Abstract
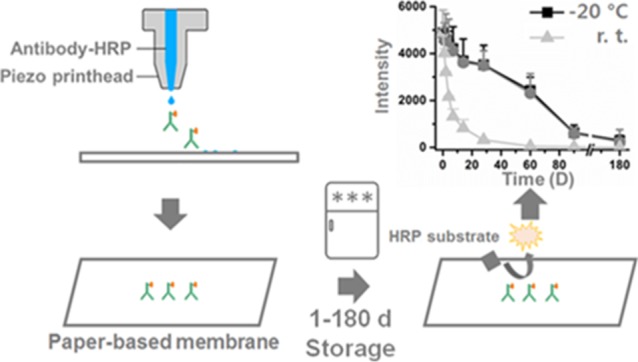
Monitoring of long-term stability of proteins on paper-based membranes is important as it is directly related to paper-based sensor fabrication. By using a simple piezo printhead inkjet printer, recombinant proteins and antibodies were printed on paper-based membranes to test their stability and sensitivity under varying lengths of storage and temperature conditions. Our data show that a printed IgG-HRP antibody on simple printing paper maintains >50% functionality up to ∼2 months under 4 and −20 °C storage. Antibodies printed on polyvinylidene difluoride (PVDF) and nitrocellulose showed 5.3 and 9.7% decreases, respectively, in initial signal intensities compared to printing paper. Prostate-specific membrane antigen and tumor necrosis factor alpha recombinant proteins printed on paper-based membranes can be detected by antibodies, and antibody signal intensities can be detected up to 28 days after storage at 4 and −20 °C when printed on PVDF membrane or printing paper. These data suggest that printed proteins on simple printing paper and PVDF membrane can maintain their functionality up to few months when stored at 4 °C or lower and can be potentially applied in paper-based sensor development.
Introduction
Detection and sensing of viruses, pollutants, and other related proteins have received much attention with the Zika and MERS outbursts in recent years.1−5 To be considered a good detection and sensing platform, stability and detection sensitivity as well as ease of use and maintenance should be guaranteed.6,7 There are several careful studies of paper-based sensing membranes that provide a cheap and convenient option for virus/pollutant detection.8−12 To date, a large number of novel paper-based sensing technologies, including nanoparticle-based and electronic-based sensors, have been developed to increase detection sensitivity.13−16 However, many of these technologies require a significant amount of detection time, function only under certain storage conditions and are quite often expensive. As alternatives to these high technology sensing platforms, colorimetric and immunosensors are still popular in clinical use.17−19 These immunosensors are based on immobilized capturing antibodies, and virus particles/antigens captured on antibodies were detected by detection antibodies.20,21 Colorimetric/immunosensing platforms are widely used in various viruses, antigens, and pollutant detection systems; however, most of the studies of these paper-based sensors focus on detection sensitivity and not stability or storage conditions.22−24 In addition, a large number of paper-based sensing membrane systems have adopted chemically treated membranes to enhance protein/antibody adsorption and stability,25−27 but comparative studies of protein stability and detection sensitivity on different paper-based membranes are rare. To verify the stability of printed proteins on paper-based membranes and to fill the gaps in research, we conducted systematic studies of protein stability and sensitivity on three different paper-based membranes. We also conducted long-term monitoring of printed proteins/antibodies on paper-based platforms with varying storage temperature conditions to identify an optimal storing period and temperature for printed proteins.
Results
Printing and Detection of Anti-IgG-HRP Antibody on a Paper-Based Platform
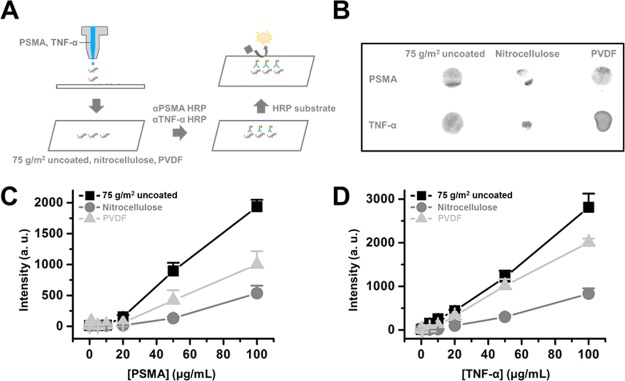
First, 1, 5, 10, 20, 50, and 100 μg/mL aliquots of the anti-IgG-HRP antibody were loaded on a Piezo printhead inkjet printer and printed on printing paper and nitrocellulose and polyvinylidene difluoride (PVDF) membranes (Figure 1A,B). Data suggest that IgG-HRP antibodies were successfully printed on the paper-based membrane (Figure 1B). The printed IgG-HRP antibodies on paper-based membranes were then incubated with the HRP substrate, and the HRP signal intensity was recorded. Chemiluminescence intensities from 100 μg/mL of the printed IgG-HRP antibody gave values of 4123 ± 1214, 4325 ± 385, and 4936 ± 325 (arbitrary unit) for printing paper, nitrocellulose membrane, and PVDF membrane, respectively (Figure 1C).
Figure 1.
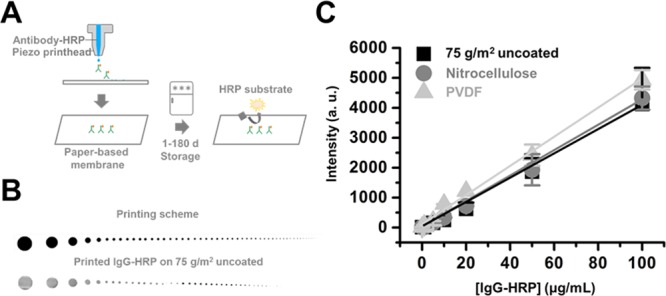
Anti IgG-HRP antibody printing on a paper-based platform. (A) Printing of anti-IgG-HRP antibody on a paper-based membrane, (B) printing scheme and printed IgG-HRP antibody on printing paper, (C) concentration of the anti-IgG-HRP antibody vs chemiluminescence intensity from printed anti-IgG-HRP. Each experiment was performed in triplicate, and values are expressed as the mean ± SD.
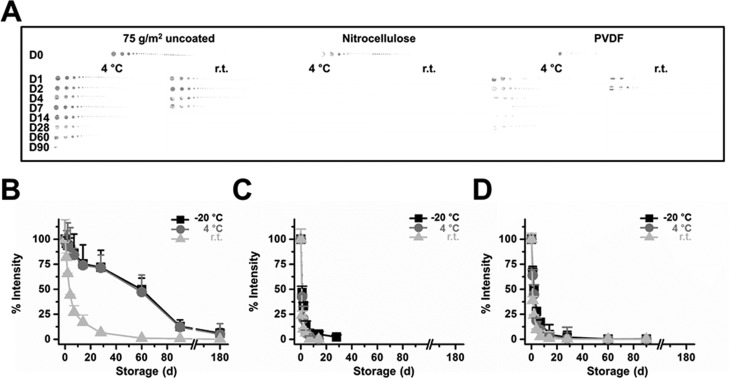
Stability Measurement of Membrane-Printed IgG-HRP
Three different paper-based membranes printed with the IgG-HRP antibody were stored at room temperature (r.t.), 4 °C or −20 °C for 1–180 days, and the HRP signal was monitored (Figure 2A). The data suggest that the signal intensity from IgG-HRP printed on printing paper decreased by half on days 56.3 and 57.1 (from 0.8 cm diameter dot) under 4 and −20 °C storage conditions, respectively, while that at r.t. was 6.7 days (Figure 2B–D). The chemiluminescence intensity of IgG-HRP printed on both nitrocellulose and PVDF membranes decreased by half on day 1.3 and day 2.5, respectively. Storage temperature does not induce >10% changes in the signal for nitrocellulose and PVDF membranes. No chemiluminescence signals from the printed IgG-HRP antibody were detected after 6 months of storage for printing paper or nitrocellulose and PVDF membranes.
Figure 2.
Stability of the printed anti-IgG-HRP antibody. (A) Chemiluminescence data from the printed IgG-HRP antibody (r.t. and −4 °C). −20 °C not shown. The chemiluminescence intensity profile from the printed anti-IgG-HRP antibody on (B) printing paper (C) nitrocellulose (D) PVDF after the designated storage period. Each experiment was performed in triplicate, and values are expressed as mean ± SD.
Detection of Printed PSMA and TNF-α on a Paper-Based Platform
Prostate-specific membrane antigen (PSMA) and tumor necrosis factor alpha (TNF-α) recombinant proteins were printed on printing paper and nitrocellulose and PVDF membranes (Figure 3A,B). After the designated storage period, PSMA/TNF-α printed paper strips were incubated with antibodies-HRP, and HRP signals were monitored. The chemiluminescence signal intensity from anti-PSMA and anti-TNF-α antibodies conjugated with HRP increased as the concentration of printed PSMA and TNF-α increased (Figure 3C,D). PSMA and TNF-α printed on printing paper showed the highest signal intensity from antibody-HRP (2016 and 2786, respectively, from a 0.8 cm diameter dot, arbitrary unit), followed by PVDF (1073 and 1932, respectively) and nitrocellulose membrane (496 and 693, respectively).
Figure 3.
Printed TNF-α and PSMA detection on a paper-based platform. (A) Schematic image of the printed TNF-α and PSMA detection model, (B) chemiluminescence from printed TNF-α and PSMA. Chemiluminescence intensity profile from printed (C) PSMA and (D) TNF-α on a paper-based platform. Each experiment was performed in triplicate, and values are expressed as mean ± SD.
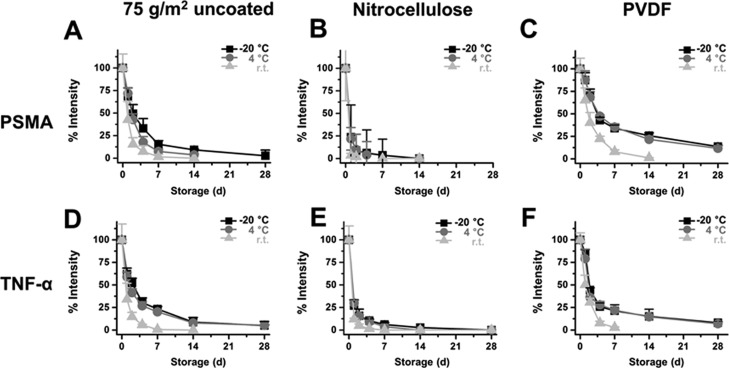
Stability Monitoring of Paper-Based Sensors of PSMA and TNF-α
Printed PSMA and TNF-α were stored at r.t., 4, or −20 °C for 28 days, and chemiluminescence signal intensities from antibody-HRP were monitored. Signal intensity from printed PSMA was reduced by half on days 3.3, 3.1, and 2.6 for −20, 4 °C, and r.t. storage, respectively, for printing paper (Figure 4A), while that of nitrocellulose was 0.9 ± 0.2 (Figure 4B). Times to reach half intensities compared to the original signal for printed PSMA on the PVDF membrane were 4.1, 3.8, and 2.9 days for −20, 4 °C, and r.t. storage (Figure 4C). Signal intensity from printed TNF-α was similar to printed PSMA, and the time to reach half intensities of the initial signal was the longest for the PVDF membrane followed by printing paper and nitrocellulose membrane (Figure 4D–F). No signals were detected.
Figure 4.
Stability of printed PSMA and TNF-α on a paper-based platform. The chemiluminescence profile from printed PSMA on (A) printing paper, (B) nitrocellulose, and (C) PVDF and from TNF-α on (D) printing paper, (E) nitrocellulose, and (F) PVDF membrane. Each experiment was performed in triplicate, and values are expressed as the mean ± SD.
Discussion
We first tested whether inkjet-printed proteins on a paper-based membrane are functional. We focused on maintenance of functional ability of printed HRP and structural integrity of printed PSMA and TNF-α proteins. Varying concentrations of anti-IgG HRP antibodies (1–100 μg/mL) were printed on printing paper and nitrocellulose and PVDF membranes, and the HRP substrate was added to detect signals from the printed antibodies (Figure 1A). As low as 1 μg/mL of printed IgG-HRP antibodies can be detected on paper-based membranes (Figure 1B,C), which suggests that piezo inkjet printing does not induce changes or damage to the function of printed antibodies. The concentration of the anti-IgG-HRP antibody versus the chemiluminescence signal intensity from the printed IgG-HRP plot showed a linear relationship, with R-squared values of 0.952, 0.931, and 0.946 for printing paper, nitrocellulose membrane, and PVDF membrane, respectively (Figure 1C). These data suggest that the signal intensity from printed IgG-HRP is a good reflection of the concentration of IgG-HRP. IgG-HRP-printed printing paper and nitrocellulose and PVDF membranes were stored at three different temperature conditions: r.t. (∼22 °C), 4, and −20 °C, to test the effect of storage temperature on printed protein functionality and stability. Chemiluminescence signal intensities from printed IgG-HRP antibodies on printing paper and nitrocellulose and PVDF membranes showed less than 10% variation, which suggests that the binding capacity of the three paper-based membranes tested are quite similar. The HRP signal from printed IgG-HRP on printing paper decreased rapidly during the initial 5 days when stored at r.t. However, 4 and −20 °C significantly increased printed protein stability, and the signal intensity from HRP remained >50% up to 60 days. Signals from printed HRP on printing paper can be detected up to 180 days when stored at −20 °C. These data suggest that storing temperature is critical in maintaining printed protein functionalities. However, when the same protein was printed on the nitrocellulose membrane, the signal intensity from HRP dropped rapidly over the first 2 days, regardless of storage temperature (Figure 2C). Although protein molecules bind to nitrocellulose membranes through hydrophobic interactions, printed proteins on nitrocellulose membranes lose functionality in a very short amount of time. IgG-HRP antibodies printed on PVDF also showed significant signal intensity drops over the first 5 days; however, signals from HRP can be detected up to 6 months when stored at 4 and −20 °C.
We next tested whether printed proteins on paper-based membranes can be detected by antibodies. Two recombinant proteins, PSMA and TNF-α, were printed and HRP conjugated antibodies against the two proteins were used to detect the printed proteins. Although signals from anti-PSMA-HRP and anti-TNF-α HRP were lower compared to the directly printed IgG-HRP antibody, printed PSMA and TNF-α were still detected when printed on all three paper-based membranes. Signal intensities from the detection antibody were highest on printing paper followed by PVDF and nitrocellulose membrane; however, they did not show a linear relationship with the concentration of printed PSMA and TNF-α (Figure 3C,D). These data may suggest that the signal intensities from colorimetric/immunosensors may not exactly reflect the actual concentration of antigens. We again tested whether printed proteins can be detected by detection antibodies after storage at various storage temperatures. It took ∼3 days for signals to be reduced to half of the initial intensity from printed proteins on printing paper (Figure 3A,D). Storage temperatures have a minimal effect on signal intensity in the first 3 days; however, storing at −20 °C increases printed protein stability after 7 days of storage.
Although the signals from printed PSMA and TNF-α on PVDF membranes were lower than those from printing paper, signal intensity from printed proteins remains longer at 4 °C compared to that from printing paper (Figure 4C,F). Storing at 4 and −20 °C also increases printed protein stability compared to r.t. Printed PSMA and TNF-α antibodies on the PVDF membrane and printing paper retains >50% activity for 4.1 and 3.5 days, respectively, when stored lower than 4 °C. Two antibodies on the PVDF membrane and printing paper retains >25% activity for 14.2 and 5.8 days when stored at −20 °C. PSMA and TNF-α printed on the nitrocellulose membrane showed significantly reduced signal intensities from detection antibodies compared to printing paper and PVDF membranes (Figure 4B,E). Low-temperature storage increases the chemiluminescence signal upon storing; however, the signal only lasts up to 7 and 14 days for PSMA and TNF-α, respectively. These data suggest that although nitrocellulose membranes are quite often used for various protein detection purposes, immobilized protein stability upon storing cannot be guaranteed compared to the PVDF membrane or even simple printing paper.
However, our studies focus more on protein stability and storage temperature and not on detection sensitivity. Paper-based sensing platforms adopting nanoparticles (plasmonic gold and quantum dot) and microelectronics have detection limits up to pictograms of proteins.28 Although this study adopted a simple colorimetric detection method using HRP, we believe that nanoparticles and even electronic or mechanistic sensing methods can be applied in a paper-based platform.29−34 Overall, our data suggest that proteins and antibodies printed on simple printing paper can be stable up to several months upon storage at low temperature. Our results can be further applied to developing a simple and cost-effective antigen/virus/pollutant test based on paper membranes.
Overall, our data also show that proteins printed on simple printing paper retain functionality for a long duration of time compared to nitrocellulose or PVDF membranes when stored at low temperature.
Experimental Section
Materials
Hybond-P PVDF and ECL nitrocellulose membranes were purchased from GE Healthcare (Chicago, IL). The 75 g/m2 uncoated print paper (printing paper) was purchased from Hankuk Paper (Seoul, Republic of Korea). The antimouse IgG-HRP antibody was purchased from Cell Signaling Technology (Danvers, MA). The recombinant human PSMA protein was purchased from Abcam (Cambridge, UK). Recombinant human TNF-α protein was obtained from Sigma-Aldrich (St. Louis, MO). Anti-PSMA antibody-HRP was obtained from Lifespan Biosciences (Washington, WA). Anti-TNF-α antibody-HRP was purchased from Abcam. The ECL western blot detection system was purchased from GE Healthcare. The piezo printhead printer (XP-310) was obtained from Epson (Suwa, Japan).
Printing and Detection of Anti-IgG-HRP Antibody
First, 1–100 μg/mL IgG-HRP antibody diluted with molecular biology grade water (Corning, Corning, NY) was loaded on the piezo printhead printer and printed on printing paper or PVDF and nitrocellulose membranes. IgG-HRP printed paper-based platforms were then cut into 1.5 × 10 cm strips. Finally, 1 mL of ECL western blotting detection reagents and the ImageQuant LAS 500 system (GE Healthcare) were used to record chemiluminescence intensity from printed IgG-HRP (intensity from 0.8 cm diameter circular dot).
Stability Measurement of Membrane-Printed IgG-HRP
IgG-HRP-printed paper-based platforms (PVDF, nitrocellulose, and printing paper) were stored at r.t. or 4 or −20 °C for the designated storage period. After 1, 2, 4, 7, 14, 28, 60, 90, and 180 days of storage, chemiluminescence intensity from printed IgG-HRP was monitored for printed IgG-HRP stability.
Stability Monitoring of Paper-Based Sensors of PSMA and TNF-α
PSMA (1–100 μg/mL) and TNF-α recombinant proteins in molecular biology grade water were printed on paper-based platforms and stored at three different conditions for 1–180 days. After the designated storage period, PSMA and TNF-α printed paper sensors were incubated with 1:2000 diluted anti-TNF-α HRP and anti-PSMA HRP followed by exposure to ECL western blotting detection reagents. Chemiluminescence intensities were recorded and analyzed with an ImageQuant LAS 500 and Bio-Rad Image Lab software (Hercules, CA).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (version 6, GraphPad Software, Inc., CA and USA) and Origin 8.5 (OriginLab Corporation, Northampton, MA). Each experiment was performed in triplicate, and values are expressed as the mean ± standard deviation (SD).
Acknowledgments
This research was supported by the Korea Research Institute of Chemical Technology core project (SKO-1930-20).
Author Contributions
The manuscript was written through contributions of all the authors. All the authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Woo P. C. Y.; Lau S. K. P.; Chen Y.; Wong E. Y. M.; Chan K.-H.; Chen H.; Zhang L.; Xia N.; Yuen K.-Y. Rapid Detection of MERS Coronavirus-like Viruses in Bats: Potential for Tracking MERS Coronavirus Transmission and Animal Origin. Emerging Microbes Infect. 2018, 7, 1. 10.1038/s41426-017-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theel E. S.; Hata D. J. Diagnostic Testing for Zika Virus: A Postoutbreak Update. J. Clin. Microbiol. 2018, 56, e01972–17. 10.1128/JCM.01972-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regla-Nava J. A.; Viramontes K. M.; Vozdolska T.; Huynh A.-T.; Villani T.; Gardner G.; Johnson M.; Ferro P. J.; Shresta S.; Kim K. Detection of Zika Virus in Mouse Mammary Gland and Breast Milk. PLoS Neglected Trop. Dis. 2019, 13, e0007080. 10.1371/journal.pntd.0007080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.; Wang H.; Cao Z.; Jin H.; Chi H.; Zhao J.; Yu B.; Yan F.; Hu X.; Wu F.; et al. A Rapid and Specific Assay for the Detection of MERS-CoV. Front. Microbiol. 2018, 9, 1101. 10.3389/fmicb.2018.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee K.; Green A. A.; Takahashi M. K.; Braff D.; Lambert G.; Lee J. W.; Ferrante T.; Ma D.; Donghia N.; Fan M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. 10.1016/j.cell.2016.04.059. [DOI] [PubMed] [Google Scholar]
- Afsahi S.; Lerner M. B.; Goldstein J. M.; Lee J.; Tang X.; Bagarozzi D. A.; Pan D.; Locascio L.; Walker A.; Barron F.; et al. Novel Graphene-Based Biosensor for Early Detection of Zika Virus Infection. Biosens. Bioelectron. 2018, 100, 85–88. 10.1016/j.bios.2017.08.051. [DOI] [PubMed] [Google Scholar]
- Nidzworski D.; Siuzdak K.; Niedziałkowski P.; Bogdanowicz R.; Sobaszek M.; Ryl J.; Weiher P.; Sawczak M.; Wnuk E.; Goddard W. A.; et al. A Rapid-Response Ultrasensitive Biosensor for Influenza Virus Detection Using Antibody Modified Boron-Doped Diamond. Sci. Rep. 2017, 7, 15707. 10.1038/s41598-017-15806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. H.; Kim S. J.; Jafry A. T.; Lee B.; Heo J. H.; Yoon S.; Jeong S. H.; Kang S. I.; Lee J. H.; Lee J. A Paper-Based Platform for Long-Term Deposition of Nanoparticles with Exceptional Redispersibility, Stability, and Functionality. Part. Part. Syst. Charact. 2019, 36, 1800483. 10.1002/ppsc.201800483. [DOI] [Google Scholar]
- López-Marzo A. M.; Merkoçi A. Paper-Based Sensors and Assays: A Success of the Engineering Design and the Convergence of Knowledge Areas. Lab Chip 2016, 16, 3150–3176. 10.1039/C6LC00737F. [DOI] [PubMed] [Google Scholar]
- Singh A.; Lantigua D.; Meka A.; Taing S.; Pandher M.; Camci-Unal G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. 10.3390/s18092838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J.; Lee B.-H.; Kim S.-Y.; Park J.-Y.; Bae H.; Choi Y.-K.; Ahn J.-H. Nanoscale FET-Based Transduction toward Sensitive Extended-Gate Biosensors. ACS Sens. 2019, 4, 1724–1729. 10.1021/acssensors.9b00731. [DOI] [PubMed] [Google Scholar]
- Pardee K.; Green A. A.; Ferrante T.; Cameron D. E.; DaleyKeyser A.; Yin P.; Collins J. J. Paper-Based Synthetic Gene Networks. Cell 2014, 159, 940–954. 10.1016/j.cell.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihar E.; Wustoni S.; Pappa A. M.; Salama K. N.; Baran D.; Inal S. A Fully Inkjet-Printed Disposable Glucose Sensor on Paper. npj Flex. Electron. 2018, 2, 30. 10.1038/s41528-018-0044-y. [DOI] [Google Scholar]
- Markina M.; Stozhko N.; Krylov V.; Vidrevich M.; Brainina K. Nanoparticle-Based Paper Sensor for Thiols Evaluation in Human Skin. Talanta 2017, 165, 563–569. 10.1016/j.talanta.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Parrilla M.; Guinovart T.; Ferré J.; Blondeau P.; Andrade F. J. A Wearable Paper-Based Sweat Sensor for Human Perspiration Monitoring. Adv. Healthcare Mater. 2019, 8, e1900342. 10.1002/adhm.201900342. [DOI] [PubMed] [Google Scholar]
- Cinti S.; Moscone D.; Arduini F. Preparation of Paper-Based Devices for Reagentless Electrochemical (Bio)Sensor Strips. Nat. Protoc. 2019, 14, 2437. 10.1038/s41596-019-0186-y. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Ghosh A.; Jose D. A. A Simple Colorimetric Sensor for the Detection of Moisture in Organic Solvents and Building Materials: Applications in Rewritable Paper and Fingerprint Imaging. Analyst 2019, 144, 594–601. 10.1039/C8AN01042K. [DOI] [PubMed] [Google Scholar]
- Lin C.; Zhu Y.; Yu J.; Qin X.; Xian X.; Tsow F.; Forzani E. S.; Wang D.; Tao N. Gradient-Based Colorimetric Sensors for Continuous Gas Monitoring. Anal. Chem. 2018, 90, 5375–5380. 10.1021/acs.analchem.8b00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva Sharma A.; SasiKumar T.; Ilanchelian M. A Rapid and Sensitive Colorimetric Sensor for Detection of Silver Ions Based on the Non-Aggregation of Gold Nanoparticles in the Presence of Ascorbic Acid. J. Cluster Sci. 2018, 29, 655–662. 10.1007/s10876-018-1375-5. [DOI] [Google Scholar]
- Byrne B.; Stack E.; Gilmartin N.; O’Kennedy R. Antibody-Based Sensors: Principles, Problems and Potential for Detection of Pathogens and Associated Toxins. Sensors 2009, 9, 4407–4445. 10.3390/s90604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.; Byrne H.; O’Kennedy R. J. Antibodies and Antibody-Derived Analytical Biosensors. Essays Biochem. 2016, 60, 9–18. 10.1042/EBC20150002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Broyles D.; Hunt E. A.; Dikici E.; Daunert S.; Deo S. K. A Paper-Based Platform for Detection of Viral RNA. Analyst 2017, 142, 815–823. 10.1039/c6an02452a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. J.; Negrete O. A.; Van Rompay K. K. Engineering Paper-Based Sensors for Zika Virus. Trends Mol. Med. 2016, 22, 529–530. 10.1016/j.molmed.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Li X.; Scida K.; Crooks R. M. Detection of Hepatitis B Virus DNA with a Paper Electrochemical Sensor. Anal. Chem. 2015, 87, 9009–9015. 10.1021/acs.analchem.5b02210. [DOI] [PubMed] [Google Scholar]
- Noroozi M.; Keypour H. Novel Mefenamic Acid PVC Membrane Sensor Based on a New Cd Schiff’s Base Complex Containing a Phenanthroline Unit. RSC Adv. 2017, 7, 39118–39126. 10.1039/C7RA06821B. [DOI] [Google Scholar]
- Li Q.-Y.; Li Y.-A.; Guan Q.; Li W.-Y.; Dong X.-J.; Dong Y.-B. UiO-68-PT MOF-Based Sensor and Its Mixed Matrix Membrane for Detection of HClO in Water. Inorg. Chem. 2019, 58, 9890. 10.1021/acs.inorgchem.9b01032. [DOI] [PubMed] [Google Scholar]
- Azmi N. A.; Ahmad S. H.; Low S. C. Detection of Mercury Ions in Water Using a Membrane-Based Colorimetric Sensor. RSC Adv. 2018, 8, 251–261. 10.1039/C7RA11450H. [DOI] [Google Scholar]
- Lee J. H.; Kang S.; Lee J. Y.; Jaworski J.; Jung J. H. Instant Visual Detection of Picogram Levels of Trinitrotoluene by Using Luminescent Metal-Organic Framework Gel-Coated Filter Paper. Chem.—Eur. J. 2013, 19, 16665–16671. 10.1002/chem.201301507. [DOI] [PubMed] [Google Scholar]
- Yang T.; Mativetsky J. M. Paper-Based Mechanical Sensors Enabled by Folding and Stacking. ACS Appl. Mater. Interfaces 2019, 11, 26339. 10.1021/acsami.9b06071. [DOI] [PubMed] [Google Scholar]
- Sher M.; Zhuang R.; Demirci U.; Asghar W. Paper-Based Analytical Devices for Clinical Diagnosis: Recent Advances in the Fabrication Techniques and Sensing Mechanisms. Expert Rev. Mol. Diagn. 2017, 17, 351–366. 10.1080/14737159.2017.1285228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebretsadik T.; Belayneh T.; Gebremichael S.; Linert W.; Thomas M.; Berhanu T. Recent Advances in and Potential Utilities of Paper-Based Electrochemical Sensors: Beyond Qualitative Analysis. Analyst 2019, 144, 2467–2479. 10.1039/C8AN02463D. [DOI] [PubMed] [Google Scholar]
- Liana D. D.; Raguse B.; Gooding J. J.; Chow E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. 10.3390/s120911505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güder F.; Ainla A.; Redston J.; Mosadegh B.; Glavan A.; Martin T. J.; Whitesides G. M. Paper-Based Electrical Respiration Sensor. Angew. Chem., Int. Ed. 2016, 55, 5727–5732. 10.1002/anie.201511805. [DOI] [PubMed] [Google Scholar]
- Li M.; Then W. L.; Li L.; Shen W. Paper-Based Device for Rapid Typing of Secondary Human Blood Groups. Anal. Bioanal. Chem. 2014, 406, 669–677. 10.1007/s00216-013-7494-9. [DOI] [PubMed] [Google Scholar]