Abstract
This study was conducted to explore the in vitro fermentation characteristics for different ratios of soluble to insoluble dietary fiber in pig fecal microbiota. The fermentation substrates consisted of inulin and a non-starch polysaccharide mixture and were divided into five groups according to different soluble dietary fiber (SDF) to insoluble dietary fiber (IDF) ratios (SDF 25, 50, 75, and 100%). With the increased SDF ratio, the total gas production increased, and the pH in the substrate decreased as the fermentation proceeded. The concentrations of lactic acid, formic acid, and acetic acid increased in the high SDF ratio group, whereas the concentrations of propionic acid and butyric acid increased in the low SDF ratio group. The genera Clostridium_sensu_stricto_1, Ruminococcaceae_NK4A214_group, Christensenellaceae_R-7_group, and Rikenellaceae_RC9_gut_group were enriched in the high SDF ratio group. Correlation analysis indicated that these differential bacteria had the potential to degrade polysaccharides. These results revealed that high SDF ratios could stimulate the proliferation of fibrolytic bacteria, which in turn degrade fibers to produce organic acids and monosaccharides. Collectively, these findings add to our understanding of the mechanisms responsible for interaction between SDF and intestinal microbiota and provide new ideas for the rational use of dietary fiber.
Introduction
Dietary fiber (DF) represents the fraction of carbohydrates and lignin not digested by endogenous digestive enzymes of animals.1 Although DF has a negative impact on energy and nutrient digestibility, it increasingly attracts interests due to its fermentable fractions and beneficial effects on gut health.2 According to its solubility, DF is usually divided into two categories: soluble dietary fiber (SDF) and insoluble dietary fiber (IDF). The SDF includes pectin, gum, and inulin, which could slow down the digestion rate, regulate the immune system function, promote the discharge of toxic heavy metals from the body, lengthen the time it takes for the stomach to empty, slowing the absorption of glucose, lower blood cholesterol levels, and reduce the retention time of excreta in the intestine.3 The IDF includes cellulose, hemicellulose, and lignin, which are reported to be a contributing factor for body fluids and blood circulation and could reduce the risk of bowel cancer, increase the volume of feces, smooth bowel movements, prevent constipation, and lessen the toxins from bacteria in the digestive tract.4 These beneficial functions are partially mediated by the SCFAs, which serve as the major energy source for intestinal epithelial cells and could promote intestinal mucosal growth and improve intestinal health.5 There are great variations in the compositions and contents of DF in the feedstuffs, which determine their physicochemical properties in the intestinal tract of pigs and in turn affect the fermentation characteristics of the intestinal microbiota.6 Therefore, the selective addition of DF to the feedstuff can be used as a nutritional strategy to optimize the intestinal health in pigs.
Bacteria account for the majority in the gut microbiota of pigs. The number of microorganisms per gram of large intestine content in pigs is about 1010–1011, including more than 50 genera and 500 species of bacteria.7 The structure and composition of the diet as well as the solubility, amount, and type of available substrates have important influences on the quantity and viability of the gut microbes.8 Dietary fiber can affect the digestive site and the intestinal microenvironment, thereby affecting the microbial proliferation in the gut. In addition, changes in the chemical structure of DF could affect their utilization by gut microbiota.8 In recent years, studies on various animal models have shown that different types of DFs have different effects on the digestion of nutrients in different parts of the intestine and on the fermentation process and intestinal microbiota.9−11 At present, the influence of DF on intestinal health of humans and animals and its interaction with intestinal microbiota are receiving increasing attention.1,6,12
Previous studies have shown that feeding different DFs has an inconsistent effect on intestinal health and productive phenotypes in pigs.13,14 There is evidence that pigs that consumed corn bran and wheat bran have better weight gain and feed efficiency than those fed soybean hulls. We speculated that the reason for this phenomenon may be the difference in the ratio of SDF to IDF in diets. Therefore, the objective of this study was to explore the fermentation characteristics of SDF/IDF with different ratios and their interaction with intestinal microbiota. Due to the single research variable, the controllable process, and the ability to monitor the fermentation dynamics in real time, numerous studies have adopted the in vitro fermentation to investigate the interaction between nutrients and gut microbiota in recent years.15,16 In the current study, the method of in vitro fermentation was also used to explore the dynamic changes of intestinal microbiota and DFs at different fermentation time points, using the microbiota in fresh feces of growing pigs as inocula and different SDF/IDF ratios as fermentation substrates.
Results
Gas Production and Changes in pH
In this experiment, we monitored the total gas production and real-time pH at eight time points including the start and endpoint of the fermentation. Gas production data at different time points are reported in Table 1. The different proportions of SDF produced similar amounts of gas when compared to each other at 4 h (P > 0.05). From 4 to 48 h, different ratios of SDF caused significant differences in gas production, which increased with the increased SDF ratio (P < 0.05). Similar to the trend of gas production, the pH decreased as the fermentation proceeded (P < 0.05; Table 2). For all five groups, the pH showed a slow decrease during the first 16 h of fermentation (P < 0.05). However, the rate of pH change increased as the SDF ratio increased within 16–48 h of fermentation (P < 0.05). Besides, there were significant differences in the pH of the five groups at all time points of fermentation (P < 0.05).
Table 1. Total Gas Production from Different Ratios of SDF Substrates at the Different Time Points of the in Vitro Fermentation (mL/g)a.
| item | SDF 0% | SDF 25% | SDF 50% | SDF 75% | SDF 100% | P value |
|---|---|---|---|---|---|---|
| 4 h | 5.72 ± 0.09 | 6.36 ± 0.10 | 6.30 ± 0.07 | 6.14 ± 0.07 | 6.16 ± 0.13 | 0.2 |
| 8 h | 5.98 ± 0.12c | 7.22 ± 0.04b | 7.50 ± 0.06b | 7.26 ± 0.09b | 7.48 ± 0.16b | <0.01 |
| 12 h | 5.98 ± 0.12e | 6.96 ± 0.10de | 7.64 ± 0.13cd | 8.04 ± 0.09bc | 8.84 ± 0.10b | <0.01 |
| 16 h | 5.98 ± 0.12e | 6.96 ± 0.10de | 8.10 ± 0.26cd | 8.64 ± 0.12c | 11.02 ± 0.16b | <0.01 |
| 24 h | 6.24 ± 0.09d | 12.28 ± 0.10cd | 18.02 ± 1.13cd | 21.48 ± 1.32c | 36.36 ± 2.99b | <0.01 |
| 32 h | 7.02 ± 0.09f | 22.16 ± 0.19e | 35.18 ± 1.73d | 57.94 ± 1.14c | 73.80 ± 1.88b | <0.01 |
| 40 h | 7.32 ± 0.09f | 23.74 ± 0.14e | 43.66 ± 1.60d | 64.72 ± 0.45c | 90.72 ± 1.64b | <0.01 |
| 48 h | 7.48 ± 0.09f | 24.20 ± 0.11e | 47.04 ± 1.17d | 68.14 ± 0.31c | 95.06 ± 0.89b | <0.01 |
Data are presented as mean ± SEM (n = 6), and values in the same row with different letter superscripts (b–f) means a significant difference (P < 0.05). SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
Table 2. pH Value in Fermentation Broth with Different Ratios of SDF Substrates and at the Different Time Points of the In Vitro Fermentationa.
| item | SDF 0% | SDF 25% | SDF 50% | SDF 75% | SDF 100% | P value |
|---|---|---|---|---|---|---|
| 4 h | 7.84 ± 0.04b | 7.70 ± 0.07bc | 7.82 ± 0.08bc | 7.76 ± 0.01bc | 7.56 ± 0.05c | 0.04 |
| 8 h | 7.84 ± 0.04b | 7.68 ± 0.07bc | 7.78 ± 0.05b | 7.75 ± 0.01b | 7.53 ± 0.05c | 0.01 |
| 12 h | 7.84 ± 0.04b | 7.63 ± 0.08bc | 7.71 ± 0.04bc | 7.65 ± 0.01bc | 7.48 ± 0.06c | 0.01 |
| 16 h | 7.83 ± 0.04b | 7.42 ± 0.11cd | 7.54 ± 0.05c | 7.08 ± 0.03e | 7.22 ± 0.05de | <0.01 |
| 24 h | 7.79 ± 0.03b | 7.14 ± 0.11c | 6.41 ± 0.09d | 6.33 ± 0.05e | 6.21 ± 0.24de | <0.01 |
| 32 h | 7.79 ± 0.06b | 7.11 ± 0.10c | 6.55 ± 0.06d | 6.12 ± 0.10e | 5.45 ± 0.02f | <0.01 |
| 40 h | 7.78 ± 0.05b | 7.14 ± 0.11bc | 6.62 ± 0.01c | 6.46 ± 0.10c | 5.54 ± 0.30d | <0.01 |
| 48 h | 7.76 ± 0.06b | 7.15 ± 0.13c | 6.68 ± 0.02d | 6.57 ± 0.10d | 5.59 ± 0.13e | <0.01 |
Data are presented as mean ± SEM (n = 6), and values in the same row with different letter superscripts (b–f) means a significant difference (P < 0.05). SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
Changes of Microbial Metabolites
Lactic acid and SCFA production data at different time points are reported in Table 3. In general, the SCFAs concentration increased with the increase in the proportion of SDF (P < 0.05). The concentration of formic acid and acetic acid among the five groups was significantly different at all time points (P < 0.05). The concentration of propionic acid was markedly different at 16 h (P < 0.05), whereas the concentration of butyric acid did not change significantly at 16 h of fermentation (P > 0.05). In addition, there were certain concentrations of propionic acid and butyric acid in the SDF 0% group, but these two acids were not detected in the other four groups at 32 and 48 h. Moreover, our results showed that the lactic acid contents between the five groups were significantly different at all time points (P < 0.05), and the differences among these groups increased as the proportion of SDF increased.
Table 3. Lactic Acid and SCFA Concentrations (mM) in Fermentation Broth with Different Ratios of SDF Substrates and at 16, 32, and 48 h of In Vitro Fermentationa.
| item | SDF 0% | SDF 25% | SDF 50% | SDF 75% | SDF 100% | P value |
|---|---|---|---|---|---|---|
| 16 h | ||||||
| lactic acid | 1.04 ± 0.03d | 1.68 ± 0.13c | 1.74 ± 0.10c | 2.25 ± 0.02b | 1.75 ± 0.07c | <0.01 |
| formic acid | 0.12 ± 0.01d | 1.32 ± 0.36c | 1.17 ± 0.07c | 2.46 ± 0.05b | 1.30 ± 0.08c | <0.01 |
| acetic acid | 0.47 ± 0.03d | 0.81 ± 0.11c | 0.77 ± 0.05c | 1.23 ± 0.02b | 0.79 ± 0.04c | <0.01 |
| propionic acid | 0.46 ± 0.03d | 4.14 ± 0.97c | 3.83 ± 0.27c | 7.19 ± 0.10b | 4.13 ± 0.36c | <0.01 |
| butyric acid | 0.06 ± 0.03 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.00 | 0.08 ± 0.02 | 0.69 |
| 32 h | ||||||
| Lactic acid | 1.21 ± 0.04e | 1.54 ± 0.16de | 6.79 ± 0.80cd | 9.20 ± 0.25bc | 14.53 ± 2.72b | <0.01 |
| Formic acid | 1.14 ± 0.01d | 9.54 ± 0.20cd | 11.28 ± 2.48c | 25.78 ± 0.99b | 31.72 ± 3.83b | <0.01 |
| Acetic acid | 3.49 ± 0.20d | 10.11 ± 0.26c | 12.43 ± 0.54c | 21.60 ± 1.26b | 26.58 ± 2.67b | <0.01 |
| Propionic acid | 0.36 ± 0.10 | ND | ND | ND | ND | |
| Butyric acid | 0.13 ± 0.13 | ND | ND | ND | ND | |
| 48 h | ||||||
| Lactic acid | 0.13 ± 0.13d | 1.40 ± 0.12d | 6.30 ± 0.11c | 10.86 ± 1.19b | 12.69 ± 1.14b | <0.01 |
| Formic acid | 1.05 ± 0.09e | 10.45 ± 0.40d | 16.06 ± 1.30d | 28.16 ± 1.62c | 39.13 ± 2.86b | <0.01 |
| Acetic acid | 3.70 ± 0.09e | 11.11 ± 0.14d | 12.28 ± 0.82d | 24.16 ± 0.93c | 28.64 ± 1.07b | <0.01 |
| Propionic acid | 0.35 ± 0.11 | ND | ND | ND | ND | |
| Butyric acid | 0.13 ± 0.11 | ND | ND | ND | ND | |
Data are presented as mean ± SEM (n = 6), and values in the same row with different letter superscripts (b–e) means a significant difference (P < 0.05). ND means that the metabolites are undetectable and multiple comparisons cannot be made. SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
Summary of 16S rRNA Gene Profiles and Diversity
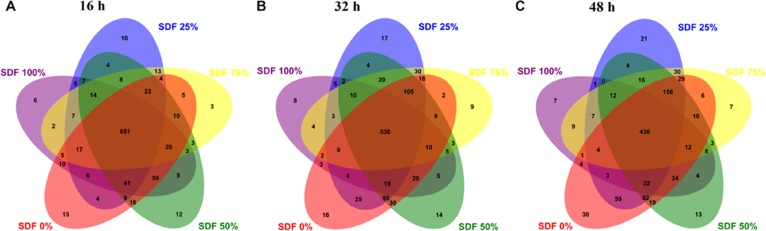
We totally collected 2,222,884 high-quality sequences of the V3–V4 region in 45 fermentation broth samples after quality control. The average numbers of high-quality sequence generated per sample were 49,397 from microbial populations. A total of 651, 536, and 436 core OTUs in microbial communities were identified at 16, 32, and 48 h, respectively. At 16 h, only 15 OTUs were found in the SDF 0% group, whereas 10, 3, 12, and 6 OTUs were specifically identified in the SDF 25%, SDF 50%, SDF 75%, and SDF 100% groups, respectively (Figure 1A). At 32 h, 16 OTUs were only found in the SDF 0% group, whereas 17, 14, 9, and 8 OTUs were specifically identified in the SDF 25%, SDF 50%, SDF 75%, and SDF 100% groups, respectively (Figure 1B). At 48 h, 30 OTUs were only found in the SDF 0% group, whereas 21, 13, 7, and 7 OTUs were specifically identified in the SDF 25%, SDF 50%, SDF 75%, and SDF 100% groups, respectively (Figure 1C).
Figure 1.
Venn diagrams for bacterial OTU compositions after fermentation for (A) 16, (B) 32, and (C) 48 h. SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
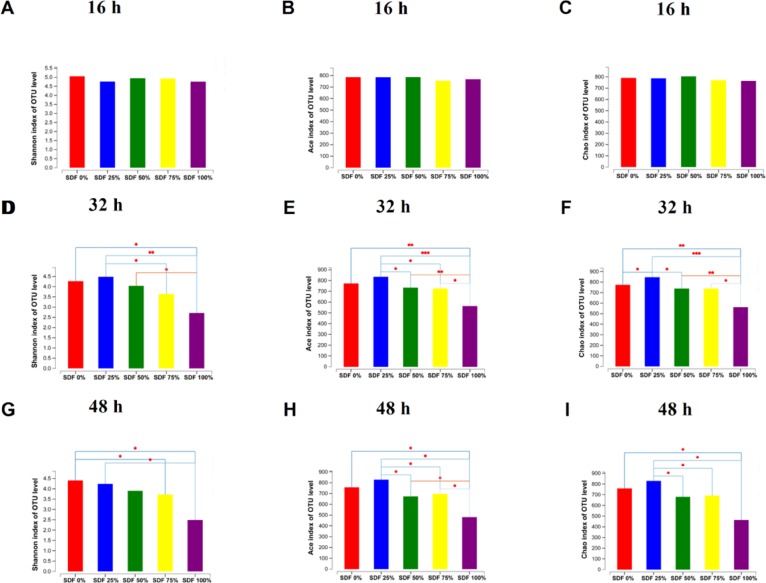
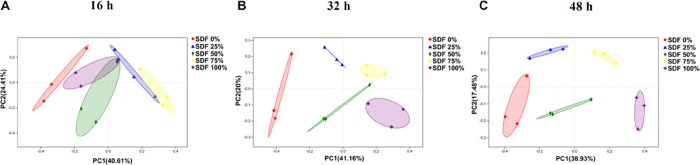
The difference in microbial α-diversity of fermentation broths from the five groups is shown in Figure 2. At 16 h, the Shannon, ACE, and Chao indexes did not change among the five groups (P > 0.05; Figure 2A–C). At 32 and 48 h, the Shannon, ACE, and Chao indexes decreased as the SDF ratio increased (P < 0.05; Figure 2D–I). Microbial β-diversity had little shift at 16 h (Figure 3A). However, PCoA based on the Bray–Curtis distances showed a shift in the microbial β-diversity of fermentation broths at 32 and 48 h (Figure 3B,C).
Figure 2.
Bacterial α-diversity after fermentation for (A–C) 16, (D–F) 32 h, and (G–I) 48 h. Data are represented as mean (n = 3; *P < 0.05, **P < 0.01, ***P < 0.001). SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
Figure 3.
Principal coordinates analysis (PCoA) based on the total operational taxonomic units (OTUs). PCoA after fermentation for (A) 16, (B) 32, and (C) 48 h. SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
Differential Analysis of Bacterial Communities
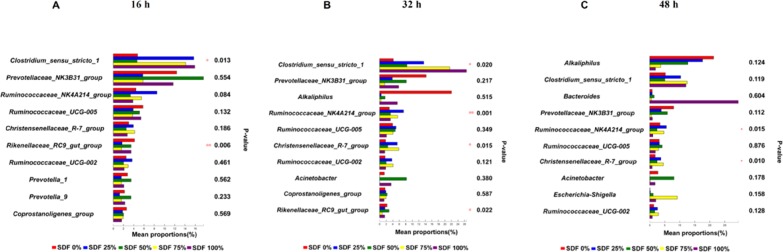
Significant differences in the relative abundance of genera in fermentation broths among the five groups at a certain time point were further identified using the multiple comparison analysis (Figure 4). We performed a differential analysis of the top 10 bacteria among the five groups at the genus level. At 16 h, the differential bacteria were Clostridium_sensu_stricto_1 and Rikenellaceae_RC9_gut_group (P < 0.05; Figure 4A). At 32 h, the differential bacteria were Clostridium_sensu_stricto_1, Ruminococcaceae_NK4A214_group, Christensenellaceae_R-7_group, and Rikenellaceae_RC9_gut_group (P < 0.05; Figure 4B). At 48 h, the differential bacteria were Ruminococcaceae_NK4A214_group and Christensenellaceae_R-7_group (P < 0.05; Figure 4C).
Figure 4.
Multiple comparisons at the genus level after fermentation for (A) 16, (B) 32, and (C) 48 h. Data are represented as mean (n = 3; *P < 0.05,**P < 0.01). SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
We further performed LEfSe analysis to identify bacteria that differed significantly among different SDF ratio groups in this study. At 16 h, the genera Clostridium_sensu_stricto_1 and Rikenellaceae_RC9_gut_group were significantly enriched in the SDF 100 and 0% groups, respectively (Figure 5A). At 32 h, the genera Clostridium_sensu_stricto_1, Ruminococcaceae_NK4A214_group, and Christensenellaceae_R-7_group were significantly enriched in the SDF 100, 25, and 75% group, respectively (Figure 5B). At 48 h, Ruminococcaceae_NK4A214_group and Christensenellaceae_R-7_group were significantly enriched in the SDF 25 and 75% groups, respectively (Figure 5C).
Figure 5.
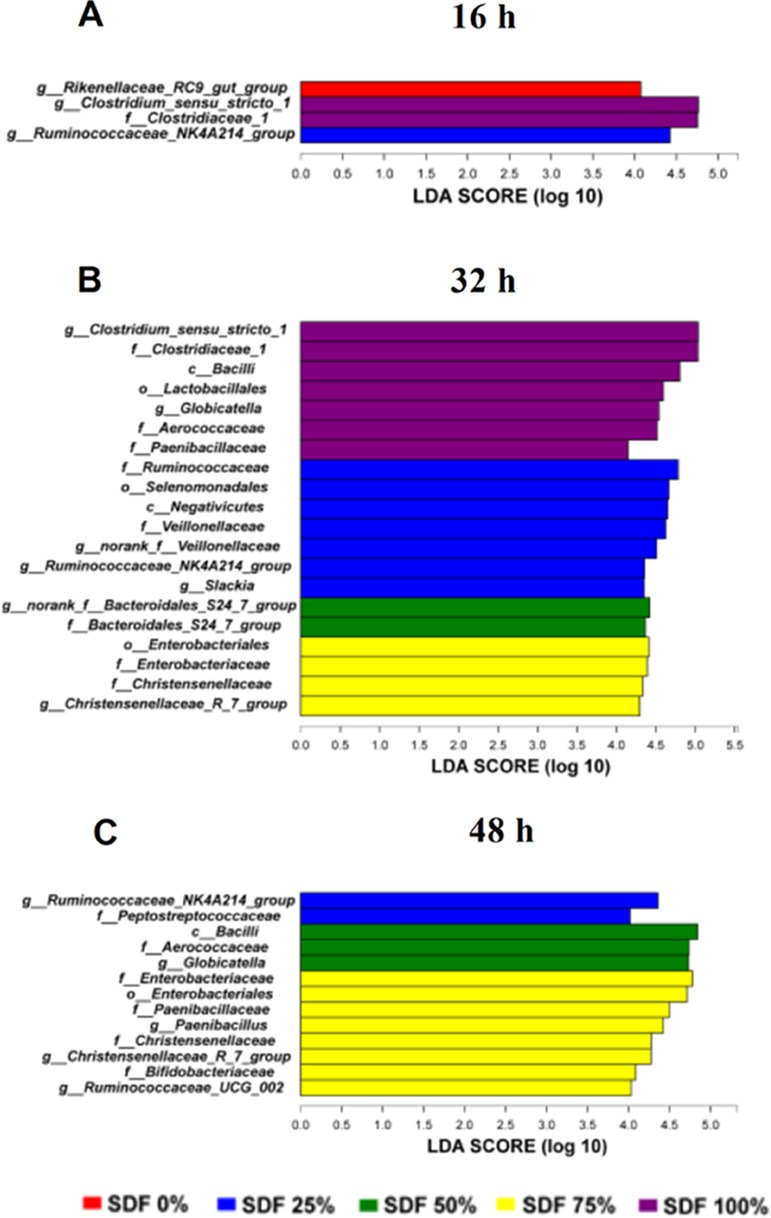
Histograms of a linear discriminant analysis (LDA) score (threshold ≥4) after fermentation for (A) 16, (B) 32, and (C) 48 h. SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
Monosaccharide Production and Correlation Analysis
We tested the concentrations of isodulcite, arabinose, galactose, glucose, mannose, and fructose at 16, 32, and 48 h in five different SDF ratio groups (Table 4). The isodulcite concentration in the five groups dramatically changed at 16 h of fermentation (P < 0.05); however, there was no significant alteration at 32 and 48 h (P > 0.05). The arabinose and fructose concentrations in the five groups dramatically changed at 16 and 32 h of fermentation (P < 0.05); however, there was no significant alteration at 48 h (P > 0.05). The galactose concentration in the five groups did not change significantly at 16, 32, and 48 h of fermentation (P > 0.05). The glucose and mannose concentration in the five groups did not change significantly at 16 h of fermentation (P > 0.05); however, they dramatically changed at 32 and 48 h of fermentation (P < 0.05).
Table 4. Monosaccharide Concentrations (μg/mL) in Fermentation Broth with Different Ratios of SDF Substrates and at 16, 32, and 48 h of In Vitro Fermentationa.
| item | SDF 0% | SDF 25% | SDF 50% | SDF 75% | SDF 100% | P value |
|---|---|---|---|---|---|---|
| 16 h | ||||||
| isodulcite | 0.16 ± 0.01bc | 0.21 ± 0.02bc | 0.23 ± 0.01b | 0.16 ± 0.02bc | 0.15 ± 0.02c | 0.02 |
| arabinose | 0.01 ± 0.00d | 0.17 ± 0.14d | 0.68 ± 0.06c | 0.91 ± 0.20bc | 1.23 ± 0.04b | <0.01 |
| galactose | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.12 ± 0.05 | 0.21 |
| glucose | 0.02 ± 0.01 | 0.03 ± 0.00 | 0.05 ± 0.02 | 0.10 ± 0.04 | 0.19 ± 0.09 | 0.13 |
| mannose | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.12 ± 0.05 | 0.07 ± 0.01 | 0.06 ± 0.02 | 0.15 |
| fructose | 0.08 ± 0.02c | 0.27 ± 0.13c | 0.58 ± 0.23c | 2.65 ± 2.15bc | 4.72 ± 0.30b | <0.01 |
| 32 h | ||||||
| isodulcite | 0.17 ± 0.00 | 0.19 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.03 | 0.28 ± 0.05 | 0.09 |
| arabinose | 0.31 ± 0.27c | 0.37 ± 0.24c | 1.43 ± 0.28bc | 2.18 ± 0.39bc | 2.66 ± 0.83b | 0.02 |
| galactose | 0.09 ± 0.01 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.76 ± 0.65 | 0.44 |
| glucose | 0.04 ± 0.03c | 0.03 ± 0.00c | 0.07 ± 0.02bc | 0.12 ± 0.03bc | 0.28 ± 0.08b | 0.01 |
| mannose | 0.04 ± 0.01c | 0.03 ± 0.02c | 0.05 ± 0.03c | 0.12 ± 0.02bc | 0.29 ± 0.09b | 0.01 |
| fructose | 1.19 ± 1.14bc | 0.08 ± 0.04c | 0.01 ± 0.00c | 0.29 ± 0.15c | 19.03 ± 8.09b | 0.02 |
| 48 h | ||||||
| isodulcite | 0.52 ± 0.35 | 0.20 ± 0.00 | 0.21 ± 0.01 | 0.18 ± 0.02 | 0.25 ± 0.03 | 0.63 |
| arabinose | 1.47 ± 0.77 | 1.06 ± 0.62 | 1.06 ± 0.29 | 1.58 ± 1.36 | 1.46 ± 0.48 | 0.98 |
| galactose | 0.05 ± 0.03 | 0.01 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.03 | 0.09 ± 0.05 | 0.42 |
| glucose | 0.06 ± 0.03bc | 0.01 ± 0.01c | 0.08 ± 0.02bc | 0.12 ± 0.04bc | 0.24 ± 0.07b | 0.02 |
| mannose | 0.05 ± 0.03cd | 0.02 ± 0.02d | 0.20 ± 0.04cd | 0.30 ± 0.08bc | 0.50 ± 0.10b | <0.01 |
| fructose | 2.53 ± 1.32 | 0.14 ± 0.14 | 0.59 ± 0.20 | 1.68 ± 0.93 | 1.90 ± 0.96 | 0.36 |
Data are presented as mean ± SEM (n = 6), and values in the same row with different letter superscripts (b–d) means a significant difference (P < 0.05). SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and 4SDF 100% means SDF/IDF = 1:0.
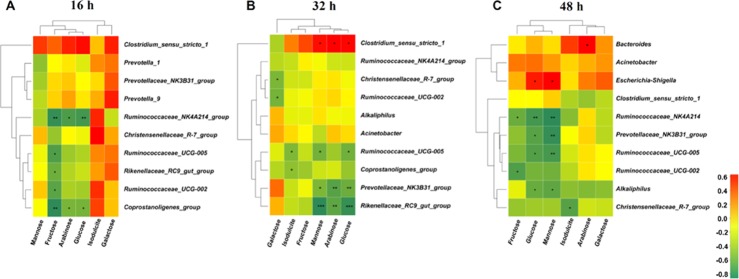
A Spearman’s correlation matrix was generated to explore the correlation between the top 10 bacterial genera (Figure 4) and monosaccharides (Table 4). As shown in Figure 6, significant associations were identified between the microbiota and the monosaccharides at 16, 32, and 48 h of fermentation. At 16 h (Figure 6A), the correlation analysis revealed that the fructose level was negatively correlated with the genera Ruminococcaceae_NK4A214_group, Ruminococcaceae_UCG-005, Rikenellaceae_RC9_gut_group, Ruminococcaceae_UCG-002, and Coprostanoligenes_group. The arabinose and glucose levels were negatively correlated with the genera Ruminococcaceae_NK4A214_group and Coprostanoligenes_group. At 32 h (Figure 6B), the correlation analysis revealed that the galactose level was negatively correlated with the genera Christensenellaceae_R-7_group and Ruminococcaceae_UCG-002. The isodulcite level was negatively correlated with the genera Ruminococcaceae_UCG-005 and Coprostanoligenes_group. The mannose and glucose levels were positively correlated with the genus Clostridium_sensu_stricto_1 and negatively correlated with the genera Prevotellaceae_NK3B31_group, Ruminococcaceae_UCG-005, and Christensenellaceae_R-7_group. The arabinose level was positively correlated with the genus Clostridium_sensu_stricto_1, whereas it was negatively correlated with the genera Prevotellaceae_NK3B31_group and Christensenellaceae_R-7_group. At 48 h (Figure 6C), the correlation analysis revealed that the isodulcite level was negatively correlated with the genus Christensenellaceae_R-7_group. The arabinose level was positively correlated with the genus Bacteroides. The fructose level was negatively correlated with the genera Ruminococcaceae_NK4A214_group and Ruminococcaceae_UCG-002. The glucose and mannose levels were positively correlated with the genus Escherichia–Shigella, whereas they were negatively correlated with the genera Ruminococcaceae_NK4A214_group, Prevotellaceae_NK3B31_group, Ruminococcaceae_UCG-005, and Alkaliphilus.
Figure 6.
Spearman correlation analysis between top 10 bacterial genera and monosaccharide concentrations after fermentation for (A) 16, (B) 32, and (C) 48 h. Asterisks indicate significant correlations between bacteria and monosaccharide. Cells are colored based upon the Spearman correlation coefficient between the significantly altered genera and monosaccharide; the red represents a significantly positive correlation (P < 0.05), the green represents a significantly negative correlation (P < 0.05), and the yellow represents no significant correlation (P > 0.05). n = 3 per group. SDF 0% means SDF/IDF = 0:1; SDF 25% means SDF/IDF = 1:3; SDF 50% means SDF/IDF = 1:1; SDF 75% means SDF/IDF = 3:1; and SDF 100% means SDF/IDF = 1:0.
Discussion
In recent years, many studies have shown that DF has an excellent effect on the intestinal health and production performance of pigs.9,10 Therefore, there is growing interest in its fermentable fraction in dietary composition. In this study, we used the fresh feces of pigs as inocula and different ratios of SDF and IDF in substrates to simulate an in vivo intestinal microenvironment using in vitro fermentation of intestinal microbiota. The aim was to investigate the interactions between different proportions of SDF and the intestinal microbiome. Our results revealed that with the increase of SDF ratios and the prolongation of fermentation time, the total gas production of the fermentation broths gradually increased, and the pH value gradually decreased and reached the plateau at 48 h of fermentation. Besides, the SCFAs concentration and the generation of monosaccharides varied with the SDF ratios and the fermentation time. Importantly, the gut microbiome in different fermentation times also underwent dramatic changes with different proportions of SDF groups. We also observed that certain specific microbes were closely related to the monosaccharide production and fiber utilization.
The generation of gas and the change of pH are two classic indicators reflecting the degree of fermentation.17 Previous studies have shown that different types of fiber-rich foods have significantly different fermentation kinetics in the gut of pigs. In addition, the fermentation of DF by pig intestinal microbes depends mainly on the specific composition of the DF, such as the content and proportion of SDF and IDF.18 In the current study, the gas production of all groups increased gradually during the first 16 h. The gas production in 16–32 h showed a jump increase, and the increase in gas production gentled within 32–48 h. In addition, the difference in pH is mainly related to the ratio of SDF, and the difference in pH among the five groups became larger as the fermentation time increased. Consistently, previous studies have shown that different components of DF have different fermentability in the intestine. The SDF could be rapidly fermented, and a higher ratio of IDF could reduce the degradability of DF.19,20
Dietary fiber could be metabolized by bacteria to produce lactic acid and SCFAs, and the rate of hydrolysis of dietary fiber determines the production of lactic acid and SCFAs.21 In this study, we tested the concentrations of lactic acid, formic acid, acetic acid, propionic acid, and butyric acid in substrates with different ratios of SDF at 16, 32, and 48 h of the in vitro fermentation. In general, the SCFA concentration increased with the increase in the proportion of SDF. Previous studies have found that d-tagatose could be fermented to produce formic acid in the large intestine of pigs.22 Our data revealed that the concentration of formic acid among the five groups was significantly different at all time points, and the differences among these groups increased as the proportion of SDF increased. This result indicated that certain components of SDF could be metabolized to produce formic acid by microorganisms. Acetic acid, propionic acid, and butyric acid are the major SCFAs in the human hindgut, which account for 90–95% of the total SCFAs.23 Our results showed that the concentration of acetic acid increased significantly in the high proportion of SDF groups (≥25%). This may be because soluble substrates are more susceptible to microbial degradation.24 As the major source of energy, propionic acid and butyric acid can be utilized by the intestinal epithelial cells.25 In the current study, there were certain concentrations of propionic acid and butyric acid in the 0% SDF group, but these two acids were not detected in the other four groups at the late fermentation stage. Besides, our results showed that the concentration of lactic acid was significantly higher in the high SDF proportion group than that in the low SDF proportion group. Previous study has shown that the concentration of acetic acid in the cecum of pigs fed inulin was significantly lower than that of the control group and the concentration of propionic acid was significantly increased.26 It is suggested that inulin has the potential to change the proportion of short-chain fatty acids in the hindgut. In the present study, the concentrations of propionic acid and butyric acid in the fermentation broths were not detected after 32 h of fermentation. There is evidence that some components of short-chain fatty acids were significantly lower than fermentation for 12 h after 24 h of in vitro fermentation of inulin.27 Therefore, we speculate that some of the short-chain fatty acid components in the fermentation system could be consumed by microorganisms, but further exploration is needed in the future studies. Studies have shown that inulin could be used as a functional food to promote intestinal health.28 However, there was evidence that inulin has the potential to induce cholestatic liver cancer.29 Therefore, the effects of fermentation of high proportion of SDF on animal health need to be studied further.
There is evidence that DFs with different structures have different effects on the structure and composition of the pig intestinal microbiota.6 Our results indicated that the number of OTUs shared by different SDF ratios decreased with the extension of fermentation time. Besides, the change of the α-diversity indicated that the microorganisms were proliferating in the early stage of fermentation, and the decrease of microbial richness and diversity was caused by the proceeding of fermentation and the increase of SDF ratio. In our study, the relative abundance of bacteria at the phylum level among all samples was evaluated. At 16, 32, and 48 h, OTUs were assigned to four phyla (relative abundance >99%), including Firmicutes, Bacteroidetes, Spirochaetae, and Proteobacteria (Figure S1A–C). Similarly, the four dominant taxonomic phyla were also found in other studies on the interaction between pig gut microbiota and dietary fiber.21 Firmicutes is thought to be beneficial bacteria to metabolize plant polysaccharides to SCFAs.30 In the present study, the relative abundance of Firmicutes was the most predominant in all groups, which was consistent with the previous study.6 Bacteroidetes and Proteobacteria were also the dominant flora in our study, which play a major role in organic matter degradation and C cycling.31 Besides, Spirochaetae was also found in pig feces samples.32 At the genus level, we revealed predominant genera in Figure S1D–F (relative abundance of >99%). A previous study has shown that Clostridium could ferment polysaccharides to SCFAs.33 In our study, the relative abundance of Clostridium_sensu_stricto_1 was significantly increased in the relatively high proportion of SDF groups (≥ 25%) at 16 and 32 h. Rikenellaceae_RC9_gut_group has an impact on carbohydrate,32 and its relative abundance has changed significantly in the five groups at 16 and 32 h. Ruminococcaceae belongs to the Firmicutes phylum, which was considered as fibrolytic bacteria to ferment the complex component of the plant cell wall.13 In addition, Christensenellaceae was regarded as potential beneficial bacteria because it participated in the positive regulation of the intestinal environment and linked to immunomodulation and healthy homeostasis.34 We also observed that the Ruminococcaceae_NK4A214_group and Christensenellaceae_R-7_group markedly changed in the five groups at 32 and 48 h. These results indicated that the effects of different SDF ratios on the gut microbiome are mainly involved in fiber degradation, SCFAs production, and maintenance of the intestinal health. In addition, LEfSe analysis suggested that a relatively high proportion of SDF (≥25%) may be more conducive to improving the ability of intestinal microbes to degrade fibers. Recent studies have shown that gut microbiota regulated the metabolism of monosaccharides such as fructose, mannose, and galactose.35 Similarly, our data indicated that some of the differential bacteria enriched in the high SDF ratio group were closely related to the changes of certain monosaccharides. In addition, other non-differential bacteria also have a significant correlation with monosaccharides, suggesting that they may have the potential to degrade polysaccharides. However, due to the large differences in molecular weight, structure, and conformation of different monosaccharides, the mechanism by which microorganisms produced and utilized them was still unclear. Therefore, the relationship between the structure of monosaccharide and specific microbial function needs to be further studied.
In summary, the fermentation characteristics of different DFs were mainly affected by the SDF ratio and fermentation time. Although microbial diversity was reduced, a high proportion of SDF (≥ 25%) was beneficial to the proliferation of fibrolytic bacteria. Besides, the dominant bacteria in the group with high proportion of SDF were closely related to polysaccharide degradation and monosaccharide production, but the underlying mechanism still needs to be further explored. Our findings may help to better understand the fermentation characteristics of different proportions of SDF and the interaction between SDF and intestinal microbiota and may provide new ideas for the rational formulation of nutrition intervention strategies.
Materials and Methods
Preparation of Inocula and Substrates
The pigs (Landrace ×Large White) originated from an antibiotic-free herd were housed in a temperature-controlled room with no exposure to antibiotics during the whole process of this study. All pigs consumed a standard corn-soybean meal basal diet formulated to meet their growth requirements for 2 weeks prior to fecal collection. Three healthy pigs (approximately 30 kg) were selected and served as sources of feces from which the inoculum was prepared. Feces (approximately 100–200 g) were manually collected directly from the rectum of pigs within 1 h after feeding, immediately stored in a plastic container, which was pre-flushed with CO2 and placed in an ice box, and transferred to the laboratory within 1 h after collection. The substrates were formulated with SDF (inulin, Hebei Vilof Agricultural Technology Co., China) and IDF (commodity fiber,36 a mixture of various non-starch polysaccharides, the main ingredients are glucan, galactan, bhamnosan, araban, xylan and mannan) and were divided into five groups according to the different SDF to IDF ratios: 1:0 (SDF 100% group), 3:1 (SDF 75% group), 1:1 (SDF 50% group), 1:3 (SDF 25% group), and 0:1 (SDF 0% group).
In Vitro Fermentation Trial
The medium for the in vitro fermentation trial was prepared according to the previous study.37 The ratio of the inocula, substrates, and medium was prepared as described by the previous report.38 The fermentation system includes 3 g of substrate, 492 mL of medium, and 30 mL of inoculum. Sterile nitrogen was continuously supplied during the fermentation period, the temperature was maintained at 39 ± 0.5 °C, and the stirring shaft speed was 80 rpm. The fermentation broth was sampled after 8, 16, 24, 32, 40, and 48 h. Fermentation residues were sampled at 48 h. Changes of pH were monitored throughout the fermentation process.
Cumulative Gas Profiles
Following the same experimental design, glass bottles (volume capacity of 120 mL) containing substrates (0.5 mg), medium (82 mL), and inoculum (5 mL) were anaerobically incubated at 39 °C for 48 h. Throughout the whole incubation, all the bottles were sealed with Hungate’s stoppers and screw caps and connected to gas channel inlets of an automated trace gas recording system (AGRS-III, China Agricultural University, Beijing, China) through medical transfusion tubes and needles to continuously record cumulative gas production.39
Analysis of SCFAs
SCFA concentrations in the fermentation broths were analyzed using the method described in a previous report.40 In brief, the fermentation broth was diluted with ultrapure water, and then the diluent was filtered using a 0.20 mm nylon membrane filter (Millipore, Bedford, OH) and poured into a gas chromatograph system (GC-14B; Shimadzu, Tokyo, Japan; capillary column: 30 m × 0.32 mm × 0.25 mm film thickness; column temperature of 110 °C; injector temperature of 180 °C; and detector temperature of 180 °C). The analyses were conducted with a gas chromatograph equipped with a flame ionization detector and a peak profile integration quantification integrator (Shimadzu Corp., Columbia, MD). Each sample peak profile was integrated and quantified relative to an internal standard of methyl butyric acid placed in the same sample. Analyses were conducted at an oven temperature of 200 °C and a flow rate of 85 mL/min.
Analysis of Monosaccharides
The monosaccharides in fermentation broths were determined as alditol acetates by gas–liquid chromatography (GLC) for neutral sugars and uronic acids by a colorimetric method using a modification of the Uppsala method according to a previous study.41 The GLC analysis of the monosaccharides was performed on an Agilent GC 6890 with a flow rate of 20 mL/min and split 40:1. A 30 m × 0.25 mm × 0.25 mm column (Agilent DB-225, film thickness 0.25 μm) was used. The column temperature was 220 °C, and the injector and detector temperature was 250 °C. Determination of fructose content in fermentation broths was performed using a commercial kit (Product number: ml077215; Shanghai Enzyme-linked Biotechnology Co. Ltd., Shanghai, China), according to the manufacturer’s instructions.
DNA Extraction, 16S rRNA Gene Amplification, and Analysis of Sequencing Data
Total microbial genomic DNA in the fermentation broths was extracted using the QIAamp Fast DNAStool Mini Kit (Qiagen Ltd., Germany) in accordance with the manufacturer’s instructions. The V3–V4 region of the 16S rRNA gene was amplified with universal primers 341F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT), as described by previous study.42 The amplified products were detected using agarose gel electrophoresis (2% agarose), recovered using the AxyPrepDNA gel recovery kit (Axygen Biosciences, Union City, CA,United States), and then quantified using a Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, United States) to pool into equimolar amounts. Amplicon libraries were sequenced on the Illumina HiSeq 2500 platform (Illumina, San Diego, CA, United States) for paired-end reads of 250 base pairs.
In order to obtain more accurate and reliable results in subsequent bioinformatics analysis, the raw data from Illumina Hiseq high-throughput sequencing were pre-processed to eliminate the adapter pollution and low quality for obtaining clean reads.43 The paired-end clean reads with overlap were merged to tags using Connecting Overlapped Pair-End (COPE, V1.2.1) software. Subsequently, bacterial tags were clustered into operational taxonomic units (OTUs) at 97% sequence similarity by scripts of USEARCH (v7.0.1090) software. Bacterial OTU representative sequences were taxonomically classified by scripts of Ribosomal Database Project (RDP) Classifier v.2.2 software based on the Ribosomal Database Project (RDP) database. The data was analyzed on the free online platform of Majorbio I-Sanger Cloud Platform (www.i-sanger.com).
Statistical Analysis
The statistical analyses were carried out with tests using the SPSS software package (SPSS v. 20.0, SPSS Inc., Chicago, IL, USA). Differences between means were determined using Tukey’s honest significance test. Statistical variation was also estimated by the standard error of the mean. All statistical analyses were considered significant at P < 0.05.
Glossary
Abbreviations
- DF
dietary fiber
- GLC
gas–liquid chromatography
- IDF
insoluble dietary fiber
- SCFAs
short-chain fatty acids
- SDF
soluble dietary fiber
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01849.
Bar graph of bacterial composition (PDF)
Author Contributions
† S.T. and Y.B. contributed equally.
Author Contributions
S.T., Y.B., and J.W. designed the research. S.T., Y.B., X.Z., and J.Z. conducted the research. S.T., Y.B., and H.Y. analyzed the data. The manuscript was mainly written by S.T. and edited by Y.B., H.Y., S.Z., and J.W. All the authors have read and approved the final manuscript.
This research was financially supported by the National Natural Science Foundation of China (31630074), the Beijing Municipal Natural Science Foundation (no. S170001), the China Postdoctoral Science Foundation (2018 M641549), the 111 Project (B16044), and the Jinxinnong Animal Science Developmental Foundation.
The authors declare no competing financial interest.
Supplementary Material
References
- Holscher H. D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood M.; Kritchevsky D. Dietary fiber: how did we get where we are?. Annu. Rev. Nutr. 2005, 25, 1–8. 10.1146/annurev.nutr.25.121304.131658. [DOI] [PubMed] [Google Scholar]
- Wanders A. J.; Mars M.; Borgonjen-van den Berg K. J.; de Graaf C.; Feskens E. J. M. Satiety and energy intake after single and repeated exposure to gel-forming dietary fiber: post-ingestive effects. Int J Obes. 2014, 38, 794–800. 10.1038/ijo.2013.176. [DOI] [PubMed] [Google Scholar]
- Nomura A. M. Y.; Hankin J. H.; Henderson B. E.; Wilkens L. R.; Murphy S. P.; Pike M. C.; Le Marchand L.; Stram D. O.; Monroe K. R.; Kolonel L. N. Dietary fiber and colorectal cancer risk: the multiethnic cohort study. Cancer, Causes Control 2007, 18, 753–764. 10.1007/s10552-007-9018-4. [DOI] [PubMed] [Google Scholar]
- Puertollano E.; Kolida S.; Yaqoob P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 139–144. 10.1097/MCO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- Tian G.; Wu X.; Chen D.; Yu B.; He J. Adaptation of gut microbiome to different dietary nonstarch polysaccharide fractions in a porcine model. Mol. Nutr. Food Res. 2017, 61, 1700012. 10.1002/mnfr.201700012. [DOI] [PubMed] [Google Scholar]
- Haenen D.; Zhang J.; Souza da Silva C.; Bosch G.; van der Meer I. M.; van Arkel J.; van den Borne J. J.; Pérez Gutiérrez O.; Smidt H.; Kemp B.; Müller M.; Hooiveld G. J. E. J. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 2013, 143, 274. 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- Högberg A.; Lindberg J. E.; Leser T.; Wallgren P. Influence of cereal non-starch polysaccharides on ileo-caecal and rectal microbial populations in growing pigs. Acta Vet. Scand. 2004, 45, 87–98. 10.1186/1751-0147-45-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Abbeele P.; Gérard P.; Rabot S.; Bruneau A.; El Aidy S.; Derrien M.; Kleerebezem M.; Zoetendal E. G.; Smidt H.; Verstraete W.; Van de Wiele T.; Possemiers S. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ. Microbiol. 2011, 13, 2667–2680. 10.1111/j.1462-2920.2011.02533.x. [DOI] [PubMed] [Google Scholar]
- Pluschke A. M.; Williams B. A.; Zhang D.; Gidley M. J. Dietary pectin and mango pulp effects on small intestinal enzyme activity levels and macronutrient digestion in grower pigs. Food Funct. 2018, 9, 991–999. 10.1039/C7FO00602K. [DOI] [PubMed] [Google Scholar]
- Knudsen K. E. B.; Jensen B. B.; Andersen J. O.; Hansen I. Gastrointestinal implications in pigs of wheat and oat fractions. 2. Microbial activity in the gastrointestinal tract. Br. J. Nutr. 1991, 65, 233–248. 10.1079/BJN19910083. [DOI] [PubMed] [Google Scholar]
- Cheng C.; Wei H.; Xu C.; Xie X.; Jiang S.; Peng J. Maternal Soluble Fiber Diet during Pregnancy Changes the Intestinal Microbiota, Improves Growth Performance, and Reduces Intestinal Permeability in Piglets. Appl. Environ. Microbiol. 2018, 84, e01047 10.1128/AEM.01047-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.; Liu P.; Wu Y.; Guo P.; Liu L.; Ma N.; Levesque C.; Chen Y.; Zhao J.; Zhang J.; Ma X. Dietary Fiber Increases Butyrate-Producing Bacteria and Improves the Growth Performance of Weaned Piglets. J. Agric. Food Chem. 2018, 66, 7995–8004. 10.1021/acs.jafc.8b02545. [DOI] [PubMed] [Google Scholar]
- He B.; Bai Y.; Jiang L.; Wang W.; Li T.; Liu P.; Tao S.; Zhao J.; Han D.; Wang J. Effects of Oat Bran on Nutrient Digestibility, Intestinal Microbiota, and Inflammatory Responses in the Hindgut of Growing Pigs. Int. J. Mol. Sci. 2018, 19, 2407. 10.3390/ijms19082407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guergoletto K. B.; Costabile A.; Flores G.; Garcia S.; Gibson G. R. In vitro fermentation of juçara pulp (Euterpe edulis) by human colonic microbiota. Food Chem. 2016, 196, 251–258. 10.1016/j.foodchem.2015.09.048. [DOI] [PubMed] [Google Scholar]
- Mao B.; Tang H.; Gu J.; Li D.; Cui S.; Zhao J.; Zhang H.; Chen W. In vitro fermentation of raffinose by the human gut bacteria. Food Funct. 2018, 9, 5824–5831. 10.1039/C8FO01687A. [DOI] [PubMed] [Google Scholar]
- Day L.; Gomez J.; Øiseth S. K.; Gidley M. J.; Williams B. A. Faster fermentation of cooked carrot cell clusters compared to cell wall fragments in vitro by porcine feces. J. Agric. Food Chem. 2012, 60, 3282–3290. 10.1021/jf204974s. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Bai Y.; Tao S.; Zhang G.; Wang J.; Liu L.; Zhang S. Fiber-rich foods affected gut bacterial community and short-chain fatty acids production in pig model. J Funct Foods 2019, 57, 266–274. 10.1016/j.jff.2019.04.009. [DOI] [Google Scholar]
- Gutierrez N. A.; Serão N. V. L.; Patience J. F. Effects of distillers’ dried grains with solubles and soybean oil on dietary lipid, fiber, and amino acid digestibility in corn-based diets fed to growing pigs. J. Anim. Sci. 2016, 94, 1508–1519. 10.2527/jas.2015-9529. [DOI] [PubMed] [Google Scholar]
- Gutierrez N. A.; Kerr B. J.; Patience J. F. Effect of insoluble-low fermentable fiber from corn-ethanol distillation origin on energy, fiber, and amino acid digestibility, hindgut degradability of fiber, and growth performance of pigs. J. Anim. Sci. 2013, 91, 5314–5325. 10.2527/jas.2013-6328. [DOI] [PubMed] [Google Scholar]
- Cheng P. H.; Liang J. B.; Wu Y. B.; Wang Y.; Tufarelli V.; Laudadio V.; Liao X. D. In vitro fermentative capacity of swine large intestine: comparison between native Lantang and commercial Duroc breeds. Anim Sci J 2017, 88, 1141–1148. 10.1111/asj.12723. [DOI] [PubMed] [Google Scholar]
- Laerke H. N.; Jensen B. B.; Højsgaard S. In vitro fermentation pattern of D-tagatose is affected by adaptation of the microbiota from the gastrointestinal tract of pigs. J Nutr. 2000, 130, 1772–1779. 10.1093/jn/130.7.1772. [DOI] [PubMed] [Google Scholar]
- Melbye P.; Olsson A.; Hansen T. H.; Søndergaard H. B.; Bang Oturai A. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand. 2019, 208. 10.1111/ane.13045. [DOI] [PubMed] [Google Scholar]
- Bliss D. Z.; Weimer P. J.; Jung H.-J. G.; Savik K. In vitro degradation and fermentation of three dietary fiber sources by human colonic bacteria. J. Agric. Food Chem. 2013, 61, 4614–4621. 10.1021/jf3054017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G.; Lange K.; Havinga R.; van Dijk T. H.; Gerding A.; van Eunen K.; Müller M.; Groen A. K.; Hooiveld G. J.; Bakker B. M.; Reijngoud D. J. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J Physiol. 2013, 305, G900–G910. 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- Wu W.; Xie J.; Zhang H. Dietary fibers influence the intestinal SCFAs and plasma metabolites profiling in growing pigs. Food Funct. 2016, 7, 4644–4654. 10.1039/C6FO01406B. [DOI] [PubMed] [Google Scholar]
- Carlson J. L.; Erickson J. M.; Hess J. M.; Gould T. J.; Slavin J. L. Prebiotic Dietary Fiber and Gut Health: Comparing the in Vitro Fermentations of Beta-Glucan, Inulin and Xylooligosaccharide. Nutrients 2017, 9, 1361. 10.3390/nu9121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C.-H.; Cao J.-H.; Zhang F.-C. The prebiotic inulin as a functional food - a review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3262–3265. [PubMed] [Google Scholar]
- Singh V.; Yeoh B. S.; Chassaing B.; Xiao X.; Saha P.; Aguilera Olvera R.; Lapek J. D. Jr.; Zhang L.; Wang W.- B.; Hao S.; Flythe M. D.; Gonzalez D. J.; Cani P. D.; Conejo-Garcia J. R.; Xiong N.; Kennett M. J.; Joe B.; Patterson A. D.; Gewirtz A. T.; Vijay-Kumar M. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e22. 10.1016/j.cell.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattimer J. M.; Haub M. D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S.; Fang C.; Sun X.; Han L.; He X.; Huang G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour. Technol. 2018, 259, 221–227. 10.1016/j.biortech.2018.03.054. [DOI] [PubMed] [Google Scholar]
- Liu P.; Zhao J.; Guo P.; Lu W.; Geng Z.; Levesque C. L.; Johnston L. J.; Wang C.; Liu L.; Zhang J.; Ma N.; Qiao S.; Ma X. Dietary Corn Bran Fermented by Bacillus subtilis MA139 Decreased Gut Cellulolytic Bacteria and Microbiota Diversity in Finishing Pigs. Fron.t Cell. Infect. Microbiol. 2017, 7, 526. 10.3389/fcimb.2017.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Q.; Li P.; Hao S.; Zhang Y.; Kim S. W.; Li H.; Ma X.; Gao S.; He L.; Wu W.; Huang X.; Hua J.; Zhou B.; Huang R. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci. Rep. 2015, 5, 9938. 10.1038/srep09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F.; Hua Y.; Zeng B.; Ning R.; Li Y.; Zhao J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. 10.1016/j.cub.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Del Chierico F.; Abbatini F.; Russo A.; Quagliariello A.; Reddel S.; Capoccia D.; Caccamo R.; Ginanni Corradini S.; Nobili V.; De Peppo F.; Dallapiccola B.; Leonetti F.; Silecchia G.; Putignani L. Gut Microbiota Markers in Obese Adolescent and Adult Patients: Age-Dependent Differential Patterns. Front. Microbiol. 2018, 9, 1210. 10.3389/fmicb.2018.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; Wang W.; Zhu X.; Sun X.; Xiao J.; Li D.; Cui Y.; Wang C.; Shi Y. Response of Gut Microbiota to Dietary Fiber and Metabolic Interaction With SCFAs in Piglets. Front. Microbiol. 2018, 9, 2344. 10.3389/fmicb.2018.02344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson G.; Vaja S.; Evans C.; Chesters C. A.; Pettit R.; Evans W.; Thomas D.; Seed P. T.; Fraser W. D. Comparison of the humoral markers of bone turnover and bone mineral density in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephron 2002, 91, 94–102. 10.1159/000057610. [DOI] [PubMed] [Google Scholar]
- Molinari F.; Bankier A. A.; Eisenberg R. L. Fat-containing lesions in adult thoracic imaging. Am. J. Roentgenol. 2011, 197, W795–W813. 10.2214/AJR.11.6932. [DOI] [PubMed] [Google Scholar]
- Bai S.; Cao Z. J.; Cao B.-B.; Yang H.-J.; Li S.-L.; Liu J.-X. Effects of different forage combinations in total mixed rations on in vitro gas production kinetics, ruminal and milk fatty acid profiles of lactating cows. Anim. Sci. J. 2018, 89, 1261–1270. 10.1111/asj.13036. [DOI] [PubMed] [Google Scholar]
- Dunkley K. D.; Dunkley C. S.; Njongmeta N. L.; Callaway T. R.; Hume M. E.; Kubena L. F.; Nisbet D. J.; Ricke S. C. Comparison of in vitro fermentation and molecular microbial profiles of high-fiber feed substrates incubated with chicken cecal inocula. Poult. Sci. 2007, 86, 801–10. 10.1093/ps/86.5.801. [DOI] [PubMed] [Google Scholar]
- Yu C.; Zhang S.; Yang Q.; Peng Q.; Zhu J.; Zeng X.; Qiao S. Effect of high fibre diets formulated with different fibrous ingredients on performance, nutrient digestibility and faecal microbiota of weaned piglets. Arch. Anim. Nutr. 2016, 70, 263–277. 10.1080/1745039X.2016.1183364. [DOI] [PubMed] [Google Scholar]
- Hong X.; Chen J.; Liu L.; Wu H.; Tan H.; Xie G.; Xu Q.; Zou H.; Yu W.; Wang L.; Qin N. Metagenomic sequencing reveals the relationship between microbiota composition and quality of Chinese Rice Wine. Sci. Rep. 2016, 6, 26621. 10.1038/srep26621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella A. P.; Bartram A. K.; Truszkowski J. M.; Brown D. G.; Neufeld J. D. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics 2012, 13, 31. 10.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.