Abstract
Background
MicroRNAs (miRNAs) are noncoding RNA molecules heavily involved in human tumors, in which few of them circulating the human body. Finding a tumor-associated signature of miRNA, that is, the minimum miRNA entities to be measured for discriminating both different types of cancer and normal tissues, is of utmost importance. Feature selection techniques applied in machine learning can help however they often provide naive or biased results.
Results
An ensemble feature selection strategy for miRNA signatures is proposed. miRNAs are chosen based on consensus on feature relevance from high-accuracy classifiers of different typologies. This methodology aims to identify signatures that are considerably more robust and reliable when used in clinically relevant prediction tasks. Using the proposed method, a 100-miRNA signature is identified in a dataset of 8023 samples, extracted from TCGA. When running eight-state-of-the-art classifiers along with the 100-miRNA signature against the original 1046 features, it could be detected that global accuracy differs only by 1.4%. Importantly, this 100-miRNA signature is sufficient to distinguish between tumor and normal tissues. The approach is then compared against other feature selection methods, such as UFS, RFE, EN, LASSO, Genetic Algorithms, and EFS-CLA. The proposed approach provides better accuracy when tested on a 10-fold cross-validation with different classifiers and it is applied to several GEO datasets across different platforms with some classifiers showing more than 90% classification accuracy, which proves its cross-platform applicability.
Conclusions
The 100-miRNA signature is sufficiently stable to provide almost the same classification accuracy as the complete TCGA dataset, and it is further validated on several GEO datasets, across different types of cancer and platforms. Furthermore, a bibliographic analysis confirms that 77 out of the 100 miRNAs in the signature appear in lists of circulating miRNAs used in cancer studies, in stem-loop or mature-sequence form. The remaining 23 miRNAs offer potentially promising avenues for future research.
Electronic supplementary material
The online version of this article (10.1186/s12859-019-3050-8) contains supplementary material, which is available to authorized users.
Keywords: MicroRNAs, miRNA, Feature selection, Machine learning, Classifiers, Dataset
Background
Cancer is difficult to diagnose and classify at early stages, and is one of the top leading causes of death worldwide [1]. Therefore, several attempts have been made to identify possible biomarkers for cancer detection. MicroRNAs (miRNAs) represent a class of small noncoding RNA molecules, with a critical role in the post-transcriptional regulation of gene expression. miRNAs also act on several cellular processes, such as cell differentiation, cell cycle progression, and apoptosis. Additionally, in tumors, some miRNAs can function as oncogenes, while others suppress tumors [2]. Succeeding the earliest evidence of miRNA involvement in human cancer by Croce et al. [3], various studies have demonstrated that miRNA expressions are deregulated in human cancer through a variety of mechanisms [4]. Since ectopic modulation of specific miRNAs compromise the hallmarks of cancer, several efforts have been spent to generate scaffold-mediated miRNA-based delivery systems trying to demonstrate the potential of miRNA-mediated therapies.
In comparison to invasive methods currently used for cancer diagnosis, there is an ongoing debate on the use of circulating miRNAs as possible biomarkers due to the fact that they can be detected directly from biological fluids, such as blood, urine, saliva and pleural fluid [5]. MiRNAs possess other qualities of good candidate biomarkers such as: a) they are useful for the identification of cancer types, b) their availability of high-quality measurement techniques for miRNAs and c) they present good conservation between practical and preclinical models [6].
Several studies have shown the properties of miRNAs as oncogenes and tumor suppressors genes [7–9]. Since then, techniques such as microarray (Affymetrix, Agilent) and sequencing techniques (Illumina), have been proposed for their identification [10]. In the context of increasing availability of data, it is of utmost practical importance to build databases of miRNA expressions data for cancer research [11–13] and to extract features that could be used as cancer biomarkers [14–16]. For example, the expression levels of miRNA hsa-miR-21 change for different cancer types such as: squamous cell lung carcinoma [17], astrocytoma [18], breast cancer [19], and gastric cancer [20]. Following this idea, the scientific community is currently looking for miRNA signatures (a subset of miRNAs), representing the minimal number of miRNAs to be measured for discriminating between different stages and types of cancer.
Thousands of miRNAs have been identified, and currently miRBase (v22.1) contains 1917 stem-loop sequences, and 2657 mature sequences for human microRNA [13]. Although a classification of cancer tumor type is possible using isomirs [21], not all of the miRNAs listed are available in every study, and only a few of them have been shown to work as circulating biomarkers [6]. Obtaining a minimal list of miRNAs able to correctly classify tumors is of utmost practical importance, because it would reduce the measurements needed and improve the likelihood of validation across multiple studies.
Several approaches in the literature propose the use of machine learning techniques for feature selection involving miRNAs. For example, feature selection for identifying miRNA targets [22], for prediction of specific biomarkers for tumor origin [23] and to learn subset of features for tumor classification [24]. In this study, the objective was to use feature selection and to uncover a small miRNAs signature with the aim to correctly classify cancer tumor types, and distinguish between normal and tumor tissue reducing the necessary features by an order of magnitude.
We propose an ensemble feature selection method, starting from a subset of The Cancer Genome Atlas dataset (TCGA) [25], containing 8023 cases, with 28 different types of cancer, and 1046 different stem-loop miRNA expressions (miRBase V161, summarized in Table 10). Typically, classifiers trained on a dataset do not use the whole set of available features to separate classes, but only a subset which could be ordered by relative importance, with a different meaning given to the list by the specific technique, pushing for simpler models. Using 8 state-of-the-art classifiers implemented in the scikit-learn toolbox [26], the most relevant miRNAs are extracted to be used as features for cancer classification. The top k features in the list are then evaluated as a potential reduced signature for classification. In this work, after preliminary tests, we select k=100 to reduce the original features by an order of magnitude. Because other feature selection methods require the user to specify a desired number of features, this also allows for a fair and meaningful comparison with these methods.
Table 10.
Summary of the TCGA dataset used in the study
| Tumor Type | Acronym | Tumor Tissue | Normal Tissue | Class |
|---|---|---|---|---|
| Adrenocortical carcinoma | ACC | 80 | 0 | 0 |
| Bladder Urothelial Carcinoma | BLCA | 411 | 19 | 1 |
| Breast invasivex carcinoma | BRCA | 777 | 87 | 2 |
| Cervical squamous cell carcinoma | CESC | 306 | 3 | 3 |
| Cholangiocarcinoma | CHOL | 36 | 9 | 4 |
| Lymphoid Neoplasm Diffuse Large B-cell Lymphoma | DLBC | 47 | 0 | 5 |
| Esophageal carcinoma | ESCA | 187 | 13 | 6 |
| Head and Neck squamous cell carcinoma | HNSC | 487 | 44 | 7 |
| Kidney Chromophobe | KICH | 66 | 25 | 8 |
| Kidney renal clear cell carcinoma | KIRC | 260 | 71 | 9 |
| Kidney renal papillary cell carcinoma | KIRP | 291 | 34 | 10 |
| Lower Grade Glioma | LGG | 528 | 0 | 11 |
| Liver hepatocellular carcinoma | LIHC | 374 | 50 | 12 |
| Lung adenocarcinoma | LUAD | 458 | 46 | 13 |
| Lung squamous cell carcinoma | LUSC | 341 | 45 | 14 |
| Mesothelioma | MESO | 86 | 0 | 15 |
| Pancreatic adenocarcinoma | PAAD | 154 | 4 | 16 |
| Pheochromocytoma and Paraganglioma | PCPG | 184 | 3 | 17 |
| Prostate adenocarcinoma | PRAD | 494 | 52 | 18 |
| Sarcoma | SARC | 260 | 0 | 19 |
| Skin Cutaneous Melanoma | SKCM | 450 | 2 | 20 |
| Stomach adenocarcinoma | STAD | 399 | 45 | 21 |
| Testicular Germ Cell Tumors | TGCT | 156 | 0 | 22 |
| Thyroid carcinoma | THCA | 513 | 59 | 23 |
| Thymoma | THYM | 124 | 2 | 24 |
| Uterine Corpus Endometrial Carcinoma | UCEC | 417 | 21 | 25 |
| Uterine Carcinosarcoma | UCS | 57 | 0 | 26 |
| Uveal Melanoma | UVM | 80 | 0 | 27 |
| Total | 8023 | 634 |
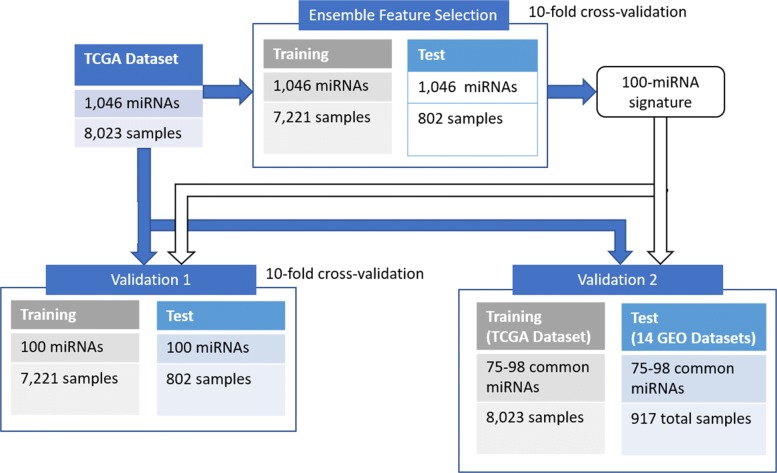
The obtained 100-miRNA signature is first tested to classify the initial TCGA dataset, and later applied on 14 Gene Expression Omnibus (GEO) datasets obtained with different platforms (Affymetrix Multispecies Array miRNA-1, miRNA-2 and miRNA-3, Illumina 2000, and Agilent-021827 Human miRNA Microarray V3), for different cancer tumor types (Prostate, Liver, Breast, Esophageal, Head and Neck Squamous and Lung). A summary of this validation is presented in Fig. 1. Furthermore, the proposed methodology is compared to popular feature selection methods in bioinformatics, such as Univariate Feature Selection, Recursive Feature Elimination, Genetic Algorithms, Least Absolute Shrinkage and Selection Operator, Random Selection, Elastic Net and Ensemble Feature Selection with Complete Linear Aggregation. Next, we use the same signature to try to distinguish molecular subtypes in breast cancer, both for the TCGA dataset and a set of GEO datasets. Finally, the 100 miRNAs included in the signature are evaluated through a meta-analysis based on the medical literature. Because this meta-analysis reveals known relationships between features selected by our approach, relative to the type of cancer considered, it has the potential to yield insight into the biological processes and relationships combinedly affecting miRNAs and cancer.
Fig. 1.
Summary of the different datasets and their use in the experiments
Results
Feature selection and validation on the tCGA dataset
Table 1 compares the classification accuracy on a 10-fold cross-validation for each classifier, using the full 1046 features, and then employing the reduced 100-miRNA signature. It is interesting to notice how the accuracy is, for most cases, unchanged, providing empirical evidence that a 100-miRNA signature is enough to obtain good classification results, with a small statistically significant (T-test, p<0.05) difference of 1.4%.
Table 1.
Accuracy of classifiers used in the experiments on the TCGA dataset
| Classifier | Accuracy (10-fold CV) | |||||
|---|---|---|---|---|---|---|
| 1046 Features | 100 Features | Hyper parameters | Feature selection method | |||
| avg | std | avg | std | |||
| Gradient Boosting | 0.9398 | 0.0076 | 0.9359 | 0.0086 | 300 predictors | Decision Trees |
| Random Forest | 0.9351 | 0.0071 | 0.9324 | 0.0073 | 300 predictors | Decision Trees |
| Logistic Regression | 0.9178 | 0.0096 | 0.9237 | 0.0067 | - | Coefficients |
| Passive Aggressive | 0.9117 | 0.0104 | 0.8831 | 0.0115 | - | Coefficients |
| SGD | 0.91 | 0.0074 | 0.9035 | 0.0152 | - | Coefficients |
| SVC | 0.9211 | 0.0122 | 0.9154 | 0.0065 | Linear kernel | Coefficients |
| Ridge | 0.8971 | 0.0138 | 0.8305 | 0.0062 | - | Coefficients |
| Bagging | 0.9151 | 0.0120 | 0.9110 | 0.0077 | 300 predictors | Decision Trees |
| Average | 0.918463 | - | 0.9044 | - | - | - |
In the case a classifier is not using standard values for its hyperparameters, the relevant variations are summarized in the corresponding column
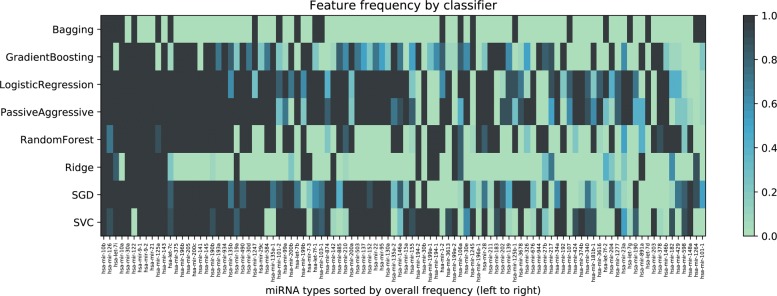
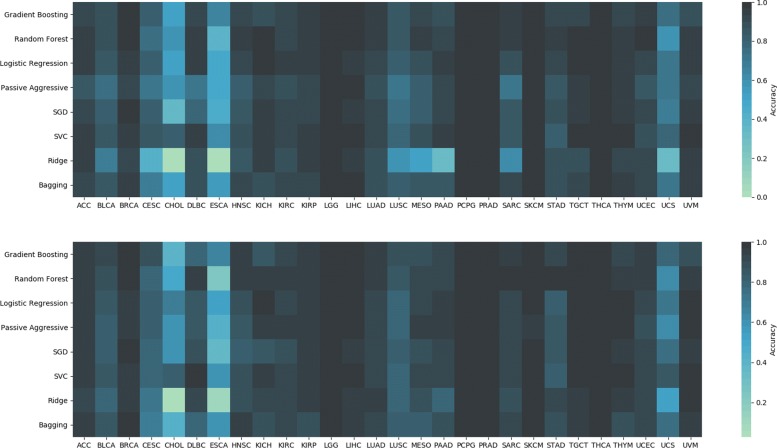
Figure 2 shows a heatmap comparing the relative frequency of the overall top 100 most frequent miRNA features, for each considered classifier. As expected, not all classifiers used the same features to separate the types of cancer, and thus, evaluating their consensus is more robust than just relying upon a single algorithm, as it is commonly accepted in the field of machine learning [27]. It is interesting to notice that while the most common biomarkers appear among the top for most classifier, others make use of only a few. For example, Bagging and Ridge do not use the vast majority of the features exploited by other techniques to discriminate between classes. A further difference between the two classifiers is that features used by Bagging that also appear in the top 100 are clearly important for the classifier, being used in almost 100% of its 10 runs; while it is noticeable how Ridge probably bases its discrimination on features that do not appear among the top 100. This would also explain why Ridge is the only algorithm that presents a decrease in performance when using the 100-miRNA signature. It’s important to note that, while the results emerging from the heatmap suggest that this is indeed the case, Ridge’s decision boundaries should be analyzed more in-depth, for each class and multiple instances, in order to have absolute certainty, a task that is outside of the scope of the current work. Figure 3 shows the difference between 1046 features and 100 features for each cancer type and classifier.
Fig. 2.
Heatmap with the frequency of the overall top 100 most frequent features, divided by classifier. Features are sorted from overall most to least frequent, from left to right, using information from the whole ensemble. For example, the most frequent is mir-10b, that is considered important by all classifiers. Color intensity is computed using information from instances of the same classifier, only. This shows the different importance that different classifiers assign to each feature
Fig. 3.
Heatmap of the accuracy by cancer type, by classifier using the 1046 features (top) and the 100-miRNA signature (bottom)
Normal vs tumor tissue classification
We compared Tumor Tissue (TT) vs Normal Tissue (NT) in a 10-cross fold validation, using stratified cross-validation to maintain the proportions for the two classes inside the folds. The global score and the classification accuracy by class are reported in Table 2. All of the classifiers have fair quality for differentiating between normal tissue and tumor tissue, except Ridge, which is more sensitive to the unbalanced number of examples.
Table 2.
Accuracy for each classifier in a 10-fold cross-validation for the comparison between Tumor Tissue (TT) and Normal Tissue (NT) for 1046 and 100 features
| Classifier | 100-NT | 100-TT | 1046-NT | 1046-TT | 100-Global | 1046-Global |
|---|---|---|---|---|---|---|
| Gradient Boosting | 0.8612 | 0.9944 | 0.8707 | 0.9950 | 0.9846 | 0.9859 |
| Random Forest | 0.8091 | 0.9978 | 0.7256 | 0.9985 | 0.9839 | 0.9785 |
| Logistic Regression | 0.8423 | 0.9908 | 0.8659 | 0.9764 | 0.9799 | 0.9683 |
| Passive Aggressive | 0.7177 | 0.9798 | 0.8123 | 0.9728 | 0.9606 | 0.9611 |
| SGD | 0.8060 | 0.9902 | 0.7445 | 0.9936 | 0.9767 | 0.9754 |
| SVC(linear) | 0.8517 | 0.9892 | 0.8218 | 0.9771 | 0.9791 | 0.9657 |
| Ridge | 0.2997 | 0.9981 | 0.5994 | 0.9923 | 0.9470 | 0.9635 |
| Bagging | 0.8028 | 0.9953 | 0.7792 | 0.9966 | 0.9812 | 0.9807 |
Comparison to established feature selection methods
Several feature selection techniques have been proposed for microarray data [28]. The most effective approaches include Univariate Feature Selection (UFS), Recursive Feature Elimination (RFE), Elastic Net (EN), Genetic Algorithms (GALGO), Least Absolute Shrinkage and Selection Operator (LASSO) and Ensemble Feature Selection with Complete Linear Aggregation (EFS-CLA). UFS aims at finding the best features, scoring them using univariate statistical tests, such as the ANOVA F-value [29], and ultimately taking the k features with the highest scores. RFE runs several times a machine learning algorithm capable of scoring features, such as SVC, iteratively removing the feature with the lowest score [30] until it reaches the user-specified k features. EN simply runs the machine learning algorithm Elastic Net [31], and takes the k highest-scored features. As Elastic Net is trying to balance accuracy and weight size in a linear model, exploiting L1 and L2 regularization, it is a popular choice for feature selection in bio-informatics [32, 33], because it tends to create sparse models with few weights different from zero. LASSO is a regression analysis method, performing variable selection and regularization to improve prediction accuracy and interpretability of the statistical model it produces [34], so it can be easily used for feature selection, only. All considered feature selection methods are implemented in the machine learning package scikit-learn, already used in the previous experiments. GALGO is a genetic algorithms-based feature selection library in R that ranks the features using several calls to a classifier and choosing the features that appear the most after evolving a subset several times [35]. EFS-CLA is a method that uses instances of SVM with several calls to a subsample of the data, ranks the features by weight value and reduces a percentage at each iteration [36].
As some of these techniques require the user to specify the number of features k to be taken, to provide a comparison with the approach presented in this paper, we have selected k=100 features using all the formerly described feature selection methods and compared classification accuracy on the considered classifiers with a 10-fold cross validation. For RFE, we have decided to use SVC, as not only it is commonly adopted for feature selection in bioinformatics [30, 37], but also represents a good compromise between accuracy and speed of convergence on our specific dataset. For EN, we have chosen the ElasticNetCV scikit-learn method, which exploits a 3-fold cross-validation to automatically adapt the internal parameter α, balancing the importance of L1 and L2 regularization in the model. For the same reasons, the LassoCV scikit-learn method is selected for LASSO. For EFS-CLA, we use percentage of reduction E=20%, 40 as SVM calls per step, and k =100. Finally, we add a random selection of 100 features, as a baseline reference to portray the efficiency of the feature selection algorithms.
From the results presented in Table 3, it is immediately clear that the 100 features selected by UFS are much less informative than the ones found by the proposed approach. RFE performs better, especially when considering SVC as the classifier used for the cross validation, but overall the performance for the other classifiers is lower. It must also be noted that, among all the methods, RFE is the most computationally expensive, as it calls the considered classifier, SVC in this case, N−k=1,046−100=946 times, where N is the original number of features. All feature selection algorithms, as expected, perform much better than the baseline random selection of features.
Table 3.
Comparison between different feature selection techniques and the proposed ensemble method for k=100, on the TCGA dataset
| Classifier | Random | GALGO | EFS-CLA | UFS | EN | LASSO | RFE | EFS |
|---|---|---|---|---|---|---|---|---|
| Gradient Boosting | 0.8588 | 0.8782 | 0.8871 | 0.9028 | 0.9208 | 0.9315 | 0.9309 | 0.9359 |
| Random Forest | 0.8515 | 0.8787 | 0.8824 | 0.8929 | 0.9224 | 0.9341 | 0.9288 | 0.9324 |
| Logistic Regression | 0.8015 | 0.8295 | 0.8832 | 0.8813 | 0.8988 | 0.8996 | 0.9088 | 0.9237 |
| Passive Aggressive | 0.6986 | 0.7235 | 0.8111 | 0.8091 | 0.8406 | 0.8424 | 0.8506 | 0.8831 |
| SGD | 0.7278 | 0.764 | 0.8446 | 0.8334 | 0.8649 | 0.8648 | 0.8824 | 0.9035 |
| SVC | 0.8077 | 0.8348 | 0.8706 | 0.885 | 0.9049 | 0.9008 | 0.9103 | 0.9154 |
| Ridge | 0.6534 | 0.6614 | 0.7422 | 0.7504 | 0.7753 | 0.7751 | 0.7954 | 0.8305 |
| Bagging | 0.822 | 0.8382 | 0.8562 | 0.8719 | 0.8889 | 0.9078 | 0.9061 | 0.911 |
| Global Average | 0.7777 | 0.8010 | 0.8472 | 0.8534 | 0.8771 | 0.8820 | 0.8892 | 0.9044 |
| Calls to Classifier | - | 60,000 | 480 | - | - | 10 | 946 | 80 |
A qualitative analysis of the features selected by each method shows that the highest-scoring ones are easily found by all considered approaches. In particular, from the 100 features found by our approach, 8 are in common with Random, 11 with GALGO, 29 with EFS-CLA, 38 are common to the group obtained through UFS, 44 are shared with the group found by LASSO, 48 again are found by EN, and 54 are in common with RFE.
Cross-Platform validation on gEO datasets
As different datasets present distinctive sets of miRNAs, it is important to assess the performance of the signature we identified on unseen data. Using the methodology previously described, the proposed approach is validated on the 14 GEO datasets. Each run of a classifier on a dataset was repeated 10 times, to compensate possible random elements that appear during the training phase of specific algorithms, e.g. RandomForest. It is worth noticing how this validation presents considerable challenges. As we are dealing with different platforms, not all of the 100 features in the signature were available everywhere. For most GEO datasets 98 were available, while for GSE62182 featured 75 of them. Furthermore, despite the transformation needed to bring the samples of the GEO datasets in the TCGA dataset space, samples measured by platforms used in the GEO datasets might prove particularly difficult to tackle for classifiers trained on TCGA samples, as most GEO datasets use microarray technology while TCGA uses sequencing. The properties of the used GEO datasets are summarized in Table 4.
Table 4.
Summary of the used GEO datasets, and the number of features in common with our 100-miRNA signature
| Dataset ID | Platform | Tumor Type | #Samples | Total Feats. | Common Feats. | Reference |
|---|---|---|---|---|---|---|
| GSE34496 | GPL8786 | HNSC | 44 | 847 | 98 | - |
| GSE36802 | GPL8786 | PRAD | 21 | 847 | 98 | [38] |
| GSE67138 | GPL8786 | LIHC | 57 | 847 | 98 | - |
| GSE67139 | GPL8786 | LIHC | 115 | 847 | 98 | - |
| GSE45604 | GPL14613 | PRAD | 50 | 2143 | 98 | [39] |
| GSE48088 | GPL14613 | BRCA | 33 | 2143 | 98 | [40] |
| GSE55856 | GPL14613 | ESCA | 108 | 2143 | 98 | [41] |
| GSE86277 | GPL14613 | BRCA | 72 | 2143 | 98 | [42] |
| GSE116182 | GPL14613 | LIHC | 64 | 2143 | 98 | - |
| GSE86278 | GPL16384 | BRCA | 49 | 3,242 | 98 | [42] |
| GSE86281 | GPL16384 | BRCA | 50 | 3,242 | 98 | [42] |
| GSE31164 | GPL10850 | LIHC | 110 | 851 | 98 | [43] |
| GSE105134 | GPL10850 | BRCA | 50 | 851 | 98 | - |
| GSE62182 | GPL11154 | LUAD | 94 | 3,242 | 75 | [44] |
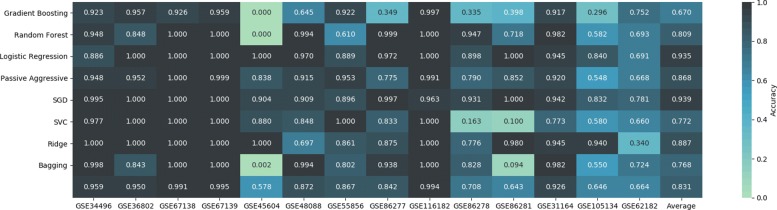
Figure 4 shows the outcomes of the validation for all classifiers. In spite of the difficulties, most algorithms yielded good classification results, with Logistic and SGD in particular featuring over 93% average accuracy on all GEO datasets. Several classifiers, on the other hand, show poor performance on specific datasets, probably due to the way their decision boundaries for that specific class were learned on the TCGA dataset. In this sense, dataset GSE45604 proves to be the overall hardest to classify correctly for most algorithms. GSE86277,GSE86278 and GSE86281, deal with different molecular subtypes of BRCA, that could explain some of the performance issues. Finally the average performance in GSE62182, is because the classifiers have problems differentiating LUAD and LUSC. In general, however, different algorithms seem to have difficulties for different classes and datasets, which suggests that an ensemble approach for classification could compensate local issues.
Fig. 4.
Results with the 100 selected features in the GEO datasets, using a 10-fold cross-validation. From the average accuracy and standard deviation, SGD proves to be significantly better than the rest using a Kolmogorov-Smirnov test (p<0.05)
To the best of our knowledge, the most similar work in literature that we can compare our results to is Telonis et al. [21], where isoform quantification was adopted to classify three of the GEO datasets used in this study (GSE36802, GSE67138, GSE67139), training SVC on a TCGA-derived dataset. For GSE36802, [21] reports an accuracy of 76%, that is surpassed by all of the classifiers. Considering GSE67138, for which an accuracy of 91% is reported, all the algorithms in our case perform better. Finally, for GSE67139, a 96% accuracy, again all the algorithms outperform that value. It must be noted, however, that even this comparison is made difficult by differences in how data was treated: for example, [21] reduced the number of classes to 6 and tested on 4 different types of tumors. In our study, we keep all 28 classes for testing.
Tumor subtype
To further test our approach, we use the 100-miRNA signature to classify tumor subtypes. As a comparison with GEO datasets is important for our validation, we select molecular subtype in breast cancer (BRCA), as it’s the only tumor class for which molecular subtype information is available in the GEO datasets. From the information in [45, 46], we are able to label 764 of the 777 BRCA samples in the TCGA dataset in 5 different subtypes (Luminal A, Luminal B, Triple-negative/basal-like, HER2-enriched and Normal-like). More information on the subtypes can be found in [47]. Next, we calculate the accuracy in a 10-fold cross validation for the 1046 TCGA features and the 100-miRNA signature, with results reported in Tables 5 and 6 respectively.
Table 5.
Molecular subtype classification accuracy of Breast Cancer for the 1046 features
| Normal | LumA | LumB | TNBC | Her2 | Global | |
|---|---|---|---|---|---|---|
| #Samples | 33 | 399 | 139 | 135 | 58 | 764 |
| Gradient Boosting | 0.1818 | 0.9348 | 0.5396 | 0.9333 | 0.5172 | 0.7987 |
| Random Forest | 0.0606 | 0.9724 | 0.4532 | 0.9630 | 0.0345 | 0.7657 |
| Logistic Regression | 0.1212 | 0.8747 | 0.5540 | 0.9259 | 0.4483 | 0.7606 |
| Passive Aggressive | 0.1515 | 0.8622 | 0.5612 | 0.9111 | 0.4483 | 0.7539 |
| SGD | 0.3030 | 0.9073 | 0.4604 | 0.9556 | 0.4655 | 0.7752 |
| SVC | 0.2727 | 0.8797 | 0.5252 | 0.9185 | 0.5345 | 0.7697 |
| Ridge | 0.1515 | 0.7293 | 0.4317 | 0.3704 | 0.2759 | 0.5524 |
| Bagging | 0.3333 | 0.9298 | 0.5108 | 0.9704 | 0.4310 | 0.7973 |
| Average | 0.1970 | 0.8863 | 0.5045 | 0.8685 | 0.3944 | 0.7467 |
Table 6.
Molecular subtype classification accuracy of Breast Cancer for the 100 features
| Normal | LumA | LumB | TNBC | Her2 | Global | |
|---|---|---|---|---|---|---|
| #Samples | 33 | 399 | 139 | 135 | 58 | 764 |
| Gradient Boosting | 0.2424 | 0.9248 | 0.5324 | 0.9333 | 0.5517 | 0.7975 |
| Random Forest | 0.2121 | 0.9599 | 0.4029 | 0.9704 | 0.2069 | 0.7712 |
| Logistic Regression | 0.2727 | 0.8997 | 0.4892 | 0.9037 | 0.5517 | 0.7727 |
| Passive Aggressive | 0.3939 | 0.8546 | 0.4460 | 0.8667 | 0.5000 | 0.7358 |
| SGD | 0.4545 | 0.8897 | 0.4460 | 0.8444 | 0.4310 | 0.7475 |
| SVC | 0.5152 | 0.8446 | 0.5108 | 0.9037 | 0.5517 | 0.7581 |
| Ridge | 0.0606 | 0.9474 | 0.4388 | 0.8593 | 0.3966 | 0.7594 |
| Bagging | 0.2727 | 0.9173 | 0.4964 | 0.9481 | 0.3793 | 0.7777 |
| Average | 0.3030 | 0.9048 | 0.4703 | 0.9037 | 0.4461 | 0.7650 |
The best classification results are obtained for subtypes Triple-Negative Breast Cancer (TNBC) and Luminal A (LumA), due to the scarcity of samples for other subtypes (especially Normal and Her2). Luminal B (LumB) presents considerable similarities to LumA, and the classifiers have difficulty separating the two subtypes using the data at our disposal. For these reasons, and the practical concern that TNBC is the subtype of BRCA with the worst prognosis, we decide to tackle the issue as a binary classification problem, separating TNBC from the other classes. TNBC is a subtype of cancer where the cells have tested negative for estrogen receptors (ER), hormone epidermal growth factor receptor 2 (Her2), and progesterone receptors (PR). This subtype of cancer has limited treatment options and poor prognosis, as hormone therapies or targeted drugs do not work on it. Results of the binary classification problem on TCGA are reported in Table 7.
Table 7.
TNBC classification from the other molecular subtypes in the TCGA dataset, using 1046 features and 100 signature
| TNBC-100 | TNBC-1046 | Other-100 | Other-1046 | Global-100 | Global-1046 | |
|---|---|---|---|---|---|---|
| #Samples | 135 | 135 | 629 | 629 | 764 | 764 |
| Gradient Boosting | 0.9111 | 0.8963 | 0.9857 | 0.9857 | 0.9725 | 0.9699 |
| Random Forest | 0.8889 | 0.8815 | 0.9905 | 0.9905 | 0.9725 | 0.9712 |
| Logistic Regression | 0.8963 | 0.9630 | 0.9793 | 0.9587 | 0.9647 | 0.9593 |
| Passive Aggressive | 0.8815 | 0.9630 | 0.9714 | 0.9523 | 0.9556 | 0.9540 |
| SGD | 0.8000 | 0.8222 | 0.9809 | 0.9841 | 0.9490 | 0.9555 |
| SVC | 0.8444 | 0.8963 | 0.9666 | 0.9825 | 0.9451 | 0.9673 |
| Ridge | 0.8000 | 0.7259 | 0.9825 | 0.9237 | 0.9503 | 0.8888 |
| Bagging | 0.8444 | 0.8963 | 0.9793 | 0.9825 | 0.9555 | 0.9673 |
| Average | 0.8583 | 0.8806 | 0.9795 | 0.9700 | 0.9582 | 0.9542 |
Finally, we test the binary subtype classification of BRCA for the GEO datasets, using just the 100-miRNA signature. We create a single dataset composed of 4 series (GSE86281, GSE86277, GSE86278, GSE46823), with 2 classes: TNBC, featuring 139 samples, and all other molecular subtypes (LumA, LumB, and Her2), with 32 samples in total. Using the stem-loop sequences from platform GPL14613, and GPL1368, we use the 98 common stem-loop miRNAs of the 100 in the signature signature for the classification. In Table 8, we show the results of the classification in a 10-fold cross validation, and the accuracy by class.
Table 8.
Molecular subtype classification of Breast Cancer to separate TNBC from other breast cancer subtypes using the 100-miRNA signature, on the GEO dataset
| TNBC | Other | Global | |
|---|---|---|---|
| #Samples | 139 | 44 | 183 |
| Gradient Boosting | 0.9353 | 0.7500 | 0.8909 |
| Random Forest | 0.9424 | 0.6136 | 0.8634 |
| Logistic Regression | 0.9065 | 0.6590 | 0.8476 |
| Passive Aggressive | 0.8561 | 0.7045 | 0.8197 |
| SGD | 0.9065 | 0.5227 | 0.8145 |
| SVC | 0.8561 | 0.7727 | 0.8355 |
| Ridge | 0.8993 | 0.6136 | 0.8300 |
| Bagging | 0.9496 | 0.7727 | 0.9070 |
| Average | 0.9065 | 0.6761 | 0.8511 |
Discussion
The results of the five experiments performed with the 100-miRNA signature (Tumor Type Classification, Tumor Tissue vs Normal Tissue, GEO datasets, BRCA subtype in TCGA, and BRCA subtype in GEO datasets), are reported in Table 9. All classifiers show high levels of accuracy over all trials, with the validation on the GEO datasets (both tumor type and subtype classification) proving to be the hardest task.
Table 9.
Comparison of the 8 classifiers, for the different experiments with the 100-miRNA signature
| TT vs | TCGA | GEO | ||||
|---|---|---|---|---|---|---|
| Classifier | TCGA | NT | GEO | (Subtype) | (Subtype) | Global |
| Gradient Boosting | 0.9359 | 0.9846 | 0.6697 | 0.9725 | 0.8909 | 0.8907 |
| Random Forest | 0.9324 | 0.9839 | 0.8085 | 0.9725 | 0.8634 | 0.9121 |
| Logistic Regression | 0.9237 | 0.9799 | 0.9351 | 0.9647 | 0.8476 | 0.9302 |
| Passive Aggressive | 0.8831 | 0.9606 | 0.8678 | 0.9556 | 0.8197 | 0.8974 |
| SGD | 0.9035 | 0.9767 | 0.9393 | 0.9490 | 0.8145 | 0.9166 |
| SVC | 0.9154 | 0.9791 | 0.7724 | 0.9451 | 0.8355 | 0.8895 |
| Ridge | 0.8305 | 0.9470 | 0.8867 | 0.9503 | 0.8300 | 0.8889 |
| Bagging | 0.9110 | 0.9812 | 0.7682 | 0.9555 | 0.9070 | 0.9046 |
Logistic Regression was the best across all experiments, and Ridge has the worst accuracy
As miRNAs have been shown to regulate approximately 30% of the human genes, and because their dysregulation has been associated with the development and progression of cancer, miRNAs have been found to have the potential to play a critical role in computational oncology. Nevertheless, their analysis and their employment in clinically relevant settings still faces various, specific technical challenges: a) the extremely small size of the miRNAs leads to diverse complications for example with respect to hybridization techniques, b) there is a lack of specificity in detection because of the high similarity of several miRNA family members, and c) the low expression of various miRNAs requires detection methods of utmost sensitivity [48]. To date, most new miRNAs are discovered through cloning, despite these methods being time-consuming, low-throughput, and being biased toward the discovery of abundant miRNAs [49, 50].
Nevertheless, we can conclude from our results that the extracted 100-miRNA signature is able to reliably classify the 28 different types of cancer in the TCGA dataset, and distinguish between normal and tumor tissue. In addition, it is sufficiently stable to be applicable across platforms, such as the ones such as the ones used in the ten GEO datasets and ahich show a good accuracy in differentiating TNBC from other molecular subtypes of BRCA. Looking ahead into the possibility of classifying tumor types using miRNAs, we need to consider circulating miRNAs, and their relationship to cancer studies.
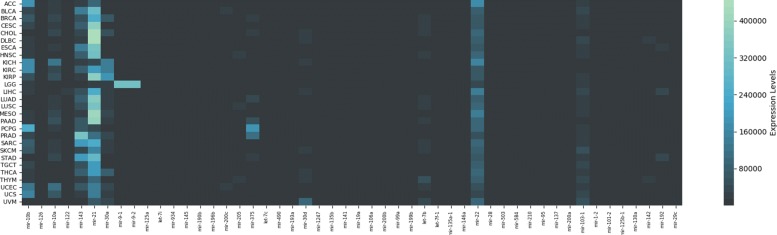
For the miRNAs included in the signature, we performed a bibliographic meta-analysis of specialized literature. The proposed meta-analysis is mainly based on 5 surveys of circulating miRNAs for cancer studies [6, 7, 51–53]. Out of the 100 miRNAs in the signature, 77 appear as circulatory miRNAs, either in their stem-loop form or mature sequence. The complete list for the 100-miRNAs is reported in Annex A of the online Additional file 1, in Fig. 5 shows the expression levels by type of cancer of the top 50 miRNAs.
Fig. 5.
miRNAs mean expression levels (RPMs) of the top 50 miRNAs for each type of cancer tumor tissue
Across all surveys analyzed, hsa-miR-21, included in our signature in stem-loop form, appears to be the most commonly over-expressed miRNA for all classes of tumors, as we would expect of a known oncomarker. In Annex B of the Additional file 1, we present a detailed analysis of the top 50 miRNAs in the signature, showing cancer study type, reference and circulating sample type used for measuring the expression. 23 miRNAs in the signature do not appear in the surveys, but they are mentioned in recent research papers, as promising research leads whose role may need further corroboration (we put the mature sequence as they appear in the study): miR-211 [54], miR-135a [55], miR-3678-3p [56], miR-204 [57], miR-1228 [58], miR-374b [59], miR-424 [60] miR-217-5p [60] miR-3613-5p [61], miR-124 [62], miR-1277-5p [63] miR-190 [64], miR-934 [65], miR-490 [66], miR-1247 [67], miR-199b [68], miR-135a [55], miR-503 [69], miR-584 [70], miR-137-3p [71], and miR-103 [72].
Interestingly, hsa-mir-135a-1 and hsa-mir-135a-2, located inside chromosomes 3 and 12, respectively, generate the same mature active sequence [73]. In the same manner, hsa-mir-124-1, hsa-mir-124-2, and hsa-mir-124-3, generate the same mature sequence hsa-miR-124-5p, and miR-124 is known as a tumor suppressor in head and neck squamous cell carcinoma [74], hepatocellular carcinoma [75] and breast cancer [76]. All of them were identified by our feature selection approach, indicting the presence of miRNA pathways shared across different tumor types. Targeting these miRNA pathways with anti-miRNA-based approaches such as infection with viral particles (having antisense sequence against the specific miRNA) or even drug design of small molecules inhibitors of miRNAs (SMIRs) which can be considered potential anti-tumoral therapy. On the other hand, the down regulation of tumor suppressor miRNAs also contributes to the acquisition of malignant features. For example, by ectopic expression of hsa-miR-944 which decreases malignant features in gastric [77], colorectal [78] and endometrial [79] cancers. Strikingly, miR-944 and other understudied miRNAs could have been detected by our approach analizing 28 different types of cancer, suggesting that they could play a key role in the biology of cancer. Future works will include further analyses of the 100-miRNA signature, crossing the information with genetic sources, assessing measures of gene quality and biomarker stability, using tools such as sigQC [80].
Conclusions
miRNAs fine-tune the regulation of the transcriptome [81, 82]. Alterations in miRNA expression profiles are associated with several diseases, such as cancer. On the other hand, the altered miRNA expression profiles present in cancer could be used as prognostic and/or diagnostic markers. In summary, several miRNA signatures are associated with clinically relevant factors [83, 84]. Therefore, our miRNA signature, which we obtained by using data from different types of cancers, can highlight the presence of so far underestimated miRNA’s such as miR-944, and overall has the potential to be used in the frame of microarray based assays, as a potential building block in clinical decision support. Of course, further experimental validation on cancer patient samples will be required to weigh the biological significance of the signature in terms of diagnosing, treating and prognosing the outcome of cancer.
In this study, we developed a new machine-learning approach to obtain a robust, reduced miRNA signature, from a TCGA dataset containing 28 different types of cancer. When tested against other datasets, our system provided good classification accuracy using only the reduced 100-feature signature, despite significant differences in the platforms used to gather the data. A further meta-analysis of literature on the miRNA in the identified signature showed both well-known oncogenic and underestimated miRNA types. The results of this work could potentially be used to uncover new, promising leads of research for a better understanding of miRNA behavior. Furthermore, personal-directed anti-tumoral therapy could be achieved by measurement of the specific, minimal miRNA signature, identified in this work.
Methods
Ensemble feature selection
As the objective is to discover and validate a reduced list of miRNAs to be used as a signature for tumor classification, we need to select features that could optimally assist in distinguishing between different cancer types and tumor tissue. In this sense, popular approaches used for feature selection range from univariate statistical considerations, to iterated runs of the same classifier with a progressively reduced number of features in order to assess the contribution of the features to the overall result. As the considered problem is particularly complex, relying upon simple statistical analyses might not suffice. Furthermore, features extracted using an iterative method on one classifier are likely to work well only for that specific classifier. Following the idea behind ensemble feature selection [36, 37, 85], we propose the use of multiple algorithms to obtain a more robust and general predictive performance. An ensemble approach has the advantage of obtaining features that will be effective across several classifiers, with a better likelihood of being more representative of the data, and not just of the inner workings of a single classifier.
For this purpose, we train a set of classifiers in order to extract a sorted list of the most relevant features from each. Intuitively, as a feature considered important by the majority of classifiers in the set is also likely to be relevant for our objective, then information from all classifiers is compiled to find the most common relevant features. Starting from a comparison of 22 different state-of-the-art classifiers on the considered dataset, presented in [86], a subset of those classifiers was selected considering both; high accuracy and a way to extract the relative importance of the features from the trained classifier. After preliminary tests to set algorithms’ hyperparameters, 8 classifiers were chosen, all featuring an average accuracy higher than 90% on a 10-fold cross-validation: Bagging [87], Gradient Boosting [88], Logistic Regression [89], Passive Aggressive [90], Random Forest [91], Ridge [92], SGD (Stochastic Gradient Descent on linear models) [93], SVC (Support Vector Machines Classifier with a linear kernel) [94]. All considered classifiers are implemented in the scikit-learn Python toolbox.
Overall, the selected classifiers fall into two broad typologies: those exploiting ensembles of classification trees [95] (Bagging, Gradient Boosting, Random Forest), and those optimizing the coefficients of linear models to separate classes (Logistic Regression, Passive Aggressive, Ridge, SGD, SVC). Depending on classifier typology, there are two different ways of extracting relative feature importance. For classifiers based on classification trees, the features used in the splits are counted and sorted by frequency, from the most to the least common. For classifiers based on linear models, the values of the coefficients associated to each feature can be used as a proxy of their relative importance, sorting coefficients from the largest to the smallest in absolute value. As the two feature extraction methods return heterogeneous numeric values, only the relative sorting of features provided by each classifier was considered. Furthermore, we decide to extract the top 100 most relevant features as a reduction of about an order of magnitude, so we assign to each feature f a simple score sf=Nt/Nc, where Nt is the number of times that specific feature appears among the top 100 of a specific classifier instance, while Nc is the total number of classifiers instances used; for instance, a feature appearing among the 100 most relevant in 73% of the classifiers used would obtain a score sf=0.73. We select 100 features because we wanted to compress the dataset at least 90%, thus from 1046 we reduce it to 100. In order to increase the generality of our results, each selected classifier was run 10 times, using a 10-fold stratified cross-validation, so that each fold preserves the percentage of samples of each class in the original dataset. Thus, Nc=80 (8 types of classifiers, run 10 times each). The complete procedure is summarized by Algorithm 1. Different approaches to the aggregation of heterogeneous feature importance from various sources are also possible (see for example [36, 37, 85]), such as assigning to each feature a weight proportional to its relative importance. However, most alternatives would require adding and tuning extra parameters, so we decided to opt for a simpler approach.

TCGA dataset
The data was downloaded from the TCGA Data Portal2, on September 1, 2016. The used data is miRNA-SEQ files (*.mirna.quantification.txt) a total of 1046 miRNA expression features for each sample in format mirbase V16 for stem-loop sequences3. We consider the read per million (RPM) values in the file and we remove all of the samples where the item does not meet the study protocol as stated in the file annotations. In summary, the dataset used in the following experiments includes 28 types of tumors, 1046 miRNA features, and 8023 patient samples. Information on the dataset is summarized in Table 10. We standardized the data by removing the mean and scaling to unit variance (specifying that we had learned the standardization on the training set, and applied it to the test set, so that knowledge of the whole dataset did not bias the performance on the test set). In addition, we created a second dataset that differentiates between normal tissue (NT) and tumor tissue (TT) that consists of 8657 samples; 8023 TT and 634 NT.
Geo datasets
To validate our results, we use 14 datasets from the GEO repository4, from 5 different platforms. We use 2 types of miRNA discovery technologies: microarrays and sequencing. miRNAs expression levels are platform and technology dependent [96–98]. Therefore, we need to consider if the information is in stem-loop or mature sequence and then calculate the contributions to make a direct comparison.
In the TCGA dataset, stem-loop sequences were directly measured in raw read counts. When reading a mature sequence, the protocol that was followed assigns a read count to it, and then randomly assigns a read count to one of the stem-loop sequences that share the same mature sequence [99].
GPL8786, gPL10850
Affymetrix Multispecies miRNA-1 Array (GPL8786) and Agilent-021827 Human miRNA Microarray V3 (GPL10850) cannot read stem-loop sequences, so the corresponding GEO datasets only show information for mature sequences. Thus, in order to perform a fair comparison, we consider the raw read count for stem-loop sequences as a linear function of the read counts of the mature sequences. If we call the read counts of a specific stem-loop sequence Xi, for hsa-mir-10b we have for example:
| 1 |
Where a0 and a1 are two coefficients to be set. The mapping between the values of two different platforms P1 and P2 can then be written as:
| 2 |
To reduce the problem, we consider only relationships between a stem-loop sequence and its most common corresponding mature sequence e.g hsa-mir-10b to hsa-miR-10b, disregarding hsa-miR-10b*. From Eq. 1 and 2 we then have:
where becomes the only coefficient to be found, and it represents the transformation between platforms for that specific sequence. A different linear function will be found for each pair of platforms, as we assume that each machine will have unique properties.
For GPL8786 GEO datasets, we consider the linear gene expression values given by the function rmasummary from the Matlab bioinformatics toolbox, which is a normalized robust multi-array average procedure, as a z-score [100, 101]. The equation of a z-score is:
| 3 |
where X is the value of a feature; μ and σ are the average and the standard deviation for a feature. Next, by considering the linear expression values as z-scores, the GEO datasets are mapped to corresponding intensities in the TCGA dataset space, by solving for X:
| 4 |
where Xi is the intensity of miRNA i in the TCGA dataset space, Zi is the linear gene expression value given by the scaled rmasummary summary function, and are the average value and the standard deviation for miRNA i, both computed on the original TCGA dataset, and is a scale value, dependent on the platform. The value is computed using a subset of all the GEO datasets from the same platform, by minimizing the error between actual class and predicted class, using a model trained in the TCGA dataset with Root Mean Squared Error (RMSE).
| 5 |
where S is the total number of samples in the dataset, and aP is a vector containing the values of for each feature i. A state-of-the-art numerical optimizer [102] is applied to this task, to find the 98 parameters represented by aP.
For GPL10850 we use the MatLab function agferead from the Bioinformatics Toolbox and use the value of gTotalGeneSignal as value for each of the probes and calculate the contributions and as for GPL8786.
GPL14613, gPL16384
Affymetrix Multispecies miRNA-2 Array (GPL14613) and Affymetrix Multispecies miRNA-3 Array (GPL16384) measure the stem-loop sequences directly, and denote them by hp_hsa. The linear relationship between the TCGA dataset and the corresponding subset of GEO datasets is thus represented by Eq. 2, and the parameters to be found are reduced to the a2i
As remarked by Telonis et al. [21], for these datasets, not all the types of cancer are available, or present the necessary quality standards. Thus, we reduce our analysis to 6 different types of cancer; Prostate, Liver, Breast, Esophageal, Head and Neck Squamous Cell and Lung. For the sequencing data, extra mapping is not necessary besides the sample normalization (platform GPL11154), and we use only stem-loop sequences.
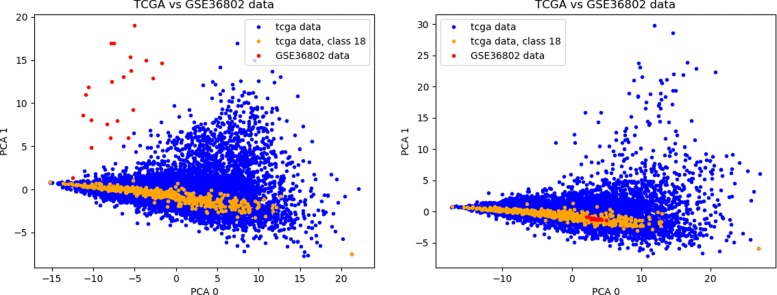
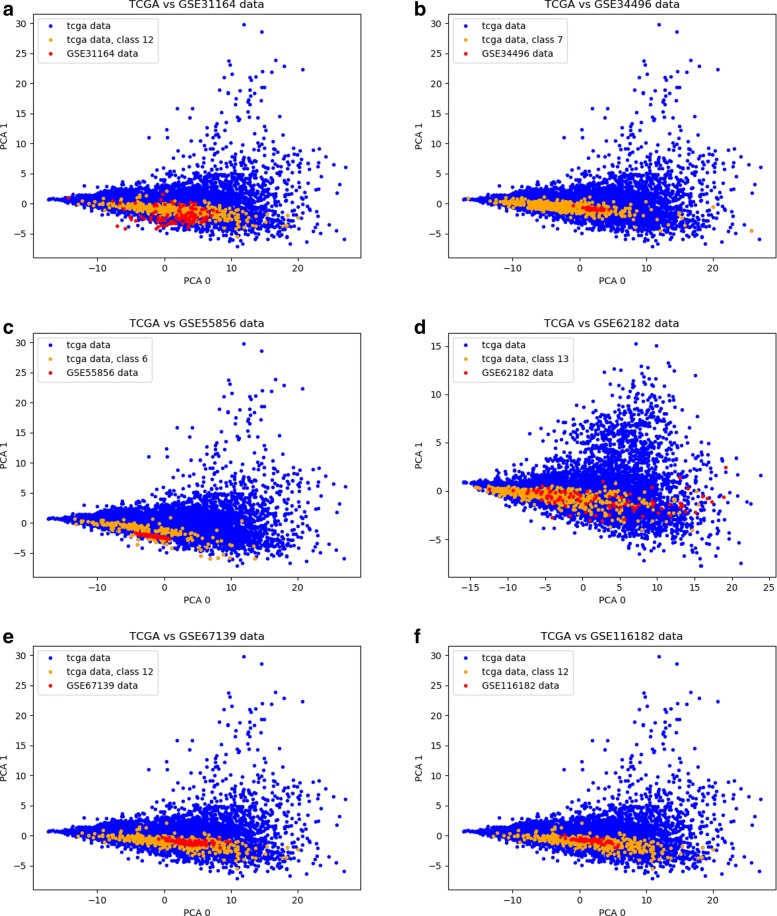
Using this procedure, we are able to map the GEO repository measurements into the TCGA dataset space as seen in Fig. 6. Other examples are shown in Fig. 7, where plots were created using the first two dimensions of a Principal Component Analysis (PCA) computed on the TCGA dataset and applied to the GEO datasets, to provide a comparison between the cancer type in each GEO and the corresponding class in TCGA. Remarkably, samples from GEO datasets are often considerably close to samples of the corresponding class in TCGA. During validation, we selected the common features between each GEO dataset and the 100-miRNA signature obtained using the ensemble approach. The accuracy of the classification algorithms was then evaluated by training them on the TCGA dataset and testing them on each GEO dataset. A summary of the experiments is presented in Fig. 1.
Fig. 6.
Example of mapping GSE microarray data into TCGA space (GSE36802)
Fig. 7.
Examples of PCA projections of GEO datasets transformed into the TCGA dataset space. Orange data points represent samples from the target class from the TCGA dataset, the blue data points are other samples in TCGA, and the red points are the projected samples from GEO datasets
Additional file
Annex B-Table comparing the top 50 most frequent features extracted by the machine learning algorithms with existing biomarkers references in literature.
Annex C- Figure with all PCA projections of GEO datasets.
Annex D- Explanation of n-fold cross-validation. (PDF 505 kb)
Acknowledgements
The results published here are based upon data generated by The Cancer Genome Atlas and GSM.
Abbreviations
- ACC
Adrenocortical carcinoma
- BLCA
Bladder Urothelial carcinoma
- BRCA
Breast invasive carcinoma
- CESC
Cervical squamous cell carcinoma
- CHOL
Cholangiocarcinoma
- DLBC
Lymphoid neoplasm diffuse large B-cell lymphoma
- EFS-CLA
Ensemble feature selection with complete linear aggregation
- EN
Elastic net
- ESCA
Esophageal carcinoma
- GEO
Gene expression omnibus
- HNSC
Head and neck squamous cell carcinoma
- KICH
Kidney chromophobe
- KIRC
Kidney renal clear cell carcinoma
- KIRP
Kidney renal papillary cell carcinoma
- LASSO
Least absolute shrinkage and selection operator
- LGG
Lower grade glioma
- LIHC
Liver hepatocellular carcinoma
- LumA
Luminal A
- LUAD
Lung adenocarcinoma
- LumB
Luminal B
- LUSC
Lung squamous cell carcinoma
- MESO
Mesothelioma
- miRNA
microRNA
- NT
Normal tissue
- PAAD
Pancreatic adenocarcinoma
- PCA
Principal component analysis
- PCPG
Pheochromocytoma and paraganglioma
- RMSE
Root mean squared error
- PRAD
Prostate adenocarcinoma
- RFE
Recursive feature elimination
- RPM
Read per million
- SARC
Sarcoma
- SGD
Stochastic gradient descent
- SKCM
Skin cutaneous melanoma
- STAD
Stomach adenocarcinoma
- SVC
Support vector machines classifier
- TCGA
The cancer genome atlas
- TGCT
Testicular germ cell tumors
- THCA
Thyroid carcinoma
- THYM
Thymoma
- TNBC
Triple negative breast cancer
- TT
Tumor tissue
- UCEC
Uterine corpus endometrial carcinoma
- UCS
Uterine carcinosarcoma
- UFS
Univariate feature selection
- UVM
Uveal melanoma
Authors’ contributions
ALR suggested the problem, wrote, built the datasets and coded. MMA and GMR helped with the writing, miRNAs concepts, and the bibliographic analysis. AS wrote and conceived the cross-platform validation, and comparison to other methods. AT got funding, wrote, and coded. All authors have read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The code and the datasets are available at https://github.com/steppenwolf0/miRNAs100
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
ftp://mirbase.org/pub/mirbase/16/
https://tcga-data.nci.nih.gov/docs/publications/tcga/
ftp://mirbase.org/pub/mirbase/16/genomes/hsa.gff
https://www.ncbi.nlm.nih.gov/gds
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alejandro Lopez-Rincon, Email: alejandro.lopez@iscpif.fr.
Marlet Martinez-Archundia, Email: mtmartineza@ipn.mx.
Gustavo U. Martinez-Ruiz, Email: gustavobambam@gmail.com
Alexander Schoenhuth, Email: a.schoenhuth@cwi.nl.
Alberto Tonda, Email: alberto.tonda@grignon.inra.fr.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136(5):359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Tanase C, Ogrezeanu I, Badiu C. Molecular Pathology of Pituitary Adenomas: Elsevier Insights; 2012, p. 130.
- 3.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al. Frequent deletions and down-regulation of micro-rna genes mir15 and mir16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci. 2002;99(24):15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng Y, Croce CM. The role of microRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauter ER, Patel N. Body fluid micro (mi) rnas as biomarkers for human cancer. J Nucleic Acids Investig. 2011;2(1):1. doi: 10.4081/jnai.2011.2160. [DOI] [Google Scholar]
- 6.He Y., Lin J., Kong D., Huang M., Xu C., Kim T.-K., Etheridge A., Luo Y., Ding Y., Wang K. Current State of Circulating MicroRNAs as Cancer Biomarkers. Clinical Chemistry. 2015;61(9):1138–1155. doi: 10.1373/clinchem.2015.241190. [DOI] [PubMed] [Google Scholar]
- 7.Calore F, Lovat F, Garofalo M. Non-coding rnas and cancer. Int J Mol Sci. 2013;14(8):17085–110. doi: 10.3390/ijms140817085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferracin M, Veronese A, Negrini M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2010;10(3):297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- 9.Redis Roxana S., Calin George A. Non-coding RNAs and Cancer. New York, NY: Springer New York; 2013. Beyond miRNAs: Role of Other Noncoding RNAs in Cancer; pp. 247–264. [Google Scholar]
- 10.Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2012;15(1):1–19. doi: 10.1093/bib/bbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar MM, Micolucci L, Islam MS, Olivieri F, Procopio AD. Bioinformatic tools for microRNA dissection. Nucleic Acids Res. 2015;44(1):24–44. doi: 10.1093/nar/gkv1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya A, Ziebarth JD, Cui Y. Somamir: a database for somatic mutations impacting microRNA function in cancer. Nucleic Acids Res. 2012;41(D1):977–82. doi: 10.1093/nar/gks1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozomara A, Griffiths-Jones S. mirbase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2010;39(suppl_1):152–7. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin Chem. 2009;55(4):623–31. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 15.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. a comprehensive review. EMBO Mol Med. 2012;4(3):143–59. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao W, Shen H, Liu L, Xu J, Xu J, Shu Y. Mir-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137(4):557–66. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhi F, Chen X, Wang S, Xia X, Shi Y, Guan W, Shao N, Qu H, Yang C, Zhang Y, et al. The use of hsa-mir-21, hsa-mir-181b and hsa-mir-106a as prognostic indicators of astrocytoma. Eur J Cancer. 2010;46(9):1640–9. doi: 10.1016/j.ejca.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Yan L-X, Huang X-F, Shao Q, Huang M-Y, Deng L, Wu Q-L, Zeng Y-X, Shao J-Y. MicroRNA mir-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. Rna. 2008;14(11):2348–60. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Fan Z, Liu F, Zuo J. Hsa-mir-21 and hsa-mir-29 in tissue as potential diagnostic and prognostic biomarkers for gastric cancer. Cell Physiol Biochem. 2015;37(4):1454–62. doi: 10.1159/000438514. [DOI] [PubMed] [Google Scholar]
- 21.Telonis AG, Magee R, Loher P, Chervoneva I, Londin E, Rigoutsos I. Knowledge about the presence or absence of miRNA isoforms (isomirs) can successfully discriminate amongst 32 tcga cancer types. Nucleic Acids Res. 2017;45(6):2973–85. doi: 10.1093/nar/gkx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yousef M, Allmer J, Khalifa W. Feature selection for microRNA target prediction comparison of one-class feature selection methodologies. Conference Paper. DSpace@IZTECH. 2016. 10.5220/0005701602160225.
- 23.Tang W, Wan S, Yang Z, Teschendorff AE, Zou Q. Tumor origin detection with tissue-specific miRNA and dna methylation markers. Bioinformatics. 2017;34(3):398–406. doi: 10.1093/bioinformatics/btx622. [DOI] [PubMed] [Google Scholar]
- 24.Piao Y, Piao M, Ryu KH. Multiclass cancer classification using a feature subset-based ensemble from microRNA expression profiles. Comput Biol Med. 2017;80:39–44. doi: 10.1016/j.compbiomed.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein JN, Collisson EA, Mills GB, Shaw KRM, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM, Network CGAR, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, et al. Scikit-learn: Machine learning in python. J Mach Learn Res. 2011;12(Oct):2825–30. [Google Scholar]
- 27.Altman N, Krzywinski M. Points of Significance: Ensemble methods: bagging and random forests. Nat Publ Group. 2017;14(10):933–4. [Google Scholar]
- 28.Hira ZM, Gillies DF. A review of feature selection and feature extraction methods applied on microarray data. Adv Bioinforma. 2015;2015:1–13. doi: 10.1155/2015/198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazo AV, Rathie P. On the entropy of continuous probability distributions (corresp.) IEEE Trans Inf Theory. 1978;24(1):120–2. doi: 10.1109/TIT.1978.1055832. [DOI] [Google Scholar]
- 30.Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46(1-3):389–422. doi: 10.1023/A:1012487302797. [DOI] [Google Scholar]
- 31.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokolov A, Carlin DE, Paull EO, Baertsch R, Stuart JM. Pathway-based genomics prediction using generalized elastic net. PLoS Comput Biol. 2016;12(3):1004790. doi: 10.1371/journal.pcbi.1004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu A, Mitra R, Liu H, Schreiber SL, Clemons PA. Rwen: Response-weighted elastic net for prediction of chemosensitivity of cancer cell lines. Bioinformatics. 2018;1:8. doi: 10.1093/bioinformatics/bty199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B Methodol. 1996;58(1):267–88. [Google Scholar]
- 35.Trevino V, Falciani F. Galgo: an r package for multivariate variable selection using genetic algorithms. Bioinformatics. 2006;22(9):1154–6. doi: 10.1093/bioinformatics/btl074. [DOI] [PubMed] [Google Scholar]
- 36.Abeel T, Helleputte T, Van de Peer Y, Dupont P, Saeys Y. Robust biomarker identification for cancer diagnosis with ensemble feature selection methods. Bioinformatics. 2009;26(3):392–8. doi: 10.1093/bioinformatics/btp630. [DOI] [PubMed] [Google Scholar]
- 37.Seijo-Pardo B., Porto-Díaz I., Bolón-Canedo V., Alonso-Betanzos A. Ensemble feature selection: Homogeneous and heterogeneous approaches. Knowledge-Based Systems. 2017;118:124–139. doi: 10.1016/j.knosys.2016.11.017. [DOI] [Google Scholar]
- 38.Lin P-C, Chiu Y-L, Banerjee S, Park K, Mosquera JM, Giannopoulou E, Alves P, Tewari AK, Gerstein MB, Beltran H, et al. Epigenetic repression of mir-31 disrupts androgen receptor homeostasis and contributes to prostate cancer progression. Cancer Res. 2013;73(3):1232–44. doi: 10.1158/0008-5472.CAN-12-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casanova-Salas I, Rubio-Briones J, Calatrava A, Mancarella C, Masiá E, Casanova J, Fernández-Serra A, Rubio L, Ramírez-Backhaus M, Armiñán A, et al. Identification of mir-187 and mir-182 as biomarkers of early diagnosis and prognosis in patients with prostate cancer treated with radical prostatectomy. J Urol. 2014;192(1):252–9. doi: 10.1016/j.juro.2014.01.107. [DOI] [PubMed] [Google Scholar]
- 40.Peña-Chilet M, Martínez MT, Pérez-Fidalgo JA, Peiró-Chova L, Oltra SS, Tormo E, Alonso-Yuste E, Martinez-Delgado B, Eroles P, Climent J, et al. MicroRNA profile in very young women with breast cancer. BMC Cancer. 2014;14(1):529. doi: 10.1186/1471-2407-14-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang H-J, Lee H-S, Burt BM, Lee GK, Yoon K-A, Park Y-Y, Sohn BH, Kim SB, Kim MS, Lee JM, et al. Integrated genomic analysis of recurrence-associated small non-coding rnas in oesophageal cancer. Gut. 2017;66(2):215–25. doi: 10.1136/gutjnl-2015-311238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero-Cordoba SL, Rodriguez-Cuevas S, Bautista-Pina V, Maffuz-Aziz A, D’Ippolito E, Cosentino G, Baroni S, Iorio MV, Hidalgo-Miranda A. Loss of function of mir-342-3p results in mct1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci Rep. 2018;8(1):12252. doi: 10.1038/s41598-018-29708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami Y, Tamori A, Itami S, Tanahashi T, Toyoda H, Tanaka M, Wu W, Brojigin N, Kaneoka Y, Maeda A, et al. The expression level of mir-18b in hepatocellular carcinoma is associated with the grade of malignancy and prognosis. BMC Cancer. 2013;13(1):99. doi: 10.1186/1471-2407-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vucic EA, Thu KL, Pikor LA, Enfield KS, Yee J, English JC, MacAulay CE, Lam S, Jurisica I, Lam WL. Smoking status impacts microRNA mediated prognosis and lung adenocarcinoma biology. BMC Cancer. 2014;14(1):778. doi: 10.1186/1471-2407-14-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Network CGA, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM, Castiglioni I, et al. Tcgabiolinks: an r/bioconductor package for integrative analysis of tcga data. Nucleic Acids Res. 2015;44(8):71. doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss M. Your guide to the breast cancer pathology report. Breastcancer.org. 2016. https://www.breastcancer.org.
- 48.Li X, Ni M, Zhang C, Ma W, Zhang Y. A convenient system for highly specific and sensitive detection of miRNA expression. RNA. 2014;20(2):252–9. doi: 10.1261/rna.040220.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Gelfond JA, McManus LM, Shireman PK. Reproducibility of quantitative rt-pcr array in miRNA expression profiling and comparison with microarray analysis. BMC Genomics. 2009;10(1):407. doi: 10.1186/1471-2164-10-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Ruan K. MicroRNA detection by microarray. Anal Bioanal Chem. 2009;394(4):1117–24. doi: 10.1007/s00216-008-2570-2. [DOI] [PubMed] [Google Scholar]
- 51.Larrea E, Sole C, Manterola L, Goicoechea I, Armesto M, Arestin M, Caffarel MM, Araujo AM, Araiz M, Fernandez-Mercado M, et al. New concepts in cancer biomarkers: circulating miRNAs in liquid biopsies. Int J Mol Sci. 2016;17(5):627. doi: 10.3390/ijms17050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev. 2015;81:75–93. doi: 10.1016/j.addr.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Zhang K-Y, Liu S-M, Sen S. Tumor-associated circulating microRNAs as biomarkers of cancer. Molecules. 2014;19(2):1912–38. doi: 10.3390/molecules19021912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margue C, Reinsbach S, Philippidou D, Beaume N, Walters C, Schneider JG, Nashan D, Behrmann I, Kreis S. Comparison of a healthy mirnome with melanoma patient mirnomes: are microRNAs suitable serum biomarkers for cancer? Oncotarget. 2015;6(14):12110. doi: 10.18632/oncotarget.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koga Y, Yasunaga M, Takahashi A, Kuroda J, Moriya Y, Akasu T, Fujita S, Yamamoto S, Baba H, Matsumura Y. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res. 2010;3(11):1435–42. doi: 10.1158/1940-6207.CAPR-10-0036. [DOI] [PubMed] [Google Scholar]
- 56.Giulietti M, Occhipinti G, Principato G, Piva F. Identification of candidate miRNA biomarkers for pancreatic ductal adenocarcinoma by weighted gene co-expression network analysis. Cell Oncol. 2017;40(2):181–92. doi: 10.1007/s13402-017-0315-y. [DOI] [PubMed] [Google Scholar]
- 57.Mengual L, Lozano JJ, Ingelmo-Torres M, Gazquez C, Ribal MJ, Alcaraz A. Using microRNA profiling in urine samples to develop a non-invasive test for bladder cancer. Int J Cancer. 2013;133(11):2631–41. doi: 10.1002/ijc.28274. [DOI] [PubMed] [Google Scholar]
- 58.Tan Y, Ge G, Pan T, Wen D, Chen L, Yu X, Zhou X, Gan J. A serum microRNA panel as potential biomarkers for hepatocellular carcinoma related with hepatitis b virus. PloS ONE. 2014;9(9):107986. doi: 10.1371/journal.pone.0107986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Summerer I, Unger K, Braselmann H, Schuettrumpf L, Maihoefer C, Baumeister P, Kirchner T, Niyazi M, Sage E, Specht H, et al. Circulating microRNAs as prognostic therapy biomarkers in head and neck cancer patients. Br J Cancer. 2015;113(1):76. doi: 10.1038/bjc.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giráldez MD, Lozano JJ, Ramírez G, Hijona E, Bujanda L, Castells A, Gironella M. Circulating microRNAs as biomarkers of colorectal cancer: results from a genome-wide profiling and validation study. Clin Gastroenterol Hepatol. 2013;11(6):681–8. doi: 10.1016/j.cgh.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Matamala N, Vargas MT, González-Cámpora R, Miñambres R, Arias JI, Menéndez P, Andrés-León E, Gómez-López G, Yanowsky K, Calvete-Candenas J, et al. Tumor microRNA expression profiling identifies circulating microRNAs for early breast cancer detection. Clin Chem. 2015;61(8):1098–106. doi: 10.1373/clinchem.2015.238691. [DOI] [PubMed] [Google Scholar]
- 62.Medina-Villaamil V, Martínez-Breijo S, Portela-Pereira P, Quindós-Varela M, Santamarina-Cainzos I, Antón-Aparicio L, Gómez-Veiga F. Circulating microRNAs in blood of patients with prostate cancer. Actas Urol Esp (Engl Ed) 2014;38(10):633–9. doi: 10.1016/j.acuro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Zheng X-H, Cui C, Ruan H-L, Xue W-Q, Zhang S-D, Hu Y-Z, Zhou X-X, Jia W-H. Plasma microRNA profiling in nasopharyngeal carcinoma patients reveals mir-548q and mir-483-5p as potential biomarkers. Chin J Cancer. 2014;33(7):330. doi: 10.5732/cjc.013.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheffer A-R, Holdenrieder S, Kristiansen G, von Ruecker A, Müller SC, Ellinger J. Circulating microRNAs in serum: novel biomarkers for patients with bladder cancer? World J Urol. 2014;32(2):353–8. doi: 10.1007/s00345-012-1010-2. [DOI] [PubMed] [Google Scholar]
- 65.Tsuchiya N, Ogata H, Okusaka T, Nakagama H. Method for detecting pancreatic cancer and detection kit. Google Patents. US Patent APP. 14/410,408. 2015. https://www.google.com.
- 66.Jiang Y, Luan Y, Chang H, Chen G. The diagnostic and prognostic value of plasma microRNA-125b-5p in patients with multiple myeloma. Oncol Lett. 2018;16(3):4001–7. doi: 10.3892/ol.2018.9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Raimondo M, Guha S, Chen J, Diao L, Dong X, Wallace MB, Killary AM, Frazier ML, Woodward TA, et al. Circulating microRNAs in pancreatic juice as candidate biomarkers of pancreatic cancer. J Cancer. 2014;5(8):696. doi: 10.7150/jca.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montalbo R, Izquierdo L, Ingelmo-Torres M, Lozano JJ, Capitán D, Alcaraz A, Mengual L. Prognostic value of circulating microRNAs in upper tract urinary carcinoma. Oncotarget. 2018;9(24):16691. doi: 10.18632/oncotarget.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin VY, Ng EK, Chan VW, Kwong A, Chu K-M. A three-miRNA signature as promising non-invasive diagnostic marker for gastric cancer. Mol Cancer. 2015;14(1):202. doi: 10.1186/s12943-015-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Peng R, Wang J, Qin Z, Xue L. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin Epigenetics. 2018;10(1):59. doi: 10.1186/s13148-018-0492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu C-M, Lin P-M, Wang Y-M, Chen Z-J, Lin S-F, Yang M-Y. Circulating miRNA is a novel marker for head and neck squamous cell carcinoma. Tumor Biol. 2012;33(6):1933–42. doi: 10.1007/s13277-012-0454-8. [DOI] [PubMed] [Google Scholar]
- 72.Jiang X, Du L, Duan W, Wang R, Yan K, Wang L, Li J, Zheng G, Zhang X, Yang Y, et al. Serum microRNA expression signatures as novel noninvasive biomarkers for prediction and prognosis of muscle-invasive bladder cancer. Oncotarget. 2016;7(24):36733. doi: 10.18632/oncotarget.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tribollet V, Barenton B, Kroiss A, Vincent S, Zhang L, Forcet C, Cerutti C, Perian S, Allioli N, Samarut J, et al. mir-135a inhibits the invasion of cancer cells via suppression of err alpha. PloS ONE. 2016;11(5):0156445. doi: 10.1371/journal.pone.0156445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Ling Z, Hao Y, Pang X, Han X, Califano JA, Shan L, Gu X. Mir-124 acts as a tumor suppressor by inhibiting the expression of sphingosine kinase 1 and its downstream signaling in head and neck squamous cell carcinoma. Oncotarget. 2017;8(15):25005. doi: 10.18632/oncotarget.15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai QQ, Dong YW, Wang R, Qi B, Guo JX, Pan J, Liu YY, Zhang CY, Wu XZ. Mir-124 inhibits the migration and invasion of human hepatocellular carcinoma cells by suppressing integrin αv expression. Sci Rep. 2017;7:40733. doi: 10.1038/srep40733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Chen L, Wu Z, Wang M, Jin F, Wang N, Hu X, Liu Z, Zhang C-Y, Zen K, et al. mir-124-3p functions as a tumor suppressor in breast cancer by targeting cbl. BMC Cancer. 2016;16(1):826. doi: 10.1186/s12885-016-2862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan T, Chen W, Yuan X, Shen J, Qin C, Wang L. mir-944 inhibits metastasis of gastric cancer by preventing the epithelial–mesenchymal transition via macc1/met/akt signaling. FEBS Open Bio. 2017;7(7):905–14. doi: 10.1002/2211-5463.12215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Wen L, Li Y, Jiang Z, Zhang Y, Yang B, Han F. mir-944 inhibits cell migration and invasion by targeting macc1 in colorectal cancer. Oncol Rep. 2017;37(6):3415–22. doi: 10.3892/or.2017.5611. [DOI] [PubMed] [Google Scholar]
- 79.He Z, Xu H, Meng Y, Kuang Y. mir-944 acts as a prognostic marker and promotes the tumor progression in endometrial cancer. Biomed Pharmacother. 2017;88:902–10. doi: 10.1016/j.biopha.2017.01.117. [DOI] [PubMed] [Google Scholar]
- 80.Dhawan A, Barberis A, Cheng W-C, Domingo E, West C, Maughan T, Scott J, Harris AL, Buffa FM. sigQC: A procedural approach for standardising the evaluation of gene signatures. 10.1101/203729. https://www.biorxiv.org/content/10.1101/203729v2.
- 81.Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17(10):1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muniyappa M, Dowling P, Henry M, Meleady P, Doolan P, Gammell P, Clynes M, Barron N. MiRNA-29a regulates the expression of numerous proteins and reduces the invasiveness and proliferation of human carcinoma cell lines. Eur J Cancer. 2009;45(17):3104–18. doi: 10.1016/j.ejca.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Lamberti M, Capasso R, Lombardi A, Di Domenico M, Fiorelli A, Feola A, Perna AF, Santini M, Caraglia M, Ingrosso D. Two different serum miRNA signatures correlate with the clinical outcome and histological subtype in pleural malignant mesothelioma patients. PloS ONE. 2015;10(8):0135331. doi: 10.1371/journal.pone.0135331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sathipati SY, Ho S-Y. Identifying the miRNA signature associated with survival time in patients with lung adenocarcinoma using miRNA expression profiles. Sci Rep. 2017;7(1):7507. doi: 10.1038/s41598-017-07739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saeys Yvan, Abeel Thomas, Van de Peer Yves. Machine Learning and Knowledge Discovery in Databases. Berlin, Heidelberg: Springer Berlin Heidelberg; 2008. Robust Feature Selection Using Ensemble Feature Selection Techniques; pp. 313–325. [Google Scholar]
- 86.Lopez-Rincon Alejandro, Tonda Alberto, Elati Mohamed, Schwander Olivier, Piwowarski Benjamin, Gallinari Patrick. Evolutionary optimization of convolutional neural networks for cancer miRNA biomarkers classification. Applied Soft Computing. 2018;65:91–100. doi: 10.1016/j.asoc.2017.12.036. [DOI] [Google Scholar]
- 87.Breiman L. Pasting small votes for classification in large databases and on-line. Mach Learn. 1999;36(1-2):85–103. doi: 10.1023/A:1007563306331. [DOI] [Google Scholar]
- 88.Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat. 2001;29(5):1189–232. doi: 10.1214/aos/1013203451. [DOI] [Google Scholar]
- 89.Cox DR. The regression analysis of binary sequences. J R Stat Soc Ser B Methodol. 1958;20(2):215–32. [Google Scholar]
- 90.Crammer K, Dekel O, Keshet J, Shalev-Shwartz S, Singer Y. Online passive-aggressive algorithms. J Mach Learn Res. 2006;7(Mar):551–85. [Google Scholar]
- 91.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 92.Tikhonov AN. On the stability of inverse problems. Cr Acad Sci Urss. 1943;39:195–8. [Google Scholar]
- 93.Zhang T. Proceedings of the Twenty-first International Conference on Machine Learning. New York: ACM; 2004. Solving large scale linear prediction problems using stochastic gradient descent algorithms. [Google Scholar]
- 94.Hearst MA, Dumais ST, Osman E, Platt J, Scholkopf B. Support vector machines. IEEE Intell Syst Appl. 1998;13(4):18–28. doi: 10.1109/5254.708428. [DOI] [Google Scholar]
- 95.Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and Regression Trees: Chapman and Hall/ CRC press; 1984, p. 368.
- 96.Leshkowitz D, Horn-Saban S, Parmet Y, Feldmesser E. Differences in microRNA detection levels are technology and sequence dependent. RNA. 2013;19(4):527–38. doi: 10.1261/rna.036475.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Del Vescovo V, Meier T, Inga A, Denti MA, Borlak J. A cross-platform comparison of affymetrix and agilent microarrays reveals discordant miRNA expression in lung tumors of c-raf transgenic mice. PloS ONE. 2013;8(11):78870. doi: 10.1371/journal.pone.0078870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bassani N, Ambrogi F, Biganzoli E. Assessing agreement between miRNA microarray platforms. Microarrays. 2014;3(4):302–21. doi: 10.3390/microarrays3040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chu A, Robertson G, Brooks D, Mungall AJ, Birol I, Coope R, Ma Y, Jones S, Marra MA. Large-scale profiling of microRNAs for the cancer genome atlas. Nucleic Acids Res. 2015;44(1):3. doi: 10.1093/nar/gkv808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 101.Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using z score transformation. J Mol Diagn. 2003;5(2):73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hansen N, Müller SD, Koumoutsakos P. Reducing the time complexity of the derandomized evolution strategy with covariance matrix adaptation (cma-es) Evol Comput. 2003;11(1):1–18. doi: 10.1162/106365603321828970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Annex B-Table comparing the top 50 most frequent features extracted by the machine learning algorithms with existing biomarkers references in literature.
Annex C- Figure with all PCA projections of GEO datasets.
Annex D- Explanation of n-fold cross-validation. (PDF 505 kb)
Data Availability Statement
The code and the datasets are available at https://github.com/steppenwolf0/miRNAs100