Abstract
Background:
The benefits of palliative care (PC) in critical illness are validated across a range of diseases, yet it remains underutilized in surgical patients. This study analyzed patient and hospital factors predictive of PC utilization for elderly patients with colorectal cancer (CRC) requiring emergent surgery.
Methods:
The National Inpatient Sample was queried for patients aged ≥65 years admitted emergently with CRC from 2009–2014. Patients undergoing colectomy, enterectomy or ostomy formation were included and stratified according to documentation of PC consultation during admission. Chi-squared testing identified unadjusted group differences, and multivariable logistic regression identified predictors of PC.
Results:
Of 86,573 discharges meeting inclusion criteria, only 3,598 (4.2%) had PC consultation. Colectomy (86.6%) and ostomy formation (30.4%) accounted for the operative majority. PC frequency increased over time (2.9% in 2009 to 6.2% in 2014, P<0.001) and was nearly twice as likely to occur in the West compared with the Northeast (5.7% vs 3.3%, P<0.001) and in not-for-profit compared with proprietary hospitals (4.5% vs 2.3%, P<0.001). PC patients were more likely to have metastases (60.1% vs 39.9%, P<0.001) and die during admission (41.5% vs 6.4%, P<0.001). On multivariable logistic regression, PC predictors (P<0.05) included region outside the Northeast, increasing age, more recent year, and metastatic disease.
Conclusions:
In the U.S., PC consultation for geriatric patients with surgically-managed complicated CRC is low. Regional variation appears to play an important role. With mounting evidence that PC improves quality of life and outcomes, understanding the barriers associated with its provision to surgical patients is paramount.
Keywords: Palliative care, Colorectal neoplasms, Geriatrics, Emergency treatment
Introduction
Palliative care (PC) is a holistic treatment approach aimed at reducing suffering, preserving quality of life, and facilitating medical decisions and end of life planning for patients and families affected by serious illness. A 2014 World Health Organization (WHO) report approximated 19 million people worldwide in need of PC at the end of life, of which 13% were concentrated in the Americas. Ample evidence demonstrates that PC improves pain and symptom control, raises satisfaction with care, lengthens survival in select cohorts, and reduces intensive care unit stays, procedures, and health care costs across numerous disease processes.[1–3] Conservation of cost is of particular interest in the United States, where health care expenditures account for nearly a fifth of the Gross Domestic Product and are partially driven by individuals aged 65 years and older in the final year of life.[4] As a result of the benefits to patients, families, and health systems, multidisciplinary healthcare authorities including the WHO, Institute of Medicine, American Heart/Stroke Associations, American Geriatrics Society, and the National Comprehensive Cancer Network strongly recommend PC for the seriously ill.[1–6]
On the surface, the goals of PC appear to conflict with those of invasive surgery. Pain, reduced quality of life, and the need for serial procedures are often byproducts of major surgery. The disparity may be more pronounced in patients requiring urgent operations, who may unexpectedly convert from healthy to seriously ill or experience rapid progression of concomitant diseases.[7,8] Indeed, studies suggest that palliative and hospice care are less likely to be administered to patients who undergo major surgery in the final year of life.[9] The well-known Institute of Medicine report, Dying in America, found that from 2008–2012, the proportion of accredited PC specialist-surgeons was a miniscule 1%.[1,10] Enormous effort has been made over the last two decades to integrate these specialties in appropriate patient populations.[11–15]
National databases have previously been used to illuminate practice gaps in PC utilization for heart failure, stroke, cirrhosis, end-stage renal disease, and cancer.[16–23] To our knowledge, no such studies have been conducted on emergency surgical patients, specifically those with a poor prognosis who would be logical candidates for PC consultation. Colorectal cancer (CRC), a common indication for palliative treatment, manifests in surgical emergencies including malignant bowel obstruction and perforation in up to a third of cases. Morbidity and mortality in emergent resection exceeds those in elective resection, and for elderly patients, complication rates have been reported as high as 40–70% and in-hospital mortality as high as 30%.[13,24–31] The present study uses a large administrative dataset to investigate PC utilization practices in this relatively common high-risk surgical population for whom the use of palliative treatment is clearly warranted.
Methods
The Healthcare Cost and Utilization Project’s (HCUP) Nationwide Inpatient Sample (NIS) from 2009–2014 served as the primary data source for this study. Developed by the Agency for Healthcare Research and Quality (AHRQ), the NIS is an approximate 20% stratified cluster sample of acute-care hospitalizations from 44 states and is the largest publicly available all-payer healthcare database in the United States.[32] Research members with access to the NIS data have completed online training and signed Data User Agreements with HCUP. This NIS-based study was classified as non-human subjects research, thus formal review was not required by the Institutional Review Board.
Cohort Selection
The NIS contains de-identified patient information at the level of hospital discharges. Each discharge contains up to 30 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes, and 15 ICD-9-CM procedural codes. To facilitate the use of multiple diagnosis codes, the AHRQ developed Clinical Classification Software (CCS) codes that collapse approximately 14,000 ICD-9-CM diagnosis codes into roughly 300 CCS codes.[33]
To study trends in PC utilization in an appropriate surgical population, we selected elderly patients with CRC admitted emergently for gastrointestinal surgery, a population projected to have high short and long-term morbidity. To identify this cohort in the NIS, discharges were selected for patients aged ≥65 years with a primary diagnosis of colorectal cancer (ICD-9-CM 154.0, 154.1) who underwent enterectomy (ICD-9-CM procedure codes 45.61, 45.62, 45.90, 45.91), colectomy (ICD-9-CM 17.3X, 45.7X) and/or ostomy formation (ICD-9-CM procedure codes 46.0X – 46.3X).[34] These specific procedures were chosen to isolate patients likely to have been primarily managed by surgical teams. To select for emergent procedures, only admissions classified as urgent/emergent or arranged from the emergency department were included.
Primary and Secondary Outcomes
Primary outcomes of interest were rates and determinants of PC consultation. PC consultation was identified by the presence of the ICD-CM-9 code for “Encounter for Palliative Care” (V66.7). According to ICD-CM-9 guidelines, this billable code applies to “end-of-life care,” “terminal care,” and “hospice care,” and is intended for secondary coding alongside the primary underlying disease code.[34] V66.7 is often used for research purposes to capture PC consultation in inpatient settings.[16–19, 34,35]
Secondary outcomes of interest were mean hospital LOS and costs, surrogate markers for healthcare utilization, compared between PC and non-PC groups. Other studies examining these outcomes adjust for confounding differences in illness severity between PC and non-PC groups by restricting analysis to patients who expired during a specific time-frame.[3,17,36–38] Likewise, we examined LOS and costs among the subset of patients who died during hospital admission.
Variables of Interest
Patient age, race, income quartile, comorbidity score, and year of admission were grouped into discrete categorical variables. Age was categorized into five-year intervals: 65–69, 70–74, 75–79, 80–84, 85–90, and 90 or more years. Race was classified according to HCUP race groupings as Asian or Pacific Islander, black, Hispanic, white, and other. Because of the extent of missing race data, particularly in the earlier years of the dataset, missing race was included as its own race category in analyses. Income quartiles were calculated for each patient based upon median income of the patient’s zip code and compared to national values for the given discharge year. We used the Elixhauser comorbidity index (ECI) to adjust for the effect of comorbid disease. The ECI is a 29-item comorbidity index calculated from ICD-9 diagnosis codes using HCUP software and is present within the NIS.[32] Patients were classified according to the following groups: ECI 0, ECI 1, ECI 2, and ECI 3 or more. To account for cancer progression, and because the NIS does not contain granular oncologic data, the ECI item for metastatic cancer was included as a separate covariate in all regression models. Finally, discharge year was included in regression models to adjust for annual changes in the frequency of PC consultation.
Hospital factors of interest included urbanicity, teaching status, bed size, census region, and control. Hospitals were classified as urban or rural based on proximity to a metropolitan area, and teaching or non-teaching based on the presence of residency training programs. Region was classified according to the four U.S. Census Bureau regions of Northeast, Midwest, South, and West. Bed size, classified as small, medium or large, is determined in HCUP databases with location-specific characteristics. Hospital control was classified as either not-for-profit, government, or proprietary.
Statistical Analysis
All analyses used appropriate methods for complex surveys, consistent with the stratified cluster design of the NIS. As such, all estimates are nationally representative. In 2012 the NIS underwent a change in sampling design, transitioning from a national sample of hospitals to a sample of discharges. To account for this change, HCUP provided “trend weight files” with updated sample weights for all years prior to 2012, which were appropriately used in our analysis.[39] Significant differences between groups were tested using the Rao-Scott Chi-square test for categorical variables. We used multivariable logistic regression, adjusted for patient age group, sex, race, ECI, presence of metastases, income quartile, discharge year, hospital region, urbanicity, hospital control, teaching status, and bed size to determine predictors of PC consultation. All statistical tests were two-sided, and statistical significance was set at a p-value of 0.05. All analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Univariable Comparisons of Palliative Care Use
A total of 86,573 patients were identified, of which only 3,598 (4.2%) received PC consultation. The rate of PC consultation increased over time, starting at 2.9% in 2009 and increasing to 6.2% in 2014 (p<0.001 for year). Females constituted 54.7% of the total cohort. There were no differences in sex, insurance status, or income quartile between the PC and non-PC groups.[Table 1]
Table 1.
Univariable analysis of demographic, clinical, and hospital characteristics of geriatric patients with colorectal cancer who underwent emergent gastrointestinal surgery, as recorded in the NIS from 2009–2014. PC = palliative care.
| No PC | PC | P-VALUE | |||||
|---|---|---|---|---|---|---|---|
| N | COLUMN % | ROW% | N | COLUMN % | R0W% | ||
| TOTAL | 82,975 | 100.0% | 95.8% | 3,598 | 100.0% | 4.2% | |
| PROCEDURE | |||||||
| Enterectomy | 7,053 | 8.5% | 93.2% | 512 | 14.2% | 6.8% | <0.001 |
| 72,310 | 87.1% | 96.5% | 2,620 | 72.8% | 3.5% | <0.001 | |
| 24,285 | 29.3% | 92.3% | 2,020 | 56.1% | 7.7% | <0.001 | |
| SEX | |||||||
| Male | 37,552 | 45.3% | 95.8% | 1,656 | 46.0% | 4.2% | |
| 45,419 | 54.7% | 95.9% | 1,942 | 54.0% | 4.1% | ||
| AGE (years) | |||||||
| 65–69 | 14,176 | 17.1% | 97.0% | 437 | 12.1% | 3.0% | |
| 14,472 | 17.4% | 96.1% | 588 | 16.3% | 3.9% | ||
| 15,554 | 18.7% | 96.3% | 599 | 16.6% | 3.7% | ||
| 17,396 | 21.0% | 96.2% | 696 | 19.3% | 3.8% | ||
| 14,160 | 17.1% | 95.0% | 748 | 20.8% | 5.0% | ||
| 7,218 | 8.7% | 93.2% | 530 | 14.7% | 6.8% | ||
| RACE | |||||||
| Asian | 2,202 | 2.7% | 95.1% | 113 | 3.1% | 4.9% | |
| 8,537 | 10.3% | 96.1% | 350 | 9.7% | 3.9% | ||
| 5,658 | 6.8% | 97.1% | 167 | 4.6% | 2.9% | ||
| 5,007 | 6.0% | 96.2% | 200 | 5.6% | 3.8% | ||
| 2,226 | 2.7% | 97.6% | 55 | 1.5% | 2.4% | ||
| 59,346 | 71.5% | 95.6% | 2,713 | 75.4% | 4.4% | ||
| PRIMARY PAYER | |||||||
| Medicaid | 1,561 | 1.9% | 96.7% | 53 | 1.5% | 3.3% | |
| 73,572 | 88.7% | 95.8% | 3,211 | 89.3% | 4.2% | ||
| 574 | 0.7% | 95.0% | 30 | 0.8% | 5.0% | ||
| 6,594 | 7.9% | 96.3% | 253 | 7.0% | 3.7% | ||
| 606 | 0.7% | 93.8% | 40 | 1.1% | 6.2% | ||
| INCOME QUARTILE | |||||||
| Bottom | 20,510 | 24.7% | 96.4% | 764 | 21.2% | 3.6% | |
| 20,240 | 24.4% | 96.0% | 834 | 23.2% | 4.0% | ||
| 20,875 | 25.2% | 95.4% | 1,000 | 27.8% | 4.6% | ||
| 19,962 | 24.1% | 95.5% | 950 | 26.4% | 4.5% | ||
| YEAR | |||||||
| 2009 | 15,114 | 18.2% | 97.1% | 452 | 12.6% | 2.9% | |
| 13,691 | 16.5% | 96.8% | 449 | 12.5% | 3.2% | ||
| 14,125 | 17.0% | 95.9% | 596 | 16.6% | 4.1% | ||
| 13,545 | 16.3% | 95.4% | 655 | 18.2% | 4.6% | ||
| 13,560 | 16.3% | 95.8% | 590 | 16.4% | 4.2% | ||
| 12,940 | 15.6% | 93.8% | 855 | 23.8% | 6.2% | ||
| ELIXHAUSER COMORBIDITY (non-cancer) | |||||||
| 0 | 2,993 | 3.6% | 97.5% | 77 | 2.1% | 2.5% | |
| 9,371 | 11.3% | 97.3% | 259 | 7.2% | 2.7% | ||
| 14,696 | 17.7% | 96.4% | 546 | 15.2% | 3.6% | ||
| 55,916 | 67.4% | 95.4% | 2,716 | 75.5% | 4.6% | ||
| METASTATIC CANCER | 33,092 | 39.9% | 93.9% | 2,163 | 60.1% | 6.1% | <0.001 |
| DISPOSITION | |||||||
| Routine Discharge | 21,797 | 26.3% | 99.1% | 203 | 5.6% | 0.9% | <0.001 |
| 20,007 | 24.1% | 97.0% | 623 | 17.3% | 3.0% | <0.001 | |
| 34,909 | 42.1% | 96.5% | 1,251 | 34.8% | 3.5% | <0.001 | |
| 5,319 | 6.4% | 78.1% | 1,492 | 41.5% | 21.9% | <0.001 | |
| HOSPITAL REGION | |||||||
| Northeast | 20,917 | 25.2% | 96.7% | 703 | 19.5% | 3.3% | |
| 31,082 | 37.5% | 96.4% | 1,166 | 32.4% | 3.6% | ||
| 15,180 | 18.3% | 95.2% | 767 | 21.3% | 4.8% | ||
| 15,797 | 19.0% | 94.3% | 962 | 26.7% | 5.7% | ||
| HOSPITAL CONTROL | |||||||
| Government | 7,494 | 9.0% | 95.9% | 322 | 8.9% | 4.1% | |
| 63,379 | 76.4% | 95.5% | 2,989 | 83.1% | 4.5% | ||
| 11,745 | 14.2% | 97.7% | 274 | 7.6% | 2.3% | ||
| URBANICITY | |||||||
| Rural | 7,894 | 9.5% | 97.2% | 231 | 6.4% | 2.8% | |
| 74,724 | 90.1% | 95.7% | 3,354 | 93.2% | 4.3% | ||
| TEACHING STATUS | |||||||
| Non-Teaching | 47,502 | 57.2% | 96.4% | 1,770 | 49.2% | 3.6% | |
| 35,117 | 42.3% | 95.1% | 1,815 | 50.4% | 4.9% | ||
| HOSPITAL BEDSIZE | |||||||
| Small | 10,898 | 13.1% | 96.5% | 399 | 11.1% | 3.5% | |
| 22,254 | 26.8% | 95.7% | 1,005 | 27.9% | 4.3% | ||
| 49,467 | 59.6% | 95.8% | 2,181 | 60.6% | 4.2% | ||
Compared with non-PC patients, the PC group was older (mean age 80.2 years vs. 78.1 years, p<0.001) and had a slightly different racial composition (p=0.044 for race), comprised of more whites (75.4% vs 71.5%) and Asians (3.1% vs 2.7%). Among all age and racial groups, patients aged ≥90 years and Asians had the highest rates of PC (6.8% and 4.9%, respectively). The PC group carried a higher comorbidity burden, with more than 3/4 affected by ≥3 comorbidities, versus 2/3 of the non-PC group (p<0.001). PC patients had higher rates of metastatic disease (60.1% vs 39.9%, p<0.001) and were much more likely to die during hospital admission (41.5% vs 6.4%, p<0.001).
The most common operation performed overall was colectomy (86.6%), followed by ostomy formation (30.4%) and enterectomy (8.7%). These frequencies were almost identical in the non-PC group; however, in PC patients, colectomy was performed less often (72.8%, p<0.001), while ostomy formation (56.1%, p<0.001) and enterectomy (14.2%, p<0.001)were performed almost twice as often.
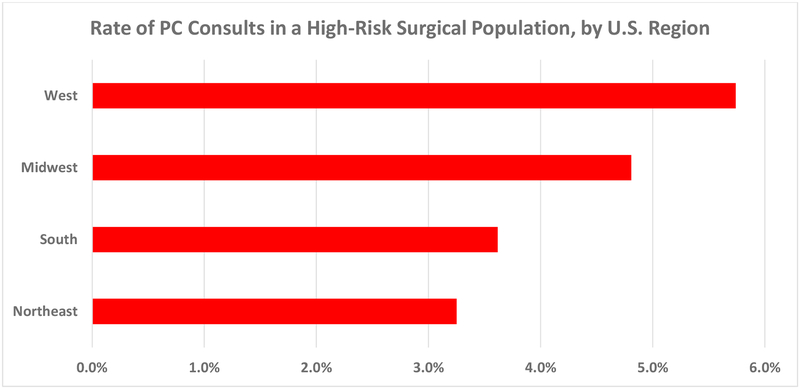
PC consultation varied significantly by hospital type and region. The Northeast had the lowest rate of PC consultation at 3.3%, increasing to 3.6% in the South, 4.8% in the Midwest, and peaking at 5.7% in the West (p<0.001 for region).[Figure 1] PC was used more in urban vs. rural settings, (4.3% vs 2.8%, p=0.010), teaching vs. non-teaching hospitals (4.9% vs 3.6%, p<0.001), and not-for-profit vs. proprietary hospitals (4.5% vs 2.3%, p<0.001 for hospital control).
Figure 1.
Regional rates of palliative care consultations among geriatric patients with colorectal cancer who underwent emergent gastrointestinal surgery. Rate range is from 0 to 6% (x-axis). PC = palliative care.
Multivariable Predictors of Palliative Care Consultation
A fully adjusted analysis revealed many of these factors to be predictive of PC consultation. Age was the strongest predictor: as age group increased from a baseline of 65–69 years, so did the odds of PC consultation, peaking in patients ≥90 years old at OR 3.3 (95%CI 2.4–4.5). Year of hospitalization was also striking. Compared with the reference year of 2009, the latter three years of the study period predicted PC use, with odds doubling by 2014. (OR 2.1, 95%CI 1.5–2.8).[Table 2]
Table 2.
Multivariable logistic regression showing strength of association between palliative care consultation and demographic, clinical, and hospital factors.
| OR | 95% CONFIDENCE INTERVAL | |
|---|---|---|
| PROCEDURE | ||
| Enterectomy | 1.6 | 1.3 – 2.0 |
| Colectomy | 0.7 | 0.6 – 0.9 |
| Ostomy | 2.6 | 2.2 – 3.1 |
| YEAR | ||
| 2009 | 1.0 | --- |
| 2010 | 1.1 | 0.8 – 1.5 |
| 2011 | 1.4 | 1.0 – 1.9 |
| 2012 | 1.6 | 1.2 – 2.2 |
| 2013 | 1.4 | 1.0 – 1.9 |
| 2014 | 2.1 | 1.5 – 2.8 |
| AGE (years) | ||
| 65–69 | 1.0 | --- |
| 70–74 | 1.4 | 1.1 – 1.9 |
| 75–79 | 1.4 | 1.1 – 1.9 |
| 80–84 | 1.6 | 1.2 – 2.1 |
| 85–89 | 2.3 | 1.7 – 3.0 |
| ≥90 | 3.3 | 2.4 – 4.5 |
| SEX | ||
| Male | 1.0 | --- |
| Female | 1.0 | 0.8 – 1.1 |
| RACE | ||
| Asian | 0.8 | 0.5 – 1.3 |
| Black | 0.9 | 0.7 – 1.2 |
| Hispanic | 0.7 | 0.5 – 0.9 |
| Missing | 0.9 | 0.6–1.3 |
| Other | 0.6 | 0.3 – 1.1 |
| White | 1.0 | --- |
| INCOME QUARTILE | ||
| Bottom | 1.0 | --- |
| 2nd | 1.1 | 0.8 – 1.3 |
| 3rd | 1.2 | 1.0 – 1.6 |
| Top | 1.2 | 0.9 – 1.5 |
| REGION | ||
| Northeast | 1.0 | --- |
| Midwest | 1.7 | 1.3 – 2.2 |
| South | 1.5 | 1.2 – 1.9 |
| West | 2.3 | 1.7 – 2.9 |
| HOSPITAL CONTROL | ||
| Not-for-profit | 1.0 | --- |
| Government | 0.9 | 0.7 – 1.3 |
| Proprietary | 0.5 | 0.4 – 0.7 |
| TEACHING STATUS | ||
| Non-teaching | 1.0 | --- |
| Teaching | 1.3 | 1.0 – 1.5 |
| HOSPITAL BEDSIZE | ||
| Small | 1.0 | --- |
| Medium | 1.4 | 1.0 – 1.8 |
| Large | 1.3 | 1.0 – 1.7 |
| RURAL/URBAN | ||
| Rural | 1.0 | --- |
| Urban | 1.2 | 0.9 – 1.7 |
| ELIXHAUSER COMORBIDITY (non-cancer) | ||
| 0 | 1.0 | --- |
| 1 | 1.1 | 0.6 – 2.0 |
| 2 | 1.6 | 0.9 – 2.8 |
| ≥3 | 1.9 | 1.1 – 3.3 |
| METASTATIC DISEASE | 2.2 | 1.9 – 2.6 |
P-value for reference categories in groups with 3 or more levels refers to the F-test for group effect (i.e., whether overall variable is significantly associated with outcome, rather than significant differences between levels).
Clinical factors that remained associated with PC included procedural type, number of comorbidities, and presence of metastatic disease. Colectomy was negatively associated with PC (OR 0.7, 95%CI 0.6–0.9), while enterectomy (OR 1.6, 95%CI 1.3–2.0) and especially ostomy formation (OR 2.6, 95%CI 2.2–3.1) were predictive. ECI ≥3 and presence of metastatic disease each conferred roughly twice the odds of PC consultation (OR 1.9, 95% CI 1.1–3.3 and OR 2.2, 95%CI 1.9–2.6, respectively).
Hospital factors that remained associated with PC included teaching status, hospital control, and region. Teaching status increased the odds of PC slightly (OR 1.3, 95%CI 1.0–1.5), and for-profit status reduced odds by half (OR 0.5, 95%CI 0.4–0.7). The strength of association between PC use and the four geographic zones mirrored the regional findings on univariable analysis. The Northeast, which had the lowest frequency of PC utilization, served as baseline for comparison. Relative to it, the South had 50% increased odds (OR 1.5, 95%CI 1.2–1.9), the Midwest 70% increased odds (OR 1.7, 95%CI 1.3–2.2), and the West 130% increased odds of PC consultation (OR 2.3, 95%CI 1.7–2.9).
Extent of Healthcare Utilization
Mean hospital LOS and costs were examined in a subset of patients who died before discharge. Mean LOS was 14.9 days for the PC group versus 14.8 days for the non-PC group, while mean total costs were $49,902 for the PC group and $48,173 for the non-PC group (P-values not significant).
Given distinct geographical utilization patterns identified on uni- and multivariable analyses, we further sub-stratified the cohort by region in order to assess for differential outcomes between PC and non-PC patients relating to region. Both endpoints were found to fluctuate between regions; however, differences between PC and non-PC groups within each region were not significant. Mean LOS was highest in the Northeast at 17.7 days, followed by the South at 14.4 days, the West at 13.8 days, and the Midwest at 12.4 days (p<0.01). Within individual regions, LOS was similar for PC and non-PC patients. Mean total hospital costs were highest in the West at $61,138 and lowest in the Midwest at $41,905, bracketing the Northeast at $52,744 and South at $43,291(p<0.01). Within each region, costs were similar for PC and non-PC groups.[Table 3]
Table 3.
Mean length of stay and mean total hospital costs for patients who died during hospital admission, stratified by region and palliative care receipt. PC = palliative care. LOS = length of stay.
| NORTHEAST | SOUTH | MIDWEST | WEST | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO PC | PC | P-VALUE | NO PC | PC | P-VALUE | NO PC | PC | P-VALUE | NO PC | PC | P-VALUE | |
|
MEAN LOS (Days) |
17.8 | 16.9 | 0.62 | 14.1 | 16.0 | 0.09 | 11.9 | 13.9 | 0.17 | 14.4 | 12.6 | 0.22 |
|
MEAN COSTS (USD) |
54,039 | 47,383 | 0.22 | 43,015 | 44,517 | 0.68 | 40,471 | 46,469 | 0.18 | 61,069 | 61,283 | 0.98 |
Discussion
The mission to integrate palliative care with surgical treatment was adopted by the American College of Surgeons (ACS) over a decade ago. In 2005, the ACS Committee on Ethics partnered with a newly formed Surgical Palliative Care Task Force to publish a statement of commitment to the core principles of PC in treating patients throughout their life cycles and at the end of life. This set off a cascade of initiatives aimed at increasing PC utilization by augmenting PC certification among surgeons, encouraging PC specialist referrals, and providing education about palliative treatment strategies in daily surgical practice.[7–9,11–17]
Still, contemporary literature suggests that surgeons fall short of utilization goals. Surgeons make up just a fraction of certified palliative care specialists in the United States, likely due to time constraints during training and practice. They refer patients to palliative specialists less often than non-surgeon providers. In intensive care unit settings, they are less likely to initiate end of life planning with patients and families. Reasons for these patterns have been explored by in-depth surveys and interviews, which highlight persistent gaps in education and awareness, discomfort leading end of life conversations, and overestimations of patient outcomes.[1,8–10,40,41] Beyond these barriers indigenous to surgery as a whole, disparities that exist across surgical practices have not been closely examined.
We sought in this study to identify specific factors related to patients and healthcare systems that make surgeons more or less likely to invoke PC expertise in clinical care. As a test population, we chose a highly specific cohort whose treatment was likely to be guided by a surgical team. We hypothesized that elderly patients with CRC who suffered a life-threatening complication were almost universally appropriate for palliative referral, and thus deviations might reveal factors predictive of PC consultation. Our study demonstrated unacceptably low nationwide use of PC consultation, in addition to striking regional differences in utilization across the United States.
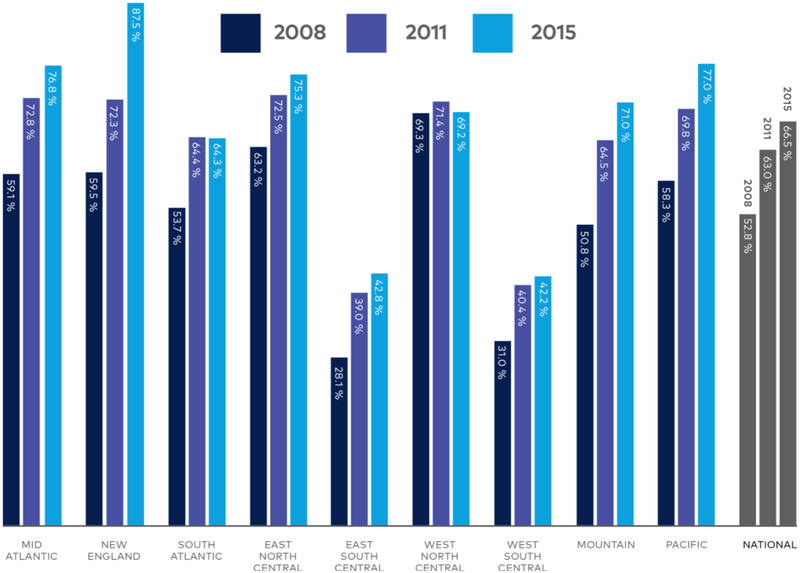
The nationwide rate of PC consultation in this surgical cohort was roughly 4%, alarming given that other studies of PC referral rates in advanced malignancy have cast estimates in the 12–40% range, depending on the year and health system.[9,42–44] PC use increased as the study period progressed, as would be expected given the growing number of PC programs in U.S. hospitals during recent years, and perhaps also reflecting the ACS’s efforts to augment utilization over the last decade.[45,46] Still, the 4% PC rate found in this study period may now be an underestimate. As shown in Figure 2, PC programs in the U.S. are continually proliferating over time. Since 2010, evidence has continued to emerge linking PC use with improved outcomes like survival and cost in cancer patients, buttressing a growing movement toward wider PC utilization.[3,38]
Figure 2.
Percent of United States hospitals with a Palliative Care program, by census region. Copied with permission from the Center to Advance Palliative Care and the National Palliative Care Research Center’s 2015 State-by-State Report Card. Available at: https://registry.capc.org/wp-content/uploads/2016/01/2015-State-by-state-Report-Card.pdf.
Concordant with studies of non-surgical cohorts, patients who died during admission or who were at highest risk for death, including those with advanced age, more comorbid conditions, and metastatic cancer, had more PC utilization.[45–47] Hospitals that were academic, not-for-profit, and of larger bedsize also had higher rates of use, consistent with multiple prior studies showing higher prevalence of PC programs, and naturally higher utilization of PC, in hospitals with these same features.[17,18,23,44,45]
Unexpected was the lack of difference in cost and LOS between PC and non-PC patients. Prior studies of non-surgical cohorts have reported improved outcomes among PC patients in healthcare utilization, including cost and number of procedures at the end of life.[18,37,38,44] In our surgical cohort, such advantages were not seen, possibly due to the fact that significant costs and resource utilization were necessitated by the surgical intervention itself. Additional studies of PC use in surgical populations are needed to further investigate this discrepancy.
Also unexpected was the association of Western and Midwestern regionality with higher PC utilization than the Northeast. According to the 2015 report by the Center to Advance Palliative Care, which draws data from the American Hospital Association Annual Survey of Hospitals Database and the National Palliative Care Registry, the highest concentration of PC programs is in New England, followed by the Mid-Atlantic and Pacific, the Midwest, and finally the South.[Figure 2] [45] In 2016, Roeland and colleagues used SEER-Medicare data to show that for elderly patients with cancer, PC consultations coded by ICD-9’s V66.7 were highest in the Northeast and West. Unequal access to PC services across the country has been cited as a critical barrier to care delivery; it was thus surprising that the Northeast, where PC resources are most abundant, ranked lowest in utilization in our study’s surgical cohort.[44–47]
What is the reason for this disparity? The lack of clinical granularity in the NIS database, a ubiquitous shortcoming in national clinical databases, makes this difficult to answer definitively. Root causes might lie at any level of care delivery, including the hospital, surgeon, and patient. Given the robust infrastructure of PC programing in the Northeast, lack of hospital resources or access are unlikely. Geographic differences and biases in goals of care, decision-making, and expectations of surgical outcomes among surgeons or patients appear more plausible.
Ethnic, religious, and cultural traditions have been linked with guiding end of life healthcare priorities and decision-making, and should be considered as possible contributors to our findings.[48–50] An intriguing study by Matlock and colleagues found that regional variability in PC use among cardiologists was associated with physician practice patterns. Among cardiologists polled about PC utilization in theoretical clinical scenarios, those practicing in regions with the highest end of life expenditures reported the lowest PC utilization.[51] Analogously, in our analyses of care utilization among patients who died during admission, Northeastern states had the longest LOS and utilized PC the least. This relationship did not extend to end of life expenditures, however, which were lowest in Western states that utilized PC the most. Beyond regional variations in surgical care utilization at the end of life, other practitioner qualities that could not be measured from our dataset, including attitudes toward PC and expectations of surgical outcomes, should be further explored in future studies.
Conclusions
Among a nationally representative cohort of elderly patients with complicated colorectal cancer requiring emergent gastrointestinal surgery, use of palliative care as an adjunct to treatment is alarmingly low. Patients with highest mortality risk, marked by older age, more comorbidities, and metastatic disease, are more likely to receive palliative care, and academic and non-proprietary hospitals are more likely to provide it. For this surgical cohort, availability of palliative care programs does not necessarily translate to utilization, as demonstrated by higher palliative care consultation rates in regions outside the Northeast. Future studies should explore this unexpected finding to address disparities in care that may relate to surgical biases and practice patterns.
Acknowledgements
This publication was made possible by CTSA Grant Numbers TL1 TR001864 and UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), NIH roadmap for Medical Research, and by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number T35DK104689. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. We also acknowledge the Center to Advance Palliative Care and the National Palliative Care Research Center for generously permitting use of an original illustration from the “2015 State-by-State Report Card” [Figure 2].
Footnotes
This study will be presented at: Digestive Diseases Week, Society for the Surgery of the Alimentary Tract, June 2018, Washington, DC
References
- 1.Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 2.World Health Organization. Global atlas of palliative care at the end of life. 2014. http://www.who.int/nmh/Global_Atlas_of_Palliative_Care.pdf. Accessed April 20th, 2018.
- 3.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 4.Riley GF, Lubitz JD. Long-Term Trends in Medicare Payments in the Last Year of Life. Health Services Research. 2010;45(2):565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dans M, Smith T, Back A, et al. NCCN Guidelines Insights: Palliative Care, Version 2.2017. J Natl Compr Canc Netw 2017;15:989–997 [DOI] [PubMed] [Google Scholar]
- 6.Braun LT, Grady KL, Kutner JS, et al. Palliative Care and Cardiovascular Disease and Stroke: A Policy Statement from the American Heart Association/American Stroke Association. Circ 2016;134:e198–e225 [DOI] [PubMed] [Google Scholar]
- 7.Lilley EJ, Cooper Z, Schwartze M, Mosenthal AC. Palliative Care in Surgery: Defining the Research Priorities. Ann Surg 2017/May/03, EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suwanabol PA, Kanters AE, Reichstein AC, et al. Characterizing the role of U.S. surgeons in the provision of Palliative Care: A systematic review and mixed-methods meta-synthesis. J Pain Symp Man 2018;55(4):1196–1215. [DOI] [PubMed] [Google Scholar]
- 9.Olmsted CL, Johnson AM, Kaboli P, Cullen J, Vaughan-Sarrazin MS. Use of Palliative Care and Hospice Among Surgical and Medical Specialties in the Veterans Health Administration. JAMA Surg. 2014;149(11):1169–1175. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Hospice and Palliative Medicine. Number of Certified Hospice and Palliative Medicine Physicians by Cosponsoring Specialty Board. http://aahpm.org/hpm/number-certified. Accessed on April 21, 2018.
- 11.Task Force on Surgical Palliative care; Committee on Ethics. Statement of principles of palliative care. Bull Am Coll Surg 2005;90:34–5. [PubMed] [Google Scholar]
- 12.Dunn GP. Surgery, palliative care, and the American College of Surgeons. Ann Pall Med 2015;4(1):5–9 [DOI] [PubMed] [Google Scholar]
- 13.Dunn GP, Martensen R, Weissman D. Surgical Palliative Care: A Resident’s Guide. American College of Surgeons/ Cunniff-Dixon Foundation: Chicago, IL/Essex, CT, 2009 [Google Scholar]
- 14.Mustafa R, Lisa O, Leigh N, et al. Prospective evaluation of surgical palliative care immersion training for general surgery residents. Am J Surg 2017;214(2):378–383. [DOI] [PubMed] [Google Scholar]
- 15.Ernst KF, Hall DE, Schmid KK, Seever G, Lavedan P, Lynch TG, Johanning JM. Surgical Palliative Care Consultations Over Time in Relationship to Systemwide Frailty Screening. JAMA Surg. 2014;149(11):1121–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Washington J, Al-Kindi SG, Oliveira GH, Robinson MR. “Inpatient Palliative Care Utilization in Elderly Patients Admitted with Heart Failure in the United States.” J Cardiac Failure 2017;23(8)S118 [Google Scholar]
- 17.Tarvinder S, Peters SR, Tirschwell DL, Creutzfeldt CJ. “Palliative Care for Hospitalized Patients with Stroke: Results from the 2010 to 2012 National Inpatient Sample.” Stroke 2017;48:2534–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel AA, Walling AM, Ricks-Oddie J, May FP, Saab S, Wenger N. “Palliative Care and Health Care Utilization for Patients with End-Stage Liver Disease at the End of Life.” Clin Gastroent Hep 2017;15(10):1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong K, Silver SA, Long J, et al. “Infrequent Provision of Palliative Care to Patients with Dialysis-Requiring AKI.” Clin J Am Soc Neph 2017/October/17 CJASN ePress [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammad AY, Robbins JR, Turaga KK, Christians KK, Gamblin TC, Johnston FM. “Palliative interventions for hepatocellular carcinoma patients: analysis of the National Cancer Database.” Ann Palliat Med 2017;6(1):26–35. [DOI] [PubMed] [Google Scholar]
- 21.Krell RW, Regenbogen SE, Wong SL. Variation in hospital treatment patterns for metastatic colorectal cancer. Cancer. 2015;121(11):1755–1761. doi: 10.1002/cncr.29253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulaylat AS, Rivet EB, Hollenbeak CS, Stewart DB. Palliative therapy for stage IV rectal adenocarcinoma: how frequently is it used? J Surg Res. 2017;218:1–8. [DOI] [PubMed] [Google Scholar]
- 23.Kulaylat AS, Mirkin KA, Hollenbeak CS, Wong J. Utilization and trends in palliative therapy for stage IV pancreatic adenocarcinoma patients: a U.S. population-based study. J Gastrointest Oncol 2017;8(4):710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossein M, Kang CY, Chen A, et al. Predictive Factors of In-Hospital Mortality in Colon and Rectal Surgery. JACS. 2012;215(2):255–261. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JH, Hole D, McArdle CS. Elective versus emergency surgery for patients with colorectal cancer. Br J Surg. 1992;79:706–709. [DOI] [PubMed] [Google Scholar]
- 26.Bayar B, Yilmaz KB, Akinci M, et al. An evaluation of treatment results of emergency versus elective surgery in colorectal cancer patients. Ulus Cerrahi Deg. 2016;32:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuman HB, O’Connor ES, Weiss J, LoConte NK, Greenblatt DY, Greenberg CC and Smith MA (2013), Surgical treatment of colon cancer in patients aged 80 years and older. Cancer, 119: 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong SK, Jalaludin BB, Berthelsen AS, et al. Tumor pathology and long-term survival in emergency colorectal cancer. Dis Colon Rectum. 2008;51(2):223–230. [DOI] [PubMed] [Google Scholar]
- 29.Hossein M, Kang CY, Chaudhry O, et al. Predictive Factors of Early Bowel Obstruction in Colon and Rectal Surgery: Data from the Nationwide Inpatient Sample, 2006–2008. JACS. 2012;214(5):831–837. [DOI] [PubMed] [Google Scholar]
- 30.Ming-gao G, Jian-zhong D, Yu W, et al. Colorectal cancer treatment in octogenarians: elective or emergency surgery? World J Surg Onc. 2014;12(386):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. Accessed May 1st, 2018. [Google Scholar]
- 32.HCUP Databases. Healthcare Cost and Utilization Project (HCUP). February 2018. Agency for Healthcare Research and Quality, Rockville, MD: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed April 30, 2018. [PubMed] [Google Scholar]
- 33.HCUP CCS. Healthcare Cost and Utilization Project (HCUP). March 2017. Agency for Healthcare Research and Quality, Rockville, MD: www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed April 30, 2018. [PubMed] [Google Scholar]
- 34.2015 ICD-9 CM for Physicians, Volumes 1 & 2. American Medical Association. 2015 Saunders, an imprint of Elsevier Inc, pp 980. [Google Scholar]
- 35.Feder SL, Redeker NS, Sangchoon J, et al. Validation of the ICD-9 diagnostic code for palliative care in patients hospitalized with heart failure within the Veterans Health Administration. Am J Hosp & Pall Med. 2017. 1–7. DOI: 10.1177/1049909117747519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horton JR, Morrison RS, Capezuti E, Hill J, Lee EJ, Kelley AS. Impact of Inpatient Palliative Care on Treatment Intensity for Patients with Serious Illness. Journal of Palliative Medicine. 2016;19(9):936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obermeyer Z, Makar M, Abujaber S, et al. Association Between the Medicare Hospice Benefit and Health Care Utilization and Costs for Patients with Poor-Prognosis Cancer. JAMA 2014;312(18):1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung MC, Earle CC, Rangrej J, et al. “Impact of Aggressive Management and Palliative Care on Cancer Costs in the Final Month of Life.” Cancer 2015;121(18):3307–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.HCUP NIS Trend Weights. Healthcare Cost and Utilization Project (HCUP). May 2015. Agency for Healthcare Research and Quality, Rockville, MD: www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed on May 3rd, 2018. [PubMed] [Google Scholar]
- 40.Galante JM, Bowles TL, Khatri VP, Schneider PD, Goodnight JE, Bold RJ. Experience and Attitudes of Surgeons Toward Palliation in Cancer. Arch Surg. 2005;140(9):873–880 [DOI] [PubMed] [Google Scholar]
- 41.Cauley CE, Block SD, Koritsanszky LA, et al. Surgeons’ perspectives on avoiding nonbeneficial treatments in seriously ill older patients with surgical emergencies: a qualitative study. J Palliat Med 2016;19(5):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gidwani R, Joyce N, Kinosian B, et al. Gap between recommendations and practice of palliative care and hospice in cancer patients. 957–963. J Palliat Med. 2016;19(9) [DOI] [PubMed] [Google Scholar]
- 43.Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016. March 1;122(5):782–90. [DOI] [PubMed] [Google Scholar]
- 44.Roeland EJ, Triplett DP, Matsuno RK, et al. Patterns of palliative care consultation among elderly patients with cancer. J Natl Compr Canc Netw. 2016;14:439–445. [DOI] [PubMed] [Google Scholar]
- 45.Dumanovsky T, Augustin R, Rogers M, et al. The growth of palliative care in U.S. hospitals: A status report. J Palliat Med. 2016;19(1)8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison SR, Meier DE. America’s care of serious illness: 2015 state-by-state report card on access to palliative care in our Nation’s Hospitals. Available at: https://registry.capc.org/wp-content/uploads/2016/01/2015-State-by-state-Report-Card.pdf. Accessed May 6, 2018. [DOI] [PMC free article] [PubMed]
- 47.Hawley P Barriers to access to palliative care. Palliat Care. 2017;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cain CL, Surbone A, Elk R, Kagawa-Singer M. Culture and palliative care: Preferences, Communication, meaning, and mutual decision making. J Pain & Symp Man. 2018;55(5):1408–1419. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg SM. Cultural and religious aspects of palliative care. Int J Crit Illn Inj Sci. 2011;1(2)154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care. 2007. May; 45(5):386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matlock DD, Peterson PN, Sirovich BE, et al. Regional variations in palliative care: Do cardiologists follow guidelines? J Palliat Med. 2010;13(11):1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]