Abstract
Background:
The objective of this study was to evaluate the efficacy and safety of repeated low-dose rituximab treatment guided by monitoring circulating CD19+ B cells in patients with refractory myasthenia gravis (MG).
Methods:
Patients with refractory MG who had received rituximab treatment at two teaching hospitals between September 2013 and January 2017 were reviewed retrospectively. The treatment protocol consisted of an induction treatment with low-dose rituximab (375 mg/m2 twice with a 2-week interval), followed by retreatment (375 mg/m2 once). Retreatment was based on either circulating CD19+ B-cell repopulation or clinical relapse. Outcome measures included the MG Foundation of America (MGFA) clinical classification and postintervention status, prednisolone dose, CD19+ B-cell counts, clinical relapse, and adverse effects.
Results:
Of 17 patients, 11 (65%) achieved the primary endpoint, defined as the minimal manifestation or better status with prednisolone ⩽5 mg/day, after median 7.6 months (range, 2–17 months) following rituximab treatment. Over a median follow up of 24 months (range, 7–49 months), a total of 30 retreatments were undertaken due to clinical relapse without B-cell repopulation (n = 6), on the basis of B-cell repopulation alone (n = 16) and both (n = 8). B-cell recovery appeared to be in parallel with clinical relapse on the group level, although the individual-level association appeared to be modest, with B-cell repopulation observed only at 57% (8/14) of clinical relapses.
Conclusions:
The repeated low-dose rituximab treatment based on the assessment of circulating B-cell depletion could be a cost-effective therapeutic option for refractory MG. Further studies are needed to verify the potentially better cost-effectiveness of low-dose rituximab, and to identify biomarkers that help optimize treatment in MG patients.
Keywords: neuromuscular junction disorders, refractory myasthenia gravis, rituximab
Introduction
Autoimmune myasthenia gravis (MG) is a neuromuscular junction disorder, mostly caused by autoantibodies to the nicotinic acetylcholine receptor (AChR) or muscle-specific tyrosine kinase (MuSK).1 Standard pharmacotherapy includes acetylcholine esterase inhibitors, corticosteroids, and nonsteroidal immunosuppressive agents, including azathioprine, mycophenolate mofetil, methotrexate, cyclosporine, and tacrolimus.2 Extended trans-sternal thymectomy was recently demonstrated in a randomized controlled trial to improve clinical outcomes in nonthymomatous generalized AChR MG.3 Although MG is usually treated effectively with standard therapy, a proportion of patients (10–15% from tertiary referral clinics) have very difficult-to-control disease.4,5 Treatment options are limited for refractory MG, and may include chronic intravenous immunoglobulin (IVIG) or plasma exchange (PLEX), high-dose cyclophosphamide, rituximab, and eculizumab (C5 inhibitor).6–8
B cells are major effector cells in autoimmunity, and B-cell targeted therapy has emerged as a promising approach to treat autoimmune diseases.9,10 Rituximab, a chimeric monoclonal antibody directed against CD20, is the most-used B-cell-depleting agent, and its use is approved in non-Hodgkin’s lymphoma, rheumatoid arthritis (RA), and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis.11–14 In MG, case reports and retrospective studies have demonstrated the efficacy and safety of rituximab.7,15–20 The optimal dosing, however, has not been determined, and the most commonly used regimen was that adopted from non-Hodgkin’s lymphoma (375 mg/m2 weekly for 4 weeks).21,22 Emerging evidence suggests that low-dose rituximab may be noninferior in efficacy compared with the conventional high-dose in autoimmune conditions, including RA and pemphigus.23–25 Two MG research groups have also observed the potential benifits of low-dose rituximab in retrospective observational studies.15,20 Given the reduced costs and possibly lower risk of adverse effects, further studies are warranted to elucidate the variability and durability of response in patients treated with low-dose rituximab.
In previous MG studies, a single cycle of rituximab treatment did not appear to be sufficient to induce sustained clinical remission.21,22 Repeated rituximab infusions are often required as maintenance therapy in relapsing neuroinflammatory diseases, and usually administered at 6-month intervals.26 This dosing schedule is based on data obtained from RA, but has not been validated in MG. Clinical response to rituximab correlates with the degree of B-cell depletion, and the circulating B-cell population may be linked to increased disease activity.27–29 Importantly, the degrees and durations of B-cell depletion are highly variable in different individuals following rituximab treatment.27,30,31 Therefore, an individualized approach to retreatment with rituximab based on the assessment of circulating B cells could optimize its efficacy while limiting costs and adverse effects. This study was performed to assess the long-term clinical efficacy and safety of repeated treatment with low-dose rituximab in patients with refractory MG, and also to evaluate the correlation between circulating B-cell depletion and clinical response.
Methods
Standard protocol approvals
This study was approved by the Institutional Review Boards and the Pharmacy and Therapeutic committees at two university-affiliated teaching hospitals (Seoul National University Hospital, and Seoul Metropolitan Government Boramae Medical Center), and also by the Health Insurance Review and Assessment Service in South Korea (H-1612-138-821, No. 30-2018-36). Written informed consent was obtained from all patients.
Patient characteristics
Patients with refractory MG who had been treated with low-dose rituximab between September 2013 and January 2017 were included in this study. MG was diagnosed based on the clinical features of exertional weakness, and laboratory tests including repetitive nerve stimulation, autoantibodies against AChR or MuSK, and, in cases of double seronegative MG, pharmacological test and exclusion of other diagnoses. Patients were defined as having refractory MG if they had moderate-to-severe weakness, the Myasthenia Gravis Foundation of America (MGFA) clinical classification class III or worse despite conventional immunosuppressive treatment with prednisolone, plus one or more immunosuppressive agents over at least 1 year.32 Standard therapy involved oral prednisolone and adjunctive steroid-sparing immunosuppressive agents, including azathioprine, tacrolimus, and mycophenolate mofetil. Thymectomy was offered as an option in nonthymomatous generalized MG patients with antibodies to AChR. IVIG or PLEX was used for acute exacerbations, crisis, or preoperatively before thymectomy.
Rituximab dosing regimen
Treatment protocol consisted of an induction cycle with low-dose rituximab (375 mg/m2 twice with a 2-week interval) followed by additional single infusions (375 mg/m2 once) as indicated (Figure 1). The retreatment with low-dose rituximab was based on either circulating B-cell repopulation or clinical relapse. The B-cell repopulation was defined as an increase of the circulating CD19+ B-cell proportion to >1% of total lymphocytes. Clinical relapse was defined as significant clinical worsening, as judged by treating neurologists (YH Hong and JJ Sung). The circulating CD19+ B cells and MGFA postintervention status (PIS) were monitored on a regular basis (1 month after induction therapy, and thereafter at least every 3 months).
Figure 1.
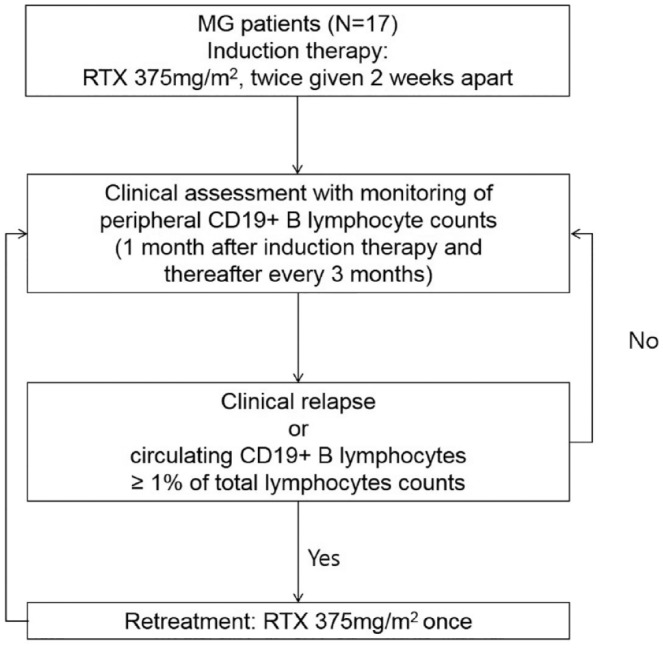
Repeated low-dose rituximab treatment protocol.
Concerning the risk of Pneumocystis pneumonia (PcP), there is no guidance on the use of prophylactic antibiotics for MG patients. Trimethoprim/sulfamethoxazole (TMP/SMX) were provided at the discretion of the treating physicians, based on individual risk factors such as prolonged high-dose steroid therapy and simultaneous use of other immunosuppressive agents (azathioprine, tacrolimus, and mycophenolate mofetil).
Adjudicated endpoints
The primary endpoint was to achieve the MGFA-PIS minimal manifestation or better status with low-dose prednisolone (⩽5 mg per day).33 Patients were monitored for abnormal infusion reactions and adverse effects of rituximab. Oral prednisolone was gradually tapered in steps with clinical improvement. Adjuvant steroid-sparing immunosuppressants were maintained in all patients.
Statistical analysis
Descriptive summaries are presented as frequency, proportion, median, and range as appropriate. The time-weighted average prednisolone dose (mg/day) was calculated for each 4-week period between weeks –16 and 24; four and six time bins before and after rituximab treatment, respectively. The time-weighted average dose over each 4-week period was chosen to reflect inter and intraindividual variations in the interval and degree of dosage changes. One-way repeated measures analysis of variance (ANOVA) was conducted to analyze the effect of rituximab on the time-weighted average prednisolone dose. Kaplan–Meyer curves for cumulative proportions of achieving the primary endpoint were estimated in AChR and MuSK-MG patient groups, and compared using the log-rank test. Kaplan–Meyer analysis was also performed to assess the effect of rituximab on B-cell repopulation and clinical relapse separately, with the other event treated as censoring; p < 0.05 was considered to indicate statistical significance. All statistical analyses were performed with R version 3.4.3.34
Results
Patient characteristics
Of 17 patients (11 women, 6 men; mean age 51 years), 9 were positive for AChR antibody, 6 for MuSK antibody, and the other 2 were negative for both antibodies (Table 1). Median disease duration (time from diagnosis to rituximab treatment) was 10 years (range, 3–26 years). MG severity at the initiation of rituximab treatment corresponded to the MGFA class III in nine patients, IV in five, and V in three. Three patients had been in myasthenic crisis, and treated with rituximab after 2–4 weeks following IVIG or PLEX (patients nos. 3, 16, and 17). All patients were taking oral prednisolone and adjunctive immunosuppressive agents when initiating rituximab treatment; tacrolimus in 11 patients, mycophenolate mofetil in 4, and azathioprine in 2. A total of 13 patients had been treated with IVIG (1 patient on a regular basis), and 9 with PLEX due to either acute exacerbation or crisis. Thymectomy was performed only in the four patients with thymoma.
Table 1.
Clinical features, previous treatments and clinical outcomes of rituximab treatment.
| Patient number | Sex | Age | Disease duration (years) | Antibodies | MGFA class (worst) | MGFA class (at the start of rituximab treatment) | Previous treatment | Follow-up duration (months) | MGFA PIS | Time to achieving primary endpoint (months) | Relapse (Number of relapses) | Ongoing treatment after RTX | Number of RTX retreatments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 73 | 3 | AChR | IVa | IIIa | PD, TAC, IVIG | 25 | PR | 9 | Yes (1) | PD, TAC | 2 |
| 2 | M | 50 | 23 | AChR | IVb | IIIb | PD, TAC, IVIG | 7 | MM | – | No | PD, TAC | 0 |
| 3 | F | 75 | 16 | AChR | V | V | PD, TAC, IVIG, PLEX, SPT | 31 | Improved | – | No | TAC | 0 |
| 4 | F | 64 | 19 | DN | IVb | IVb | PD, AZA, IVIG, PLEX | 49 | PR | 3 | No | PD, AZA | 4 |
| 5 | F | 41 | 5 | AChR | V | IVb | PD, MMF, IVIG, PLEX, SPT | 34 | Died | – | Yes (3) | MMF | 3 |
| 6 | M | 51 | 26 | AChR | V | IVb | PD, TAC, IVIG, PLEX, SPT | 19 | MM | 7 | Yes (1) | TAC | 1 |
| 7 | M | 57 | 17 | AChR | IVb | IVb | PD, TAC, IVIG, PLEX, SPT | 28 | MM | 17 | Yes (1) | TAC | 1 |
| 8 | F | 34 | 11 | AChR | IVb | IIIb | PD, TAC, IVIG, PLEX | 25 | PR | 6 | Yes (1) | PD, TAC | 2 |
| 9 | M | 38 | 6 | DN | IVb | IIIb | PD, TAC | 13 | MM | 3 | Yes (2) | PD, TAC | 2 |
| 10 | F | 41 | 11 | MuSK | IVb | IIIb | PD, AZA | 34 | PR | 6 | No | AZA | 2 |
| 11 | F | 46 | 7 | AChR | IVa | IIIa | PD, MMF | 19 | Improved | – | No | MMF | 2 |
| 12 | F | 60 | 7 | MuSK | IVb | IVb | PD, MMF, IVIG, PLEX | 40 | PR | 12 | No | PD, MMF | 4 |
| 13 | M | 41 | 7 | MuSK | IVb | IIIb | PD, MMF | 22 | MM | 2 | Yes (1) | MMF | 1 |
| 14 | F | 22 | 4 | AChR | IVb | IIIb | PD, TAC, IVIG | 22 | PR | 17 | No | TAC | 2 |
| 15 | F | 63 | 3 | MuSK | IVa | IIIa | PD, TAC, IVIG | 7 | MM | – | No | PD, TAC | 0 |
| 16 | F | 32 | 4 | MuSK | V | V | PD, TAC, IVIG, PLEX | 13 | PR | 2 | Yes (1) | TAC | 1 |
| 17 | M | 71 | 4 | MuSK | V | V | PD, TAC, IVIG(c), PLEX | 28 | Improved | – | Yes (3) | TAC | 3 |
AChR, acetylcholine receptor; AZA, azathioprine; DN, double seronegative; F, Female; IVIG, intravenous immunoglobulin; IVIG(c) IVIG therapy, chronic on a regular basis; M, male; MM, minimal manifestation; MMF, mycophenolate mofetil; MuSK, muscle-specific tyrosine kinase; MGFA, Myasthenia Gravis Foundation of America; PD, oral prednisolone; PLEX, plasma exchange; PR, pharmacologic remission; RTX, rituximab; SPT, status post-thymectomy; TAC, tacrolimus.
The thymus of patient 15 was not assessed.
Clinical outcome
A total of 11 patients (65%) achieved the primary endpoint (4 of 6 MuSK MG, 5 of 9 AChR MG, and 2 double seronegative); 7 patients reached remission, and 4 minimal manifestation status with prednisolone dose ⩽5 mg/day (Table 1). Nine patients reached the primary endpoint after an induction cycle of rituximab, while two patients had to receive one additional infusion (retreatment) due to B-cell repopulation before achieving the primary endpoint.
The median time to the primary endpoint was 7.6 months (range 2–17 months) following rituximab treatment. Two patients improved to minimal manifestation status, but prednisolone doses could not be lowered below 20 mg per day without worsening of weakness (patients nos. 2 and 15). Three patients also improved to varying degrees, albeit not to remission or minimal manifestation status (patients nos. 3, 11, and 17). The other one patient died due to complications of invasive thymoma for which the patient underwent thymectomy 1 year before rituximab treatment.
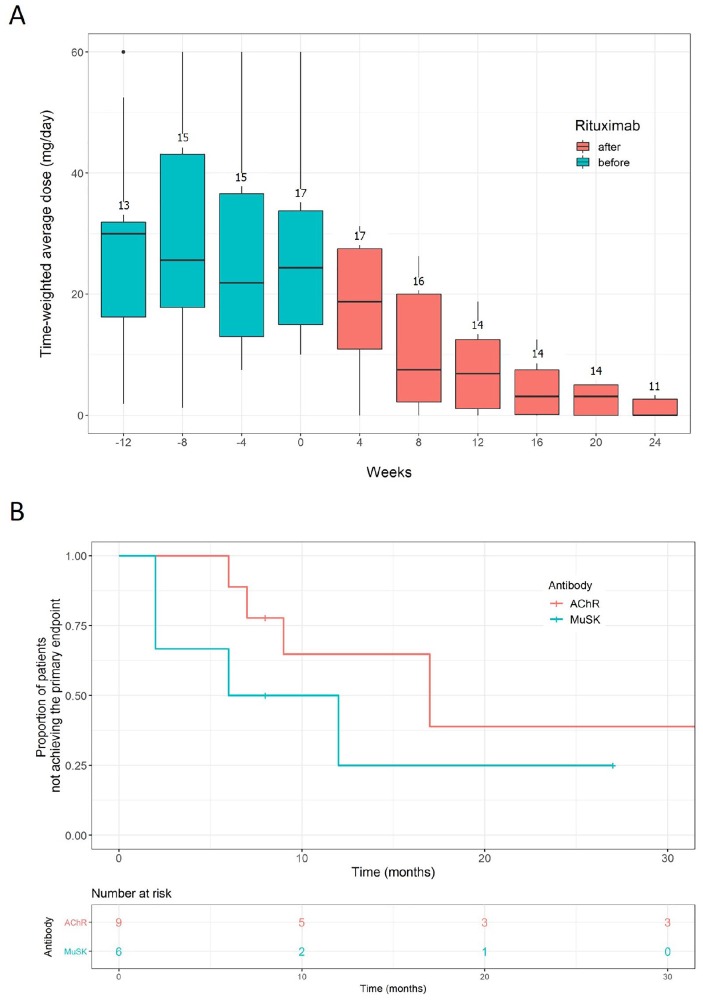
The time-weighted average prednisolone dose did not change over the 4 month period before rituximab treatment, but was significantly reduced over the next 6 months following rituximab induction treatment; from 28 mg/day (SD 17 mg/day, for the first 4-week after rituximab) to 7.5 mg/day (SD 6.8 mg/day, for the last 4-week period) (one-way repeated measures ANOVA, p < 0.001, Figure 2a). Although the primary endpoint was not significantly different between AChR and MuSK MG patients, rituximab treatment tended to be more effective in patients with MuSK MG compared with AChR MG (Figure 2b). There was no significant difference between groups in the baseline characteristics, including age, sex, disease duration, thymoma, disease severity, prednisolone dose, and the use of immunosuppressants.
Figure 2.
Changes of prednisolone dose (a) and the Kaplan–Meier curves for achieving the primary endpoint in AChR and MuSK MG patients following repeated treatment with low-dose rituximab (b, log-rank test, p = 0.3). The dose of prednisolone was calculated as time-weighted average over the 4 weeks preceding the depicted time points. The numbers above the boxes represent the number of patients from whom the time-weighted average prednisolone dose was calculated for each time period.
AChR, acetylcholine receptor; MG, myasthenia gravis; MuSK, muscle-specific tyrosine kinase.
In three patients (patients nos. 3, 16, and 17), rituximab was administered 2–4 weeks after IVIG treatment for MG crisis, and glucocorticoids were rapidly tapered-off over a couple of months because of intolerable side effects. The acute rescue therapy could explain a short-term symptom improvement, but probably not the long-term maintenance of remission or improved clinical status observed in these patients.
Retreatment
Over a median follow up of 24 months (range, 7–49 months), a total of 30 retreatments were undertaken in 14 patients. Retreatments were given due to clinical relapse without B-cell repopulation (n = 6), on the basis of B-cell repopulation alone (n = 16) and both (n = 8); four retreatments were undertaken in two patients, three retreatments in two patients, two retreatments in six patients, and one retreatment in four patients. There was no patient who did not receive retreatment with rituximab at B-cell repopulation, although retreatment was delayed for up to 3 months in some patients because of various reasons such as follow-up interval, laboratory turn-around time, and patients’ request. The median time interval was 10 months (range, 1–19 months) between the induction and first retreatment, 10.5 months (range, 6–15 months) between the first and second retreatments, 7.5 months (range, 6–15 months) between the second and third retreatments, and 12 months between the third and fourth retreatments. Retreatment with a single infusion of low-dose rituximab induced prompt B-cell depletion, and also led to clinical improvement in relapsing cases.
Correlation between circulating B-cell repopulation and clinical relapse
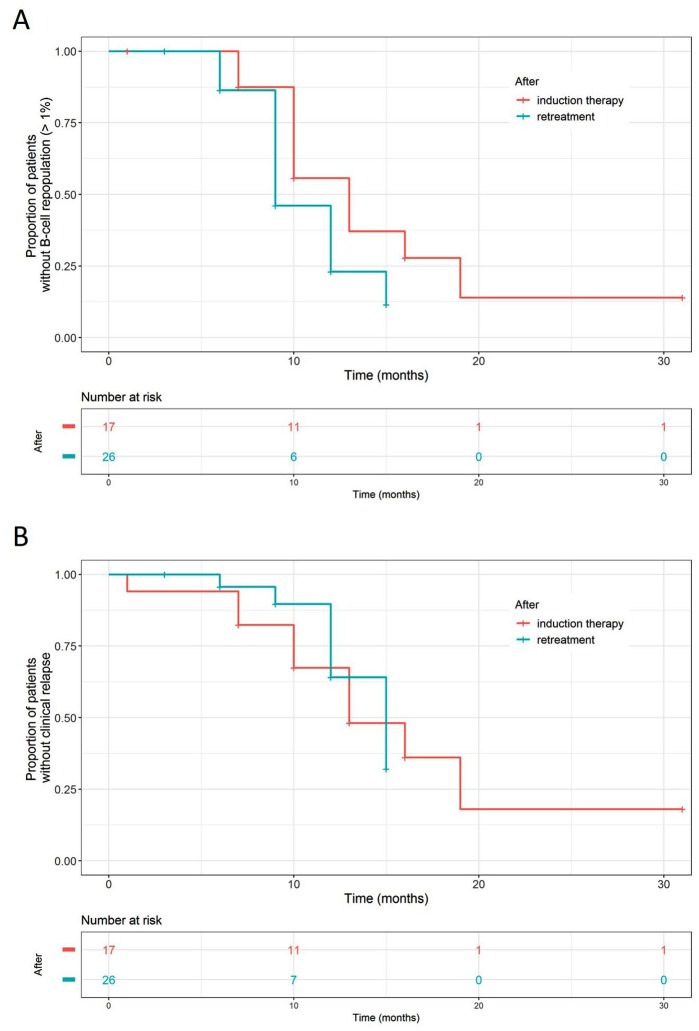
Monitoring at 1 month following initial treatment revealed substantial B-cell depletion in the peripheral blood from all patients tested (n = 15). To examine whether repeated treatments lead to more prolonged effects over time, Kaplan–Meier analysis was performed to assess the effects of rituximab on B-cell repopulation and clinical relapse. B-cell recovery appeared to occur earlier after retreatment than initial treatment, but the difference was not significant (median time to B-cell repopulation 9 months versus 13 months, p = 0.093, log-rank test, Figure 3a). The opposite trend was observed in the time to clinical relapse, with clinical relapse occurring earlier after initial treatment than retreatment (13 months versus 15 months, p = 0.76, log-rank test, Figure 3b).
Figure 3.
Kaplan–Meier curves for circulating CD19+ B-cell repopulation (a, log-rank test, p = 0.093) and clinical relapse (b, log-rank test, p = 0.76) following an induction treatment with low-dose rituximab (375 mg/m2 twice with a 2-week interval, depicted in red) and retreatment (375 mg/m2 once, in green). The outcome of clinical relapse and B-cell repopulation would be confounded by retreatment decision which was based on either event. To mitigate this confounding effect, when we analyzed the effect of rituximab on B-cell repopulation, the event of clinical relapse was treated as censoring. The same approach for the effect on clinical relapse with the event of B-cell repopulation being treated as censoring.
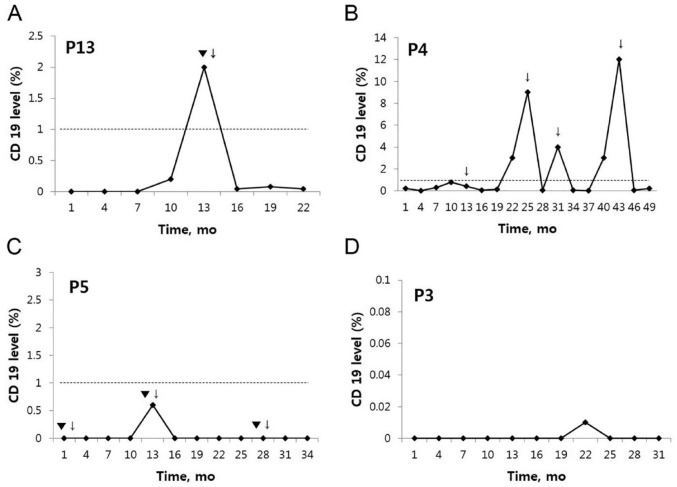
Of note, B-cell repopulation appeared to be in parallel with clinical relapse on the group level. However, the individual-level association appeared to be weak, with B-cell repopulation observed only at 57% (8/14) of clinical relapses. As illustrated in Figure 4, clinical relapse was not observed in some cases at the time of B-cell repopulation (Figure 4b), and the numbers of B cells were kept low at clinical relapse in others (Figure 4c). Intriguingly, an exceptionally prolonged B-cell depletion observed in one patient was sustained for 31 months following an induction treatment with low-dose rituximab (Figure 4d). The time data for clinical relapse, B-cell recovery and retreatment in all patients are depicted in the Supplemental Figure.
Figure 4.
Representative examples illustrating the associations between clinical relapse and the number of circulating CD19+ B cells (expressed as percentage relative to total lymphocytes). The cut-off level of B-cell repopulation was set at 1%. Retreatment was given preemptively at the time of B-cell repopulation, or at clinical relapse (▼: clinical relapse, ↓: retreatment). Clinical relapse was observed to occur typically with B-cell repopulation (as depicted in a), but the B-cell repopulation was not always associated with clinical relapse (as in b and c). An exceptionally prolonged B-cell depletion for 31 months in a patient following an induction therapy (d).
Adverse effects and safety
Two patients experienced infusion reactions, chest discomfort in one patient, and skin rash in the other. During follow up, one patient was affected by herpes zoster, and one patient died due to complications of invasive thymoma. Otherwise, there was no case with serious adverse events including severe infections other drug-related and laboratory abnormalities. Since immunoglobulin levels had not been checked routinely, we were not able to report on the incidence in our cohort of hypogammaglobulinemia, which might be associated with an increased risk of serious infections. We did not observe any worsening related to the use of prophylactic antibiotics (TMP/SMX) for PcP which was given to five patients (patients nos. 5, 7, 14, 16, and 17).
Discussion
This study provides support to the efficacy of low-dose rituximab in refractory MG for improving clinical outcomes and reducing the need for corticosteroid. Our results also suggest that repeated treatment based on the assessment of B-cell depletion in the peripheral blood could help to maintain clinical efficacy of rituximab with acceptable long-term safety profiles.
In the present study, 65% (11 of 17) of patients achieved the treatment goal, defined as MGFA minimal manifestation or better status with low dose prednisolone (⩽5 mg per day). Consistent with previous studies, the therapeutic effect of rituximab tended to be better in MuSK MG compared with AChR, with higher, albeit not statistically significant, response rate in MuSK MG (4/6) compared with AChR MG (5/9).21,22
There is great heterogeneity concerning rituximab dosing regimen; some protocols use fixed doses (as in RA), while others use body surface area (BSA)-based dosing (as in lymphoma). Furthermore, dosing frequency and interval for induction therapy varied across studies, which were usually either once weekly for 4 weeks (as in lymphoma) or twice separated by 2 weeks (as in RA). Our induction regimen is basically a composite of lymphoma and RA regimens; BSA-based dosing was adopted from lymphoma, while the dosing frequency and interval were adopted from RA. We assumed that a half-dose regimen might be noninferior to the full-dose, and also that BSA-based dosing might reflect better the interindividual pharmacokinetic variation than flat-fixed dosing.
As for the use of low dose rituximab, there have been different schemes in autoimmune diseases. In a study of ANCA-associated vasculitis, Takakuwa and colleagues defined two once-weekly doses of 375 mg/m2 as low-dose induction therapy.35 Jing and colleagues described 600 mg rituximab (100 mg on the 1st day and 500 mg on the 2nd day) as low-dose treatment for MG.20 In a study of Blum and colleagues, most MG patients received 1 g rituximab in two divided doses.15 Additionally, there are large cohorts of rituximab-treated patients with multiple sclerosis or neuromyelitis optica spectrum disorder. In a Swedish cohort, patients were treated with 500–1000 mg rituximab every 6–12 months, similar to a German cohort in which dosages ranged from 250 to 2000 mg.36,37
There is a large variation in reported response rates in the literature, which may be explained by variability of patient selection (MG subtypes and refractory MG) and the primary endpoint.21,22 Despite strict criteria for the primary endpoint in our study, the response rate is comparable with previous data (30–89%) from high-dose RTX treatment studies. To our knowledge, two MG research groups reported the efficacy of low-dose rituximab, with a response rate similar to ours.15,20 Taken together, it is likely that low-dose rituximab may be noninferior in clinical efficacy compared with high-dose treatment. This notion is supported by increasing evidence in other autoimmune diseases.23–25 For instance, low-dose (2 × 500 mg) rituximab is noninferior to high-dose (2 × 1000 mg) regimen for most clinical efficacy outcomes in RA.23,24 Pemphigus is another autoimmune condition for which low-dose RTX may have better cost-effectiveness.25
This study indicates that the initial efficacy of low-dose rituximab could not be retained, with 14 clinical relapses occurring in nine patients over a median follow up of 24 months. There are only a few studies addressing the issue of durability of rituximab response in MG patients.17,18 In a study by Diaz-Manera and colleagues, 6 of 10 AChR MG patients relapsed after 17 months on average following high-dose rituximab treatment (375 mg/m2 every week for 4 consecutive weeks followed by two monthly additional infusions).17 In another long-term study by Robeson and colleagues, clinical relapse occurred in 56% of patients after 3 years on average following 3–5 cycles of repeated treatment at 6-monthly intervals.18 Although the relapse rate in our cohort (53%, 9/17) is similar to previous observations, the durability of rituximab response seems to be different, with clinical relapse occurring much earlier in our cohort than previously reported. The discrepancy may be explained by differences in the definition of clinical relapse, corticosteroid tapering schedule and rituximab dosing regimen.38
Previous studies mostly described the short-term follow ups of patients treated with single or a couple of cycles of rituximab.21,22 There is no consensus on how to guide retreatment, which was based on clinical relapse or given at regular intervals (6 months) in previous MG studies. Repeat dosing based purely on clinical relapse, however, may reduce the therapeutic efficacy, as demonstrated in RA.39 On the other hand, treating at fixed intervals may lead to overtreatment in cases of sustained suppression of disease activity. Emerging evidence suggests that monitoring CD19 and CD27 positive B-cell depletion to guide retreatment allow for a lower dosing frequency and cumulative dose without apparent loss of clinical efficacy in patients with neuromyelitis optica.40–42
The present study demonstrates an overlap between the time to B-cell repopulation and to clinical relapse, suggesting a correlation between the two at the group level. Preemptive retreatment at the time of B-cell repopulation might have helped to maintain the efficacy of rituximab, preventing clinical relapse. Our results, however, indicate that B-cell repopulation, defined as CD19+ B cells more than 1% of total lymphocyte counts, may not be a good predictor of clinical relapse at the individual level, with B-cell repopulation observed only at 57% of clinical relapses. Further studies are warranted to identify other biomarkers, and promising approaches may include monitoring specific B-cell subsets such as transitional B cells, CD27+ memory B cells and plasmablasts, which may help to predict clinical relapse and guide optimal retreatment.43–45
There have been contradictory results in the literature regarding the change of AChR antibody levels after rituximab treatment and its association with clinical response. Blum and colleagues argued that serial measurements of AChR antibody levels were unhelpful for purposes of monitoring rituximab effect.15 Jing and colleagues also mentioned that AChR antibody levels were independent of clinical response and not influenced by rituximab.20 In contrast, Robeson and colleagues reported that anti-AChR antibody levels were decreased after cycles of rituximab in patients followed for 18–84 months, although no significant increase in the antibody levels was noted at the time of relapse.19 Because systematic monitoring of antibody levels were not part of the present study, we were unable to comment about the role of monitoring antibody levels in treating MG patients with rituximab. However, our anecdotal observations were consistent with earlier works in that even marked clinical response to rituximab may not accompany reduced autoantibodies.46 This may be explained by differences in the specificities of AChR antibodies and immunoglobulin subtypes, as well as differences in serum and tissue antibody concentrations.47
This study was limited by its uncontrolled design and retrospective analysis, which precluded comparison with other dosing regimens. A bias in selecting patients could not be completely ruled out, and laboratory parameters such as autoantibodies and immunoglobulin levels were not systematically monitored. Other limitations may include small sample size and the failure of serological confirmation in two double seronegative patients (e.g. anti-LRP4, anti-Agrin). Nonetheless, considering the limited data on the optimal rituximab dose and retreatment schedule, our results are encouraging and have therapeutic implications for refractory MG. Given the reduced costs and possibly lower risk of adverse effects, we propose that repeated treatment with low-dose rituximab guided by monitoring circulating B cells could be a cost-effective therapeutic option in patients with refractory MG. Further studies are needed to verify the potentially better cost-effectiveness of low-dose rituximab, and to identify biomarkers that help optimize treatment in MG patients.
Supplemental Material
Supplemental material, Supplemental_Figure for Repeated low-dose rituximab treatment based on the assessment of circulating B cells in patients with refractory myasthenia gravis by Kyomin Choi, Yoon-Ho Hong, So-Hyun Ahn, Seol-Hee Baek, Jun-Soon Kim, Je-Young Shin and Jung-Joon Sung in Therapeutic Advances in Neurological Disorders
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number; HI17C-0200-020017)
Conflict of interest statement: Kyomin Choi, Yoon-Ho Hong, So-Hyun Ahn, Seol-Hee Baek, Jun-Soo Kim and Je-Youn Shin and Jung-Joo Sung report no disclosures.
ORCID iD: Kyomin Choi  https://orcid.org/0000-0001-9730-3363
https://orcid.org/0000-0001-9730-3363
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kyomin Choi, Department of Neurology, Konkuk University School of Medicine, Konkuk University Medical Center, Seoul, Republic of Korea.
Yoon-Ho Hong, Department of Neurology, Neuroscience Research Institute, Seoul National University Medical Research Council, Seoul National University College of Medicine, Seoul Metropolitan Boramae Medical Center, Seoul, Republic of Korea.
So-Hyun Ahn, Department of Neurology, Hallym University College of Medicine, Kangnam Sacred Heart Hospital, Seoul, Republic of Korea.
Seol-Hee Baek, Department of Neurology, Korea University College of Medicine, Korea University Medical Center, Seoul, Republic of Korea.
Jun-Soon Kim, Department of Neurology, Seoul National University Bundang Hospital, Seongnam, Republic of Korea.
Je-Young Shin, Department of Neurology, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Republic of Korea.
Jung-Joon Sung, Department of Neurology, Neuroscience Research Institute, Seoul National University Medical Research Council, Seoul National University College of Medicine, Seoul National University Hospital, 28 Yeongeon-dong, Jongno-gu, Seoul, 03080, Republic of Korea.
References
- 1. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015; 14: 1023–1036. [DOI] [PubMed] [Google Scholar]
- 2. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology 2016; 87: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016; 375: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suh B, Goldstein JM, Nowak RJ. Clinical characteristics of refractory myasthenia gravis patients. Yale J Biol Med 2013; 86: 255–260. [PMC free article] [PubMed] [Google Scholar]
- 5. Mantegazza R, Antozzi C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disord 2018; 11: 175628561774913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drachman DB, Jones RJ, Brodsky RA. Treatment of refractory myasthenia: “rebooting” with high-dose cyclophosphamide. Ann Neurol 2003; 53: 29–34. [DOI] [PubMed] [Google Scholar]
- 7. Zebardast N, Patwa HS, Novella SP, et al. Rituximab in the management of refractory myasthenia gravis. Muscle Nerve 2010; 41: 375–378. [DOI] [PubMed] [Google Scholar]
- 8. Howard JF, Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 2017; 16: 976–986. [DOI] [PubMed] [Google Scholar]
- 9. Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev 2010; 237: 264–283. [DOI] [PubMed] [Google Scholar]
- 10. Musette P, Bouaziz JD. B cell modulation strategies in autoimmune diseases: new concepts. Front Immunol 2018; 9: 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrison AM, Thalji NM, Greenberg AJ, et al. Rituximab for non-Hodgkin’s lymphoma: a story of rapid success in translation. Clin Trans Sci 2014; 7: 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy. Arthritis Rheum 2006; 54: 2793–2806. [DOI] [PubMed] [Google Scholar]
- 13. Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013; 369: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blum S, Gillis D, Brown H, et al. Use and monitoring of low dose rituximab in myasthenia gravis. J Neurol Neurosurg Psychiatry 2011; 82: 659–663. [DOI] [PubMed] [Google Scholar]
- 16. Nowak RJ, DiCapua DB, Zebardast N, et al. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther Adv Neurol Disord 2011; 4: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Díaz-Manera J, Martínez-Hernández E, Querol L, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology 2012; 78: 189–193. [DOI] [PubMed] [Google Scholar]
- 18. Anderson D, Phan C, Johnston WS, et al. Rituximab in refractory myasthenia gravis: a prospective, open-label study with long-term follow-up. Ann Clin Transl Neurol 2016; 3: 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robeson KR, Kumar A, Keung B, et al. Durability of the rituximab response in acetylcholine receptor autoantibody-positive myasthenia gravis. JAMA Neurol 2016; 06520: 60–66. [DOI] [PubMed] [Google Scholar]
- 20. Jing S, Song Y, Song J, et al. Responsiveness to low-dose rituximab in refractory generalized myasthenia gravis. J Neuroimmunol 2017; 311: 14–21. [DOI] [PubMed] [Google Scholar]
- 21. Iorio R, Damato V, Alboini PE, et al. Efficacy and safety of rituximab for myasthenia gravis: a systematic review and meta-analysis. J Neurol 2015; 262: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 22. Tandan R, Hehir MK, Waheed W, et al. Rituximab treatment of myasthenia gravis: a systematic review. Muscle Nerve 2017; 56: 185–196. [DOI] [PubMed] [Google Scholar]
- 23. Bredemeier M, De Oliveira FK, Rocha CM. Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2014; 66: 228–235. [DOI] [PubMed] [Google Scholar]
- 24. Bredemeier M, Campos GG, de Oliveira FK. Updated systematic review and meta-analysis of randomized controlled trials comparing low- versus high-dose rituximab for rheumatoid arthritis. Clin Rheumatol 2015; 34: 1801–1805. [DOI] [PubMed] [Google Scholar]
- 25. Wang HH, Liu CW, Li YC, et al. Efficacy of rituximab for pemphigus: a systematic review and meta-analysis of different regimens. Acta Derm Venereol 2015; 95: 928–932. [DOI] [PubMed] [Google Scholar]
- 26. Whittam DH, Tallantyre EC, Jolles S, et al. Rituximab in neurological disease: principles, evidence and practice. Pract Neurol. Epub ahead of print 29 November 2018. DOI: 10.1136/practneurol-2018-001899. [DOI] [PubMed] [Google Scholar]
- 27. Vital EM, Rawstron AC, Dass S, et al. Reduced-dose rituximab in rheumatoid arthritis: efficacy depends on degree of B-cell depletion. Arthritis Rheum 2011; 63: 603–608. [DOI] [PubMed] [Google Scholar]
- 28. Trouvin AP, Jacquot S, Grigioni S, et al. Usefulness of monitoring of B cell depletion in rituximab-treated rheumatoid arthritis patients in order to predict clinical relapse: a prospective observational study. Clin Exp Immunol 2015; 180: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pellkofer HL, Krumbholz M, Berthele A, et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 2011; 76: 1310–1315. [DOI] [PubMed] [Google Scholar]
- 30. Leandro MJ, Cambridge G, Ehrenstein M, et al. Reconstitution of peripheral blood Bcells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 2006; 54: 613–620. [DOI] [PubMed] [Google Scholar]
- 31. Ellwardt E, Ellwardt L, Bittner S, et al. Monitoring B-cell repopulation after depletion therapy in neurologic patients. Neurol Neuroimmunol Neuroinflamm 2018; 5: e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaretzki A, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Ann Thorac Surg 2000; 70: 327–334. [DOI] [PubMed] [Google Scholar]
- 33. Utsugisawa K, Suzuki S, Nagane Y, et al. Health-related quality-of-life and treatment targets in myasthenia gravis. Muscle Nerve 2014; 50: 493–500. [DOI] [PubMed] [Google Scholar]
- 34. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 35. Takakuwa Y, Hanaoka H, Kiyokawa T, et al. Low-dose rituximab as induction therapy for ANCA-associated vasculitis. Clin Rheumatol 2019; 38: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 36. Salzer J, Svenningsson R, Alping P, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology 2016; 87: 2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ellrichmann G, Bolz J, Peschke M, et al. Peripheral CD19(+) B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J Neurol 2019; 266: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cortés-Vicente E, Rojas-Garcia R, Díaz-Manera J, et al. The impact of rituximab infusion protocol on the long-term outcome in anti-MuSK myasthenia gravis. Ann Clin Transl Neurol 2018; 5: 710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Emery P, Mease PJ, Rubbert-Roth A, et al. Retreatment with rituximab based on a treatment-to-target approach provides better disease control than treatment as needed in patients with rheumatoid arthritis: a retrospective pooled analysis. Rheumatology 2011; 50: 2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim SH, Huh SY, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol 2013; 70: 1110–1117. [DOI] [PubMed] [Google Scholar]
- 41. Kim SH, Jeong IH, Hyun JW, et al. Treatment outcomes with rituximab in 100 patients with neuromyelitis optica: influence of FCGR3A polymorphisms on the therapeutic response to rituximab. JAMA Neurol 2015; 72: 989–995. [DOI] [PubMed] [Google Scholar]
- 42. Cohen M, Romero G, Bas J, et al. Monitoring CD27+ memory B-cells in neuromyelitis optica spectrum disorders patients treated with rituximab: results from a bicentric study. J Neurol Sci 2017; 373: 335–338. [DOI] [PubMed] [Google Scholar]
- 43. Roll P, Dörner T, Tony HP. Anti-CD20 therapy in patients with rheumatoid arthritis: predictors of response and b cell subset regeneration after repeated treatment. Arthritis Rheum 2008; 58: 1566–1575. [DOI] [PubMed] [Google Scholar]
- 44. Hall RP, Streilein RD, Hannah DL, et al. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. J Invest Dermatol 2013; 133: 2786–2788. [DOI] [PubMed] [Google Scholar]
- 45. Stathopoulos P, Kumar A, Nowak RJ, et al. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight 2017; 2: e94263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chan A, Lee DH, Linker R, et al. Rescue therapy with anti- CD20 treatment in neuroimmunologic breakthrough disease. J Neurol 2007; 254: 1604–1606. [DOI] [PubMed] [Google Scholar]
- 47. Meriggioli MN, Sanders DB. Muscle autoantibodies in myasthenia gravis: beyond diagnosis? Expert Rev Clin Immunol 2012; 8: 427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Figure for Repeated low-dose rituximab treatment based on the assessment of circulating B cells in patients with refractory myasthenia gravis by Kyomin Choi, Yoon-Ho Hong, So-Hyun Ahn, Seol-Hee Baek, Jun-Soon Kim, Je-Young Shin and Jung-Joon Sung in Therapeutic Advances in Neurological Disorders