Abstract
Glucocorticoids (GCs) are secreted into the blood by the adrenal glands and are also locally-produced by organs such as the lymphoid organs (bone marrow, thymus, and spleen). Corticosterone is the primary circulating GC in many species, including mice, rats and birds. Within lymphoid organs, corticosterone can be locally produced from the inactive metabolite, 11-dehydrocorticosterone (DHC). However, very little is known about endogenous DHC levels, and no immunoassays are currently available to measure DHC. Here, we developed an easy-to-use and inexpensive immunoassay to measure DHC that is accurate, precise, sensitive, and specific. The DHC immunoassay was validated in multiple ways, including comparison with a mass spectrometry assay. After assay validations, we demonstrated the usefulness of this immunoassay by measuring DHC (and corticosterone) in mice, rats and song sparrows. Overall, corticosterone levels were higher than DHC levels across species. In Study 1, using mice, we measured steroids in whole blood and lymphoid organs at postnatal day (PND) 5, PND23, and PND90. Corticosterone and DHC showed distinct tissue-specific patterns across development. In Studies 2 and 3, we measured circulating corticosterone and DHC in adult rats and song sparrows, before and after restraint stress. In rats and song sparrows, restraint stress rapidly increased circulating levels of both steroids. This novel DHC immunoassay revealed major changes in DHC concentrations during development and in response to stress, which have important implications for understanding GC physiology, effects of stress on immune function, and regulation of local GC levels.
Keywords: 11β-Hydroxysteroid dehydrogenase, Immunosteroid, Glucocorticoid, Development, ELISA, Sex difference
1. Introduction
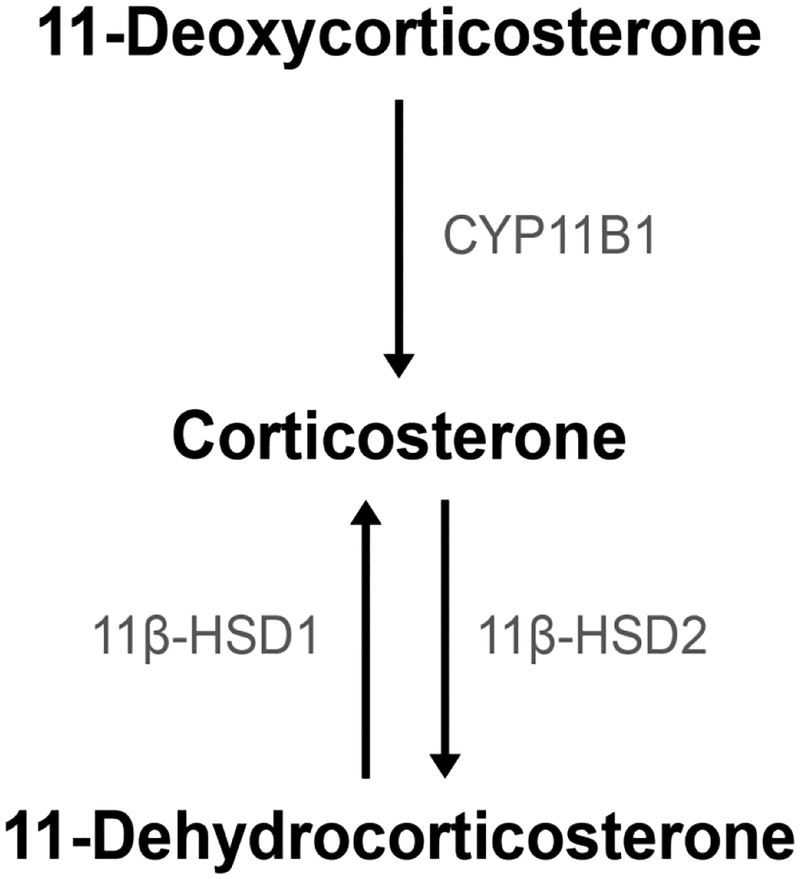
Glucocorticoids (GCs) are steroid hormones secreted by the adrenal glands to coordinate a myriad of physiological processes (Landys et al., 2006; Munck et al., 1984; Sapolsky et al., 2000; Toufexis et al., 2014; Wada, 2008). Corticosterone is the predominant circulating GC in many species, including mice, rats, and songbirds (Gong et al., 2015; Landys et al., 2006; Taves et al., 2017; Taves et al., 2016a; Taves et al., 2015; van Weerden et al., 1992). Corticosterone can be converted to the inactive 11-dehydrocorticosterone (DHC) within tissues by the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) (Cottrell and Seckl, 2009). Conversely, corticosterone can be regenerated from DHC by 11β-HSD1 (Chapman et al., 2013) (Figure 1). During development, 11β-HSD1 and 2 show marked changes in expression in tissues, including lymphoid organs (Taves et al., 2016b). Furthermore, during neonatal development, rodents display the stress hyporesponsive period (SHRP), in which they have very low levels of circulating GCs and minimal effects of stressors on circulating GCs (Gunnar and Donzella, 2002; Sapolsky and Meaney, 1986; Stanton and Levine, 1990; Wood and Walker, 2015). In mice, the SHRP begins at postnatal day (PND) 2 and ends at PND12 (D’Amato et al., 1992; Schmidt et al., 2003).
Figure 1:
Simplified GC production pathway, with steroid names in black and steroidogenic enzyme names in grey italics.
GCs are powerful regulators of immunity, and bone marrow, thymus, and spleen are particularly sensitive to the effects of GCs. For example, GCs modulate thymocyte selection via glucocorticoid receptor binding (Iwata et al., 1991; Vacchio et al., 1999). In addition, short-term increases in systemic GCs enhance immunity by promoting proliferation and trafficking of immune cells in the early stages of an immune response (Bowers et al., 2008; Dhabhar et al., 1996; Wiegers et al., 1994). In contrast, long-term elevation of GCs suppresses the immune system, as demonstrated by involution of the thymus and spleen (Selye, 1936). GCs promote thymic involution, in part, by increasing apoptosis of developing and mature T cells (Ashwell et al., 2000).
In mice, lymphoid organs such as bone marrow, thymus, and spleen locally produce GCs, allowing for tissue-specific regulation of GC levels (Schmidt et al., 2008; Schmidt and Soma, 2008; Taves et al., 2011a). Evidence for the local production of corticosterone in mice includes expression of all GC-synthetic and -regenerative enzymes, in vitro production of corticosterone by thymic epithelial cells, and local elevation of corticosterone during the SHRP (Mittelstadt et al., 2018; Pazirandeh et al., 1999; Qiao et al., 2009; Taves et al., 2016b; Taves et al., 2015; Vacchio et al., 1994). Interestingly, when either the corticosterone precursor, 11-deoxycorticosterone, or the corticosterone metabolite, DHC, are provided in vitro at a high concentration (1μM) to bone marrow, thymus, or spleen, all three tissues produce more corticosterone via regeneration from DHC than via synthesis from 11-deoxycorticosterone (Taves et al., 2016b). However, little is known about endogenous DHC levels in mice during early development, although a few studies have examined adult mice (Alberts et al., 2005; Cobice et al., 2013; Harris et al., 2001; Hundertmark et al., 2002b; Tagawa et al., 2007; Verma et al., 2018; Yau et al., 2001). Thus, it remains unclear to what extent corticosterone is locally regenerated from DHC in vivo, especially during the SHRP. Importantly, very little is known about endogenous DHC levels because no immunoassays are currently available to measure DHC.
In addition to mice, rats and songbirds are important animal models for the study of stress physiology. Numerous studies in rats and birds have demonstrated that circulating corticosterone levels rise in response to acute stressors (Romero, 2002; Sapolsky et al., 2000), but few studies have measured DHC in rats (Hay and Mormède, 1997; Hundertmark et al., 2002a; Obut et al., 2004; Tagawa et al., 2007), and no study has measured DHC in birds.
Here, we developed and validated a sensitive and specific enzyme-linked immunosorbent assay (ELISA) to measure DHC. After assay validation, we measured DHC in circulation and in tissues of mice, rats, and songbirds. In Study 1, corticosterone and DHC were measured in the blood and lymphoid organs of mice at three ages across development. In Studies 2 and 3, circulating corticosterone and DHC were measured before and after restraint stress in adult rats and songbirds, respectively, to determine whether an acute stressor increases DHC levels.
2. Materials and Methods
2.1. DHC assay materials
Sheep anti-11-dehydrocorticosterone-3-carboxymethoxyoxime-thyroglobin (anti-DHC) and biotin-labeled DHC were produced by Dr. Celso Gomez-Sanchez. DHC (Q3690–000) was purchased from Steraloids Inc. (Newport, USA) and dissolved in 100% ethanol (and stored at −80 C). High-binding microtiter plates (82050–720) were purchased from VWR (Edmonton, Canada). Boric acid (B6768), bovine serum albumin (BSA) (A1933), tetramethylbenzidine liquid substrate solution (TMB) (T4444), sulfuric acid (28105), Tween 20 (P1379), ProClin 200 (48171-U), and sodium chloride (S5886) were purchased from Sigma-Aldrich (Oakville, Canada). Tris (161–0716) was purchased from Bio-Rad Laboratories (Mississauga, Canada). Avidin horseradish peroxidase (HRP-Avidin) (A-2014) was purchased from Vector Labs (Burlingame, USA). Sheep IgG (7409005) and donkey anti-sheep IgG (7453800) were purchased from Lampire Biological Laboratories (Pipersville, USA).
2.2. DHC assay development and validation
We validated the DHC immunoassay for sensitivity, accuracy, precision, and specificity using multiple approaches.
2.2.1. Standard curve development
To develop a standard curve with excellent sensitivity, various concentrations of DHC, primary and secondary antibodies, and biotin-labeled DHC were tested. The standards in the standard curve were 0, 1, 2, 4, 8, 16, 32, and 64 pg/well.
2.2.2. Accuracy and intra- and inter-assay variation
To assess accuracy and precision, we extracted (using solid phase extraction (SPE), see Section 2.9 below) and measured two quality controls (QCs) (2pg and 16pg, n=10 each) across multiple assays (n=10).
2.2.3. Antibody cross-reactivity
Cross-reactivity of the anti-DHC antibody was tested for closely-related steroids: progesterone, 11-deoxycorticosterone, corticosterone, 11-deoxycortisol, cortisol, and cortisone (Table 1). Steroids were dissolved in ethanol, diluted to 5% ethanol using ELISA buffer, and then extracted and resuspended as described in Section 2.9.
Table 1:
Cross-reactivities of the anti-11-dehydrocorticosterone (DHC) antibody.
| Progesterone | 11-Deoxycorticosterone | 11-Deoxycortisol | Corticosterone | Cortisol | Cortisone | |
|---|---|---|---|---|---|---|
| 100pg | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1000pg | n.d. | 5.10 | n.d. | 3.56 | n.d. | 4.89 |
| Cross-reactivity | < 0.1% | 0.51% | < 0.1% | 0.36% | < 0.1% | 0.49% |
Data represent the mean (n=5) relative to DHC binding (100%), n.d. = not detectable.
2.2.4. Recovery
Matrix effects were assessed by creating blood, serum, plasma, and tissue pools and then comparing unspiked and spiked samples from the same pool (Table 2). Blood, serum, plasma and tissue pools were divided into 2 groups, unspiked and spiked. Exogenous DHC and corticosterone were added to spiked pools; vehicle was added to unspiked pools. Pools were then extracted and resuspended as described in Section 2.9.
Table 2:
Steroid recovery from different sample types.
| Whole Blood | Serum/Plasma | Bone Marrow | Thymus | Spleen | |
|---|---|---|---|---|---|
| Mouse PND5 Corticosterone | 129% | n.a. | 64% | 103% | 94% |
| Mouse PND23 and PND90 Corticosterone | 81% | n.a. | 81% | 76% | 90% |
| Rat Corticosterone | n.a. | 50% | n.a. | n.a. | n.a. |
| Song sparrow Corticosterone | n.a. | 63% | n.a. | n.a. | n.a. |
| Mouse DHC | 94% | 79% | 124% | 106% | 97% |
| Rat DHC | n.a. | 49% | n.a. | n.a. | n.a. |
| Song sparrow DHC | n.a. | 47% | n.a. | n.a. | n.a. |
Data represent the mean steroid recovery (n=3). PND = postnatal day; DHC = 11-dehydrocorticosterone; n.a. = not applicable. For mouse DHC, values represent recovery for all ages.
2.2.5. Serum serial dilution
Matrix effects were also assessed by serial diluting one pool of mouse serum and examining parallelism with the standard curve (n=2/dilution). Serially diluted serum was extracted via SPE and resuspended as described in Section 2.9.
2.2.6. Comparison with liquid chromatography tandem mass spectrometry (LC-MS/MS)
We compared the immunoassay with a LC-MS/MS assay. We serially diluted one pool of mouse serum, and serum was extracted via SPE, as described in Section 2.9. DHC was measured by both ELISA and LC-MS/MS (n=2/method/dilution). For LC-MS/MS analysis, dried SPE eluates were resuspended in 50μl 50% MeOH and injected into a Nexera X2 UHPLC system, as previously described (Tobiansky et al., 2018). Steroids were measured by scheduled multiple reaction monitoring, and data were acquired on a Sciex 6500 Qtrap triple quadrupole tandem mass spectrometer (Tobiansky et al., 2018). Picograms of DHC measured by both ELISA and LC-MS/MS were compared. The DHC concentration at each serum dilution was also calculated (n=8/method).
2.3. DHC assay procedure
High-binding 96-well microtiter plates were coated with 100μl/well sheep IgG (5μg/μl) in coating buffer (deionized H2O, 0.1M borate, pH 9.6). Plates were placed on an orbital shaker for 1h at room temperature, and then left overnight at 4°C. Plates were washed three times using a plate washer with 200μl/well washing buffer (phosphate buffered saline (PBS), 0.05% Tween 20). Plates were then blocked with 200μl/well blocking buffer (PBS, 3% BSA) and incubated for 1h at room temperature. Blocking buffer was removed, and 100μl/well donkey anti-sheep IgG in ELISA buffer (deionized H2O, 1.2% Tris, 0.05% Tween 20, 0.05% ProClin 200, 0.29% NaCl, 0.25% BSA, pH 7.4) was added and plates were incubated for 1h at room temperature. Plates were washed three times with washing buffer. Then 50μl/well of anti-DHC diluted in ELISA buffer was added to all wells, except for the non-specific binding (NSB) wells, in which 50μl/well of ELISA buffer was added. Afterwards, 50μl/well of standards, controls, and samples were added to appropriate wells. Blank and NSB wells received 50μl/well of ELISA buffer. Next 50μl/well of biotin-labeled DHC in ELISA buffer was added to all wells. Plates were shaken for 1h at room temperature. Plates were washed three times with washing buffer. Then, 100μl/well (HRP-Avidin) in ELISA buffer was added to all wells and plates were shaken for 30min at room temperature. Plates went through a final wash as above. 100μl/well TMB solution was added to all wells and plates were incubated in the dark for 30min at room temperature. The reaction was halted by 100μl/well 1N sulfuric acid. Absorbance at 450nm was read using a plate reader (Victor3 multilabel reader, Perkin Elmer) within 10min of stopping the reaction.
2.4. Corticosterone assay
Corticosterone was quantified using a radioimmunoassay (07120103) from MP Biomedicals (Santa Ana, USA). The lowest standard was further diluted to increase assay sensitivity to 1.56pg/tube (Schmidt and Soma, 2008). Corticosterone recovery was estimated by creating blood, serum, plasma, and tissue pools and then comparing unspiked and spiked samples from the same pool (Table 2). Due to the low concentration of corticosterone in PND5 mice, recovery was estimated separately because a greater tissue amount was used than for PND23 and PND90 mice (Table 2).
2.5. Subjects
Study 1.
Subjects were male and female C57BL/6J mice at PND5, PND23, and PND90 (PND86–90) (n=10 for PND5 and PND23, n=9 for PND90), with PND0 defined as the first day pups were present in the cage. PND5 was selected because it is within the SHRP, and at PND5, corticosterone levels are higher in lymphoid organs than in whole blood (hereafter “blood”) (Taves et al., 2015). Sex of PND5 mice was determined via genotyping at the University of British Columbia Genotyping Facility. After weaning at PND19, mice were housed with 2–5 same-sex mice per cage. Mice were housed in a specific pathogen-free colony in the Centre for Disease Modeling at the University of British Columbia. Colony rooms were maintained between 20–22°C with 40–70% relative humidity. Mice were housed in filter top cages, with beta-chip bedding, under a 14:10 light:dark cycle (lights on 0600–2000h), with free access to water (purified by reverse osmosis and sterilized by chlorination) and food (Teklad Diet 2919 for breeders and Teklad Diet 2918 after weaning at PND19). A red translucent hut and nestlet were placed in each cage for enrichment.
Study 2.
Subjects were adult (9-weeks-old) male and female Long-Evans rats purchased from Charles River (n=9 and 10, respectively). Rats were housed in a conventional animal facility at the University of British Columbia. Rats were housed in same-sex pairs in polyurethane cages, with aspen-chip bedding, under a 12:12 light:dark cycle (lights on 0700–1900h), with free access to tap water and food (Rat Diet 4012, Land O’Lakes, Inc). A small polyvinyl chloride tube was placed in each cage for enrichment.
Study 3.
Subjects were free-living adult male song sparrows, Melospiza melodia (n=7). They were captured using conspecific song playback and mist nets in October to November (nonbreeding season) in Vancouver, BC (49° 12N, 123° 01W).
All procedures were in compliance with the Canadian Council on Animal Care and protocols were approved by the University of British Columbia Animal Care Committee.
2.6. Study 1. Mouse tissue collection
Mouse tissues were collected between 0900–1100h to reduce diurnal variation in steroids. Mice were rapidly and deeply anesthetized with 5% isoflurane (2L/min) and euthanized by rapid decapitation (<3 min from touching the cage). Blood, femurs, tibia, thymus, and spleen were collected in microcentrifuge tubes and immediately frozen on dry ice. Tissues were stored at − 80°C until steroids were extracted. Due to the low concentrations of DHC, separate age-matched cohorts of mice were used to quantify corticosterone and DHC. Steroids were extracted from 0.42–25mg or μl of tissue or blood, depending on the age of the subject and the steroid being measured. Bone marrow was extracted from femurs prior to steroid extraction.
2.7. Study 2. Rat restraint stress and serum collection
Animals were handled 5min/day for 5 days before serum collection. Prior to restraint, rats were moved to a separate holding room and allowed to habituate for 2h. Individual cages were then transported to an adjacent room, where rats were placed into acrylic restraint tubes (PLAS Labs, Lansing, MI). Restraint tubes for males (6.35cm inner diameter, 21.6cm length) were larger than restraint tubes for females (5.1cm inner diameter, 20.3cm length). Blood samples were obtained by placing the rat’s tail in warm water, making a small incision on a visible tail vein using a scalpel (No. 11), and collecting blood in microcentrifuge tubes. Baseline blood was collected after rats were put into restraint tubes, but within 3min from approaching the cage. Rats remained in restraint rubes, were placed back into their home cages and moved to another room for the duration of the restraint period. An additional blood sample was obtained for all animals after 60min of restraint. Serum was isolated by centrifuging blood at 10,000g for 1min and frozen within 30min of collection, then stored at ‒ 80°C until steroids were extracted. Steroids were extracted from 0.1–3μl of serum, depending on the sex of the subject and steroid being measured.
2.8. Study 3. Song sparrow restraint stress and plasma collection
Blood samples were obtained from the brachial vein by venipuncture using a sterile needle into heparinized capillary tubes as previously described (Newman et al., 2008). Initial blood samples were collected within 3min from the time of capture. Birds were then restrained in an opaque cloth bag for 30min and a second blood sample was collected. Capillary tubes were kept cool until centrifugation (within 5h). After centrifugation, plasma was stored at − 80°C until steroids were extracted. Steroids were extracted from 2–4μl of plasma, depending on the steroid being measured.
2.9. Steroid extraction
Steroids were extracted from all samples by solid-phase extraction (SPE) using C18 columns as previously described in detail (Tobiansky et al., 2018). Briefly, tissue samples were weighed and then homogenized in acetonitrile with 0.01% formic acid using a bead mill homogenizer at 4m/s for 30sec. After centrifugation of homogenates, supernatants were transferred to 7mL glass scintillation vials, dried at 40°C in a vacuum centrifuge, and resuspended in 500μL HPLC-grade methanol. SPE columns were primed with 3mL HPLC-grade hexane, 3mL HPLC-grade acetone, and then 3mL HPLC-grade methanol prior to sample loading. Samples (500μL) were loaded onto columns, and the eluates were collected into glass vials. Then 2mL HPLC-grade methanol was added to the columns, and the eluates were collected into the same vials. The total eluate (2.5mL) was dried at 40°C in a vacuum centrifuge. Samples were resuspended by adding ethanol directly to the dried residues, vortexed briefly, and diluted with buffer to 5% ethanol. Resuspended samples were stored at ‒ 20°C overnight.
Corticosterone was quantified using a radioimmunoassay (07120103) from MP Biomedicals (Santa Ana, USA), and DHC was quantified using an enzyme-linked immunosorbent assay (ELISA) as described above.
2.10. Statistical analysis
Non-detectable samples (samples below the lowest standard on the standard curve) were set to the average of the water blanks + 2 standard deviations (Taves et al., 2015). To make comparisons between tissues, 1mL of blood was considered to weigh 1g (Schmidt and Soma, 2008; Taves et al., 2011b). The correlation between ELISA and LC-MS/MS values of DHC was determined by Pearson correlation. When necessary, data were log transformed prior to analysis. In Study 1, male and female mice showed similar steroid levels, so sexes were pooled and subjects were analyzed by one-way analysis of variance separately at each age, followed by Dunnett’s multiple comparison test. In Study 2, data were analyzed by two-way analysis of variance, and in Study 3, data were analyzed by paired t-tests. All data were analyzed using GraphPad Prism 6 and R version 3.3.3 (Another Canoe). All graphs are presented using the non-transformed data.
3. Results
3.1. DHC ELISA development and validations
To develop the DHC ELISA, we established a standard curve, and then validated our assay by examining intra- and inter-assay variation, cross-reactivity, recovery, serum serial dilution, and comparison with LC-MS/MS.
3.1.1. Standard curve development
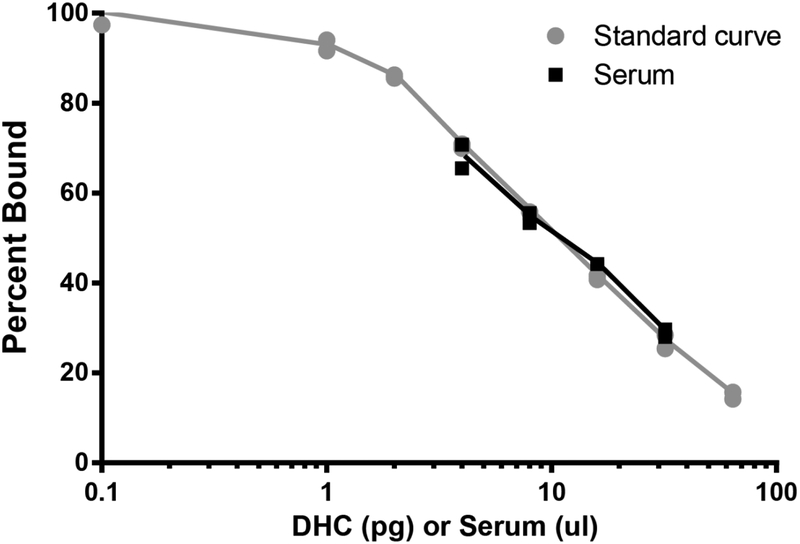
The range of the standard curve was optimized to be 1 to 64 pg/well (Figure 2), which demonstrates excellent sensitivity. The extracted water blanks across all assays (n=10) were consistently below the lowest standard (1 pg/well).
Figure 2:
Representative 11-dehydrocorticosterone (DHC) standard curve and serum serial dilution. Curves were generated by plotting the percent of biotin-labeled DHC bound versus the concentration of DHC/well on a logarithmic scale. Standard curves and serum were run in duplicate and mean percent bound was determined for each concentration. The lowest standard was 1pg/well, demonstrating excellent assay sensitivity. Serial diluted serum was parallel to the DHC standard curve, indicating a lack of matrix effects. n=2/dilution for both the standard curve and serum serial diluiton.
3.1.2. Intra- and inter- assay variation
We used two QCs (2pg and 16pg) and observed average values of 2.08pg and 15.59pg, demonstrating excellent accuracy even for the low QC. The intra-assay variation for the two QCs was 5% and 9%, respectively. To assess inter-assay variation, we used the same two QCs and measured them across multiple assays. Inter-assay variation for the two QCs was 15% and 10%, respectively.
3.1.3. Antibody cross-reactivity
Cross-reactivity was tested for six closely-related steroids: progesterone, 11-deoxycorticosterone, corticosterone, 11-deoxycortisol, cortisol, and cortisone, at 100pg and 1000pg. These steroids were all non-detectable at 100pg. At 1000pg, we detected slight cross-reactivity for 11-deoxycorticosterone (0.51%), corticosterone (0.36%), and cortisone (0.49%) (Table 1).
3.1.4. Recovery
Recovery was calculated by subtracting unspiked tissue values from spiked tissue values and dividing by the amount of exogenous steroid added. Corticosterone and DHC recovery was species- and tissue-specific (Table 2), as seen previously (Taves et al., 2015).
3.1.5. Serum serial dilution
Serial diluted serum was plotted as percent bound and was parallel to the standard curve, demonstrating a lack of matrix effects (Figure 2).
3.1.6. Comparison with liquid chromatography tandem mass spectrometry (LC-MS/MS)
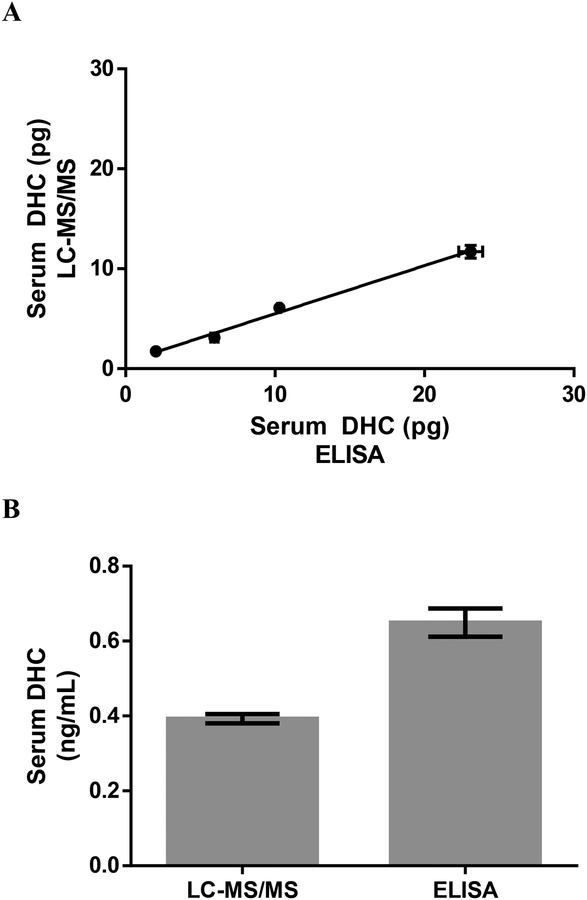
Analysis of DHC in mouse serum by ELISA and LC-MS/MS showed a significant positive linear correlation between the methods (r2=0.9928; p=0.0036; Figure 3A). The serum DHC concentration as measured by ELISA (0.65 ± 0.04 ng/mL) was slightly higher than the serum DHC concentration as measured by LC-MS/MS (0.39 ±0.01 ng/mL) (Figure 3B). This is consistent with previous studies that compare immunoassays and LC-MS/MS for other steroids (Fanelli et al., 2011; Moal et al., 2007). The primary antibody used in the ELISA shows a slight cross-reactivity with 11-deoxycorticosterone (0.51%), corticosterone (0.36%), and cortisone (0.49%). Only corticosterone (76.4 ± 1.5 ng/mL) and 11-deoxycorticosterone (4.5 ± 0.03 ng/mL) levels were high enough in this pool of serum, as measured by LC-MS/MS, to significantly affect DHC ELISA values. Cortisone was non-detectable. This explains the slight difference in DHC values between LC-MS/MS and ELISA.
Figure 3:
Picograms of 11-dehydrocorticosterone (DHC) in serial diluted mouse serum measured by LC-MS/MS and ELISA (n=2/method/dilution) (A) and concentraton of DHC in the same mouse serum measured by LC-MS/MS and ELISA (n=8/method) (B). DHC concentration for each method was determeined by first calculating the average conentration of each volume of serum volume and then calculating the average of all serum DHC concentrations. Data are expressed as mean ± SEM.
3.2. Study 1: Systemic and local levels of corticosterone and DHC in mice
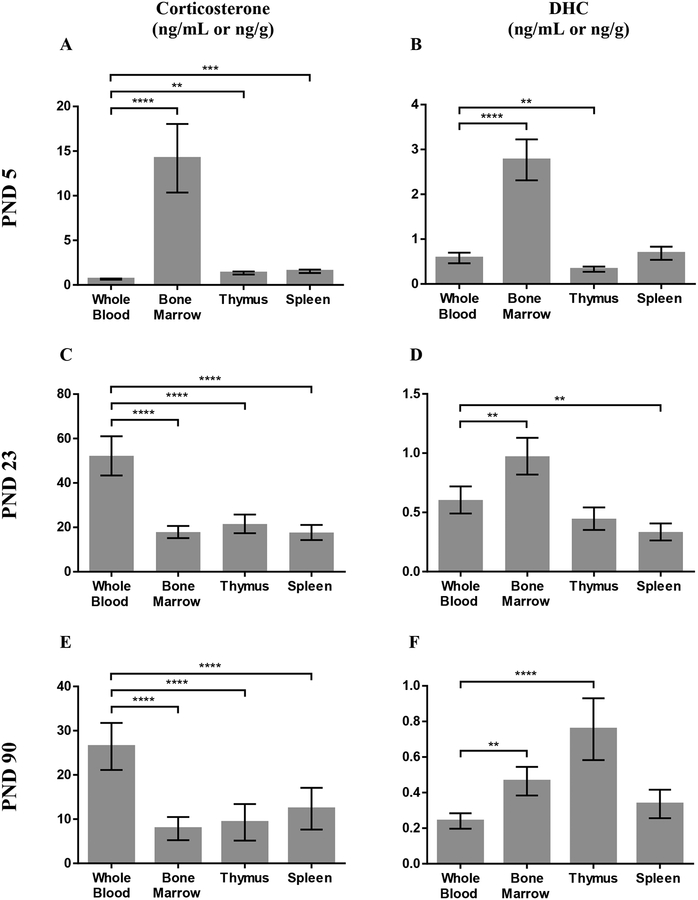
In PND5 mice, as expected, corticosterone levels were higher in bone marrow, thymus and spleen than in blood (F3,27=90.53; p<0.0001; Figure 4A). Also at PND5, DHC levels were higher in bone marrow than in blood, and DHC levels were lower in thymus than in blood (F3,27=79.70; p<0.0001; Figure 4B).
Figure 4:
Concentrations of corticosterone (A, C, E) or 11-dehydrocorticosterone (DHC) (B, D, F) in whole blood, bone marrow, thymus, and spleen of mice at PND5 (A and B), PND23 (C and D), and PND90 (E and F). Data are shown as mean ± SEM and tissue levels different from blood levels are indicated as follows * P≤0.05, ** P≤0.01, *** P≤0.001, **** P≤0.0001. n=10 for both steroids and all tissues at PND5 and PND23, n=9 for both steroids and all tissues at PND90.
In PND23 mice, corticosterone levels were higher in blood than in bone marrow, thymus, and spleen (F3,27=92.41; p<0.0001; Figure 4C). Also at PND23, DHC levels were higher in bone marrow and lower in spleen than in blood (F3,27=18.29; p<0.0001; Figure 4D).
In PND90 mice, corticosterone levels were higher in blood than in bone marrow (F3,24=44.41; p<0.0001; Figure 4E). Also at PND90, DHC levels were higher in bone marrow and thymus than in blood (F3,24=10.87; p<0.0001; Figure 4F).
Circulating corticosterone levels were lowest at PND5, which is consistent with PND5 being in the SHRP (Figure 4A, C, E). Unlike corticosterone, DHC levels in blood and tissues generally decreased with age (Figure 4B, D, F).
Corticosterone concentrations were generally higher than DHC concentrations. At PND5, corticosterone was approximately 1.5-fold higher than DHC in blood and 2- to 5-fold higher than DHC in lymphoid organs. At PND23, corticosterone was approximately 85-fold higher than DHC in blood and 18- to 53-fold higher than DHC in lymphoid organs. At PND90, corticosterone was approximately 110-fold higher than DHC in blood and 12- to 37-fold higher than DHC in lymphoid organs. Thus, the relationship between corticosterone and DHC is tissue-and age-specific.
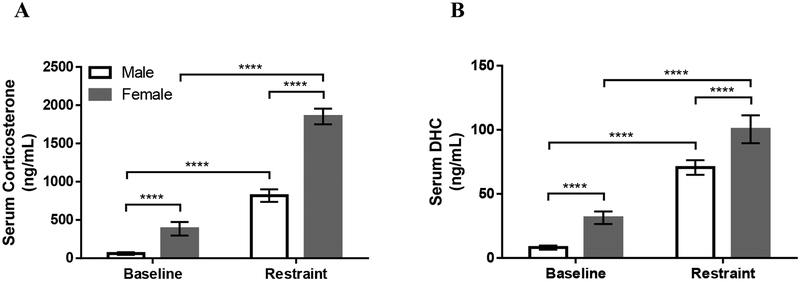
3.3. Study 2: Systemic levels of corticosterone and DHC in rats
For serum corticosterone levels in rats, there were main effects of Sex (F1,16=30.85; P<0.0001) and Restraint (F1,16=119.8; p<0.0001) and there was a Sex × Restraint interaction (F1,16=6.403; p=0.0223; Figure 5A). Corticosterone levels were higher in females than in males and increased more in females than in males after restraint (Figure 5A).
Figure 5:
The effects of sex and restraint for 60min on concentrations of corticosterone (A) or 11-dehydrocorticosterone (DHC) (B) in serum of adult rats. Data are shown as mean ± SEM. **** P≤0.0001. n=9 for males and females for baseline and restraint.
For serum DHC levels in rats, there were main effects of Sex (F1,16=42.04; p<0.0001) and Restraint (F1,16=148.4; p<0.0001) and there was a Sex × Restraint interaction (F1,16=14.61; p=0.0015; Figure 5B). DHC levels were higher in females than in males and increased more in females than in males after restraint (Figure 5B).
At baseline, corticosterone levels were approximately 7-fold higher than DHC levels in male rats and 13-fold higher than DHC levels in female rats. After restraint, corticosterone levels were approximately 12-fold higher than DHC levels in male rats and 20-fold higher than DHC levels in female rats.
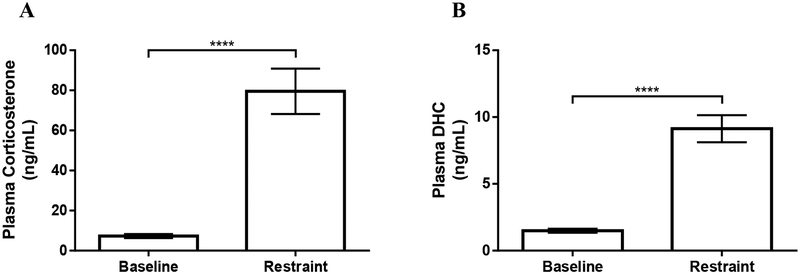
3.4. Study 3: Systemic levels of corticosterone and DHC in song sparrows
Plasma corticosterone levels in male song sparrows increased in response to restraint stress (t6=11.08; p<0.0001; Figure 6A). Plasma DHC levels also increased in response to restraint stress (t6=11.07; p<0.0001; Figure 6B). Overall, corticosterone levels were approximately 5-fold higher than DHC levels at baseline and approximately 9-fold higher than DHC levels after restraint stress.
Figure 6:
The effect of restraint for 30min on concentrations of corticosterone (A) or 11-dehydrocorticosterone (DHC) (B) in plasma of free-living non-breeding male song sparrows. Data are shown as mean ± SEM. **** P≤0.0001. n=7 for both baseline and restraint.
4. Discussion
Despite the importance of DHC for local regeneration of corticosterone and thus for stress physiology, DHC levels in circulation have rarely been reported in rodents and have never been reported in birds. No immunoassays are currently available to measure DHC. Here, we developed and validated a sensitive and specific ELISA to measure DHC. In Study 1, we quantified corticosterone and DHC in mouse blood, bone marrow, thymus, and spleen across 3 ages. Interestingly, each organ showed a specific pattern of local steroid regulation. In Study 2, we measured circulating corticosterone and DHC in rats at baseline and after restraint stress. Corticosterone levels were higher than DHC levels in both males and females, and both corticosterone and DHC levels increased more in females than in males after restraint. In Study 3, we measured circulating corticosterone and DHC in male songbirds at baseline and after restraint stress. Both corticosterone and DHC levels increased after restraint. To our knowledge, this is the first study to report 1) DHC levels in neonatal mice, 2) DHC levels in lymphoid organs, 3) local regulation of DHC levels, 4) a sex-specific increase in DHC levels in response to an acute stressor in rats, and 5) DHC levels in any bird.
4.1. DHC ELISA development and validations
We validated our DHC ELISA on four key elements: accuracy, precision, sensitivity, and specificity. To assess accuracy, we measured two standards (2pg and 16pg) and observed values within 4% of expected values. To assess precision, we measured the same two standards repeatedly. The intra-assay variation was 5% and 9% for the 2pg and 16pg standards, respectively, and the inter-assay variation was 15% and 10%, respectively. The sensitivity of our ELISA is indicated by the low detection limit of 1pg/well (Figure 2). To assess specificity, we used six closely-related steroids. We detected low cross-reactivity to 11-deoxycorticosterone, corticosterone, and cortisone at 0.51%, 0.36%, and 0.49%, respectively. Progesterone, 11-deoxycortisol, and cortisol were non-detectable, even at the 1000pg level (Table 1). Moreover, a serial dilution of serum showed parallelism with the standard curve, demonstrating that the extraction protocol effectively removed possible matrix effects.
To further assess the accuracy, precision, and specificity of our ELISA, we directly compared it against a LC-MS/MS assay. One pool of mouse serum was serial diluted and measured by both LC-MS/MS and ELISA. The amount of DHC in serum samples as measured by both methods showed a significant positive linear correlation (r2=0.9928; p=0.0036). As expected, the DHC concentration in mouse serum was slightly higher when measured by ELISA, relative to LC-MS/MS, as seen in comparisons of other steroid assays (Fanelli et al., 2011; Moal et al., 2007). Higher DHC concentrations obtained by ELISA than LC-MS/MS may be explained by the slight cross-reactivity with corticosterone (0.36%), which is present at high levels, and with 11-deoxycorticosterone (0.51%), which is present at low levels. Nonetheless, all of these validations taken together indicate that the DHC ELISA is accurate, precise, sensitive, and specific, and it is a useful tool for measuring DHC levels. This immunoassay is especially useful because there are currently no other DHC immunoassays available.
4.2. Study 1: Local regulation of corticosterone and DHC in mouse immune organs
Local production of GCs may be of particular importance during the SHRP, when circulating GCs are low and tissues are rapidly developing. A growing body of evidence suggests many tissues locally regulate GCs (Taves et al., 2017). GCs play an important role in lymphocyte development, most commonly studied in T-cells. Studies over the last three decades have shown that GCs modulate T-cell affinity for antigens, responsiveness, and proliferation (Mittelstadt et al., 2012; Pazirandeh et al., 1999; Vacchio and Ashwell, 1997; Vacchio et al., 1999). Furthermore, recent studies with mice lacking CYP11B1 in either thymocytes or thymic epithelial cells reveal that thymic epithelial cells, rather than thymocytes, are responsible for most of the locally-produced, bioactive corticosterone. However, this study examined only synthesis of corticosterone from 11-deoxycorticosterone, not regeneration of corticosterone from DHC (Mittelstadt et al., 2018). GCs are less studied in B-cell development and function, but GCs modulate B-cell development and cause apoptosis of B-cells at high concentrations (Cortez et al., 1996; Garvy et al., 1993; Gruver-Yates et al., 2014; Trottier et al., 2008). Finally, in vitro experiments administering a high concentration (1μM) of either 11-deoxycortiocsterone or DHC suggest that corticosterone is preferentially produced from DHC rather than 11-deoxycorticosterone (Taves et al., 2016b), but DHC levels in blood and lymphoid organs were previously unknown.
The current data demonstrate that DHC is present in the blood and lymphoid organs of mice. In bone marrow, both corticosterone and DHC were locally elevated at PND5, but only DHC was locally elevated at PND23 and neither were locally elevated at PND90. Corticosterone levels were lower in bone marrow than blood at PND23 and PND90. In thymus, corticosterone was locally elevated only at PND5 and was lower than in blood at PND23 and PND90. In contrast, DHC levels were lower in thymus than in blood at PND5. DHC was also elevated in thymus at PND90. In spleen, corticosterone was locally elevated at only PND5 and lower than blood at all other ages. DHC was not locally elevated in spleen at any age and was lower in spleen than blood at PND90 (Figure 4). The local elevation of corticosterone in all lymphoid organs at PND5 is consistent with previous findings (Taves et al., 2015). Additionally, our DHC levels at PND90 are consistent with recently reported DHC levels in circulation of adult mice measured by LC-MS/MS and our own LC-MS/MS data (Verma et al., 2018).
The local elevation of DHC in bone marrow and the local reduction of DHC in thymus at PND5 could be explained in three different ways. 1) Tissues might increase local DHC levels by sequestering DHC from blood, in order to be locally regenerated to corticosterone. In this scenario, tissues create a “reservoir” of DHC that can be locally regenerated to corticosterone via 11β-HSD1. However, there are no known mechanisms to sequester DHC. 2) Local DHC levels might be a result of local corticosterone synthesis and subsequent metabolism to DHC via 11β-HSD2. In this scenario, high local levels of DHC simply reflect high local inactivation of corticosterone. 3) Local DHC levels might be a result of both uptake of DHC from blood and local corticosterone synthesis with subsequent metabolism to DHC via 11β-HSD2. This scenario allows for the greatest control over local corticosterone levels by providing a mechanism for both local synthesis and regeneration of corticosterone.
In vitro experiments suggest that tissues increasingly regenerate corticosterone from DHC with age (Taves et al., 2016b), but until now, DHC levels across age in mice were unknown. As expected, baseline corticosterone levels in the blood were very low at PND5 and increased approximately 75-fold at PND23. Surprisingly, DHC levels did not increase in the blood or in tissues after PND5 and were lowest in blood at PND90. These data indicate that DHC is present in circulation and tissues, but might not be at high enough levels outside of the SHRP to substantially contribute to local corticosterone production. It is possible that tissues rely more heavily on regeneration from DHC to contribute to local elevation of corticosterone during the SHRP and more on synthesis afterwards. These data are also in agreement with the idea that the SHRP reduces the harmful effects of circulating GCs on developing tissues, and thus allows for tissue-specific upregulation of corticosterone based on need (Sapolsky and Meaney, 1986; Taves et al., 2015).
Corticosterone locally synthesized from 11-deoxycorticosterone by thymic epithelial cells modulates T-cell development (Mittelstadt et al., 2018), but no study has examined whether corticosterone locally regenerated from DHC is also biologically active. Studies using genetic models, such as 11β-HSD1−/− mice, will be helpful in determining if intracellular regeneration is important for lymphocyte selection (Cobice et al., 2013). Additionally, studies using a combination of genetically altered mice and labeled GC precursors or DHC will be beneficial in determining if locally-derived GCs are a result of synthesis, regeneration, or both.
4.3. Study 2: Corticosterone and DHC levels after restraint stress in rats
Despite the widespread use of rats in studies of stress physiology, few studies have measured DHC in rats and only one has measured DHC after a stressor (Hundertmark et al., 2002a; Obut et al., 2004; Tagawa et al., 2007). Here, we present data on circulating corticosterone and DHC in rats at baseline and after 60min of restraint stress (Figure 5). Our data demonstrate that both corticosterone and DHC levels increase in response to restraint in a sex-specific fashion. Similar to corticosterone, DHC increased in response to stress and increased to a greater extent in females than in males. Additionally, our reported DHC levels at baseline are in line with previously reported values in rats of the same age (Tagawa et al., 2007).
These data indicate that DHC increases in serum in response to restraint stress. Systemic DHC levels may increase in response to stress for two reasons: 1) increased conversion of corticosterone to the inactive DHC, thus protecting tissues from harmful effects of corticosterone or 2) provide a reservoir for specific tissues to intracellularly regenerate DHC to corticosterone. Evidence for the latter includes high levels of 11β-HSD1 in the rat brain (Diaz et al., 1998; Holmes and Seckl, 2006; Wyrwoll et al., 2011) and prolonged stress-induced increases in blood corticosterone in 11β-HSD1-deficient mice (Harris et al., 2001). Together, the data suggest that 11β-HSD1 plays a pivotal role in HPA axis regulation by utilizing systemic DHC to intracellularly regenerate corticosterone, to provide increased negative feedback on the HPA axis and prevent a heightened and prolonged stress response.
4.4. Study 3: Corticosterone and DHC levels after restraint stress in song sparrows
Song sparrows show increases in corticosterone levels in response to restraint stress (Newman et al., 2008; Newman and Soma, 2009). While the corticosterone response to restraint is well characterized in song sparrows and many other birds, DHC has yet to be measured in any bird species. Here, we present data on both corticosterone and DHC in male song sparrows at baseline and 30min after restraint. Corticosterone levels were higher than DHC levels at baseline and restraint, and both corticosterone and DHC increased in response to restraint stress (Figure 6).
Little is known about 11β-HSD1 in song sparrow tissues, but a recent study in zebra finches was unable to detect 11β-HSD1 mRNA in adult brain, while mRNAs for 11β-HSD2, mineralocorticoid receptor, and glucocorticoid receptors were all detected in brain regions (Rensel et al., 2018). Another study in birds found no evidence of local GC production in the brain of European starlings but did report evidence of local GC production in lymphoid organs of zebra finches (Schmidt et al., 2009). These data may indicate that inactivation of corticosterone to DHC by 11β-HSD2, to keep local corticosterone levels low, may be important in the brain of birds, but not in immune tissues. Additional studies in birds of DHC across season, age, and species will be useful.
4.5. Possible implications for human health
Cortisol, and its metabolite cortisone, are the predominant circulating GCs in humans (Levine et al., 2007). A wide array of human tissues express 11β-HSD1—including liver, adipose tissue, and brain—and intracellular regeneration of cortisol from cortisone via 11β-HSD1 might be important in type 2 diabetes mellitus and neurodegenerative diseases (Rask et al., 2001; Walker, 2007). Interestingly, corticosterone and DHC are also present at low levels in human blood, and human neuronal cells can convert DHC to corticosterone in vitro (Branchaud et al., 1969; Morris and Williams, 1953; Rajan et al., 1996; Weichselbaum and Margraf, 1960). These data raise the possibility that DHC and corticosterone are relevant for human health.
4.6. Conclusions
We developed and validated a sensitive and specific ELISA for measurement of DHC and compared our immunoassay with a LC-MS/MS assay. After validation, we used this DHC immunoassay to measure circulating and local DHC levels in mice and circulating DHC levels in rats and song sparrows. In mice, DHC levels in the blood and lymphoid organs vary by age and tissue. For example, at PND5, DHC was locally elevated in bone marrow, relative to the blood. DHC levels decreased with age, in contrast to corticosterone. These data suggest that age-related changes in DHC levels impact local regeneration of corticosterone from DHC in lymphoid organs. In rats and song sparrows, circulating DHC and corticosterone levels increase in response to restraint stress. This novel assay and these fundamental data on DHC levels provide a basis for future studies of DHC, an important but relatively neglected component of GC physiology.
We developed an immunoassay for 11-dehydrocorticosterone, an important steroid.
We validated the assay for accuracy, precision, specificity, and matrix effects.
In mice, corticosterone and 11-dehydrocorticosterone levels vary with age and tissue.
In rats, restraint increases corticosterone and 11-dehydrocorticosterone levels.
In song sparrows, restraint increases corticosterone and 11-dehydrocorticosterone levels.
Acknowledgments
We thank Katherine Gray, Dr. Daniel Tobiansky, and Dr. Cathy Ma for assistance in data collection and analysis. We would also like to thank the Centre for Disease Modeling staff for animal husbandry and the UBC Genotyping Facility. This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (RGPIN-2014–04884) to KKS, a Canadian Institutes of Health Research Project Grant (360035) to JSS, grants from the National Heart, Lung, and Blood Institute (R01 HL27255) and the National Institute of General Medical Sciences (U54 GM115428) to CEG-S, a Four Year Doctoral Fellowship from the University of British Columbia to JEH, a Natural Sciences and Engineering Research Council of Canada Undergraduate Student Research Award to MS, and a fellowship from the Uruguay Agencia Nacional de Investigación e Innovación to CJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none.
References
- Alberts P, Rönquist-Nii Y, Larsson C, Klingström G, Engblom L, Edling N, Lidell V, Berg I, Edlund PO, Ashkzari M, Sahaf N, Norling S, Berggren V, Bergdahl K, Forsgren M, Abrahmsén L, 2005. Effect of high-fat diet on KKAy and ob/ob mouse liver and adipose tissue corticosterone and 11-dehydrocorticosterone concentrations. Horm Metab Res 37, 402–407. [DOI] [PubMed] [Google Scholar]
- Ashwell JD, Lu FW, Vacchio MS, 2000. Glucocorticoids in T cell development and function. Annu Rev Immunol 18, 309–345. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ, 2008. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun 22, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchaud C, Schweitzer M, Giroud CJ, 1969. Characterization of the 21-yl sulfates of 11-beta, 17-alpha, 21-trihydroxypregn-4-ene-3,20-dione; 17-alpha, 21-dihydroxypregn-4-ene-3, 11,20-trione; 11-beta, 21-dihydroxyregn-4-ene-3,20-dione; 21-hydroxypregn-4-ene-3, 11,20-trione and 21-hydroxypregn-4-ene, 3,20-dione in human cord plasma. Steroids 14, 179–190. [DOI] [PubMed] [Google Scholar]
- Chapman K, Holmes M, Seckl J, 2013. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93, 1139–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobice DF, Mackay CL, Goodwin RJ, McBride A, Langridge-Smith PR, Webster SP, Walker BR, Andrew R, 2013. Mass spectrometry imaging for dissecting steroid intracrinology within target tissues. Anal Chem 85, 11576–11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Stoica G, Pierce JH, Pendergast AM, 1996. The BCR-ABL tyrosine kinase inhibits apoptosis by activating a Ras-dependent signaling pathway. Oncogene 13, 2589–2594. [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR, 2009. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR, Cabib S, Puglisi-Allegra S, Patacchioli FR, Cigliana G, Maccari S, Angelucci L, 1992. Effects of acute and repeated exposure to stress on the hypothalamo-pituitary-adrenocortical activity in mice during postnatal development. Horm Behav 26, 474–485. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL, 1996. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol 157, 1638–1644. [PubMed] [Google Scholar]
- Diaz R, Brown RW, Seckl JR, 1998. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci 18, 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli F, Belluomo I, Di Lallo VD, Cuomo G, De Iasio R, Baccini M, Casadio E, Casetta B, Vicennati V, Gambineri A, Grossi G, Pasquali R, Pagotto U, 2011. Serum steroid profiling by isotopic dilution-liquid chromatography-mass spectrometry: comparison with current immunoassays and reference intervals in healthy adults. Steroids 76, 244–253. [DOI] [PubMed] [Google Scholar]
- Garvy BA, King LE, Telford WG, Morford LA, Fraker PJ, 1993. Chronic elevation of plasma corticosterone causes reductions in the number of cycling cells of the B lineage in murine bone marrow and induces apoptosis. Immunology 80, 587–592. [PMC free article] [PubMed] [Google Scholar]
- Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH, 2015. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 10, e0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruver-Yates AL, Quinn MA, Cidlowski JA, 2014. Analysis of glucocorticoid receptors and their apoptotic response to dexamethasone in male murine B cells during development. Endocrinology 155, 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B, 2002. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology 27, 199–220. [DOI] [PubMed] [Google Scholar]
- Harris HJ, Kotelevtsev Y, Mullins JJ, Seckl JR, Holmes MC, 2001. Intracellular regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase (11beta-HSD)-1 plays a key role in regulation of the hypothalamic-pituitary-adrenal axis: analysis of 11beta-HSD-1-deficient mice. Endocrinology 142, 114–120. [DOI] [PubMed] [Google Scholar]
- Hay M, Mormède P, 1997. Improved determination of urinary cortisol and cortisone, or corticosterone and 11-dehydrocorticosterone by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Biomed Sci Appl 702, 33–39. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Seckl JR, 2006. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol 248, 9–14. [DOI] [PubMed] [Google Scholar]
- Hundertmark S, Dill A, Bühler H, Stevens P, Looman K, Ragosch V, Seckl JR, Lipka C, 2002a. 11beta-hydroxysteroid dehydrogenase type 1: a new regulator of fetal lung maturation. Horm Metab Res 34, 537–544. [DOI] [PubMed] [Google Scholar]
- Hundertmark S, Dill A, Ebert A, Zimmermann B, Kotelevtsev YV, Mullins JJ, Seckl JR, 2002b. Foetal lung maturation in 11beta-hydroxysteroid dehydrogenase type 1 knockout mice. Horm Metab Res 34, 545–549. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hanaoka S, Sato K, 1991. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur J Immunol 21, 643–648. [DOI] [PubMed] [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC, 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148, 132–149. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A, 2007. Measuring cortisol in human psychobiological studies. Physiol Behav 90, 43–53. [DOI] [PubMed] [Google Scholar]
- Mittelstadt PR, Monteiro JP, Ashwell JD, 2012. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest 122, 2384–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstadt PR, Taves MD, Ashwell JD, 2018. Cutting edge: De novo glucocorticoid synthesis by thymic epithelial cells regulates antigen-specific thymocyte selection. J Immunol 200, 1988–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moal V, Mathieu E, Reynier P, Malthièry Y, Gallois Y, 2007. Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clin Chim Acta 386, 12–19. [DOI] [PubMed] [Google Scholar]
- Morris CJ, Williams DC, 1953. The polarographic estimation of steroid hormones. VI. Determination of individual adrenocortical hormones in human peripheral blood. Biochem J 54, 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ, 1984. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5, 25–44. [DOI] [PubMed] [Google Scholar]
- Newman AE, Pradhan DS, Soma KK, 2008. Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinology 149, 2537–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AE, Soma KK, 2009. Corticosterone and dehydroepiandrosterone in songbird plasma and brain: effects of season and acute stress. Eur J Neurosci 29, 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obut TA, Ovsyukova MV, Cherkasova OP, 2004. Ratio between the contents of 11-dehydrocorticosterone and corticosterone after acute and repeated stress: effect of dehydroepiandrosterone sulfate. Bull Exp Biol Med 138, 137–139. [DOI] [PubMed] [Google Scholar]
- Pazirandeh A, Xue Y, Rafter I, Sjövall J, Jondal M, Okret S, 1999. Paracrine glucocorticoid activity produced by mouse thymic epithelial cells. FASEB J 13, 893–901. [DOI] [PubMed] [Google Scholar]
- Qiao S, Okret S, Jondal M, 2009. Thymocyte-synthesized glucocorticoids play a role in thymocyte homeostasis and are down-regulated by adrenocorticotropic hormone. Endocrinology 150, 4163–4169. [DOI] [PubMed] [Google Scholar]
- Rajan V, Edwards CR, Seckl JR, 1996. 11 beta-Hydroxysteroid dehydrogenase in cultured hippocampal cells reactivates inert 11-dehydrocorticosterone, potentiating neurotoxicity. J Neurosci 16, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask E, Olsson T, Söderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR, 2001. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab 86, 1418–1421. [DOI] [PubMed] [Google Scholar]
- Rensel MA, Ding JA, Pradhan DS, Schlinger BA, 2018. 11β-HSD types 1 and 2 in the songbird brain. Front Endocrinol (Lausanne) 9, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM, 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128, 1–24. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ, 1986. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res 396, 64–76. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21, 55–89. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Chin EH, Shah AH, Soma KK, 2009. Cortisol and corticosterone in immune organs and brain of European starlings: developmental changes, effects of restraint stress, comparison with zebra finches. Am J Physiol Regul Integr Comp Physiol 297, R42–51. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Pradhan DS, Shah AH, Charlier TD, Chin EH, Soma KK, 2008. Neurosteroids, immunosteroids, and the Balkanization of endocrinology. Gen Comp Endocrinol 157, 266–274. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Soma KK, 2008. Cortisol and corticosterone in the songbird immune and nervous systems: local vs. systemic levels during development. Am J Physiol Regul Integr Comp Physiol 295, R103–110. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS, 2003. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci 21, 125–132. [DOI] [PubMed] [Google Scholar]
- Selye H, 1936. A syndrome produced by diverse nocuous agents. 1936. Nature 10, 230–231. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S, 1990. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev Psychobiol 23, 411–426. [DOI] [PubMed] [Google Scholar]
- Tagawa N, Ikariko N, Fukumura K, Kobayashi Y, 2007. Development of an enzyme-linked immunosorbent assay for serum 11-dehydrocorticosterone in rat and mouse. Biol Pharm Bull 30, 403–409. [DOI] [PubMed] [Google Scholar]
- Taves MD, Gomez-Sanchez CE, Soma KK, 2011a. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab 301, E11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves MD, Hamden JE, Soma KK, 2017. Local glucocorticoid production in lymphoid organs of mice and birds: Functions in lymphocyte development. Horm Behav 88, 4–14. [DOI] [PubMed] [Google Scholar]
- Taves MD, Losie JA, Rahim T, Schmidt KL, Sandkam BA, Ma C, Silversides FG, Soma KK, 2016a. Locally elevated cortisol in lymphoid organs of the developing zebra finch but not Japanese quail or chicken. Dev Comp Immunol 54, 116–125. [DOI] [PubMed] [Google Scholar]
- Taves MD, Ma C, Heimovics SA, Saldanha CJ, Soma KK, 2011b. Measurement of steroid concentrations in brain tissue: methodological considerations. Front Endocrinol (Lausanne) 2, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves MD, Plumb AW, Korol AM, Van Der Gugten JG, Holmes DT, Abraham N, Soma KK, 2016b. Lymphoid organs of neonatal and adult mice preferentially produce active glucocorticoids from metabolites, not precursors. Brain Behav Immun 57, 271–281. [DOI] [PubMed] [Google Scholar]
- Taves MD, Plumb AW, Sandkam BA, Ma C, Van Der Gugten JG, Holmes DT, Close DA, Abraham N, Soma KK, 2015. Steroid profiling reveals widespread local regulation of glucocorticoid levels during mouse development. Endocrinology 156, 511–522. [DOI] [PubMed] [Google Scholar]
- Tobiansky DJ, Korol AM, Ma C, Hamden JE, Jalabert C, Tomm RJ, Soma KK, 2018. Testosterone and corticosterone in the mesocorticolimbic system of male rats: effects of gonadectomy and caloric restriction. Endocrinology 159, 450–464. [DOI] [PubMed] [Google Scholar]
- Toufexis D, Rivarola MA, Lara H, Viau V, 2014. Stress and the reproductive axis. J Neuroendocrinol 26, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier MD, Newsted MM, King LE, Fraker PJ, 2008. Natural glucocorticoids induce expansion of all developmental stages of murine bone marrow granulocytes without inhibiting function. Proc Natl Acad Sci U S A 105, 2028–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio MS, Ashwell JD, 1997. Thymus-derived glucocorticoids regulate antigen-specific positive selection. J Exp Med 185, 2033–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio MS, Lee JY, Ashwell JD, 1999. Thymus-derived glucocorticoids set the thresholds for thymocyte selection by inhibiting TCR-mediated thymocyte activation. J Immunol 163, 1327–1333. [PubMed] [Google Scholar]
- Vacchio MS, Papadopoulos V, Ashwell JD, 1994. Steroid production in the thymus: implications for thymocyte selection. J Exp Med 179, 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schröder FH, 1992. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci 50, 857–861. [DOI] [PubMed] [Google Scholar]
- Verma M, Kipari TMJ, Zhang Z, Man TY, Forster T, Homer NZM, Seckl JR, Holmes MC, Chapman KE, 2018. 11β-hydroxysteroid dehydrogenase-1 deficiency alters brain energy metabolism in acute systemic inflammation. Brain Behav Immun 69, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, 2008. Glucocorticoids: mediators of vertebrate ontogenetic transitions. Gen Comp Endocrinol 156, 441–453. [DOI] [PubMed] [Google Scholar]
- Walker BR, 2007. Extra-adrenal regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1: physiological regulator and pharmacological target for energy partitioning. Proc Nutr Soc 66, 1–8. [DOI] [PubMed] [Google Scholar]
- Weichselbaum TE, Margraf HW, 1960. Isolation and identification of adrenocortical steroids in human peripheral plasma. I. A naturally occurring C21 steroid acetate (11-dehydrocorticosterone acetate) and “free” tetrahydrocortisone in normal plasma. J Clin Endocrinol Metab 20, 1341–1350. [DOI] [PubMed] [Google Scholar]
- Wiegers GJ, Reul JM, Holsboer F, de Kloet ER, 1994. Enhancement of rat splenic lymphocyte mitogenesis after short term preexposure to corticosteroids in vitro. Endocrinology 135, 2351–2357. [DOI] [PubMed] [Google Scholar]
- Wood CE, Walker CD, 2015. Fetal and neonatal HPA axis. Compr Physiol 6, 33–62. [DOI] [PubMed] [Google Scholar]
- Wyrwoll CS, Holmes MC, Seckl JR, 2011. 11β-hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front Neuroendocrinol 32, 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Noble J, Kenyon CJ, Hibberd C, Kotelevtsev Y, Mullins JJ, Seckl JR, 2001. Lack of tissue glucocorticoid reactivation in 11beta-hydroxysteroid dehydrogenase type 1 knockout mice ameliorates age-related learning impairments. Proc Natl Acad Sci USA 98, 4716–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]