Abstract
Background
The asymptomatic nature and suboptimal screening rates of sexually transmitted diseases (STD) call for implementation of successful interventions to improve screening in community-based clinic settings with attention to cost and resources.
Methods
We used MEDLINE to systematically review comparative analyses of interventions to improve STD (chlamydia, gonorrhea, or syphilis) screening or rescreening in clinic-based settings that were published between January 2000 and January 2014. Absolute differences in the percent of the target population screened between comparison groups or relative percent increase in the number of tests or patients tested were used to score the interventions as highly effective (>20% increase) or moderately effective (5%–19% increase) in improving screening. Published cost of the interventions was described where available and, when not available, was estimated.
Results
Of the 4566 citations reviewed, 38 articles describing 42 interventions met the inclusion criteria. Of the 42 interventions, 16 (38.1%) were categorized as highly effective and 14 (33.3%) as moderately effective. Effective low-cost interventions (<$1000) included the strategic placement of specimen collection materials or automatic collection of STD specimens as part of a routine visit (7 highly effective and 1 moderately effective) and the use of electronic health records (EHRs; 3 highly effective and 4 moderately effective). Patient reminders for screening or rescreening (via text, telephone, and postcards) were highly effective (3) or moderately effective (2) and low or moderate cost (<$1001–10,000). Interventions with dedicated clinic staff to improve STD screening were highly effective (2) or moderately effective in improving STD screening (1) but high-cost ($10,001–$100,000).
Conclusions
Successful interventions include changing clinic flow to routinely collect specimens for testing, using EHR screening reminders, and reminding patients to get screened or rescreened. These strategies can be tailored to different clinic settings to improve screening at a low cost.
Sexually transmitted diseases (STDs) are among the most commonly reported diseases in the United States and impose an estimated $15.6 billion dollars in lifetime direct medical costs per year.1 Bacterial STDs including chlamydia (CT), gonorrhea (GC), and syphilis can be asymptomatic at genital, oropharyngeal, and rectal sites in men and women.2 Screening asymptomatic individuals, promptly treating patients and partners, and rescreening-infected persons to detect repeat infection are key components of STD control and prevention.2
Screening and treatment of CT and GC prevents sequelae in women such as pelvic inflammatory disease, chronic pelvic pain, tubal factor infertility, and ectopic pregnancy.3–5 Sexually transmitted diseases facilitate HIV transmission and acquisition.6,7 Among men who have sex with men (MSM), screening for rectal CT/GC may be a cost-saving strategy to prevent HIV infection.8 Therefore, STD screening and treatment are key components of reproductive health as well as comprehensive HIV prevention.
For more than a decade, both the Centers for Disease Control and Prevention and the United States Preventive Services Task Force have recommended routine STD screening for sexually active young women.2,9 The Centers for Disease Control and Prevention also recommends repeat screening for CT and GC approximately 3 months after treatment2 to identify reinfection. Despite national recommendations, numerous studies have demonstrated suboptimal annual CT screening and rescreening rates, ranging from 22.3% to 44%, in a variety of clinical settings.10–13 Although national recommendations exist for annual STD screening among MSM, screening rates are also unacceptably low: 2% to 10% for rectal and pharyngeal GC, 14% to 29% for urethral GC, and 66% to 86% for syphilis.14,15
Clinics can implement interventions to improve screening and rescreening to reduce STD prevalence, reduce long-term sequelae, and meet federal requirements to demonstrate improvement in health outcomes.16 Opportunities to leverage expanding insurance coverage of persons for preventive services can further maximize public health resources for STD screening. Successful interventions require investing financial and staff resources and overcoming barriers at the system, provider, and patient levels.17 To guide STD programs, primary care providers, clinics, and other public health programs in prioritizing cost-effective STD screening interventions, we performed a literature review of recent interventions to increase STD screening and rescreening in community-based clinics.
METHODS
Publication Review and Inclusion Criteria
We conducted a systematic review of published articles evaluating interventions to improve STD screening in clinic-based settings using MEDLINE for studies published between January 1, 2000, and January 31, 2014. Publications describing clinic-based interventions to increase STD screening (CT, GC, and syphilis) and rescreening were reviewed for inclusion. The MEDLINE search was performed using the following key words: STD/STI, chlamydia, gonorrhea, MSM, or syphilis. These key words were individually combined with testing or screening intervention and community clinics or primary care. Chlamydia and GC retesting was entered as a separate search term (Fig. 1). Publications with abstracts not containing descriptions of interventions to improve STD/sexually transmitted infection (STI) screening were not reviewed. Citation lists of published studies were reviewed for additional relevant publications. Review articles summarizing interventions to improve STD screening were used to obtain additional studies for inclusion.18–21 Additional inclusion criteria were publication in the English language and analysis using a control group or a before-and-after comparison. Analyses published as meeting proceedings or in conference abstracts were excluded. We also excluded studies that reported on patient or provider intent to screen but not actual screening performance, and interventions conducted outside community clinic settings (i.e., home-based testing, online testing services, outreach settings, and case investigation situations).
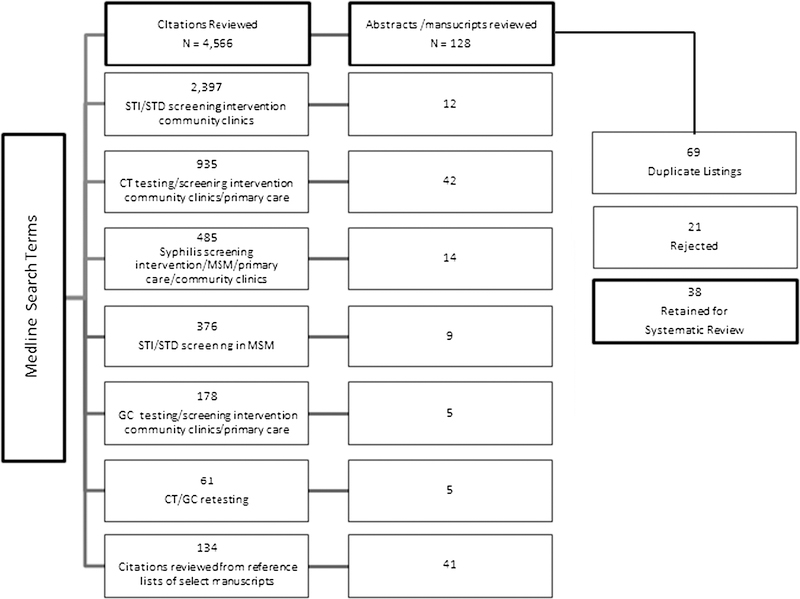
Figure 1.
MEDLINE STD screening interventions search results (n = 4566).
Assessing Quality of Randomized Controlled Trials
A modified Jadad score (total possible 7 points) was used to assess the quality of the randomized controlled trials (RCTs) included in our analysis (n = 11).22 Two reviewers (J.F.W. and I.U.P) independently reviewed and scored each trial. Trials scoring 5.5–7/7 were considered high quality studies, those scoring 4 or 5/7 were of moderate quality, and those scoring 3/7 or worse were considered of poor quality.
Elements of Data Collection
Following MEDLINE review, selected studies were organized into 4 intervention types based on target population and intervention methods: (1) structural interventions and clinic incentives, (2) screening/testing reminders incorporated into electronic health records (EHR), (3) provider education, and (4) patient education and reminders. Intervention types 1 to 3 aimed to increase STD screening among patients seen in clinic for routine care. Intervention type 4 sought to bring patients into a clinic specifically to undergo STD screening or rescreening. Proportions of persons screened and rescreened in intervention and control groups or preintervention and postintervention periods were summarized. Studies that evaluated multiple interventions were listed more than once in the tables. The primary outcome of interest was the absolute percent increase in the number of tests performed or patients screened. Where absolute percent increase was not available or calculable, relative percent increase was used.
Evaluation of Effectiveness
The absolute difference in the percent of the target population screened (percent screened in intervention group minus percent screened in comparison group) was used to score effectiveness of the interventions. Where absolute difference was not calculable, relative percent increase in the number of tests performed or patients tested preintervention and postintervention was used ([(number of tests or patients tested in intervention group minus tests or patients tested in comparison group)/number of tests or patients tested in the comparison group] × 100). Categories used to rank intervention effectiveness were as follows: highly effective was a ≥20% absolute increase in percent screened or at least 100% relative increase in number of tests or patients tested; moderately effective was a 5% to 19% absolute increase in percent screened or 10% to 99% relative increase in number of tests or patients tested, regardless of reported statistical significance; and not effective was a <5% absolute increase in percent screened or <10% relative increase in number of tests or patients tested, regardless of reported statistical significance.
Cost Analysis
Overall cost estimates for each intervention were assigned to each study by the authors based on reported costs, or estimated based on the type of intervention that was used. Where reported, actual costs were included in Tables 1–4. Categories used to rank intervention cost were as follows: (1) less than $1000, included electronic health reminders and text messaging reminders, environmental practice changes (placing test kits on examination table or universal urine collection), clinical in-service lectures, or total cost reported in publication as less than $1000; (2) $1000 to $10,000, included quality improvement projects with structured education and feedback for patients or providers inclusive of continuing medical education or Web-based education, or total cost reported in publication as ranging from $1000 to $10,000; (3) $10,001 to $100,000, included incentives for patients or providers or total cost reported in the publication as $10,001 to $100,000; and (4) more than $100,000, included quality improvement projects that hired staff or assigned focused staff, or total cost reported in the publication as more than $100,000.
TABLE 1.
Structural Interventions and Clinic-Based Incentives for STD Screening
| Table Position | Author (Year) Country, Study Design | Population Characteristics | Objective | Intervention | Main Findings | % Difference* | Effect Category† | Cost‡ |
|---|---|---|---|---|---|---|---|---|
| Adolescents/Young adults | ||||||||
| 1A | Andersen et al.23 (2005), Denmark, NRCT | Males aged 16–25 y attending general practice clinics | Improve CT screening | Offered urine-based testing for CT to men by their provider regardless of reason for visit. Control clinics performed testing based on symptoms or known exposure. | During the intervention 29.4% (239/814) of males were tested in the intervention practices as compared with 3.8% (583/15,419) in the control practices (P < 0.001). The intervention clinics detected 6 infections before the intervention and 14 during the intervention (P = 0.08). | 25.6 | ★★ | $ |
| 1B | Armstrong et al.43 (2003), Scotland, NRCT | Females and males aged ≥15 y | Improve CT screening | Placed a health adviser in intervention clinic to raise awareness of CT testing guidelines | The number of tests performed in the intervention clinic increased 120% (from 152 to 335) as compared with the increase in the control clinic (11%) (from 336 to 374). | 109 (R) | ★★ | $$$$ |
| 1C | Bilardi et al.25 (2010), Australia, RCT | Females aged 16–24 y attending 12 general practice clinics | Improve CT screening | Provided intervention clinics $5 (AUD) payments per CT test. | There was no significant increase in CT testing in the intervention group (11.5%–13.4%) tested compared with the control group (6.2%–8.8%). | 1.9 | ☆☆ | $$$ |
| 1D | Burstein et al.26 (2005), USA, PPI | Females aged ≤26 y seen in OB/Gyn and primary care clinics | Improve CT screening | Placed CT swabs next to Pap collection materials for collection at the same time. | The proportion of patients screened for CT increased from 57% (2939/5127) to 81% (4067/5014) (P < 0.001). The number of newly diagnosed patients increased 10% overall. | 24 | ★★ | $ |
| 1E | Kalwij et al.,27 (2012), UK, PPI | Sexually active males and females aged 15–24 y attending clinics in 2 Primary Care Trusts in Lambeth and Southwark | Improve CT screening | Clinic incentives were offered based on proportion of patients aged 15–24 y screened for CT. In Lambeth, providers were paid £100–£2600 based on practice size and aggregate screening rates at financial year-end In Southwark, clinics were paid £6–£15 per CT test performed according to baseline screening coverage. A full time CT screening coordinator and a general practice doctor (8 h/mo) were hired to promote screening. | CT tests performed in general practice increased from 23 tests in 2003–2004 to 4813 tests in 2010–2011 in Lambeth and from 5 tests in 2003–2004 to 4321 in 2010–2011 in Southwark The total cost of incentives was £54,566 ($85,607). | 32,521 (R) | ★★ | $$$$ |
| 1 F | Kettinger28 (2013), USA, PPI | Sexually active females age 25 and under attending women’s health center | Increase CT screening | Placed CT specimen collection kits next to Pap kits or collected urine among females not getting vaginal examination. Implemented site screening policy inclusive of provider flags for CT screening after nursing interview at check-in. Conducted in-service teaching regarding CT screening guidelines to providers and nursing staff. | CT testing coverage increased from 44.4% (59/133 eligible patients) before the intervention to 64.6% (84–130 eligible patients) after the intervention. Two CT cases were identified in the preintervention group as compared with 6 in the postintervention group. | 20.2 | ★★ | $ |
| 1G | Lawton et al.29 (2010), New Zealand, NRCT | Males and females aged 16–24 y in 3 general practice settings | Increase CT screening | Focused on systems approach, with educational team, identification of practice gaps, regular monitoring and feedback. Offered screening to all patients. Implement a recall system to offer 3-mo testing for patients that tested positive. | The intervention practices had significantly higher screening rates (20.9%; 158/756) as compared with the control practice (5.4%; 29/536) (P < 0.001). Screening rates declined at both intervention practices 6 months following the end of intervention activities with screening rates returning to baseline (2–5%). There were 17 cases detected before the intervention and 14 cases detected during the intervention. | 15.5 | ★☆ | $ |
| 1H | Scholes et al.30 (2006), USA, RCT | Sexually active females aged 14–25 y in 23 clinics | Improve CT screening | Established clinic-based opinion leaders, provided individual level measurement and feedback, and implemented examination room reminders. | The clinic-level intervention did not significantly affect overall CT testing, 42.0% (2373/5650) in intervention clinics vs. 40.1% (2488/6105) in control (P = 0.31). | 1.9 | ☆☆ | $ |
| 1I | Shafer et al.31 (2002), USA, RCT | Females aged 14–18 y in 10 pediatric clinics in HMOs | Improve CT screening | Implemented universal urine collection at check-in from all adolescent females aged 14–18 y before provider examination. Leadership monitored progress and screening performance. | Intervention clinics demonstrated a greater increase in screening coverage from 5% to 65% at 18 mo as compared with the increase in control clinics, 14% to 21% during the study period (overall P value for entire study period was <0.001). The intervention clinics identified 23 CT cases as compared with 12 CT cases in control clinics. | 60 | ★★ | $ |
| 1J | Tebb et al.,32 (2005), USA, RCT | Males aged 14–18 y attending 10 HMO clinics | Increase CT screening in young males | Quality improvement team established protocols for gathering sexual histories and collecting urine samples of all males before a clinician visit. | After the intervention, the percentages of males screened at intervention clinics (48%; 58/121) was significantly greater than the percentage screened at control clinics (9%; 11/128) (P = 0.007). | 39 | ★★ | $ |
| MSM and HIV-infected patients | ||||||||
| 1K | Bissessor et al.33 (2010), Australia, PPI | HIV-positive MSM attending sexual health center | Improve detection of asymptomatic syphilis | Included syphilis screening with every HIV clinical monitoring blood test | The number of MSM that received 3 or more syphilis screens increased from 80 in the 18 mo before the intervention to 228 after the intervention (P < 0.001). Detection of asymptomatic syphilis increased from 3 to 41 cases after the intervention. | 185 (R) | ★★ | $ |
| 1L | Botes et al.34 (2011), Australia, PPI | HIV-positive MSM attending urban HIV primary care clinic | Increase comprehensive STI screening in HIV-positive MSM | Incorporated comprehensive STI testing (including nongenital sites) into a routine anal cytology screening program | Before the intervention, 20.4% (67/328) of MSM completed STI testing as compared with 34.8% (123/353) after the intervention. Before the intervention 9 STIs were detected as compared with 12 STIs detected, after the intervention. | 14.4 | ★☆ | $ |
| 1M | Cohen et al.35 (2005), UK, PPI | HIV-positive MSM | Improve syphilis screening among asymptomatic HIV-infected MSM | Included syphilis screening in routine HIV follow-up care | Compared with the year preceding the protocol implementation syphilis screening of men undergoing CD4 testing increased from 3% to 85% and median time between syphilis tests decreased from 6 to 3 mo. In the year after the intervention, 40 syphilis cases were identified as compared with 26 in the year prior. | 82 | ★★ | $ |
| 1N | Ryder et al.36 (2005), Australia, PPI | MSM attending urban sexual health center | Improve comprehensive STI screening | Adoption of updated country guidelines recommending STI screening among MSM during annual comprehensive screening | Comprehensive screening for rectal GC, rectal CT, pharyngeal GC, urine CT, and syphilis increased from 46% to 61% after the release of the updated guidelines (P < 0.05). | 15 | ★☆ | $ |
| 1O | Snow et al.37 (2013), UK, PPI | MSM attending gay men’s health clinic | Improve comprehensive HIV and STD testing among MSM clients | Placed a sexual health practice nurse in the clinic | The proportion of HIV-negative MSM receiving comprehensive HIV/STD testing increased from 41% (411/1011) to 47% (489/1042) (P < 0.01). The proportion of HIV-positive MSM that received comprehensive STD testing increased from 27% (100/374) to 43% (180/418) (P < 0.001) after nurse placement. | 6 HIV− 16 HIV+ |
★☆ | $$$$ |
| 1P | Tu et al.38 (2013), Canada, PPI | HIV-positive aboriginal patients in 2 urban community health centers | Improve syphilis screening | Implemented chronic care model in which syphilis screening was one of 7 clinic practice guidelines. Dedicated staff to improving patient follow-up, screening, and vaccination. | After the implementation of the model, syphilis screening increased (91%; 199/219) when compared with the period before implementation (56%; 123/219) (P < 0.001). | 35 | ★★ | $$$$ |
Percent difference was calculated using absolute difference in screening between the intervention and comparison groups (percent screened in intervention group minus percent screened in comparison group). Where absolute difference was not calculable, relative percent increase in the number of tests performed or patients tested preintervention and postintervention was calculated ([(number of tests or patients tested in intervention group minus tests or patients tested in comparison group)/number of tests or patients tested in the comparison group] × 100). Relative differences are indicated with (R).
Effect categories: ★★ = >20% absolute increase in percent screened or >100% relative increase in number of tests, ★☆ = 5%–19% absolute increase in percent screened or 10%–99% relative increase in number of tests, and ☆☆ = <5% absolute increase in percent screened or <10% relative increase in number of tests.
Cost categories (estimated): $ = <$1000, $$ = $1000–$10,000, $$$ = $10,001–$100,000, and $$$$ > $100,000.
NRCT indicates nonrandomized controlled trial; PPI, premtervention/postintervention comparison; HMO = health maintenance organization.
TABLE 4.
Patient-Level Interventions Including Education, Incentives, and Testing Reminders
| Table Position | Author (Year) Country, Study Design | Population Characteristics | Objective | Intervention | Main Findings | Absolute % Difference* | Effect Category† | Cost‡ |
|---|---|---|---|---|---|---|---|---|
| Adolescents/Young adults | ||||||||
| 4A | Bilardi et al.53 (2009), Australia, PPI | Patients aged 16–24 y attending 3 general practice clinics | Increase CT screening | Providers referred all patients aged 16–24 y to educational Web site www.checkyourrisk.org.au after clinical consultation | There was no significant increase in testing among males during the intervention (3.0%; 30/995) compared with baseline (2.7%; 20/752) or females during intervention (6.4%; 129/2002) and at baseline (6.3%; 98/1548). | 0.3 males 0.1 females |
☆☆ | $ |
| 4B | Chacko et al.54 (2010), USA, RCT | Females aged 16–22.5 y attending reproductive health clinic | Improve STI retesting | Offered client-centered motivational behavioral counseling to females attending clinic, encouraging them to return for STI testing in response to high-risk sexual behavior | There was no improvement in the percent of females that returned for an STI testing visit in the intervention group (46%; 78/168) as compared with the control group (54%; 90/168). | −8.0 | ☆☆ | $$ |
| 4C | Malotte et al.55 (2004), USA, RCT | Patients aged 14–30 y treated for CT or GC at 1 STD clinic | Improve CT and GC repeat testing after initial diagnosis and treatment | Conducted motivational interviewing | Retesting was not significantly increased among patients that received motivational interviewing (12.0%) as compared with those receiving an appointment card at the treatment visit (3.4%). | 8.6 | ★☆ | $$ |
| 4D | Malotte et al.55 (2004), USA, RCT | Patients aged 14–30 y treated for CT or GC at 1 STD clinic | Improve CT and GC repeat testing after initial diagnosis and treatment | Provided motivational interviewing plus telephone or letter reminders | Retesting was significantly increased among patients that received motivational interviewing along with a reminder as compared with patients receiving an appointment card at the treatment visit (23.9% vs. 11.4%). | 12.5 | ★☆ | $$ |
| 4E | Malotte et al.55 (2004), USA, RCT | Patients aged 14–30 y treated for CT or GC at 1 STD clinic | Improve CT and GC repeat testing after initial diagnosis and treatment | Provided phone reminders to return to STD clinic for repeat testing | Retesting was significantly higher (33.3%) among patients that received a telephone reminder for rescreening as compared with those receiving an appointment card at the treatment visit (3.4%). | 29.9 | ★★ | $ |
| 4F | Malotte et al.55 (2004), USA, RCT | Patients aged 14–30 y treated for CT or GC at 2 STD clinics | Improve CT and GC repeat testing after initial diagnosis and treatment | Paid $20 patient incentive for return for repeat testing | Retesting was not significantly different among patients that received the $20 incentive (13.2%) as compared with those who did not (11.4%). | 1.8 | ☆☆ | $$ |
| 4G | Morgan and Haar56 (2009), New Zealand, NRCT | Patients aged <25 y attending 49 clinics serving Māori, low socioeconomic, or rural populations | Increase CT screening | Offered free sexual health consultations and testing in 20 clinics | Among females aged 18–24 y, screening coverage increased from 13.9% to 16.8% in the intervention group, and from 13.0% to 13.2% in the control group. No change in screening coverage was observed among males, with 13% and 13.2% in the intervention and control groups, respectively For all age and gender groups, intervention practices had higher test positivity (8.7%) compared with nonintervention practices (5.9%) (P < 0.01). | 2.9 females 0.2 males |
☆☆ | $$$ |
| 4H | Paneth-Pollak et al.57 (2010), USA, CO | Patients diagnosed as having CT or GC at 10 STD clinics | Improve CT and GC repeat testing after initial diagnosis and treatment | Mailed reminder postcards for repeat testing to patients | Retesting was higher among patients that received a reminder postcard (14.1 %; 179/1267) compared with patients that did not receive reminder postcards (7.7%; 382/4953). There was a decrease in reinfection among persons retesting during intervention (12.3%; 22/179) compared with preintervention period (25.5%; 24/94) (P = 0.015) and compared with nonintervention clinics (20.1%; 58/288) (P = 0.05). | 6.4 | ★☆ | $ |
| 4I | Zenner et al.58 (2012), UK, NRCT | Patients aged 15–24 y attending medical care in 84 Primary Care Trusts (PCTs) | Increase CT screening | Used patient incentives including prize draws (£50 voucher, £2000 vacation), condoms, tokens and vouchers (£5–£10) to increase uptake of CT screening | Among PCTs using incentives, the mean CT screening coverage rate increased 1.08% as compared with 0.41% in matched PCTs not using incentives (P = 0.02). Positivity did not change (0.07%, P = 0.9) among PCTs using incentives. | N/A | N/A | $$$ |
| MSM and HIV-infected patients | ||||||||
| 4J | Bourne et al.59 (2011), Australia, PPI | HIV-negative MSM at a community STI clinic | Increase STI rescreening at 3- to 6-mo intervals | Sent SMS (text) reminders to MSM for repeat STI screening | In the SMS group, 64.4% (n = 714) were retested as compared with 29.7% (322/1084) in the non-SMS group (P < 0.001). SMS cost was AU$0.05 each. | 34.7 | ★★ | $ |
| 4K | Zou et al.60 (2013) Australia, NRCT | MSM at a sexual health center | Improve asymptomatic STD screening among MSM | Sent MSM computer-generated, automated text message and e-mail reminders | MSM who chose SMS reminders had higher testing rates (49.2%; 435/885) for complete testing visits (GC and CT at genital and nongenital sites, syphilis and HIV) than those who did not choose text reminders (25.5%, 249/97S)(P < 0.001). Men receiving reminders had higher positivity rectal CT (6.6% vs. 2.8%), rectal GC (3.7% vs. 1.2%), urethral CT (3.1% vs. 1.4%), and early latent syphilis (1.4% vs. 0.4%) compared with controls. (P < 0.03 for all) | 23.7 | ★★ | $ |
Absolute difference in the percent screened (intervention group minus the comparison group).
Effect categories: ★★ = >20% absolute increase, ★☆ = 5%–19% absolute increase, and ☆☆ = <5% absolute increase.
Cost categories (estimated): $ = <$1000, $$ = $1000–$10,000, $$$ = $10,001–$100,000, and $$$$ > $100,000.
NRCT indicates nonrandomized controlled trial; PPI, preintervention/postintervention evaluation; CO, controlled observational.
RESULTS
Search and Review Outcomes
We reviewed 4566 citations and identified 128 abstracts or articles for further review. Of those, 69 had been identified using previous search terms, 23 were rejected, and 38 met the inclusion criteria (Fig. 1). One study evaluated 2 interventions and another evaluated 4 total interventions. Thus, 42 interventions were summarized alphabetically by author and target population category in Tables 1–4.
Sites of Interventions
Interventions were conducted in the United States (n = 17), Australia (n = 13), United Kingdom (n = 6), New Zealand (n = 2), Scotland (n = 1), Denmark (n = 1), Belgium (n = 1), and Canada (n = 1).
Design of Studies
All of the 42 interventions included in this review compared STD screening or rescreening rates as the main outcome of a RCT (n = 14), a nonrandomized controlled trial (n = 9), an evaluation of a preintervention and postintervention period comparison (n = 18), or a controlled observational study (n = 1).
Of the 42 interventions evaluated, 16 described structural interventions (including staff positions dedicated to STD screening activities) or clinic incentives for screening (Table 1)23–38; 9 evaluated electronic and medical chart reminders (Table 2)30,39–46; 6 evaluated provider-level interventions focusing on education (Table 3) 47–52; and 11 evaluated patient-level interventions, one of which described multiple interventions (Table 4).53–60 One article described both a quality improvement program and a chart prompt and is thus listed in Tables 1 and 2.39 One article described 4 patient-level interventions, each of which is listed separately in Table 4.55
TABLE 2.
Interventions Incorporating EHR Reminders for Providers to Perform STD Screening
| Table Position | Author (Year) Country, Study Design | Population Characteristics | Objective | Intervention | Main Findings | Absolute % Difference* | Effect Category† | Cost‡ |
|---|---|---|---|---|---|---|---|---|
| Adolescents/Young adults | ||||||||
| 2A | McNulty et al39 (2014), UK, RCT | Patients aged 15–24 y attending general practice clinics | Improve CT screening | Implemented practice-based education and resources, including EHR reminders in some settings. | Clinics with EHR reminders had a 2.81 times higher testing rate (6.52/100 patients) than control practices (1.4/100 patients) (P < 0.001). | N/A | N/A | $ |
| 2B | Riley et al.40 (2011), USA, PPI | Pregnant females attending family medicine teaching clinics | Improve CT/GC repeat screening during the third trimester in accordance with standards of prenatal care | Implemented automated CT/GC/HIV testing reminders in EHR | Compared with the preintervention period either completion of the test or documentation of the lack of need for repeat GC/CT testing increased from 33% at baseline to 97% when prompts were active (P < 0.001). Screening rates went back to 34% in the postintervention period when reminders were turned off. | 64 | ★★ | $ |
| 2C | Rudd et al.41 (2013), USA, PPI | American Indian females attending one Indian Health Service (IHS) Clinic | Improve performance of IHS national quality screening indicators for CT screening | Implement a prompt in EHR for CT screening of women aged ≥25 y | The number of females receiving CT screening increased from 56 to 200 unique patients after the intervention. CT screening coverage increased from 13% to 48.9% of eligible females. CT screening positivity increases from 9.5% before the intervention to 11.2% after the intervention. | 35.9 | ★★ | $ |
| 2D | Scholes et al.30 (2006), USA, RCT | Sexually active females aged 14–20 in 23 HMO clinics | Improve CT screening | Placed chart prompts (stickers) at the front of paper medical charts to screen for CT in a random sample of females. | Screening among patients with a paper chart prompt (42.6%, 757/1,777) was not significantly different compared with those without a prompt (40.8%, 706/1,732) (P = 0.27). | 1.8 | ☆☆ | $ |
| 2E | Walker et al.42 (2006), Australia, RCT | Females aged 16–24 attending 68 general practice clinics | Improve CT screening | Implemented a computerized alert advising the medical provider to consider offering CT testing | The intervention group had a greater increase in CT screening (from 8.3% to 12.2%) compared with control clinics (8.8%−10.6%) that did not have the alert. Screening positivity did not change as a result of the intervention. | 3.9 | ☆☆ | $ |
| MSM and HIV-infected patients | ||||||||
| 2F | Bissessor et al.43 (2011), Australia, PPI | MSM with >10 partners in prior 12 mo | Improve syphilis screening | Implemented a computer alert to remind clinicians to order syphilis test on high-risk MSM | The percent of high-risk MSM screened for syphilis increased from 77% (1559/2017) to 89% (1282/1445) (P = 0.001) and the percent of MSM identified with asymptomatic syphilis increased from 16% (5/31) to 53% (31/58) after the intervention (P = 0.001). | 12 | ★☆ | $ |
| 2G | Callander et al.44 (2013), Australia, PPI | HIV-positive MSM attending HIV primary care clinic | Increase frequency of syphilis screening | Altered EHR to include syphilis testing as part of the battery of tests automatically requested for patients at 4–6-mo intervals | The number of patients who had at least 1 syphilis test increased (from 73% to 97%, P < 0.001) and the average number of syphilis tests performed per year per male patient increased (from 1.1 to 2.3, P < 0.001).after the intervention. The proportion of patients meeting testing target of 3 tests per year rose from 10% to 41% (P < 0.001). | 24 | ★★ | $ |
| 2H | Hotton et al45 (2011), USA, PPI | MSM aged ≥18 y previously diagnosed as having syphilis attending an LGBT health center | Improve syphilis retesting | Implemented EHR allowing follow-up appointments for serologic testing to be tracked | The proportion of patients retested for syphilis 6 mo after initial diagnosis and treatment increased from 64% (56/87) to 81% (38/47) after the implementation of the EHR. | 17 | ★☆ | $ |
| 21 | Lister et al.46 (2005), Australia, PPI | MSM clients attending sexual health clinic | Improve rectal GC and CT screening | Listed new STD screening recommendations for MSM at nongenital sites in an electronic reminder (dialogue box) for providers. | After the introduction of the screening guidelines reminder, there was an increase in the proportion of MSM screened at any site from 78% (339/435) to 83% (928/1119) (P = 0.023). Screening positivity did not change (7% vs. 7%). Rectal GG screening remained constant (61%) and rectal CT screening increased from 55% (185/435) to 67% (619/1,119) (P < 0.001). | 5 Any site 0 rectal GC 12 rectal CT |
★☆ | $ |
Absolute difference in the percent screened (intervention group minus the comparison group).
Effect categories: ★★ = >20% absolute increase, ★☆ = 5%–19% absolute increase, and ☆☆ = <5% absolute increase.
Cost categories (estimated): $ = <$1000, $$ = $1000–$10,000, $$$ = $10,001–$100,000, and $$$$ > $10.
N/A indicates not applicable; NRCT, nonrandomized controlled trial; PPI, preintervention/postintervention evaluation; HMO, health maintenance organization.
TABLE 3.
Interventions With Focus on Provider-Level Education and Performance Feedback for STD Screening
| Table Position | Author (Year) Country, Study Design | Population Characteristics | Objective | Intervention | Main Findings | Absolute % Difference* | Effect Category† | Cost‡ |
|---|---|---|---|---|---|---|---|---|
| Adolescents and young adults | ||||||||
| 3A | Allison et al.47 (2005), USA, RCT | Females aged 16–26 y | Improve CT screening | Randomized primary care physicians’ offices to receive 4 continuing medical education modules for CT screening via Internet | CT screening rates decreased significantly in both the control arm (from 18.9% to 12.4%) and the intervention arm (from 16.2% to 15.5%) (P = 0.044). | −0.7 | ☆☆ | $$ |
| 3B | Bowden et al.48 (2008), Australia, RCT | Females aged 16–39 y attending 31 general practice clinics | Improve CT screening | Asked doctors in intervention clinics to routinely offer combined CT screening with Pap screening. Asked doctors in control groups to implement CT screening based on sexual risk assessment | CT screening occurred more often among females who attended a visit at intervention clinic (6.9%, 1107/16,082) as compared with control clinic (4.5%; 486/10,794). | 2.4 | ☆☆ | $ |
| 3C | Gooding et al.49 (2012), USA, NRCT | Females aged 16–26 y | Improve CT and HIV screening | Provided medical residents with workshops on disease screening in adolescents including US Preventative Task Force CT screening recommendations. Included adolescent instructors reporting behavioral and sexual risk factors in a role play exercise with residents | CT screening rates did not increase significantly in the intervention group. Medical residents in the intervention group screened 39.7% (25/63) of eligible females for CT as compared with 34.8%o (8/23) in the control group. | 4.9 | ☆☆ | $$ |
| 3D | Relly et al.50 (2014), UK, PPI | Heterosexual patients aged >16 y at 12 general practices | Increase comprehensive STI screening (GC/CT/syphilis/HIV) | Provided training and resource packages to general practice providers and nurses in accordance with British Society for Sexual Health and HIV standards. Required providers to complete 6 core e-learning models and half-day practice-based training day | There was a nonsignificant increase in the number and proportion of patients aged 16–25 y tested before the intervention (31%; 30/97) compared with after (40%; 52/131) (P = 0.2). CT positivity among those aged 16–25 y was 9.9% (14/142) in the intervention clinics as compared with 14.7% (36/244) in the nonintervention clinics. | 9 | ★☆ | $$$ |
| 3E | Merritt et al.51 (2007), Australia, PPI | Males and females aged 15–24 y from 10 general practice clinics | Increase CT screening | Convened educational meetings for providers every 8 wk presenting CT epidemiology, testing guidelines, strategies for improving detection and management, and identification of barriers | The number of tests performed on those aged 15–24 y increased from 227 before the intervention to 442 during the intervention. The percent tested increased from 6.7% to 10.2% for females and from 4.5% to 6.3% for males. Increases were not sustained after the intervention. | 3.5 Females 1.8 Males |
☆☆ | $ |
| 3 F | Verhoeven et al.52 (2005), Belgium, RCT | Females aged <35 y attending 36 general practice clinics | Improve CT screening | Delivered educational package including information on performing a sexual history and a video simulating a provider offering CT testing to a patient. | The proportion of patients appropriately tested for CT, after adjusting for study design, was significantly higher in the intervention group (86.7%; 108/126) than the control group (67;, 57/85) (P = 0.029). | 19.7 | ★☆ | $$ |
Absolute difference in the percent screened (intervention group minus the comparison group).
Effect categories: ★★ = >20% absolute increase, ★☆ = 5%–19% absolute increase, and ☆☆ = <5% absolute increase.
Cost categories (estimated): $ <$1,000, $$ = $1,000–$10,000, $$$ = $10,001–$100,000, $$$$ > $100,000
NRCT indicates nonrandomized controlled trial; PPI, preintervention/postintervention evaluation.
Quality of the Studies
Of the 11 randomized controlled studies, 4 studies were rated as high quality,31,32,39,48,54 6 studies were rated as moderate quality25,30,42,47 (multiple),55 and 1 study was of poor quality.52
Target Populations and Outcomes
Of the 42 interventions evaluated, 15 (35.7%) evaluated screening of young women for CT and other STDs 24,25,27,28,30,31,41,42,47–49,51,52,54 and 2 studied screening of pregnant women.26,40 Two (4.5%) interventions targeted young men for STD screening,23,32 and 6 interventions were for both young men and women.29,39,50,53,56,58 A total of 11 interventions targeted MSM (n = 7)36,37,43,45,46,59,60 or HIV-infected MSM (n = 4)33–35,44 for STD screening. One study targeted both and male and female HIV-infected patients for STD screening.38 There were 5 interventions that targeted CT or GC rescreening among male and female patients after initial diagnosis55,57 and one intervention that targeted repeat syphilis screening among MSM treated for syphilis.45
Outcomes by Intervention Strategy
Of the 42 individual interventions, 16 (38.1%) were rated as highly effective in increasing STD screening and 14 (33.3%) were moderately effective in increasing screening. The remaining 12 (28.6%) interventions did not demonstrate notable or sustainable increases in STD screening as a result of the intervention.
Among 8 structural interventions that included strategic placement of specimen collection materials or automatic collection of urine or blood as part of a routine visit,23,26,28,31–35 7 were highly effective and 1 was moderately effective (Table 1). Among 8 studies that modified EHRs to remind providers to screen, 3 were highly effective and 4 were moderately effective in improving STD screening (Table 2).
Patient-level interventions such as incentives and testing reminders increased screening (Table 4). Of 5 patient-level interventions (including text, telephone, or postcard reminders), 3 were highly effective and 2 were moderately effective in improving STD screening or rescreening.55,57,59,60 Offering free STD testing services in a setting where STD services had previously required payment was moderately effective in improving STD screening.56
Three studies described interventions that included dedicated staff devoted to increasing STD screening24,27,37; all 3 demonstrated improvements in screening, 2 were highly effective, and 1 was moderately effective (Table 2). Both of the studies that described financial incentives for clinics per CT test performed reported an increase in screening25,27 (Table 1). In 1 of these 2 studies, dedicated staff devoted to STD screening was combined with financial incentives to clinics for screening.27
Provider-level interventions had limited success in sustainably increasing STD screening. In this group (n = 6), only 2 of the interventions were categorized as moderately effective.50,52 All 6 provider-level interventions described continuing medical education modules, meetings, simulation videos, workshops, resource packages, or guideline updates (Table 3).
Additional interventions that did not improve STD screening included monetary55 and nonmonetary58 incentives to patients and clinics,25 motivational counseling to patients,54 paper chart reminders,30 and referring patients to an educational Web site.53
Cost Estimates
Costs for each intervention were estimated or reported as less than $1000 for 28 (66.7%) of the interventions, $1001 to $10,000 for 7 (16.7%), $10,001 to $100,000 for 4 (9.5%), and more than $100,000 for 3 (7.1%) (Tables 1–4). Actual costs associated with intervention implementation were reported for only 5 interventions (12%; Tables 1 and 4).25,27,55,58,59 Each of these 5 interventions included incentives for clinics, providers, or patients.
Combining Effect and Cost
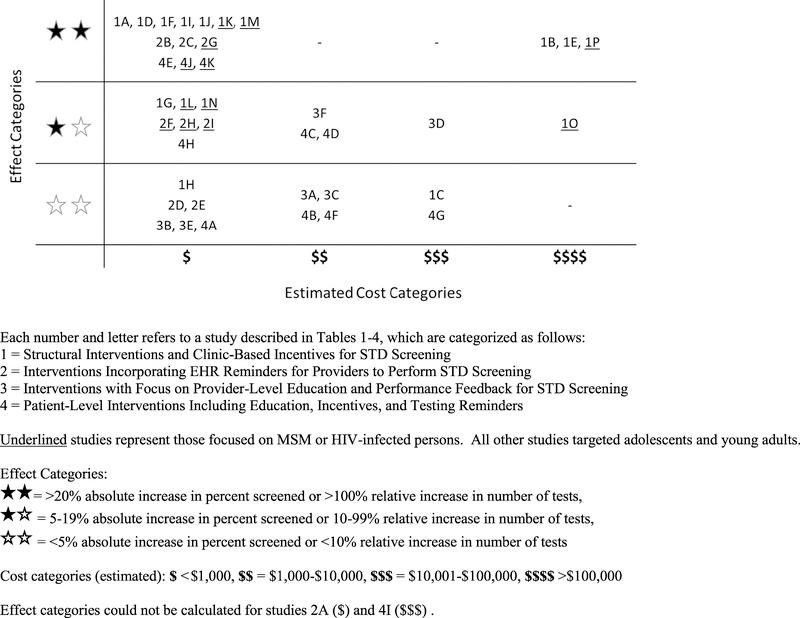
Interventions that included the automatic collection of specimens as part of a routine or follow-up visit (Table 1),23,26,28,31–35 use of EHR reminders (Table 2),39–46 or the use of patient reminders (Table 4)55,57,59,60 had the greatest improvements in STD screening at the lowest cost (Fig. 2). Dedicating staff to improve STD screening was associated with the largest improvement in screening at the highest estimated cost (Table 1).24,27,37
Figure 2.
Screening effectiveness by cost.
Case Finding and Positivity
Of the 42 interventions in this review, 18 (43%) included before-and-after data on case finding, 9 (21.4%) or the percent of attendees that were positive, 9 (21.4%). Of the 9 interventions that reported on percent of tests that were positive, 3 reported no change,42,46,58 4 reported an increase in percent positive,41,43,56,60 and 2 reported a decrease50,57 after the intervention. Of the 9 interventions that included case identification, 8 reported an absolute increase in STD cases identified,23,26,28,31,33–35,39 and 1 reported an absolute decrease in cases identified29 after the intervention (Tables 1–4).
DISCUSSION
We systematically reviewed clinic-based interventions developed to improve CT, GC, and syphilis screening. Interventions evaluated in this review incorporated systems-level programs, EHR reminders, and patient- and provider-level activities to improve STD screening. Effects varied by type of intervention, but improvements in screening were consistent for interventions that incorporated the automatic collection of STD specimens in association with a routine or follow-up visit. The EHR reminders also led to notable improvements in STD screening. Patient reminders for testing or retesting were effective in improving screening, whereas motivational counseling and interviewing showed minimal improvement. Overall, patient and provider education was of limited benefit in improving STD screening. Cost estimates varied depending on the intervention but were lower for interventions that included the universal collection of STD testing specimens or the use of EHR reminders and were greater for those where dedicated staff was assigned to improve STD screening or incentives were provided for screening.
Increasing screening through program and protocol development (with or without assignment of a dedicated staff) was often done using the automatic collection of specimens in association with a patient visit, usually before the patient saw the provider.23,26,28,31–35 Others strategically placed specimen collection kits such that providers would remember to perform the test along with other tests due at that appointment. Routinizing collection of specimens at a variety of visit types (even visits for unrelated issues) for patient in target age group/demographic reduces missed opportunities and standardizes an effective approach to STD screening at a lower cost. Quality improvement efforts that placed dedicated staff members to facilitate STD screening also demonstrated increases in STD screening,24,27,37 albeit at a much higher cost.
Reminders in EHRs were effective at increasing STD screening39–46 and have been used to improve performance of other routine screening measures in different clinical settings.61,62 With the increase in use of EHR in community clinics and primary care settings, this may be an opportune time for clinical and public health programs to focus on EHR reminders especially for screening that has been recommended by the US Preventive Services Task Force9 such as CT screening among adolescent girls and young women. Many EHR platforms have programmable provider reminders that can be tailored to correspond to health screening recommendations.39–46,61,62
Direct provider education related to appropriate management of health conditions remains a mainstay of efforts to advance medical knowledge and clinical practice. These efforts are often the easiest to put in place, are an accepted method of acquiring information about a medical topic, and can be done quickly at a reasonable cost. However, studies we reviewed found education led to limited improvements in STD screening.53–60 These findings suggest that provider education should be considered only in combination with other highly successful methods such as EHR reminders or clinic-based protocol development.
Patient-level interventions that used incentives and motivational interviewing had little success at increasing STD screening or rescreening. Patient reminders via telephone call, text messaging, or letter increased STD screening and rescreening at a relatively low cost.55,57,59,60 The use of texting to communicate health reminders with youth has been evaluated in other clinical settings63,64 but would require knowing the patient’s cell phone number and may be most appropriate for rescreening or for frequent screening of high-risk persons.
Costs are often a major component of choosing and implementing an intervention. However, few of these publications reported costs associated with implementation. Many of the multilevel interventions involved the systematic collection of STD testing specimens from eligible clients before, or as a part of, a clinician encounter. Those interventions could be done at minimal cost by changing clinic policy. Similarly, programming clinical reminders into EHRs may be done with limited additional cost and those reminders may be adapted or expanded to address local STD epidemiology. Patient reminders, some of which used cellular messaging technologies or other accessible reminder systems, were low-cost strategies that could be adapted in community clinic settings. Clinic-level advocacy for opportunistic STD screening as a standard of care for eligible patients according to national guidelines2,9 may be needed in some clinical settings to improve screening performance even in the absence of concern for cost.
There are several limitations related to the interpretation of these data. Our search did not include abstracts, meeting proceedings, or other “gray” literature; thus, unpublished studies that reported negative results are likely underrepresented. Effectiveness categories were chosen to identify high- and low-performing interventions. Programs should carefully consider moderately effective interventions in relation to their clinical care setting and patient population. The effects of the interventions we studied may be different in other clinical settings. Some multilevel interventions demonstrated significant improvements in STD screening; however, it was not possible to discern which element of the intervention was responsible in part or in full for the improvement. Cutoff values for categories of effectiveness were determined by the authors and may not reflect patient or population-level impact. For example, we did not fully account for baseline screening performance when calculating changes, so sites with low baseline screening had a greater opportunity to increase screening compared with clinics where baseline screening was already high. Interventions were not weighted or stratified based on rigor of the study methods. Our cost estimates may underestimate or overestimate the true costs of the interventions; only 5 studies reported actual costs. We did not include ancillary costs of screening such as staff time associated with collection of STD specimens in our estimates. Also, we did not include estimates of insurance reimbursement to the clinics. Thus, cost categories used in this review should be viewed as general estimates and not specific values. There were only 4 high-quality RCTs in our systematic review; therefore, our conclusions about effectiveness are largely based on observational data. Finally, although case identification is the ultimate goal of screening, we assessed the number of tests done; STD case identification, or positivity, was reported for less than half of the interventions in this review.
Screening in clinics improved the most when interventions used protocols for the universal collection of specimens or strategic placement of collection kits, electronic health reminders for providers, or reminders for patients to improve STD screening and rescreening. These interventions might be implemented in clinical settings at a minimal cost, but often require clinic- and provider-level advocacy, protocol development, and feedback on performance. Only research studies were included in our review, so it will be important for programs that implement these interventions in real-world settings evaluate and share their experience. The ability of STD programs to influence clinics to implement successful interventions depends on collaborative partnerships. Establishing those partnerships is a new task for many STD programs, one that also requires study.
Footnotes
Conflict of interest: None declared.
Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent views of the Centers for Disease Control and Prevention, Arizona Department of Health Services, and California Department of Public Health.
REFERENCES
- 1.Owusu-Edusei K Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013; 40:197–201. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2010. MMWR 2010; 59:44–45.20094027 [Google Scholar]
- 3.Haggerty CL, Gottlieb SL, Taylor BD, et al. Risk of sequelae after Chlamydia trachomatis infection genital infection in women. J Infect Dis 2010; 201:s134–s155. [DOI] [PubMed] [Google Scholar]
- 4.Scholes D, Stergachis A, Heidrick FE, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydia infection. N Engl J Med 1996; 334:1362–1366. [DOI] [PubMed] [Google Scholar]
- 5.Anschuetz GL, Asbel L, Spain CV et al. Association between enhanced screening for Chlamydia trachomatis and Neisseria gonorrhoeae and reductions in sequelae among women. J Adolesc Health 2012; 51:80–85. [DOI] [PubMed] [Google Scholar]
- 6.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted disease to sexual transmission of HIV infection. Sex Transm Infect 1999; 75:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sexton J, Garnett G, Rottingen JA. Meta-analysis and metaregression in interpreting study variability in the impact of sexually transmitted disease to sexual transmission on susceptibility to HIV infection. Sex Transm Dis 2005; 32:351–357. [DOI] [PubMed] [Google Scholar]
- 8.Chesson HW, Bernstein KT, Gift TL, et al. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Preventive Services Task Force. USPTF Recommendations for STI Screening. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/methods/stinfections.htm. Accessed September 12, 2014.
- 10.Tao G, Hoover KW, Kent CK. 2009 cervical cytology guidelines and chlamydia testing among sexually active young women. Obstet Gynecol 2010; 116:1319–1323. [DOI] [PubMed] [Google Scholar]
- 11.Tao G, Hoover KW, Kent CK. Chlamydia testing patterns for commercially insured women, 2008. Am J Prev Med 2012; 42:337–341. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen-Lindquist L, Tao G, Hoover K, et al. Chlamydia screening of young sexually active, Medicaid-insured women by race and ethnicity, 2002–2005. Sex Transm Dis 2009; 36:642–646. [DOI] [PubMed] [Google Scholar]
- 13.Hoover KW, Tao G, Nye MB, et al. Suboptimal adherence to repeat testing recommendations for men and women with positive chlamydia tests in the United States, 2008–2010. Clin Infect Dis 2013; 56:51–57. [DOI] [PubMed] [Google Scholar]
- 14.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis 2010;37:771–776. [DOI] [PubMed] [Google Scholar]
- 15.National HIV Qual data (Ryan White Care). 2013. Available at: http://www.nationalqualitycenter.org/index.cfm/35778/index.cfm/22/82627. Accessed June 14, 2014.
- 16.Center for Medicare & Medicaid Services. 2014. Clinical Quality Measures(CQMs) Pediatric Recommended Core Measures. Available at: http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/2014_CQM_PrediatricRecommended_CoreSetTable.pdf. Accessed February 3, 2014.
- 17.Atherly A, Blake SC. Efforts by commercial health plans to increase Chlamydia trachomatis screening among their members. Sex Transm Dis 2013; 40:55–60. [DOI] [PubMed] [Google Scholar]
- 18.Ginige S, Fairley CK, Hocking JS, et al. Interventions for increasing chlamydia screening in primary care: A review. BMC Public Health 2007; 7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy R, Hocking J, Low N, et al. Interventions to increase rescreening for repeat chlamydial infection. Sex Transm Dis 2012; 39:136–146. [DOI] [PubMed] [Google Scholar]
- 20.Guy RJ, Ali H, Liu B, et al. Efficacy of interventions to increase the uptake of chlamydia screening in primary care: A systematic review. BMC Infect Dis 2011; 11:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou H, Fairley CK, Guy R, et al. The efficacy of clinic-based interventions aimed at increasing screening for bacterial sexually transmitted infections among men who have sex with men: a systematic review. Sex Transm Dis 2012; 39:382–387. [DOI] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 23.Andersen B, Eidner PO, Hagensen D, et al. Opportunistic screening of young men for urogenital chlamydia trachomatis infection in general practice. Scand J Infect Dis 2005; 37:35–39. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong B, Kinn S, Scoular A, et al. Shared care in the management of genital Chlamydia trachomatis infection in primary care. Sex Transm Infect 2003; 79:369–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilardi JE, Fairley CK, Temple-Smith MJ, et al. Incentive payments to general practitioners aimed at increasing opportunistic testing of young women for chlamydia: A pilot cluster randomized controlled trial. BMC Public Health 2010; 10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burstein GR, Snyder MH, Conley D, et al. Chlamydia screening in a Health Plan before and after a national performance measure introduction. Obstet Gynecol 2005; 106:327–334. [DOI] [PubMed] [Google Scholar]
- 27.Kalwij S, French S, Mugezi R, et al. Using educational outreach and a financial incentive to increase general practices’ contribution to chlamydia screening in South-East London. BMC Public Health 2012; 12:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kettinger LD. A practice improvement intervention increases chlamydia screening among young women at a women’s health practice. J Obstet Gynecol Neonatal Nurs 2013; 42:81–90. [DOI] [PubMed] [Google Scholar]
- 29.Lawton BA, Rose SB, Elley CR, et al. Increasing the uptake of opportunistic chlamydia screening: A pilot study in general practice. J Prim Health Care 2010; 2:199–207. [PubMed] [Google Scholar]
- 30.Scholes D, Grothaus L, McClure J, et al. A randomized trial of strategies to increase chlamydia screening in young women. Prev Med 2006; 43:343–350. [DOI] [PubMed] [Google Scholar]
- 31.Shafer MA, Tebb KP, Pantell RH, et al. Effect of a clinical practice improvement intervention on chlamydial screening among adolescent girls. JAMA 2002; 288:2846–2852. [DOI] [PubMed] [Google Scholar]
- 32.Tebb KP, Pantell RH, Wibbelsman CJ, et al. Screening sexually active adolescents for Chlamydia trachomatis: What about the boys? Am J Public Health 2005; 95:1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bissessor M, Fairley CK, Leslie D, et al. Frequent screening for syphilis as part of HIV monitoring increases the detection of early asymptomatic syphilis among HIV-positive homosexual men. J AIDS 2010; 55:211–216. [DOI] [PubMed] [Google Scholar]
- 34.Botes LP, McAllister J, Ribbons E, Jin F, et al. Significant increase in testing rates of sexually transmissible infections following the introduction of an anal cytological screening program, targeting HIV-positive men who have sex with men. Sexual Health 2011; 8:76–78. [DOI] [PubMed] [Google Scholar]
- 35.Cohen CE, Winston A, Asboe D, et al. Increasing detection of asymptomatic syphilis in HIV patients. Sex Transm Infect 2005; 81:217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryder N, Bourne C, Rohrsheim R, et al. Clinical audit: Adherence to sexually transmitted infection screening guidelines for men who have sex with men. Int J STD AIDS 2005; 16:446–449. [DOI] [PubMed] [Google Scholar]
- 37.Snow AF, Vodstrcil LA, Fairley CK, et al. Introduction of a sexual health practice nurse is associated with increased STI testing of men who have sex with men. BMC Infect Dis 2013; 13:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu D, Belda P, Littlejohn D, et al. Adoption of the chronic care model to improve HIV care: In a marginalized, largely aboriginal population. Can Fam Physician 2013; 59:650–657. [PMC free article] [PubMed] [Google Scholar]
- 39.McNulty CA, Hogan AH, Ricketts EJ, et al. Increasing chlamydia screening tests in general practice: A modified Zelen prospective Cluster Randomized Controlled Trial evaluating a complex intervention based on the Theory of Planned Behaviour. Sex Transm Infect 2014; 90:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riley M, Galang S, Green LA, et al. The impact of clinical reminders on prenatal care. Fam Med 2011; 43:560–565. [PubMed] [Google Scholar]
- 41.Rudd S, Gemelas J, Reilley B, et al. Integrating clinical decision support to increase HIV and chlamydia screening. Prev Med 2013; 57: 908–909. [DOI] [PubMed] [Google Scholar]
- 42.Walker J, Fairley CK, Walker SM, et al. Computer reminders for chlamydia screening in general practice: A randomized controlled trial. Sex Transm Dis 2010; 37:445–450. [DOI] [PubMed] [Google Scholar]
- 43.Bissessor M, Fairley CK, Leslie D, et al. Use of a computer alert increases detection of early, asymptomatic syphilis among higher-risk men who have sex with men. Clin Infect Dis 2011; 53:57–58. [DOI] [PubMed] [Google Scholar]
- 44.Callander D, Baker D, Chen M, et al. Including syphilis testing as partner of standard HIV management checks and improved syphilis screening in primary care. Sex Trans Dis 2013; 40:338–340. [DOI] [PubMed] [Google Scholar]
- 45.Hotton AL, Gratzer B, Pohl D, et al. Factors associated with repeat syphilis testing at a large urban LGBT health clinic: Chicago, IL 2002–2008. Sex Transm Dis 2011; 38:205–209. [DOI] [PubMed] [Google Scholar]
- 46.Lister NA, Smith A, Fairley CK, et al. an STD clinic, the Melbourne Sexual Health Centre, Australia. Sex Health 2005; 2:241–244. [DOI] [PubMed] [Google Scholar]
- 47.Allison JJ, Kiefe CI, Wall T, et al. Multicomponent Internet continuing medical education to promote chlamydia screening. Am J Prev Med 2005; 28:285–290. [DOI] [PubMed] [Google Scholar]
- 48.Bowden FJ, Currie MJ, Toyne H, et al. Screening for Chlamydia trachomatis at the time of routine Pap smear in general practice: A cluster randomized controlled trial. Med J Aust 2008; 188:76–80. [DOI] [PubMed] [Google Scholar]
- 49.Gooding HC, Blood EA, Sharma N, et al. An educational intervention to increase internists’ confidence with provision of preventive services to adolescents and young adults. Teach Learn Med 2012; 24:321–326. [DOI] [PubMed] [Google Scholar]
- 50.Kelly C, Johnston J, Carey F, et al. Evaluation of a partnership between primary and secondary care providing an accessible level 1 sexual health service in the community. Int J STD AIDS 2014; 25:751–757. [DOI] [PubMed] [Google Scholar]
- 51.Merritt TD, Durrheim DN, Hope K, et al. General practice intervention to increase opportunistic screening for chlamydia. Sex Health 2007; 4: 249–251. [DOI] [PubMed] [Google Scholar]
- 52.Verhoeven V, Avonts D, Vermeire E, et al. A short educational intervention on communications skills improves the quality of screening for chlamydia in GPs in Belgium: A cluster randomized controlled trial. Patient Educ Couns 2005; 57:101–105. [DOI] [PubMed] [Google Scholar]
- 53.Bilardi JE, Sanci LA, Fairley CK, et al. The experience of providing young people attending general practice with an online risk assessment tool to assess their own sexual health risk. BMC Infect Dis 2009; 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chacko MR, Wiemann CM, Kozinetz CA, et al. Efficacy of a motivational behavioral intervention to promote chlamydia and gonorrhea screening in young women: A randomized controlled trial. J Adolesc Health 2010; 46:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malotte CK, Ledsky R, Hogben M, et al. Comparison of methods to increase repeat testing in persons treatment for gonorrhea and/or chlamydia at public sexually transmitted disease clinics. Sex Transm Dis 2004; 31:637–642. [DOI] [PubMed] [Google Scholar]
- 56.Morgan J, Haar J. General practice funding to improve provision of adolescent primary sexual health care in New Zealand: Results from an observational intervention. Sex Health 2009; 6:203–207. [DOI] [PubMed] [Google Scholar]
- 57.Paneth-Pollak R, Klingler EJ, Blank S, et al. The elephant never forgets: Piloting a chlamydia and gonorrhea retesting reminder postcard in an STD clinic setting. Sex Transm Dis 2010; 37:365–368. [DOI] [PubMed] [Google Scholar]
- 58.Zenner D, Molinar D, Nichols T, et al. Should young people be paid for getting tested? A national comparative study to evaluate patient financial incentives for chlamydia screening. BMC Pubic Health 2012; 12:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourne C,Knight V,Guy R,et al. Shortmessageservicereminderintervention doublessexuallytransmittedinfection/HIV re-testingratesamongmenwho havesex with men. SexTransmInfect 2011; 87:229–231. [Google Scholar]
- 60.Zou H, Fairley CK, Guy R, et al. Automated, computer generated reminders and increased detection of gonorrhea, chlamydia and syphilis in men who have sex with men. PLoS One 2013; 8:e61972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sequist TD, Gandhi TK, Karson AS, et al. A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. J Am Med Inform Assoc 2005; 12:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holt TA, Thorogood M, Griffiths F, et al. Changing clinical practice through patient specific reminders available at the time of the clinical encounter: Systematic review and meta-analysis. J Gen Int Med 2012; 27:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matheson EC, Derouin A, Gagliano M, et al. Increasing HPV vaccination series completion rates via text message reminders. J Pediatr Health Care 2014; 28:e35–e39. [DOI] [PubMed] [Google Scholar]
- 64.Stockwell MS, Kharbanda EO, Martinez RA, et al. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: A randomized controlled trial. JAMA 2012; 307:1702–1708. [DOI] [PubMed] [Google Scholar]