Abstract
Introduction:
The incidence of biliary tract cancer (BTC) is increasing, and the disease is frequently diagnosed during advanced stages, leading to poor overall survival. Limited treatment options are currently available and novel therapeutic approaches are needed. A number of completed clinical trials have evaluated the role of chemotherapy for BTC, demonstrating a marginal benefit. Thus, there is increased interest in applying targeted therapies for this disease.
Areas Covered:
This review article summarizes the role of chemotherapeutic regimens for the treatment of BTC, and highlights key signal transduction pathways of interest for targeted inhibition. Of particular interest are the MEK or MAP2K (mitogen-activated protein kinase kinase), phosphatidylinositol-3 kinase (PI3K) and signal transducer and activator of transcription-3 (STAT3) pathways. We discuss the available data on several promising inhibitors of these pathways, both in the pre-clinical and clinical settings.
Expert Opinion:
Future treatment strategies should address targeting of MEK, PI3K and STAT3 for BTC, with a focus on combined therapeutic approaches.
Keywords: biliary tract cancer, cholangiocarcinoma, targeted therapy, STAT3, PI3K, MEK
1.0. Introduction
Biliary tract cancer (BTC) is comprised of intra- and extrahepatic cholangiocarcinoma and cancers of the gallbladder. This malignancy results from the transformation of epithelial cells that line the bile duct tree. The 5-year survival rate for this rare malignancy is dismal at only 3.2% [1]. Surgery represents an option for a subset of patients with BTC, especially those without metastases or invasion of nearby tissues. Approximately 65% of patients with this malignancy are eligible to undergo surgical resection, although only 50% of those who underwent surgery achieved curative or margin-free resection (R0) [2]. Gallbladder cancers present with distant metastatic disease in approximately 85% of patients upon recurrence, whereas 60% of cholangiocarcinoma patients present primarily with a local regional pattern upon disease recurrence [3]. These data underscore the urgency for developing improved therapeutic options in this disease. Here, we review approaches for the treatment of BTC and highlight ongoing pre-clinical studies that may provide new therapeutic opportunities, with a focus on signal transduction pathways.
2.0. Combination chemotherapy treatment options in BTC
Over the last three decades, more than 50 articles reporting the results of clinical trials in BTC have been published (Table 1) [4–66]. Of these, the majority have focused on evaluation of chemotherapeutic agents. Thirty trials utilized gemcitabine (Gem)-based regimens: 7 trials included combination therapy with cisplatin (Cis), 4 with capecitabine (Cap), 9 with oxaliplatin (Ox) and 4 with S-1, oral fluoropyrimidine. In a single arm phase II study, Knox et al evaluated the combination of gemcitabine and capecitabine for 75 patients with BTC, of which 22 had objective responses with a median PFS and OS of 6.2 and 12.7 months, respectively [15]. Gemcitabine plus oxaliplatin (GEMOX) has also been evaluated in a phase II study of 56 patients (n = 19 GBC, n = 5 ECC, n = 3 ampulla of vater, n = 29 ICC). This trial reported a response rate of 36% in 33 patients who had not received prior treatment. These individuals demonstrated median PFS and OS of 5.7 and 15.4 months, respectively [24]. Based on the promising activity observed with the combination of gemcitabine and platinum based therapy in earlier trials, ABC-02, the largest randomized phase III trial in BTC to date, was conducted to investigate the efficacy of these agents in patients with unresectable BTC. In this study, 410 patients with locally advanced or metastatic disease, including all anatomic subgroups (cholangiocarcinoma, gallbladder and ampullary) were randomized to receive gemcitabine and cisplatin (GemCis) or gemcitabine alone, with overall survival (OS) as the primary endpoint. The combination of GemCis resulted in increased median OS (11.7 months) compared to patients treated with single agent Gem (8.1 months). GemCis also resulted in an increased median progression-free survival (PFS) of 8 months in patients receiving the combination as compared to 5 months for patients treated with Gem alone [59]. However, a more recent pooled analysis of 104 trials did not demonstrate any significant benefit of GemCis in either time to tumor progression (TTP), or median OS as compared to GemCap or GEMOX [67]. Though a phase III randomized trial would be necessary to access clinical advantages between the different gemcitabine-based regimens, GemCis has become the standard approach in treating locally advanced or metastatic BTC based on the data from the ABC-02 trial. Finally, clinical activity has been observed for advanced BTC with single agent, oral fluorpyrimidine, S-1 in the setting of a Phase II trial [12]. The combination of S-1 with gemcitabine also showed favorable activity in a randomized phase II trial versus S-1 alone with an acceptable safety profile [65]. These data have led to a randomized Phase III study of gemcitabine and S-1 that is powered to assess non-inferiority against the current standard of care consisting of gemcitabine and cisplatin [65]. Taken together, there are a number of ongoing clinical trials utilizing chemotherapy that will provide important data upon completion (Table 2).
Table 1.
Published clinical trials on BTC.
| Single agent trials | Reference |
|---|---|
| Capecitabine | [4] |
| Docetaxel | [5, 6] |
| Erlotinib | [7] |
| Gemcitabine | [8, 9] |
| Irinotecan | [10] |
| MK2206 | [11] |
| S-l | [12] |
| Sunitinib | [13] |
| Gemcitabine (Gem) combination based trials | Reference |
| Gem + capecitabine | [14–18] |
| Gem + cetuximab | [19] |
| Gem + cisplatin | [20–22] |
| Gem + docetaxel | [23] |
| Gem + oxaliplatin | [24–27] |
| Gem + oxaliplatin + bevacizumab | [28] |
| Gem + oxaliplatin + cetuximab | [29] |
| Gem + cisplatin + S-l | [30, 31] |
| Fluorouracil (5-FU) combination based trials | Reference |
| 5-FU + cisplatin | [32] |
| 5-FU + IFNα−2b | [33] |
| 5-FU + leucovorin | [34–36] |
| 5-FU + carboplatin + leucovorin | [37] |
| 5-FU + cisplatin + epirubicin | [38] |
| 5-FU + cisplatin + leucovorin | [39] |
| 5-FU + doxorubicin + mitomycin C | [40] |
| 5-FU + epirubicin + leucovorin + methotrexate | [41] |
| FOLFIRI + bevacizumab | [42] |
| Gemcitabine + 5-FU combination based trials | Reference |
| Gem + 5-FU + leucovorin | [43] |
| Gem + 5-FU + cisplatin + epirubicin | [44] |
| Gem + oxaliplatin + 5-FU | [45] |
| Other combination trials | Reference |
| Bevacizumab + erlotinib | [46] |
| Capecitabine + cisplatin | [47, 48] |
| Capecitabine + oxaliplatin | [49] |
| Capecitabine + cisplatin + epirubicin | [50] |
| S-l + oxaliplatin | [51] |
| S-l + valproic acid | [52] |
| 2-arm Comparison trials | Reference |
| 5-FU vs. 5-FU + doxorubicin + mitomycin C | [53] |
| Gem vs. Gem + 5-FU + leucovorin | [54] |
| Mitomycin C + Gem vs. mitomycin C + capecitabine | [55] |
| 5-FU + etoposide + leucovorin vs. 5-FU + cisplatin + epirubicin, | [56] |
| Gem vs. Gem + cisplatin | [57–59] |
| Gem + oxaliplatin vs. Gem + oxaliplatin + erlotinib | [60, 61] |
| Gem + oxaliplatin vs. Gem + oxaliplatin + cetuximab | [62] |
| Gem vs. Gem + sorafenib | [63] |
| 5-FU vs. capecitabine vs. 5-FU + irinotecan vs. 5-FU + oxaliplatin vs. 5-FU + cisplatin vs. sunitinib | [64] |
| S-l vs. S-l + Gem | [65] |
| Photodynamic therapy (PDT) vs. PDT + S-l | [66] |
5-FU - fluorouracil, FOLFIRI - irinotecan with fluorouracil and folinic acid, Gem - gemcitabine
Table 2.
Completed clinical trials in BTC.
| Type of Trial | Patient Characteristics | Treatments | Outcome | Reference/ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Phase I | Patients at risk of CCA recurrence | Gem + cisplatin + sirolimus (rapamycin) | Completed, results not yet posted | |
| Phase I/II | CCA | Gem + cisplatin + floxuridine | Completed, results not yet posted | |
| Phase II | Advanced and/or inoperable CCA | Gem + capecitabine | Completed, results not yet posted | |
| Phase II | Metastatic or unresectable biliary cancers | Erlotinib + bevacizumab | Median survival: 9.9 months; TTP: 4.4 months | |
| Phase II | CCA | Gem + capecitabine + bevacizumab | Completed, results not yet posted | |
| Phase I/II | CCA | Gem + cisplatin | Completed, results not yet posted | |
| Phase II | Advanced or metastatic BTC | Imatinib + 5-FU + leucovorin | Completed, results not yet posted | |
| Phase II | BTC | Gem + oxaliplatin vs. panitumumab + gem + oxaliplatin | Completed, results not yet posted |
5-FU - fluorouracil, CCA - cholangiocarcinoma, TTP - time-to-progression
3.0. Novel targeted therapies in the treatment of BTC
While up to 80% of BTC patients experience some benefit from chemotherapy, the great majority ultimately develop resistance, emphasizing the need to identify more efficacious therapies. Further, novel targeted therapies may offer greater tolerability in cancer patients. This premise is centered around the idea that malignant cells may be more dependent on the activation of the targeted pathways [68–70]. Chemotherapeutic agents typically target rapidly proliferating cells irrespective of whether they are malignant or normal. For this reason, adverse events often manifest as fatigue, diarrhea, and nausea [71], and are prevalent in the majority of patients during the course of chemotherapy. Ongoing efforts to improve BTC treatment regimens can be seen in the context of numerous ongoing clinical trials (summarized in Table 3). A growing number of trials are focused on small molecule or Ab-based targeted therapies that inhibit intracellular signal transduction pathways, kinases, survival pathways or receptors (i.e. VEGF, EGFR) [28] [46].
Table 3.
Ongoing clinical trials in BTC.
| Type of Trial | Treatments | ClinicalTrials.gov Identifier |
|---|---|---|
| Phase 0 | HAI of FURD + dexamethasone vs. HAI of FURD + dexamethasone + gem vs. HAI of FURD + dexamethasone + gem + oxaliplatin | |
| Phase I | Acelarin + Cisplatin | |
| Phase I | DKN-01 + gem + cisplatin | |
| Phase I | HAI of FURD + dexamethasone + gem | |
| Phase I | Pazopanib + GSK1120212 | |
| Phase I/II | Amphinex + gem vs. gem + cisplatin | |
| Phase I/II | ARQ 087 | |
| Phase I/II | CX-4945 + gem + cisplatin | |
| Phase I/II | Cytokine induced killer cells | |
| Phase I/II | Gem + cisplatin + MEK162 | |
| Phase II | Abraxane + gem + cisplatin | |
| Phase II | ASLAN001 | |
| Phase II | BGJ398 (pan FGFR kinase inhibitor) | |
| Phase II | Cabozantinib | |
| Phase II | Ceritinib (LDK378) | |
| Phase II | FOLFIRINOX | |
| Phase II | Gem + cisplatin + selumetinib | |
| Phase II | Gem + oxaliplatin + capecitabine vs. gem + oxaliplatin + capecitabine + panitumumab | |
| Phase II | Gem + oxaliplatin + capecitabine + panitumumab vs. gem + oxaliplatin + catecitabine + bevacizumab | |
| Phase II | HAI of FURD + dexamethasone + gem + oxaliplatin | |
| Phase II | Low-dose radiation + gem + cisplatin | |
| Phase II | Nab-paclitaxel + gem | |
| Phase II | Pembrolizumab | |
| Phase II | Ramucirumab | |
| Phase II | Refametinib | |
| Phase II | Regorafenib | |
| Phase II | RRx-001 + gem + cisplatin | |
| Phase II | Sunitinib | |
| Phase Ha | Trametinib | |
| Phase II/III | Radiofrequency ablation + cytokine-induced killer cells (CIK) transfusion | |
| Phase III | Gem + cisplatin | |
| Phase III | Gem + oxaliplatin vs. capecitabine | |
| Phase III | Oxaliplatin |
5-FU - fluorouracil, FOLFIRI - irinotecan with fluorouracil (5FU) and folinic acid, FOLFIRINOX - FOLFIRI plus oxaliplatin, FURD - floxuridine, Gem - gemcitabine, HAI - hepatic arterial infusion.
However, resistance to targeted therapy remains a concern, due to the cross-talk and redundancy in the maintenance of key pathways in this disease [72]. Various studies looking at known oncogenic drivers in BTC (summarized in Table 4) [73–91], as well as whole-exome sequencing of responders to single-agent selumetinib (MEK inhibitor) [92], all failed to identify any common mutational signatures, highlighting the heterogeneous nature of the disease.
Table 4.
Genetic alterations and abnormal protein expression found in BTC.
| Gene | Percentage of patients | Function/Alterations | Reference |
|---|---|---|---|
| Proliferation | |||
| KRAS | 24.6% of BTC | Mutation at codon 12 or 13, constitutively active protein | [73] |
| 54% of BTC | [74] | ||
| 45% of BTC | [75] | ||
| 33% of BTC | [76] | ||
| 7.4% ICC | [77] | ||
| 5% of ICC and 23% of ECC | [78] | ||
| BRAF | 22% of BTC | Mutations at various codons | [75] |
| 7.4 of ICC | V600E substitution, constitutively | [77] | |
| 3% of ICC | active protein | [78] | |
| EGFR | 13.6% of BTC | Mutations at various codons | [79] |
| 13–15% of BTC | [80] | ||
| 26.5% of BTC | [81] | ||
| RASSF1A | 69% of BTC | Promoter hypermethylation, decreased transcriptional activity | [82] |
| 68.75% of ECC | [83] | ||
| 27% of BTC | [84] | ||
| PIK3CA | 8.2% of BTC | Gain of function mutation | [81] |
| 9% of ICC and 0% of ECC | [85] | ||
| Cell-cycle regulation | |||
| SMAD4 | 45.2% of ICC | Loss of protein expression | [86] |
| CDKN2A (p16) | 35.7% of ICC | Loss of protein expression | [86] |
| 16.1%, 57.1%, 20% of BTC | Hypermethylation, mutation, LOH | [87] | |
| TP53 | 5% of ICC and 14% of ECC | G245S and R175H substitution, loss of protein function | [78] |
| 37% of ICC | Loss of protein function | [88] | |
| Chronic inflammation | |||
| SOCS3 | 27% of ICC | Promoter hypermethylation | [89] |
| Metabolism | |||
| IDH1/2 | 23% of ICC and 0% of ECC | Mutation decrease protein function | [78] |
| 10% of ICC | [90] | ||
| 28% of ICC and 7% of ECC | [91] | ||
BTC - biliary tract cancer, CDKN2A - cyclin-dependent kinase inhibitor 2A, ECC - extrahepaticcholangiocarcinoma, EGFR - epidermal growth factor receptor, ICC - intrahepatic cholangiocarcinoma, IDH1/2 - isocitrate dehydrogenase, LOH - loss of heterozygosity, PIK3CA – phosphatidylinositol−4,5-bisphosphate 3-kinase, RASSF1A - Ras association domain family 1 isoform A, SMAD4- mothers against decapentaplegic homolog 4, SOCS3 - suppressor of cytokine signaling 3,TP53- tumor suppressor protein p53
Since current treatment options for BTC patients have shown limited improvement in PFS and OS, testing of new potential targeted therapies continues. Candidate pathways of interest for targeted therapy will be discussed in further detail below, with a focus on inhibitors targeting the MAPK, PI3K, and JAK/STAT pathways.
3.1. MEK Inhibitors
The Ras/Raf/MEK/ERK signaling cascade is among the most commonly dysregulated pathways in human cancers [93]. MEK, also known as MAP2K (mitogen-activated protein kinase kinase), is part of the Ras/Raf/MEK/ERK (MAPK) pathway. Depending on the stimulus, activation of this pathway can result in context-dependent effects on apoptosis or the cell cycle [94]. Aberrant activation of this pathway is frequently observed in BTC, as well as in melanoma, lung carcinoma, pancreatic, and colon cancers, among others [81, 95]. Although several studies have documented BRAF mutations in BTC [75, 77, 78], the existing evidence indicates a number of potential advantages to targeting MEK rather than its upstream mediators of activation, such as B-Raf. First, inhibition of MEK signaling can be accomplished without genetic testing to identify mutations leading to the aberrant activation of this pathway, as certain B-Raf inhibitors in the presence of RAS mutations can lead to reactivation of Raf and development of resistance necessitating such genetic screening [96]. Second, MEK1/2 have a narrow substrate specificity [95], and are only known to activate ERK1/2 [97], whereas there are 3 families of Raf proteins and ERK1/2 has numerous downstream targets [98]. Accounting for the properties of the proteins involved, MEK represents a point of convergence for many signaling pathways, thereby making it an attractive target for mitigating the effect of pathway activation.
With the exception of E6201, most MEK inhibitors do not target ATP binding. This allows a relatively higher specificity, as ATP binding sites tend to be highly conserved [99]. Indeed, the structure of MEK1 and MEK2 allows allosteric inhibitors to bind in a hydrophobic pocket which does not overlap with the ATP-binding site [100]. A summary of the MEK inhibitors being used in both preclinical studies and in clinical trials is provided in Table 5 [95, 96, 99, 101–166]. Trametinib, a well-studied MEK inhibitor, was approved by the FDA in 2013 after a phase III trial demonstrated superior efficacy over standard chemotherapy in melanoma patients with BRAF V600E/K mutations. Patients who received trametinib had a median PFS of 4.8 months, versus 1.5 months for those on chemotherapy (p < 0.001). The OS at 6 months was 81% on trametinib compared to 67% on standard chemotherapy [134]. This MEK inhibitor is currently being evaluated in the setting of a phase I clinical trial for BTC patients ().
Table 5.
Inhibitors of MEK/PI3K/STAT3
| Pathway | Name | Target | Clinical trial | Cancers | References |
|---|---|---|---|---|---|
| MAPK | PD098059 | MEK1/2 | Preclinical | Advanced hematological and advanced solid cancers | [101–104] |
| U0126 | MEK1/2 | Preclinical | Advanced hematological and advanced solid cancers | [103–105, 107, 108] | |
| AZD8330 | MEK1/2 | Phase I | Advanced solid tumors | [109] | |
| E6201 | MEK1 | Phase I | Melanoma | [99, 110, 111] | |
| PD-0325901 | MEK1/2 | Phase I | Melanoma, NSCLC | [110, 112, 113] | |
| Pimasertib (AST03026) | MEK1/2 | Phase I | Colorectal, multiple myeloma | [115] | |
| RO4987655 | MEK1 | Phase I | Melanoma | [116] | |
| RO5126766 | Raf/MEKl/2 | Phase I | Melanoma | [117] | |
| TAK733 | MEK1/2 | Phase I | Melanoma, NSCLC, colorectal, breast | [114, 118] | |
| MEK162 | MEK1/2 | Phase I/II | NRAS mutant melanoma, NSCLC, pancreatic, BTC | [119] | |
| Selumetinib (AZD6244) | MEK1 | Phase I, II | Melanoma HCC, pancreatic, colon, lung, breast, NSCLC | [120–133] | |
| Refametinib (RDEA119) | MEK1/2 | Phase II | HCC, melanoma, colorectal | [95] | |
| WX-554 | MEK1/2 | Phase II | Advanced solid tumors | [95] | |
| Trametinib (GSK1120212) | MEK1/2 | Phase III | Melanoma, colorectal | [134] | |
| Cobimetinib (GDC-0973) | MEK1 | Phase I, II, III | Advanced solid tumors, melanoma | [135–138] | |
| PI3K | LY294002 | PI3K and other related kinases | Preclinical | Fibrosarcoma | [105, 107, 139–141] |
| PI-103 | PI3K, mTORC1/2, DNA-PK | Preclinical | Glioma prostate, colon, NSCLC | [142–146] | |
| PWT-458 | PI3K | Preclinical | NSCLC, glioblastoma, renal | [147, 148] | |
| Wortmannin | PI3K, mTOR, DNA-PK, MAPK | Preclinical | Advanced hematological and advanced solid cancers | [135, 142, 149] | |
| ZSTK474 | PI3Ks | Preclinical | NSCLC, melanoma, ovarian, prostate | [150, 151] | |
| BAY 80–6946 | PI3K(p110α, p) | Phase I | Lymphoma, esophageal, pancreatic | [135] | |
| GDC-0032 | PI3K(pl00α,-δ,-γ) | Phase I | Breast, NSCLC | [135] | |
| GSK-2126458 | PI3K, mTOR | Phase I | Renal cell, bladder | [152] | |
| IPI-145 | PI3K(p110 δ, γ) | Phase I | Leukemia, lymphoma | [135] | |
| BEZ-235 | PI3K, mTOR | Phase I, II | Breast, glioma, melanoma, pancreatic | [135, 153–156] | |
| BGT-226 | PI3K, mTOR | Phase I/II | Solid tumors, breast | [157] | |
| BKM120 (Buparlisib) | PI3K | Phase I/II | Breast, glioblastoma, NSCLC | [135,158] | |
| BYL-719 | PBK(p110 α) | Phase I/II | Breast, cervical, ovarian, head and neck | [135] | |
| GDC-0941 | PI3K(p110α),Flt3 | Phase I/II | Lymphoma, NSCLC, breast, melanoma, pancreatic endometrial | [135, 159–161] | |
| GDC-0980 | PI3K, mTOR | Phase I/II | Prostate | [135] | |
| PX-866 | PI3K | Phase I/II | Glioblastoma, breast, colon, prostate, NSCLC, pancreatic, ovarian | [135, 162, 163] | |
| XL-147 | PI3K | Phase I/II | NSCLC, solid tumors, glioblastoma | [96, 135] | |
| XL-765 | PI3K, mTOR | Phase I/II | Glioma, NSCLC | [164] | |
| PF-04691502 | PI3K, mTOR | Phase II | Endometrial | [135] | |
| PF-05212384 | PI3K(p110 α, γ), mTOR | Phase II | Solid tumors, colon | [135] | |
| CAL-101 (Idelalisib) | PI3K(p110δ) | Phase III | Leukemias, lymphomas, myeloma | [135] | |
| STAT3 | OPB-31121 | STAT3 | Phase I | Advanced HCC | [165] |
| AZD9150 | STAT3 | Phase I/II | Lymphoma, HCC, ovarian, GI | , , , , [166] |
|
| BBI6018 (napabucasin) | STAT3 | Phase I/II/III | Hematologic malignancies, colorectal, GI, pancreatic, HCC, glioblastoma, NSCLC, mesothelioma, | , , , , , , , , , , , , , , , |
BTC - biliary tract cancer, DNA-PK - DNA-dependent protein kinase, Flt3 - Fms-related tyrosine kinase 3, GI - gastrointestinal, HCC - hepatocellular carcinoma, MEK - extracellular signal-regulated kinase (ERK) kinase, mTOR - mammalian target of rapamycin, NSCLC – non-small cell lung cancer,PI3K - phosphoinositide 3-kinase, STAT3 - signal transducer and activator of transcription 3
The MEK inhibitor MEK162 has been evaluated for safety in a phase I dose-escalation study of advanced solid tumors, and showed signs of clinical efficacy and desirable pharmacokinetics. This agent had an acceptable safety profile at 60 mg twice daily [167]. This small molecule is currently under investigation for BTC in combination with GemCis in a phase I/II clinical trial (NCT 01828034). One encouraging phase II study in metastatic BTC patients using selumetinib (another MEK inhibitor), observed clinical activity in 28 patients, with a median PFS of 3.7 months, and median OS of 9.8 months. Interestingly, the clinical activity of selumetinib in this cohort of patients was not associated with BRAF or KRAS mutations (as assessed by pre-treatment sequencing of tumor biopsies)[168]. Another notable observation from this clinical trial was the fact that administration of selumetinib led to a gain in lean muscle mass in BTC patients. These data imply that MEK inhibitors may provide some benefit to patients by limiting the cancer cachexia syndrome that accompanies BTC and other advanced malignancies. Pre-clinical studies using MEK162 in a classic model of colon-26 cancer cachexia confirmed that the muscle sparing effects of MEK inhibitors can occur in a manner independent of their action on the tumor cells [169].
MEK inhibition appears to be an effective therapy in a small subset of BTC patients. However, the overall effects on clinical response have been modest. That said, several ongoing clinical trials using MEK inhibitors in combination with chemotherapeutic agents in BTC may establish their role in the treatment of this disease and its utility as an agent to combat the cancer cachexia syndrome deserves further investigation (Table 3).
3.2. PI3K Inhibitors
Phosphotidylinositol-3 kinase (PI3K) is the first of the downstream proteins regulated in the PI3K/Akt pathway following activation of associated receptors. This pathway is important for numerous biological functions in malignant cells, including proliferation, senescence, and survival [170]. Disruption in the regulation of this pathway has been associated with up to one-third of all cancers [171–173], and has demonstrated a prominent role in BTC [174–178]. Constitutive activation of PI3K can occur via several mechanisms including genomic alterations in PIK3CA or PTEN [179, 180]. Due to the extensive involvement of the PI3K pathway in different components of cell-cycle regulation and survival, there are ongoing efforts to develop and test inhibitors specific to this pathway. Table 5 lists both PI3K inhibitors in pre-clinical testing and those currently in clinical trials [96, 105, 107, 135, 139–164, 181]. Buparlisib is one orally bioavailable pan-class I PI3K inhibitor that was well tolerated in patients as a single agent. In combination with other chemotherapeutic agents, buparlisib demonstrated clinical activity in patients with advanced breast cancer [135]. Another PI3K inhibitor GDC-0941 has been tested concurrently with GDC-0973 (a MEK1/2 inhibitor). The regimen was well-tolerated, and also showed clinical responses in patients with melanoma, pancreatic cancer, NSCLC, prostate cancer, and endometrioid cancer [135]. Results from this combination are encouraging for future studies incorporating inhibitors of MEK and PI3K.
To date, inhibitors directly targeting PI3K activity have not been utilized extensively as either single agents, or as combination treatment in the context of clinical trials for BTC patients. However, one clinical trial in BTC patients was recently completed (), utilizing an Akt inhibitor (MK2206) to limit the activation of this pro-survival pathway. Results from this trial indicated this drug was tolerable, but no clinical activity was observed [11]. Thus, targeting the PI3K/Akt signaling pathway may be a potential strategy to overcome resistance if combined with other agents. Thus this pathway represents an interesting target for BTC that deserves more rigorous pre-clinical and potentially clinical evaluation.
3.3. STAT3 Inhibitors
Another pathway relevant to BTC is the JAK/STAT signaling cascade [89, 182, 183]. There are 7 Signal Transducer and Activator of Transcription (STAT) family members (STAT1–4, STAT5a, STAT5b, STAT6), all transcription factors. Though structurally similar, these proteins have distinct cellular functions. Of the STAT proteins, STAT3 and STAT5 most frequently undergo constitutive activation in malignancy [184–186]. These proteins act in an oncogenic manner and promote the expression of genes that enhance metastatic spread and survival [187–189]. STAT3 activation has been observed in numerous human cancer cell lines, including BTC, and is thought to act downstream of IL-6 or other cytokines to promote progression of the disease [190–195]. The diverse pro-tumorigenic cellular functions regulated by STAT3 signaling makes this pathway an attractive target. Agents targeting upstream JAK have also been of interest as a way of mediating STAT3 signaling, especially in myeloproliferative neoplasms given a high frequency of activating JAK2V617F mutations. Ruxolitinib is a JAK1/2 inhibitor, and has undergone several phase I, II, and III clinical trials and is approved in the treatment of myelofibrosis based on significant improvement in splenomegaly [196, 197]. However, in a randomized, double-blind phase II trial in patients with metastatic pancreatic cancer that failed gemcitabine therapy, ruxolitinib combined with capecitabine did not demonstrate a significant improvement in OS or PFS [198]. Furthermore, a recent phase III trial combining Jakafi (JAK1/2) with capecitabine was discontinued after interim analyses in pancreatic cancer patients (). To date, JAK/STAT inhibitors have not been evaluated in human clinical trials for BTC.
Natural products have been one driving force in the development of STAT3 inhibitors, as many synthetic products are modified from natural compounds that were identified to inhibit STAT3 [199]. The majority of STAT3 inhibitors have been designed in an effort to prevent its phosphorylation or STAT:STAT dimerization. A variety of naturally-derived and synthetic STAT3 inhibitors that target various STAT3 interactions are summarized in Table 5 [165, 166]. One major limitation to natural product-derived compounds has been their bioavailability, which has limited their in vivo efficacy.
Likewise, only two STAT3 small molecule inhibitors have undergone investigation in the clinical setting. OPB-31121 is an inhibitor of STAT3 phosphorylation developed by Otsuka Pharmaceuticals Co. This molecule was evaluated in a phase I clinical trial in patients with advanced HCC [165]. Twenty-four patients were enrolled, and 26% experienced stable disease≥ 8 weeks. The second,, BBI-608, is a small molecule STAT3 inhibitor developed by Boston Biomedical, Inc. BBI-608 inhibits STAT3 and can inhibit expression of genes involved in the cancer stem cell phenotype. This approach may be of interest for BTC, given the role of cancer stem cells in this disease [200–203]. Although not investigated formally for BTC, this aspect has been the primary focus of preclinical studies using BBI-608. In vitro studies demonstrate this agent has activity against prostate and pancreatic cancer cell lines. BBI-608 down-regulated β-catenin and c-Myc in pancreatic cancer at the level of protein expression [204] and decreased expression of these same mRNAs in prostate cancer cell lines [205]. Further studies showed that in vivo treatment of mice with BBI-608 strongly inhibited the growth of PC-3 prostate cancer xenografts [205]. In pancreatic cancer xenograft models using the PaCa-2 cell line, BBI-608 likewise slowed tumor growth as compared to mice treated with gemcitabine [204]. Several ongoing phase III studies are investigating the combination of BBI-608 with chemotherapy in multiple GI malignancies, including gastric, colon and pancreatic cancer (Table 5).
In addition to the role of STAT3 and β-catenin in regulating stemness, these proteins each play important roles in regulating immune evasion in advanced malignancy. This property may also be of interest in BTC, given the immunosuppressive features of disease. As mentioned previously, STAT3 plays an important role in regulating T cell phenotypes and the expansion of immunosuppressive myeloid cells [206]. With regards to β-catenin, a recent study by Spranger et al. reported that activated β-catenin signaling was associated with gene expression signatures in patient melanoma tumors indicative of limited T cell infiltration [207]. This and other studies suggest that targeting STAT3 and β-catenin may augment the efficacy immunotherapy. However, it is also appreciated that Wnt/β-catenin signaling plays a key, T-cell intrinsic role in balancing the generation of CD8+ memory T cells that may be instrumental in maintenance of effective anti-tumor immune responses [208, 209]. Several ongoing clinical trials are utilizing BBI-608 in combination with chemotherapy, inhibitors of VEGF or MEK, and immune checkpoint inhibitors (Table 5) and will provide valuable information.
Another clinically relevant approach to STAT3 inhibition is using AZD9150, an antisense oligonucelotide. This agent has been tested in patients with advanced lymphoma and solid tumors. With promising results observed in two-thirds of patients with diffuse large B-cell lymphoma (DLBCL), AZD9150 is currently under evaluation in several other clinical trials. For example, phase I studies of AZD9150 as a single agent are ongoing in HCC patients (), as a single agent in phase II studies in ovarian and GI cancers () and in combination with immunotherapies such as MEDI4736, an anti-programmed death-ligand 1 (PD-L1) inhibitor and tremelimumab, an anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody () in diffuse large B cell lymphoma. Though no results from these clinical trials are available yet, the outcome will surely have significant implications on targeting STAT3 in the setting of BTC, given the role of this pathway in its pathology.
4.0. Resistance to Targeted Inhibition
Resistance to targeted, small-molecule therapies represents an ongoing challenge for numerous malignancies including BTC. Inhibition of the MEK and PI3K signaling pathways are particularly subject to resistance based on several published studies. For example, resistance to single agent MEK inhibition is mediated via both PI3K and STAT3 signaling pathways in BTC and other cancers, providing rational approaches to combined therapy [72, 210, 211]. Further, MEK inhibition results in MYC-dependent transcriptional upregulation of ERBB3, leading to cell intrinsic drug resistance in KRAS mutant tumors [212]. Similarly, resistance to PI3K inhibitors can occur via multiple mechanisms. These include cell-intrinsic events such as negative feedback loops mediated via downstream mTOR signaling or MYC accumulation resulting activation [213, 214]. Alternatively, upstream receptor tyrosine kinase activation can also occur, maintaining the signaling through this pathway [215].
Further contributing to resistance to these inhibitors are the inherent crosstalk between each of these signaling pathways. This multi-directional communication occurs by virtue of common features of both the receptors and their ligands. For instance, IL-6, GM-CSF, and EGF can activate MEK, PI3K, and STAT3 signal transduction [216, 217] as these cytokines activate kinases associated with each pathway. IL-6, an inflammatory cytokine, is highly elevated in patient BTC tumors and circulating blood, and is secreted from human BTC cell lines. This and other gp130 interacting cytokines can also function in an autocrine manner to signal simultaneously via the MEK, PI3K, and STAT3 pathways (Fig. 1). Thus, inhibition of any individual pathway has the potential to promote compensatory activation of other signaling nodes, ultimately leading to drug resistance. Other proteins can behave in a manner that activates redundant signal transduction between these pathways. For example, Src can activate STAT3, Raf-1, and A-Raf, while B-Raf can be phosphorylated independently of Src [94]. Similarly, activated Ras can interact with PI3K, leading to its activation [218], while the PI3K-TOR and STAT3 signaling pathways are functionally linked [219]. Besides signaling to downstream Raf, Ras can also activate PI3K by directly binding to the catalytic subunit [220–222], thus bypassing the need for EGFR activation, and rendering EGFR inhibition ineffective.
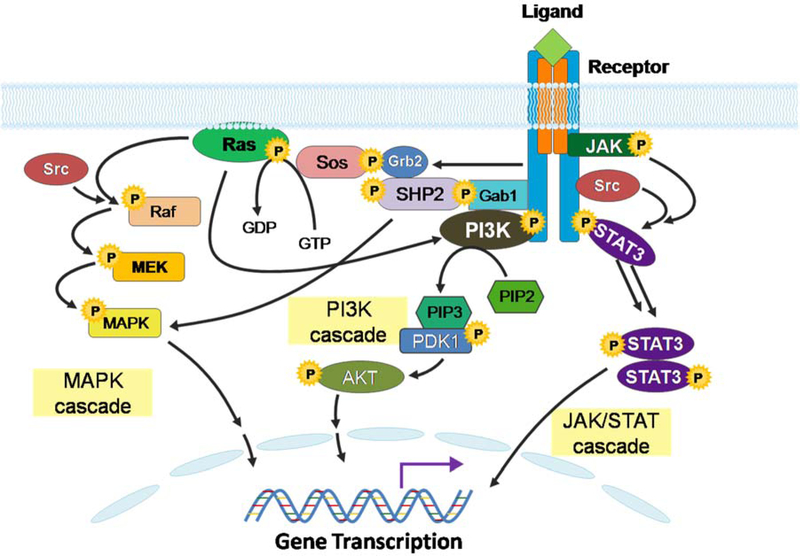
Figure 1.
Network of gp130-interacting signaling pathways. This figure illustrates the network of integral signaling pathways that are aberrantly activated in a variety of cancers, as well as in BTC. Mitogen-activated protein kinases (MAPK), phosphoinositide 3-kinase (PI3K), and Janus kinase/signal transducer and activator of transcription (Jak/STAT) signaling cascades cross talk and contribute to tumor growth, proliferation and treatment resistance.
Inhibition of multiple signaling pathways, notably those that crosstalk and can act to compensate one another, represents a rational strategy in the treatment of BTC. Several recent studies have examined the mechanisms by which cancers may bypass the inhibitory effects of these drugs. In vitro resistance to the MEK inhibitor AZD6244 in BTC was overcome with the addition of Akt or mTOR inhibitors (MK-2206 and AZD8055), and was most likely effective due to inhibition of the feedback mechanism on the PI3K pathway [210]. Such resistance has also been observed in cancers with KRAS mutations [211]. Notably, the STAT3 pathway also functions as a mechanism of resistance to MEK inhibition in KRAS mutated pancreatic and colon cancers [72]. Such compensatory signaling mechanisms showcase the need for concurrent targeting of these pathways to circumvent resistance.
5.0. Conclusion
Applying targeted therapeutic approaches to the treatment of BTC represents an area that remains largely unexplored. Given the complicated pathology and heterogeneity of BTC, inhibiting a single oncogenic signaling pathway is unlikely to elicit durable complete responses. Combined targeting of multiple signaling pathways may be an important strategy to improve efficacy and limit resistance. The small molecule inhibitors discussed also have the potential to be tested in combination with other modalities, including radiotherapy and immunotherapy. Given that MEK, PI3K, and JAK/STAT pathways may be involved in the radio-resistance or immune suppression observed in cancer patients, concurrent inhibition of these pathways may impact efficacy.
Expert Opinion
BTC is a refractory tumor that has poor outcomes. Chemotherapeutic regimens have been evaluated extensively in clinical trials and have provided only incremental benefit. Recent data has uncovered a role for many key pro-survival and inflammatory pathways that are candidates for targeting therapeutically. These findings hold strong potential for identification of new treatment approaches that may produce durable clinical activity in this aggressive malignancy. It is likely that targeting constitutively active signal transduction pathways could benefit patients with advanced biliary cancer, when they are administered together with chemotherapeutic approaches, or when applied in combination based on supportive pre-clinical data. Among the most notable of these pathways which can be subject to pharmacologic inhibition are the MEK, PI3K and STAT3 pathways. These pathways are particularly interesting based on their role in the malignant phenotype, the tumor microenvironment and immune dysregulation that occurs in BTC patients. However, additional pre-clinical data will be necessary to achieve the goal of translating combination therapy approaches into human clinical trials. In the coming years, it will be critical to adapt our understanding of cross-talk between these and other pathways to identify the most promising therapeutic combinations to move forward. Given the recent renaissance in immunotherapy, it is also desirable to identify small molecules that can be administered with the goal of potentiating the efficacy of these immune stimulatory modalities. This is especially important in light of a recent report indicating that adoptive immunotherapy can produce clinical activity in BTC [223].
One of many challenges to date in advancing therapy for BTC has been a lack of in vivo pre-clinical models that approximate human disease. The fact that most BTC patients diagnosed already possess metastatic disease also leaves little time to attempt multiple lines of treatment and learn in the clinical setting. Further, it is likely that because these tumors have heterogeneous etiology, genetic profiles, and anatomic location, development of relevant pre-clinical models will remain a challenge. These factors necessitate the need for a collaborative, coordinated effort within the field to collect and study patient material whenever possible to gain the most information about this disease.
It is conceivable that small molecule inhibitors targeting MEK, PI3K and STAT3 will have utility as second line therapy in patients who receive limited benefit from chemotherapy and radiotherapy. This particularly relevant as the activation of these pathways could be altered in response to many of these conventional therapy approaches. However, a key endeavor will be to more carefully define how the order of treatment alters activation of these pathways, and importantly, the mechanisms of resistance to these modalities. It will also be critical to gain further information about resistance of patient tumors on an individual level, which could allow for prioritizing among the many pathways that could be targeted. As new treatment combinations targeting these pathways enter clinical trials, we must take several factors into consideration when planning both patient assessment and laboratory correlative studies. These include characterizing the safety profile, optimizing dose, schedule and potential for synergy or antagonism on tumor, immune and stromal cell compartments.
Several opportunities are available to fine-tune the use of these and other drugs to improve clinical outcomes for BTC patients in the future. First, we need to continue to improve our ability to classify patients in a more personalized manner via unique mutation profiles in the tumor. This may identify distinct subsets of patients with greater likelihood to respond to these targeted agents. In the coming years, coordinated efforts to conduct both targeted and unsupervised sequencing may identify key genetic features that could be used as prognostic or predictive markers in the clinical setting.
Article Highlight Box.
Poor survival and limited treatment options for biliary tract cancer (BTC) demonstrate the need for novel therapeutic approaches.
A summary of the various chemotherapeutic regiments of both published and ongoing clinical trials in BTC has been presented.
Key signal transduction pathways are of interest for targeted therapy in BTC, with particular focus and rationale for targeting mitogen-activated protein kinase kinase (MEK), phosphatidylinositol-3 kinase (PI3K), and signal transducer and activator of transcription-3 (STAT3) pathways.
Several small molecule inhibitors designed to inhibit MEK, PI3K, and STAT3 are available for potential application to BTC.
References
* Denotes references of importance
** Denotes references of considerable importance
- 1.Patel T Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagorney DM, Kendrick ML. Hepatic resection in the treatment of hilar cholangiocarcinoma. Adv Surg. 2006;40:159–71. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–700. [DOI] [PubMed] [Google Scholar]
- 4.Patt YZ, Hassan MM, Aguayo A, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101:578–86. [DOI] [PubMed] [Google Scholar]
- 5.Pazdur R, Royce ME, Rodriguez GI, et al. Phase II trial of docetaxel for cholangiocarcinoma. Am J Clin Oncol. 1999;22:78–81. [DOI] [PubMed] [Google Scholar]
- 6.Papakostas P, Kouroussis C, Androulakis N, et al. First-line chemotherapy with docetaxel for unresectable or metastatic carcinoma of the biliary tract. A multicentre phase II study. Eur J Cancer. 2001;37:1833–8. [DOI] [PubMed] [Google Scholar]
- 7.Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24:3069–74. [DOI] [PubMed] [Google Scholar]
- 8.Penz M, Kornek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001;12:183–6. [DOI] [PubMed] [Google Scholar]
- 9.Park JS, Oh SY, Kim SH, et al. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a phase II study. Jpn J Clin Oncol. 2005;35:68–73. [DOI] [PubMed] [Google Scholar]
- 10.Sanz-Altamira PM, O’Reilly E, Stuart KE, et al. A phase II trial of irinotecan (CPT-11) for unresectable biliary tree carcinoma. Ann Oncol. 2001;12:501–4. [DOI] [PubMed] [Google Scholar]
- 11.Ahn DH, Li J, Wei L, et al. Results of an abbreviated phase-II study with the Akt Inhibitor MK-2206 in Patients with Advanced Biliary Cancer. Sci Rep. 2015;5:12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno H, Okusaka T, Ikeda M, et al. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer. 2004;91:1769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi JH, Thongprasert S, Lee J, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer. 2012;48:196–201. [DOI] [PubMed] [Google Scholar]
- 14.Cho JY, Paik YH, Chang YS, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer. 2005;104:2753–8. [DOI] [PubMed] [Google Scholar]
- 15.Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23:2332–8. [DOI] [PubMed] [Google Scholar]
- 16.Riechelmann RP, Townsley CA, Chin SN, et al. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110:1307–12. [DOI] [PubMed] [Google Scholar]
- 17.Koeberle D, Saletti P, Borner M, et al. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2008;26:3702–8. [DOI] [PubMed] [Google Scholar]
- 18.Iqbal S, Rankin C, Lenz HJ, et al. A phase II trial of gemcitabine and capecitabine in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma: Southwest Oncology Group study S0202. Cancer Chemother Pharmacol. 2011;68:1595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borbath I, Ceratti A, Verslype C, et al. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: a phase II study of the Belgian Group of Digestive Oncology. Ann Oncol. 2013;24:2824–9. [DOI] [PubMed] [Google Scholar]
- 20.Giuliani F, Gebbia V, Maiello E, et al. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell’Italia Meridionale (GOIM). Ann Oncol. 2006;17 Suppl 7:vii73–7. [DOI] [PubMed] [Google Scholar]
- 21.Meyerhardt JA, Zhu AX, Stuart K, et al. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci. 2008;53:564–70. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki T, Isayama H, Nakai Y, et al. Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs. 2011;29:1488–93. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn R, Hribaschek A, Eichelmann K, et al. Outpatient therapy with gemcitabine and docetaxel for gallbladder, biliary, and cholangio-carcinomas. Invest New Drugs. 2002;20:351–6. [DOI] [PubMed] [Google Scholar]
- 24.Andre T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–43. [DOI] [PubMed] [Google Scholar]
- 25.Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95:848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manzione L, Romano R, Germano D. Chemotherapy with gemcitabine and oxaliplatin in patients with advanced biliary tract cancer: a single-institution experience. Oncology. 2007;73:311–5. [DOI] [PubMed] [Google Scholar]
- 27.Andre T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. 2008;99:862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48–54. [DOI] [PubMed] [Google Scholar]
- 29.Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11:1142–8. [DOI] [PubMed] [Google Scholar]
- 30.Uwagawa T, Sakamoto T, Abe K, et al. Phase I trial of S-1 every other day in combination with gemcitabine/cisplatin for inoperable biliary tract cancer. Cancer Chemother Pharmacol. 2015;75:191–6. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe A, Kida M, Miyazawa S, et al. Phase I trial of combination chemotherapy with gemcitabine, cisplatin, and S-1 in patients with advanced biliary tract cancer. World J Gastroenterol. 2015;21:5979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducreux M, Rougier P, Fandi A, et al. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol. 1998;9:653–6. [DOI] [PubMed] [Google Scholar]
- 33.Patt YZ, Jones DV Jr., Hoque A, et al. Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol. 1996;14:2311–5. [DOI] [PubMed] [Google Scholar]
- 34.Chen JS, Jan YY, Lin YC, et al. Weekly 24 h infusion of high-dose 5-fluorouracil and leucovorin in patients with biliary tract carcinomas. Anticancer Drugs. 1998;9:393–7. [DOI] [PubMed] [Google Scholar]
- 35.Choi CW, Choi IK, Seo JH, et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol. 2000;23:425–8. [DOI] [PubMed] [Google Scholar]
- 36.Malik IA, Aziz Z. Prospective evaluation of efficacy and toxicity of 5-fu and folinic acid (Mayo Clinic regimen) in patients with advanced cancer of the gallbladder. Am J Clin Oncol. 2003;26:124–6. [DOI] [PubMed] [Google Scholar]
- 37.Sanz-Altamira PM, Ferrante K, Jenkins RL, et al. A phase II trial of 5-fluorouracil, leucovorin, and carboplatin in patients with unresectable biliary tree carcinoma. Cancer. 1998;82:2321–5. [PubMed] [Google Scholar]
- 38.Ellis PA, Norman A, Hill A, et al. Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer. 1995;31A:1594–8. [DOI] [PubMed] [Google Scholar]
- 39.Taieb J, Mitry E, Boige V, et al. Optimization of 5-fluorouracil (5-FU)/cisplatin combination chemotherapy with a new schedule of leucovorin, 5-FU and cisplatin (LV5FU2-P regimen) in patients with biliary tract carcinoma. Ann Oncol. 2002;13:1192–6. [DOI] [PubMed] [Google Scholar]
- 40.Harvey JH, Smith FP, Schein PS. 5-Fluorouracil, mitomycin, and doxorubicin (FAM) in carcinoma of the biliary tract. J Clin Oncol. 1984;2:1245–8. [DOI] [PubMed] [Google Scholar]
- 41.Kajanti M, Pyrhonen S. Epirubicin-sequential methotrexate-5-fluorouracil-leucovorin treatment in advanced cancer of the extrahepatic biliary system. A phase II study. Am J Clin Oncol. 1994;17:223–6. [DOI] [PubMed] [Google Scholar]
- 42.Guion-Dusserre JF, Lorgis V, Vincent J, et al. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:2096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alberts SR, Al-Khatib H, Mahoney MR, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer. 2005;103:111–8. [DOI] [PubMed] [Google Scholar]
- 44.Cereda S, Passoni P, Reni M, et al. The cisplatin, epirubicin, 5-fluorouracil, gemcitabine (PEFG) regimen in advanced biliary tract adenocarcinoma. Cancer. 2010;116:2208–14. [DOI] [PubMed] [Google Scholar]
- 45.Wagner AD, Buechner-Steudel P, Moehler M, et al. Gemcitabine, oxaliplatin and 5-FU in advanced bile duct and gallbladder carcinoma: two parallel, multicentre phase-II trials. Br J Cancer. 2009;101:1846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol. 2010;28:3491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim TW, Chang HM, Kang HJ, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol. 2003;14:1115–20. [DOI] [PubMed] [Google Scholar]
- 48.Woo SM, Lee WJ, Han SS, et al. Capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer: a retrospective single-center study. Chemotherapy. 2012;58:225–32. [DOI] [PubMed] [Google Scholar]
- 49.Nehls O, Oettle H, Hartmann JT, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park SH, Park YH, Lee JN, et al. Phase II study of epirubicin, cisplatin, and capecitabine for advanced biliary tract adenocarcinoma. Cancer. 2006;106:361–5. [DOI] [PubMed] [Google Scholar]
- 51.Kim KP, Jang G, Hong YS, et al. Phase II study of S-1 combined with oxaliplatin as therapy for patients with metastatic biliary tract cancer: influence of the CYP2A6 polymorphism on pharmacokinetics and clinical activity. Br J Cancer. 2011;104:605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwahashi S, Utsunomiya T, Imura S, et al. Effects of valproic acid in combination with S- 1 on advanced pancreatobiliary tract cancers: clinical study phases I/II. Anticancer Res. 2014;34:5187–91. [PubMed] [Google Scholar]
- 53.Takada T, Kato H, Matsushiro T, et al. Comparison of 5-fluorouracil, doxorubicin and mitomycin C with 5-fluorouracil alone in the treatment of pancreatic-biliary carcinomas. Oncology. 1994;51:396–400. [DOI] [PubMed] [Google Scholar]
- 54.Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol. 2001;19:4089–91. [DOI] [PubMed] [Google Scholar]
- 55.Kornek GV, Schuell B, Laengle F, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004;15:478–83. [DOI] [PubMed] [Google Scholar]
- 56.Rao S, Cunningham D, Hawkins RE, et al. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer. 2005;92:1650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Demonstrated that chemotherapy prolonged overall survival in advanced biliary cancer.
- 57.Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study -The UK ABC-01 Study. Br J Cancer. 2009;101:621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Established cisplatin and gemcitabine as a chemotherapy for biliary tract cancer that improves overall survival.
- 58.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. [DOI] [PubMed] [Google Scholar]
- 60.Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–8. [DOI] [PubMed] [Google Scholar]
- 61.Kim ST, Jang KT, Lee SJ, et al. Tumour shrinkage at 6 weeks predicts favorable clinical outcomes in a phase III study of gemcitabine and oxaliplatin with or without erlotinib for advanced biliary tract cancer. BMC Cancer. 2015;15:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moehler M, Maderer A, Schimanski C, et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer. 2014;50:3125–35. [DOI] [PubMed] [Google Scholar]
- 64.Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer. 2015;121:3290–7. [DOI] [PubMed] [Google Scholar]
- 65.Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci. 2013;104:1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park DH, Lee SS, Park SE, et al. Randomised phase II trial of photodynamic therapy plus oral fluoropyrimidine, S-1, versus photodynamic therapy alone for unresectable hilar cholangiocarcinoma. Eur J Cancer. 2014;50:1259–68. [DOI] [PubMed] [Google Scholar]
- 67.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23 Suppl 7:vii155–66. [DOI] [PubMed] [Google Scholar]
- 69.Institute NC. Targeted Cancer Therapies [cited 2016 14 Feb]. Available from: http://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet.
- 70.Zhang Q, Wang Z, Guo J, et al. Comparison of single-agent chemotherapy and targeted therapy to first-line treatment in patients aged 80 years and older with advanced non-small-cell lung cancer. Onco Targets Ther. 2015;8:893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lotfi-Jam K, Carey M, Jefford M, et al. Nonpharmacologic strategies for managing common chemotherapy adverse effects: a systematic review. J Clin Oncol. 2008;26:5618–29. [DOI] [PubMed] [Google Scholar]
- 72.Zhao C, Xiao H, Wu X, et al. Rational combination of MEK inhibitor and the STAT3 pathway modulator for the therapy in K-Ras mutated pancreatic and colon cancer cells. Oncotarget. 2015;6:14472–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–31 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Identified previously unrecognized subclasses ofpatients who may benefit from multi-target tyrosine kinase inhibitors.
- 74.Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Showed inactivation of p16 gene is frequent in cholangiocarcinoma.
- 75.Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Distinguished mutations in BRAF as a common event in cholangiocarcinoma but not hepatocellular carcinoma.
- 76.Ahrendt SA, Rashid A, Chow JT, et al. p53 overexpression and K-ras gene mutations in primary sclerosing cholangitis-associated biliary tract cancer. J Hepatobiliary Pancreat Surg. 2000;7:426–31. [DOI] [PubMed] [Google Scholar]
- 77.Robertson S, Hyder O, Dodson R, et al. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum Pathol. 2013;44:2768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gwak GY, Yoon JH, Shin CM, et al. Detection of response-predicting mutations in the kinase domain of the epidermal growth factor receptor gene in cholangiocarcinomas. J Cancer Res Clin Oncol. 2005;131:649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leone F, Cavalloni G, Pignochino Y, et al. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin Cancer Res. 2006;12:1680–5. [DOI] [PubMed] [Google Scholar]
- 81.Pignochino Y, Sarotto I, Peraldo-Neia C, et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong N, Li L, Tsang K, et al. Frequent loss of chromosome 3p and hypermethylation of RASSF1A in cholangiocarcinoma. J Hepatol. 2002;37:633–9. [DOI] [PubMed] [Google Scholar]
- 83.Chen YJ, Tang QB, Zou SQ. Inactivation of RASSF1A, the tumor suppressor gene at 3p21.3 in extrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tozawa T, Tamura G, Honda T, et al. Promoter hypermethylation of DAP-kinase is associated with poor survival in primary biliary tract carcinoma patients. Cancer Sci. 2004;95:736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riener MO, Bawohl M, Clavien PA, et al. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosomes Cancer. 2008;47:363–7. [DOI] [PubMed] [Google Scholar]
- 86.Kang YK, Kim WH, Jang JJ. Expression of G1-S modulators (p53, p16, p27, cyclin D1, Rb) and Smad4/Dpc4 in intrahepatic cholangiocarcinoma. Hum Pathol. 2002;33:877–83. [DOI] [PubMed] [Google Scholar]
- 87.Ueki T, Hsing AW, Gao YT, et al. Alterations of p16 and prognosis in biliary tract cancers from a population-based study in China. Clin Cancer Res. 2004;10:1717–25. [DOI] [PubMed] [Google Scholar]
- 88.Tannapfel A, Weinans L, Geissler F, et al. Mutations of p53 tumor suppressor gene, apoptosis, and proliferation in intrahepatic cholangiocellular carcinoma of the liver. Dig Dis Sci. 2000;45:317–24. [DOI] [PubMed] [Google Scholar]
- 89.Isomoto H, Mott JL, Kobayashi S, et al. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43:1552–8. [DOI] [PubMed] [Google Scholar]
- 92.Ahn DH, Ozer HG, Hancioglu B, et al. Whole-exome tumor sequencing study in biliary cancer patients with a response to MEK inhibitors. Oncotarget. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao Y, Adjei AA. The clinical development of MEK inhibitors. Nat Rev Clin Oncol. 2014;11:385–400. [DOI] [PubMed] [Google Scholar]; * Provides a comprehensive review of MEK inhibitors.
- 94.Chang F, Steelman LS, Lee JT, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–93. [DOI] [PubMed] [Google Scholar]
- 95.Akinleye A, Furqan M, Mukhi N, et al. MEK and the inhibitors: from bench to bedside. J Hematol Oncol. 2013;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chappell WH, Steelman LS, Long JM, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wortzel I, Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer. 2011;2:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. [DOI] [PubMed] [Google Scholar]
- 99.Goto M, Chow J, Muramoto K, et al. E6201 [(3S,4R,5Z,8S,9S,11E)-14-(ethylamino)-8, 9,16-trihydroxy-3,4-dimethyl-3,4,9,19-tetrahydro-1H-2-benzoxacyclotetradecine-1,7 (8H)- dione], a novel kinase inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK)-1 and MEK kinase-1: in vitro characterization of its anti-inflammatory and antihyperproliferative activities. J Pharmacol Exp Ther. 2009;331:485–95. [DOI] [PubMed] [Google Scholar]
- 100.Ohren JF, Chen H, Pavlovsky A, et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol. 2004;11:1192–7. [DOI] [PubMed] [Google Scholar]
- 101.Dudley DT, Pang L, Decker SJ, et al. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1995;92:7686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle. 2009;8:1896–900. [DOI] [PubMed] [Google Scholar]
- 103.Brognard J, Dennis PA. Variable apoptotic response of NSCLC cells to inhibition of the MEK/ERK pathway by small molecules or dominant negative mutants. Cell Death Differ. 2002;9:893–904. [DOI] [PubMed] [Google Scholar]
- 104.Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–32. [DOI] [PubMed] [Google Scholar]
- 105.Abrams SL, Steelman LS, Shelton JG, et al. Enhancing therapeutic efficacy by targeting non-oncogene addicted cells with combinations of signal transduction inhibitors and chemotherapy. Cell Cycle. 2010;9:1839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abrams SL, Steelman LS, Shelton JG, et al. The Raf/MEK/ERK pathway can govern drug resistance, apoptosis and sensitivity to targeted therapy. Cell Cycle. 2010;9:1781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle. 2009;8:1883–7. [DOI] [PubMed] [Google Scholar]
- 108.Rieber M, Rieber MS. Signalling responses linked to betulinic acid-induced apoptosis are antagonized by MEK inhibitor U0126 in adherent or 3D spheroid melanoma irrespective of p53 status. Int J Cancer. 2006;118:1135–43. [DOI] [PubMed] [Google Scholar]
- 109.Cohen RB, Aamdal S, Nyakas M, et al. A phase I dose-finding, safety and tolerability study of AZD8330 in patients with advanced malignancies. Eur J Cancer. 2013;49:1521–9. [DOI] [PubMed] [Google Scholar]
- 110.Sheth PR, Liu Y, Hesson T, et al. Fully activated MEK1 exhibits compromised affinity for binding of allosteric inhibitors U0126 and PD0325901. Biochemistry. 2011;50:7964–76. [DOI] [PubMed] [Google Scholar]
- 111.Narita Y, Okamoto K, Kawada MI, et al. Novel ATP-competitive MEK inhibitor E6201 is effective against vemurafenib-resistant melanoma harboring the MEK1-C121S mutation in a preclinical model. Mol Cancer Ther. 2014;13:823–32. [DOI] [PubMed] [Google Scholar]
- 112.Haura EB, Ricart AD, Larson TG, et al. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2010;16:2450–7. [DOI] [PubMed] [Google Scholar]
- 113.Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281–93. [DOI] [PubMed] [Google Scholar]
- 114.von Euw E, Atefi M, Attar N, et al. Antitumor effects of the investigational selective MEK inhibitor TAK733 against cutaneous and uveal melanoma cell lines. Mol Cancer. 2012;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Macarulla T, Cervantes A, Tabernero J, et al. Phase I study of FOLFIRI plus pimasertib as second-line treatment for KRAS-mutated metastatic colorectal cancer. Br J Cancer. 2015;112:1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leijen S, Middleton MR, Tresca P, et al. Phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of the MEK inhibitor RO4987655 (CH4987655) in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4794–805. [DOI] [PubMed] [Google Scholar]
- 117.Martinez-Garcia M, Banerji U, Albanell J, et al. First-in-human, phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of RO5126766, a first-in-class dual MEK/RAF inhibitor in patients with solid tumors. Clin Cancer Res. 2012;18:4806–19. [DOI] [PubMed] [Google Scholar]
- 118.Dong Q, Dougan DR, Gong X, et al. Discovery of TAK-733, a potent and selective MEK allosteric site inhibitor for the treatment of cancer. Bioorg Med Chem Lett. 2011;21:1315–9. [DOI] [PubMed] [Google Scholar]
- 119.Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249–56. [DOI] [PubMed] [Google Scholar]
- 120.Bennouna J, Lang I, Valladares-Ayerbes M, et al. A Phase II, open-label, randomised study to assess the efficacy and safety of the MEK1/2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Invest New Drugs. 2011;29:1021–8. [DOI] [PubMed] [Google Scholar]
- 121.Bodoky G, Timcheva C, Spigel DR, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30:1216–23. [DOI] [PubMed] [Google Scholar]
- 122.Hainsworth JD, Cebotaru CL, Kanarev V, et al. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5:1630–6. [DOI] [PubMed] [Google Scholar]
- 123.Hayes DN, Lucas AS, Tanvetyanon T, et al. Phase II efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18:2056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012;18:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–83. [DOI] [PubMed] [Google Scholar]
- 126.Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tentler JJ, Nallapareddy S, Tan AC, et al. Identification of predictive markers of response to the MEK1/2 inhibitor selumetinib (AZD6244) in K-ras-mutated colorectal cancer. Mol Cancer Ther. 2010;9:3351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCubrey JA, Steelman LS, Abrams SL, et al. Emerging MEK inhibitors. Expert Opin Emerg Drugs. 2010;15:203–23. [DOI] [PubMed] [Google Scholar]
- 129.Huynh H, Soo KC, Chow PK, et al. Targeted inhibition of the extracellular signal-regulated kinase kinase pathway with AZD6244 (ARRY-142886) in the treatment of hepatocellular carcinoma. Mol Cancer Ther. 2007;6:138–46. [DOI] [PubMed] [Google Scholar]
- 130.Haass NK, Sproesser K, Nguyen TK, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res. 2008;14:230–9. [DOI] [PubMed] [Google Scholar]
- 131.Friday BB, Yu C, Dy GK, et al. BRAF V600E disrupts AZD6244-induced abrogation of negative feedback pathways between extracellular signal-regulated kinase and Raf proteins. Cancer Res. 2008;68:6145–53. [DOI] [PubMed] [Google Scholar]
- 132.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19. [DOI] [PubMed] [Google Scholar]
- 133.Chung EJ, Brown AP, Asano H, et al. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 kinase. Clin Cancer Res. 2009;15:3050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14. [DOI] [PubMed] [Google Scholar]
- 135.Akinleye A, Avvaru P, Furqan M, et al. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boespflug A, Thomas L. Cobimetinib and vemurafenib for the treatment of melanoma. Expert Opin Pharmacother. 2016;17:1005–11. [DOI] [PubMed] [Google Scholar]
- 137.Rosen LS, LoRusso P, Ma WW, et al. A first-in-human phase I study to evaluate the MEK1/2 inhibitor, cobimetinib, administered daily in patients with advanced solid tumors. Invest New Drugs. 2016;34:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Signorelli J, Shah Gandhi A. Cobimetinib: A Novel MEK Inhibitor for Metastatic Melanoma. Ann Pharmacother. 2016. [Google Scholar]
- 139.Vlahos CJ, Matter WF, Hui KY, et al. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 140.Stein RC. Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment.Endocr Relat Cancer. 2001;8:237–48. [DOI] [PubMed] [Google Scholar]
- 141.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zou ZQ, Zhang XH, Wang F, et al. A novel dual PI3Kalpha/mTOR inhibitor PI-103 with high antitumor activity in non-small cell lung cancer cells. Int J Mol Med. 2009;24:97–101. [DOI] [PubMed] [Google Scholar]
- 145.Prevo R, Deutsch E, Sampson O, et al. Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res. 2008;68:5915–23. [DOI] [PubMed] [Google Scholar]
- 146.Chiarini F, Fala F, Tazzari PL, et al. Dual inhibition of class IA phosphatidylinositol 3-kinase and mammalian target of rapamycin as a new therapeutic option for T-cell acute lymphoblastic leukemia. Cancer Res. 2009;69:3520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu K, Lucas J, Zhu T, et al. PWT-458, a novel pegylated-17-hydroxywortmannin, inhibits phosphatidylinositol 3-kinase signaling and suppresses growth of solid tumors. Cancer Biol Ther. 2005;4:538–45. [DOI] [PubMed] [Google Scholar]
- 148.Zhu T, Gu J, Yu K, et al. Pegylated wortmannin and 17-hydroxywortmannin conjugates as phosphoinositide 3-kinase inhibitors active in human tumor xenograft models. J Med Chem. 2006;49:1373–8. [DOI] [PubMed] [Google Scholar]
- 149.Walker EH, Pacold ME, Perisic O, et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–19. [DOI] [PubMed] [Google Scholar]
- 150.Kong D, Okamura M, Yoshimi H, et al. Antiangiogenic effect of ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor. Eur J Cancer. 2009;45:857–65. [DOI] [PubMed] [Google Scholar]
- 151.Yaguchi S, Fukui Y, Koshimizu I, et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst. 2006;98:545–56. [DOI] [PubMed] [Google Scholar]
- 152.Knight SD, Adams ND, Burgess JL, et al. Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. ACS Med Chem Lett. 2010;1:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–30. [DOI] [PubMed] [Google Scholar]
- 154.Liu TJ, Koul D, LaFortune T, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. [DOI] [PubMed] [Google Scholar]
- 156.Chiarini F, Grimaldi C, Ricci F, et al. Activity of the novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 against T-cell acute lymphoblastic leukemia. Cancer Res. 2010;70:8097–107. [DOI] [PubMed] [Google Scholar]
- 157.Markman B, Tabernero J, Krop I, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol. 2012;23:2399–408. [DOI] [PubMed] [Google Scholar]
- 158.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. [DOI] [PubMed] [Google Scholar]
- 159.Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–32. [DOI] [PubMed] [Google Scholar]
- 160.Raynaud FI, Eccles SA, Patel S, et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther. 2009;8:1725–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yao E, Zhou W, Lee-Hoeflich ST, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009;15:4147–56. [DOI] [PubMed] [Google Scholar]
- 162.Ihle NT, Williams R, Chow S, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3:763–72. [PubMed] [Google Scholar]
- 163.Howes AL, Chiang GG, Lang ES, et al. The phosphatidylinositol 3-kinase inhibitor, PX- 866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther. 2007;6:2505–14. [DOI] [PubMed] [Google Scholar]
- 164.Molckovsky A, Siu LL. First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J Hematol Oncol. 2008;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Okusaka T, Ueno H, Ikeda M, et al. Phase 1 and pharmacological trial of OPB-31121, a signal transducer and activator of transcription-3 inhibitor, in patients with advanced hepatocellular carcinoma. Hepatol Res. 2015;45:1283–91. [DOI] [PubMed] [Google Scholar]; * Provides one of the first clinical trials with a pharmcologic inhibitor of STAT3.
- 166.Furqan M, Akinleye A, Mukhi N, et al. STAT inhibitors for cancer therapy. J Hematol Oncol. 2013;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Finn RS JM, Tan BR, Weekes CD, Bendell JC, Patnaik A, Naaz Khan G, Laheru D, Anderson L, Christy-Bittel JL, Guthrie K, Litwiler KS, Bekaii-Saab TS. A phase I study of MEK inhibitor MEK162 (ARRY-438162) in patients with biliary tract cancer. Journal of Clinical Oncology. 2012;30 suppl 4:abstr 220. [Google Scholar]
- 168.Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011;29:2357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Clinical trial shows that MEK inhibitors have clinical activity in metastatic biliary cancer.
- 169.Talbert EE, Yang J, Mace TA, et al. Dual Inhibition of MEK and PI3K/Akt Rescues Cancer Cachexia through Both Tumor Extrinsic and Intrinsic Activities. Mol Cancer Ther. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Comprehensive review of the PI3K pathway and consequences of its targeting.
- 171.Arteaga CL. Clinical development of phosphatidylinositol-3 kinase pathway inhibitors. Curr Top Microbiol Immunol. 2010;347:189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. [DOI] [PubMed] [Google Scholar]
- 173.Zitzmann K, De Toni EN, Brand S, et al. The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology. 2007;85:54–60. [DOI] [PubMed] [Google Scholar]
- 174.Schmitz KJ, Lang H, Wohlschlaeger J, et al. AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2007;13:6470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tanno S, Ohsaki Y, Nakanishi K, et al. Small cell lung cancer cells express EGFR and tyrosine phosphorylation of EGFR is inhibited by gefitinib (“Iressa”, ZD1839). Oncol Rep. 2004;12:1053–7. [PubMed] [Google Scholar]
- 176.Yoon H, Min JK, Lee JW, et al. Acquisition of chemoresistance in intrahepatic cholangiocarcinoma cells by activation of AKT and extracellular signal-regulated kinase (ERK)1/2. Biochem Biophys Res Commun. 2011;405:333–7. [DOI] [PubMed] [Google Scholar]
- 177.Yothaisong S, Dokduang H, Techasen A, et al. Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol. 2013;34:3637–48. [DOI] [PubMed] [Google Scholar]
- 178.Wilson JM, Kunnimalaiyaan S, Kunnimalaiyaan M, et al. Inhibition of the AKT pathway in cholangiocarcinoma by MK2206 reduces cellular viability via induction of apoptosis. Cancer Cell Int. 2015;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. [DOI] [PubMed] [Google Scholar]; ** Sentinel report establishing the relevance of PIK3CA mutations in human cancer.
- 180.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. [DOI] [PubMed] [Google Scholar]; ** Sentinel report establishing the relevance of PTEN mutations in human cancer.
- 181.Demidenko ZN, Blagosklonny MV. Quantifying pharmacologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential. Aging (Albany NY). 2009;1:1008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dokduang H, Techasen A, Namwat N, et al. STATs profiling reveals predominantly- activated STAT3 in cholangiocarcinoma genesis and progression. J Hepatobiliary Pancreat Sci. 2014;21:767–76. [DOI] [PubMed] [Google Scholar]
- 184.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–54. [PubMed] [Google Scholar]
- 185.Lavecchia A, Di Giovanni C, Novellino E. STAT-3 inhibitors: state of the art and new horizons for cancer treatment. Curr Med Chem. 2011;18:2359–75. [DOI] [PubMed] [Google Scholar]
- 186.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. [DOI] [PubMed] [Google Scholar]; * Comprehensive review of STAT proteins and their role in cancer.
- 187.Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–15. [DOI] [PubMed] [Google Scholar]
- 188.Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR- Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159:4720–8. [PubMed] [Google Scholar]
- 189.Grandis JR, Drenning SD, Chakraborty A, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wei D, Le X, Zheng L, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–29. [DOI] [PubMed] [Google Scholar]
- 191.Sartor CI, Dziubinski ML, Yu CL, et al. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997;57:978–87. [PubMed] [Google Scholar]
- 192.Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–10. [DOI] [PubMed] [Google Scholar]
- 193.Corvinus FM, Orth C, Moriggl R, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cai L, Zhang G, Tong X, et al. Growth inhibition of human ovarian cancer cells by blocking STAT3 activation with small interfering RNA. Eur J Obstet Gynecol Reprod Biol. 2010;148:73–80. [DOI] [PubMed] [Google Scholar]
- 195.Abdulghani J, Gu L, Dagvadorj A, et al. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172:1717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sonbol MB, Firwana B, Zarzour A, et al. Comprehensive review of JAK inhibitors in myeloproliferative neoplasms. Ther Adv Hematol. 2013;4:15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hurwitz HI, Uppal N, Wagner SA, et al. Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination With Capecitabine in Patients With Metastatic Pancreatic Cancer for Whom Therapy With Gemcitabine Has Failed. J Clin Oncol. 2015;33:4039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136–54. [DOI] [PubMed] [Google Scholar]
- 200.Kokuryo T, Yokoyama Y, Nagino M. Recent advances in cancer stem cell research for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:606–13. [DOI] [PubMed] [Google Scholar]
- 201.Romano M, De Francesco F, Gringeri E, et al. Tumor Microenvironment Versus Cancer Stem Cells in Cholangiocarcinoma: Synergistic Effects? J Cell Physiol. 2016;231:768–76. [DOI] [PubMed] [Google Scholar]
- 202.Roskams T Liver stem cells and their implication in hepatocellular and holangiocarcinoma. Oncogene. 2006;25:3818–22. [DOI] [PubMed] [Google Scholar]
- 203.Nanashima A, Hatachi G, Tsuchiya T, et al. Clinical significances of cancer stem cells markers in patients with intrahepatic cholangiocarcinoma who underwent hepatectomy. Anticancer Res. 2013;33:2107–14. [PubMed] [Google Scholar]
- 204.Li Y, Rogoff HA, Keates S, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112:1839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang Y, Jin Z, Zhou H, et al. Suppression of prostate cancer progression by cancer cell stemness inhibitor napabucasin. Cancer Med. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Review highlighting the importance of STAT protiens as modulators of both tumor cell biology and immunity.
- 207.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5. [DOI] [PubMed] [Google Scholar]
- 208.Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16:4695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ewald F, Norz D, Grottke A, et al. Dual Inhibition of PI3K-AKT-mTOR- and RAF- MEK-ERK-signaling is synergistic in cholangiocarcinoma and reverses acquired resistance to MEK-inhibitors. Invest New Drugs. 2014;32:1144–54. [DOI] [PubMed] [Google Scholar]
- 211.Wee S, Jagani Z, Xiang KX, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–93. [DOI] [PubMed] [Google Scholar]
- 212.Sun C, Hobor S, Bertotti A, et al. Intrinsic resistance to MEK inhibition in KRAS mutant lung and colon cancer through transcriptional induction of ERBB3. Cell Rep. 2014;7:86–93. [DOI] [PubMed] [Google Scholar]
- 213.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tan J, Lee PL, Li Z, et al. B55beta-associated PP2A complex controls PDK1-directed myc signaling and modulates rapamycin sensitivity in colorectal cancer. Cancer Cell. 2010;18:459–71. [DOI] [PubMed] [Google Scholar]
- 215.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fahmi A, Smart N, Punn A, et al. p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation. Cell Signal. 2013;25:898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ogata A, Chauhan D, Teoh G, et al. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–21. [PubMed] [Google Scholar]; * Article shows how IL-6, a key cytokine can stimulate MAPK signaling.
- 218.Katso R, Okkenhaug K, Ahmadi K, et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–75. [DOI] [PubMed] [Google Scholar]
- 219.Vogt PK, Hart JR. PI3K and STAT3: a new alliance. Cancer Discov. 2011;1:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang HW, Shin MG, Lee S, et al. Cooperative activation of PI3K by Ras and Rho family small GTPases. Mol Cell. 2012;47:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes Cancer. 2011;2:261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Demonstrates biliary cancers may be susceptible to immunotherapy.