Abstract
A novel formic acid-assisted rapid and efficient route for C–S bond construction via the thiol–ene reaction has been reported. Exclusively, the anti-Markovnikov product was obtained in good to excellent yield using the developed protocol. Various styrenes and thiols bearing different functionalities were well tolerated. The reaction also provided a good yield of sulfones in a one-pot two-step protocol. The developed method is operationally simple, green, metal-free, solvent-free, and having a high atom economy with high regioselectivity.
1. Introduction
Organosulfur compounds serve as synthons for the various chemical transformations in organic synthesis. Organosulfur compounds are found in several biologically active natural products, bioactive peptides, food products, and flavoring agents.1,2 These compounds are also an important intermediate in pharmaceuticals, as well as in material chemistry.3 The thiol–ene click reaction produces the thioether following the free-radical as well as ionic mechanism.4−7 The reaction provided two types of products following Markovnikov and anti-Markovnikov additions.8 Previously, various metal-catalyzed (Ru, Cu, In) methods have been developed to construct the C–S bond.9−12 However, the use of an expensive and toxic metal catalyst and hazardous reaction conditions limit the scope of these methodologies. Few metal-free approaches were also reported using ionic liquids, silica nanoparticles, and Amberlyst as catalyst.13−16 Cumbersome catalyst synthesis, tedious recyclability, and limited substrate scope are the drawbacks of the previously reported protocols. To overcome these challenges, we report a green, base-free, metal-free formic acid-mediated protocol for the synthesis of thioethers at room temperature (Scheme 1).
Scheme 1. Thiol–Ene Reaction.
2. Results and Discussion
To begin our investigation, styrene and 4-methoxy thiophenol were chosen as model substrates. Initially, the reaction was tested in various solvents in the presence of potassium tert-butoxide as a base at room temperature, but no reaction was observed (Table 1, entries 1–5). Further, the reaction was attempted with different bases unsuccessfully (entries 6–8). A reaction in formic acid with potassium tert-butoxide provided the desired thioether (entry 9) in moderate yield. However, when the reaction was performed in acetic acid, a slight decrease in the yield was observed (entry 10). The presence of base resulted in the formation of disulfide, which decreases selectivity for the desired product. Next, when a reaction was performed in the absence of base, an optimal yield of the desired product was obtained (entry 11). Similarly, the reaction in acetic acid has also proceeded well in the absence of base with slightly lower yield (entry 12). The reaction performed in BF3·O(C2H5)2 and Cu(OTf)2 resulted in no reaction (entries 13 and 14). However, a reaction without formic acid showed a considerable decrease in yield due to the formation of disulfides. Formic acid was found to be efficient for the rapid conversion of styrene and thiol into their anti-Markovnikov products at room temperature.
Table 1. Optimization of the Reaction Conditions.
| entry | additive | solvent (1 mL) | yield (%)a |
|---|---|---|---|
| 1 | tBuOK | ACN | NR |
| 2 | tBuOK | ethanol | NR |
| 3 | tBuOK | DMF | NR |
| 4 | tBuOK | toluene | NR |
| 5 | tBuOK | chloroform | NR |
| 6 | tBuOLi | ACN | NR |
| 7 | K2CO3 | ACN | NR |
| 8 | NEt3 | ACN | NR |
| 9 | tBuOK | formic acid | 65 |
| 10 | tBuOK | acetic acid | 60 |
| 11 | formic acidb | 92 | |
| 12 | acetic acidc | 89 | |
| 13 | BF3·O(C2H5)2d | NR | |
| 14 | Cu(OTf)2e | NR | |
| 15 | 66 |
Reaction conditions: 1a (1.0 equiv), 2 (1.0 equiv), additive (2.0 equiv), rt, 15 min.
Formic acid (10.0 equiv)
Acetic acid (10.0 equiv).
BF3·O(C2H5)2 (10.0 equiv).
Cu(OTf)2 (20 mol %).
With optimized reaction conditions in hand, the substrate scope for various styrene and thiol derivatives was tested. Initially, styrene was reacted with 4-methoxy thiophenol and provided 92% yield of the desired product (Scheme 2, 3a). The styrenes bearing electron-donating groups were well tolerated and delivered the product in good to excellent yield (Scheme 2, 3b−d). The sterically hindered substrate such as 2,4,6-trimethylstyrene also gave the product in good yield (Scheme 2, 3d). The halogen-substituted styrene also reacted well and resulted in good to excellent yield of the desired product (Scheme 2, 3e−g). Styrenes bearing electron-withdrawing groups such as nitrile, nitro, and carboxylic acid were tolerated well and provided good yields (Scheme 2, 3h−j). The O-acetyl group-containing styrene also tolerated well and provided a good yield (Scheme 2, 3ak). Cyclic styrene systems such as indene and 1,2-dihydronaphthalene also reacted well and provided a good yield of the required product (Scheme 2, 3l, 3m). The heterocyclic styrenes transformed into the desired thioether in good yields (Scheme 2, 3n, 3o). Naphthalene and biphenyl derivatives were also reacted well and provided an excellent yield of the desired product (Scheme 2, 3p, 3q). The bulkier hydrocarbon like 9-vinyl anthracene gave a good yield of the product (Scheme 2, 3r). The reaction was not observed with allylic and aliphatic alkenes (Scheme 2, 3s, 3t).
Scheme 2. Substrate Scope for Various Styrenes,
Reaction conditions: 1 (1.0 equiv), 2 (1.0 equiv), formic acid (10.0 equiv), rt, 15–60 min.
Isolated yield, NR: no reaction.
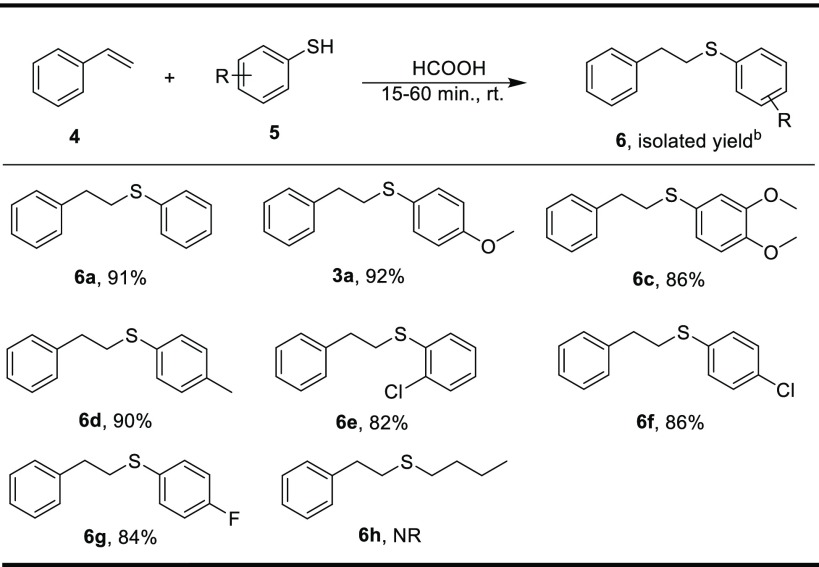
Next, reactions were performed with different thiols. Thiophenol gave an excellent yield of thioether with styrene (Scheme 3, 6a). Various thiols bearing electron-donating groups reacted well and provided good to excellent yield of the desired thioether (Scheme 3, 6b−d). The thiol-containing electron-withdrawing group furnishes the corresponding product in good to excellent yield (Scheme 3, 6e−g). However, aliphatic thiol did not yield the desired product (Scheme 3, 6h).
Scheme 3. Substrate Scope for Various Thiols,
Reaction conditions: 4 (1.0 equiv), 5 (1.0 equiv), formic acid (10.0 equiv), rt, 15–60 min.
Isolated yield, NR: no reaction.
The established protocol was further investigated for the thiol alkyne reaction, which provided the E/Z mixture of the desired product. The synthesized products and methodology developed are important for several chemical transformations and material chemistry.17−19 When the reaction of phenylacetylene (1 equiv), 4-methoxy thiophenol (1 equiv), and formic acid (10 equiv) was subjected to the optimized reaction conditions, an excellent yield of the product was obtained. The resulting product was found to be an E/Z mixture in 20:80 ratio (Scheme 4, 8). To demonstrate the feasibility and sustainability of the developed protocol, we performed the gram-scale synthesis on substrate 9 and a good yield of the desired product was obtained (Scheme 4, 11). The sulfone derivative of the desired thioether was synthesized in good yield (Scheme 4, 12).
Scheme 4. Reaction with Alkyne and Gram-Scale Reaction and Synthesis of Sulfone.
Reaction conditions: 7 (1 equiv), 2 (1 equiv), formic acid (10 equiv), rt, 15 min.
Isolated yield.
E/Z ratio was calculated using 1H NMR, 9 (1 equiv), 10 (1 equiv), rt, 60 min.
Isolated yield in the gram-scale reaction: 1 (1 equiv), 2 (1 equiv), formic acid (10 equiv), H2O2 (5 equiv) rt, 1.5 h.
To understand the mechanism of the developed protocol, following control experiments were performed. Initially, the reaction with disulfide furnished no product, which ruled out the formation of disulfide intermediate during the reaction (Scheme 5, A). The reaction of styrene and 4-methoxy thiophenol provided a good yield of the required product in the presence of a radical quencher (TEMPO), which ruled out a free-radical pathway (Scheme 5, B). Next, the isotopic experiment was done to gain the mechanistic insight into the reaction. The reaction with deuterated formic acid provided a collective yield of 86% for the desired product having the D/H ratio of 6:4 (Scheme 5, C). This result indicates that the hydrogen atom of formic acid plays an important role in the reaction by transferring the hydrogen atom as well as by thiol activation via electrostatic attraction (hydrogen bonding). To identify the participation of hydrogen atom during the reaction, we performed the reaction in deuterated acetic acid and found a similar result, which confirmed that the hydrogen atom of the carboxylic group is responsible for the initiation and rapid conversion of the reaction. The reaction of styrene with sodium thiophenolate also gave the desired product in good yield, which confirmed the role of formic acid in the reaction (Scheme 5, D and Scheme 6).
Scheme 5. Control Experiment,
Isolated yield
D/H ratio was calculated using 1H NMR.
Scheme 6. Plausible Mechanism.
To support it further, we carried out the infrared analysis of the reaction. Formic acid showed a strong band at 1726 cm–1, which corresponds to the carbonyl group (Figure S1 in the Supporting Information).20,21 The IR spectra of 4-methoxy thiophenol gave a signal at 2559 cm–1, corresponding to the S–H group (Figure S2 in the Supporting Information).7 When formic acid and 4-methoxy thiophenol were mixed, a shift was observed in both formic acid (1726 to 1730 cm–1) and 4-methoxy thiophenol (2559 to 2567 cm–1) bands in IR spectra, which shows the interaction between thiol and formic acid (Figure S3 in the Supporting Information).
3. Conclusions
In summary, we have developed a green, metal-free, and solvent-free methodology for the synthesis of thioethers. The developed protocol has wide functional group tolerance and found to be operational for gram-scale synthesis. Besides, the mechanistic study was performed to ascertain the mechanism of this important protocol.
4. Experimental Section
4.1. General Information
High-purity solvents were used for all reactions. Silica gel (60–120, 230–400 mesh, S.D. Fine and Spectrochem make) was used for column chromatography. All of the reactions were monitored by thin-layer chromatography using precoated silica plates (Merck Silica gel 60 F254, 0.25 mm thickness). NMR solvents were purchased from Sigma-Aldrich. 1H NMR and 13C NMR experiments were performed on Bruker Avance-300 and 600 spectrometers. Mass spectra were recorded on a Water Q-TOF mass spectrometer. The melting points were recorded using the LABINDIA visual melting range apparatus.
4.2. General Procedure for the Synthesis of Compounds
Styrene (50 mg, 1 equiv), 4-methoxy thiophenol (1 equiv), and formic acid (10 equiv) were mixed and stirred at room temperature for 15–60 min; then, the resulting mixture was diluted with ethyl acetate and water, extracted three times with ethyl acetate, dried over sodium sulfate, and purified using silica gel column chromatography.
4.3. General Procedure for the Synthesis of Compound 12
Styrene (50 mg, 1 equiv), 4-methoxy thiophenol (67 mg, 1 equiv), and formic acid (10 equiv) were stirred at room temperature for 15 min. To this solution was added hydrogen peroxide (5 equiv), and the solution was stirred again for 1.5 h at the same temperature. The resulting mixture was diluted with ethyl acetate and water, extracted three times with ethyl acetate, dried over sodium sulfate, and purified using column chromatography.
4.4. Characterization Data for the Compounds
4.4.1. (4-Methoxyphenyl)(phenethyl)sulfane (3a)
Colorless oil, (107 mg, 92%). 1H NMR (600 MHz, CDCl3): δ 7.42–7.38 (m, 2H), 7.32–7.30 (m, 2H), 7.25–7.22 (m, 1H), 7.19 (d, J = 7.0 Hz, 2H), 6.90–6.88 (m, 2H), 3.83 (s, 3H), 3.11–3.08 (m, 2H), 2.91–2.88 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 158.9, 140.3, 133.2, 128.4, 128.4, 126.3, 114.5, 55.3, 37.2, 35.8.
4.4.2. (4-Methoxyphenyl)(4-methylphenethyl)sulfane (3b)
Colorless oil, (98 mg, 90%). 1H NMR (600 MHz, CDCl3): δ 7.45–7.43 (m, 2H), 7.17 (d, J = 7.9 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 6.94–6.91 (m, 2H), 3.86 (s, 3H), 3.13–3.10 (m, 2H), 2.91–2.89 (m, 2H), 2.39 (s, 3H). 13C NMR (151 MHz, CDCl3): δ 158.8, 137.2, 135.7, 133.0, 129.0, 128.3, 126.3, 114.5, 55.2, 37.2, 35.3, 20.9. ESI-MS: m/z calcd for C16H18OS [M + H]+: 259.1151, found: 259.1163.
4.4.3. (4-(tert-Butyl)phenethyl)(4-methoxyphenyl)sulfane (3c)
Colorless oil (82 mg, 88%). 1H NMR (600 MHz, CDCl3): δ 7.43–7.40 (m, 2H), 7.36 (d, J = 8.2 Hz, 2H), 7.16 (d, J = 8.2 Hz, 2H), 6.92–6.89 (m, 2H), 3.84 (s, 3H), 3.13–3.10 (m, 2H), 2.91–2.88 (m, 2H), 1.36 (s, 9H). 13C NMR (151 MHz, CDCl3): δ 158.8, 149.0, 137.2, 133.0, 128.1, 126.3, 125.2, 114.5, 55.2, 37.1, 35.3, 34.3, 31.3. ESI-MS: m/z calcd for C19H24OS [M + H]+: 301.1621, found: 301.1611.
4.4.4. (4-Methoxyphenyl)(2,4,6-trimethylphenethyl)sulfane (3d)
Colorless oil, (84 mg, 86%). 1H NMR (600 MHz, CDCl3): δ 7.47 (d, J = 12.0 Hz, 2H), 6.92 (d, J = 6.0 Hz, 2H), 6.85 (s, 2H), 3.85 (s, 3H), 2.89 (s, 4H), 2.28 (s, 3H), 2.22 (s, 6H). 13C NMR (151 MHz, CDCl3): δ 159.0, 136.0, 135.5, 133.9, 133.6, 128.9, 126.2, 114.4, 55.2, 34.5, 29.8, 20.7, 19.5. ESI-MS: m/z calcd for C18H22OS [M + H]+: 287.1464, found: 287.1449.
4.4.5. (4-Chlorophenethyl)(4-methoxyphenyl)sulfane (3e)
Light yellow oil, (82 mg, 82%). 1H NMR (600 MHz, CDCl3): δ 7.41–7.39 (m, 2H), 7.29–7.27 (m, 2H), 7.13–7.11 (m, 2H), 6.91–6.89 (m, 2H), 3.84 (s, 3H), 3.08–3.06 (m, 2H), 2.88–2.85 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 158.9, 138.6, 133.3, 131.9, 129.8, 128.4, 125.9, 114.5, 55.2, 37.0, 35.0. ESI-MS: m/z calcd for C15H15ClOS [M + H]+: 279.0605, found: 279.0608.
4.4.6. (4-Bromophenethyl)(4-methoxyphenyl)sulfane (3f)
Yellow oil, (74 mg, 84%). 1H NMR (600 MHz, CDCl3): δ 7.42–7.40 (m, 2H), 7.39–7.36 (m, 2H), 7.06–7.04 (m, 2H), 6.89–6.86 (m, 2H), 3.82 (s, 3H), 3.06–3.03 (m, 2H), 2.84–2.82 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 159.0, 139.2, 133.4, 131.4, 130.2, 125.9, 120.0, 114.6, 55.3, 37.0, 35.1. ESI-MS: m/z calcd for C15H15BrOS [M + H]+: 323.0100, found: 323.0119.
4.4.7. (3,5-Bis(trifluoromethyl)phenethyl)(4-methoxyphenyl)sulfane (3g)
Colorless oil, (63 mg, 80%). 1H NMR (600 MHz, CDCl3): δ 7.74 (s, 1H), 7.61 (s, 2H), 7.38–7.35 (m, 2H), 6.89–6.86 (m, 2H), 3.83 (s, 3H), 3.13–3.10 (m, 2H), 3.02–2.99 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 159.3, 142.5, 133.8, 131.8, 131.6, 131.4, 131.2, 128.8, 126.0, 125.1, 124.2, 122.4, 120.6, 120.4, 120.4, 120.4, 120.3, 114.7, 55.3, 36.6, 35.4. ESI-MS: m/z calcd for C17H14F6OS [M + H]+: 381.0742, found: 381.0731.
4.4.8. 4-(2-((4-Methoxyphenyl)thio)ethyl)benzonitrile (3h)
Yellow oil, (84 mg, 81%). 1H NMR (600 MHz, CDCl3): δ 7.59 (d, J = 6.0 Hz, 2H), 7.38–7.36 (m, 2H), 7.28 (d, J = 6.0 Hz, 2H), 6.89–6.87 (m, 2H), 3.83 (s, 3H), 3.09–3.06 (m, 2H), 2.94–2.92 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 159.1, 145.7, 133.6, 132.1, 129.3, 125.4, 118.8, 114.6, 110.2, 55.3, 36.6, 35.7. ESI-MS: m/z calcd for C16H15NOS [M + H]+: 270.0947, found: 270.0961.
4.4.9. (4-Methoxyphenyl)(3-nitrophenethyl)sulfane (3i)
Yellow oil, (83 mg, 86%). 1H NMR (600 MHz, CDCl3): δ 8.09–8.07 (m, 1H), 8.03–8.03 (m, 1H), 7.51 (d, J = 6.0 Hz, 1H), 7.47–7.45 (m, 1H), 7.39–7.37 (m, 2H), 6.89–6.87 (m, 2H), 3.82 (s, 3H), 3.12–3.10 (m, 2H), 2.99–2.96 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 159.1, 148.2, 142.1, 134.8, 133.6, 129.2, 125.3, 123.4, 121.46, 114.6, 55.2, 36.7, 35.2.
4.4.10. 4-(2-((4-Methoxyphenyl)thio)ethyl)benzoic acid (3j)
Mp 157–159 °C, Colorless powder, (79 mg, 78%). 1H NMR (600 MHz, CDCl3): δ 8.06 (d, J = 8.0 Hz, 2H), 7.40 (d, J = 8.5 Hz, 2H), 7.29 (d, J = 8.1 Hz, 2H), 6.89 (d, J = 8.5 Hz, 2H), 3.84 (s, 3H), 3.13–3.08 (m, 2H), 2.96 (t, J = 7.7 Hz, 2H). 13C NMR (151 MHz, CDCl3): δ 171.9, 159.1, 146.7, 133.5, 130.4, 128.7, 127.4, 125.8, 114.7, 55.3, 36.8, 35.8. ESI-MS: m/z calcd for C16H16O3S [M + H]+: 289.0893, found: 289.0899.
4.4.11. 4-(2-((4-Methoxyphenyl)thio)ethyl)phenyl acetate (3k)
Yellow oil, (76 mg, 82%). 1H NMR (600 MHz, CDCl3): δ 7.39 (d, J = 8.7 Hz, 2H), 7.19 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 8.4 Hz, 2H), 6.89 (d, J = 8.7 Hz, 2H), 3.82 (s, 3H), 3.09–3.06 (m, 2H), 2.89–2.86 (m, 2H), 2.31 (s, 3H). 13C NMR (151 MHz, CDCl3): δ 169.5, 158.9, 149.0, 137.8, 133.2, 129.3, 126.0, 121.4, 114.6, 55.2, 37.0, 35.2, 21.0.
4.4.12. (2,3-Dihydro-1H-inden-2-yl)(4-methoxyphenyl)sulfane (3l)
Yellow oil, (84 mg, 76%). 1H NMR (600 MHz, CDCl3): δ 7.45–7.43 (m, 2H), 7.22–7.17 (m, 4H), 6.91–6.88 (m, 2H), 4.00–3.95 (m, 1H), 3.84 (s, 3H), 3.31–3.27 (m, 2H), 3.01–2.98 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 159.3, 141.6, 134.7, 132.6, 126.5, 125.7, 124.4, 114.5, 114.5, 55.3, 47.1, 39.9. ESI-MS: m/z calcd for C16H16OS [M + H]+: 257.0995, found: 257.0988.
4.4.13. (4-Methoxyphenyl)(1,2,3,4-tetrahydronaphthalen-2-yl)sulfane (3m)
Colorless oil, (75 mg, 72%). 1H NMR (600 MHz, CDCl3): δ 7.49 (d, J = 8.5 Hz, 2H), 7.16–7.10 (m, 3H), 7.07–7.06 (m, 1H), 6.91 (d, J = 8.6 Hz, 2H), 3.85 (s, 3H), 3.41–3.38 (m, 1H), 3.12 (dd, J = 16.5, 4.9 Hz, 1H), 2.98 (dt, J = 16.8, 4.9 Hz, 1H), 2.87–2.82 (m, 2H), 2.23–2.20 (m, 1H), 1.84–1.77 (m, 1H). 13C NMR (151 MHz, CDCl3): δ 159.5, 135.6, 135.6, 135.1, 128.9, 128.7, 125.9, 125.7, 124.3, 114.4, 55.2, 44.3, 36.0, 29.4, 28.6. ESI-MS: m/z calcd for C17H18OS [M + H]+: 271.1151, found: 271.1155.
4.4.14. 4-(2-((4-Methoxyphenyl)thio)ethyl)pyridine (3n)
Brown oil, (103 mg, 88%). 1H NMR (600 MHz, CDCl3): δ 8.53 (s, 2H), 7.37 (d, J = 8.7 Hz, 2H), 7.14 (d, J = 4.0 Hz, 2H), 6.87 (d, J = 8.7 Hz, 2H), 3.81 (s, 3H), 3.08–3.05 (m, 2H), 2.88–2.85 (m, 2H).13C NMR (151 MHz, CDCl3): δ 159.1, 149.8, 148.8, 133.7, 125.3, 124.2, 114.6, 55.2, 35.9, 34.9. ESI-MS: m/z calcd for C14H15NOS [M + H]+: 246.0947, found: 246.0938.
4.4.15. 2-(2-((4-Methoxyphenyl)thio)ethyl)pyridine (3o)
Yellow oil, (96 mg, 82%). 1H NMR (600 MHz, CDCl3): δ 8.52–8.52 (m, 1H), 7.58–7.56 (m, 1H), 7.37 (d, J = 8.7 Hz, 2H), 7.12–7.11 (m, 2H), 6.84 (d, J = 8.7 Hz, 2H), 3.78–3.77 (m, 3H), 3.24–3.22 (m, 2H), 3.05–3.03 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 159.7, 158.8, 149.2, 136.2, 133.2, 126.0, 123.1, 121.3, 114.4, 55.1, 37.9, 35.1. ESI-MS: m/z calcd for C14H15NOS [M + H]+: 246.0947, found: 246.0937.
4.4.16. (4-Methoxyphenyl)(2-(naphthalen-2-yl)ethyl)sulfane (3p)
Mp 58–61 °C, Colorless powder, (87 mg, 93%). 1H NMR (600 MHz, CDCl3): δ 7.85 (d, J = 7.8 Hz, 1H), 7.82 (d, J = 8.2 Hz, 2H), 7.65 (s, 1H), 7.52–7.44 (m, 4H), 7.34 (d, J = 8.4 Hz, 1H), 6.92 (d, J = 8.6 Hz, 2H), 3.84 (s, 3H), 3.22–3.19 (m, 2H), 3.10–3.07 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 158.9, 137.7, 133.4, 133.3, 132.1, 128.0, 127.5, 127.4, 127.0, 126.7, 126.2, 125.9, 125.3, 114.5, 55.2, 37.1, 36.0. ESI-MS: m/z calcd for C19H18OS [M + H]+: 295.1151, found: 295.1144.
4.4.17. (2-([1,1′-Biphenyl]-4-yl)ethyl)(4-methoxyphenyl)sulfane (3q)
Colorless powder, (82 mg, 92%). 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 8.1 Hz, 2H), 7.55 (d, J = 8.0 Hz, 2H), 7.46 (t, J = 7.6 Hz, 2H), 7.42 (d, J = 8.6 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H), 7.27 (d, J = 8.0 Hz, 2H), 6.90 (d, J = 8.6 Hz, 2H), 3.84 (s, 3H), 3.15–3.13 (m, 2H), 2.96–2.93 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 158.9, 140.9, 139.4, 139.2, 133.3, 128.9, 128.7, 127.1, 127.1, 126.9, 126.2, 114.6, 55.3, 37.1, 35.5.
4.4.18. (2-(Anthracen-9-yl)ethyl)(4-methoxyphenyl)sulfane (3r)
Mp 113–115 °C, Yellow solid, (69 mg, 82%). 1H NMR (600 MHz, CDCl3): δ 8.36 (s, 1H), 8.04–7.99 (m, 4H), 7.57–7.54 (m, 2H), 7.48–7.45 (m, 4H), 6.98–6.96 (m, 2H), 3.88 (s, 3H), 3.87–3.85 (m, 2H), 3.21–3.18 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 159.3, 134.1, 132.2, 131.5, 129.5, 129.2, 126.3, 126.0, 125.8, 124.8, 123.8, 114.6, 55.4, 36.4, 28.5. ESI-MS: m/z calcd for C23H20NOS [M + H]+: 345.1308, found: 345.1300.
4.4.19. Phenethyl(phenyl)sulfane (6a)
Colorless oil, (94 mg, 91%). 1H NMR (600 MHz, CDCl3): δ 7.44–7.43 (m, 2H), 7.37–7.35 (m, 4H), 7.31–7.25 (m, 4H), 3.25–3.23 (m, 2H), 3.01–2.99 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 140.1, 136.3, 129.1, 128.8, 128.4, 126.4, 125.9, 35.5, 35.0.
4.4.20. (3,4-Dimethoxyphenyl)(phenethyl)sulfane (6c)
Yellow oil, (113 mg, 86%). 1H NMR (600 MHz, CDCl3): δ 7.33–7.30 (m, 2H), 7.25–7.22 (m, 1H), 7.20–7.19 (m, 2H), 7.03 (dd, J = 8.3, 2.1 Hz, 1H), 6.97 (d, J = 2.1 Hz, 1H), 6.84 (d, J = 8.3 Hz, 1H), 3.90–3.90 (m, 6H), 3.13–3.11 (m, 2H), 2.92–2.90 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 149.0, 148.3, 140.2, 128.4, 128.4, 126.7, 126.3, 124.1, 114.8, 111.6, 55.9, 37.0, 35.8. ESI-MS: m/z calcd for C16H18OS [M + H]+: 275.1100, found: 275.1111.
4.4.21. Phenethyl(p-tolyl)sulfane (6d)
Colorless oil, (99 mg, 90%). 1H NMR (600 MHz, CDCl3): δ 7.39–7.36 (m, 4H), 7.31–7.26 (m, 3H), 7.20 (d, J = 8.0 Hz, 2H), 3.22–3.19 (m, 2H), 3.00–2.97 (m, 2H), 2.41 (s, 3H). 13C NMR (151 MHz, CDCl3): δ 140.2, 136.1, 133.3, 132.4, 130.0, 129.6, 128.4, 128.4, 126.3, 35.7, 35.6, 20.9.
4.4.22. (2-Chlorophenyl)(phenethyl)sulfane (6e)
Colorless oil, (98 mg, 82%). 1H NMR (600 MHz, CDCl3): δ 7.42–7.41 (m, 1H), 7.37–7.33 (m, 3H), 7.29–7.24 (m, 4H), 7.17–7.14 (m, 1H), 3.24–3.21 (m, 2H), 3.03–3.00 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 139.9, 135.8, 133.5, 129.7, 128.5, 128.4, 128.3, 127.0, 126.5, 126.4, 35.1, 33.8.
4.4.23. (4-Chlorophenyl)(phenethyl)sulfane (6f)
Colorless oil, (103 mg, 86%). 1H NMR (600 MHz, CDCl3): δ 7.36–7.27 (m, 7H), 7.24–7.23 (m, 2H), 3.20–3.18 (m, 2H), 2.97–2.95 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 139.8, 134.8, 131.9, 130.5, 129.0, 128.5, 128.4, 126.5, 35.4, 35.3.
4.4.24. (4-Fluorophenyl)(phenethyl)sulfane (6g)
Colorless oil, (94 mg, 84%). 1H NMR (600 MHz, CDCl3): δ 7.43–7.41 (m, 2H), 7.37–7.35 (m, 2H), 7.30–7.27 (m, 1H), 7.24 (d, J = 7.5 Hz, 2H), 7.07 (t, J = 8.6 Hz, 2H), 3.19–3.16 (m, 2H), 2.97–2.94 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 162.5, 160.9, 139.9, 135.8, 135.8, 133.1, 133.0, 132.3, 132.2, 131.0, 128.4, 128.4, 127.9, 127.7, 126.4, 116.0, 115.9, 115.8, 36.3, 35.6.
4.4.25. (4-Methoxyphenyl)(styryl)sulfane E/Z Isomer 80:20 (9)
Colorless powder, (108 mg, 91%). Z isomer 1H NMR (600 MHz, CDCl3): δ 7.60 (d, J = 7.8 Hz, 2H), 7.50–7.44 (m, 3H), 7.35 (d, J = 4.0 Hz, 1H), 7.33–7.31 (m, 1H), 6.98–6.94 (m, 2H), 6.55 (d, J = 10.8 Hz, 1H), 6.47 (d, J = 10.8 Hz, 1H), 3.85 (s, 3H). 13C NMR (151 MHz, CDCl3): δ 159.4, 136.5, 132.8, 128.8, 128.6, 128.2, 127.1, 126.8, 125.6, 114.7, 55.2. E isomer 1H NMR (600 MHz, CDCl3): δ 7.50–7.44 (m, 6H), 7.27–7.25 (m, 1H), 6.98–6.94 (m, 2H), 6.90 (d, J = 15.4 Hz, 1H), 6.58 (d, J = 15.4 Hz, 1H), 3.86 (s, 3H). 13C NMR (151 MHz, CDCl3): δ 159.4, 136.6, 133.3, 128.8, 128.5, 128.2, 126.7, 125.7, 124.3, 114.8, 55.2. ESI-MS: m/z calcd for C15H14OS [M + H]+: 243.0838, found: 243.0830.
4.4.26. 2-((2-(Pyridin-2-yl)ethyl)thio)pyridine (11)
Reddish brown oil, (1.55 g, 76%). 1H NMR (600 MHz, CDCl3): δ 8.53 (d, J = 4.6 Hz, 1H), 8.42–8.41 (m, 1H), 7.58 (td, J = 7.6, 1.7 Hz, 1H), 7.43 (td, J = 7.8, 1.8 Hz, 1H), 7.19 (d, J = 7.8 Hz, 1H), 7.15 (d, J = 8.1 Hz, 1H), 7.11 (dd, J = 7.1, 5.2 Hz, 1H), 6.95–6.93 (m, 1H), 3.56–3.54 (m, 2H), 3.20–3.18 (m, 2H). 13C NMR (151 MHz, CDCl3): δ 159.8, 158.7, 149.3, 149.1, 136.3, 135.7, 123.2, 122.1, 121.4, 119.2, 37.8, 29.4. ESI-MS: m/z calcd for C12H12N2S [M + H]+: 217.0794, found: 217.0786.
4.4.27. 1-Methoxy-4-(phenethylsulfonyl)benzene (12)
Colorless oil, (96 mg, 72%). 1H NMR (300 MHz, CDCl3): δ 7.89–7.86 (m, 2H), 7.31–7.19 (m, 3H), 7.13 (d, J = 8.0 Hz, 2H), 7.06–7.03 (m, 2H), 3.91 (s, 3H), 3.39–3.33 (m, 2H), 3.07–3.02 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 163.7, 137.5, 130.1, 128.7, 128.2, 126.8, 114.4, 57.7, 55.6, 28.8. ESI-MS: m/z calcd for C15H16O3S [M + H]+: 277.0893, found: 277.0909.
Acknowledgments
We are thankful to the Director, CSIR-IHBT, Palampur (H.P.), for the necessary infrastructure. S.K.M. is thankful to the SERB, Government of India, for an early career research award (ECR/2016/000134) and R.R. thanks CSIR, New Delhi, for SRF. CSIR-IHBT communication no. is 4399.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01968.
Mechanistic study including the IR studies of formic acid, 4-methoxy thiophenol, and mixture of formic acid and thiol and IR spectrum of formic acid + thiol; copies of 1H and 13C NMR (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kazemi M.; Kohzadi H.; Abdi O. Alkylation of Thiols in Green Mediums. J. Mater. Environ. Sci. 2015, 6, 1451–1456. [Google Scholar]
- Devendar P.; Yang G. F. Sulfur-Containing Agrochemicals. Top. Curr. Chem. 2017, 375, 82. 10.1007/s41061-017-0169-9. [DOI] [PubMed] [Google Scholar]
- Omar S. H.; Al-Wabel N. A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58. 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S.; Chung J.; Park J. E.; Chung Y. K. Hydrothiolation of Alkenes and Alkynes Catalyzed by 3,4- Dimethyl-5-vinylthiazolium iodide and Poly(3,4-dimethyl-5-vinylthiazolium) iodide. ChemCatChem 2016, 8, 2476–2481. 10.1002/cctc.201600363. [DOI] [Google Scholar]
- Limnios D.; Kokotos C. G. Photoinitiated Thiol-ene “Click” Reaction: An Organocatalytic Alternative. Adv. Synth. Catal. 2017, 359, 323–328. 10.1002/adsc.201600977. [DOI] [Google Scholar]
- Subramanian H.; Moorthy R.; Sibi M. P. Thiyl Radicals: From Simple Radical Additions to Asymmetric Catalysis. Angew. Chem., Int. Ed. 2014, 53, 13660–13662. 10.1002/anie.201408781. [DOI] [PubMed] [Google Scholar]
- Chan J. W.; Hoyle C. E.; Lowe A. B. Sequential Phosphine-Catalyzed, Nucleophilic Thiol-Ene/Radical-Mediated Thiol-Yne Reactions and the Facile Orthogonal Synthesis of Polyfunctional Materials. J. Am. Chem. Soc. 2009, 131, 5751–5753. 10.1021/ja8099135. [DOI] [PubMed] [Google Scholar]
- Cabrero-Antonino J. R.; Perez A. L.; Cormaa A. Iron-Catalysed Markovnikov Hydrothiolation of Styrenes. Adv. Synth. Catal. 2012, 354, 678–687. 10.1002/adsc.201100731. [DOI] [Google Scholar]
- Tyson E. L.; Ament M. S.; Yoon T. P. Transition Metal Photoredox Catalysis of Radical Thiol-Ene Reactions. J. Org. Chem. 2013, 78, 2046–2050. 10.1021/jo3020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Boyer C. Visible Light Photocatalytic Thiol–Ene Reaction: An Elegant Approach for Fast Polymer Postfunctionalization and Step-Growth Polymerization. Macromolecules 2015, 48, 520–529. 10.1021/ma502460t. [DOI] [Google Scholar]
- Munro-Leighton C.; Delp S. A.; Alsop N. M.; Blue E. D.; Gunnoe T. B. Anti-Markovnikov hydroamination and hydrothiolation of electron deficient vinylarenes catalyzed by well-defined monomeric copper(I) amido and thiolate complexes. Chem. Commun. 2008, 111–113. 10.1039/B715507G. [DOI] [PubMed] [Google Scholar]
- Ranu B. C.; Mandal T. Indium(I) iodide promoted cleavage of dialkyl/diaryl disulfides and subsequent anti-Markovnikov addition to styrenes: a new route to linear thioethers. Tetrahedron Lett. 2006, 47, 6911–6914. 10.1016/j.tetlet.2006.07.017. [DOI] [Google Scholar]
- Kumar R.; Saima; Shard A.; Andhare N. H.; Richa; Sinha A. K. Thiol–Ene “Click” Reaction Triggered by Neutral Ionic Liquid: The “Ambiphilic” Character of [hmim]Br in the Regioselective Nucleophilic Hydrothiolation. Angew. Chem., Int. Ed. 2015, 54, 828–832. 10.1002/anie.201408721. [DOI] [PubMed] [Google Scholar]
- Qureshi Z. S.; Deshmukh K. M.; Dhake K. P.; Bhanage B. M. Brønsted acidic ionic liquid: a simple, efficient and recyclable catalyst for regioselective alkylation of phenols and anti-Markovnikov addition of thiols to alkenes. RSC Adv. 2011, 1, 1106–1112. 10.1039/c1ra00401h. [DOI] [Google Scholar]
- Banerjee S.; Das J.; Santra S. Native silica nanoparticle catalyzed anti-Markovnikov addition of thiols to inactivated alkenes and alkynes: a new route to linear and vinyl thioethers. Tetrahedron Lett. 2009, 50, 124–127. 10.1016/j.tetlet.2008.10.110. [DOI] [Google Scholar]
- Lanke S. R.; Bhanage B. M. Amberlyst-15©: An efficient heterogeneous reusable catalyst for selective anti-Markovnikov addition of thiols to alkenes/alkynes and for thiolysis of epoxides. Catal. Commun. 2013, 41, 29–33. 10.1016/j.catcom.2013.06.032. [DOI] [Google Scholar]
- Lowe A. B. Thiol-yne ‘click’/coupling chemistry and recent applications in polymer and materials synthesis and modification. Polymer 2014, 55, 5517–5549. 10.1016/j.polymer.2014.08.015. [DOI] [Google Scholar]
- Kade M. J.; Burke D. J.; Hawker C. J. The Power of Thiol-ene Chemistry. J. Polym. Sci., Part A: Polym. Chem. 2010, 48, 743–750. 10.1002/pola.23824. [DOI] [Google Scholar]
- Nguyen V. H.; Nishino H.; Kajikawa S.; Kurosawa K. Mn(lll)-Based Reactions of Alkenes and Alkynes with Thiols. An Approach toward Substituted 2,3-Dihydro-l,4-oxathiins and Simple Route to (E)-Vinyl Sulfides. Tetrahedron 1998, 54, 11445–11460. 10.1016/S0040-4020(98)00707-8. [DOI] [Google Scholar]
- Thakur M. S.; Nayal O. S.; Upadhyay R.; Kumar N.; Maurya S. K. 2-Aminoquinazolin-4(3H)-one as an Organocatalyst for the Synthesis of Tertiary Amines. Org. Lett. 2018, 20, 1359–1362. 10.1021/acs.orglett.8b00127. [DOI] [PubMed] [Google Scholar]
- Gantenberg M.; Halpuka M.; Sander W. Dimerization of formic acid- An example of a “noncovalent” reaction mechanism. Chem. - Eur. J. 2000, 6, 1865–1869. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.