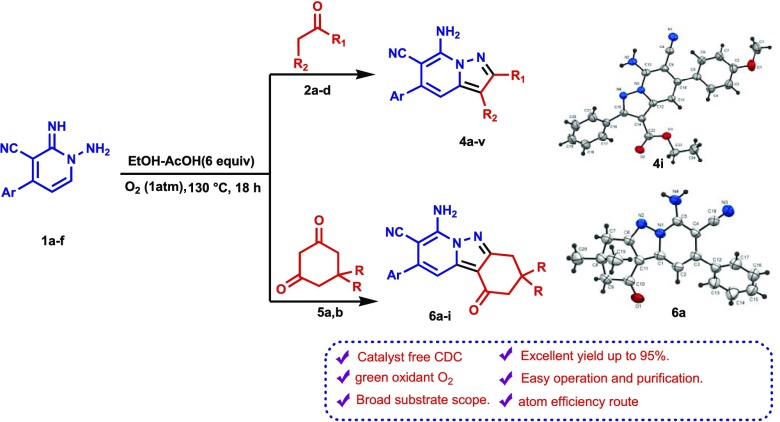
Abstract
An efficient method has been developed for the synthesis of uniquely substituted pyrazolo[1,5-a]pyridine and pyrido[1,2-b]indazole derivatives, which involves acetic acid and molecular oxygen promoted cross-dehydrogenative coupling reactions of respective β-ketoesters and β-diketones (like ethyl acetoacetate, ethyl benzoylacetate, methyl propionylacetate, acetylacetone, dimedone, 1,3-cyclohexanedione, and 1,3-cyclopentanedione) with N-amino-2-iminopyridines. The proposed tentative mechanism involves formal acetic acid-promoted oxidative C(sp3)–C(sp2) dehydrogenative coupling followed by dehydrative cyclization under a catalyst-free condition within high atom economy processes.
Introduction
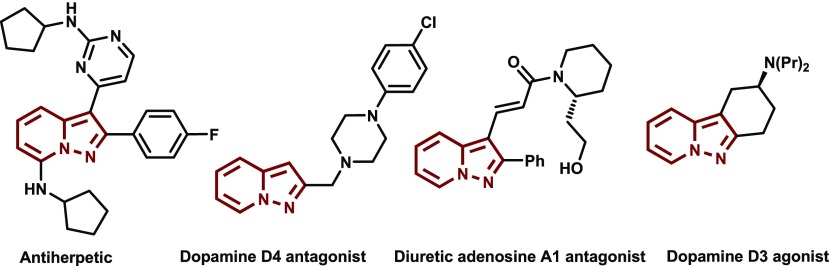
Fused nitrogen containing heterocyclic ring systems is a key structural motif in many natural products and pharmaceutically active compounds.1−3 As a result, these ring systems are often considered as important backbones in the design of new drugs. Among fused N-heteroaromatic substances, those containing pyrazolo[1,5-a]pyridine and pyrido[1,2-b]indazole ring systems are medicinally important owing to their anti-viral,4 -cancer,5 -inflammatory,6 -fungal,7 and -tubercular activities.8,9 Moreover, the pyrazolopyridine ring system is present in the known dopamine D3 agonist and D4 antagonist shown in Figure 1.5 Consequently, drugs containing this structural framework have been developed for the treatment of many neurological and central nervous system disorders like Parkinson’s disease, schizophrenia, anxiety, unipolar major depression, and attention deficit disorder.10−12 In addition, substituted pyrazolo[1,5-a]pyridines and pyrido[1,2-b]indazoles have been observed to have potent antiherpetic13 and diuretic activities (Figure 1).14 Finally, several pyrazolo[1,5-a]pyridine derivatives are known to be selective p38 inhibitors.15
Figure 1.
Examples of biologically active pyrazolo[1,5-a]pyridines and pyrido[1,2-b]indazoles.
The elegant conceptual basis for and applications of cross-dehydrogenative coupling (CDC) reactions has greatly expanded in recent years. As a result, great progress has been made in developing new approaches for forming different types of bonds that are difficult to construct by using older classical methods.16−25 The CDC concept has been employed to devise new strategies to construct an assortment of bonds as part of sequences to generate complex organic substances. An important example is C–C bond formation, which can be carried out using intramolecular26−29 or intermolecular30−39 CDC reactions between two C–H bond bearing moieties. In addition, CDC reactions have been developed for C–O,40,41 C–N,42−46 S–N,47,48 C–S,49,50 S-P,51 and C–P52,53 bond formation.
In comparison with their traditional cross-coupling counterparts, cross-dehydrogenative coupling reactions are sustainable owing to their green characteristics, including atom efficiency, step economy, and lack of prefunctionalization.54,55 C(sp2)–H–C(sp3)–H cross-dehydrogenative coupling reactions are now among the most important methods for construction of C–C bonds in a single step. However, the methods developed for CDC to date usually require a catalyst to activate feebly reactive C(sp3)–H bonds. The catalysts typically used for this purpose include complexes of transition metals, like copper (Cu),56 palladium (Pd),57 gold (Au),58 ruthenium (Ru),23,59 iron (Fe),60 iridium (Ir),,61 vanadium (V),62 and cobalt (Co).63 In addition, some of the CDC processes are activated by nonmetal catalysts, such as iodine,50,64 rose bengal,65 1,4-benzoquinone,66 phenyliodonium diacetate,67 and eosin.68
In a continuation of efforts aimed at developing methods for the synthesis of novel fused N-heterocycles,69−74 our recent attention became focused on members of the pharmaceutically relevant pyrazolo[1,5-a]pyridine and pyrido[1,2-b]indazole families. Particular attention was given to devising novel aerobic, catalyst-free CDC processes that would produce these targets in an efficient and atom economical manner. The investigation described below demonstrates that simple cross-dehydrogenative coupling reactions of N-amino-2-iminopyridine derivatives with β-ketoesters and β-diketones, promoted by utilizing the green oxidant molecular oxygen (O2) and the mild reagent acetic acid, efficiently generate respective pyrazolo[1,5-a]pyridines and pyrido[1,2-b]indazoles.
Results and Discussion
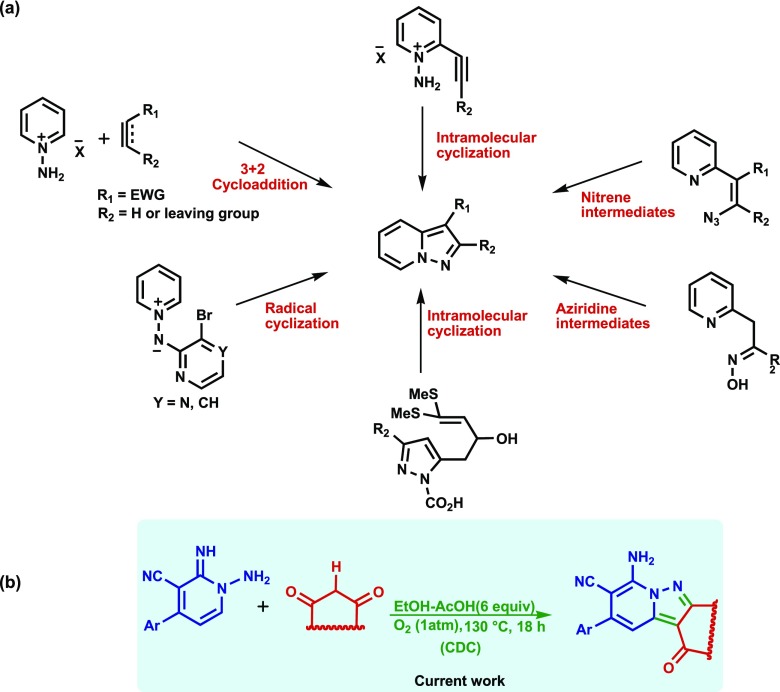
The most commonly utilized synthetic protocol to prepare pyrazolo[1,5-a]pyridines involves intermolecular [3 + 2] cycloaddition of N-iminopyridinium ylides with alkenes or alkynes as dipolarophiles (Scheme 1a).75 Also, synthesis of these substances has been accomplished via intramolecular cyclizations of transient nitrenes15b,76 and ethynylpyridines.77,78 We envisaged that a novel and potentially more simple strategy for synthesis of substituted pyrazolo[1,5-a]pyridines and related pyrido[1,2-b]indazoles would involve cross-dehydrogenative coupling reactions between N-amino-2-iminopyridine derivatives and β-ketoesters and β-diketones (Scheme 1b).
Scheme 1. Previous (a) and Newly Developed (b) Routes for the Synthesis of Substituted Pyrazolo[1,5-a]pyridines.
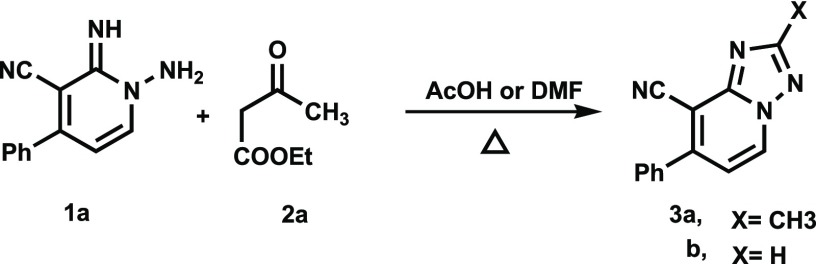
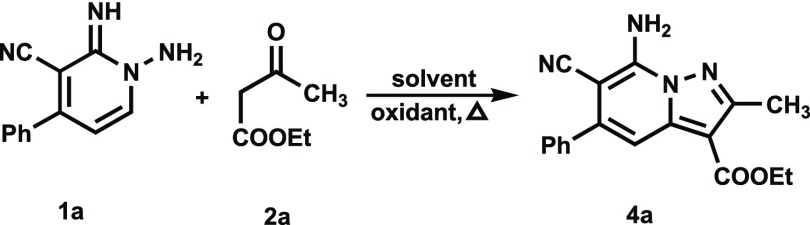
In an initial study designed to assess the viability of this approach, we explored the CDC reaction between N-aminopyridine 1a and ethyl acetoacetate (2a). We observed that the reaction of 1-amino-2-imino-4-phenyl-1,2-dihydropyridine-3-carbonitrile (1a) (3 mmol) and 2a (3 mmol) in refluxing ethanol (10 mL) under an air atmosphere for up to 48 h leads to quantitative recovery of the starting materials. Also, no reaction takes place between 1a with 2a under the same conditions when either Pd(OAc)2 (10 mol %) or Cu(OAc)2 is present. A screening effort showed that no reaction occurs between 1a and 2a when various other solvents, including acetonitrile, methanol, dioxane, propanol, H2O, and toluene, are employed. Interestingly, when acetic acid or DMF is used as solvent and Pd(OAc)2 (10 mol %) is employed as catalyst, reactions do take place to form the respective triazolo[1,5-a]pyridine derivatives 3a (72%) and 3b (74%). These substances presumably arise by reaction of the N-amino-2-iminopyridine 1a with either acetic acid or DMF, Scheme 2.
Scheme 2. Reactions of N-aminopyridine 1a and Ethyl acetoacetate (2a) in HOAc and DMF in the Presence of Pd(OAc)2 To Form Triazolo[1,5-a]pyridines 3a and 3b, Respectively.
Rewardingly, when acetic acid (0.36 g, 2 equiv) is present, the reaction of 1a and 2a in ethanol under an air atmosphere does take place to form the desired 7-amino-6-cyano-2-methyl-5-phenylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4a, 34%) (Table 1) (Table 1, entry 1). The structure of 4a was assigned using spectroscopic methods and comparisons of the data to those of related substances that are characterized by using X-ray crystallographic analysis (see below). For example, the 1H NMR spectrum of 4a contains resonances corresponding to ethyl (1.32 ppm, t) and (4.30 ppm, q), CH3 (2.62 ppm, s), H-5 pyridine (7.20 ppm, s), NH2 (7.76 ppm, s), and phenyl [7.48–7.54 (3H, m) and 7.58–7.59 (2H, m)] moieties. Moreover, as anticipated for 4a, the 13C NMR spectrum contains 16 characteristic resonances and the HRMS contains a mass peak at m/z 320.1268 associated with the molecular formula C18H16N4O2.
Table 1. Reaction of N-aminopyridine 1a and Ethyl acetoacetate (2a) in Ethanol Containing Acetic Acid To Form Pyrazolo[1,5-a]pyridine 4aa.
| entry | molar equiv. acid | atmosphere | percent yield 4a |
|---|---|---|---|
| 1 | (HOAc) 2 | air | 34 |
| 2 | (HOAc) 4 | air | 52 |
| 3 | (HOAc) 6 | air | 74 |
| 4 | (HOAc) 6 | O2 | 94 |
| 5 | (HOAc) 6 | Ar | 6 |
| 6 | (p-TSA) 1 | O2 | 39 |
| 7 | (p-TSA) 2 | O2 | 41 |
| 8 | (TFA) 1 | O2 | 48 |
| 9 | (TFA) 2 | O2 | 55 |
Reaction conditions: N-amino-2-imino-pyridine 1a (3 mmol), ethyl acetoacetate (2a) (3 mmol), in ethanol (10 mL), under an atmosphere of air or O2 1 atm at 130 °C for 18 h.
Experiments were conducted to determine the effect of acetic acid loading on the efficiency of the 4a forming reaction of 1a and 2a under an air atmosphere. The results (Table 1, entries 2 and 3) show that increasing the loading of acetic acid from 2 to 4 to 6 equivalents leads to increases in the yield of 4a from 34 to 52 to 74%, respectively. Moreover, using higher amounts of acetic acid (e. g., 8 equivalent) causes the process to become complicated by competitive formation of triazolo[1,5-a]pyridine derivatives 3a. As a result, the maximum loading of acetic acid was maintained at 6 equivalents to prevent the formation of the undesired by-product (compound 3a). Importantly, the reaction between 1a and 2a carried out under molecular oxygen rather than an air atmosphere (1 atm) takes place to form 4a in near quantitative yield (94%, Table 1, entry 4). As expected, the yield of this process is significantly diminished (6%, Table 1, entry 5) when an Ar atmosphere is utilized. This implies that the reaction is mostly driven by oxygen, thus confirming the proposed oxidative CDC route. Furthermore, utilizing a catalytic amount of strong Brønsted acids like p-toluenesulfonic acid (p-TSA) or trifluoroacetic acid (TFA) was found to be less effective under the optimal condition (Table 1, entries 6–9). It is worth mentioning that increasing the loading of TFA above 2 equivalents leads also to a side reaction between TFA and N-aminopyridine 1a.
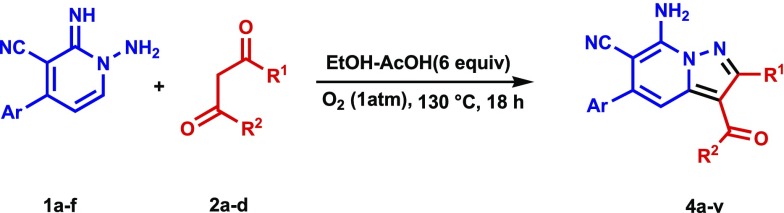
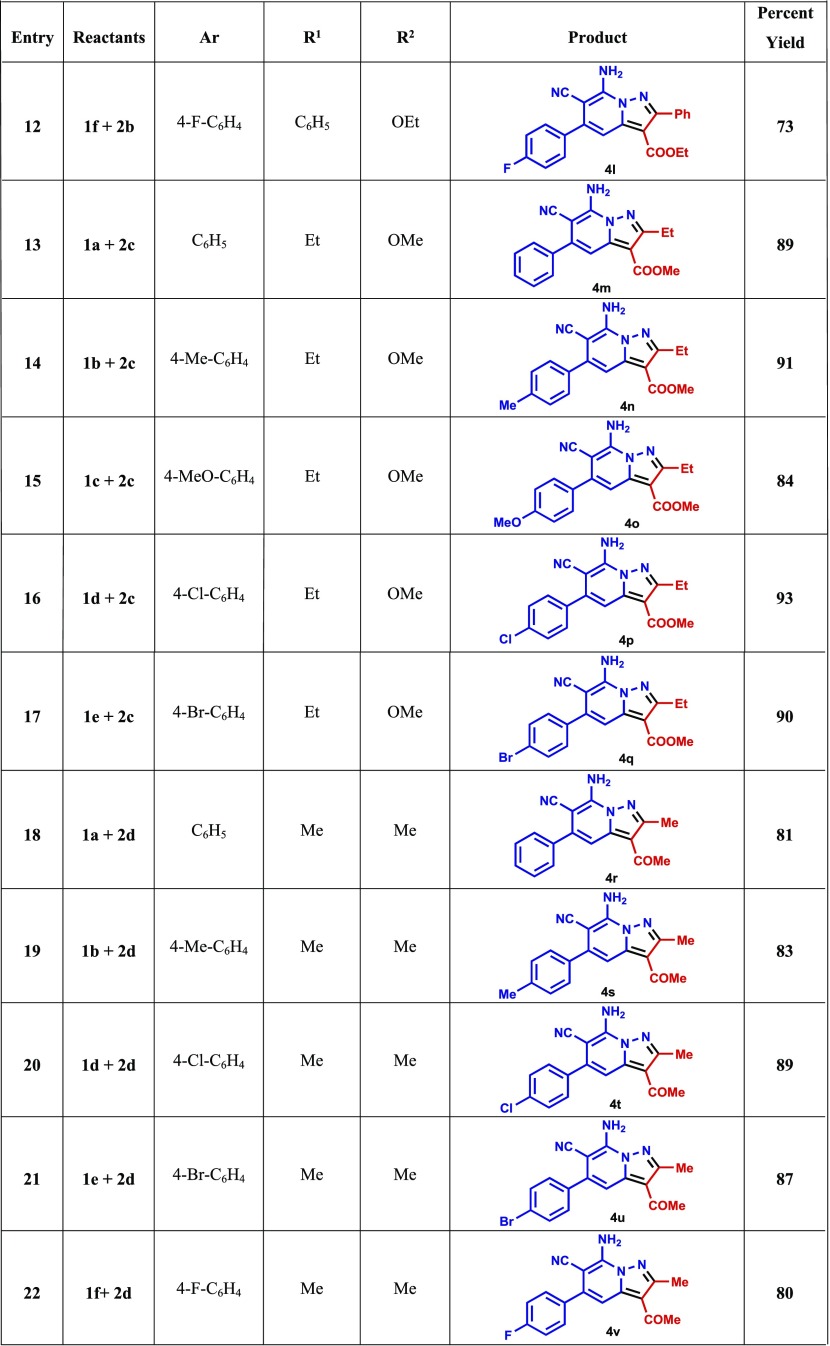
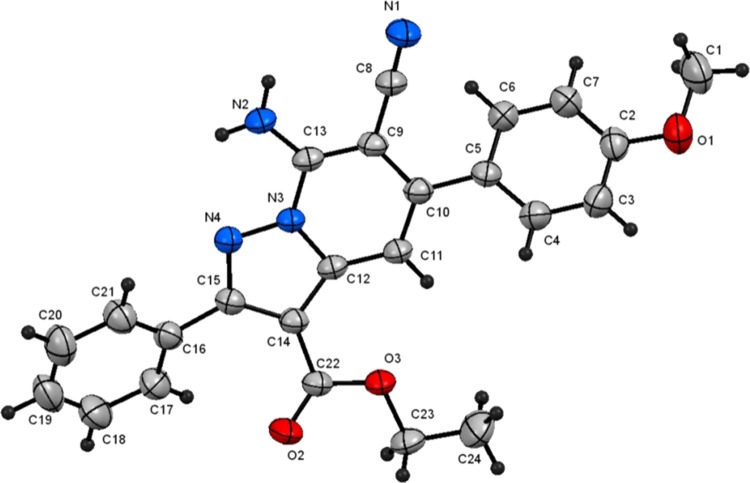
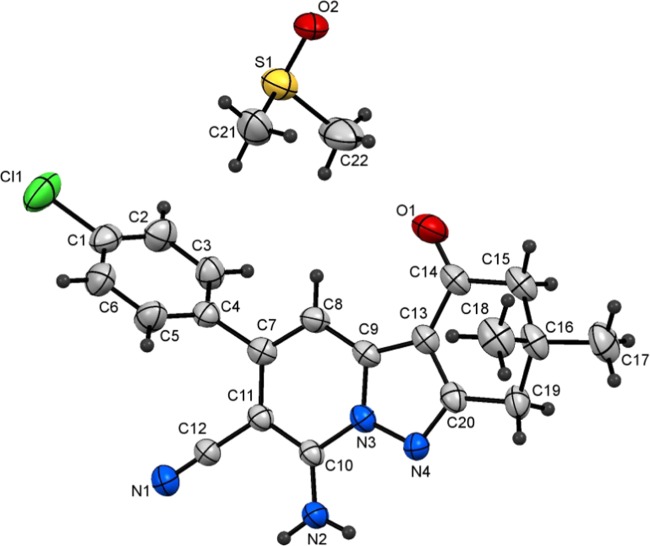
A study of the CDC reaction was conducted to explore its substrate scope. For this purpose, an assortment of N-amino-2-iminopyridines, including 1a–f, and β-dicarbonyl compounds, including ethyl acetoacetate (2a), ethyl benzoylacetate (2b), methyl propionylacetate (2c), and acetylacetone (2d) were subjected to the optimal reaction conditions uncovered in the exploratory investigation (entry 4, Table 1). The results (Table 2) show that reactions between 1a–f and 2a–d generate the corresponding pyrazolo[1,5-a]pyridines 4a–v and that the presence of either electron-withdrawing or electron-donating substituents on the aryl moiety in the N-amino-2-iminopyridine substrate does not have a remarkable effect on the course of the CDC process. In addition, because of their crystalline nature, 4i and 4r were subjected to X-ray crystallographic analysis, the results of which confirm their assigned structures (cf. Figures 2 and 3).
Table 2. Acetic Acid-Promoted CDC Reactions of N-Amino-2-iminopyridines 1a–f and β-Ketoesters/β-Diketones 2a–da.
Reaction conditions: N-amino-2-iminopyridine 1a–f (3 mmol), β-ketoesters/β-diketones 2a–d (3 mmol), in ethanol (10 mL) containing acetic acid (6 equiv), under an O2 atmosphere (1 atm) at 130 °C for 18 h.
Figure 2.
Plot of X-ray crystallographic data for 4i.
Figure 3.
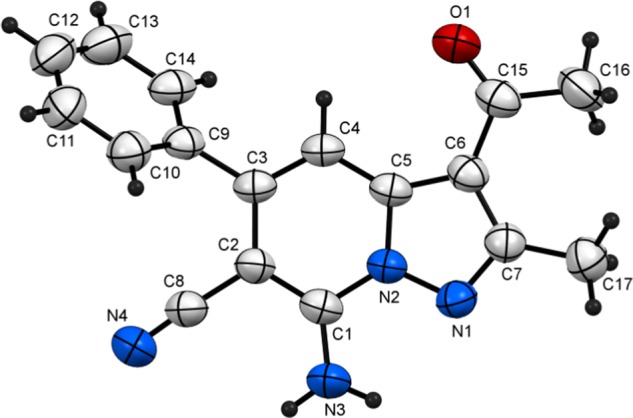
Plot of X-ray crystallographic data for 4r.
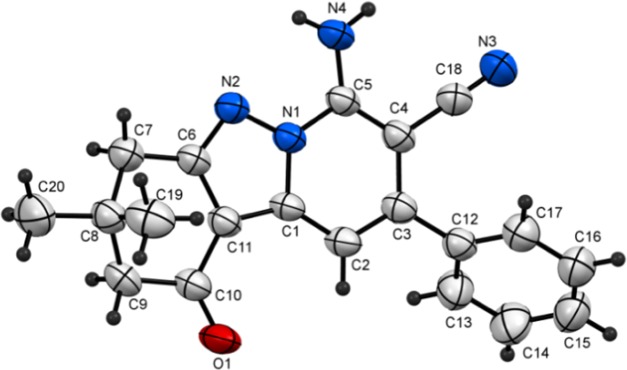
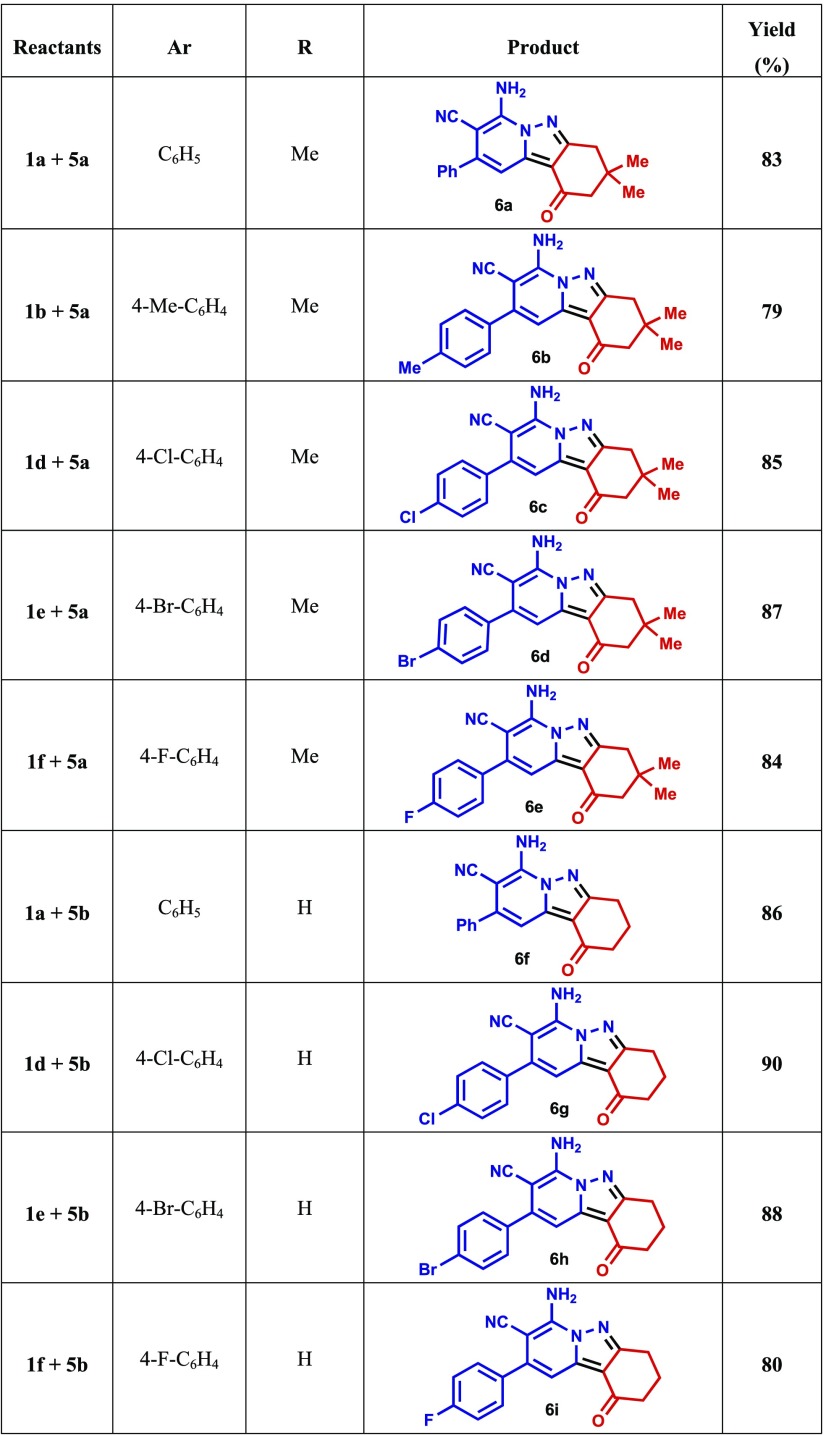
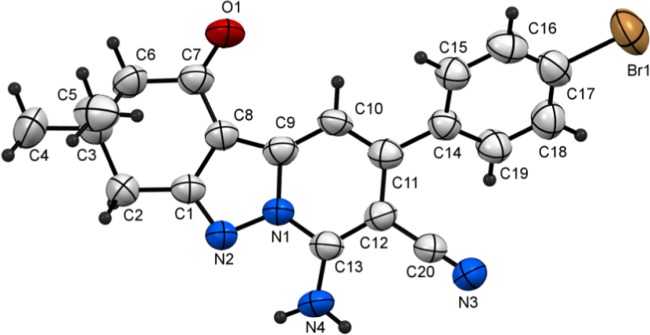
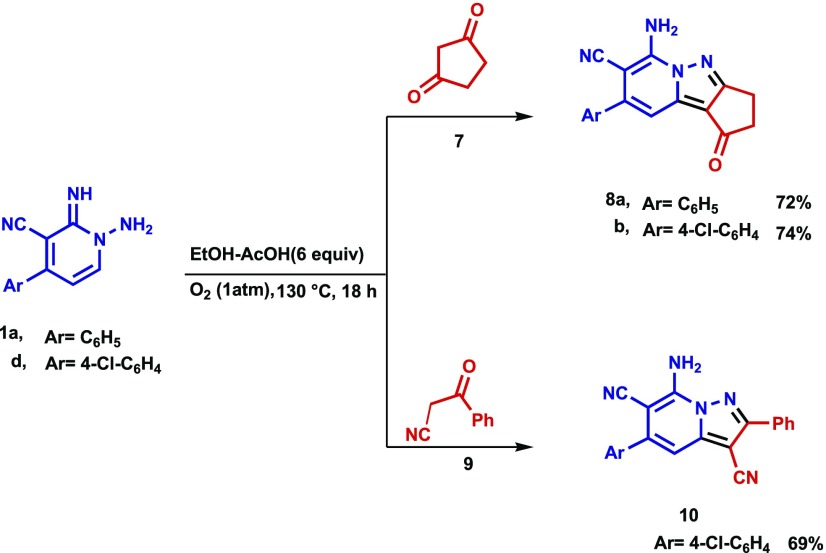
The results of further studies demonstrated that the CDC reaction also occurs when cyclic 1,3-diketones are utilized as substrates. For example, the reaction of N-amino-2-iminopyridine 1a and 5,5-dimethylcyclohexane-1,3-dione (dimedone, 5a) under the optimized reaction conditions described above produces 7-aminotetrahydropyrido[1,2-b]indazole-8-carbonitrile (6a) in 83% yield. The structure of 6a was unambiguously assigned using spectrometric and X-ray crystallographic analysis (Figure 4). Moreover, tetrahydropyrido[1,2-b]indazoles 6b–e are efficiently formed (79–87%) in reactions of the respective N-aminopyridines 1b and 1d–f with dimedone (5a), and analogous CDC reactions take place between 1,3-cyclohexanedione (5b) and N-amino-2-iminopyridines 1a, 1d, 1e, and 1f to form the corresponding tetrahydropyrido[1,2-b]indazoles 6f–j in high yields (80–90%) (Table 3). The structures of two of these products, 6c and 6d, were assigned using X-ray crystallographic methods (Figures 5 and 6). Finally, N-amino-2-iminopyridines 1a and 1b react with 1,3-cyclopentanedione 7 and 3-oxo-3-phenylpropionitrile 9 under CDC reaction conditions to form the corresponding 2,3-dihydrocyclopenta[3,4]pyrazolo[1,5-a]pyridin-1-ones 8a and 8b (72 and 74% respective yields) and pyrazolo[1,5-a]pyridine-3,6-dicarbonitrile 10 (Scheme 3). Although the preparation of tetrahydropyrido[1,2-b]indazoles has been reported previously,79 to the best of our knowledge, the CDC process developed in the current effort is the first to generate these substances in a direct (single step), versatile (large substrate scope), and environmentally friendly manner.
Figure 4.
Plot of X-ray crystallographic data for 6a.
Table 3. CDC Reaction of N-amino-2-iminopyridines 1a–f with Cyclic β-Diketones 5a,ba.
Reaction conditions: N-amino-2-iminopyridine 1a–f (3 mmol), cyclic β-diketones 5a,b (3 mmol), in ethanol (10 mL) containing acetic acid (6 equiv), under an O2 atmosphere (1 atm) at 130 °C for 18 h.
Figure 5.
Plot of X-ray crystallographic data for 6c.
Figure 6.
Plot of X-ray crystallographic data for 6d.
Scheme 3. CDC Reactions of N-amino-2-iminopyridines 1a and 1b with 1,3-Cyclopentanedione (7) and 3-Oxo-3-phenylpropionitrile 9.
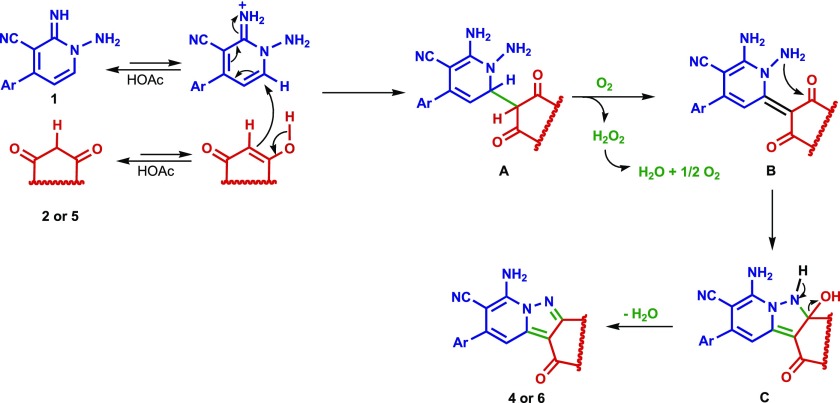
A plausible mechanistic pathway for the CDC coupling reactions80 described above is illustrated in Scheme 4. In one possible route, proton transfer from acetic acid activates N-amino-2-iminopyridine 1 for nucleophilic addition of the enol form of the β-dicarbonyl substrates 2 or 5. The resulting adduct A then undergoes oxidative dehydrogenation via a reaction with molecular oxygen to form intermediate B, which cyclizes to generate C that loses water to produce the pyrazolo[1,5-a]pyridine 4 or pyrido[1,2-b]indazole 6 product. In an alternative mechanistic route, dehydration/cyclization of A precedes oxidative dehydrogenation.
Scheme 4. Plausible Mechanistic Pathway for the Formation of Pyrazolo[1,5-a]pyridines 4 and Pyrido[1,2-b]indazoles 6.
Conclusions
In summary, the investigation described above led to the development of a unique CDC reaction between N-amino-2-iminopyridines derivatives and 1,3-dicarbonyl compounds. The process, which takes place under mild environmentally compatible conditions, is promoted by the Bronstead acid catalyst AcOH and uses O2 as the oxidant. Significantly, the new CDC reaction exhibits many features required for classification as a green process, including high atom economy, superior substrates scope, lack of toxic or harsh reagents, and simple purification and work-up procedures. Owing to the frequent occurrence of pyrazolo[1,5-a]pyridine and pyrido[1,2-b]indazole ring systems in drugs, the new methodology could have significance in the context of developing new pharmaceutically active substances.
Experimental Section
General
Melting points were recorded on a Griffin melting point apparatus and are uncorrected. IR spectra were recorded using KBr disks using a Jasco FT-IR-6300 spectrophotometer. 1H NMR (600 MHz) and 13C NMR (150 MHz) spectra were recorded at 25 °C using dimethyl sulfoxide (DMSO)-d6 as solvent with TMS as an internal standard on a Bruker DPX 600 superconducting NMR spectrometer. Chemical shifts are reported in ppm. Low-resolution electron impact mass spectra [MS (EI)] and high-resolution electron impact mass spectra [HRMS (EI)] were performed using a high-resolution gas chromatography-MS (DFS) thermo-spectrometer at 70.1 eV and a magnetic sector mass analyzer. Monitoring reactions and checking the homogeneity of compounds were performed using thin layer chromatography. X-ray crystal structures were determined using a Rigaku R-AXIS RAPID diffractometer and Bruker X8 Prospector, and the collection of single crystal data was made at room temperature by using Cu Kα radiation. The structures were solved by using direct methods and expanded using Fourier techniques. The nonhydrogen atoms were refined anisotropically. The structures were solved and refined using the Bruker SHELXTL Software Package (Structure solution program- SHELXS-97 and Refinement program- SHELXL-97).81 Data were corrected for the absorption effects using the multiscan method (SADABS). N-amino-2-iminopyridines 1a–f were prepared according to the literature procedure.75d,82
2-Methyl-7-phenyl[1,2,4]triazolo[1,5-a]pyridine-8-carbonitrile (3a)
Recrystallized from EtOH/dioxane mixture (5:1), as yellow white crystals, yield: 0.51 g (74%), mp 155–156 °C; IR (KBr): ν/cm–1 2218 (CN); 1H NMR (600 MHz, DMSO-d6): δ 2.55 (s, 3H), 7.37 (d, J = 6.8 Hz, 1H), 7.59–7.63 (m, 3H), 7.75 (d, J = 8.4 Hz, 2H), 9.19 (d, J = 6.8 Hz, 1H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.1, 96.4, 114.6, 114.6, 128.8, 129.0, 130.2, 132.5, 135.5, 149.1, 150.3, 165.0; MS (EI): m/z (%) 235 (M+ + 1, 26.78), 234 (M+, 100). HRMS (EI): m/z calcd. for C14H10N4 (M+) 234.0899, found 234.0898.
7-Phenyl[1,2,4]triazolo[1,5-a]pyridine-8-carbonitrile (3b)
Recrystallized from EtOH/dioxane mixture (4:1), as yellow crystals, yield: 0.47 g (72%), mp 159–160 °C; IR (KBr): ν/cm–1 2221 (CN); 1H NMR (600 MHz, DMSO-d6): δ 7.49 (d, J = 7.2 Hz, 1H), 7.62–7.67 (m, 3H), 7.79 (d, J = 8.4 Hz, 2H), 8.75 (s, 1H), 9.35 (d, J = 7.2 Hz, 1H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 97.6, 114.4, 115.6, 128.9, 129.0, 130.3, 133.3, 135.4, 149.6, 149.7, 155.4; MS (EI): m/z (%) 221 (M+ + 1, 17.65), 220 (M+, 100). HRMS (EI): m/z calcd. for C13H8N4 (M+) 220.0743, found 220.0743.
General Procedure for the Preparation of Pyrazolo[1,5-a]pyridines 4a–v, 10, Pyrido[1,2-b]indazoles 6a–i and Cyclopenta[3,4]pyrazolo[1,5-a]pyridin-1-one 8a,b
Independent solutions of 1-amino-2-imino-pyridines 1a–f (3 mmol), and the 1,3-dicarbonyl compounds 2a–d, 5a,b, 7, or 3-oxo-3-phenylpropionitrile 9 (3 mmol), in ethanol (10 mL) containing acetic acid (1.08 g, 6 equiv) under an O2 atmosphere (1 atm) were stirred at 130 °C for 18 h. Crystals formed upon cooling to room temperature were collected by filtration and recrystallized from an appropriate solvent to give pure pyrazolo[1,5-a]pyridines 4a–v, 10, pyrido[1,2-b]indazoles 6a–i, and cyclopenta[3,4]pyrazolo[1,5-a]pyridin-1-one 8a,b.
7-Amino-6-cyano-2-methyl-5-phenylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4a)
Recrystallized from EtOH as yellow white crystals, yield: 0.90 g (94%), mp 228–229 °C; IR (KBr): ν/cm–1 3454, 3325 (NH2), 2216 (CN), 1701 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.32 (t, J = 7.2 Hz, 3H), 2.62 (s, 3H), 4.30 (q, J = 7.2 Hz, 2H), 7.20 (s, 1H), 7.48–7.54 (m, 3H), 7.58–7.59 (m, 2H), 7.76 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 13.6, 13.7, 59.0, 75.4, 102.0, 103.8, 115.7, 127.8, 128.1, 128.5, 137.2, 141.9, 142.6, 147.4, 155.5, 162.4; MS (EI): m/z (%) 321 (M+ + 1, 18.95), 320 (M+, 100). HRMS (EI): m/z calcd. for C18H16N4O2 (M+) 320.1268, found 320.1268.
7-Amino-6-cyano-2-methyl-5-p-tolylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4b)
Recrystallized from EtOH as yellow white crystals, yield: 0.95 g (95%), mp 214–215 °C; IR (KBr): ν/cm–1 3462, 3323 (NH2), 2216 (CN), 1703 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.29 (t, J = 7.2 Hz, 3H), 2.38 (s, 3H), 258 (s, 3H), 4.25 (q, J = 7.2 Hz, 2H), 7.11 (s, 1H), 7.32 (d, J = 8.4 Hz, 2H), 7.45 (d, J = 8.4 Hz, 2H), 8.11 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.2, 14.3, 20.8, 59.5, 75.3, 102.0, 103.6, 116.6, 128.2, 129.2, 134.7, 138.7, 142.3, 143.2, 147.9, 155.9, 162.9; MS (EI): m/z (%) 335 (M+ + 1, 22.08), 334 (M+, 100). HRMS (EI): m/z calcd. for C19H18N4O2 (M+) 334.1424, found 334.1423.
7-Amino-6-cyano-5-(4-methoxyphenyl)-2-methylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4c)
Recrystallized from EtOH as yellow white crystals, yield: 0.92 g (88%), mp 215–216 °C; IR (KBr): ν/cm–1 3462, 3313 (NH2), 2218 (CN), 1699 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.30 (t, J = 7.2 Hz, 3H), 2.58 (s, 3H), 3.84 (s, 3H), 4.26 (q, J = 7.2 Hz, 2H), 7.08 (d, J = 8.4 Hz, 2H), 7.10 (s, 1H), 7.53 (d, J = 8.4 Hz, 2H), 8.10 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.2, 14.3, 55.3, 59.5, 75.4, 101.8, 103.4, 114.0, 116.7, 129.67, 129.70, 142.3, 142.9, 147.8, 155.8, 159.9, 162.9; MS (EI): m/z (%) 351 (M+ + 1, 22.25), 350 (M+, 100). HRMS (EI): m/z calcd. for C19H18N4O3 (M+) 350.1373, found 350.1373.
7-Amino-5-(4-chlorophenyl)-6-cyano-2-methylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4d)
Recrystallized from EtOH as yellow white crystals, yield: 0.96 g (90%), mp 245–246 °C; IR (KBr): ν/cm–1 3456, 3317 (NH2), 2216 (CN), 1709 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.29 (t, J = 7.2 Hz, 3H), 2.59 (s, 3H), 4.26 (q, J = 7.2 Hz, 2H), 7.12 (s, 1H), 7.60 (s, 4H), 8.22 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.25, 14.28, 59.6, 75.1, 102.3, 103.8, 116.4, 128.7, 130.2, 134.0, 136.4, 141.9, 142.2, 147.9, 155.9, 162.9; MS (EI): m/z (%) 356 (M+ + 2, 33.12), 355 (M+ + 1, 20.05), 354 (M+, 100). HRMS (EI): m/z calcd. for C18H15ClN4O2 (M+) 354.0878, found 354.0878.
7-Amino-5-(4-bromophenyl)-6-cyano-2-methylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4e)
Recrystallized from EtOH/dioxane mixture (3:1), as yellow white crystals, yield: 1.00 g (84%), mp 246–247 °C; IR (KBr): ν/cm–1 3448, 3325 (NH2), 2214 (CN), 1720 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.30 (t, J = 7.2 Hz, 3H), 2.60 (s, 3H), 4.27 (q, J = 7.2 Hz, 2H), 7.13 (s, 1H), 7.53 (d, J = 8.4 Hz, 2H), 7.74 (d, J = 8.4 Hz, 2H), 8.22 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.2, 14.3, 59.6, 75.0, 102.3, 103.7, 116.4, 122.6, 130.5, 131.6, 136.8, 141.9, 142.2, 147.9, 155.9, 162.9; MS (EI): m/z (%) 400 (M+ + 2, 96.33), 399 (M+ + 1, 19.98), 398 (M+, 100). HRMS (EI): m/z calcd. for C18H15BrN4O2 (M+) 398.0373, found 398.0373.
7-Amino-6-cyano-5-(4-fluorophenyl)-2-methylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4f)
Recrystallized from EtOH/dioxane mixture (4:1), as white crystals, yield: 0.80 g (78%), mp 199–200 °C; IR (KBr): ν/cm–1 3451, 3321 (NH2), 2216 (CN), 1707 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.30 (t, J = 7.2 Hz, 3H), 2.61 (s, 3H), 4.28 (q, J = 7.2 Hz, 2H), 7.14 (s, 1H), 7.36–7.39 (m, 2H), 7.63–7.65 (m, 2H), 8.20 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.76, 14.79, 60.1, 75.9, 102.7, 104.3, (116.04, 116.18) (d, 2JCF = 21.0 Hz), 117.0, (131.08, 131.14) (d, 3JCF = 9.0 Hz), (134.47, 134.49) (d, 4JCF = 3.0 Hz), 142.69, 142.73, 148.4, 156.4, (162.22, 163.85 ppm) (d, 1JCF = 244.5 Hz), 163.4; MS (EI): m/z (%) 339 (M+ + 1, 23.89), 338 (M+, 100). HRMS (EI): m/z calcd. for C18H15FN4O2 (M+) 338.1174, found 338.1175.
7-Amino-6-cyano-2,5-diphenylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4g)
Recrystallized from EtOH/DMF mixture (3:1), as buff crystals, yield: 0.94 g (82%), mp 201–202 °C; IR (KBr): ν/cm–1 3444, 3323 (NH2), 2212 (CN), 1687 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.18 (t, J = 7.2 Hz, 3H), 4.20 (q, J = 7.2 Hz, 2H), 7.29 (s, 1H), 7.48–7.50 (m, 4H), 7.52–7.54 (m, 3H), 7.55 (d, J = 8.4 Hz, 2H), 7.80–7.82 (m, 2H), 8.29 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.0, 59.7, 75.8, 101.6, 104.4, 116.4, 124.2, 127.6, 128.3, 128.7, 129.1, 129.9, 131.9, 137.6, 142.9, 143.3, 148.2, 156.9, 162.4; MS (EI): m/z (%) 383 (M+ + 1, 19.98), 382 (M+, 100). HRMS (EI): m/z calcd. for C23H18N4O2 (M+) 382.1424, found 382.1424.
7-Amino-6-cyano-2-phenyl-5-p-tolylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4h)
Recrystallized from EtOH/DMF mixture (3:1), as pale yellow crystals, yield: 1.00 g (87%), mp 273–274 °C; IR (KBr): ν/cm–1 3460, 3323 (NH2), 2216 (CN), 1714 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.18 (t, J = 7.2 Hz, 3H), 2.40 (s, 3H), 4.19 (q, J = 7.2 Hz, 2H), 7.27 (s, 1H), 7.36 (d, J = 7.8 Hz, 2H), 7.48–7.52 (m, 5H), 7.80–7.81 (m, 2H), 8.26 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.0, 20.8, 59.7, 75.8, 101.5, 104.2, 116.5, 127.6, 128.2, 129.1, 129.3, 129.9, 131.9, 134.7, 138.8, 143.0, 143.4, 148.2, 156.9, 162.4; MS (EI): m/z (%) 397 (M+ + 1, 24.15), 3 96 (M+, 100). HRMS (EI): m/z calcd. for C24H20N4O2 (M+) 396.1581, found 396.1580.
7-Amino-6-cyano-5-(4-methoxyphenyl)-2-phenylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester (4i)
Recrystallized from EtOH/DMF mixture (3:1), as yellow crystals, yield: 1.05 g (85%), mp 260–261 °C; IR (KBr): ν/cm–1 3438, 3321 (NH2), 2212 (CN), 1714 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.18 (t, J = 7.2 Hz, 3H), 3.84 (s, 3H), 4.19 (q, J = 7.2 Hz, 2H), 7.10 (d, J = 9.0 Hz, 2H), 7.25 (s, 1H), 7.47–7.49 (m, 3H), 7.56 (d, J = 9.0 Hz, 2H), 7.79–7.80 (m, 2H), 8.23 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.0, 55.3, 59.7, 75.8, 101.3, 104.0, 114.1, 116.6, 127.6, 129.1, 129.70, 129.71, 129.9, 131.9, 143.0, 143.1, 148.1, 156.9, 160.0, 162.4; MS (EI): m/z (%) 413 (M+ + 1, 27.84), 412 (M+, 100). HRMS (EI): m/z calcd. for C24H20N4O3 (M+) 412.1530, found 412.1529. Crystal data, moiety formula: C24H20N4O3, M = 412.44, triclinic, a = 8.2355(3) Å, b = 10.4603(4) Å, c = 12.9164(4) Å, V = 1021.68(6) Å3, α = 105.604(2)°, β = 90.933(2)°, γ = 106.617(2)°, space group: P1̅, Z = 2, Dcalc = 1.341 g·cm–3, no. of reflection measured 3541, θmax = 66.56°, R1 = 0.0435 (CCDC 1916934).83
7-Amino-5-(4-chlorophenyl)-6-cyano-2-phenylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester(4j)
Recrystallized from EtOH/DMF mixture (3:1), as buff crystals, yield: 0.95 g (76%), mp 291–292 °C; IR (KBr): ν/cm–1 3435, 3307 (NH2), 2218 (CN), 1714 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.18 (t, J = 7.2 Hz, 3H), 4.21 (q, J = 7.2 Hz, 2H), 7.28 (s, 1H), 7.49–7.51 (m, 3H), 7.63–7.66 (m, 4H), 7.95 (d, J = 8.4 Hz, 2H), 8.34 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.0, 59.8, 75.6, 101.8, 104.4, 116.3, 127.6, 128.7, 129.2, 129.9, 130.3, 131.8, 134.0, 136.4, 142.0, 142.9, 148.2, 156.9, 162.4; MS (EI): m/z (%) 418 (M+ + 2, 30.58), 417 (M+ + 1, 22.89), 416 (M+, 100). HRMS (EI): m/z calcd. for C23H17ClN4O2 (M+) 416.1035, found 416.1035.
7-Amino-5-(4-bromophenyl)-6-cyano-2-phenylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester(4k)
Recrystallized from EtOH/DMF mixture (3:1), as buff crystals, yield: 1.05 g (77%), mp 259–260 °C; IR (KBr): ν/cm–1 3429, 3309 (NH2), 2216 (CN), 1714 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.18 (t, J = 7.2 Hz, 3H), 4.20 (q, J = 7.2 Hz, 2H), 7.28 (s, 1H), 7.48–7.50 (m, 3H), 7.57 (d, J = 8.4 Hz, 2H), 7.76 (d, J = 8.4 Hz, 2H), 7.80–7.81 (m, 2H), 8.33 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.0, 59.8, 75.5, 101.8, 104.4, 116.3, 122.7, 127.6, 129.2, 129.9, 130.5, 131.7, 131.8, 136.8, 142.1, 142.9, 148.2, 156.9, 162.4; MS (EI): m/z (%) 462 (M+ + 2, 99.87), 461 (M+ + 1, 26.95), 460 (M+, 100). HRMS (EI): m/z calcd. for C23H17BrN4O2 (M+) 460.0529, found 460.0528.
7-Amino-5-(4-fluorophenyl)-6-cyano-2-phenylpyrazolo[1,5-a]pyridine-3-carboxylic acid ethyl ester(4l)
Recrystallized from EtOH/dioxane mixture (1:1), as yellow white crystals, yield: 0.88 g (73%), mp 234–235 °C; IR (KBr): ν/cm–1 3444, 3311 (NH2), 2222 (CN), 1714 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.19 (t, J = 7.2 Hz, 3H), 4.22 (q, J = 7.2 Hz, 2H), 7.28 (s, 1H), 7.41 (t, J = 9.0 Hz, 2H), 7.49–7.51 (m, 3H), 7.67–7.70 (m, 2H), 7.81–7.83 (m, 2H), 8.30 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 14.0, 59.7, 75.8, 101.7, 104.4, (115.59, 115.73) (d, 2JCF = 21.0 Hz), 116.4, 127.6, 129.2, 129.9, (130.62, 130.68) (d, 3JCF = 9.0 Hz), 131.9, (133.97, 133.99) (d, 4JCF = 3.0 Hz), 142.3, 142.9, 148.1, 156.9 (161.76, 163.40 ppm) (d, 1JCF = 246 Hz), 162.4; MS (EI): m/z (%) 401 (M+ + 1, 21.89), 400 (M+, 100). HRMS (EI): m/z calcd. for C23H17FN4O2 (M+) 400.1330, found 400.1330.
7-Amino-6-cyano-2-ethyl-5-phenylpyrazolo[1,5-a]pyridine-3-carboxylic acid methyl ester (4m)
Recrystallized from EtOH as pale yellow crystals, yield: 0.85 g (89%), mp 209–210 °C; IR (KBr): ν/cm–1 3452, 3309 (NH2), 2218 (CN), 1705 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.29 (t, J = 7.2 Hz, 3H), 3.02 (q, J = 7.2 Hz, 2H), 3.80 (s, 3H), 7.13 (s, 1H), 7.50–7.57 (m, 5H), 8.14 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 12.9, 21.3, 51.0, 75.4, 101.2, 104.0, 116.5, 128.4, 128.6, 129.0, 137.6, 142.3, 143.2, 147.9, 161.0, 163.2; MS (EI): m/z (%) 321 (M+ + 1, 18.15), 320 (M+, 100). HRMS (EI): m/z calcd. for C18H16N4O2 (M+) 320.1268, found 320.1267.
7-Amino-6-cyano-2-ethyl-5-p-tolylpyrazolo[1,5-a]pyridine-3-carboxylic acid methyl ester (4n)
Recrystallized from EtOH as yellow crystals, yield: 0.90 g (91%), mp 220–221 °C; IR (KBr): ν/cm–1 3462, 3329 (NH2), 2212 (CN), 1705 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.27 (t, J = 7.2 Hz, 3H), 2.37 (s, 3H), 3.00 (q, J = 7.2 Hz, 2H), 3.79 (s, 3H), 7.09 (s, 1H), 7.30 (d, J = 7.8 Hz, 2H), 7.42 (d, J = 7.8 Hz, 2H), 8.07 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 12.8, 20.8, 21.3, 51.0, 75.4, 101.0, 103.8, 116.6, 128.2, 129.1, 134.7, 138.6, 142.3, 143.1, 147.9, 160.9, 163.1; MS (EI): m/z (%) 335 (M+ + 1, 22.45), 334 (M+, 100). HRMS (EI): m/z calcd. for C19H18N4O2 (M+) 334.1424, found 334.1425.
7-Amino-6-cyano-2-ethyl-5-(4-methoxyphenyl)pyrazolo[1,5-a]pyridine-3-carboxylic acid methyl ester (4o)
Recrystallized from EtOH as yellow crystals, yield: 0.88 g (84%), mp 198–199 °C; IR (KBr): ν/cm–1 3471, 3363 (NH2), 2206 (CN), 1716 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.28 (t, J = 7.2 Hz, 3H), 3.00 (q, J = 7.2 Hz, 2H), 3.80 (s, 3H), 3.83 (s, 3H), 7.06–7.08 (m, 3H), 7.50 (d, J = 8.4 Hz, 2H), 8.05 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 12.8, 21.3, 51.0, 55.2, 75.4, 100.9, 103.6, 114.0, 116.7, 129.7, 142.3, 142.9, 147.9, 159.9, 160.9, 163.1; MS (EI): m/z (%) 351 (M+ + 1, 18.74), 350 (M+, 100). HRMS (EI): m/z calcd. for C19H18N4O3 (M+) 350.1373, found 350.1373.
7-Amino-5-(4-chlorophenyl)-6-cyano-2-ethylpyrazolo[1,5-a]pyridine-3-carboxylic acid methyl ester (4p)
Recrystallized from EtOH/dioxane mixture (2:1), as canary yellow crystals, yield: 0.99 g (93%), mp 238–239 °C; IR (KBr): ν/cm–13462, 3344 (NH2), 2212 (CN), 1714 cm–1 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.29 (t, J = 7.2 Hz, 3H), 3.01 (q, J = 7.2 Hz, 2H), 3.80 (s, 3H), 7.13 (s, 1H), 7.59 (s, 4H), 8.20 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 12.9, 21.3, 51.0, 75.2, 101.4, 104.0, 116.3, 128.6, 130.2, 133.9, 136.4, 141.9, 142.2, 147.9 161.0, 163.1; MS (EI): m/z (%) 356 (M+ + 2, 31.08), 355 (M+ + 1, 20.13), 354 (M+, 100). HRMS (EI): m/z calcd. for C18H15 ClN4O2 (M+) 354.0878, found 354.0879.
7-Amino-5-(4-bromophenyl)-6-cyano-2-ethylpyrazolo[1,5-a]pyridine-3-carboxylic acid methyl ester (4q)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow crystals, yield: 1.05 g (90%), mp 247–248 °C; IR (KBr): ν/cm–1 3441, 3311 (NH2), 2216 (CN), 1703 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.29 (t, J = 7.2 Hz, 3H), 3.01 (q, J = 7.2 Hz, 2H), 3.81 (s, 3H), 7.12 (s, 1H), 7.51 (d, J = 8.4 Hz, 2H), 7.72 (d, J = 8.4 Hz, 2H), 8.18 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 12.9, 21.3, 51.0, 75.1, 101.4, 103.9, 116.4, 122.6, 130.5, 131.6, 136.7, 141.9, 142.2, 147.9 161.0, 163.1; MS (EI): m/z (%) 400 (M+ + 2, 99.62), 399 (M+ + 1, 18.78), 398 (M+, 100). HRMS (EI): m/z calcd. for C18H15 BrN4O2 (M+) 398.0373, found 398.0374.
3-Acetyl-7-amino-2-methyl-5-phenylpyrazolo[1,5-a]pyridine-6-carbonitrile (4r)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow crystals, yield: 0.70 g (81%), mp 244–245 °C; IR (KBr): ν/cm–1 3458, 3330 (NH2), 2212 (CN), 1649 cm–1 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.51 (s, 3H), 2.65 (s, 3H), 7.27 (s, 1H), 7.50–7.55 (m, 3H), 7.59–7.61 (m, 2H), 8.16 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 15.4, 30.5, 75.7, 104.3, 112.1, 116.4, 128.4, 128.6, 129.0, 137.5, 141.9, 143.7, 147.7, 155.2, 191.7; MS (EI): m/z (%) 291 (M+ + 1, 9.18), 290 (M+, 46.05), 275 (M+-15, 100). HRMS (EI): m/z calcd. for C17H14N4O (M+) 290.1162, found 290.1162. Crystal data, moiety formula: C17H14N4O, M = 290.32 monoclinic, a = 4.3144(6) Å, b = 10.7340(12) Å, c = 31.118(5) Å, V = 1441.0(3) Å3, α = γ = 90°, β = 90.599(6)°, space group: P21/n (#14), Z = 4, Dcalc = 1.338 g·cm–3, no. of reflection measured 3034, 2θmax = 50.10°, R1 = 0.0783 (ref CCDC 1940357).83
3-Acetyl-7-amino-2-methyl-5-p-tolylpyrazolo[1,5-a]pyridine-6-carbonitrile (4s)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow crystals, yield: 0.75 g (83%), mp above 300 °C; IR (KBr): ν/cm–1 3464, 3330 (NH2), 2216 (CN), 1651 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.39 (s, 3H), 2.52 (s, 3H), 2.66 (s, 3H), 7.27 (s, 1H), 7.33 (d, J = 7.8 Hz, 2H), 7.50 (d, J = 7.8 Hz, 2H), 8.15 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 15.4, 20.8, 30.5, 75.7, 104.1, 112.0, 116.5, 128.3, 129.2, 134.7, 138.7, 142.0, 143.8, 147.7, 155.2, 191.7; MS (EI): m/z (%) 305 (M+ + 1, 12.36), 304 (M+, 52.12), 289 (M+-15, 100). HRMS (EI): m/z calcd. for C18H16N4O (M+) 304.1319, found 304.1319.
3-Acetyl-7-amino-5-(4-chlorophenyl)-2-methylpyrazolo[1,5-a]pyridine-6-carbonitrile (4t)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow crystals, yield: 0.86 g (89%), mp 272–273 °C; IR (KBr): ν/cm–1 3438, 3309 (NH2), 2220 (CN), 1653 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.52 (s, 3H), 2.66 (s, 3H), 7.27 (s, 1H), 7.59–7.64 (m, 4H), 8.22 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 15.4, 30.5, 75.4, 104.3, 112.2, 116.3, 128.6, 130.3, 134.0, 136.3, 141.9, 142.4, 147.7, 155.2, 191.8; MS (EI): m/z (%) 326 (M+ + 2, 15.12), 325 (M+ + 1, 9.13), 324 (M+, 46.78), 309 (M+ – 15, 100). HRMS (EI): m/z calcd. for C17H13ClN4O (M+) 324.0772, found 324.0772.
3-Acetyl-7-amino-5-(4-bromophenyl)-2-methylpyrazolo[1,5-a]pyridine-6-carbonitrile (4u)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow white crystals, yield: 0.95 g (87%), mp 284–285 °C; IR (KBr): ν/cm–1 3433, 3311 (NH2), 2218 (CN), 1653 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.52 (s, 3H), 2.66 (s, 3H), 7.27 (s, 1H), 7.56 (d, J = 8.4 Hz, 2H), 7.74 (d, J = 7.8 Hz, 2H), 8.22 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 15.4, 30.6, 75.6, 104.2, 112.2, 116.3, 122.6, 130.6, 131.6, 136.7, 141.9, 142.4, 147.7, 155.2, 191.8; MS (EI): m/z (%) 370 (M+ + 2, 57.12), 369 (M+ + 1, 11.13), 368 (M+, 57.33), 353 (M+ – 15, 100). HRMS (EI): m/z calcd. for C17H13BrN4O (M+) 368.0267, found 368.0267.
3-Acetyl-7-amino-5-(4-flurophenyl)-2-methylpyrazolo[1,5-a]pyridine-6-carbonitrile (4v)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow white crystals, yield: 0.74 g (80%), mp 223–224 °C; IR (KBr): ν/cm–1 3444, 3323 (NH2), 2218 (CN), 1651 cm–1 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.51 (s, 3H), 2.64 (s, 3H), 7.24 (s, 1H), 7.35–7.38 (m, 2H), 7.64–7.66 (m, 2H), 8.17 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 15.4, 30.5, 75.7, 104.3, 112.1, (115.46, 115.60) (d, 2JCF = 21.0 Hz), 116.4, (130.66, 130.71) (d, 3JCF = 7.5 Hz), (133.89, 133.91) (d, 4JCF = 3.0 Hz), 141.9, 142.6, 147.6, 155.2, (161.74, 163.37 ppm) (d, 1JCF = 244.5 Hz) and 191.7 ppm (CN, Ar–C and CO); MS (EI): m/z (%) 309 (M+ + 1, 8.24), 308 (M+, 43.89), 293 (M+ – 15, 100). HRMS (EI): m/z calcd. for C17H13FN4O (M+) 308.1068, found 308.1068.
7-Amino-3,3-dimethyl-1-oxo-9-phenyl-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6a)
Recrystallized from EtOH/dioxane mixture (3:1), as yellow crystals, yield: 0.82 g (83%), mp 272–273 °C; IR (KBr): ν/cm–1 3435, 3291 (NH2), 2212 (CN), 1660 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.08 (s, 6H), 2.40 (s, 2H), 2.88 (s, 2H), 7.14 (s, 1H), 7.52–7.56 (m, 3H), 7.60–7.61 (m, 2H), 8.25 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 28.0, 34.9, 36.3, 51.9, 76.6, 103.3, 108.2, 116.3, 128.3, 128.6, 129.2, 137.2, 139.1, 144.8, 148.1, 160.7, 191.9; MS (EI): m/z (%) 331 (M+ + 1, 19.04), 330 (M+, 100). HRMS (EI): m/z calcd. for C20H18N4O (M+) 330.1475, found 330.1476. Crystal data, moiety formula: C20H18N4O, M = 330.39, monoclinic, a = 5.9727(5) Å, b = 25.0246(18) Å, c = 11.9182(9) Å, V = 1727.8(2) Å3, α = γ = 90°, β = 104.090(7)°, space group: P21/c, Z = 4, Dcalc = 1.270 g·cm–3, no. of reflections measured 3034, 2θmax = 50.10°, R1 = 0.0441 (CCDC 1916935).83
7-Amino-3,3-dimethyl-1-oxo-9-p-tolyl-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6b)
Recrystallized from EtOH/dioxane mixture (3:1), as yellow white crystals, yield: 0.82 g (79%), mp 261–262 °C; IR (KBr): ν/cm–1 3444, 3292 (NH2), 2214 (CN), 1658 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.07 (s, 6H), 2.38 (s, 2H), 2.39 (s, 3H), 2.86 (s, 2H), 7.11 (s, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.49 (d, J = 8.4 Hz, 2H), 8.25 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 20.8, 28.0, 34.9, 36.3, 51.9, 76.6, 103.1, 108.1, 116.4, 128.2, 129.2, 134.3, 138.8, 139.7, 144.9, 148.1, 160.7, 191.8; MS (EI): m/z (%) 345 (M+ + 1, 23.87), 344 (M+, 100). HRMS (EI): m/z calcd. for C21H20N4O (M+) 344.1632, found 344.1632.
7-Amino-9-(4-chlorophenyl)-3,3-dimethyl-1-oxo-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6c)
Recrystallized from EtOH/dioxane mixture (3:1), as yellow crystals, yield: 0.93 g (85%), mp 223–224 °C; IR (KBr): ν/cm–1 3346, 3290 (NH2), 2214 (CN), 1655 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.07 (s, 6H), 2.39 (s, 2H), 2.87 (s, 2H), 7.12 (s, 1H), 7.59–7.63 (m, 4H), 8.32 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 27.8, 34.6, 36.3, 51.9, 76.4, 103.4, 108.3, 115.9, 128.5, 130.0, 133.9, 135.9, 139.0, 143.3, 147.9, 160.6, 191.6; MS (EI): m/z (%) 366 (M+ + 2, 33.16), 365 (M+ + 1, 23.09), 364 (M+, 100). HRMS (EI): m/z calcd. for C20H17ClN4O (M+) 364.1085, found 364.1085. Crystal data, sum formula: C22H23ClN4O2S, moiety formula: C24H22N6O, M = 364.84, monoclinic, a = 5.8529(2) Å, b = 19.0224(6) Å, c = 20.2441(6) Å, V = 2251.86(12) Å3, α = γ = 90°, β = 92.438(2)°, space group P121/c1, Z = 4, Dcalc = 1.307 g·cm–3, no. of reflections measured: 3821, θmax = 66.54°, R1 = 0.0804 (CCDC 1916936).83
7-Amino-9-(4-bromophenyl)-3,3-dimethyl-1-oxo-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6d)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow crystals, yield: 1.06 g (87%), mp 274–275 °C; IR (KBr): ν/cm–1 3444, 3288 (NH2), 2214 (CN), 1655 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.11 (s, 6H), 2.41 (s, 2H), 2.90 (s, 2H), 7.21 (s, 1H), 7.56 (d, J = 8.4 Hz, 2H), 7.72 (d, J = 8.4 Hz, 2H), 7.90 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 27.5, 34.3, 36.2, 51.8, 76.4, 103.4, 108.1, 115.4, 122.2, 129.9, 131.1, 136.1, 138.7, 142.9, 147.6, 160.3, 191.1; MS (EI): m/z (%) 410 (M+ + 2, 99.97), 409 (M+ + 1, 21.73), 408 (M+, 100). HRMS (EI): m/z calcd. for C20H17BrN4O (M+) 408.0580, found 408.0581. Crystal data, moiety formula: C20H17BrN4O, M = 409.29, monoclinic, a = 6.7934(7) Å, b = 29.438(3) Å, c = 9.5955(9) Å, V = 1880.3(3) Å3, α = γ = 90°, β = 101.519(7)°, space group P21/c, Z = 4, Dcalc = 1.446 g·cm–3, no. of reflections measured: 3294, θmax = 25.007°, R1 = 0.0701 (CCDC 1916937).83
7-Amino-9-(4-fluorophenyl)-3,3-dimethyl-1-oxo-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6e)
Recrystallized from EtOH as yellow crystals, yield: 0.88 g (84%), mp 224–225 °C; IR (KBr): ν/cm–1 3480, 3324 (NH2), 2216 (CN), 1653 (CO); 1H NMR (600 MHz, DMSO-d6): δ 1.09 (s, 6H), 2.40 (s, 2H), 2.88 (s, 2H), 7.13 (s, 1H), 7.36–7.39 (m, 2H), 7.65–7.68 (m, 2H), 8.26 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 28.0, 34.9, 36.3, 51.9, 76.6, 103.4, 108.6, (115.56, 115.70) (d, 2JCF = 21.0 Hz), 116.3, (130.65, 130.71) (d, 3JCF = 9.0 Hz), (133.63, 133.65) (d, 4JCF = 3.0 Hz), 139.2, 143.8, 148.0, 160.7, (161.80, 163.43 ppm) (d, 1JCF = 244.5 Hz), 191.9; MS (EI): m/z (%) 349 (M+ + 1, 38.95), 348 (M+, 100). HRMS (EI): m/z calcd. for C20H17FN4O (M+) 348.1381, found 348.1381.
7-Amino-1-oxo-9-phenyl-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6f)
Recrystallized from EtOH/dioxane mixture (3:1), as yellow crystals, yield: 0.78 g (86%), mp 261–262 °C; IR (KBr): ν/cm–1 3429, 3298 (NH2), 2213 (CN), 1654 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.14 (pentet, J = 6.0 Hz, 2H), 2.50 (t, J = 6.0 Hz, 2H), 2.99 (t, J = 6.0 Hz, 2H), 7.16 (s, 1H), 7.53–7.57 (m, 3H), 7.60–7.62 (m, 2H), 8.29 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 22.8, 23.1, 38.1, 76.7, 103.4, 109.4, 116.3, 128.4, 128.7, 129.2, 137.2, 139.4, 144.8, 148.0, 161.6, 192.6; MS (EI): m/z (%) 303 (M+ + 1, 21.72), 302 (M+, 100). HRMS (EI): m/z calcd. for C18H14N4O (M+) 302.1162, found 302.1162.
7-Amino-9-(4-chlorophenyl)-1-oxo-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6g)
Recrystallized from EtOH/dioxane mixture (3:1), as yellow crystals, yield: 0.91 g (90%), mp 299–300 °C; IR (KBr): ν/cm–1 3435, 3319 (NH2), 2214 (CN), 1655 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.11 (pentet, J = 6.0 Hz, 2H), 2.48 (t, J = 6.0 Hz, 2H), 2.97 (t, J = 6.0 Hz, 2H), 7.11 (s, 1H), 7.58–7.62 (m, 4H), 8.31 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 22.8, 23.1, 38.1, 76.5, 103.4, 109.5, 116.2, 128.7, 130.3, 134.1, 136.0, 139.2, 143.4, 148.0, 161.6, 192.6; MS (EI): m/z (%) 338 (M+ + 2, 32.78), 337 (M+ + 1, 21.27), 336 (M+, 100). HRMS (EI): m/z calcd. for C18H13ClN4O (M+) 336.0772, found 336.0772.
7-Amino-9-(4-bromophenyl)-1-oxo-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6h)
Recrystallized from EtOH/dioxane mixture (2:1), as canary yellow crystals, yield: 1.00 g (88%), mp 295–296 °C; IR (KBr): ν/cm–1 3429, 3328 (NH2), 2212 (CN), 1655 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.12 (pentet, J = 6.0 Hz, 2H), 2.48 (t, J = 6.0 Hz, 2H), 2.98 (t, J = 6.0 Hz, 2H), 7.12 (s, 1H), 7.55 (d, J = 8.4 Hz, 2H), 7.74 (d, J = 8.4 Hz, 2H), 8.32 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 22.8, 23.1, 38.1, 76.4, 103.3, 109.5, 116.2, 122.8, 130.5, 131.6, 136.4, 139.3, 143.5, 148.0, 161.6, 192.6; MS (EI): m/z (%) 382 (M+ + 2, 99.65), 381 (M+ + 1, 20.08), 380 (M+, 100). HRMS (EI): m/z calcd. for C18H13BrN4O (M+) 380.0267, found 380.0269.
7-Amino-9-(4-fluorophenyl)-1-oxo-1,2,3,4-tetrahydropyrido[1,2-b]indazole-8-carbonitrile (6i)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow crystals, yield: 0.77 g (80%), mp 275–276 °C; IR (KBr): ν/cm–1 3436, 3294 (NH2), 2214 (CN), 1656 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.13 (pentet, J = 6.0 Hz, 2H), 2.50 (t, J = 6.0 Hz, 2H), 2.99 (t, J = 6.0 Hz, 2H), 7.14 (s, 1H), 7.37–7.41 (m, 2H), 7.65–7.68 (m, 2H), 8.31 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 22.8, 23.1, 38.1, 76.7, 103.4, 109.4, (115.56, 115.71) (d, 2JCF = 22.5 Hz), 116.3, (130.66, 130.71) (d, 3JCF = 7.5 Hz), (133.60, 133.62) (d, 4JCF = 3.0 Hz), 139.3, 143.8, 148.0, 161.6, (161.80, 163.43) (d, 1JCF = 244.5 Hz), 192.6; MS (EI): 321 (M+ + 1, 21.02), 320 (M+, 100). HRMS (EI): m/z calcd. for C18H13FN4O (M+) 320.1068, found 320.1067.
6-Amino-1-oxo-8-phenyl-2,3-dihydro-1H-cyclopenta[3,4]pyrazolo[1,5-a]pyridine-7-carbonitrile (8a)
Recrystallized from EtOH/dioxane mixture (1:1), as pale yellow crystals, yield: 0.62 g (72%), mp 231–232 °C; IR (KBr): ν/cm–1 3413, 3288 (NH2), 2215 (CN), 1652 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.62 (t, J = 6.0 Hz, 2H), 2.89 (t, J = 6.0 Hz, 2H), 7.13 (s, 1H), 7.55–7.59 (m, 3H), 7.69–7.72 (m, 2H), 8.33 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 24.9, 33.5, 76.3, 103.7, 109.5, 116.4, 128.5, 129.0, 129.4, 136.9, 139.1, 144.5, 147.9, 161.5, 192.1; MS (EI): m/z (%) 289 (M+ + 1, 26.01), 288 (M+, 100). HRMS (EI): m/z calcd. for C17H12N4O (M+) 288.1006, found 288.1005.
6-Amino-8-(4-chlorophenyl)-1-oxo-2,3-dihydro-1H-cyclopenta[3,4]pyrazolo[1,5-a]pyridine-7-carbonitrile (8b)
Recrystallized from EtOH/dioxane mixture (2:1), as yellow crystals, yield: 0.71 g (74%), mp 253–254 °C; IR (KBr): ν/cm–1 3422, 3314 (NH2), 2216 (CN), 1656 (CO); 1H NMR (600 MHz, DMSO-d6): δ 2.59 (t, J = 6.0 Hz, 2H), 2.83 (t, J = 6.0 Hz, 2H), 7.14 (s, 1H), 7.61–7.63 (m, 4H), 8.36 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 24.7, 33.3, 76.7, 103.5, 109.8, 115.9, 129.0, 129.9, 134.2, 135.9, 139.5, 143.1, 147.8, 161.5, 192.2; MS (EI): m/z (%) 324 (M+ + 2, 36.02), 323 (M+ + 1, 24.15), 322 (M+, 100). HRMS (EI): m/z calcd. for C17H11ClN4O (M+) 322.0616, found 322.0617
7-Amino-5-(4-chlorophenyl)-2-phenylH-pyrazolo[1,5-a]pyridine-3,6-dicarbonitrile (10)
Recrystallized from dioxane, as beige crystals, yield: 0.76 g (69%), mp 270–271 °C; IR (KBr): ν/cm–1 3415, 3298 (NH2), 2214, 2219 (2CN); 1H NMR (600 MHz, DMSO-d6): δ 7.35 (s, 1H), 7.44–7.47 (m, 3H), 7.65–7.67 (m, 4H), 7.91 (d, J = 8.4 Hz, 2H), 8.36 (s, 2H); 13C{1H} NMR (150 MHz, DMSO-d6): δ 76.1, 102.0, 113.6, 116.2, 127.8, 128.5, 129.6, 129.8, 130.8, 132.0, 133.8, 136.6, 141.9, 143.0, 148.5, 156.8, 162.8; MS (EI): m/z (%) 371 (M+ + 2, 33.26), 370 (M+ + 1, 23.12), 369 (M+, 100). HRMS (EI): m/z calcd. for C21H12ClN5 (M+) 369.0776, found 369.0776.
Acknowledgments
Financial support for this study was provided by the University of Kuwait through a research grant (SC06/18). The facilities of Analab/SAF supported by research grants GS01/01, GS01/05, GS01/03, and GS03/08 are gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02430.
The authors declare no competing financial interest.
Supplementary Material
References
- Eicher T.; Hauptmann S.. The Chemistry of Heterocycles: Structures, Reactions. Synthesis, and Applications, 2nd ed.; Wiley-VCH: Weinheim, 2003. [Google Scholar]
- Pozharskii A. F.; Soldatenkov A. T.; Katritzky A. R.. Heterocycles in Life and Society; John Wiley & Sons, Ltd: West Sussex, 1997. [Google Scholar]
- a Dugger R. W.; Ragan J. A.; Ripin D. H. B. Survey of GMP Bulk Reactions Run in a Research Facility between 1985 and 2002. Org. Process Res. Dev. 2005, 9, 253–258. 10.1021/op050021j. [DOI] [Google Scholar]; b Taylor A. P.; Robinson R. P.; Fobian Y. M.; Blakemore D. C.; Jones L. H.; Fadeyi O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. 10.1039/C6OB00936K. [DOI] [PubMed] [Google Scholar]; c Kumar D.; Jain S. K. A Comprehensive Review of N-Heterocycles as Cytotoxic Agents. Curr. Med. Chem. 2016, 23, 4338–4394. 10.2174/0929867323666160809093930. [DOI] [PubMed] [Google Scholar]
- Rashad A. E.; Hegab M. I.; Abdel-Megeid R. E.; Micky J. A.; Abdel-Megeid F. M. Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Bioorg. Med. Chem. 2008, 16, 7102–7106. 10.1016/j.bmc.2008.06.054. [DOI] [PubMed] [Google Scholar]
- a Burja B.; Čimbora-Zovko T.; Tomic S.; Jelušić T.; Kočevar M.; Polanc S.; Osmak M. Pyrazolone-fused combretastatins and their precursors: synthesis, cytotoxicity, antitubulin activity and molecular modeling studies. Bio. Med. Chem. 2010, 18, 2375–2387. 10.1016/j.bmc.2010.03.006. [DOI] [PubMed] [Google Scholar]; b Ravi C.; Qayum A.; Mohan D. C.; Singh S. K.; Adimurthy S. Design, synthesis and cytotoxicity studies of novel pyrazolo[1,5-a]pyridine derivatives. Eur. J. Med. Chem. 2017, 126, 277–285. 10.1016/j.ejmech.2016.11.037. [DOI] [PubMed] [Google Scholar]; c Timári G.; Soos T.; Hajos G.; Messmer A.; Nacsa J.; Molnar J. Synthesis of novel ellipticine analogues and their inhibition of Moloney leukaemia reverse transcriptase. Bioorg. Med. Chem. Lett. 1996, 6, 2831–2836. 10.1016/S0960-894X(96)00521-5. [DOI] [Google Scholar]
- Kojima A.; Takita S.; Sumiya T.; Ochiai K.; Iwase K.; Kishi T.; Ohinata A.; Yageta Y.; Yasue T.; Kohno Y. Phosphodiesterase inhibitors. Part 6: Design, synthesis, and structure–activity relationships of PDE4-inhibitory pyrazolo[1,5-a]pyridines with anti-inflammatory activity. Bioorg Med. Chem. Lett. 2013, 23, 5311–5316. 10.1016/j.bmcl.2013.07.069. [DOI] [PubMed] [Google Scholar]
- Mamolo M. G.; Zampieri D.; Falagiani V.; Vio L.; Fermeglia M.; Ferrone M.; Pricl S.; Banfi E.; Scialino G. Antifungal and antimycobacterial activity of new N1-[1-aryl-2-(1H-Imidazol-1-yl and 1H-1,2,4-triazol-1-yl)ethylidene]pyridine-2-carboxamidrazone derivatives: A combined experimental and computational approach. ARKIVOC 2003, 2004, 231–250. 10.3998/ark.5550190.0005.521. [DOI] [Google Scholar]
- Tang J.; Wang B.; Wu T.; Wan J.; Tu Z.; Njire M.; Wan B.; Franzblauc S. G.; Zhang T.; Lu X.; Ding K. Design, synthesis, and biological evaluation of pyrazolo[1,5-a]pyridine-3-carboxamides as novel antitubercular agents. ACS Med. Chem. Lett. 2015, 6, 814–818. 10.1021/acsmedchemlett.5b00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lu X. Y.; Tang J.; Cui S. Y.; Wan B. J.; Franzblauc S. G.; Zhang T. Y.; Zhang X. T.; Ding K. Pyrazolo[1,5-a]pyridine-3-carboxamide hybrids: Design, synthesis and evaluation of antitubercular activity. Eur. J. Med. Chem. 2017, 125, 41–48. 10.1016/j.ejmech.2016.09.030. [DOI] [PubMed] [Google Scholar]; b Hu X.; Wan B.; Liu Y.; Shen J.; Franzblau S. G.; Zhang T.; Ding K.; Lu X. Identification of Pyrazolo[1,5-a]pyridine-3-carboxamide Diaryl Derivatives as Drug Resistant Antituberculosis Agents. ACS Med. Chem. Lett. 2019, 10, 295–299. 10.1021/acsmedchemlett.8b00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Löber S.; Hubner H.; Gmeiner P. Fused azaindole derivatives: molecular design, synthesis and In vitro pharmacology leading to the preferential dopamine D3 receptor agonist FAUC 725. Bioorg. Med. Chem. Lett. 2002, 12, 2377–2380. 10.1016/S0960-894X(02)00390-6. [DOI] [PubMed] [Google Scholar]; b Elsner J.; Boeckler F.; Heinemann F. W.; Hübner H.; Gmeiner P. Pharmacophore-guided drug discovery investigations leading to bioactive 5-aminotetrahydropyrazolopyridines. Implications for the binding mode of heterocyclic dopamine D3 receptor agonists. J. Med. Chem. 2005, 48, 5771–5779. 10.1021/jm0503805. [DOI] [PubMed] [Google Scholar]
- a Prante O.; Tietze R.; Hocke C.; Löber S.; Hübner H.; Kuwert T.; Gmeiner P. Synthesis, Radiofluorination, and In Vitro Evaluation of Pyrazolo[1,5-a]pyridine-Based Dopamine D4 Receptor Ligands: Discovery of an Inverse Agonist Radioligand for PET. J. Med. Chem. 2008, 51, 1800–1810. 10.1021/jm701375u. [DOI] [PubMed] [Google Scholar]; b Bettinetti L.; Schlotter K.; Hubner H.; Gmeiner P. Interactive SAR studies: rational discovery of super-potent and highly selective dopamine D3 receptor antagonists and partial agonists. J. Med. Chem. 2002, 45, 4594–4597. 10.1021/jm025558r. [DOI] [PubMed] [Google Scholar]
- Umei K.; Nishigaya Y.; Kondo A.; Tatani K.; Tanaka N.; Kohno Y.; Seto S. Novel pyrazolo[1,5-a]pyridines as orally active EP1 receptor antagonists: Synthesis, structure-activity relationship studies, and biological evaluation. Bioorg. Med. Chem. 2017, 25, 2635–2642. 10.1016/j.bmc.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Johns B. A.; Gudmundsson K. S.; Turner E. M.; Allen S. H.; Samano V. A.; Ray J. A.; Freeman G. A.; Boyd F. L. Jr.; Sexton C. J.; Selleseth D. W.; Creech K. L.; Moniri K. R. Pyrazolopyridine antiherpetics: SAR of C2′ and C7 amine substituents. Bioorg. Med. Chem. 2005, 13, 2397. 10.1016/j.bmc.2005.01.044. [DOI] [PubMed] [Google Scholar]
- a Kuroda S.; Akahane A.; Itani H.; Nishimura S.; Durkin K.; Kinoshita T.; Tenda Y.; Sakane K. Discovery of FR166124, a novel water-soluble pyrazolo[1,5-a]pyridine adenosine A1 receptor antagonist. Bioorg. Med. Chem. Lett. 1999, 9, 1979–1984. 10.1016/S0960-894X(99)00304-2. [DOI] [PubMed] [Google Scholar]; b Akahane A.; Katayama H.; Mitsunaga T.; Kato T.; Kinoshita T.; Kita Y.; Kusunoki T.; Terai T.; Yoshida K.; Shiokawa Y. Discovery of 6-Oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-1(6H)-pyridazinebutanoic acid (FK 838): A novel non-xanthine adenosine A1 receptor antagonist with potent diuretic activity. J. Med. Chem. 1999, 42, 779–883. 10.1021/jm980671w. [DOI] [PubMed] [Google Scholar]
- a Cheung M.; Harris P. A.; Badiang J. G.; Peckham G. E.; Chamberlain S. D.; Alberti M. J.; Jung D. K.; Harris S. S.; Bramson N. H.; Epperly A. H.; Stimpson S. A.; Peel M. R. The identification of pyrazolo[1,5-a]pyridines as potent p38 kinase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 5428–5430. 10.1016/j.bmcl.2008.09.040. [DOI] [PubMed] [Google Scholar]; b Stevens K. L.; Jung D. K.; Alberti M. J.; Badiang J. G.; Peckham G. E.; Veal J. M.; Cheung M. P.; Harris A. S.; Chamberlain D.; Peel M. R. Pyrazolo[1,5-a]pyridines as p38 Kinase Inhibitors. Org. Lett. 2005, 7, 4753–4756. 10.1021/ol0519745. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Kumar R.; Chandra D.; Sharma U. Cp*CoIII–catalyzed alkylation of primary and secondary C(sp3)-H bonds of 8-alkylquinolines with maleimides. J. Org. Chem. 2019, 84, 1542–1552. 10.1021/acs.joc.8b02974. [DOI] [PubMed] [Google Scholar]
- Akulov A. A.; Varaksin M. V.; Charushin V. N.; Chupakhin O. N. Direct functionalization of C(sp2)-H bond in nonaromatic azaheterocycles: palladium-catalyzed cross-Dehydrogenative coupling (CDC) of 2H-imidazole 1-oxides with pyrroles and thiophenes. ACS Omega 2019, 4, 825–834. 10.1021/acsomega.8b02916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mane K. D.; Mukherjee A.; Vanka K.; Suryavanshi G. Metal-free regioselective cross dehydrogenative coupling of cyclic ethers and aryl carbonyls. J. Org. Chem. 2019, 84, 2039–2047. 10.1021/acs.joc.8b03048. [DOI] [PubMed] [Google Scholar]
- Ren L.; Nan G.; Wang Y.; Xiao Z. Carboxylic acid-promoted single-step indole construction from simple anilines and ketones via aerobic cross-dehydrogenative coupling. J. Org. Chem. 2018, 83, 14472–14488. 10.1021/acs.joc.8b02180. [DOI] [PubMed] [Google Scholar]
- Shaikh M. M.; Patel A. P.; Chikhalia K. H. Copper catalyzed cross-dehydrogenative coupling (CDC) reaction of 4-thiazolidinone with terminal alkyne. Tetrahedron 2019, 75, 475–485. 10.1016/j.tet.2018.10.068. [DOI] [Google Scholar]
- Shakoor S. M. A.; Agarwal D. S.; Kumar A.; Sakhuja R. Copper catalyzed direct aerobic double-oxidative crossdehydrogenative coupling of imidazoheterocycles with aryl acetaldehydes: an articulate approach for icarbonylation at C-3 position. Tetrahedron 2016, 72, 645–652. 10.1016/j.tet.2015.12.012. [DOI] [Google Scholar]
- Liu X.; Zhang J.; Ma S.; Ma Y.; Wang R. Oxidative cross-dehydrogenative coupling between N-aryl tetrahydroisoquinolins and 5H-oxazol-4-ones through two methodologies: copper catalysis or a metal-free strategy. Chem. Commun. 2014, 50, 15714–15717. 10.1039/C4CC04508D. [DOI] [PubMed] [Google Scholar]
- Dana S.; Chowdhury D.; Mandal A.; Chipem F. A. S.; Baidya M. Ruthenium(II) catalysis/noncovalent interaction synergy for cross-dehydrogenative coupling of arene carboxylic acids. ACS Catal. 2018, 8, 10173–10179. 10.1021/acscatal.8b03392. [DOI] [Google Scholar]
- Zhang R.; Jin S.; Liu Q.; Lin S.; Yan Z. Transition metal-free cross-dehydrogenative coupling reaction of coumarins with acetonitrile or acetone. J. Org. Chem. 2018, 83, 13030–13035. 10.1021/acs.joc.8b01508. [DOI] [PubMed] [Google Scholar]
- Wei W.; Wang L.; Yue H.; Bao P.; Liu W.; Hu C.; Yang D.; Wang H. Metal-free visible-light-induced C-H/C-H cross-dehydrogenative-coupling of quinoxalin-2(H)-ones with simple ethers. ACS Sustainable Chem. Eng. 2018, 6, 17252–17257. 10.1021/acssuschemeng.8b04652. [DOI] [Google Scholar]
- Ghosh A.; Patra A.; Mukherjee S.; Biju A. T. Synthesis of 2-aryl naphthoquinones by the cross-dehydrogenative coupling involving an NHC-catalyzed endo-stetter reaction. J. Org. Chem. 2019, 84, 1103–1110. 10.1021/acs.joc.8b02931. [DOI] [PubMed] [Google Scholar]
- Nandwana N. K.; Singh R. P.; Patel O. P. S.; Dhiman S.; Saini H. K.; Jha P. N.; Kumar A. Design and synthesis of imidazo/benzimidazo[1,2-c]quinazoline derivatives and evaluation of their antimicrobial activity. ACS Omega 2018, 3, 16338–16346. 10.1021/acsomega.8b01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.-T.; Yu Z.-Z.; Zhang Y.-K.; Wang B. N-Bromosuccinimide-induced C-H bond functionalization: An intramolecular cycloaromatization of electron withdrawing group substituted 1-biphenyl-2-ylethanone for the synthesis of 10-phenanthrenol. Org. Lett. 2018, 20, 3728–3731. 10.1021/acs.orglett.8b01160. [DOI] [PubMed] [Google Scholar]
- Lee A.; Betori R. C.; Crane E. A.; Scheidt K. A. An enantioselective cross-dehydrogenative coupling catalysis approach to substituted tetrahydropyrans. J. Am. Chem. Soc. 2018, 140, 6212–6216. 10.1021/jacs.8b03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvatkar P. T.; Manetsch R.; Banik B. K. Metal-free cross-dehydrogenative coupling (CDC): molecular iodine as a versatile catalyst/reagent for CDC reactions. Chem. Asian J. 2019, 14, 6–30. 10.1002/asia.201801237. [DOI] [PubMed] [Google Scholar]
- Dong J.; Xia Q.; Lv X.; Yan C.; Song H.; Liu Y.; Wang Q. Photoredox-mediated direct cross-dehydrogenative coupling of heteroarenes and amines. Org. Lett. 2018, 20, 5661–5665. 10.1021/acs.orglett.8b02389. [DOI] [PubMed] [Google Scholar]
- Kibriya G.; Bagdi A. K.; Hajra A. Visible-light-promoted C(sp3)-C(sp2) cross-dehydrogenative coupling of tertiary amine with imidazopyridine. J. Org. Chem. 2018, 83, 10619–10626. 10.1021/acs.joc.8b01433. [DOI] [PubMed] [Google Scholar]
- Yang K.; Bao X.; Yao Y.; Qu J.; Wang B. Iodine-mediated cross-dehydrogenative coupling of pyrazolones and alkenes. Org. Biomol. Chem. 2018, 16, 6275–6283. 10.1039/C8OB01645C. [DOI] [PubMed] [Google Scholar]
- Yang F.; Li Y.; Floreancig P. E.; Li X.; Liu L. Copper-catalyzed oxidative cross-dehydrogenative coupling of 2H-chromenes and terminal alkynes. Org. Biomol. Chem. 2018, 16, 5144–5149. 10.1039/C8OB00949J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z.-Q.; Xiao L.-J.; Chen Y.; Xie Z.-B.; Zhu H.-B.; Le Z.-G. A highly efficient copper(II)-catalyzed cross-dehydrogenative-coupling reaction of N-arylglycine esters with 2-arylimidazo[1,2-a]pyridines. Synthesis 2018, 50, 2775–2783. 10.1055/s-0036-1609845. [DOI] [Google Scholar]
- Li Y.; Peng J.; Chen X.; Mo B.; Li X.; Sun P.; Chen C. Copper-catalyzed synthesis of multisubstituted indoles through tandem Ullmann-type C–N formation and cross-dehydrogenative coupling reactions. J. Org. Chem. 2018, 83, 5288–5294. 10.1021/acs.joc.8b00353. [DOI] [PubMed] [Google Scholar]
- Doan S. H.; Nguyen V. H. H.; Nguyen T. H.; Pham P. H.; Nguyen N. N.; Phan A. N. Q.; Tu T. N.; Phan N. T. S. Cross-dehydrogenative coupling of coumarins with Csp3-H bonds using an iron–organic framework as a productive heterogeneous catalyst. RSC Adv. 2018, 8, 10736–10745. 10.1039/C8RA00872H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Lei B.; Ma L.; Zhu L.; Zhang X.; Zuo H.; Zhuang D.; Li Z. Cobalt-catalyzed cross-dehydrogenative C(sp2)-C(sp3) coupling of oxazole/thiazole with ether or cycloalkane. Chem. Asian J. 2017, 12, 2799–2803. 10.1002/asia.201701258. [DOI] [PubMed] [Google Scholar]
- Zhu C.; Zhu R.; Zeng H.; Chen F.; Liu C.; Wu W.; Jiang H. Copper-catalyzed C(sp3)-H/C(sp3)-H cross-dehydrogenative coupling with internal oxidants: synthesis of 2-trifluoromethyl-substituted dihydropyrrol-2-ols. Angew. Chem., Int. Ed. 2017, 56, 13324–13328. 10.1002/anie.201707719. [DOI] [PubMed] [Google Scholar]
- Bityukov O. V.; Matveeva O. K.; Vil V. A.; Kokorekin V. A.; Nikishin G. I.; Terent’ev A. O. Electrochemically induced intermolecular cross-dehydrogenative C-O coupling of β-diketones and β-ketoesters with carboxylic acids. J. Org. Chem. 2019, 84, 1448–1460. 10.1021/acs.joc.8b02791. [DOI] [PubMed] [Google Scholar]
- Xu X.; Li P.; Huang Y.; Tong C.; Yan Y.; Xie Y. Atmospheric oxidative catalyst-free cross-dehydrogenative coupling of aldehydes with N-hydroxyimides. Tetrahedron Lett. 2017, 58, 1742–1746. 10.1016/j.tetlet.2017.03.064. [DOI] [Google Scholar]
- Kramer S. Synthesis of α-substituted primary benzylamines through copper-catalyzed cross-dehydrogenative coupling. Org. Lett. 2019, 21, 65–69. 10.1021/acs.orglett.8b03505. [DOI] [PubMed] [Google Scholar]
- Wei W.; Wang L.; Bao P.; Shao Y.; Yue H.; Yang D.; Yang X.; Zhao X.; Wang H. Metal-free C(sp2)-H/N-H cross-dehydrogenative coupling of quinoxalinones with aliphatic amines under visible-light photoredox catalysis. Org. Lett. 2018, 20, 7125–7130. 10.1021/acs.orglett.8b03079. [DOI] [PubMed] [Google Scholar]
- Sun B.; Yan Z.; Jin C.; Su W. (Diacetoxyiodo)benzene-mediated transition-metal-free amination of C(sp3)-H bonds adjacent to heteroatoms with azoles: synthesis of N-alkylated azoles. Synlett 2018, 29, 2432–2436. 10.1055/s-0037-1610293. [DOI] [Google Scholar]
- Kumar P.; Kumar S. A.; Singh R.; Guntreddi T.; Nand S. K. Nickel catalyzed ipso-hydroxylation and Subsequent cross dehydrogenative coupling of arylboronic acids with tertiary amines: A facile access to α-phenolated tertiary amines. Adv. Synth. Catal. 2018, 360, 1786–1789. 10.1002/adsc.201701625. [DOI] [Google Scholar]
- Ahmad A.; Dutta H. S.; Khan B.; Kant R.; Koley D. Cu(I)-Catalyzed site selective acyloxylation of indoline using O2 as the sole oxidant. Adv. Synth. Catal. 2018, 360, 1644–1649. 10.1002/adsc.201800040. [DOI] [Google Scholar]
- Rattanangkool E.; Krailat W.; Vilaivan T.; Phuwapraisirisan P.; Sukwattanasinitt M.; Wacharasindhu S. Hypervalent iodine(III)-promoted metal-free S–H activation: An approach for the construction of S–S, S–N, and S–C bonds. Eur. J. Org. Chem. 2014, 2014, 4795–4804. 10.1002/ejoc.201402180. [DOI] [Google Scholar]
- Dou Y.; Huang X.; Wang H.; Yang L.; Li H.; Yuan B.; Yang G. Reusable cobalt-phthalocyanine in water: Efficient catalytic aerobic oxidative coupling of thiols to construct S–N/S–S bonds. Green Chem. 2017, 19, 2491–2495. 10.1039/C7GC00401J. [DOI] [Google Scholar]
- Huang X.; Chen Y.; Zhen S.; Song L.; Gao M.; Zhang P.; Li H.; Yuan B.; Yang G. Cobalt-catalyzed aerobic cross-dehydrogenative coupling of C-H and thiols in water for C-S formation. J. Org. Chem. 2018, 83, 7331–7340. 10.1021/acs.joc.7b02718. [DOI] [PubMed] [Google Scholar]
- Yang D.; Sun P.; Wei W.; Meng L.; He L.; Fang B.; Jiang W.; Wang H. Metal-free iodine-catalyzed direct cross-dehydrogenative coupling (CDC) between pyrazoles and thiols. Org. Chem. Front. 2016, 3, 1457–1461. 10.1039/C6QO00407E. [DOI] [Google Scholar]
- Chang C.-Z.; Liu X.; Zhu H.; Wu L.; Dong Z.-B. Copper-catalyzed and air-mediated mild cross-dehydrogenative coupling of aryl thioureas and dialkyl H-phosphonates: The synthesis of thiophosphonates. J. Org. Chem. 2018, 83, 13530–13535. 10.1021/acs.joc.8b02011. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Liu Z.; Zhang Y.; Xiong C. Manganese(III) acetylacetonate-mediated phosphorylation of enamides at room temperature. Adv. Synth. Catal. 2018, 360, 3492–3496. 10.1002/adsc.201800585. [DOI] [Google Scholar]
- Deng L.; Wang Y.; Mei H.; Pan Y.; Han J. Electrochemical dehydrogenative phosphorylation of alcohols for the synthesis of organophosphinates. J. Org. Chem. 2019, 84, 949–956. 10.1021/acs.joc.8b02882. [DOI] [PubMed] [Google Scholar]
- Song G.; Li X. Substrate activation strategies in rhodium (III)-catalyzed selective functionalization of arenes. Acc. Chem. Res. 2015, 48, 1007–1020. 10.1021/acs.accounts.5b00077. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Lan J.; You J. Oxidative C–H/C–H coupling reactions between two (hetero) arenes. Chem. Rev. 2017, 117, 8787–8863. 10.1021/acs.chemrev.6b00567. [DOI] [PubMed] [Google Scholar]
- a Perepichka I.; Kundu S.; Hearne Z.; Li C.-J. Efficient merging of copper and photoredox catalysis for the asymmetric cross-dehydrogenativecoupling of alkynes and tetrahydroisoquinolines. Org. Biomol. Chem. 2015, 13, 447–451. 10.1039/C4OB02138J. [DOI] [PubMed] [Google Scholar]; b Wang F.-F.; Luo C.-P.; Deng G.; Yang L. C(sp3)–C(sp3) bond formation via copper/Brønsted acid co-catalyzed C(sp3)–H bond oxidative cross-dehydrogenative-coupling (CDC) of azaarenes. Green Chem. 2014, 16, 2428–2431. 10.1039/c4gc00038b. [DOI] [Google Scholar]; c Liu X.; Zhang J.; Ma S.; Ma Y.; Wang R. Oxidative cross-dehydrogenative coupling between N-aryl tetrahydroisoquinolins and 5H-oxazol-4-ones through two methodologies: copper catalysis or a metal-free strategy. Chem. Commun. 2014, 50, 15714–15717. 10.1039/C4CC04508D. [DOI] [PubMed] [Google Scholar]
- Xuan J.; Zeng T.-T.; Feng Z.-J.; Deng Q.-H.; Chen J.-R.; Lu L.-Q.; Xiao W.-J.; Alper H. Redox-neutral α-allylation of amines by combining palladium catalysis and visible-light photoredox catalysis. Angew. Chem., Int. Ed. 2015, 54, 1625–1628. 10.1002/anie.201409999. [DOI] [PubMed] [Google Scholar]
- Xie J.; Li H.; Zhou J.; Cheng Y.; Zhu C. A highly efficient gold-catalyzed oxidative C–C coupling from C–H bonds using air as oxidant. Angew. Chem., Int. Ed. 2012, 51, 1252–1255. 10.1002/anie.201107605. [DOI] [PubMed] [Google Scholar]
- Meng Q.-Y.; Liu Q.; Zhong J.-J.; Zhang H.-H.; Li Z.-J.; Chen B.; Tung C.-H.; Wu L. Z. Graphene-supported RuO2 nanoparticles for efficient aerobic cross-dehydrogenative coupling reaction in water. Org. Lett. 2012, 14, 5992–5995. 10.1021/ol3028785. [DOI] [PubMed] [Google Scholar]
- a Yu J.-B.; Zhang Y.; Jiang Z.-J.; Su W.-K. Mechanically induced Fe(III) catalysis at room temperature: solvent-free cross-dehydrogenative coupling of 3-benzylic indoles with methylenes/indoles. J. Org. Chem. 2016, 81, 11514–11520. 10.1021/acs.joc.6b02197. [DOI] [PubMed] [Google Scholar]; b Ratnikov M. O.; Xu X.; Doyle M. P. Simple and sustainable iron-catalyzed aerobic C–H functionalization of N,N-dialkylanilines. J. Am. Chem. Soc. 2013, 135, 9475–9479. 10.1021/ja402479r. [DOI] [PubMed] [Google Scholar]
- a Condie A. G.; Gozález-Gómez J. C.; Stephenson C. R. J. Visible-light photoredox catalysis: aza-Henry reactions via C–H functionalization. J. Am. Chem. Soc. 2010, 132, 1464–1465. 10.1021/ja909145y. [DOI] [PubMed] [Google Scholar]; b Feng Z.-J.; Xuan J.; Xia X.-D.; Ding W.; Guo W.; Chen J.-R.; Zou Y.-Q.; Lu L.-Q.; Xiao W.-J. Direct sp3 C-H acroleination of N-aryltetrahydroisoquinolines by merging photoredox catalysis with nucleophilic catalysis. Org. Biomol. Chem. 2014, 12, 2037–2040. 10.1039/C3OB42453G. [DOI] [PubMed] [Google Scholar]
- Jones K. M.; Karier P.; Klussmann M. C1-Substituted N-alkyl tetrahydroisoquinoline derivatives through V-catalyzed oxidative coupling. ChemCatChem 2012, 4, 51–54. 10.1002/cctc.201100324. [DOI] [Google Scholar]
- Li Q.; Hu W.; Hu R.; Lu H.; Li G. Cobalt-catalyzed cross-dehydrogenative coupling reaction between unactivated C(sp2)-H and C(sp3)-H bonds. Org. Lett. 2017, 19, 4676–4679. 10.1021/acs.orglett.7b02316. [DOI] [PubMed] [Google Scholar]
- Majji G.; Guin S.; Rout S. K.; Behera A.; Patel B. K. Cyclic ethers to esters and monoesters to bis-esters with unconventional coupling partners under metal free conditions via sp3 C–H functionalization. Chem. Commun. 2014, 50, 12193–12196. 10.1039/C4CC05050A. [DOI] [PubMed] [Google Scholar]
- a Pan Y.; Wang S.; Kee C. W.; Dubuisson E.; Yang Y.; Loh K. P.; Tan C.-H. Graphene oxide and rose bengal: oxidative C–H functionalisation of tertiary amines using visible light. Green Chem. 2011, 13, 3341–3344. 10.1039/c1gc15865a. [DOI] [Google Scholar]; b Zhong J.-J.; Meng Q.-Y.; Liu B.; Li X.-B.; Gao X.-W.; Lei T.; Wu C.-J.; Li Z.-J.; Tung C.-H.; Wu L.-Z. Cross-coupling hydrogen evolution reaction in homogeneous solution without noble metals. Org. Lett. 2014, 16, 1988–1991. 10.1021/ol500534w. [DOI] [PubMed] [Google Scholar]
- Franz J. F.; Kraus W. B.; Zeitler K. No photocatalyst required-versatile, visible light mediated transformations with polyhalomethanes. Chem. Commun. 2015, 51, 8280–8283. 10.1039/C4CC10270C. [DOI] [PubMed] [Google Scholar]
- Huang L.; Zhao J. Iodo-Bodipys as visible-light-absorbing dual-functional photoredox catalysts for preparation of highly functionalized organic compounds by formation of C–C bonds via reductive and oxidative quenching catalytic mechanisms. RSC Adv. 2013, 3, 23377–23388. 10.1039/c3ra43299h. [DOI] [Google Scholar]
- Liu Q.; Li Y.-N.; Zhang H.-H.; Chen B.; Tung C.-H.; Wu L.-Z. Reactivity and mechanistic insight into visible-light-induced aerobic cross-dehydrogenative coupling reaction by organophotocatalysts. Chem. – Eur. J. 2012, 18, 620–627. 10.1002/chem.201102299. [DOI] [PubMed] [Google Scholar]
- Behbehani H.; Ibrahim H. M. A strategy for the synthesis of 2-aryl-3-dimethylamino- pyrazolo[3,4-c]pyridines that utilizes [4+1] cycloaddition reactions of 5-arylazo-2,3,6-trisubstituted pyridines. Tetrahedron 2013, 69, 10535–10543. 10.1016/j.tet.2013.10.061. [DOI] [Google Scholar]
- Behbehani H.; Ibrahim H. M.; Makhseed S.; Elnagdi M. H.; Mahmoud H. 2-Aminothiophenes as Building Blocks in Heterocyclic Synthesis: Synthesis and Antimicrobial Evaluation of a New Class of Pyrido[1,2-a]thieno[3,2-e]pyrimidine, quinoline and Pyridin-2-one Derivatives. Eur. J. Med. Chem. 2012, 52, 51–65. 10.1016/j.ejmech.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Ibrahim H. M.; Arafa W. A. A.; Behbehani H. L-Proline catalyzed one-pot synthesis of polysubstituted pyridine system incorporating benzothiazole moiety via sustainable sonochemical approach. RSC Adv. 2018, 8, 37606–37617. 10.1039/C8RA07013J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani H.; Ibrahim H. M.; Elnagdi M. H. Non-concerted nucleophilic [4+1] cycloaddition of (dimethylamino)methoxycarbene to arylazonicotinates in the synthesis of a pyrazolo[3,4-c]pyridines and pyrazolo[4′,3′:4,5]pyrido[2,3-d]pyrimidines. Tetrahedron 2013, 69, 6176–6184. 10.1016/j.tet.2013.05.040. [DOI] [Google Scholar]
- Behbehani H.; Ibrahim H. M. Microwave assisted synthesis in water. First one pot synthesis of a novel class of polysubstituted benzo[4,5]imidazo[1,2-b]pyridazines via intramolecular SNAr. RSC Adv. 2015, 5, 89226–89237. 10.1039/C5RA17313B. [DOI] [Google Scholar]
- Behbehani H.; Ibrahim H. M. An efficient ultrasonic-mediated one-pot synthesis of 2,3,6,7,9- pentaazabicyclo- [3.3.1]nonanes via a N,N-dimethylformamide dimethylacetal catalyzed Mannich-like reaction. RSC Adv. 2016, 6, 52700–52709. 10.1039/C6RA09210A. [DOI] [Google Scholar]
- a Koike T.; Takai T.; Hoashi Y.; Nakayama M.; Kosugi Y.; Nakashima M.; Yoshikubo S.; Hirai K.; Uchikawa O. Synthesis of a novel series of tricyclic dihydrofuran derivatives: discovery of 8,9-dihydrofuro[3,2-c]pyrazolo[1,5-a]pyridines as melatonin receptor (MT1/MT2) ligands. J. Med. Chem. 2011, 54, 4207–4218. 10.1021/jm200385u. [DOI] [PubMed] [Google Scholar]; b Mousseau J. J.; Bull J. A.; Ladd C. L.; Fortier A.; Roman D. S.; Charette A. B. Synthesis of 2- and 2,3-substituted pyrazolo[1,5-a]pyridines: scope and mechanistic considerations of a domino direct alkynylation and cyclization of N-iminopyridinium ylides using alkenyl bromides, alkenyl iodides, and alkynes. J. Org. Chem. 2011, 76, 8243–8261. 10.1021/jo201303x. [DOI] [PubMed] [Google Scholar]; c Elsner J.; Boeckler F.; Davidson K.; Sugden D.; Gmeiner P. Bicyclic melatonin receptor agonists containing a ring-junction nitrogen: Synthesis, biological evaluation, and molecular modeling of the putative bioactive conformation. Bioorg. Med. Chem. 2006, 14, 1949–1958. 10.1016/j.bmc.2005.10.042. [DOI] [PubMed] [Google Scholar]; d Ibrahim H. M.; Behbehani H.; Mostafa N. S. Scalable sonochemical synthetic strategy for pyrazolo[1,5-a]pyridine derivatives: First catalyst free concerted [3+2] cycloaddition of alkyne and alkene derivatives to 2-imino-1H-pyridin-1-amines. ACS Omega 2019, 4, 7182–7193. 10.1021/acsomega.9b00562. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Yu X.; Ye S.; Wu J. Facile assembly of H-pyrazolo[5,1,a]isoquinolines via silver triflate-catalyzed one-pot tandem reaction of 2-alkynyl-benzaldehyde, sulfonohydrazide, and ketone or aldehyde. Adv. Synth. Catal. 2010, 352, 2050–2056. 10.1002/adsc.201000176. [DOI] [Google Scholar]
- Chen Z.; Wu J. Efficient generation of biologically active H-Pyrazolo[5,1-a] isoquinolines via multicomponent reaction. Org. Lett. 2010, 12, 4856–4859. 10.1021/ol101988q. [DOI] [PubMed] [Google Scholar]
- Johns B. A.; Gudmundsson K. S.; Turner E. M.; Allen S. H.; Jung D. K.; Sexton C. J.; Boyd F. L.; Peel M. R. Pyrazolo[1,5-a]pyridines: synthetic approaches to a novel class of antiherpetics. Tetrahedron 2003, 59, 9001–9011. 10.1016/j.tet.2003.02.003. [DOI] [Google Scholar]
- Hoashi Y.; Takai T.; Kotani E.; Koike T. Synthesis of pyrazolo[1,5-a]pyridines by thermal intramolecular cyclization. Tetrahedron Lett. 2013, 54, 2199–2202. 10.1016/j.tetlet.2013.02.078. [DOI] [Google Scholar]
- a Abramovitch R. A.; Kalinowski J. Pyrido[1,2-b] indazole and its derivatives. J. Heterocycl. Chem. 1974, 11, 857–861. 10.1002/jhet.5570110603. [DOI] [Google Scholar]; b Abramovitch R. A.; Adams K. A. H. Tryptamines, carbolines, and related compounds: part ix. the cyclization of some nitro- and azido-phenylpyridines. pyrido[1,2-b]indazole. Can. J. Chem. 1961, 39, 2516–2528. 10.1139/v61-332. [DOI] [Google Scholar]; c Abramovitch R. A.; Ahmad Y.; Newman D. The formation of a nitrene intermediate in the reaction of nitro compounds with ferrous oxalate. Tetrahedron Lett. 1961, 2, 752–757. 10.1016/S0040-4039(01)99261-5. [DOI] [Google Scholar]; d Zhao J.; Wu C.; Li P.; Ai W.; Chen H.; Wang C.; Larock R. C.; Shi F. Synthesis of pyrido[1,2-b]indazoles via aryne [3 + 2] cycloaddition with n-tosylpyridinium imides. J. Org. Chem. 2011, 76, 6837–6843. 10.1021/jo200863e. [DOI] [PubMed] [Google Scholar]
- Ibrahim H. M.; Behbehani H. Sustainable synthetic approach for (pyrazol-4-ylidene)pyridines by metal catalyst-free aerobic C(sp2)–C(sp3) coupling reactions between 1-amino-2-imino-pyridines and 1-aryl-5-pyrazolones. ACS Omega 2019, 4, 11701–11711. 10.1021/acsomega.9b01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Schmidpeter A.; Steinmüller F.; Zabotina E. Y. Four and fivemembered phosphorus heterocycles. LXXXIV. [1,5]-anellated 1,2,4,3-triazaphospholes. J. Prakt. Chem. A. 1993, 335, 458–460. 10.1002/prac.19933350512. [DOI] [Google Scholar]
- The crystallographic data for 4i (ref. CCDC 1916934), 4r (ref. CCDC 1940357), 6a (ref. CCDC 1916935), 6c (ref. CCDC 1916936), and 6d (ref. CCDC 1916937) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, U.K.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.