on the upper chamber of transwell assay. All the experiments were repeated 3 times independently.
Colony-Forming Assay
The logarithmic phase cells were digested with trypsin and blowed them into single. Cell suspension was prepared and counted. The cells were inoculated in 60-mm-depth dish that was prefilled with 5 mL warm medium with a density of 400 cells/well. Then the cells were cultured at 37°C, 5% CO2 incubator for approximately 2 weeks. When visible clones appear in the dish, the culture was stopped. The cells were fixed with 5 mL of 4% paraformaldehyde for 30 minutes. Then removed the fixed solution, added appropriate 0.1% crystal violet dye for 30 min, and then slowly washed the dye solution with running water and dried in the air. Cells were fixed and stained with crystal violet to visualize colonies. Experiments were executed in triplicate.
Cell Apoptosis Assay
After transfection, the AGS cells were collected into centrifuge tubes to centrifuge 2 times at 1000 rpm for 5 minutes. The supernatant was aspirated and resuspended by 1× binding buffer to adjust the cells density into 1 to 5 × 106/mL; 100 μL cell suspension and 5 μL Annexin V/FITC (Beyotime, Shanghai, China) were well mixed to block in darkroom for 5 minutes. For machine detection, cells were exposed to the staining mixture with 10 μL propidium iodide and 400 μL PBS. Flowjo software (version 7.6.1) was used to analyze the results.
Statistical Analysis
SPSS22.0 statistical analysis and GraphPad Prism 6.0 software were used to analyze the experimental data. The comparison in 2 groups was analyzed using t test, while multiple comparison was demonstrated by 1-way analysis of variance followed by a Dunnett t test. The correlation between FNDC1 expression and clinicopathological correlation was measured by χ2 test. The OS curves were calculated through Kaplan-Meier method and log-rank test. *P < .05 and **P < .01 express statistically significant differences.
Results
Overexpression of FNDC1 and Correlates With Poor Prognosis in Patients With GC
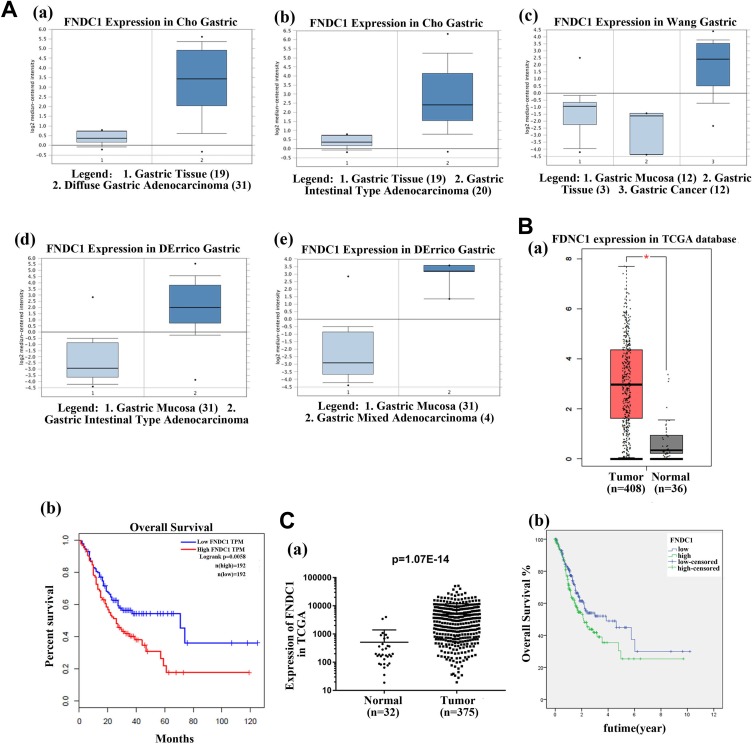
All the data about the FNDC1 expression were extracted from Oncomine and TCGA databases, and the results suggested that the FNDC1 was highly expressed in gastric tissues compared with normal tissue, no matter the data sets from Cho, Wang, and Derrico (Figure 1A, **P < .01). Patients with high FNDC1 expression have shorter OS compared to those with lower FNDC1 expression (Figure 1B and C, **P < .01). Subsequently, we analyzed the relationship between the expression of FNDC1 and clinicopathological correlation (Table 1). As shown in Table 1, significant associations were detected between FNDC1 expression and grade (G1 + G2, P = .015) of patients with GC and tumor pathologic-T (P = .002). But, the expression of FNDC1 was not related to age (P = .543), gender (P = .442), pathologic-stage (P = .182), pathologic-N (P = .373), and pathologic-M (P = 0.812). The results showed that FNDC1 was overexpressed in GC tissues and a significant connection between FNDC1 expression and level resulted in worse OS poor prognosis of GC. (The data of Figure 1B were from TCGA and analyzed by GEPIA website, the data from Figure 1C were downloaded from TCGA by ourselves and analyzed by χ2, Kaplan-Meier, and log-rank tests.)
Figure 1.
Fibronectin type III domain containing 1 was highly expressed in different GC tissues and high expression of FNDC1 correlated with poor prognosis. A, The expression of FNDC1 from different GC tissues, including Cho gastric, Wang gastric, and Derrico gastric, was highly expressed, compared with normal tissues (**P < .01). The data sets from Oncomine. B, The data were from TCGA and analyzed by GEPIA website, suggesting that FNDC1 was upregulated in patients with GC and resulted in poor prognosis, the expression of FNDC1 was upregulated in tumor tissues compared with normal tissues (** P < .01), and patients with high expression of FNDC1 had a low overall survival through analyzing data from GEPIA (**P < .01). C, The data were downloaded from TCGA database and analyzed by χ2, Kaplan-Meier, and log-rank tests, indicating that FNDC1 was highly expressed in patients with GC and led to poor prognosis (P = 1.07E−14). FNDC1 indicates fibronectin type III domain containing 1; GC, gastric cancer; TCGA, The Cancer Genome Atlas.
Table 1. Relationship.
Relationship Between FNDC1 Expression and Clinicopathologic Features of Patients With Gastric Cancer.a
| Characteristics | Expression of FNDC1 | P Value | |
|---|---|---|---|
| Low | High | ||
| Age | .543 | ||
| <60 | 57 | 52 | |
| ≥60 | 122 | 128 | |
| Gender | .442 | ||
| Female | 68 | 61 | |
| Male | 113 | 120 | |
| Grade | |||
| G1 + G2 | 81 | 59 | .015b |
| G3 + G4 | 95 | 118 | |
| Pathologic-stage | .182 | ||
| I + II | 85 | 70 | |
| III + IV | 88 | 97 | |
| Pathologic-T | .002b | ||
| T1 + T2 | 60 | 33 | |
| T3 + T4 | 120 | 141 | |
| Pathologic-N | .373 | ||
| N0 | 57 | 47 | |
| N1 | 119 | 121 | |
| Pathologic-M | .812 | ||
| M0 | 162 | 159 | |
| M1 | 12 | 13 | |
Abbreviations: FNDC1, fibronectin type III domain containing 1; M, metastasis; N, lymph nodes; T, tumor.
aIn some cases, the clinical pathological information is not complete, so the total cases of age, gender, grades, pathological classification, and lymph node metastasis were less than 407 cases.
bP < .05.
FNDC1 Is Highly Expressed in AGS Cell Line
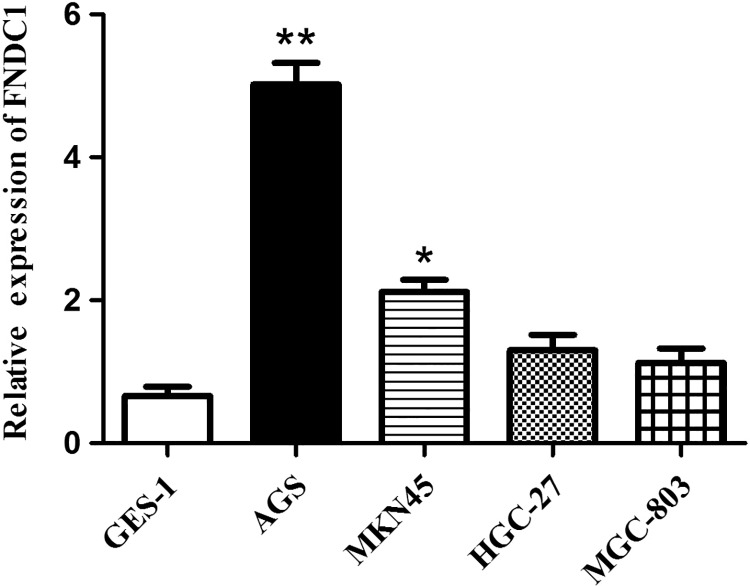
To explore the function of FNDC1 in GC, we detected the expression of FNDC1 in different GC cell lines including AGS, MKN45, HGC-27, and MGC-803 and normal control cell GES-1. The data from qRT-PCR showed that FNDC1 was highly expressed in GC cell lines, especially highest level in AGS, compared with normal control cell GES-1 (Figure 2, **P < .01). Thus, AGS cells were used in subsequent experiments to assess the biological role of FNDC1 in GC. The detection results of FNDC1 at the cell level in vitro were consistent with the results analyzed in vivo, indicating that FNDC1 was highly expressed in GC, which may indicate that FNDC1 plays an important role in the development of GC.
Figure 2.
Fibronectin type III domain containing 1 was highly expressed in GC cell lines. Quantitative real-time polymerase chain reaction (qRT-PCR) analyzed the expression of FNDC1 from GC cell lines AGS, Mkn45, HGC-27, and MGC-803 and normal control cell GES-1. *Significant difference compared with other cell lines. *P < .05, **P < .01. FNDC1 indicates fibronectin type III domain containing 1; GC, gastric cancer.
Proliferation of AGS Was Downregulated After Knockdown of FNDC1
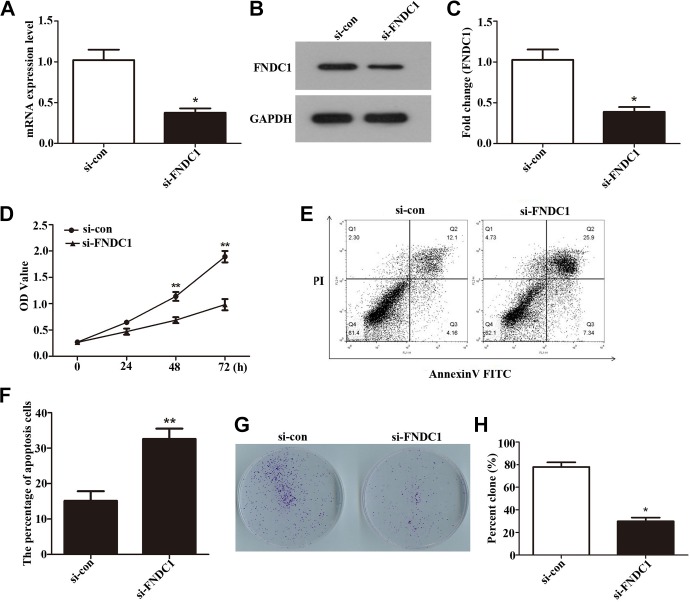
To further investigate the function of FNDC1 in GC, first, FNDC1 was knocked down by transfected siRNA into AGS and nonspecific sequences were transfected as control. Total RNA and protein of AGS cells were extracted 24 hours after siRNA transfection; qRT-PCR (Figure 3A, *P < .05) and Western blot (Figure 3B and C, *P < .05) results showed that silenced FNDC1 (si-FNDC1) transfection could significantly reduce the expression levels of FNDC1 both in RNA and protein in AGS cells, indicating that interference efficiency was successful. Next, in order to explore the effect on GC cells after knockdown of FNDC1, we used CCK-8 (Figure 3D, **P < .01) to detect the proliferation activity of AGS in the following experiment; the OD value in AGS cells was obviously decreased after transfected with si-FNDC1 for 48 and 72 hours, respectively. Subsequently, flow cytometry was performed to analyze the AGS cells apoptosis after knockdown of FNDC1. The results (Figure 3E and F, **P < .01) showed that si-FNDC1 increased the AGS cells apoptosis ability compared to the control. The data from CCK-8 and flow cytometry indicated that depletion FNDC1 decreased AGS cells proliferation and increased AGS cells apoptosis. Then, we designed plate cloning experiment to evaluate colony-forming ability after knockdown of FNDC1. Cloning experiment can directly reflect the effect of FNDC1 on GC cells after interference. After interfering with FNDC1, we found that the number of colony formation in the experimental group was significantly reduced compared to that in the control group (Figure 3G and H, *P < .05). These findings suggested that si-FNDC1 induced an inhibiting effect on GC cells proliferation and motivating function on GC cells apoptosis.
Figure 3.
Fibronectin type III domain containing 1 was knocked down by siRNA. Cell Counting Kit-8 assay was performed to explore the proliferation of AGS cells, and the colony-forming rate of AGS cells was observed after knockdown of FNDC1. A, The mRNA levels of FNDC1 expression after knock down by siRNA was analyzed using qRT-PCR. B, Western blotting was used to measure the expression of FNDC1 after knock down by siRNA. C, Western blot data were analyzed by software Quantity-One program by 3 independent repeats. *P < .05. D, The data from CCK-8 showed that knockdown FNDC1 downregulated the proliferation of AGS cells. **P < .01. E, The GC cells apoptosis ability was measured by flow cytometry, and (F) is quantification of (E). ** P < .01. G, The colony formation number was declined after knockdown of FNDC1 by siRNA. H, The colony number was counted. *P < .05. CCK-8, Cell Counting Kit-8; FNDC1, fibronectin type III domain containing 1; mRNA, messenger RNA; qRT-PCR, quantitative real-time polymerase chain reaction; siRNA, small interfering RNA.
Depletion of FNDC1 Limited the GC Cells Invasion and Migration Abilities
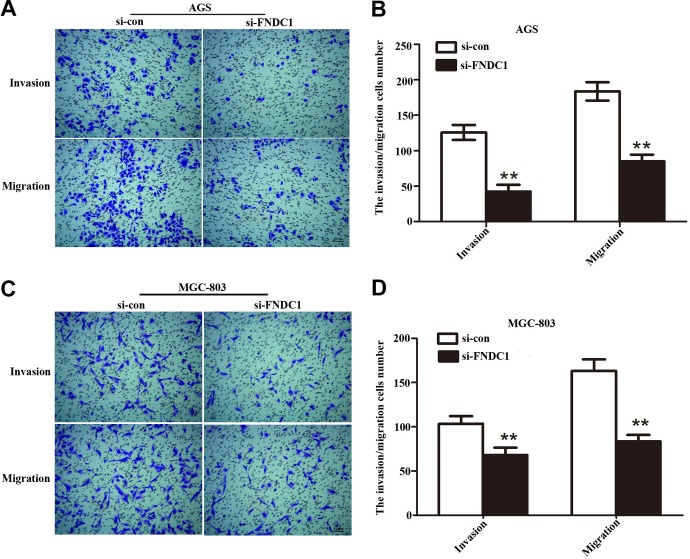
To vanish the rate of invasion and migration is very important to capture GC. To this end, transwell chamber assay was conducted to measure GC cells (AGS and MGC-803) invasion and migration capabilities after infected with si-FNDC1. Compared to the control group, the number of crystal violet staining of the AGS (Figure 4A and B, **P < .01) and MGC-803 (Figure 4C and D, **P < .01) cells significantly decreased after knockdown of FNDC1 (Figure 4A and B, **P < .01). The results indicated that invasion ability of GC cells in experimental group was obviously decreased and that the number of invasive cells was clearly lower than that of the control group. Moreover, the migration ability of GC cells was also significantly inhibited and the number of migratory cells was declined compared with si-con group. Our findings investigated that knockdown FNDC1 can repress the invasion and migration abilities of GC cells.
Figure 4.
Transwell assay was performed to evaluate the function of FNDC1 on cell invasion and migration. A, After knockdown FNDC1, the AGS cells invasion and migration abilities were downregulated compared with si-con group. B, The statistics about AGS cells invasion and migration. **P < .01. C, The MGC-803 cells invasion and migration abilities were measured by transwell after silencing FNDC1, and (D) is quantification of (C). **P < .01. Scale bars = 0.1 mm. FNDC1 indicates fibronectin type III domain containing 1.
Verified the Mechanism of FNDC1 on Phenotype
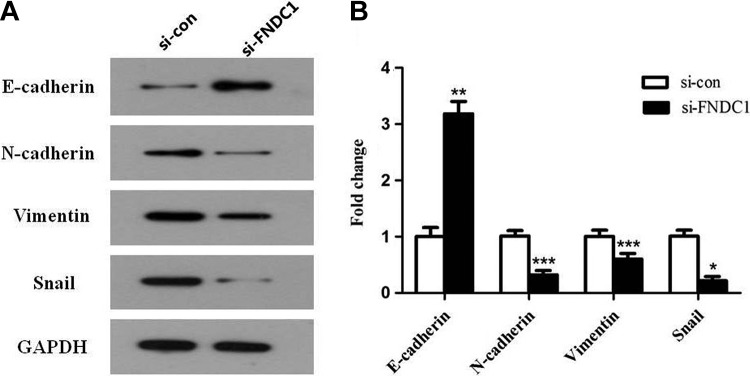
Recent studies have explored that disordered EMT in mature tissues has a vital impact on the development and progression of tumors.12 Epithelial–mesenchymal transition can promote tumor infiltration and metastasis and also cause tumor to escape apoptosis induced by some factors.13 The decrease or loss of E-cadherin is the most important landmark change of EMT, accompanied by the increase in N-cadherin. In contrast to E-cadherin, the increase in N-cadherin protein level is positively correlated with the degree of malignancy, invasion, and metastasis of various tumors and directly affects the prognosis of patients. The occurrence of EMT can significantly increase the expression level of Vimentin, an essential component of fibroblasts. By upregulating Snail transcription factor, EMT-producing cells can degrade extracellular matrix proteins and increase the invasion ability of tumor cells.
In this study, we found that after knockdown of FNDC1, the expression of E-cadherin was upregulated 3-fold, while N-cadherin, Vimentin, and Snail expression were significantly decreased in different degrees (Figure 5A and B, ***P < .001, **P < .01, *P < .05). These results indicated that knockdown of FNDC1 resulted in decrease in invasion and migration abilities of GC cells, maybe by inhibiting the occurrence of EMT.
Figure 5.
Using Western blotting to measure the related protein expression in the process of EMT. A, EMT-related key proteins E-cadherin, N-cadherin, Vimentin, and Snail were detected by Western blot assay after knockdown of FNDC1 compared with control. B, Western blot data were analyzed by software Quantity-One program with 3 independent repeats. *P < .05, **P < .01. EMT indicates epithelial–mesenchymal transition; FNDC1, fibronectin type III domain containing 1.
Discussion
Gastric cancer has become a major threat to human health due to its difficulty in early detection and poor prognosis.14 It is reported that FN is a known tumorigenic regulator and plays an important role in cell proliferation, migration, and apoptosis.15 The structural domain of FN has been found in many molecules. Acknowledgedly, FNDC1 is recognized as an important structural domain of FN, whereas its biological function remains to be further studied.16 Fibronectin type III domain containing 1, also known as AGS8 (activator of G-protein signaling8), has been reported to be expressed in many organizations, such as in the heart, kidney, ovary, and thyroid gland.17 Based on previous studies, increasing attention has been paid to associations between FNDC1 and tumor. To comprehend, the role of FNDC1 in the pathogenesis of GC, such as the differential expression of FNDC1 at various stages of GC, may contribute to speculate its application as a prognostic biomarker or a possible therapeutic target.
Due to the important structure of FNDC1, more and more researches have been done on it. Some researchers found that FNDC1 was involved in vascular epidermal growth factor–mediated signal processing during angiogenesis.18 Meanwhile, FNDC1 may mediate the G-protein signaling pathway and hold a crucial function in hypoxic-induced apoptosis of myocardial cells.8 A strong relationship between FNDC1 and prostate cancer was confirmed by determining the significant overexpression of FNDC1 in prostate cancer cells and tissues, and the overexpression of FNDC1 positively correlated with invasiveness in Das and Ogunwobi’s report.19 According to the above studies, we speculated that FNDC1 was closely related to the occurrence of cancer, as well as the proliferation, migration, and invasion abilities of cancer cells. The present study indicated that FNDC1 was highly expressed in GC tissues and AGS cells; knockdown of FNDC1 by siRNA can inhibit GC cells proliferation, migration, and invasion; based on these researches, we regard FNDC1 as a hopeful biomarker for GC.
Gastric cancer, as a kind of malignant tumor with high morbidity and mortality, can be treated with surgery and chemotherapy, although the prognosis is still a complicated problem.20 The prognosis of GC is related to many factors, such as gender, age, Borrmann type, degree of differentiation of tumor cells, and lymph node metastasis.21 Many studies about cancer in recent years have gradually shown that active and effective comprehensive treatment can bring a better prognosis.22 Recent research has shown that plasminogen Kringle 5 via regulating HIF-1α and GRP78 suppresses GC and has a positive prognostic effect; however, the research is not very complete, prompting us to explore more potential molecules as therapeutic targets. Thus, in this study, we have reported that high expression of FNDC1 was associated with poor prognosis of patients with GC. In our research, overexpression of FNDC1 was found in GC tissues compares with control samples from database; notably, its overexpression was closely linked with the shorter OS of patients with GC. On the basis of previous researches, we found that high expression of FNDC1 plays an important role in indicating the prognosis of GC, but how this molecule affects the prognosis and what role it plays in the occurrence and development of GC remains to be confirmed. This study was only a preliminary exploration of the relationship between FNDC1 and GC, and our study only focused on the in vitro level, what will happen after losing FNDC1 in patients with GC remains unclear. Further experiments are needed to demonstrate the molecular mechanism of FNDC1 expression in GC. In addition, how the changes in FNDC1 level in patients with GC after treatment was also not involved in our study. In order to obtain more authoritative experimental conclusions and devote ourselves to the application of clinical trials, in vivo experiments are indispensable and also the direction of our future efforts.
Recent studies have reported that abnormal initiation of EMT plays an important role in the development of various tumors, including GC.23 Epithelial–mesenchymal transition is responsible for the change of cell phenotype, the loss of cell polarity, and the acquisition of migration and invasion during the occurrence of cancer. These phenotype changes play an important role in the development of tumors.24 In the process of cancer formation, the lack of E-cadherin expression is considered to be a key step of EMT in the process of cancer development.25 Our study also found that the expression of FNDC1 had a certain impact on several key proteins in the development of EMT. After knockdown FNDC1, invasion and migration abilities of GC cells were significantly repressed. Then, given that EMT is strongly linked with cancer invasion and migration, we detected EMT hallmarks, including E-cadherin, N-cadherin, Vimentin, and Snail through Western blot analysis. The expression of E-cadherin increased significantly, while the expression of Vimentin, N-cadherin, and Snail positively correlated with the occurrence of EMT downregulated to varying degrees. The results observed that FNDC1 inhibited the invasion and migration abilities of GC cells may be by blocking EMT, but the specific mechanism remains to be further explored.
In summary, we found that FNDC1 was highly expressed in GC tissues or cells compared with normal samples. Knockdown of FNDC1 could inhibit the proliferation of GC cells, reduce their invasion and migration, and downregulate the expression of several important proteins that were positively correlated with the occurrence of EMT, while the negative correlation protein was increased. Meanwhile, high expression of FNDC1 has poor prognosis in GC. Therefore, we speculated that FNDC1 played an important role in the occurrence and development of GC. In conclusion, FNDC1 may be a new candidate diagnostic indicator for GC and also provides a theoretical basis for the selection of therapeutic targets in the further clinical exploration.
Abbreviations
- CCK-8
Cell Counting Kit-8
- EGF
epidermal growth factor
- EMT
epithelial–mesenchymal transition
- FN
fibronectin
- FNDC1
fibronectin type III domain containing 1
- GC
gastric cancer
- OS
overall survival
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- qRT-PCR
quantitative real-time polymerase chain reaction
- si-FNDC1
silenced FNDC1
- siRNA
small interfering RNA
- TCGA
The Cancer Genome Atlas
Footnotes
Authors’ Note: Authors regard Wei-Da Chen as the first-common author and Wen-Na Li as the second author. Wei-Da Chen and Wen-Na Li have made great contributions. Yan-Peng Liu and Wei-Da Chen contributed equally. This study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yan-Peng Liu  https://orcid.org/0000-0001-9542-1227
https://orcid.org/0000-0001-9542-1227
References
- 1. Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. [DOI] [PubMed] [Google Scholar]
- 2. Wroblewski LE, Peek RM., Jr Helicobacter pylori, cancer, and the gastric microbiota. Adv Exp Med Biol. 2016;908:393–408. [DOI] [PubMed] [Google Scholar]
- 3. Li L, Fu LQ, Wang HJ, Wang YY. CAP2 is a valuable biomarker for diagnosis and prognostic in patients with gastric cancer. Pathol Oncol Res. 2018. [DOI] [PubMed] [Google Scholar]
- 4. Sun D, Xu H, Huang J. . Prognostic factors of lymph node-negative metastasis gastric cancer [in Chinese]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20(2):190–194. [PubMed] [Google Scholar]
- 5. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo Z, Wang Q, Lau WB, et al. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett. 2016;377(2):174–182. [DOI] [PubMed] [Google Scholar]
- 7. van Ingen G, Li J, Goedegebure A, et al. Genome-wide association study for acute otitis media in children identifies FNDC1 as disease contributing gene. Nat Commun. 2016;7:12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato M, Jiao Q, Honda T, et al. Activator of G protein signaling 8 (AGS8) is required for hypoxia-induced apoptosis of cardiomyocytes: role of G betagamma and connexin 43 (CX43). J Biol Chem. 2009;284(45):31431–31440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Achim B, Diana B, Weber RS, El-Naggar AK. CpG island methylation profiling in human salivary gland adenoid cystic carcinoma. Cancer. 2011;117(13):2898–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das DK, Naidoo M, Ilboudo A, et al. miR-1207-3p regulates the androgen receptor in prostate cancer via FNDC1/fibronectin. Exp Cell Res. 2016;348(2):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhong M, Zhang Y, Yuan F, et al. High FNDC1 expression correlates with poor prognosis in gastric cancer. Exp Ther Med. 2018;16(5):3847–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeung KT, Yang J. Epithelial–mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11(1):28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H, Xu F, Li S, Zhong A, Meng X, Lai M. The tumor microenvironment: an irreplaceable element of tumor budding and epithelial–mesenchymal transition-mediated cancer metastasis. Cell Adh Migr. 2016;10(4):434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(5). doi:10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 15. Cao Y, Liu X, Lu W, et al. Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett. 2015;360(1):141–145. [DOI] [PubMed] [Google Scholar]
- 16. Gao M, Craig D, Lequin O, Campbell ID, Vogel V, Schulten K. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci U S A 2003;100(25):14784–14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sato M, Cismowski MJ, Toyota E, et al. Identification of a receptor-independent activator of G protein signaling (AGS8) in ischemic heart and its interaction with G betagamma. Proc Natl Acad Sci U S A. 2006;103(3):797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayashi H, Al Mamun A, Sakima M, Sato M. Activator of G-protein signaling 8 is involved in VEGF-mediated signal processing during angiogenesis. J Cell Sci. 2016;129(6):1210–1222. [DOI] [PubMed] [Google Scholar]
- 19. Das DK, Ogunwobi OO. A novel microRNA-1207-3p/FNDC1/FN1/AR regulatory pathway in prostate cancer. RNA Dis. 2017;4(1):e1503. [PMC free article] [PubMed] [Google Scholar]
- 20. Cho JY, Lim JY, Cheong JH, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17(7):1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao R, Wang X, Lan X, Li M. Clinicopathologic characteristics and prognosis of gastric hepatoid adenocarcinoma [in Chinese]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20(9):1035–1039. [PubMed] [Google Scholar]
- 22. Meinhold-Heerlein I, Hauptmann S. The heterogeneity of ovarian cancer. Arch Gynecol Obstet. 2014;289(2):237–239. [DOI] [PubMed] [Google Scholar]
- 23. Peng Z, Wang CX, Fang EH, Wang GB, Tong Q. Role of epithelial–mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. 2014;20(18):5403–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu J, Liu D, Niu H, et al. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial–mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res. 2017;36(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fu DG. Epigenetic alterations in gastric cancer (review). Mol Med Rep. 2015;12(3):3223–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]