Abstract
Background
Heavy menstrual bleeding (HMB) is an important cause of ill health in premenopausal women. Although surgery is often used as a treatment, a range of medical therapies are also available. Non‐steroidal anti‐inflammatory drugs (NSAIDs) reduce prostaglandin levels, which are elevated in women with excessive menstrual bleeding and also may have a beneficial effect on dysmenorrhoea.
Objectives
To determine the effectiveness, safety and tolerability of NSAIDs in achieving a reduction in menstrual blood loss (MBL) in women of reproductive years with HMB.
Search methods
We searched, in April 2019, the Cochrane Gynaecology and Fertility specialised register, Cochrane Central Register of Studies Online (CENTRAL CRSO), MEDLINE, Embase, PsycINFO, the clinical trial registries and reference lists of articles.
Selection criteria
The inclusion criteria were randomised comparisons of individual NSAIDs or combined with other medical therapy with each other, placebo or other medical treatments in women with regular heavy periods measured either objectively or subjectively and with no pathological or iatrogenic (treatment‐induced) causes for their HMB.
Data collection and analysis
We identified 19 randomised controlled trials (RCTs) (759 women) that fulfilled the inclusion criteria for this review and two review authors independently extracted data. We estimated odds ratios (ORs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes from the data of nine trials. We described in data tables the results of the remaining seven cross‐over trials with data unsuitable for pooling, one trial with skewed data, and one trial with missing variances. One trial had no data available for analysis.
Main results
As a group, NSAIDs were more effective than placebo at reducing HMB but less effective than tranexamic acid, danazol or the levonorgestrel‐releasing intrauterine system (LNG IUS). Treatment with danazol caused a shorter duration of menstruation and more adverse events than NSAIDs, but this did not appear to affect the acceptability of treatment, based on trials from 1980 to 1990. However, currently danazol is not a usual or recommended treatment for HMB. There was no clear evidence of difference between NSAIDs and the other treatments (oral luteal progestogen, ethamsylate, an older progesterone‐releasing intrauterine system and the oral contraceptive pill (OCP), but most studies were underpowered. There was no evidence of a difference between the individual NSAIDs (naproxen and mefenamic acid) in reducing HMB. The evidence quality ranged from low to moderate, the main limitations being risk of bias and imprecision.
Authors' conclusions
NSAIDs reduce HMB when compared with placebo, but are less effective than tranexamic acid, danazol or LNG IUS. However, adverse events are more severe with danazol therapy. In the limited number of small studies suitable for evaluation, there was no clear evidence of a difference in efficacy between NSAIDs and other medical treatments such as oral luteal progestogen, ethamsylate, OCP or the older progesterone‐releasing intrauterine system.
Plain language summary
Are non‐steroidal anti‐inflammatory drugs safe and effective for treating heavy menstrual bleeding?
Review question
Cochrane authors investigated whether non‐steroidal anti‐inflammatory drugs (NSAIDs) helped reduce heavy menstrual bleeding (HMB) in women before they reach the menopause.
Background
NSAIDs reduce prostaglandin levels, which are elevated in women with excessive menstrual bleeding. It was suggested that they might help with heavy bleeding and may have a beneficial effect on painful menstrual periods.
Study characteristics
Authors search medical databases and identified 19 randomised controlled trials (RCTs; clinical studies where people are randomly put into one of two or more treatment groups) with 759 women that could be included in the review, but data from only nine trials were suitable for analyses.
Key results
Women sought help for HMB when it affected their quality of life. Levels of prostaglandin (a naturally occurring hormone) are higher in women with HMB and are reduced by NSAIDs. The review of trials found that NSAIDs were modestly effective in reducing HMB, but other medicines, such as danazol, tranexamic acid and levonorgestrel‐releasing intrauterine system (LNG IUS), are more effective. These results were based on a small number of low‐ to moderate‐quality trials.
Quality of the evidence
The evidence quality ranged from low to moderate, the main limitations being poor reporting of study methods and imprecision resulting from small study numbers.
Summary of findings
Summary of findings for the main comparison. NSAIDs versus placebo (control).
| NSAIDs versus placebo (control) | ||||||
| Patient or population: women with heavy menstrual bleeding Intervention: NSAIDs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | NSAIDs | |||||
| MBL (mL/cycle) | — | The mean MBL (mL/cycle) in the intervention groups was 124 lower (186.36 to 61.64 lower) | — | 11 (1 study) | ⊕⊕⊝⊝ Lowa | — |
| Proportion of women with no subjective improvement in MBL | Study population | OR 0.08 (0.03 to 0.18) | 80 (1 study) | ⊕⊕⊝⊝ Lowb,c | — | |
| 800 per 1000 | 242 per 1000 (107 to 419) | |||||
| Moderate | ||||||
| 800 per 1000 | 242 per 1000 (107 to 419) | |||||
| Quality of life | No study reported this outcome | |||||
| Number of days' bleeding | No study reported this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MBL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded two levels for imprecision (very small trial). bDowngraded one level for imprecision (single trial). cDowngraded one level for risk of bias (no explanation was provided).
Summary of findings 2. NSAIDs versus tranexamic acid (control).
| NSAIDs versus tranexamic acid (control) | ||||||
| Patient or population: women with heavy menstrual bleeding Intervention: NSAIDs Comparison: tranexamic acid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | NSAIDs | |||||
| MBL (mL/cycle) alkaline haematin method | — | The mean MBL (mL/cycle) in the intervention groups was 73 higher (21.66 to 124.34 higher) | — | 48 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Proportion of women with no subjective improvement in MBL | Study population | OR 1.44 (0.45 to 4.61) | 49 (1 study) | ⊕⊕⊝⊝ Lowa,b | — | |
| 308 per 1000 | 390 per 1000 (167 to 672) | |||||
| Moderate | ||||||
| 308 per 1000 | 391 per 1000 (167 to 672) | |||||
| Number of days' bleeding | — | The mean duration of menstruation (days) in the intervention groups was 0.4 higher (0.47 lower to 1.27 higher) | — | 49 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Quality of life | No study reported this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MBL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias (allocation concealment not stated). bDowngraded one level for imprecision (single trial).
Summary of findings 3. NSAIDs versus ethamsylate (control).
| NSAIDs versus ethamsylate (control) | ||||||
| Patient or population: women with heavy menstrual bleeding Intervention: NSAIDs Comparison: ethamsylate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | NSAIDs | |||||
| MBL at Rx (mL/cycle) | — | The mean MBL at Rx (mL/cycle) in the intervention groups was 42.88 lower (86.25 lower to 0.5 higher) | — | 82 (2 studies) | ⊕⊕⊕⊝ Moderatea | — |
| Proportion of women with no subjective improvement in MBL | Study population | OR 0.7 (0.23 to 2.12) | 50 (1 study) | ⊕⊕⊝⊝ Lowa,b | ||
| 481 per 1000 | 394 per 1000 (176 to 663) | |||||
| Moderate | ||||||
| 482 per 1000 | 394 per 1000 (176 to 664) | |||||
| Number of days' bleeding | — | The mean duration of menstruation (days) in the intervention groups was 0.4 lower (1.56 lower to 0.76 higher) | — | 46 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Quality of life | No study reported this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MBL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug; OR: odds ratio; Rx: treatment | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias (allocation concealment not stated). bDowngraded one level for imprecision (one small study).
Summary of findings 4. NSAIDs versus danazol (control).
| NSAIDs versus danazol (control) | ||||||
| Patient or population: women with heavy menstrual bleeding Intervention: NSAIDs Comparison: danazol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | NSAIDs | |||||
| MBL (mL/cycle) | — | The mean MBL (mL/cycle) in the intervention groups was 45.06 higher (18.73 to 71.39 higher) | — | 79 (3 studies) | ⊕⊕⊕⊝ Moderatea | — |
| Number of days' bleeding | — | The mean duration of menstruation (days) in the intervention groups was 1.03 higher (0.26 to 1.8 higher) | — | 53 (2 studies) | ⊕⊕⊕⊝ Moderatea | — |
| Quality of life | No study reported this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MBL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias (allocation concealment not stated).
Summary of findings 5. NSAIDs versus oral progestogens (control).
| NSAIDs vs oral progestogens (control) | ||||||
| Patient or population: women with heavy menstrual bleeding Intervention: NSAIDs Comparison: oral progestogens | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | NSAIDs vs oral progestogens | |||||
| MBL (mL/cycle) | — | The mean MBL (mL/cycle) in the intervention groups was 22.97 lower (46.57 lower to 0.62 higher) | — | 48 (2 studies) | ⊕⊕⊝⊝ Lowa,b | — |
| Number of days' bleeding | — | The mean duration of bleeding (days) in the intervention groups was 0.41 lower (0.95 lower to 0.13 higher) | — | 48 (2 studies) | ⊕⊕⊕⊝ Moderatea | — |
| Quality of life | No study reported this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MBL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias (allocation concealment not reported). bDowngraded one level for substantial heterogeneity.
Summary of findings 6. NSAIDs versus progesterone‐releasing intrauterine system (control).
| NSAIDs versus progesterone‐releasing intrauterine system (control) | ||||||
| Patient or population: women with heavy menstrual bleeding Intervention: NSAIDs Comparison: progesterone‐releasing intrauterine system | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | NSAIDs | |||||
| MBL (mL/cycle) | — | The mean MBL (mL/cycle) in the intervention groups was 4 lower (31.23 lower to 23.23 higher) | — | 16 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Number of days' bleeding | — | The mean duration of menstruation (days) in the intervention groups was 5 lower (6.08 to 3.92 lower) | — | 16 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Quality of life | No study reported this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MBL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias (randomisation method and allocation concealment not reported). bDowngraded one level for imprecision (one small trial).
Summary of findings 7. NSAIDs versus oral contraceptive pill (control).
| NSAIDs versus oral contraceptive pill (control) | ||||||
| Patient or population: women with heavy menstrual bleeding Intervention: NSAIDs Comparison: oral contraceptive pill | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | NSAIDs | |||||
| MBL (mL/cycle) | — | The mean MBL (mL/cycle) in the intervention groups was 25.25 higher (22.34 lower to 72.84 higher) | — | 26 (1 study) | ⊕⊕⊝⊝ Lowa,b | — |
| Quality of life | No study reported this outcome | |||||
| Days of bleeding | No study reported this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MBL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias (randomisation method and allocation concealment not reported). bDowngraded one level for imprecision (one small study).
Summary of findings 8. Mefenamic acid compared to naproxen for heavy menstrual bleeding.
| Mefenamic acid compared to naproxen for heavy menstrual bleeding | ||||||
| Patient or population: women with heavy menstrual bleeding Intervention: mefenamic acid Comparison: naproxen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Naproxen | Mefenamic acid | |||||
| MBL (mL/cycle) | — | The mean MBL (mL/cycle) in the intervention groups was 21 higher (5.85 lower to 47.85 higher) | — | 61 (2 studies) | ⊕⊕⊝⊝ Lowa,b | — |
| Number of days' bleeding | — | The mean duration of menstruation (days) in the intervention groups was 0.4 lower (1.59 lower to 0.79 higher) | — | 35 (1 study) | ⊕⊕⊝⊝ Lowb,c | — |
| Quality of life | No study reported this outcome | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MBL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias (lack of blinding). bDowngraded one level for imprecision (one or two small studies). cDowngraded one level for risk of bias (attrition bias).
Background
Description of the condition
Excessively heavy menstrual bleeding (menorrhagia or HMB) is an important cause of ill health in women and a very common gynaecological problem. It causes a major burden in quality of life (Frick 2009), and uses substantial healthcare resources (Liu 2007). In the UK one in 20 women between 30 and 49 years see their doctor each year for HMB (NICE 2018). According to a European study, 27% of women in reproductive age had experienced HMB symptoms within the previous 12 months (Fraser 2014).
HMB has often been objectively defined as 80 mL or more of menstrual blood loss (MBL) per period (Cole 1971; Hallberg 1966), which is unrelated to pregnancy or any known pelvic or systemic disease. However, this definition is mainly used in research and is difficult to quantify in clinical settings. Unacceptably problematic bleeding is most commonly determined by the woman herself if the amount or frequency of blood loss interferes with her physical or psychosocial well‐being. This personal perception is often what determines the need for treatment, and the assessment of outcomes afterwards. To clarify the situation, the International Federation of Gynaecology and Obstetrics (FIGO) formally defined HMB as "the woman's perspective of increased menstrual volume, regardless of regularity, frequency or duration" (Munro 2011).
Research studies have traditionally used the alkaline haematin method to measure HMB objectively (Hallberg 1966), but a simpler method that is often used is the pictorial blood loss assessment chart (PBAC) (Higham 1990). With this method, the woman assesses the blood loss on her used sanitary pads or tampons and assigns a numerical score. It has been suggested that total menstrual fluid loss (TMFL) may be used as an assessment of HMB (Reid 2005). Measurement is determined by the difference in weight of tampons or pads before and after use. TMFL correlates well with changes in objective MBL and may be of more relevance to women concerned mainly about heavy menstrual flow (flooding).
Surgery has traditionally had a dominant role in treating HMB, but 80% of women treated by surgery have no anatomical pathology and over a third of women undergoing hysterectomy for excessive blood loss have normal uteri (wombs) removed (Clarke 1995; Gath 1982). Thus, medical therapy, with the avoidance of possibly unnecessary surgery and a bonus of preserved fertility in women who have not completed their family, is an attractive alternative.
Description of the intervention
A wide variety of medications are used to reduce HMB. The currently available medical therapies include hormonal agents, anti‐fibrinolytic drugs and non‐steroidal anti‐inflammatory drugs (NSAIDs). These agents vary in their effectiveness, tolerability and acceptability to women. When selecting a medical treatment, patient preference and need for contraception are issues that need to be taken into account, together with the benefits and risks of each treatment. NSAIDs are considered useful for women not desiring contraception, especially women with dysmenorrhoea (Fraser 2008). Individual NSAIDs used for the treatment of HMB include mefenamic acid (MFA), naproxen, ibuprofen, flurbiprofen, meclofenamic acid, diclofenac, indomethacin and acetylsalicylic acid (aspirin).
How the intervention might work
A rationale for the use of NSAIDs is given by the accumulation of data suggesting a role for the prostaglandins in the pathogenesis of HMB (Hagenfeldt 1987; Lopez 1991). The endometria of women with excessive menstrual bleeding have higher levels of prostaglandin E2 and prostaglandin F2α when compared with women with normal menses (Willman 1976). There is further evidence of deranged haemostasis (abnormal clotting) as the ratio of prostaglandin E2 to F2α (Smith 1981), and the ratio of prostacyclin (prostaglandin I2) to thromboxane A2 (Makarainen 1986), are elevated. These substances are present both in the endometrium and myometrium, although the exact mechanism by which the excessive blood loss occurs remains speculative. NSAIDs reduce prostaglandin levels by inhibiting the cyclo‐oxygenase enzyme (Rees 1987; Smith 1981).
NSAIDs are contraindicated in women with HMB and an underlying bleeding disorder because of their inhibitory effect on platelet (cell fragment) aggregation (Kadir 2005).
Why it is important to do this review
NSAIDs reduces MBL by 25% to 35% or more in about three‐quarters of women with HMB (Roy 2004). NSAIDs also can have a beneficial effect on dysmenorrhoea, a symptom often related to HMB. Adverse effects of treatment, especially gastrointestinal effects, are variable in frequency but are not usually severe. It is important to distinguish this medical option for HMB from other options with respect to effectiveness, tolerability, acceptability and safety for women to make informed choices. In addition, it is usually assumed that there are no differences in effectiveness between individual NSAIDs, even though there are individual women who seem to respond well to one agent but less well to another. This review tested this assumption with the inclusion of individual NSAID comparisons for the treatment of HMB.
Objectives
To determine the effectiveness, safety and tolerability of NSAIDs in achieving a reduction in MBL in women of reproductive years with HMB.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled comparisons of NSAID therapies or combined NSAID and other medical therapy with either placebo or other medical therapies when used to reduce HMB. Comparisons of one type of NSAID with another type of NSAID were also eligible for the review.
Types of participants
Inclusion criteria
Women of reproductive years.
Women with regular heavy periods measured either objectively (greater than 80 mL) for one or more cycles prior to the intervention or subjectively by the women (this criterion has been changed from two or more previous cycles to one or more previous cycles in the 2007 and 2012 updates).
Women recruited from primary care, family planning or specialist clinics.
Exclusion criteria
Postmenopausal bleeding (less than one year from the last period).
Irregular menses and intermenstrual bleeding.
Pathological causes of HMB.
Iatrogenic (treatment induced) causes of HMB.
Types of interventions
NSAIDs versus placebo or any other medical therapy (antifibrinolytic agents, hormone treatment, danazol, gonadotropin‐releasing hormone analogues).
Specific NSAIDs versus other NSAIDs (MFA, naproxen, ibuprofen, flurbiprofen, meclofenamic acid, diclofenac, acetylsalicylic acid).
NSAIDs combined with other medical treatment(s) versus other medical treatment(s) (this comparison was added in the 2012 update).
We considered variable doses and routes of administration of treatments.
Types of outcome measures
Each of the following outcomes were recorded where available.
Primary outcomes
-
MBL:
objective assessment of blood loss, by the alkaline haematin method (mL/cycle) (Newton 1977);
subjective assessment of blood loss by the pictorial chart method (score on PBAC chart) (Higham 1990), or patient perception (on questionnaire or survey instrument).
Quality of life: women's perceived change in quality of life where it was recorded in a reproducible and validated format. This included improvement in symptoms of dysmenorrhoea, headache, diarrhoea, depression and other menstrually related symptoms.
Secondary outcomes
Total menstrual fluid loss (TMFL: measured as difference between the original weight of the sanitary material and the returned sanitary material) (Fraser 1985; Fraser 2001).
Number of days' bleeding during the intervention menstrual cycle.
Patient adherence to treatment.
Patient acceptability of treatment.
Adverse events, of any degree, reported either spontaneously by the patient or elicited from specific questioning.
Resource use/cost.
Search methods for identification of studies
Electronic searches
The original search was performed in 1998, with updated searches undertaken in September/October 2001, April 2004, July 2007, July 2012 and April 2019. We searched the following electronic databases, trial registers and websites:
Cochrane Gynaecology and Fertility Specialised Register of Controlled Trials; PROCITE platform (searched 1 April 2019)
Cochrane Central Register of Studies Online (CENTRAL CRSO); web platform (searched 1 April 2019)
MEDLINE; OVID platform (searched from 1946 to 1 April 2019)
Embase; OVID platform (searched from 1980 to 1 April 2019)
PsycINFO; OVID platform (searched from 1806 to 1 April 2019)
trial registers for ongoing and registered trials: www.controlled‐trials.com, clinicaltrials.gov/ct/home, www.who.int/trialsearch/Default.aspx; web platform (searched 1 April 2019)
conference abstracts in the ISI Web of Knowledge: wokinfo.com/; web platform (searched 1 April 2019)
LILACS database: lilacs.bvsalud.org/en/; web platform (searched 1 April 2019)
clinical study results for clinical trial results of marketed pharmaceuticals: www.clinicalstudyresults.org/; web platform (searched 1 April 2019)
PubMed: www.ncbi.nlm.nih.gov/pubmed/; web platform (searched 1 April 2019)
OpenSIGLE database: opensigle.inist.fr; web platform (searched 1 April 2019)
GOOGLE and GOOGLE Scholar for grey literature, web platform (searched 1 April 2019).
Searching other resources
We also searched the reference lists of relevant publications and identified trials.
Data collection and analysis
Selection of studies
Two review authors (AL and MB) independently selected trials for inclusion in the review using the search strategy described previously. We used the prespecified inclusion criteria to consider titles and abstracts from the lists of potentially relevant studies. Where necessary, we obtained full‐text copies of the studies for the independent assessment. We planned to resolve differences in opinion over study selection by discussion and consensus, but this did not prove necessary.
Data extraction and management
We analysed included trials for the following quality criteria, methodological details and study characteristics.
Trial characteristics
Method of randomisation.
Presence or absence of blinding to treatment allocation.
Quality of allocation concealment.
Number of women randomised, excluded or lost to follow‐up.
Use of an intention‐to‐treat analysis.
Use of a power calculation.
Duration, timing and location of the study.
Characteristics of the study participants
Age and any other recorded characteristics of women in the study.
Methods used to define heavy MBL.
Other inclusion criteria.
Exclusion criteria.
Interventions used
Types of medical therapy used.
Dose, duration and timing of administration of medical therapy.
Outcomes
Methods used to measure MBL at or after intervention.
Methods used to evaluate patient satisfaction, symptoms and change in quality of life.
All three review authors independently extracted data using forms designed according to Cochrane guidelines. One of these review authors was a content expert and the other two had methodological expertise. Where necessary, we sought additional information on trial methodology or actual trial data from the principal author of any trials that appeared to meet the eligibility criteria. In cases where results were presented in graphs and no actual data were given, we extracted the data from the graphs.
Assessment of risk of bias in included studies
Two review authors (AL and either CA or SF) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). Individual domains included random sequence generation, allocation concealment, blinding, incomplete outcome data and other bias; each domain was separately scored as low, unclear or high risk of bias.
We resolved disagreements over quality assessments by discussion with a third review author (KD).
Measures of treatment effect
For dichotomous data (e.g. proportion of women who found the treatment unacceptable), we expressed results for each study as an odds ratio (OR) with 95% confidence intervals (CI) and combined them for meta‐analysis with Review Manager 5 using the Peto OR (Review Manager 2014). For all dichotomous outcomes, a high value had negative consequences (e.g. the proportion of women who had no subjective improvement in MBL and proportion who had no improvement in quality of life). For ease of interpretation of the graphs, this meant that results to the left of the line favoured the experimental group (i.e. NSAIDs), and results to the right of the line favoured the control or other comparison group.
There were difficulties with the reporting of continuous outcomes (e.g. MBL after treatment). Meta‐analysis with Review Manager 5 software used a mean difference (MD) to combine outcomes and required data to be presented as absolute values of post‐treatment means with their standard deviations (SD) (or change values (either absolute or percentage) between baseline and final values together with the SD of the change). For many outcomes, particularly MBL, the data are skewed and authors correctly presented their data as medians with a range. Where possible, we obtained original data from the principal authors, but post‐treatment means and SDs were not always available or calculable. Where only medians and ranges were available and there was evidence that the data were approximately normally distributed, we regarded the median as being identical to the mean and calculated an estimate of the SD from the range (range multiplied by 0.95/4). We performed sensitivity analysis with and without these studies in the meta‐analysis to check the appropriateness of this assumption. Where there was strong evidence that the data were highly skewed and the sample size was small, we considered it inappropriate to regard the median as identical to the mean and did not combine the results of the relevant study in meta‐analysis but reported them separately in the 'Other data' section of this review. For all continuous outcomes, a high value had negative consequences and so results to the left of the line in the graphs favoured the experimental group (i.e. NSAIDs), and results to the right of the line favoured the control or other comparison group.
Unit of analysis issues
All analyses were per woman randomised.
Dealing with missing data
We analysed data, where possible, on an intention‐to‐treat basis. We attempted to obtain missing data from the authors of the included studies, where necessary, but were often unsuccessful. We did not impute missing values.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. Where meta‐analysis was feasible, we examined heterogeneity between the results of different combined studies by inspecting the scatter in the data points and the overlap in their CIs. We then assessed any outliers identified by this method to determine whether the differences could be further explained. We used the results of the Chi2 tests for formal assessment of heterogeneity (with P < 0.10 being considered evidence of significant heterogeneity) and the I2 statistic for quantity (Higgins 2003).
Assessment of reporting biases
We undertook a comprehensive search to minimise the chance of publication bias in the review. We planned to use a funnel plot to explore the possibility of small‐study effects on the results, but there were insufficient studies for this to be meaningful.
Data synthesis
We undertook meta‐analysis where possible (participants, interventions, outcomes and duration of included studies sufficiently homogeneous, or where data were in an appropriate form for combination, or both). Where there was substantial heterogeneity indicated that could not be explained, we used a random‐effects model as a more appropriate method for estimating a mean treatment effect.
Several studies were cross‐over in design. In principle, cross‐over and parallel group designed RCTs estimate the same effect but there are practical difficulties, such as carryover from the first treatment period and differences in the standard errors, and so pooling the data from both types of trials was not appropriate. An option was to enter data from cross‐over trials at the end of the first treatment period so that the two comparison groups were in effect parallel groups. We attempted to locate the original data at the end of the first cross‐over trial period but for most of the cross‐over trials these were unavailable. The data from these trials were described in text form in the 'Other data' section of this review and not included in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Differences between the action of individual NSAIDs are minimal but there is considerable variation in individual patient response and in the incidence and type of adverse events. We performed subgroup analyses comparing the efficacy and adverse events of individual NSAIDs with placebo and compared the results.
Sensitivity analysis
We planned sensitivity analysis to compare potential differences in participants, interventions, outcomes and whether allocation concealment was adequate in the included studies. However, only limited sensitivity analysis could be undertaken because of the small numbers of included studies for each outcome.
Overall quality of the body of evidence; 'Summary of findings' tables
We generated 'Summary of findings' tables using GRADEpro software (GRADEpro GDT) to evaluate the overall quality of the body of evidence for both the primary review outcomes: MBL and quality of life. Two review authors (AL, KD) independently judged on the overall quality of studies for each of these outcomes, according to the GRADE criteria (study limitations, i.e. risk of bias; consistency of effect; imprecision; indirectness and publication bias). For each GRADE criterion, if there were concerns about quality, the assessment could be downgraded by one or two levels. Overall quality for each outcome could be categorised as high, moderate, low or very low according to these assessments.
Results
Description of studies
Results of the search
The search identified 25 RCTs of medical treatment with NSAIDs for regular HMB. Nineteen RCTs met the criteria for inclusion in the review (see Characteristics of included studies table). See Figure 1 for details of the screening and selection process.
1.
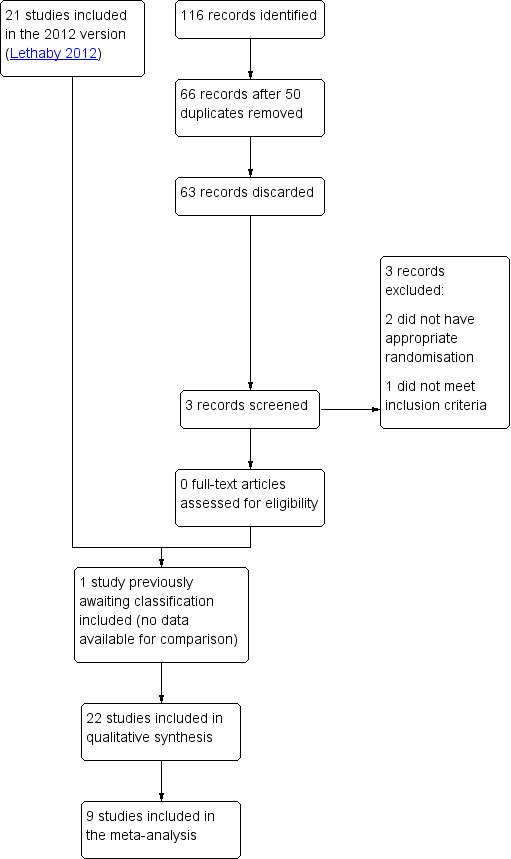
Study flow diagram.
Included studies
The studies included 759 women but not all the women could be included in each measured outcome as there were multiple comparison groups. One study that was previously awaiting classification was included on the 2019 update, but had no data available for comparisons (Jaisamrarn 2006). We contacted the authors but received no data. Thirteen studies were conducted in Europe. Six in the UK (Cameron 1987; Cameron 1990; Chamberlain 1991; Hall 1987; Muggeridge 1983; Reid 2005), two in Ireland (Bonnar 1996; Dockeray 1989), two in Finland (Makarainen 1986; Ylikorkala 1986), two in Sweden (Andersch 1988; Rybo 1981), and one in the Netherlands (van Eijkeren 1992). Two studies were conducted in India (Grover 1990; Najam 2010), two in Australia (Fraser 1981; Fraser 1991), one in Thailand (Jaisamrarn 2006), and one in Canada (Tsang 1987).
Trials included in the meta‐analysis
The data from nine trials with 419 women were included in meta‐analyses (Bonnar 1996; Cameron 1987; Cameron 1990; Chamberlain 1991; Dockeray 1989; Fraser 1991; Grover 1990; Hall 1987; van Eijkeren 1992). All comparisons were between MFA and placebo (two trials); another NSAID; naproxen (two trials) or other medical treatments: danazol, tranexamic acid, ethamsylate, oral contraceptive pill (OCP), norethisterone given during the luteal phase and the progesterone‐releasing intrauterine system (IUS).
Participants
The age of the participants in 18 of the included trials ranged from 18 to 55 years and all had sought medical assistance for HMB. One trial included women from 12 to 45 years, with 67% being over 30 years, but no further details were provided.Ten studies defined HMB objectively using the alkaline haematin method to satisfy the criteria for inclusion. Some of the studies also required the women to have regular ovulatory cycles. Common exclusion criteria were hormonal contraception, intrauterine device (IUD) use, fibroids and pelvic pathology.
Interventions
In general, the dosage regimen did not vary extensively for each type of NSAID. MFA was most commonly studied and the usual dosage was 500 mg three times a day from onset of menses for four or five days or until menstruation ceased. Two studies required administration of MFA four or five days prior to menses until its cessation (Reid 2005; van Eijkeren 1992), and one study used a slightly different dosage (500 mg initially, then 250 mg four times per day for three to five days) (Tsang 1987). Four studies used a similar regimen for naproxen: 500 mg at onset and three to five hours later, then 500 mg twice a day for five days (Ylikorkala 1986); 500 mg in the morning, 250 mg in the afternoon for days one to two, then 250 mg twice a day for up to seven days (Rybo 1981); 500 mg then 250 mg three to four times daily (Fraser 1991); 550 mg initially, then 275 mg four times daily for five days (Hall 1987). The trial that compared MBL in women treated with ibuprofen versus placebo used two different regimens of ibuprofen: 600 mg daily and 1200 mg daily.
Comparisons
The duration of medication with tranexamic acid and ethamsylate was during the five days of menstruation. Danazol administration was daily throughout the cycle. The studies gave oral progestogen as a luteal phase supplement although a longer regimen is widely accepted as having greater efficacy. The progesterone‐releasing IUS/levonorgestrel‐releasing IUS (LNG IUS) were inserted for the whole trial period and the OCP taken for three of the four weeks of the cycle. Duration of the intervention mostly ranged from two to three menstrual cycles, but for the van Eijkeren 1992 the treatment phase was only one month.
Outcomes
All trials but two, had MBL measured objectively by the alkaline haematin method as the main outcome; the remaining trials assessed the effect of treatment on MBL by the proportion of women experiencing "relief" of HMB (no details given by the author as to how this was measured) (Grover 1990), or PBAC scores (Najam 2010). One trial also measured TMFL and PBAC scores in addition to MBL measured by the alkaline haematin method. Five trials assessed the effects of treatment on number of days of menstrual bleeding, eight trials measured the incidence of adverse events, three trials measured the effects on dysmenorrhoea, two assessed treatment acceptability and women's perception of change in MBL, and one trial measured non‐adherence.
Trials used several methods to assess adverse events. Some studies recorded the numbers of women who experienced any adverse events. Other studies reported the numbers of women who experienced specific adverse events (e.g. headache, nausea, abdominal pain). One study attempted to combine specific adverse events in broad categories (e.g. gastrointestinal events and central nervous system events). The patient either mentioned adverse effects spontaneously or by responding to specific questioning such as "Do you think the treatment has upset you in any way?"
Data considered unsuitable for pooling in the meta‐analysis (cross‐over trials, trials with skewed data or no measure of variance)
The data from seven trials of cross‐over design with 99 women could not be pooled and included in the meta‐analysis but the individual results are described in data tables rather than forest plots (Andersch 1988; Fraser 1981; Makarainen 1986; Muggeridge 1983; Rybo 1981; Tsang 1987; Ylikorkala 1986). Three trials compared MBL in MFA and placebo groups, two trials compared MBL in naproxen and placebo groups, one trial compared MBL in ibuprofen and placebo groups (Analysis 1.4), and one trial compared MBL in flurbiprofen and tranexamic acid treatment groups (Analysis 2.6).
1.4. Analysis.
Comparison 1 NSAIDs versus placebo, Outcome 4 MBL and other outcomes (descriptive results).
| MBL and other outcomes (descriptive results) | |
|---|---|
| Study | |
| Mefenamic acid vs placebo | |
| Fraser 1981 | Mean menstrual blood loss (standard deviation (SD)) on placebo: 70.7 (24.9) mL Mean menstrual blood loss (SD) on mefenamic acid: 47.3 (21.7) mL P < 0.001, n = 28, paired t test, t = 6.56 Other outcomes were not given for this subgroup. |
| Muggeridge 1983 | Mean menstrual blood loss (SD) on placebo: 161 (78.5) mL
Mean menstrual blood loss (SD) on mefenamic acid: 128.3 (78.1) mL
No significant difference between placebo and mefenamic acid (MFA) cycles, n = 15, Wilcoxon Sum Rank Test Mean score of dysmenorrhoea symptoms (SD) in 2nd placebo cycle: 2.1 (2.1) Mean score of dysmenorrhoea symptoms (SD) in 2nd MFA cycle: 1.3 (1.6) No significant difference between placebo and MFA cycles, n = 15, Wilcoxon Sum Rank Test |
| Tsang 1987 | Mean menstrual blood loss (SD) on placebo: 156.5 (105.9) Mean menstrual blood loss (SD) on MFA: 140.0 (109) P < 0.05, n = 10, t test comparing MFA cycles with combined placebo and control cycles |
| Naproxen vs placebo | |
| Rybo 1981 | Mean menstrual blood loss (standard deviation (SD)) on placebo: 144 (26) mL Mean menstrual blood loss (SD) on naproxen: 107 (154) mL P < 0.02, n = 4, statistical method not given |
| Ylikorkala 1986 | Mean menstrual blood loss (SD) on placebo: 150.7 (34) mL
Mean menstrual blood loss (SD) on naproxen: 96.8 (27.3) mL
P < 0.001, n = 14, paired t test Proportion of women with adverse effects on placebo: 7% Proportion of women with adverse effects on naproxen: 0% Not tested |
| Ibuprofen vs placebo | |
| Makarainen 1986 | Median menstrual blood loss (range) on placebo: 146 (71–374) mL Median menstrual blood loss (range) on ibuprofen 600 mg: 123 (23–319) mL Median menstrual blood loss (range) on ibuprofen 1200 mg: 110 (30–288) mL P < 0.01, n = 13, Wilcoxon paired test, ibuprofen 1200 vs placebo |
2.6. Analysis.
Comparison 2 NSAIDs versus tranexamic acid, Outcome 6 MBL and other outcomes (descriptive results).
| MBL and other outcomes (descriptive results) | |
|---|---|
| Study | |
| Andersch 1988 | Mean menstrual blood loss (standard deviation (SD)) on tranexamic acid: 154.8 (127.8) mL
Mean menstrual blood loss (SD) on flurbiprofen: 223 (168.5) mL
P < 0.01, n = 15, student's t test Proportion of women with adverse effects on tranexamic acid: 47% Proportion of women with adverse effects on flurbiprofen: 27% DIfference not tested |
The data from Reid 2005 were highly skewed and the results from this study are also reported in data tables (Analysis 6.2).
6.2. Analysis.
Comparison 6 NSAIDs versus progesterone‐releasing intrauterine system (IUS), Outcome 2 MBL and other outcomes (descriptive results).
| MBL and other outcomes (descriptive results) | |||||
|---|---|---|---|---|---|
| Study | Outcomes | Results (NSAIDs) | Results (LNG IUS) | Significance test | Comment |
| Reid 2005 | Menstrual blood loss (alkaline haematin method): Total menstrual fluid loss: PBAC score: | 3 months: Median (range): 94 (29–219) mL 6 months: Median (range): 100 (46–168) mL 3 months: Median (range): 151 (57–280) mL 6 months: Median (range): 157 (76–319) 3 months: Median (range): 161 (77–262) 6 months: Median (range): 159 (50–307) | 3 months: Median (range): 12 (0‐240) mL 6 months: Median (range): 5 (0–45) mL 3 months: Median (range): 53 (0–459) mL 6 months: Median (range): 27 (0–156) mL 3 months: Median (range): 49 (0–286) 6 months: Median (range): 25 (0–402) | Wilcoxon rank sum test: P < 0.001 for all comparisons | — |
The data from Najam 2010 did not include measures of the variance and the results of this study are also reported in data tables (Analysis 9.1).
9.1. Analysis.
Comparison 9 Tranexamic acid and mefenamic acid versus tranexamic acid, Outcome 1 Pictorial blood loss assessment chart (PBAC) score at 6 months' follow‐up.
| Pictorial blood loss assessment chart (PBAC) score at 6 months' follow‐up | |||||||
|---|---|---|---|---|---|---|---|
| Study | Group | n | Baseline mean PBAC score | Baseline standard deviation (SD) | PBAC score at 6 months | SD at 6 months | Test |
| Najam 2010 | Tranexamic acid + mefenamic acid | 55 | 246 points | Not reported | 100 points | Not reported | Significant change from baseline, P < 0.01 |
| Najam 2010 | Tranexamic acid | 55 | 250 points | Not reported | 125 points | Not reported | Not significant change from baseline, P > 0.05 |
Excluded studies
Two trials were excluded in previous versions of the review. One of these assessed reduction in bleeding in the treatment and placebo groups according to the number of sanitary pads used compared to the baseline use of sanitary wear in the categories of no change, one to three less, four to six less and seven to nine less) (Martinez Alcala 1979). There is sometimes a weak positive correlation between the number of pads and tampons used and the amount of MBL, but this is often dependent on the personal hygiene of the woman and varies even between a woman's two menstruations of almost the same volume (Chimbira 1980; Fraser 1984). The other excluded trial compared meclofenamate sodium with placebo (Vargyas 1987). It included seven women (21%) with fitted IUDs, which is an exclusion criterion for this review.
In the 2019 version, we excluded three trials. Two because the women were randomised to "medical treatment" including NSAIDs. Gupta 2013 randomised women to the progesterone‐releasing IUS or to other medical treatment including NSAIDs, but they were free to choose the medical treatment. Famuyide 2017 randomised women to endometrial ablation or medical treatment, and they were also free to choose between combined OCP or NSAIDs. We excluded one trial because the comparison was MFA versus MFA plus herbal medicine (Naafe 2018).
Risk of bias in included studies
Risk of bias assessments are presented for the individual studies in the Characteristics of included studies table and summaries are presented in figures (Figure 2; Figure 3).
2.
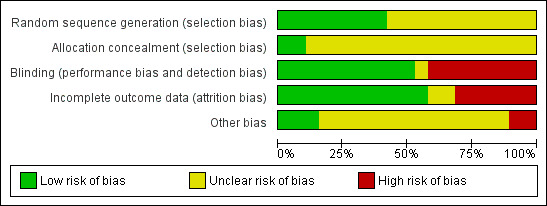
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
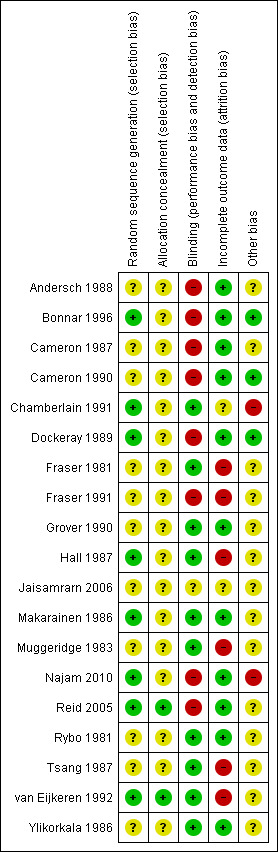
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation and allocation concealment
Eight studies described an adequate randomisation method and were at low risk of bias for this domain; the remaining 11 studies did not report how this was performed and were classified at unclear risk of bias. Only two of the 19 trials were at low risk of bias for allocation concealment (van Eijkeren 1992; Reid 2005). However, van Eijkeren 1992 had a very small sample size, had a high proportion of withdrawals and duration of treatment was for only one month. The remaining trials were at unclear risk of bias as they did not provide evidence of sufficient safeguards in place to conceal the allocation.
Blinding
Ten of 19 studies used double blinding (low risk of bias) and one trial used single blinding (unclear who was blinded; unclear risk); the remaining studies did not report whether blinding was undertaken and this was considered unlikely (high risk of bias).
Incomplete outcome data
Nine of 19 trials did not report whether there was any loss to follow‐up or exclusions or withdrawals post randomisation. The remaining 10 trials had loss to follow‐up ranging from 16% to 29% (three trials) or exclusions or withdrawals after randomisation ranging from 3% to 42% (seven trials) (low risk of bias). Two of these trials with exclusions or withdrawals had intention‐to‐treat analysis and were considered at low risk of bias.
Other potential sources of bias
Two trials reported results separately for subgroups. One trial gave results for four women with primary menorrhagia out of the total 14 who were randomised (Rybo 1981). Another study included a heterogeneous group of women with many different diagnoses: ovulatory and anovulatory menorrhagia, fibroids, IUD, tubal sterilisation, von Willebrand's disease and OCP (Fraser 1981). Reduction in blood loss was reported separately for the women with ovulatory menorrhagia but a significant proportion of this group did not have excessive bleeding, as defined by the alkaline haematin method. This subgroup of women did not report other outcomes.
Two trials provided confusing outcome data and were at high risk of this bias. Fourteen trials provided insufficient information and were considered at unclear risk of this bias. Three trials reported similar data at baseline and were at low risk of this bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
1 NSAIDs versus placebo
Table 1 provides a summary of the main findings for the comparison of NSAIDs versus placebo.
1.1 Menstrual blood loss (objective, subjective or both)
Eight trials measured MBL in different ways. One small trial with 11 women found clear evidence of difference in mean MBL favouring the MFA group compared to the placebo group (MBL in the MFA group was 124 mL less than in the placebo group; MD –124 mL/cycle, 95% CI –186 to –62; Analysis 1.1). There were no other trials with data suitable for pooling but six cross‐over trials reported a total effect at the end of the study (three trials compared MFA with placebo, two trials compared naproxen with placebo and one trial compared different dosages (600 mg and 1200 mg daily) of ibuprofen with placebo). In five of the seven post‐treatment comparisons, there was clear evidence of difference in the post‐treatment mean MBL favouring NSAIDs. However, there was no clear evidence of difference between low‐dose ibuprofen versus placebo (Makarainen 1986), or MFA versus placebo (Muggeridge 1983).
1.1. Analysis.
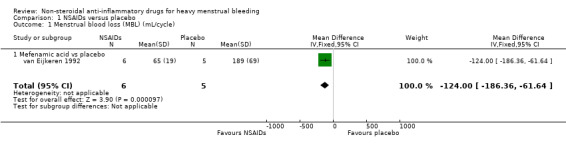
Comparison 1 NSAIDs versus placebo, Outcome 1 Menstrual blood loss (MBL) (mL/cycle).
There was clear evidence of a difference in women's perception of "relief" of HMB between MFA and placebo groups (OR 0.08, 95% CI 0.03 to 0.18; Analysis 1.2; Grover 1990). The authors provided no additional information regarding the measurement of relief of HMB. In one of the cross‐over trials not included in the meta‐analysis (Ylikorkala 1986), 79% of women indicated that naproxen was "better" compared to 21% who indicated that the placebo treatment was "better" at reducing their HMB.
1.2. Analysis.
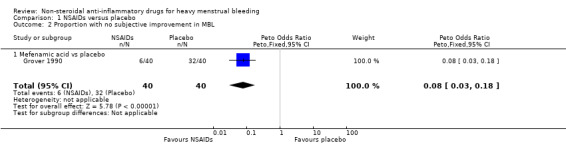
Comparison 1 NSAIDs versus placebo, Outcome 2 Proportion with no subjective improvement in MBL.
1.2 Adverse events
In the small van Eijkeren and colleagues study, total incidence of adverse events was comparable between MFA and placebo (Analysis 1.3; van Eijkeren 1992). Of the cross‐over trials identified above, there was no change in dysmenorrhoea scores between MFA and placebo (Muggeridge 1983), and no differences in the total incidence of adverse events between naproxen versus placebo and ibuprofen versus placebo (Analysis 1.4; Makarainen 1986; Ylikorkala 1986).
1.3. Analysis.
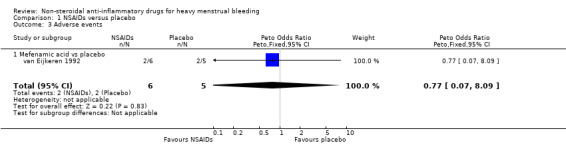
Comparison 1 NSAIDs versus placebo, Outcome 3 Adverse events.
1.3 Other outcomes
We identified no trials for the meta‐analysis to assess quality of life, TMFL, number of days of bleeding, patient adherence to treatment, patient acceptability of treatment or resource use/cost.
2 NSAIDs versus tranexamic acid
Table 2 provides a summary of the main findings for the comparison of NSAIDS versus tranexamic acid.
2.1 Menstrual blood loss (objective, subjective or both)
In the one study available for analysis with 48 women, the MD was 73 mL/cycle (95% CI 22 to 124) in the comparison of MFA and tranexamic acid (MBL in the tranexamic acid group was 73 mL less than in the MFA group; Analysis 2.1; Bonnar 1996). In the same study, there was no clear evidence of difference between the groups in the women's perception of change in their MBL (Analysis 2.2; Bonnar 1996). In one cross‐over trial, where data were not suitable for pooling, mean MBL was significantly less in the tranexamic acid cycles (155 mL) than in the flurbiprofen cycles (223 mL) (P < 0.01; Andersch 1988).
2.1. Analysis.
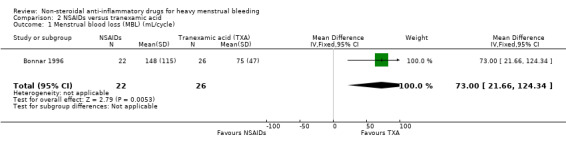
Comparison 2 NSAIDs versus tranexamic acid, Outcome 1 Menstrual blood loss (MBL) (mL/cycle).
2.2. Analysis.
Comparison 2 NSAIDs versus tranexamic acid, Outcome 2 Proportion with no subjective improvement in MBL.
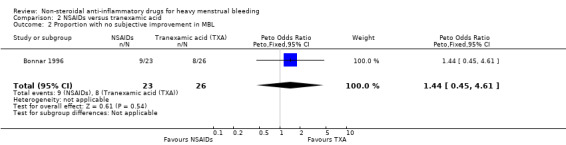
2.2 Number of days' bleeding
In the one study available for analysis, there was no clear evidence of a difference between treatment groups (Peto OR 1.44, 95% CI 0.45 to 4.61; 49 women; 1 study; Analysis 2.3).
2.3. Analysis.
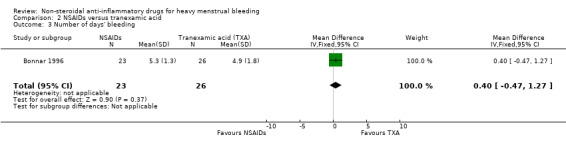
Comparison 2 NSAIDs versus tranexamic acid, Outcome 3 Number of days' bleeding.
2.3 Quality of life
There was no clear evidence of differences between groups for change in quality of life (Peto OR 1.13, 95% CI 0.27 to 4.73; 49 women; 1 study; Analysis 2.4) and treatment acceptability (Peto OR 1.17, 95% CI 0.32 to 4.27; 49 women; 1 study; Analysis 2.5), although these results were based on only one study. Another study reported outcomes in a form that was not suitable for analysis (Analysis 2.6).
2.4. Analysis.
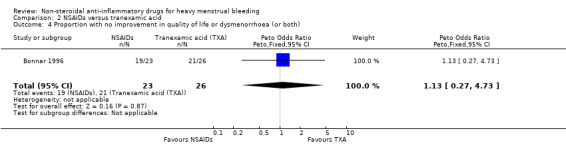
Comparison 2 NSAIDs versus tranexamic acid, Outcome 4 Proportion with no improvement in quality of life or dysmenorrhoea (or both).
2.5. Analysis.
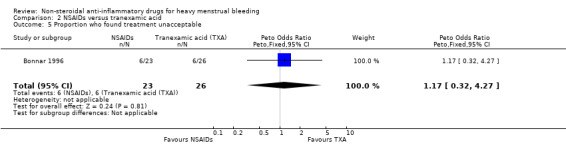
Comparison 2 NSAIDs versus tranexamic acid, Outcome 5 Proportion who found treatment unacceptable.
2.4 Other outcomes
We identified no trials for the meta‐analysis to assess TMFL, patient adherence to treatment, patient acceptability of treatment or resource use/cost.
3 NSAIDs versus ethamsylate
Table 3 provides a summary of the main findings for the comparison of NSAIDs versus ethamsylate.
Two studies contributed to this comparison (Bonnar 1996; Chamberlain 1991).
3.1 Menstrual blood loss (objective, subjective or both)
There was no evidence of a difference in MBL measured objectively both immediately after treatment in two studies (MD –42.88 mL/cycle, 95% CI –86.25 to 0.50; 82 women; I2 = 0%; Analysis 3.1), or at longer follow‐up at six months in one study (MD –70.30 mL/cycle, 95% CI –158.88 to 18.28; 31 women; Analysis 3.2). Women were unable to perceive a difference in their blood loss in one study (Peto OR 0.70, 95% CI 0.23 to 2.12; 50 women; Analysis 3.3).
3.1. Analysis.
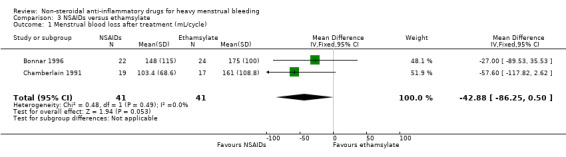
Comparison 3 NSAIDs versus ethamsylate, Outcome 1 Menstrual blood loss after treatment (mL/cycle).
3.2. Analysis.
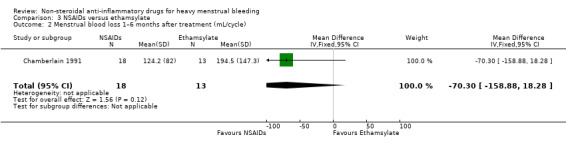
Comparison 3 NSAIDs versus ethamsylate, Outcome 2 Menstrual blood loss 1–6 months after treatment (mL/cycle).
3.3. Analysis.
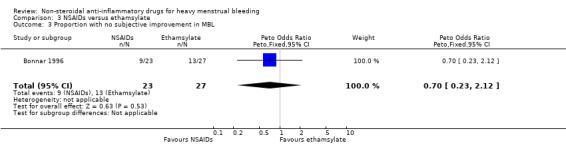
Comparison 3 NSAIDs versus ethamsylate, Outcome 3 Proportion with no subjective improvement in MBL.
3.2 Quality of life
In the one study available for analysis, there was no clear evidence of a difference between treatment groups for quality of life (Peto OR 0.83, 95% CI 0.18 to 3.72; 50 women; Analysis 3.4).
3.4. Analysis.
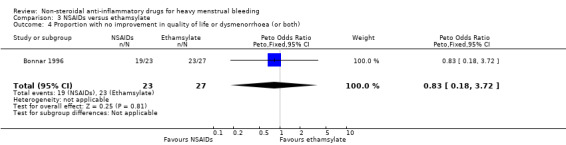
Comparison 3 NSAIDs versus ethamsylate, Outcome 4 Proportion with no improvement in quality of life or dysmenorrhoea (or both).
3.3 Number of days' bleeding
There was no clear evidence of a difference in number of treatment days of menstrual bleeding between treatment groups in one study (MD –0.40 days, 95% CI –1.56 to 0.76; 46 women; Analysis 3.5).
3.5. Analysis.
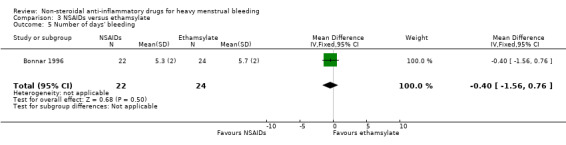
Comparison 3 NSAIDs versus ethamsylate, Outcome 5 Number of days' bleeding.
3.4 Patient acceptability of treatment
A greater proportion of women found ethamsylate unacceptable compared to NSAIDs (Peto OR 0.20, 95% CI 0.07 to 0.61; 50 women; Analysis 3.6).
3.6. Analysis.
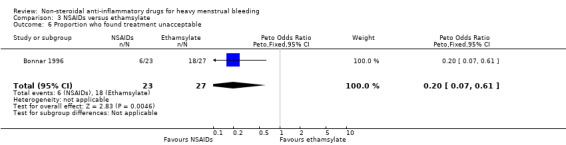
Comparison 3 NSAIDs versus ethamsylate, Outcome 6 Proportion who found treatment unacceptable.
3.5 Other outcomes
We identified no trials for the meta‐analysis to assess TMFL, patient adherence to treatment, patient acceptability of treatment or resource use/cost.
4 NSAIDs versus danazol
Table 4 provides a summary of the main findings for the comparison of NSAIDs versus danazol.
4.1 Menstrual blood loss (objective)
Reduction of HMB was significantly greater in the danazol group (MD 45.06 mL/cycle, 95% CI 18.73 to 71.39; 79 women; 3 studies; I2 = 29%; Analysis 4.1). No trials assessed women's perception of MBL after treatment.
4.1. Analysis.
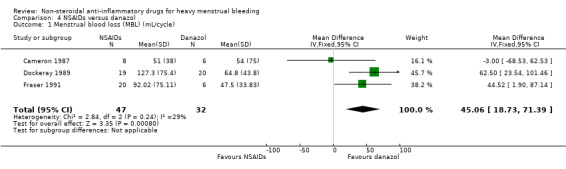
Comparison 4 NSAIDs versus danazol, Outcome 1 Menstrual blood loss (MBL) (mL/cycle).
4.2 Quality of life
There was no clear evidence of a difference between groups for quality of life (Peto OR 1.19, 95% CI 0.20 to 7.05; 28 women; 1 study; Analysis 4.2).
4.2. Analysis.
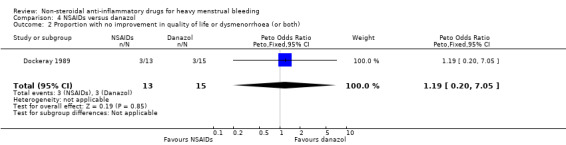
Comparison 4 NSAIDs versus danazol, Outcome 2 Proportion with no improvement in quality of life or dysmenorrhoea (or both).
4.3 Number of days' bleeding
There was clear evidence of a difference in the number of days of menstrual bleeding between danazol and the MFA groups favouring danazol (MD 1.03 days, 95% CI 0.26 to 1.80; 53 women; 2 studies; I2 = 0%; Analysis 4.3).
4.3. Analysis.
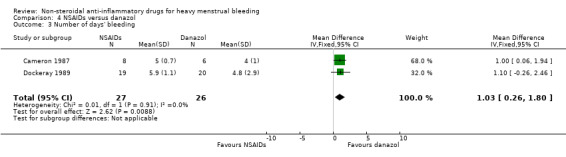
Comparison 4 NSAIDs versus danazol, Outcome 3 Number of days' bleeding.
4.4 Patient acceptability of treatment
There was no clear evidence of a difference between groups for treatment acceptability, although these results were based on one study with 40 women (Peto OR 0.82, 95% CI 0.24 to 2.80; Analysis 4.4)
4.4. Analysis.
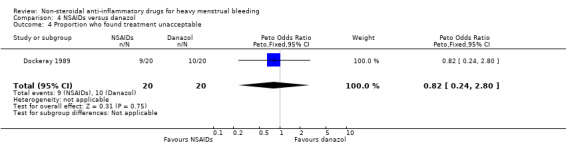
Comparison 4 NSAIDs versus danazol, Outcome 4 Proportion who found treatment unacceptable.
4.5 Adverse events
In this same study, the risk of adverse events was less in the MFA group (Peto OR 0.17, 95% CI 0.05 to 0.59; 40 women; Analysis 4.5).
4.5. Analysis.
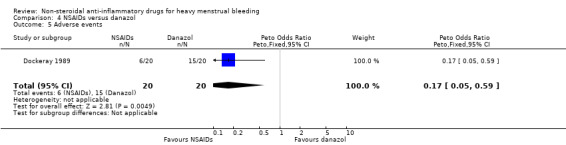
Comparison 4 NSAIDs versus danazol, Outcome 5 Adverse events.
4.6 Other outcomes
We identified no trials for the meta‐analysis to assess TMFL, patient adherence to treatment or resource use/cost.
5 NSAIDs versus oral progestogen (luteal phase)
Table 5 provides a summary of the main findings for the comparison of NSAIDs versus oral progestogen.
5.1 Menstrual blood loss (objective, subjective or both)
Two studies assessed MBL and duration of bleeding.
NSAIDs may have improved MBL compared to oral progestogens (MD –22.97 mL/cycle, 95% CI –46.57 to 0.62; 48 women; 2 studies; I2 = 71%; Analysis 5.1). The high heterogeneity could be explained by different inclusion criteria (age and bleeding level at baseline) in the two included studies.
5.1. Analysis.
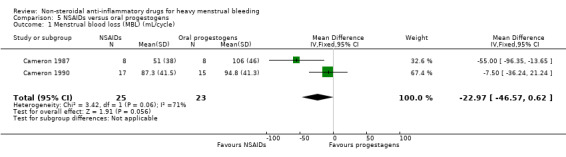
Comparison 5 NSAIDs versus oral progestogens, Outcome 1 Menstrual blood loss (MBL) (mL/cycle).
5.2 Number of days' bleeding
There was no clear evidence of a difference in duration of bleeding (MD –0.41, 95% CI –0.95 to 0.13; 48 women; 2 studies; I2 = 35%; Analysis 5.2).
5.2. Analysis.
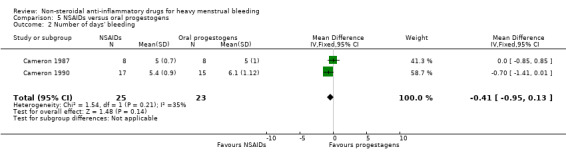
Comparison 5 NSAIDs versus oral progestogens, Outcome 2 Number of days' bleeding.
5.3 Adverse events
There was no clear evidence of a difference for non‐adherence (Peto OR 0.88, 95% CI 0.05 to 14.78; 32 women; 1 study; Analysis 5.3), total adverse events (Peto OR 0.54, 95% CI 0.13 to 2.26; 32 women; 1 study; Analysis 5.4), headache (Peto OR 0.63, 95% CI 0.14 to 2.86; 32 women; 1 study; Analysis 5.5), abdominal pain (Peto OR 0.86, 95% CI 0.15 to 4.96; 32 women; 1 study; Analysis 5.6), and nausea (Peto OR 1.79, 95% CI 0.17 to 18.65; 32 women; 1 study; Analysis 5.7). One study had no data available for comparisons (Jaisamrarn 2006).
5.3. Analysis.
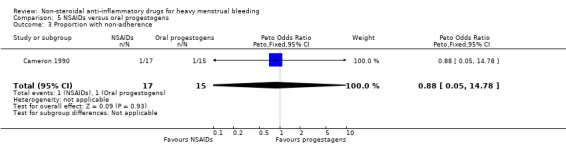
Comparison 5 NSAIDs versus oral progestogens, Outcome 3 Proportion with non‐adherence.
5.4. Analysis.
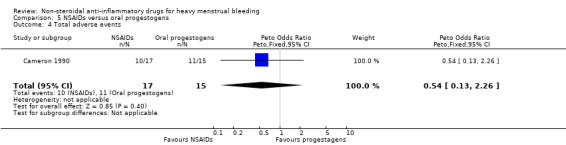
Comparison 5 NSAIDs versus oral progestogens, Outcome 4 Total adverse events.
5.5. Analysis.
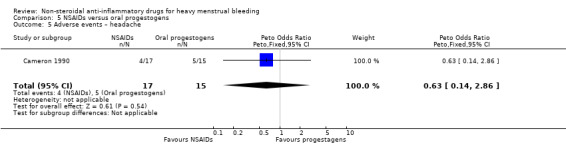
Comparison 5 NSAIDs versus oral progestogens, Outcome 5 Adverse events – headache.
5.6. Analysis.
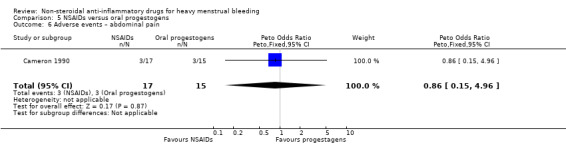
Comparison 5 NSAIDs versus oral progestogens, Outcome 6 Adverse events – abdominal pain.
5.7. Analysis.
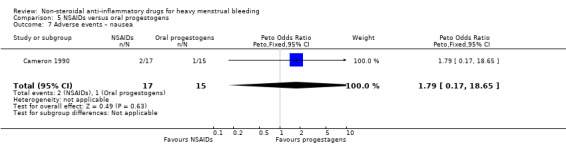
Comparison 5 NSAIDs versus oral progestogens, Outcome 7 Adverse events – nausea.
5.4 Other outcomes
We identified no trials for the meta‐analysis to assess TMFL, patient adherence to treatment, patient acceptability of treatment or resource use/cost.
6 NSAIDs versus progesterone‐releasing intrauterine system
Table 6 provides a summary of the main findings for the comparison of NSAIDs versus progesterone‐releasing IUS.
6.1 Menstrual blood loss (objective, subjective or both)
There was no clear evidence of a difference in reduction of HMB between groups (MD –4.00 mL/cycle, 95% CI –31.23 to 23.23; 16 women; 1 study; Analysis 6.1). Another larger trial that compared MFA with the LNG IUS reported highly significant differences between groups in MBL and PBAC scores (P < 0.001 for both outcomes; no summary effect measures calculated; Analysis 6.2).
6.1. Analysis.
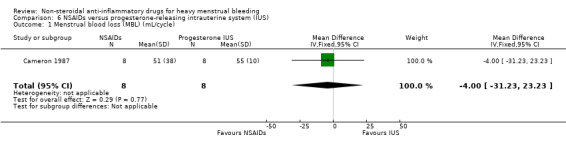
Comparison 6 NSAIDs versus progesterone‐releasing intrauterine system (IUS), Outcome 1 Menstrual blood loss (MBL) (mL/cycle).
6.2 Total menstrual fluid loss
One trial that compared MFA with the LNG IUS reported highly significant differences between groups in TMFL (P < 0.001; no summary effect measures calculated; Analysis 6.2).
6.3 Number of days' bleeding
There was clear evidence of a difference in the number of days of menstrual bleeding favouring NSAIDs compared to the progesterone‐releasing IUS (withdrawn from the market since 2001) (MD –5.00 days, 95% CI –6.08 to –3.92; 16 women; 1 study; Analysis 6.3).
6.3. Analysis.
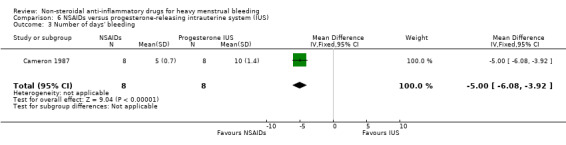
Comparison 6 NSAIDs versus progesterone‐releasing intrauterine system (IUS), Outcome 3 Number of days' bleeding.
6.4 Adverse events
There was no clear evidence of a difference for adverse events other than abdominal pain which may have been lower (Peto OR 0.22, 95% CI 0.06 to 0.87; 51 women; 1 study; Analysis 6.4).
6.4. Analysis.
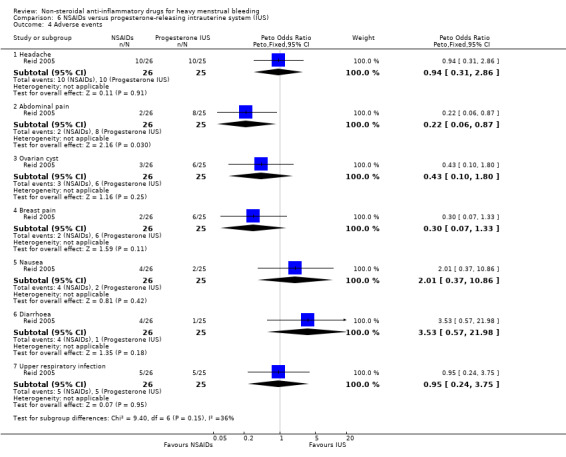
Comparison 6 NSAIDs versus progesterone‐releasing intrauterine system (IUS), Outcome 4 Adverse events.
6.5 Other outcomes
We identified no trials for the meta‐analysis to assess quality of life, patient adherence to treatment, patient acceptability of treatment or resource use/cost.
7 NSAIDs versus oral contraceptive pill
Table 7 provides a summary of the main findings for the comparison of NSAIDs versus OCP.
7.1 Menstrual blood loss (objective, subjective or both)
In the one study with data suitable for analysis, there was no clear evidence of a difference in the objective measurement of MBL between groups (MD 25.25 mL/cycle, 95% CI –22.34 to 72.84; 26 women; 1 study; Analysis 7.1).
7.1. Analysis.
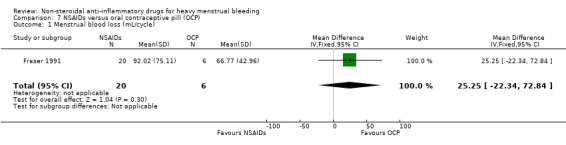
Comparison 7 NSAIDs versus oral contraceptive pill (OCP), Outcome 1 Menstrual blood loss (mL/cycle).
7.2 Other outcomes
We identified no trials for the meta‐analysis to assess quality of life, TMFL, number of days' bleeding, patient adherence to treatment, patient acceptability of treatment, adverse events or resource use/cost.
8 Mefenamic acid versus naproxen
Table 8 provides a summary of the main findings for the comparison of MFA versus naproxen.
8.1 Menstrual blood loss (objective, subjective or both)
There was no clear evidence of a difference between groups in the objective measurement of MBL (MD 21.00 mL/cycle, 95% CI –5.85 to 47.85; 61 women; 2 studies; I2 = 25%; Analysis 8.1).
8.1. Analysis.
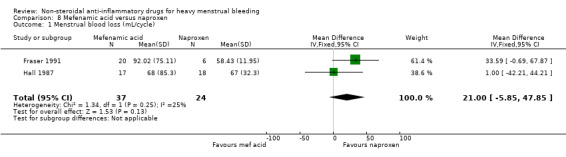
Comparison 8 Mefenamic acid versus naproxen, Outcome 1 Menstrual blood loss (mL/cycle).
8.2 Number of days' bleeding
There was no clear evidence of a difference between groups in duration of bleeding (MD –0.40 days, 95% CI –1.59 to 0.79; 35 women; 1 study; Analysis 8.2).
8.2. Analysis.
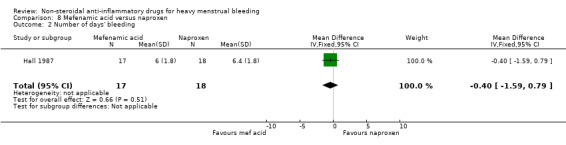
Comparison 8 Mefenamic acid versus naproxen, Outcome 2 Number of days' bleeding.
8.3 Adverse events
There was no clear evidence of difference in the total incidence of adverse events between groups (Peto OR 0.12, 95% CI 0.01 to 2.00; 35 women; 1 study; Analysis 8.3).
8.3. Analysis.
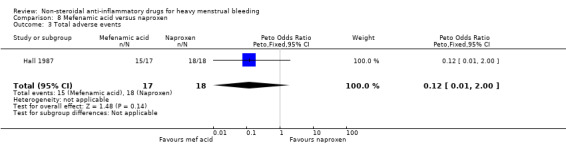
Comparison 8 Mefenamic acid versus naproxen, Outcome 3 Total adverse events.
There was clear evidence of a difference in the risk of gastrointestinal effects favouring the MFA group compared with the naproxen group (Peto OR 0.24, 95% CI 0.06 to 0.87; 35 women; 1 study; Analysis 8.4).
8.4. Analysis.
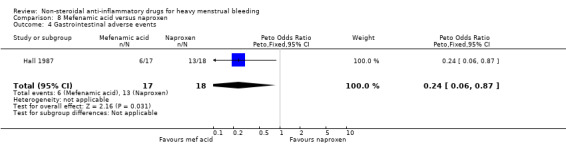
Comparison 8 Mefenamic acid versus naproxen, Outcome 4 Gastrointestinal adverse events.
There was no clear evidence of a difference in central nervous system adverse events between groups (Peto OR 1.40, 95% CI 0.34 to 5.73; 35 women; 1 study; Analysis 8.5).
8.5. Analysis.
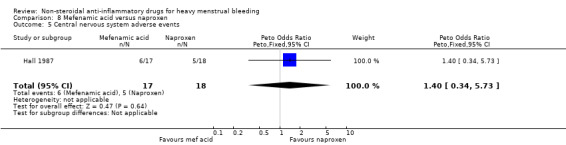
Comparison 8 Mefenamic acid versus naproxen, Outcome 5 Central nervous system adverse events.
8.4 Other outcomes
We identified no trials for the meta‐analysis to assess quality of life, TMFL, number of days' bleeding, patient adherence to treatment, patient acceptability of treatment or resource use/cost.
9 Tranexamic acid plus mefenamic acid versus tranexamic acid
9.1 Menstrual blood loss (objective, subjective or both)
There was clear evidence of a reduction in MBL from baseline in the combined tranexamic acid plus MFA group in one study with 55 women (PBAC score at baseline 246 versus PBAC score at 6 months' follow‐up 100; P < 0.01). There was no clear evidence of a difference in MBL from baseline in the tranexamic acid alone group (PBAC score at baseline 250 versus PBAC score at 6 months' follow‐up 125; P > 0.05; Analysis 9.1).
9.2 Other outcomes
We identified no trials for the meta‐analysis to assess quality of life, TMFL, number of days' bleeding, patient adherence to treatment, patient acceptability of treatment, adverse events or resource use/cost.
Discussion
Summary of main results
NSAIDs versus placebo
Evidence from the one trial in the meta‐analysis and five of the six cross‐over studies confirms that NSAIDs are more effective than placebo in reducing MBL. However, the quality of the only study in the meta‐analysis was not high; 42% of randomised patients dropped out and the analysis was not intention to treat. A highly significant difference between NSAIDs and placebo in reduction of MBL was also perceived by the patients in one study that recorded the proportion of patients who were relieved of their HMB as the primary outcome.
NSAIDs versus other medical treatments
In the comparisons of NSAIDs as a group with other medical treatments, both tranexamic acid and danazol were more effective than any of the NSAIDs in reducing MBL. One cross‐over trial confirmed results from the only study in the meta‐analysis assessing the effect NSAIDs versus tranexamic acid on MBL (Andersch 1988). There was clear evidence of a difference in the reduction of blood loss in the meta‐analysis of NSAIDs versus danazol in two included studies; the third study had non‐comparable groups at baseline with a significantly higher (almost double) pretreatment MBL in the danazol group and the results must be regarded with caution (Cameron 1987).
There was no clear evidence of a difference in reduction of MBL in the comparisons of NSAIDs with ethamsylate, oral progestogen given during the luteal phase, the progesterone‐releasing IUS and the OCP.
The study comparing the progesterone‐releasing IUS was very small and results may have been affected by the lack of baseline comparability between groups. The progesterone‐releasing IUS was withdrawn from the market in 2001. A newer progesterone‐releasing IUS delivering 20 μg of levonorgestrel daily was developed primarily as a contraceptive and reduced both MBL and TMFL more effectively in one study (Reid 2005). There is evidence that blood contributes only a third of the TMFL in normal women (Fraser 1985; Fraser 2001). Thus, it could be argued that women may seek medical advice for excess total fluid loss (i.e. blood and fluid) rather than blood loss per se. The study found high correlations between MBL and TMFL and the authors suggested that TMFL may be a more useful estimate of MBL because it is easier to measure. It remains to be demonstrated in future trials if women are more concerned about the volume rather than the composition of unacceptable menstrual loss.
In the comparisons of NSAIDs with other medical treatment, the number of days of menstrual bleeding was shorter with danazol treatment and longer with the progesterone‐releasing IUS, although this latter result was based on only one small trial. This outcome was not compared with OCP treatment.
Abdominal pain was more likely with LNG IUS than MFA in the short term (up to six months) but this adverse effect usually resolved over time (Stewart 2001). Incidence of total adverse events was more likely under danazol treatment. Although acceptability of treatment did not differ between danazol and MFA therapy (50% with danazol versus 47% with MFA refused to continue), the reasons given were not similar. About 80% of this group of danazol‐treated women refused to continue because of adverse effects, but 100% of the MFA group unwilling to continue were unhappy about the lack of efficacy of their treatment. A greater proportion of women in the ethamsylate group compared to women in the NSAID group (MFA) found their treatment unacceptable.
Comparison of individual NSAIDs
The clinical differences between individual NSAID preparations have not been previously explored thoroughly in randomised studies. Two studies in this review compared MFA with naproxen and found no differences in post‐treatment MBL or incidence of adverse events, although women treated with MFA were less likely to have gastrointestinal effects. However, this latter finding is based on only one small trial.
Although data comparing different types of NSAIDs were limited, there was no suggestion of differential efficacy, so, in line with the widely accepted assumption that NSAIDs have similar clinical efficacy, studies comparing different NSAIDs with placebo or other treatments were combined.
Tranexamic acid plus NSAIDs versus tranexamic acid alone
The authors of one trial reported that six months of combined treatment with tranexamic acid plus NSAIDs significantly reduced PBAC scores from baseline, but not treatment with tranexamic acid alone. However the data were in a form that could not be entered into analysis.
Overall comparisons
Despite the limited data, it appears that NSAIDs are more effective than placebo but less effective than tranexamic acid, danazol or the LNG IUS in reducing HMB. However, adverse events are more frequent under danazol therapy than NSAID therapy. No trials were identified with data on cost or resource use of NSAID treatments.
Overall completeness and applicability of evidence
Assessment of MBL is difficult because of cycle‐to‐cycle variation in women (Haynes 1977). Haynes and coworkers found that cycle‐to‐cycle variation was greater in women with HMB (39 mL to 271 mL) than in women with normal menses. Consequently, we included trials in this review only if MBL (measured objectively) was greater than 80 mL/cycle for two or more cycles prior to the intervention, although we also included trials where women had a subjective complaint of HMB. The alkaline haematin extraction method is the most commonly used objective method for assessment of blood loss and is used as the standard but a woman's own perception of her MBL is also important in the evaluation of effectiveness of treatment on MBL and as such is also a valid assessment tool. However, many women who seek medical help for HMB will have normal blood loss (Fraser 1984; Haynes 1977; Warner 2004a; Warner 2004b), and results from one RCT have suggested that there is little response to therapy in women with MBL less than 35 mL (Fraser 1981). Since a proportion of the study participants with a complaint of HMB had normal MBL, it is likely that some reported differences between treatment and placebo groups have been underestimated.
The studies included in this review examined effects over two or three menstrual cycles of treatment and one study with unpublished data assessed effects one month after treatment was withdrawn. There was no evidence from randomised trials of effects over a longer period but one observational study examined the effects of MFA in 34 women over a 16‐month period and reported persistent reductions of 25% to 35% in MBL and improvement in quality of life (Fraser 1983).
Moreover, different regimens for some of the medical therapies were not considered in the included trials. A longer duration of oral progestogen treatment (from days five to 26 of the menstrual cycle), and longer duration of treatment over a number of cycles with all medical therapies are necessary to assess the comparisons considered in this review adequately.
Quality of the evidence
Although 19 trials met the criteria for inclusion, the inadequacies in some of the studies must be highlighted. The trials were all small and underpowered and for many outcomes the results were based on only one trial. Reduction of MBL in two of the nine trials that were included in the meta‐analysis was correctly reported as a median and range in the publications since the distribution of data was positively skewed with one or more extremely high values (Cameron 1987; Hall 1987). Substitution of the mean for the median and the estimation of the SD for these studies has enabled their inclusion in the meta‐analysis but sensitivity analysis with and without the inclusion of Cameron 1987 has not altered results. Hall 1987 was the only trial comparing MBL after treatment with two NSAIDs, MFA and naproxen. The Mann‐Whitney U test reported in the publication of the trial agreed with the results of the meta‐analysis finding no clear evidence of a difference between the groups.
Two of the included trials had non‐comparable groups at baseline (Cameron 1987; Chamberlain 1991), but sensitivity analysis indicated that their inclusion in the meta‐analysis did not substantially alter the results.
Two trials assessed carryover effects (Fraser 1981; Hall 1987). Fraser 1981 was a cross‐over trial of MFA and placebo, and found that the MBL during placebo cycles was greater (but not significantly) in the group that received MFA first. Of considerable interest was the finding that blood loss in the MFA cycles was significantly less when placebo was taken first (P < 0.01), so that MFA appeared to have a greater beneficial effect when taken after placebo. There was evidence of a small carryover effect but this did not reach statistical significance. Hall 1987 also tested for carryover effects but found none (P = 0.96). It is generally believed that MBL returns to baseline levels very quickly after medical treatment is withdrawn and so the results from the cross‐over trials were unlikely to be affected by carryover effects.
Potential biases in the review process
A comprehensive search for relevant studies minimised the chance of publication bias, but this could not be ruled out. There were too few studies for an analysis of funnel plots to be meaningful. We used appropriate methods to select studies, extract data and make quality assessments, thus minimising the chance of review author error and bias.
Agreements and disagreements with other studies or reviews
The search identified no systematic reviews of NSAIDs. However, evidence‐based guidance from the UK National Institute for Health and Care Excellence (NICE) suggests that NSAIDs, together with tranexamic acid and OCPs, are a recommended second‐line treatment option for women with HMB in whom either hormonal or non‐hormonal treatments are acceptable. For women in whom hormonal treatment is not acceptable, NSAIDs are a first‐line option alongside tranexamic acid (NICE 2007).
Authors' conclusions
Implications for practice.
The review provides limited evidence of the efficacy of non‐steroidal anti‐inflammatory drugs (NSAIDs) (of which the most commonly studied is mefenamic acid (MFA)) as a treatment for reducing heavy menstrual bleeding (HMB) in women with menorrhagia. However, the efficacy of NSAIDs is superseded by danazol, tranexamic acid and levonorgestrel‐releasing levonorgestrel‐releasing (LNG IUS). Other medical treatments appear to be similarly effective compared to NSAIDs, although there is a suggestion that MFA may be more effective than either ethamsylate or oral progestogen. Danazol also reduces the number of days of menstrual bleeding but is more likely to cause adverse events when compared to NSAIDs. Gastrointestinal effects, which are often found with NSAID treatment, are less likely with MFA than naproxen. However, the benefits of NSAIDs that have been demonstrated in this review are modest; although MBL is reduced, a proportion of women will still have objective menorrhagia after treatment.
It is important to emphasise that a proportion of women with a convincing history of menorrhagia may not have excessive bleeding as defined by the alkaline haematin method. Treatments used to reduce blood loss in these women are not likely to be as effective.
The results of the review underscore the multiple assessments that are required in the evaluation of an optimum treatment. Efficacy is only one of these; other factors such as cost, convenience, beneficial and adverse effects on symptoms are also required.
Implications for research.
Since MFA has been most commonly studied, further randomised controlled studies (RCTs) are required to compare individual NSAIDs so that the optimum treatment can be identified.
Because many of the analyses contained only one RCT, some of the comparisons were not assessed thoroughly, in particular, the effects of NSAIDs on dysmenorrhoea, adherence to and acceptability of treatment and incidence of adverse events. However, since NSAIDs appear to be less efficacious than a number of other medical therapies, further RCTs are not likely to change the findings in this review significantly. Future studies comparing treatments for HMB should consider giving a longer duration of treatment over a number of cycles and a regimen of oral progestogen given during three of the four weeks of the cycle to assess comparative effects between medical treatments adequately. It is also possible that the addition of NSAIDs to other medical treatments may improve efficacy and further adequately designed trials are needed to explore this possibility.
What's new
| Date | Event | Description |
|---|---|---|
| 3 April 2019 | New citation required but conclusions have not changed | No new data were available at this update. Conclusions did not change. |
| 3 April 2019 | New search has been performed | One trial included with no data available for analysis (Jaisamrarn 2006); two new trials excluded (Famuyide 2017; Gupta 2013), and one new ongoing study identified (NCT02943655). |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 31 July 2012 | New search has been performed | Inclusion criteria amended, one new study added. |
| 31 July 2012 | New citation required but conclusions have not changed | New citation identified of combined treatment of NSAID with tranexamic acid. Inclusion criteria amended to include new citation. |
| 17 July 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
An updated search was performed in September and October 2001 but no new eligible trials were identified. An updated search was performed in April 2007; one published trial was included and one abstract is awaiting assessment. A further updated search was performed in July 2012; one published RCT was included. The 2019 search did not identify any new eligible trials.
Acknowledgements
The authors acknowledge the helpful comments of those who refereed previous versions of this review. Special thanks are due to Ms Ruth Jepson and Mrs Jane Clarke, Review Group Co‐ordinators, for their professionalism and help with the inevitable problems that arose; to Mrs Sue Furness and Marion Showell, Trials Search Co‐ordinators, for their assistance with identifying trials and to Mrs Sue Hall, past Secretary of the Review Group, for her secretarial help. The authors would also like to acknowledge the input of the Consumer Network who wrote a synopsis for the review. This synopsis has been added by the principal author with minor editing.
The authors of the 2012 update acknowledge the contribution of Cristina Augood to earlier versions of this review.
Appendices
Appendix 1. Gynaecology and Fertility Group specialised register search strategy
PROCITE platform
Searched 1 April 2019
Keywords CONTAINS "heavy bleeding"or"heavy menstrual bleeding" or "heavy menstrual loss" or "menometrorrhagia" or "menorrhagia" or "menorrhagia‐outcome" or "Menorrhagia‐Symptoms" or "abnormal bleeding" or "abnormal uterine bleeding" or "abnormal vaginal bleeding" or "excessive menstrual bleeding" or "excessive menstrual loss" or "dysfunctional bleeding" or "dysfunctional uterine bleeding" or Title CONTAINS "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "menometrorrhagia" or "menorrhagia" or "menorrhagia‐outcome" or "Menorrhagia‐Symptoms" or "abnormal bleeding" or "abnormal uterine bleeding" or "abnormal vaginal bleeding" or "excessive menstrual bleeding" or "excessive menstrual loss" or "dysfunctional bleeding" or "dysfunctional uterine bleeding"
AND
Keywords CONTAINS "non steroidal" or "non steroidal cytochrome inhibitor" or "NSAID" or "NSAIDs" or "naproxen" or "Naproxen Sodium" or "Ibuprofen" or "Flurbiprofen" or "Meclofenamic Acid" or "Meclofenamate" or "diclofenac" or "acetylsalicylic" or "acetyl salicylic acid" or "aspirin" or "indomethacin" or "indometacin" or "Ketoprofen" or "Piroxicam" or "Flufenamic Acid" or "nimesulide" or "COX‐2 inhibitors" or "cyclooxygenase" or "etoricoxib" or "lumiracoxib" or "parecoxib sodium" or "rofecoxib" or "valdecoxib" or Title CONTAINS"non steroidal" or "non steroidal cytochrome inhibitor" or "NSAID" or "NSAIDs" or "naproxen" or "Naproxen Sodium" or "Ibuprofen" or "Flurbiprofen" or "Meclofenamic Acid" or "Meclofenamate" or "diclofenac" or "acetylsalicylic" or "acetyl salicylic acid" or "aspirin"or "indomethacin" or "indometacin" or "Ketoprofen" or "Piroxicam" or "Flufenamic Acid" or "nimesulide" or "COX‐2 inhibitors" or "cyclooxygenase" or "etoricoxib" or "lumiracoxib" or "parecoxib sodium" or "rofecoxib" or "valdecoxib" (51 records)
Appendix 2. Cochrane Central Register of Studies Online (CRSO) search strategy
Web platform
Searched 1 April 2019
#1 MESH DESCRIPTOR Menorrhagia EXPLODE ALL TREES 338 #2 menorrhag*:TI,AB,KY 738 #3 (menstrua* adj5 disorder*):TI,AB,KY 316 #4 (heavy adj5 menstrua*):TI,AB,KY 245 #5 (iron adj5 anaem*):TI,AB,KY 483 #6 hypermenorr*:TI,AB,KY 25 #7 (dysfunction* adj2 uter*):TI,AB,KY 154 #8 (excessive* adj3 menstru*):TI,AB,KY 24 #9 (heavy adj3 menses):TI,AB,KY 4 #10 (abnormal* adj3 uterine):TI,AB,KY 304 #11 (excessive* adj2 uter*):TI,AB,KY 31 #12 (heavy adj2 period*):TI,AB,KY 15 #13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 1996 #14 MESH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal EXPLODE ALL TREES 18827 #15 MESH DESCRIPTOR Cyclooxygenase Inhibitors EXPLODE ALL TREES 14653 #16 (non‐steroidal adj2 anti‐inflammator*):TI,AB,KY 2099 #17 (nonsteroidal adj2 anti‐inflammator*):TI,AB,KY 1986 #18 ((ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid or ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid or mesalamine)):TI,AB,KY 26190 #19 ((naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin or cyclooxygenase inhibitors)):TI,AB,KY 6588 #20 (flufenamic or nimesulide):TI,AB,KY 404 #21 nsaid*:TI,AB,KY 4010 #22 MESH DESCRIPTOR Cyclooxygenase 2 EXPLODE ALL TREES 324 #23 (Cox 2):TI,AB,KY 1118 #24 (etoricoxib* or lumiracoxib*):TI,AB,KY 389 #25 parecoxib*:TI,AB,KY 355 #26 (rofecoxib* or valdecoxib*):TI,AB,KY 567 #27 sulphonanilide*:TI,AB,KY 0 #28 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 36296 #29 #13 AND #28 137
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 1 April 2019
1 exp Menorrhagia/ (4116) 2 menorrhag$.tw. (3181) 3 (menstrua$ adj5 disorder$).tw. (2727) 4 (heavy adj5 menstrua$).tw. (969) 5 (iron adj5 anaem$).tw. (3565) 6 hypermenorr$.tw. (292) 7 (dysfunction$ adj2 uter$).tw. (1141) 8 (excessive$ adj2 menstru$).tw. (203) 9 (heavy adj2 menses).tw. (45) 10 (abnormal$ adj2 uterine).tw. (2937) 11 (excessive$ adj2 uter$).tw. (199) 12 (heavy adj2 period$).tw. (468) 13 or/1‐12 (16223) 14 exp anti‐inflammatory agents, non‐steroidal/ or exp cyclooxygenase inhibitors/ (196818) 15 (non‐steroidal adj2 anti‐inflammator$).tw. (14907) 16 (non$steroidal adj2 anti$inflammator$).tw. (4360) 17 (ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid or ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid or mesalamine).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (165199) 18 (naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin or cyclooxygenase inhibitors).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (58233) 19 (flufenamic or nimesulide).tw. (2527) 20 nsaid$.tw. (23255) 21 exp Cyclooxygenase 2/ (22169) 22 Cox 2.tw. (28830) 23 (etoricoxib$ or lumiracoxib$).tw. (828) 24 parecoxib$.tw. (508) 25 (rofecoxib$ or valdecoxib$).tw. (2368) 26 sulphonanilide$.tw. (5) 27 or/14‐26 (283757) 28 13 and 27 (443) 29 randomized controlled trial.pt. (478941) 30 controlled clinical trial.pt. (92997) 31 randomized.ab. (438440) 32 placebo.tw. (201790) 33 clinical trials as topic.sh. (186485) 34 randomly.ab. (307960) 35 trial.ti. (196211) 36 (crossover or cross‐over or cross over).tw. (79677) 37 or/29‐36 (1234498) 38 exp animals/ not humans.sh. (4564068) 39 37 not 38 (1134047) 40 28 and 39 (132)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 1 April 2019
1 menstruation disorder/ or exp "menorrhagia and metrorrhagia"/ or exp hypermenorrhea/ or exp menorrhagia/ (19536) 2 (menstrua$ adj5 disorder$).tw. (2984) 3 (heavy adj5 menstrua$).tw. (1681) 4 (iron adj5 anaem$).tw. (5069) 5 menorrhag$.tw. (5085) 6 hypermenorr$.tw. (398) 7 (dysfunction$ adj2 uter$).tw. (1373) 8 (excessive$ adj2 menstru$).tw. (271) 9 (heavy adj2 menses).tw. (84) 10 (abnormal$ adj2 uterine).tw. (4583) 11 (excessive$ adj2 uter$).tw. (267) 12 (heavy adj2 period$).tw. (620) 13 or/1‐12 (32380) 14 exp anti‐inflammatory agents, non‐steroidal/ or exp cyclooxygenase inhibitors/ (684261) 15 (non‐steroidal adj5 anti‐inflammator$).tw. (20236) 16 (non$steroidal adj5 anti$inflammator$).tw. (5347) 17 (ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid or ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid or mesalamine or naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin).tw. (224432) 18 cyclooxygenase inhibitors.tw. (1769) 19 (flufenamic or nimesulide).tw. (3239) 20 nsaid$.tw. (39949) 21 exp Cyclooxygenase 2/ (42216) 22 exp cyclooxygenase 2 inhibitor/ or exp celecoxib/ or exp cimicoxib/ or exp deracoxib/ or exp etoricoxib/ or exp flosulide/ or exp lumiracoxib/ or exp meloxicam/ or exp nimesulide/ or exp parecoxib/ or exp rofecoxib/ or exp tilmacoxib/ or exp valdecoxib/ (49937) 23 cyclooxygenase$.tw. (47922) 24 Cox 2.tw. (37963) 25 sulphonanilide$.tw. (6) 26 (celecoxib$ or cimicoxib$ or deracoxib$ or etoricoxib$ or flosulide$ or lumiracoxib$ or meloxicam$ or nimesulide$ or parecoxib$ or rofecoxib$ or tilmacoxib$ or valdecoxib$).tw. (16256) 27 exp acetylsalicylic acid/ (189080) 28 aspirin.tw. (105180) 29 or/14‐28 (766152) 30 13 and 29 (2372) 31 Clinical Trial/ (941918) 32 Randomized Controlled Trial/ (534682) 33 exp randomization/ (81488) 34 Single Blind Procedure/ (34108) 35 Double Blind Procedure/ (155378) 36 Crossover Procedure/ (58329) 37 Placebo/ (316842) 38 Randomi?ed controlled trial$.tw. (197090) 39 Rct.tw. (31342) 40 random allocation.tw. (1846) 41 randomly allocated.tw. (31749) 42 allocated randomly.tw. (2402) 43 (allocated adj2 random).tw. (798) 44 Single blind$.tw. (22178) 45 Double blind$.tw. (188245) 46 ((treble or triple) adj blind$).tw. (905) 47 placebo$.tw. (279474) 48 prospective study/ (504229) 49 or/31‐48 (1985284) 50 case study/ (59663) 51 case report.tw. (364431) 52 abstract report/ or letter/ (1041323) 53 or/50‐52 (1456175) 54 49 not 53 (1935584) 55 30 and 54 (726)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 1 April 2019
1 exp Anti Inflammatory Drugs/ (5542) 2 (non‐steroidal adj5 anti‐inflammator$).tw. (524) 3 (non$steroidal adj5 anti$inflammator$).tw. (90) 4 (ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid or ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid or mesalamine).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] (3040) 5 (naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin or cyclooxygenase inhibitors).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] (522) 6 (flufenamic or nimesulide).tw. (111) 7 nsaid$.tw. (904) 8 Cox 2.tw. (833) 9 (etoricoxib$ or lumiracoxib$).tw. (28) 10 parecoxib$.tw. (23) 11 (rofecoxib$ or valdecoxib$).tw. (111) 12 or/1‐11 (9265) 13 exp Menstrual Disorders/ (1200) 14 menorrhag$.tw. (83) 15 (menstrua$ adj5 disorder$).tw. (389) 16 (heavy adj5 menstrua$).tw. (29) 17 (iron adj5 anaem$).tw. (46) 18 hypermenorr$.tw. (2) 19 (dysfunction$ adj2 uter$).tw. (32) 20 (excessive$ adj2 menstru$).tw. (8) 21 (heavy adj2 menses).tw. (1) 22 (abnormal$ adj2 uterine).tw. (30) 23 (excessive$ adj2 uter$).tw. (5) 24 (heavy adj2 period$).tw. (80) 25 or/13‐24 (1680) 26 12 and 25 (19) 27 random.tw. (54841) 28 control.tw. (422150) 29 double‐blind.tw. (22014) 30 clinical trials/ (11275) 31 placebo/ (5216) 32 exp Treatment/ (711116) 33 or/27‐32 (1123818) 34 26 and 33 (15)
Data and analyses
Comparison 1. NSAIDs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss (MBL) (mL/cycle) | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐124.0 [‐186.36, ‐61.64] |
| 1.1 Mefenamic acid vs placebo | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐124.0 [‐186.36, ‐61.64] |
| 2 Proportion with no subjective improvement in MBL | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.08 [0.03, 0.18] |
| 2.1 Mefenamic acid vs placebo | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.08 [0.03, 0.18] |
| 3 Adverse events | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.07, 8.09] |
| 3.1 Mefenamic acid vs placebo | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.07, 8.09] |
| 4 MBL and other outcomes (descriptive results) | Other data | No numeric data | ||
| 4.1 Mefenamic acid vs placebo | Other data | No numeric data | ||
| 4.2 Naproxen vs placebo | Other data | No numeric data | ||
| 4.3 Ibuprofen vs placebo | Other data | No numeric data |
Comparison 2. NSAIDs versus tranexamic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss (MBL) (mL/cycle) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 73.0 [21.66, 124.34] |
| 2 Proportion with no subjective improvement in MBL | 1 | 49 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.45, 4.61] |
| 3 Number of days' bleeding | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.47, 1.27] |
| 4 Proportion with no improvement in quality of life or dysmenorrhoea (or both) | 1 | 49 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.27, 4.73] |
| 5 Proportion who found treatment unacceptable | 1 | 49 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.32, 4.27] |
| 6 MBL and other outcomes (descriptive results) | Other data | No numeric data |
Comparison 3. NSAIDs versus ethamsylate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss after treatment (mL/cycle) | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐42.88 [‐86.25, 0.50] |
| 2 Menstrual blood loss 1–6 months after treatment (mL/cycle) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐70.3 [‐158.88, 18.28] |
| 3 Proportion with no subjective improvement in MBL | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.23, 2.12] |
| 4 Proportion with no improvement in quality of life or dysmenorrhoea (or both) | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.18, 3.72] |
| 5 Number of days' bleeding | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.56, 0.76] |
| 6 Proportion who found treatment unacceptable | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.07, 0.61] |
Comparison 4. NSAIDs versus danazol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss (MBL) (mL/cycle) | 3 | 79 | Mean Difference (IV, Fixed, 95% CI) | 45.06 [18.73, 71.39] |
| 2 Proportion with no improvement in quality of life or dysmenorrhoea (or both) | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.20, 7.05] |
| 3 Number of days' bleeding | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [0.26, 1.80] |
| 4 Proportion who found treatment unacceptable | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.24, 2.80] |
| 5 Adverse events | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.17 [0.05, 0.59] |
Comparison 5. NSAIDs versus oral progestogens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss (MBL) (mL/cycle) | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐22.97 [‐46.57, 0.62] |
| 2 Number of days' bleeding | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.95, 0.13] |
| 3 Proportion with non‐adherence | 1 | 32 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.05, 14.78] |
| 4 Total adverse events | 1 | 32 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.13, 2.26] |
| 5 Adverse events – headache | 1 | 32 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.14, 2.86] |
| 6 Adverse events – abdominal pain | 1 | 32 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.15, 4.96] |
| 7 Adverse events – nausea | 1 | 32 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [0.17, 18.65] |
Comparison 6. NSAIDs versus progesterone‐releasing intrauterine system (IUS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss (MBL) (mL/cycle) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐31.23, 23.23] |
| 2 MBL and other outcomes (descriptive results) | Other data | No numeric data | ||
| 3 Number of days' bleeding | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐6.08, ‐3.92] |
| 4 Adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Headache | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.31, 2.86] |
| 4.2 Abdominal pain | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.06, 0.87] |
| 4.3 Ovarian cyst | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.10, 1.80] |
| 4.4 Breast pain | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.07, 1.33] |
| 4.5 Nausea | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.01 [0.37, 10.86] |
| 4.6 Diarrhoea | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.53 [0.57, 21.98] |
| 4.7 Upper respiratory infection | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.24, 3.75] |
Comparison 7. NSAIDs versus oral contraceptive pill (OCP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss (mL/cycle) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 25.25 [‐22.34, 72.84] |
Comparison 8. Mefenamic acid versus naproxen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Menstrual blood loss (mL/cycle) | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 21.00 [‐5.85, 47.85] |
| 2 Number of days' bleeding | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.59, 0.79] |
| 3 Total adverse events | 1 | 35 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 2.00] |
| 4 Gastrointestinal adverse events | 1 | 35 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.06, 0.87] |
| 5 Central nervous system adverse events | 1 | 35 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.34, 5.73] |
Comparison 9. Tranexamic acid and mefenamic acid versus tranexamic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pictorial blood loss assessment chart (PBAC) score at 6 months' follow‐up | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersch 1988.
| Methods | Randomisation technique not stated Blinding not mentioned but unlikely because drugs given with different frequency. Cross‐over design over 6 menstrual cycles: 2 control cycles, then 4 Rx cycles where women received either flurbiprofen or tranexamic acid for 2 cycles followed by the alternative over the next 2 cycles. No exclusions postrandomisation or loss to follow‐up No power calculation |
|
| Participants | Sweden 15 women, mean age 40.5 years (range 34–49 years). Inclusion criteria: MBL > 80 mL/cycle for 2 periods Exclusion criteria: menorrhagia caused by uterine myomata or IUCD. |
|
| Interventions | Group 1: flurbiprofen 100 mg 2 times daily for 5 days, n = 15 Group 2: tranexamic acid 1.5 g 3 times daily for 3 days, 1 g twice daily on days 4 and 5, n = 15 Duration: 4 menstrual cycles |
|
| Outcomes | MBL (mL/cycle) Duration of menstruation (days) Adverse events |
|
| Notes | ITT uncertain Baseline comparability not stated Data were not available at the end of the first cross‐over period so results could not be included in the meta‐analysis of pooled effects. Data also not provided for duration of menstruation or adverse effects. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear how it was done exactly. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Other bias | Unclear risk | Baseline comparability not stated |
Bonnar 1996.
| Methods | Randomised controlled trial, parallel group, no evidence of blinding, randomisation by computer‐generated randomisation list 5 women withdrew during first cycle of treatment (2 from MFA group, 2 from ethamsylate group and 1 from tranexamic acid group) ITT analysis on remaining 76 women (18 of whom withdrew during the study) Power calculation for sample size made No loss to follow‐up |
|
| Participants | Dublin, Ireland 81 women, mean age 39 years (range 35–46 years) with a mean MBL > 80 mL/cycle measured over 3 consecutive menstrual periods. Exclusions: "Organic" causes of menorrhagia by gynaecological investigation (hysteroscopy, endometrial biopsy and cervical smear). History of renal or hepatic impairment, previous thromboembolic disease, inflammatory bowel disease, peptic or intestinal ulceration or coagulation or fibrinolytic disorders |
|
| Interventions | Group 1: tranexamic acid: 1 g 4 times daily for 5 days from day 1 of menses, n = 26 Group 2: ethamsylate: 500 mg 4 times daily for 5 days from day 1 of menses, n = 27 Group 3: MFA: 500 mg 3 times daily for 5 days from day 1 of menses, n = 23 Duration: 3 menstrual cycles |
|
| Outcomes | MBL (by alkaline haematin method) Duration of bleeding (days) Patient's estimation of blood loss (less, same, greater) Quality of life (dysmenorrhoea) Adverse events (type, incidence) Patient acceptability of treatment (would you be prepared to continue with this treatment?) |
|
| Notes | No ITT analysis Baseline comparability done Additional data provided by the author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/81 women withdrew (2 from MFA group, 2 from ethamsylate group and 1 from tranexamic acid group) |
| Other bias | Low risk | Groups similar at baseline |
Cameron 1987.
| Methods | Randomisation method not stated and no blinding Women randomly allocated to 4 parallel treatment groups No reported loss to follow‐up No power calculation made |
|
| Participants | Women recruited form Edinburgh Royal Infirmary, UK 30 women aged 29–50 years with MBL > 50 mL/cycle |
|
| Interventions | Group 1: MFA 500 mg 3 times daily for first 5 days of menses, n = 8 Group 2: danazol 200 mg daily, n = 6 Group 3: norethisterone 5 mg twice daily days 15–25 of cycle, n = 8 Group 4: progesterone coil 65 μg of progesterone released daily, n = 8 Duration: 2 cycles |
|
| Outcomes | MBL (measured by alkaline haematin method) | |
| Notes | Unclear if ITT analysis was done No baseline comparability Pretreatment MBL in danazol group significantly higher than in other 3 Rx groups; therefore, groups not comparable at baseline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Other bias | Unclear risk | No baseline comparability |
Cameron 1990.
| Methods | Randomisation technique not stated and no blinding MBL over 2 cycles was assessed in 102 women 20 women refused to collect their pads, 10 women had anovulatory cycles and 40 women had MBL < 80 mL/cycle. The remaining 32 women were randomised to treatment arms. No withdrawals or loss to follow‐up, no power calculation made |
|
| Participants | Edinburgh, UK 32 women with MBL > 80 mL/cycle, median age 40 years (range 21–51 years) Exclusion criteria: organic disease, anovulatory cycles and non‐compliance with collecting pads |
|
| Interventions | Group 1: MFA 500 mg 3 times daily days 1–5 of menses, n = 17 Group 2: norethisterone 5 mg 2 times daily on cycle days 19–26, n = 15 Duration over 2 cycles |
|
| Outcomes | MBL (alkaline haematin method) Number of days bleeding Adverse events Patient compliance |
|
| Notes | Unclear if ITT analysis was done. Baseline comparability was done. Additional data provided by the author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Other bias | Low risk | Baseline comparability done |
Chamberlain 1991.
| Methods | Randomisation by Taves method of minimisation Double‐blind randomised trial with parallel groups and double‐dummy technique Exclusions postrandomisation: 6 (5 fibroids, 1 IUCD) Not ITT, no power calculation made |
|
| Participants | UK 42 women, aged 18–55 years, with menorrhagia, MBL > 80 mL/cycle and regular menstrual cycles Exclusions: taking oral contraceptives, antacids, anticoagulants or protein bound drugs; hepatic impairment, inflammatory bowel disease or endocrine disorders; wish to become pregnant during trial; known allergies to prostaglandin inhibitors; anaemic (haemoglobin < 9 g/dL); IUCD fitted, uterine enlargement due to fibroids |
|
| Interventions | Group 1: MFA 500 mg 3 times daily for duration of menses, n = 19 Group 2: ethamsylate 500 mg 4 times daily for duration of menses, n = 17 Duration: 3 cycles + 1 cycle post‐Rx |
|
| Outcomes | MBL (mean of 3 Rx cycles measured by alkaline haematin method) MBL (during 1 post‐Rx cycle measured by alkaline haematin method) Adverse events |
|
| Notes | No ITT analysis Detailed data from published study not available from authors. Lorex Synthélabo was able to provide data from the same study but numbers in the groups were different from the published study. Results analysed from the unpublished data. Pretreatment MBL not comparable between the 2 groups. Adverse effects data not available from unpublished data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Taves method of minimisation |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/42 women excluded (not clear from which group: 5 for fibroids and 1 because of IUCD) |
| Other bias | High risk | Detailed data from published study not available from authors. Lorex Synthélabo was able to provide data from the same study but numbers in the groups were different from the published study. Results analysed from the unpublished data. Pretreatment MBL not comparable between the 2 groups. Adverse effects data not available from unpublished data. |
Dockeray 1989.
| Methods | Women randomised according to a randomisation code without blinding into 2 treatment groups, MFA and danazol. 1 woman (from MFA group) was withdrawn because of adverse effects. No loss to follow‐up. The duration of the study was 4 menstrual cycles, 2 untreated (control) and 2 with treatment. No power calculation made or ITT analysis |
|
| Participants | Ireland 40 women, mean age 37.8 years (range 23–48 years) with normal pelvic organs and no endometrial pathology Inclusion criteria: objective unexplained MBL > 80 mL/cycle (alkaline haematin method), history of excessive menstrual bleeding |
|
| Interventions | Group 1: MFA 500 mg 3 times daily for 3–5 days for 2 cycles, n = 19 Group 2: danazol 100 mg twice daily for 60 days for 2 cycles, n = 20 |
|
| Outcomes | MBL (alkaline haematin method) Number of days bleeding Quality of life (dysmenorrhoea) Adverse events (incidence, severity) Patient acceptability of treatment (prepared to continue Rx?) |
|
| Notes | No ITT analysis Baseline comparability done |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported; 1 woman was withdrawn because of adverse effects. |
| Other bias | Low risk | Similar at baseline |
Fraser 1981.
| Methods | Randomisation technique by identically labelled containers of identical drugs. 4 cycle double‐blind randomised placebo‐controlled cross‐over trial Not ITT and no power calculation made. 16 withdrawals |
|
| Participants | Australia 85 women (69 completed the trial), mean age 33 years (range 14–48 years) with convincing history of menorrhagia, but with a variety of menorrhagia diagnoses: ovulatory DUB (n = 28), anovulatory DUB (n = 6), IUD (n = 6), fibroids (n = 2), tubal sterilisation (n = 25), oral contraceptive pill (n = 1) and von Willebrand disease (n = 1). Results reported in review only for the subgroup of women with ovulatory menorrhagia (n = 28) |
|
| Interventions | Group 1: MFA 500 mg 3 times daily from onset to end of menstruation, n = 28 Group 2: identical dosage regimen, n = 28 |
|
| Outcomes | MBL (alkaline haematin method) Menstrual symptoms (graded 0–3): data not available for subgroup Adverse events: data not available for subgroup |
|
| Notes | No ITT analysis Inclusion criteria: subjective menorrhagia; however, only 43% had MBL > 80 mL/cycle. Separate analyses provided for > 80 mL/cycle (n = 30), < 80 mL/cycle (n = 39) and < 35 mL/cycle (n = 14). Results of subgroup with ovulatory menorrhagia given at end of trial since data not available at the end of the first cross‐over period; consequently these were not included in the meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/85 women withdrew, no ITT analysis |
| Other bias | Unclear risk | Nothing detected |
Fraser 1991.
| Methods | Randomisation technique not stated. 8‐cycle cross‐over treatment trial without blinding or placebo. 7 lost to follow‐up. Not ITT and no power calculation made. Women randomised into 3 treatment groups. All women received MFA and 1 of 3 other treatments: naproxen, oral contraceptive and danazol in a random order. 2 control cycles prior to first Rx, 2 Rx cycles, 2 control cycles prior to second Rx, 2 Rx cycles. | |
| Participants | Australia 45 women with a convincing clinical history of menorrhagia, regular periods, no hormonal therapy in the previous 3 months and with no evidence of pelvic and systemic causes of menorrhagia Inclusion criteria: subjectively defined menorrhagia (37% had MBL < 80 mL/cycle) |
|
| Interventions | MFA 500 mg every 6–8 hours from onset of menstruation until 1 day after (maximum 5 days), n = 20 Naproxen 500 mg at onset followed by 250 mg every 6–8 hours until 1 day after (maximum 5 days), n = 6 Oral contraceptive (low dose): 30 μg ethinyl and 150 μg levonorgestrel daily for 21 days out of 28, n = 6 Danazol (low dose): 200 mg daily from day 5 of the second control cycle for 8 weeks, n = 6 Group 1: MFA + naproxen, n = 14 Group 2: MFA + combined low‐dose contraceptive pill, n = 12 Group 3: MFA + danazol, n = 12 8 cycle design: 2 untreated cycles, 2 cycles with first treatment, 2 untreated cycles, 2 cycles with second treatment |
|
| Outcomes | MBL (alkaline haematin method) | |
| Notes | No ITT analysis Data made available by the author for the end of the first cross‐over period. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/45 women lost to follow‐up, no ITT analysis |
| Other bias | Unclear risk | Nothing detected |
Grover 1990.
| Methods | Randomisation method not stated Double‐blind placebo‐controlled trial with parallel groups 80 women randomised No loss to follow‐up given but details of numbers randomised sketchy No power calculation made |
|
| Participants | India 80 women aged 19–50 years with a subjective complaint of menorrhagia and normal cervical cytology and secretory endometrium (after D&C) Exclusion criteria: local pelvic causes of bleeding |
|
| Interventions | Group 1: MFA 500 mg 3 times daily for 5 days of menstruation or until menstruation ceased, n = 40 Group 2: placebo same dosage regimen, n = 40 Duration: 3 consecutive cycles |
|
| Outcomes | "Relief" of menorrhagia Number of days bleeding |
|
| Notes | Not stated if ITT analysis No details given regarding measurement of "relief" of menorrhagia No data given for number of days bleeding in placebo group Author could not be contacted for clarification |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Other bias | Unclear risk | No details given regarding measurement of "relief" of menorrhagia No data given for number of days bleeding in placebo group Author could not be contacted for clarification |
Hall 1987.
| Methods | Randomisation by Fisher's table of random numbers Double‐blind cross‐over trial comparing MFA with naproxen using a double‐dummy technique Not ITT and no power calculation made 9 women excluded postrandomisation mostly for not meeting inclusion criteria An additional 6 women withdrawn during treatment |
|
| Participants | UK 50 women aged ≥ 18 years to the menopause with a complaint of DUB Exclusion criteria: pelvic inflammation, fibroids, other local disease, gross cycle irregularities |
|
| Interventions | Group 1: MFA 500 mg 3 times daily for 5 days, n = 17 Group 2: naproxen initially 550 mg, then 275 mg 4 times daily for 5 days, n = 18 Duration: 2 + 2 cycles |
|
| Outcomes | MBL (alkaline haematin method) Adverse events (gastrointestinal, central nervous system) Duration of bleeding (days) Improvement in dysmenorrhoea Improvement in MBL (subjective) |
|
| Notes | Not ITT analysis Tests of carryover effects not significant No data provided by author for improvement in dysmenorrhoea and subjective improvement in MBL Data on MBL given at end of first Rx phase |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fisher's table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/50 women (9 women excluded post randomisation for not meeting inclusion criteria; 6 withdrawn during treatment); no ITT analysis |
| Other bias | Unclear risk | No source of funding. Nothing detected |
Jaisamrarn 2006.
| Methods | Randomised controlled trial | |
| Participants | Thailand 169 women with ovulatory menorrhagia No data available from this trial including numbers randomised to each group |
|
| Interventions | Group 1: tranexamic acid 3 g daily on days 1–5 of menstrual cycle Group 2: norethisterone 10 mg daily on days 19–26 Group 3: MFA 1.5 mg daily days 1–5 of menstrual cycle |
|
| Outcomes | PBAC Quality of life |
|
| Notes | Conference abstract Source of funding not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Other bias | Unclear risk | No source of funding. No baseline characteristics. |
Makarainen 1986.
| Methods | Randomisation by manufacturer using tables for random numbers Double‐blind, placebo‐controlled using cross‐over design No loss to follow‐up No power calculation made |
|
| Participants | Finland 13 women, mean age 39 years with a complaint of menorrhagia (subgroup from a total number of 30 women: primary menorrhagia (n = 13), myoma‐associated menorrhagia (n = 10), factor VIII deficiency (n = 1) and normal blood loss (n = 6)) No exclusions specifically mentioned |
|
| Interventions | Group 1: ibuprofen 600 mg daily throughout menstrual cycle (maximum 10 days), n = 13 Group 2: ibuprofen 1200 mg daily throughout menstrual cycle (maximum 10 days), n = 13 Group 3: placebo same dosage regimen, n = 13 |
|
| Outcomes | MBL (alkaline haematin method) Adverse events (any vs none) Duration of bleeding (days) Menstrual pain |
|
| Notes | No ITT analysis Data not available at end of first treatment period and so not suitable for pooling Data not given by author for duration of bleeding or menstrual pain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Tables of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Other bias | Unclear risk | Nothing detected |
Muggeridge 1983.
| Methods | Randomisation method not stated Double‐blind placebo‐controlled trial with cross‐over design Losses to follow‐up: 5 (1 heavy bleeding, 1 irregular bleeding, 1 nausea, 2 left the area) Not ITT and no power calculation made |
|
| Participants | UK 20 women with MBL > 75 mL/cycle Exclusion criteria: pelvic pathology |
|
| Interventions | Group 1: MFA 500 mg 3 times daily, number of days not reported, n = 15 Group 2: placebo same dosage regimen, n = 15 Duration: 2 + 2 cycles |
|
| Outcomes | MBL (alkaline haematin method) Dysmenorrhoea (numerical score) Adverse events |
|
| Notes | Data not available from author at the end of the first cross‐over period Data on adverse effects not given by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/20 women lost to follow‐up (not clear from which group: 1 for heavy bleeding, 1 irregular bleeding, 1 nausea, 2 left the area), no ITT analysis |
| Other bias | Unclear risk | Data not available from author at the end of the first cross‐over period Data on adverse effects not given by author |
Najam 2010.
| Methods | Randomisation by computer‐generated numbers Parallel‐group design, single‐centre, single‐blinded trial 110 women randomised, number of women analysed not reported, withdrawals and ITT not reported Power calculation for sample size: 30% improvement in haemoglobin concentration post‐treatment would require 110 women Source of funding not stated |
|
| Participants | India Women with abnormal uterine bleeding in Indian hospital, aged 12–45 years; transvaginal sonography (in married women) indicating endometrial thickness < 5 mm; normal Pap test, thyroid function test, renal function tests, liver function tests, coagulation profile; endometrial sampling in secretory phase (perimenopausal women) Exclusion criteria: history of recent IUD or hormonal therapy; anovulatory or irregular cycles; pregnancy or any pelvic pathology; coagulation disturbances; polycystic ovarian disease and thyroid, liver or renal dysfunction Mean age 37 years in the tranexamic acid group; 39 years in the tranexamic acid + MFA 67% of the women were older than 30 years; no further details were provided |
|
| Interventions | Group 1: tranexamic acid 500 mg daily + MFA 250 mg daily for days 1–5 of menstrual cycle Group 2: tranexamic acid 500 mg daily for days 1–5 of menstrual cycle. Rx duration 3 months |
|
| Outcomes | Haemoglobin levels PBAC scores |
|
| Notes | Measured at 3 and 6 months. Inappropriate analysis – authors measured change from baseline separately in randomised groups and reported where change was significantly different per group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only single blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Other bias | High risk | Measured at 3 and 6 months. Inappropriate analysis – authors measured change from baseline separately in randomised groups and reported where change was significantly different per group. |
Reid 2005.
| Methods | Randomisation by random permuted blocks by computer Parallel group design, single centre with no blinding 51 women randomised and analysed Number of withdrawals: MFA (4 discontinued: 1 for non‐compliance, 2 diarrhoea and 1 treatment ineffective); LNG IUS (4 discontinued: 2 partial expulsion and 2 full expulsion). ITT analysis Power calculation for sample size Source of funding: Schering |
|
| Participants | Luton and Dunstable Hospital NHS Trust, UK Women aged 18–47 years, mean 39 years; in good general health; referred by general practitioners or self‐referred following advertisements; regular, ovulatory menstrual cycles (21–35 days); objective idiopathic menorrhagia (MBL > 80 mL/cycle confirmed in at least 1 cycle up to 4 months before study by the alkaline haematin method) Exclusion criteria: undiagnosed, abnormal bleeding, anovulatory; submucous fibroids or fibroids with total volume > 5 cm3; uterine size on ultrasound > 20 cm; abnormal cervical cytology; untreated hypertension; abnormal thyroid or liver function tests; asthma; IUCD in situ; treated for menorrhagia or used hormonal contraception in previous 4 months |
|
| Interventions | Group 1: MFA 500 mg 3 times daily, days 1–4 of cycle) Group 2: LNG IUS duration 6 months |
|
| Outcomes | MBL | |
| Notes | ITT analysis Data on MBL given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted blocks by computer |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation and preparation of consecutively numbered, opaque, sealed envelopes was performed by Schering Oy (Finland). Women were allocated treatment by the author (PCR) opening the next numbered envelope, after screening, in the presence of the patient". |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/51 women (4 from MFA group: 1 for non‐compliance, 2 for diarrhoea, 1 treatment ineffective; 4 from LNGIUS group: 2 for partial expulsion, 2 for full expulsion), ITT analysis |
| Other bias | Unclear risk | Nothing detected |
Rybo 1981.
| Methods | Randomisation method not given Double‐blind placebo‐controlled trial with cross‐over design No loss to follow‐up |
|
| Participants | Sweden 18 women with a complaint of menorrhagia, ovulatory cycles and a normal coagulation test (data reported for subgroup of 4 women with primary menorrhagia, the remaining 12 had IUD‐induced menorrhagia) Exclusion criteria: organic cause of menorrhagia |
|
| Interventions | Group 1: naproxen 500 mg morning, 250 mg afternoon days 1 and 2, then 250 mg twice daily for up to 7 days, n = 4 Group 2: placebo same dosage regimen, n = 4 Duration: 2 + 2 cycles |
|
| Outcomes | MBL (alkaline haematin method) | |
| Notes | Not ITT analysis Data not available at end of first cross‐over period and so not suitable for pooling Very large standard deviation for mean MBL in Rx group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double bind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Other bias | Unclear risk | Nothing detected |
Tsang 1987.
| Methods | Randomisation method not stated Double‐blind placebo‐controlled trial with cross‐over design Losses to follow‐up: 4 Not ITT and no power calculation made |
|
| Participants | Canada 14 women aged 26–47 years with either a history of heavy menstrual bleeding or MBL > 80 mL/cycle (measured objectively) and regular menstrual cycles Exclusions: use of hormonal contraceptives or anti‐inflammatory drugs and use of IUCD |
|
| Interventions | Group 1: MFA 500 mg at onset of menses, then 250 mg 4 times daily for 3–5 days, n = 10 Group 2: placebo same dosage regimen, n = 10 Duration: 2 + 2 cycles |
|
| Outcomes | MBL (measured by alkaline haematin method) | |
| Notes | Data not available at end of first cross‐over period so not suitable for pooling in a meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/14 women lost to follow‐up, no ITT analysis |
| Other bias | Unclear risk | Nothing detected |
van Eijkeren 1992.
| Methods | Randomisation list controlled by pharmacy Double‐blind, placebo‐controlled, parallel group No ITT and no power calculation made 7 withdrawals (postponed hysterectomy, fibroids at operation, postmenstrual phase, premenstrual phase). 1 discontinuation because of adverse effects of MFA (rash and itching) |
|
| Participants | Netherlands 19 women, mean age 40 years Inclusion criteria: aged < 45 years, MBL > 80 mL/cycle, regular menstrual cycle Exclusion criteria: IUD, use of NSAIDs or other medication that could affect haemostasis, contraindications against NSAIDs, use of hormonal medication |
|
| Interventions | Group 1: MFA 500 mg 3 times daily from 5 days prior to menses to cessation of bleeding, n = 6 Group 2: placebo 3 times daily from 5 days prior to menses to cessation of bleeding, n = 5 Duration: 1 menstrual cycle |
|
| Outcomes | MBL (alkaline haematin method) Adverse events |
|
| Notes | 19 women randomised but 7 dropped out after the treatment cycle (this high dropout rate reduced the quality of the study). Data reported only for the 11 women that went on to have a hysterectomy. Requested data from the 7 withdrawals but received no answer from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list controlled by pharmacy |
| Allocation concealment (selection bias) | Low risk | Outside control of randomisation list |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/19 women withdrawn (postponed hysterectomy, fibroids at operation, postmenstrual phase, premenstrual phase, adverse effects of MFA) and 1 woman discontinuation because of adverse effects; no ITT analysis |
| Other bias | Unclear risk | Nothing detected |
Ylikorkala 1986.
| Methods | Randomisation method not stated Double‐blind placebo‐controlled trial with cross‐over design No loss to follow‐up Not ITT and no power calculation made |
|
| Participants | Finland 14 women, mean age 42 years with MBL > 80 mL/cycle, regular cycles and normal pelvic findings |
|
| Interventions | Group 1: naproxen 500 mg at onset then 3–5 hours later, then 500 mg twice daily for 5 days, n = 14 Group 2: placebo same dosage regimen, n = 14 Duration: 2 + 2 cycles |
|
| Outcomes | MBL (alkaline haematin method) Subjective perception of improvement in MBL Adverse events (any vs none) |
|
| Notes | No ITT analysis Data not available at end of the first Rx period so not suitable for pooling |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear risk |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Other bias | Unclear risk | Nothing detected |
D&C: dilation and curettage; DUB: dysfunctional uterine bleeding; MFA: mefenamic acid; ITT: intention to treat; IUCD: intrauterine contraceptive device; IUD: intrauterine device; LNG IUS: levonorgestrel‐releasing intrauterine system; MBL: menstrual blood loss; n: number of participants; NSAID: non‐steroidal anti‐inflammatory drug; PBAC: pictorial blood loss assessment chart; Rx: treatment.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Famuyide 2017 | Randomisation was endometrial ablation vs medical treatment, including NSAIDs, but women could choose if they wanted combined contraceptive pill or NSAIDs. |
| Gupta 2013 | Randomisation was progestogen‐releasing intrauterine system vs other medical treatment including NSAIDs, but women could choose their medical treatment. |
| Martinez Alcala 1979 | Double‐blind randomised controlled trial of cross‐over design assessing the effect of mefenamic acid on bleeding patterns, sanitary pad usage and adverse effects. The outcome, MBL, was measured by the change in sanitary pad usage. The number of sanitary pads used during a menstrual cycle does not correlate well with the MBL measured objectively by the alkaline haematin method and so this study was excluded. |
| Naafe 2018 | The comparison was mefenamic acid to mefenamic acid + herbal medicine. |
| Vargyas 1987 | 7/32 (21%) participants had intrauterine devices fitted, which is an exclusion criterion for this review since this is considered an iatrogenic cause of heavy menstrual bleeding. The data were not available separately for the remaining women. |
MSL: menstrual blood loss; NSAID: non‐steroidal anti‐inflammatory drug.
Characteristics of ongoing studies [ordered by study ID]
NCT02943655.
| Trial name or title | Treatment of heavy and/or prolonged menstrual bleeding without organic cause |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion criteria: regular menstrual cycles with body mass index (19–29 kg/m2). Heavy or prolonged (or both) menstrual bleeding involving ≥ 3 consecutive menstrual cycles. Exclusion criteria: postmenopausal bleeding (> 1 year since the last menstrual period); irregular menses or intermenstrual bleeding; organic causes of heavy menstrual bleeding suspected or confirmed by experienced abdominal and transvaginal ultrasound after thorough general and gynaecological examination; iatrogenic (treatment‐related) causes of heavy menstrual bleeding (e.g. non‐progestogen‐releasing intrauterine contraceptive device, oral contraceptives, other hormonal drug use or anticoagulant agent); iron‐deficiency anaemia; history of chronic diseases known to interfere with menstrual bleeding or prevent the use of any of the listed drugs, e.g. previous or current thromboembolic disease. |
| Interventions | Combined contraceptive pill Medroxyprogesterone acetate Mefenamic acid |
| Outcomes | Menstrual blood loss at 3 months |
| Starting date | November 2016, Cairo, Egypt |
| Contact information | Ahmed Abbas, bmr90@hotmail.com |
| Notes |
Differences between protocol and review
In the 2012 update, we expanded the inclusion criteria to include the clinically important comparison NSAIDs combined with other medical treatment(s) versus other medical treatment(s).
Contributions of authors
The 2019 update: MB and AL updated the search, no new trials were added. MB made minor edits to the review to meet Cochrane standards. CF approved the final version.
The 2012 update: AL performed an updated search; one RCT was identified that met the inclusion criteria.
The 2007 update: CF updated the search; one published trial was identified as eligible for inclusion and one abstract, which is awaiting assessment.
AL registered the title, wrote the draft protocol and incorporated changes, performed searches, selected trials for inclusion, assessed quality and extracted data from the included trials, entered data, wrote the draft of the full review and incorporated changes from peer review.
For the original publication of the review, Cristina Augood and Kirsten Duckitt selected trials for inclusion into the review, assessed quality and extracted data from the included trials, and commented on the draft protocol and draft review.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, University of Auckland, Auckland, New Zealand.
External sources
Health Research Council, Auckland, New Zealand.
Declarations of interest
MB: none.
AL: none.
CF: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andersch 1988 {published data only}
- Andersch B, Milsom I, Rybo G. An objective evaluation of flurbiprofen and tranexamic acid in the treatment of idiopathic menorrhagia. Acta Obstetricia et Gynecologica Scandinavica 1988;67:645‐8. [DOI] [PubMed] [Google Scholar]
- Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel‐releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. American Journal of Obstetrics and Gynecology 1991;164:879‐83. [DOI] [PubMed] [Google Scholar]
Bonnar 1996 {published data only}
- Bonnar J, Sheppard BL. Treatment of menorrhagia during menstruation: randomised controlled trial of ethamsylate, mefenamic acid, and tranexamic acid. BMJ 1996;313:579‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 1987 {published data only}
- Cameron IT. Dysfunctional uterine bleeding. Bailliere's Clinical Obstetrics and Gynaecology 1989;2:315‐27. [DOI] [PubMed] [Google Scholar]
- Cameron IT, Leask R, Kelly RW, Baird DT. The effects of danazol, mefenamic acid, norethisterone and a progesterone‐impregnated coil on endometrial prostaglandin concentrations in women with menorrhagia. Prostaglandins 1987;34:99‐110. [DOI] [PubMed] [Google Scholar]
Cameron 1990 {published data only}
- Cameron IT, Haining R, Lumsden MA, Thomas VR, Smith SK. The effects of mefenamic acid and norethisterone on measured menstrual blood loss. Obstetrics & Gynecology 1990;76:85‐8. [PubMed] [Google Scholar]
- Smith SK, Haining RH, Reed‐Thomas V, Cameron IT. The diagnosis and treatment of menorrhagia. Silver Jubilee British Congress of Obstetrics and Gynaecology; 1989 Jul 4‐7; London (UK) 1989:62.
Chamberlain 1991 {published and unpublished data}
- Chamberlain G. Comparing treatments for menorrhagia. Nursing Times 1992;88:46. [PubMed] [Google Scholar]
- Chamberlain G, Freeman R, Price F, Kennedy A, Green D, Eve L. A comparative study of ethamsylate and mefenamic acid in dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology 1991;98:707‐11. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Freeman R, Price F, Kennedy A, Green D, Eve L. Raw data from RCT. Lorex Synthélabo Laboratory, UK.
Dockeray 1989 {published data only}
- Dockeray CJ, Sheppard BL, Bonnar J. Comparison between mefenamic acid and danazol in the treatment of established menorrhagia. British Journal of Obstetrics and Gynaecology 1989;96:840‐4. [DOI] [PubMed] [Google Scholar]
Fraser 1981 {published data only}
- Fraser IS, Pearse C, Shearman RP, Elliott PM, McIlveen J, Markham R. Efficacy of mefenamic acid in patients with a complaint of menorrhagia. Obstetrics & Gynaecology 1981;58(5):543‐51. [PubMed] [Google Scholar]
Fraser 1991 {published data only}
- Fraser IS, McCarron G. Randomised trial of 2 hormonal and 2 prostaglandin‐inhibiting agents in women with a complaint of menorrhagia. Australian & New Zealand Journal of Obstetrics & Gynaecology 1991;31(1):66‐70. [DOI] [PubMed] [Google Scholar]
Grover 1990 {published data only}
- Grover V, Usha R, Gupta U, Kalra S. Management of cyclical menorrhagia with prostaglandin synthetase inhibitor. Asia‐Oceania Journal of Obstetrics & Gynaecology 1990;16:255‐9. [DOI] [PubMed] [Google Scholar]
Hall 1987 {published data only}
- Hall P, MacLachlan N, Thorn N, Nudd MW, Taylor CG, Garrioch DB. Control of menorrhagia by the cyclo‐oxygenase inhibitors naproxen sodium and mefenamic acid. British Journal of Obstetrics and Gynaecology 1987;94:554‐8. [DOI] [PubMed] [Google Scholar]
Jaisamrarn 2006 {published and unpublished data}
- Jaisamrarn J, Sitavarin S, Suwikrom S, Kamolpornwijit W. Randomized trial of medical therapies for menorrhagia: clinical efficacy and effect on quality of life. XVIII FIGO World Congress of Gynecology and Obstetrics 2006;3:83. [Google Scholar]
Makarainen 1986 {published data only}
- Makarainen L, Ylikorkala O. Primary and myoma‐associated menorrhagia: role of prostaglandin and effects of ibuprofen. British Journal of Obstetrics and Gynaecology 1986;93:974‐8. [DOI] [PubMed] [Google Scholar]
Muggeridge 1983 {published data only}
- Muggeridge J, Elder MG. Mefenamic acid in the treatment of menorrhagia. Research and Clinical Forums 1983;5:83‐8. [Google Scholar]
Najam 2010 {published data only}
- Najam R, Agarwald D, Tyagi R, Singh S. Comparison of tranexamic acid with a combination of tranexamic acid and mefenamic acid in reducing menstrual blood loss in ovulatory dysfunctional uterine bleeding. Journal of Clinical and Diagnostic Research 2010;4:3020‐5. [Google Scholar]
Reid 2005 {published data only}
- Reid PC, Virtanen‐Kari S. Randomised comparative trial of the levonorgestrel intrauterine system and mefenamic acid for the treatment of idiopathic menorrhagia: a multiple analysis using total menstrual fluid loss, menstrual blood loss and pictorial blood loss assessment charts. British Journal of Obstetrics and Gynaecology 2005;112:1121‐5. [DOI] [PubMed] [Google Scholar]
Rybo 1981 {published data only}
- Nygren KG, Rybo G. Prostaglandins and menorrhagia. Acta Obstetricia et Gynecologica Scandinavica Supplement 1983;113:101‐3. [DOI] [PubMed] [Google Scholar]
- Rybo G, Nilsson S, Sikstrom B, Nygren KG. Naproxen in menorrhagia (letter). Lancet 1981;March:608‐9. [DOI] [PubMed] [Google Scholar]
Tsang 1987 {published data only}
- Tsang BK, Domingo MT, Spence JE, Garner PR, Dudley DK, Oxorn H. Endometrial prostaglandins and menorrhagia: influence of a prostaglandin synthetase inhibitor in vivo. Canadian Journal of Physiology and Pharmacology 1987;65:2081‐4. [DOI] [PubMed] [Google Scholar]
van Eijkeren 1992 {published data only}
- Eijkeren MA, Christiaens GC, Geuze HJ, Haspels AA, Sixma JJ. Effects of mefenamic acid on menstrual hemostasis in essential menorrhagia. American Journal of Obstetrics and Gynecology 1992;166:1419‐28. [DOI] [PubMed] [Google Scholar]
Ylikorkala 1986 {published data only}
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma‐induced menorrhagia. Obstetrics & Gynaecology 1986;68:10‐2. [PubMed] [Google Scholar]
References to studies excluded from this review
Famuyide 2017 {published data only}
- Famuyide A, Laughlin‐Tommaso S, Shazly S, Hall Long K, Breitkopf D, Weaver A. Medical therapy versus radiofrequency endometrial ablation in the initial treatment of heavy menstrual bleeding (iTOM Trial): a clinical and economic analysis. PloS One 2017;12(11):e0188176. [DOI: 10.1371/journal.pone.0188176] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2013 {published data only}
- Gupta J, Kai J, Middleton L, Pattison H, Gray R, Daniels J. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. New England Journal of Medicine 2013;368:128‐37. [CRSREF: 3289371] [DOI] [PubMed] [Google Scholar]
Martinez Alcala 1979 {published data only}
- Martinez Alcala FO, Casanova Alvarez N, Manzanilla Sevilla R, Gonzalez Iniguez R, Martinez Reyes YP. Efficacy and tolerability of mefenamic acid in dysfunctional uterine bleeding [Eficacia y tolerabilidad del acido mefenamico en la hipermenorrea disfuncional]. La Prensa Médica Mexicana 1979;44(11‐12):295‐8. [PubMed] [Google Scholar]
Naafe 2018 {published data only}
- Naafe M, Kariman N, Keshavarz Z, Khademi N, Mojab F, Mohammadbeigi A. Effect of hydroalcoholic extracts of capsella bursa‐pastoris on heavy menstrual bleeding: a randomized clinical trial. Journal of Alternative and Complementary Medicine 2018;24(7):694‐700. [DOI] [PubMed] [Google Scholar]
Vargyas 1987 {published data only}
- Vargyas JM, Campeau JD, Mishell DR. Treatment of menorrhagia with meclofenamate sodium. American Journal of Obstetrics & Gynecology 1987;157:944‐50. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02943655 {published data only}
- NCT02943655. Treatment of heavy and/or prolonged menstrual bleeding without organic cause. clinicaltrials.gov/ct2/show/NCT02943655 (first received 25 October 2016).
Additional references
Chimbira 1980
- Chimbira TH, Anderson AB, Turnbull AC. Relation between measured menstrual blood loss and patient's subjective assessment of loss, duration of bleeding, number of sanitary towels used, uterine weight and endometrial surface area. British Journal of Obstetrics and Gynaecology 1980;87:603‐9. [DOI] [PubMed] [Google Scholar]
Clarke 1995
- Clarke A, Black N, Rowe P, Mott S, Howie K. Indications for and outcomes of total abdominal hysterectomy for benign disease: a prospective cohort study. British Journal of Obstetrics and Gynaecology 1995;102:611‐20. [DOI] [PubMed] [Google Scholar]
Cole 1971
- Cole S, Billewicz W, Thomson A. Sources of variation in menstrual blood loss. Journal of Obstetrics and Gynaecology of the British Commonwealth 1971;78(10):933‐9. [DOI] [PubMed] [Google Scholar]
Fraser 1983
- Fraser IS, McCarron G, Markham R, Robinson M, Smyth E. Long term treatment of menorrhagia with mefenamic acid. Obstetrics & Gynaecology 1983;61:109‐12. [PubMed] [Google Scholar]
Fraser 1984
- Fraser IS, McCarron G, Markham R. A preliminary study of factors influencing perception of menstrual blood loss volume. American Journal of Obstetrics and Gynecology 1984;149:788‐93. [DOI] [PubMed] [Google Scholar]
Fraser 1985
- Fraser IS, McCarron G, Markham R, Resta T. Blood and total fluid content of menstrual discharge. Obstetrics & Gynecology 1985;65:194‐8. [PubMed] [Google Scholar]
Fraser 2001
- Fraser IS, Warner P, Marantos PA. Estimating menstrual blood loss in women with normal and excessive menstrual fluid volume. Obstetrics & Gynecology 2001;98:806‐14. [DOI] [PubMed] [Google Scholar]
Fraser 2008
- Fraser IS, Robert JP, Kouides PA, Lukes AS. A benefit‐risk review of systemic haemostatic agents. Part 2: in excessive or heavy menstrual bleeding. Drug Safety 2008;31(4):275‐82. [DOI] [PubMed] [Google Scholar]
Fraser 2014
- Fraser I, Mansour D, Breymann C, Hoffman C et cols. Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey. Internationa Journal of Gynecology and Obstetrics 2014;128(3):196‐200. [DOI: ] [DOI] [PubMed] [Google Scholar]
Frick 2009
- Frick KD, Clark MA, Steinwachs DM, Langenberg P, Stovall D, Munro MG, et al. Financial and quality‐of‐life burden of dysfunctional uterine bleeding among women agreeing to obtain surgical treatment. Women's Health Issues 2009;19(1):70‐8. [DOI] [PubMed] [Google Scholar]
Gath 1982
- Gath D, Cooper P, Day A. Hysterectomy and psychiatric disorder. Levels of psychiatric morbidity before and after hysterectomy. British Journal of Psychiatry 1982;140:335‐40. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014.
Hagenfeldt 1987
- Hagenfeldt K. The role of prostaglandins and allied substances in uterine haemostasis. Contraception 1987;36(1):23‐35. [DOI] [PubMed] [Google Scholar]
Hallberg 1966
- Hallberg L, Hogdahl AM, Nilsson L, Rybo G. Menstrual blood loss – a population study. Acta Obstetricia et Gynecologica Scandanavica 1966;45:320‐51. [DOI] [PubMed] [Google Scholar]
Haynes 1977
- Haynes PJ, Hodgson H, Anderson AB, Turnbull AC. Measurement of menstrual blood loss in patients complaining of menorrhagia. British Journal of Obstetrics and Gynaecology 1977;84(10):763‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higham 1990
- Higham JM, O'Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. British Journal of Obstetrics and Gynaecology 1990;97:734‐39. [DOI] [PubMed] [Google Scholar]
Kadir 2005
- Kadir RA, Lukes AS, Kouides PA, Fernandez H, Goudemand J. Management of excessive menstrual bleeding in women with hemostatic disorders. Fertility and Sterility 2005;84(5):1352‐9. [DOI] [PubMed] [Google Scholar]
Liu 2007
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health‐related quality of life, work impairment and healthcare costs and utilisation in abnormal uterine bleeding. Value in Health 2007;10(8):183‐94. [DOI] [PubMed] [Google Scholar]
Lopez 1991
- Lopez BA, Buckley S, Rees CM, Marshall JM. Meclofenamate inhibits prostaglandin E binding and adenylyl cyclase activation in human endometrium. Journal of Endocrinology 1991;129(3):439‐45. [DOI] [PubMed] [Google Scholar]
Munro 2011
- Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM‐COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynaecology and Obstetrics 2011;113(1):3‐13. [DOI] [PubMed] [Google Scholar]
Newton 1977
- Newton J, Barnard G, Collins W. A rapid method for measuring menstrual blood loss using automatic extraction. Contraception 1977;16:269‐82. [Google Scholar]
NICE 2007
- National Institute for Health and Clinical Excellence (NICE). Heavy menstrual bleeding. guidance.nice.org.uk/CG44/ (accessed 21 November 2012).
NICE 2018
- National Institute for Health and Clinical Excellence (NICE). Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88 Accessed August 2019. [https://www.nice.org.uk/guidance/ng88] [PubMed]
Rees 1987
- Rees MC, DiMarzo V, Tippins JR, Morris HR, Turnbull AC. Leukotriene release by endometrium and myometrium throughout the menstrual cycle in dysmenorrhoea and menorrhagia. Journal of Endocrinology 1987;113:291‐5. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roy 2004
- Roy SN, Bhattacharya S. Benefits and risks of pharmacological agents used for the treatment of menorrhagia. Drug Safety 2004;27(2):75‐90. [DOI] [PubMed] [Google Scholar]
Smith 1981
- Smith SK, Abel MH, Kelly RW, Baird DT. Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology 1981;88:434‐42. [DOI] [PubMed] [Google Scholar]
Stewart 2001
- Stewart A, Cummins C, Gold L, Jordan R, Phillips W. The effectiveness of the levonorgestrel‐releasing intrauterine system in menorrhagia: a systematic review. British Journal of Obstetrics and Gynecology 2001;108(1):74‐86. [DOI] [PubMed] [Google Scholar]
Warner 2004a
- Warner PE, Critchley HO, Lumsden MA, Campbell‐Brown M, Douglas A, Murray GD. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow up data. American Journal of Obstetrics and Gynecology 2004;190:1216‐23. [DOI] [PubMed] [Google Scholar]
Warner 2004b
- Warner PE, Critchley HO, Lumsden MA, Campbell‐Brown M, Douglas A, Murray GD. Menorrhagia II: is the 80‐mL blood loss criterion useful in the management of complaint of menorrhagia?. American Journal of Obstetrics and Gynecology 2004;190:1224‐9. [DOI] [PubMed] [Google Scholar]
Willman 1976
- Willman EA, Collins WD, Clayton SC. Studies on the involvement of prostaglandins in uterine symptomatology and pathology. British Journal of Obstetrics and Gynaecology 1976;83:337‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lethaby 1997
- Lethaby A, Augood C, Duckitt K, Farquhar C. Nonsteroidal anti‐inflammatory drugs vs either placebo or any other medical treatment for menorrhagia. Cochrane Database of Systematic Reviews 1997, Issue 3. [DOI: 10.1002/14651858.CD000400] [DOI] [Google Scholar]
Lethaby 1998
- Lethaby A, Augood C, Duckitt K. Nonsteroidal anti‐inflammatory drugs vs either placebo or any other medical treatment for heavy menstrual bleeding (menorrhagia). Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD000400] [DOI] [Google Scholar]
Lethaby 2007
- Lethaby A, Augood C, Duckitt K, Farquhar C. Nonsteroidal anti‐inflammatory drugs for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000400.pub2] [DOI] [PubMed] [Google Scholar]
Lethaby 2012
- Lethaby A, Duckitt K, Farquhar C. Non‐steroidal anti‐inflammatory drugs for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD000400.pub3] [DOI] [PubMed] [Google Scholar]


