Key Points
Question
Does the use of 3-dimensional, virtual reality models for planning robotic-assisted partial nephrectomy improve surgical outcomes?
Findings
In this single-blind randomized clinical trial involving 92 patients, the use of 3-dimensional virtual reality models reduced the operative time, estimated blood loss, clamp time, and length of hospital stay.
Meaning
This randomized clinical trial demonstrates key outcomes improvements when using 3-dimensional, virtual reality models to plan robotic-assisted partial nephrectomy.
This single-blind randomized clinical trial examines whether 3-dimensional (3-D) virtual reality models used to plan robotic-assisted partial nephrectomy can reduce the operative time, estimated blood loss, clamp time, and length of hospital stay.
Abstract
Importance
Planning complex operations such as robotic-assisted partial nephrectomy requires surgeons to review 2-dimensional computed tomography or magnetic resonance images to understand 3-dimensional (3-D), patient-specific anatomy.
Objective
To determine surgical outcomes for robotic-assisted partial nephrectomy when surgeons reviewed 3-D virtual reality (VR) models during operative planning.
Design, Setting, and Participants
A single-blind randomized clinical trial was performed. Ninety-two patients undergoing robotic-assisted partial nephrectomy performed by 1 of 11 surgeons at 6 large teaching hospitals were prospectively enrolled and randomized. Enrollment and data collection occurred from October 2017 through December 2018, and data analysis was performed from December 2018 through March 2019.
Interventions
Patients were assigned to either a control group undergoing usual preoperative planning with computed tomography and/or magnetic resonance imaging only or an intervention group where imaging was supplemented with a 3-D VR model. This model was viewed on the surgeon’s smartphone in regular 3-D format and in VR using a VR headset.
Main Outcomes and Measures
The primary outcome measure was operative time. It was hypothesized that the operations performed using the 3-D VR models would have shorter operative time than those performed without the models. Secondary outcomes included clamp time, estimated blood loss, and length of hospital stay.
Results
Ninety-two patients (58 men [63%]) with a mean (SD) age of 60.9 (11.6) years were analyzed. The analysis included 48 patients randomized to the control group and 44 randomized to the intervention group. When controlling for case complexity and other covariates, patients whose surgical planning involved 3-D VR models showed differences in operative time (odds ratio [OR], 1.00; 95% CI, 0.37-2.70; estimated OR, 2.47), estimated blood loss (OR, 1.98; 95% CI, 1.04-3.78; estimated OR, 4.56), clamp time (OR, 1.60; 95% CI, 0.79-3.23; estimated OR, 11.22), and length of hospital stay (OR, 2.86; 95% CI, 1.59-5.14; estimated OR, 5.43). Estimated ORs were calculated using the parameter estimates from the generalized estimating equation model. Referent group values for each covariate and the corresponding nephrometry score were summed across the covariates and nephrometry score, and the sum was exponentiated to obtain the OR. A mean of the estimated OR weighted by sample size for each nephrometry score strata was then calculated.
Conclusions and Relevance
This large, randomized clinical trial demonstrated that patients whose surgical planning involved 3-D VR models had reduced operative time, estimated blood loss, clamp time, and length of hospital stay.
Trial Registration
ClinicalTrials.gov identifiers (1 registration per site): NCT03334344, NCT03421418, NCT03534206, NCT03542565, NCT03556943, and NCT03666104
Introduction
Kidney surgery, specifically robotic-assisted partial nephrectomy (RAPN), is an ideal case to test methods for surgical planning other than the current standard of care, which typically involves review of computed tomography (CT) and magnetic resonance imaging (MRI) scans. Localized renal masses are a rapidly growing subset of cancer, with the incidence of stage I disease increasing from 3.7 to 7.0 cases per 100 000 US adults in the past decade.1 Although treatment strategies such as surveillance and ablation have gained popularity of late, surgical intervention, specifically RAPN, is the established standard for kidney cancer that is amenable to nephron-sparing approaches.2,3,4,5,6,7 Successfully performing this surgery depends largely on the surgeon’s understanding of the patient’s anatomy, including the configuration of the kidney, mass, vessels, and collecting system.
Recent work8,9 has studied the impact of 3-dimensional (3-D) digital imaging on surgical planning for RAPN. Three-dimensional imaging has been shown to improve understanding of patient anatomy, influence RAPN surgical plans, and increase the use of selective clamping of renal arterial branches during RAPN.8,9 Although the previous work validated that 3-D imaging provides meaningful additional information that affects understanding and surgical planning, it remains unclear whether the use of patient-specific 3-D virtual reality (VR) models for operative planning would also affect key surgical outcomes.
In this context, we identified patients undergoing RAPN and performed a multi-institutional, single-blind, randomized clinical trial using 3-D VR models generated from CT or MRI scans. We sought to determine whether the use of these patient-specific 3-D VR models for operative planning would affect key surgical outcomes.
Methods
Participant Eligibility
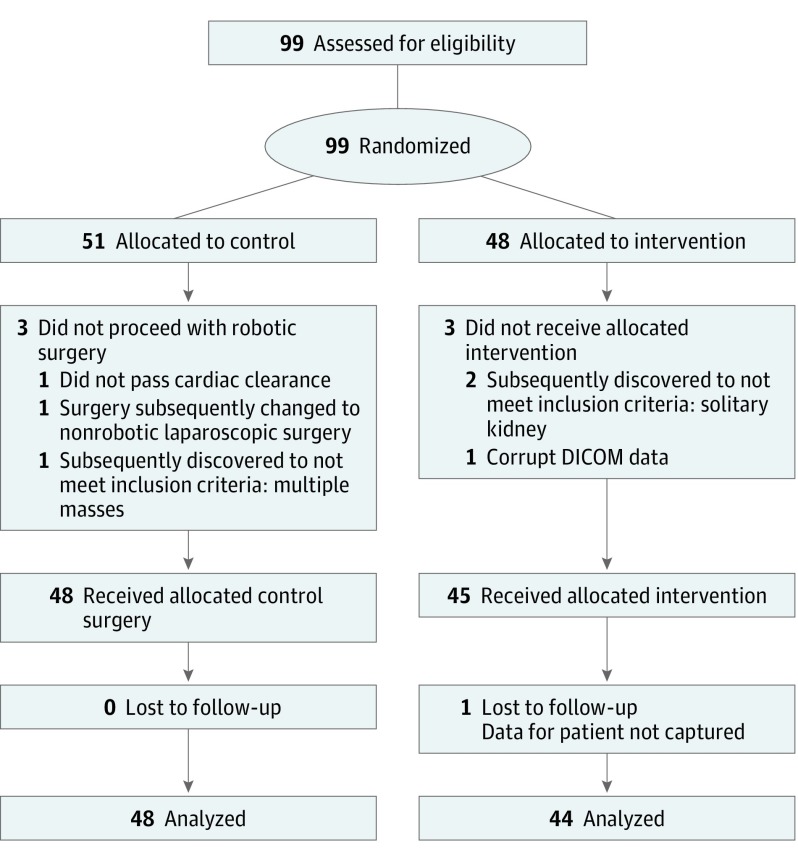
Participants were recruited from 6 large teaching hospitals. Patients with a solitary kidney, more than 1 ipsilateral tumor, a planned bilateral operation, or who lacked preoperative imaging or were unable to give informed consent were excluded from the study. There were no exclusions based on CT or MRI slice thickness. Participants signed a written informed consent document that outlined the specific risks and benefits of enrollment. The study was approved by the Western Institutional Review Board, which served as the institutional review board of record for 4 study sites. At 2 sites, the study was approved by the site’s own institutional review boards. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline (Figure 1). The trial protocol is shown in Supplement 1.
Figure 1. Flow of Participants in the 3-Dimensional Virtual Reality Models for Surgical Planning of Robotic-Assisted Partial Nephrectomy Trial.
DICOM indicates Digital Imaging and Communications in Medicine.
Randomization, Blinding, and Sample Size
Patients consented to be randomized to either a control group, in which the operation was planned with surgeon review of the CT and/or MRI scans only, or the intervention group, in which the surgeon reviewed both the CT and/or MRI scans along with a 3-D VR model of the patient’s anatomy that was created by the sponsor from the source CT and/or MRI scan. Randomization was stratified by surgeon experience, and each surgeon-specific randomization schedule yielded a randomization in a 1:1 ratio per surgeon. Eleven surgeons participated in the study. Sequentially numbered, opaque, sealed envelopes containing the treatment assignment for a single case were prepared by the sponsor and provided to each site. Patients were assigned to a study group by opening an envelope at the time of enrollment and were blinded to group assignment.
We calculated the sample size according to our pilot data,10 which showed a significant improvement in operative time when 3-D VR models were using in surgical planning. In the pilot study,10 we noted an effect size of 0.44 regarding the difference in operative time between 3-D VR model–aided and control groups. We used data from the variable of most interest, total operative time. We accounted for within-sample clustering and took 3 values of within-cluster correction (ρ = 0.3, 0.5, and 0.7) to consider low, moderate, and high levels, respectively, of within-cluster correlation. To account for multiple end points and some other unpredictable factors, we increased the sample size by 15%. We selected ρ = 0.3 given the wide variation in case complexity seen in our pilot study,10 which drastically limited within-cluster clustering.
Model Preparation, Delivery, and Use
The CT or MRI scans from patients randomized to the intervention group were deidentified, provided to the sponsor in Digital Imaging and Communications in Medicine format, and used to create a patient-specific 3-D VR model for each intervention patient (Reveal software versions 2.1-2.3; Ceevra). This model included, at a minimum, the kidney, mass, renal artery, and renal vein. Most models also included additional anatomic structures, such as the spleen, splenic artery, splenic vein, ureter and collecting system, renal cysts (if present), ribs, and spine. In rare cases, structures such as the psoas muscle or liver were included if there was any suspicion of tumor involvement of these structures (eFigure in Supplement 2).
Surgeons reviewed the 3-D VR models via a mobile application developed by the sponsor and installed on their smartphones. Parameters such as number of arteries and veins and nephrometry score with individual components were also viewable from the surgeon’s smartphone. The model could be rotated and zoomed using standard smartphone gestures, and the surgeon could show or hide each anatomic structure during the viewing session. In addition, certain structures were rendered translucent to allow viewing of embedded structures. This was an important feature to allow visualization of the mass within the kidney and, in some cases, cystic components of the mass.
For each intervention case, the surgeon viewed the 3-D VR model before the operation in both regular 3-D format from the smartphone and in VR with an off-the-shelf Google Cardboard–compatible VR headset. Intraoperative viewing of the 3-D VR models was performed at the surgeon’s discretion.
End-Point Selection and Data Collection
Enrollment and data collection occurred from October 2017 through December 2018. Demographic data, including age, sex, and race, were collected for both groups from the medical record. Clinical data collected included site, surgeon, surgeon experience level, resident or fellow involvement in the surgery, mass size, nephrometry score with individual components, and laterality of operation. Operative data collected included transperitoneal or retroperitoneal approach, use of intraoperative ultrasonography, and use of the fourth robotic arm. The nephrometry score uses the tumor’s radius, endophyticity, nearness to the collecting system, and location to describe the tumor and was used as a measure of case complexity.11,12 We identified outcome measures of interest from recent literature13,14 comparing robotic and laparoscopic partial nephrectomy. These outcomes had previously been investigated in the pilot study.10 Our primary outcome measure (operative time) and secondary outcome measures (clamp time [ie, warm ischemia time], estimated blood loss [EBL], and length of hospital stay) were collected from the medical record. Operative time was defined as incision to closure, clamp time was the total time the renal vessel(s) were clamped, EBL was estimated collaboratively by the surgeon and anesthesiologist, and discharge on postoperative day 1 was recorded as a hospital stay of 1 day. We also recorded conversion to radical nephrectomy, conversion to open surgery, margin status, intraoperative complications, postoperative complications, readmissions, and mortality.
Statistical Analysis
Data analysis was performed from December 2018 through March 2019. As an initial analytic step, we compared baseline characteristics between cases performed with (intervention) or without (control) 3-D VR models involved in preoperative planning. We used a nonparametric test (Wilcoxon rank sum test and median test) to compare nonnormally distributed operative time, clamp time, and EBL means and medians. We also dichotomized the outcomes as follows: less than or equal to the 75th percentile (low) vs greater than the 75th percentile (high) for operative time and EBL, less than or equal to 20 minutes vs longer than 20 minutes for clamp time, and less than or equal to 2 days vs longer than 2 days for hospital stay. In addition, we dichotomized length of hospital stay as less than or equal to 1 day vs greater than 1 day to assess the earliest possible discharge. These cutoffs were chosen as either having data-demonstrated adverse outcomes above the cutoff (clamp time), empirically near the standard for our surgeon sample (hospital stay), or as a measure of surgical quality (operative time and EBL).15,16 We compared the high vs low dichotomized outcome variables by group using a χ2 test.
For the multivariate analyses, we conducted a logistic regression for the dichotomous outcome measures, with group as the main independent variable and intervention as the reference group. We included nephrometry score and conducted a forward selection process, resulting in a model controlling for significant demographic and clinical variables. We then conducted a multilevel model with the addition of the surgeon to control for findings clustering around individual surgeons. From the resulting model, we calculated estimated odds ratios (ORs) for each outcome at any given nephrometry score, as well as a weighted mean OR across all nephrometry scores. We calculated the estimated ORs using the parameter estimates from the resulting generalized estimating equation model (PROC GENMOD with REPEATED statement in SAS statistical software version 9.4 [SAS Institute]). We entered the referent group value for each covariate (ie, “intervention” for group, “female” for sex, “none” for experience, “yes” for fellow involvement, “left” for side, and mean of 61.5 years for age) and the corresponding nephrometry score into the linear equation, summed across the covariates and nephrometry score, and exponentiated the sum to obtain the OR. This was all calculated in SAS using the ESTIMATE statement. For each outcome, we also calculated a weighted mean of the estimated OR weighted by sample size for each nephrometry score strata. All statistical tests were 2-sided and were performed at the 5% significance level, using SAS.
Results
Ninety-two patients (58 men [63%]; mean [SD] age, 60.9 [11.6] years) were included in the analysis, with 48 randomized to the control group and 44 randomized to the intervention group. Baseline characteristics were well matched between groups with the exception of higher mean (SD) age for the control group (64.6 [9.7] years vs 57.6 [12.3] years) (Table 1). Unadjusted mean (SD) values for operative time between control and intervention groups (172.6 [48.5] minutes vs 173.3 [49.6] minutes; P = .70), clamp time (18.0 [7.9] minutes vs 17.6 [7.8] minutes; P = .76), EBL (124.5 [90.5] mL vs 145.7 [140.4] mL; P = .71), and length of stay longer than 2 days (4 of 44 participants [9%] vs 7 of 48 participants [15%]; P = .41) showed no significant differences (Table 2).
Table 1. Baseline Characteristics Between Groups Who Underwent Robotic-Assisted Partial Nephrectomy With and Without 3-Dimensional Virtual Reality Models.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Virtual Reality Models (n = 44) | No Virtual Reality Models (n = 48) | |
| Age, mean (SD), y | 64.6 (9.7) | 57.6 (12.3) |
| Sex | ||
| Male | 29 (66) | 29 (60) |
| Female | 15 (34) | 19 (40) |
| Race | ||
| White | 36 (82) | 38 (79) |
| Nonwhite | 8 (18) | 10 (21) |
| Nephrometry score | ||
| 4 | 5 (11) | 11 (23) |
| 5 | 3 (7) | 6 (12) |
| 6 | 3 (7) | 8 (17) |
| 7 | 5 (11) | 8 (17) |
| 8 | 8 (18) | 8 (17) |
| 9 | 13 (30) | 4 (8) |
| 10 | 5 (11) | 2 (4) |
| 11 | 2 (5) | 1 (2) |
| Surgeon experience with robotic-assisted partial nephrectomy, No. of cases/y | ||
| 1-10 | 4 (9) | 8 (17) |
| 11-30 | 7 (16) | 7 (15) |
| >30 | 33 (75) | 33 (69) |
| Fellow involved in operation | 25 (57) | 25 (52) |
| Resident involved in operation | 22 (40) | 26 (54) |
| Laterality | ||
| Right | 26 (59) | 20 (42) |
| Left | 18 (41) | 28 (58) |
| Approach | ||
| Retroperitoneal | 8 (18) | 14 (29) |
| Transperitoneal | 36 (82) | 34 (71) |
| Fourth robotic arm used | 28 (64) | 25 (52) |
Table 2. Comparative Outcomes Between Groups Who Underwent Robotic-Assisted Partial Nephrectomy With and Without 3-Dimensional Virtual Reality Models.
| Outcome | Virtual Reality Models (n = 44) | No Virtual Reality Models (n = 48) | P Value |
|---|---|---|---|
| Operative time, min | |||
| Mean (SD) | 172.6 (48.5) | 173.3 (49.6) | .70a |
| Median (IQR) | 163 (146.5-200.5) | 173.5 (148.0-199.0) | .10b |
| >75th Percentile, participants, No. (%)c | 11 (25) | 11 (23) | .82 |
| Clamp time, min | |||
| Mean (SD) | 18.0 (7.9) | 17.6 (7.8) | .76a |
| Median (IQR) | 18 (15-23) | 18 (12-22) | .90b |
| >75th Percentile, participants, No. (%)c | 11 (25) | 11 (24) | .90 |
| >20 min, participants, No. (%) | 14 (32) | 16 (35) | .77 |
| Clamp time >0 min | |||
| Participants, No. | 41 | 43 | |
| Mean (SD) | 19.3 (6.3) | 18.8 (6.4) | .71a |
| Median (IQR) | 18 (16-24) | 18 (13-23) | .97b |
| >75th Percentile, participants, No. (%)c | 11 (27) | 11 (26) | .90 |
| Estimated blood loss, mL | |||
| Mean (SD) | 124.5 (90.5) | 145.7 (140.4) | .71a |
| Median (IQR) | 100 (50-200) | 100 (50-200) | .55b |
| >75th Percentile, participants, No. (%)c | 4 (9) | 6 (12) | .74d |
| Length of stay >2 d, participants, No. (%) | 4 (9) | 7 (15) | .42 |
Abbreviation: IQR, interquartile range.
Wilcoxon rank sum test of means.
Brown-mood test of medians.
The 75th percentiles are defined as follows: operative time, 199 minutes; clamp time, 22 minutes; and estimated blood loss, 200 mL.
Fisher exact test.
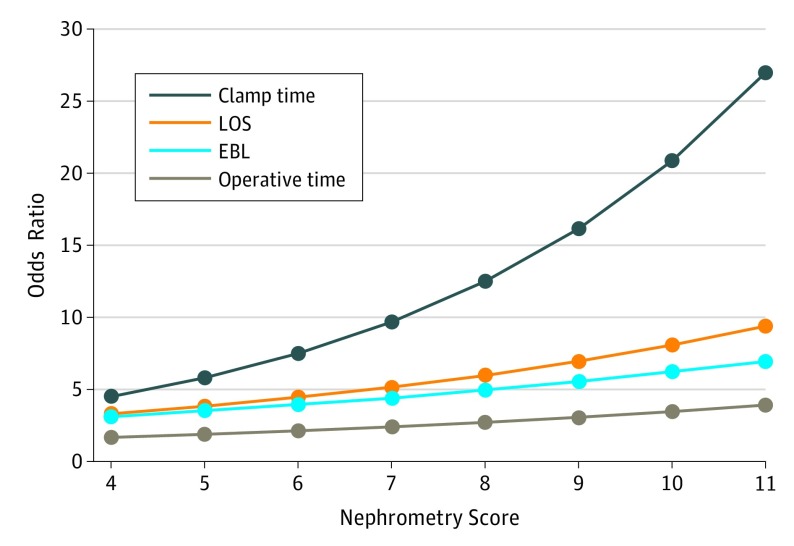
In the subsequent multilevel model, patients in the control group were significantly more likely than patients with 3-D VR–assisted surgical planning to have a length of stay longer than 2 days (OR, 2.86; 95% CI, 1.59-5.14) and EBL greater than 200 mL (OR, 1.98; 95% CI, 1.04-3.78). Operative time (OR, 1.00; 95% CI, 0.37-2.70) and clamp time (OR, 1.60; 95% CI, 0.79-3.23) were also examined but were not statistically different between groups in this model. Some secondary covariates also were significant for the outcomes, including surgeon experience (OR for clamp time, 5.22; 95% CI, 1.86-14.6) and side of operation (OR for EBL, 3.53; 95% CI, 2.05-6.10) (Table 3). The estimated ORs showed improvements in operative time (estimated OR, 2.47), EBL (estimated OR, 4.56), clamp time (estimated OR, 11.22), and hospital stay (estimated OR, 5.43) in the 3-D VR–assisted cases, as well as the association between case complexity and the odds of more adverse outcomes if standard CT or MRI surgical planning was used (Figure 2).
Table 3. Comparative Outcomes Between Groups Who Underwent Robotic-Assisted Partial Nephrectomy With and Without 3-Dimensional Virtual Reality Models, Mixed Model.
| Characteristic | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|
| Operative Time >199 min vs ≤199 min | Clamp Time >22 min vs ≤22 min | Estimated Blood Loss >200 mL vs ≤200 mL | Length of Stay | ||
| 2-5 d vs 1 d | 3-5 d vs 1-2 d | ||||
| Group (control vs intervention) | 1.00 (0.37-2.70) | 1.60 (0.79-3.23) | 1.98 (1.04-3.78)a | 1.79 (0.84-3.86) | 2.86 (1.59-5.14)b |
| Nephrometry score | 1.13 (0.77-1.65) | 1.29 (1.03-1.63)a | 1.12 (0.82-1.53) | 1.16 (1.06-1.27)a | 1.04 (0.85-1.28) |
| Age | NS | NS | NS | NS | 1.07 (1.01-1.14)a |
| Sex (male vs female) | 2.20 (1.07-4.55)a | NS | NS | NS | NS |
| Side (right vs left) | NS | NS | 3.53 (2.05-6.10)b | NS | NS |
| Fellow (no vs yes) | NS | NS | 3.70 (1.01-13.6)a | NS | NS |
| Surgeon experience | NS | 5.22 (1.86-14.6)b | NS | NS | NS |
Abbreviation: NS, not sufficient (ie, the model did not yield sufficient data to report here).
P < .05.
P < .01.
Figure 2. Estimated Odds Ratios for Outcomes With 3-Dimensional Virtual Reality Models, by Nephrometry Score.
Estimated odds ratios were calculated using the parameter estimates from the generalized estimating equation model. Referent group values for each covariate and the corresponding nephrometry score were summed across the covariates and nephrometry score, and the sum exponentiated to obtain the odds ratio. A mean of the estimated odds ratio weighted by sample size for each nephrometry score strata was then calculated. EBL indicates estimated blood loss; and LOS, length of stay.
Discussion
Cancer care over the last decade has evolved in both the surgical and medical arenas. From the surgical perspective, minimally invasive approaches have gained popularity. Although laparoscopy set the stage, a major acceleration has occurred with the advent of robotic-assisted systems, which enable many procedures to be performed without an open incision and provide the surgeon with better visibility of the operating field and finer dexterity.17
Robotic-assisted partial nephrectomy is an ideal case study to assess the advantages and disadvantages of novel forms of surgical planning because it is a complex operation, with many decision points being made according to the patient’s anatomy, as understood by the surgeon from review of the patient’s CT or MRI scan. The decisions could include, for example, whether to use a transperitoneal or retroperitoneal approach, selection of blood vessels for clamping, identification of appropriate vessels to ligate (if any), and tumor resection margin and depth. In this context, novel forms of surgical planning, such as those involving 3-D VR models, could help improve the surgeon’s understanding of the patient’s anatomy and optimal surgical approach.
For patients undergoing RAPN, we identified the metrics that define a successful surgery, including operative time, EBL, clamp time, and length of hospital stay. Operations that fare better according to these metrics are positively correlated with better patient outcomes. Higher rates of 30-day perioperative complications have been seen in cases with longer operative times and higher levels of EBL, both for RAPN and other types of operations.18,19,20 Shorter clamp times and selective clamping can reduce the level of nephron loss due to hypoxia.21 Finally, shorter hospital stays limit the potential for various in-hospital complications sometimes seen among patients undergoing RAPN.22,23 In this setting, our study has several important findings.
After controlling for the appropriate covariates for each outcome, the outcomes improved with the use of a 3-D VR model. We further observed that the outcomes of our control group were consistent with previously published data14 for RAPN, adding validity to these findings.
We also saw that as case complexity increased, so too did the magnitude of the outcomes improvements. A previous study24 indicated that complex cases are more likely than less complex ones to have adverse outcomes, both short and long term. The management of the renal hilum is particularly challenging in cases with large central tumors, and complex vascular anatomy may also lend an increased degree of difficulty to such cases. By clearly revealing the relationships among these structures, including size, distance, and configuration, the 3-D VR models appeared to help surgeons make improved preoperative decisions and may have helped minimize intraoperative challenges that occur as a result of uncertain anatomic parameters in highly complex cases.
As context for these findings, it is worth examining the human visual processing system, which is sophisticated yet highly dependent on cues to visualize 3-D spatial relationships.25 The 3-D VR application used in this study is understood to have enhanced both 3-D visualization, which is dependent on cues such as linear perspective, occlusion, shading, texture, and recognition of familiar structures. It would also have contributed to 3-D sensation, with VR simulating cues such as motion parallax (ie, foreground objects appear to move faster than background objects) and binocular disparity (ie, the difference in an image location between the right and left eyes).26 The study results imply that understanding of patient anatomy was enhanced by 3-D VR models in the intervention cases, which, in turn, may have contributed to the improvements seen in outcomes.
The 3-D VR models also may have reduced the cognitive load demanded of surgeons. The 3-D VR models simply reduced the amount of information that the surgeon needed to process. The psychophysiological factors associated with perception and interpretation of medical images have been studied extensively in the setting of radiologists’ reading of images to better understand sources of observer error; many of these factors, including the amount of extraneous information that the radiologists visually absorb during reviews, are likely to affect surgeons’ cognition during their own interpretation of imaging.27,28,29 Specifically, CT and MRI scans depict every element of the patient’s anatomy captured during the imaging process, including those irrelevant to surgical decision-making. Those cross-sectional images are visualized in gray scale, which communicates a wealth of detail requiring interpretation. The 3-D VR models, in contrast, excluded structures that were less relevant to the operation. Although subtle differences in voxel intensity on gray-scale CT and MRI scans aid radiological primary diagnosis, surgical planning may be improved by a deeper understanding of the borders and shapes of structures, the junctions between them, and the relationships between structures.30 These aspects are easily visualized in the 3-D VR models’ multicolor, multitexture format.
Limitations
Our study has several limitations. First, we used nephrometry score as a proxy for case complexity, which assumes that nephrometry scores are a reliable measure of technical difficulty. Nephrometry scores have been used to reliably stratify the complexity of cases, and higher nephrometry scores have previously been associated with greater EBL, warm ischemia time, overall operative time, and length of stay.11,12,31 However, other factors affecting case length and complexity, including body mass index, prior abdominal or retroperitoneal surgery, and the presence of sticky fat, are not captured in the nephrometry score. Second, 11 surgeons, with varying levels of robotic experience and assistance in the operating room, were involved in this study. However, we controlled for this in our analysis by controlling for both the experience level of the surgeon and the presence of a resident or fellow in the operating room. In addition, the use of a multilevel model also helped to address the issue of within-surgeon correlations. Third, we made multiple comparisons in our analysis, which increases the probability of generating a significant result. However, the careful selection of end points to correlate with both the hypothesized benefits of the model and the relatively small number of end points limit the chance of this issue. Fourth, the loss of 7 patients from our analysis may have affected our overall results.
Conclusions
Our findings may affect the care of patients undergoing RAPN in several ways and have the potential to change the way preoperative planning is performed altogether. The patient, physician, and hospital may all derive benefit from this technology, each in a different way. For patients, this benefit comes by way of improved outcomes. The 3-D VR models augment the surgeon’s ability to deliver excellent surgical care by addressing key limitations in the current surgical planning process. Hospitals will benefit from both reduced operative time and shortened patient length of stay. To improve the ability to deliver this care to patients at a population level, future work should focus on process workflows and integration to ensure that surgeons have a simple, seamless way to obtain 3-D VR models for surgical planning. Also, although this technology is clearly useful for kidney surgery, it may also improve the outcomes of patients needing surgical interventions for many other conditions.
Trial Protocol
eFigure. Computed Tomography Scan of Kidney and Mass With Corresponding 3-Dimensional, Virtual Reality Model
Data Sharing Statement
References
- 1.Tan HJ, Filson CP, Litwin MS. Contemporary, age-based trends in the incidence and management of patients with early-stage kidney cancer. Urol Oncol. 2015;33(1):-. doi: 10.1016/j.urolonc.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 2.Novick AC, Campbell SC, Belldegrun A, et al. Guideline for management of the clinical stage 1 renal mass. https://www.auanet.org/education/guidelines/renal-mass.cfm. Accessed July 3, 2014.
- 3.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735-740. doi: 10.1016/S1470-2045(06)70803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163(2):442-445. doi: 10.1016/S0022-5347(05)67896-2 [DOI] [PubMed] [Google Scholar]
- 5.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS; Urologic Diseases in America Project . Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008;112(3):511-520. doi: 10.1002/cncr.23218 [DOI] [PubMed] [Google Scholar]
- 6.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors: is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181(1):55-61. doi: 10.1016/j.juro.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629-1635. doi: 10.1001/jama.2012.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porpiglia F, Fiori C, Checcucci E, Amparore D, Bertolo R. Hyperaccuracy three-dimensional reconstruction is able to maximize the efficacy of selective clamping during robot-assisted partial nephrectomy for complex renal masses. Eur Urol. 2018;74(5):651-660. doi: 10.1016/j.eururo.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 9.Wake N, Bjurlin MA, Rostami P, Chandarana H, Huang WC. Three-dimensional printing and augmented reality: enhanced precision for robotic assisted partial nephrectomy. Urology. 2018;116:227-228. doi: 10.1016/j.urology.2017.12.038 [DOI] [PubMed] [Google Scholar]
- 10.Shirk JD, Kwan L, Saigal C. The use of 3-dimensional, virtual reality models for surgical planning of robotic partial nephrectomy. Urology. 2019;125:92-97. doi: 10.1016/j.juro.2018.02.1504 [DOI] [PubMed] [Google Scholar]
- 11.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844-853. doi: 10.1016/j.juro.2009.05.035 [DOI] [PubMed] [Google Scholar]
- 12.Ellison JS, Montgomery JS, Hafez KS, et al. Association of RENAL nephrometry score with outcomes of minimally invasive partial nephrectomy. Int J Urol. 2013;20(6):564-570. doi: 10.1111/j.1442-2042.2012.03222.x [DOI] [PubMed] [Google Scholar]
- 13.Komninos C, Shin TY, Tuliao P, et al. R-LESS partial nephrectomy trifecta outcome is inferior to multiport robotic partial nephrectomy: comparative analysis. Eur Urol. 2014;66(3):512-517. doi: 10.1016/j.eururo.2013.10.058 [DOI] [PubMed] [Google Scholar]
- 14.Choi JE, You JH, Kim DK, Rha KH, Lee SH. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol. 2015;67(5):891-901. doi: 10.1016/j.eururo.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 15.Thompson RH, Frank I, Lohse CM, et al. The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multi-institutional study. J Urol. 2007;177(2):471-476. doi: 10.1016/j.juro.2006.09.036 [DOI] [PubMed] [Google Scholar]
- 16.Becker F, Van Poppel H, Hakenberg OW, et al. Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol. 2009;56(4):625-634. doi: 10.1016/j.eururo.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 17.Sivarajan G, Taksler GB, Walter D, Gross CP, Sosa RE, Makarov DV. The effect of the diffusion of the surgical robot on the hospital-level utilization of partial nephrectomy. Med Care. 2015;53(1):71-78. doi: 10.1097/MLR.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catanzarite T, Saha S, Pilecki MA, Kim JY, Milad MP. Longer operative time during benign laparoscopic and robotic hysterectomy is associated with increased 30-day perioperative complications. J Minim Invasive Gynecol. 2015;22(6):1049-1058. doi: 10.1016/j.jmig.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 19.Singh S, Swarer K, Resnick K. Longer operative time is associated with increased post-operative complications in patients undergoing minimally-invasive surgery for endometrial cancer. Gynecol Oncol. 2017;147(3):554-557. doi: 10.1016/j.ygyno.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 20.Fardoun T, Chaste D, Oger E, et al. Predictive factors of hemorrhagic complications after partial nephrectomy. Eur J Surg Oncol. 2014;40(1):85-89. doi: 10.1016/j.ejso.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 21.Simone G, Gill IS, Mottrie A, et al. Indications, techniques, outcomes, and limitations for minimally ischemic and off-clamp partial nephrectomy: a systematic review of the literature. Eur Urol. 2015;68(4):632-640. doi: 10.1016/j.eururo.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 22.Patel A, Golan S, Razmaria A, Prasad S, Eggener S, Shalhav A. Early discharge after laparoscopic or robotic partial nephrectomy: care pathway evaluation. BJU Int. 2014;113(4):592-597. doi: 10.1111/bju.12278 [DOI] [PubMed] [Google Scholar]
- 23.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32(7):700-715. doi: 10.1097/00005650-199407000-00004 [DOI] [PubMed] [Google Scholar]
- 24.Png KS, Bahler CD, Milgrom DP, Lucas SM, Sundaram CP. The role of R.E.N.A.L. nephrometry score in the era of robot-assisted partial nephrectomy. J Endourol. 2013;27(3):304-308. doi: 10.1089/end.2012.0182 [DOI] [PubMed] [Google Scholar]
- 25.Marieb E, Hoehn K. Human Anatomy & Physiology. 9th ed. Chicago, IL: Pearson; 2012. [Google Scholar]
- 26.Okoshi T. Three-Dimensional Imaging Techniques. New York, NY: Academic Press; 1976. [Google Scholar]
- 27.Kundel HL, Nodine CF, Carmody D. Visual scanning, pattern recognition and decision-making in pulmonary nodule detection. Invest Radiol. 1978;13(3):175-181. doi: 10.1097/00004424-197805000-00001 [DOI] [PubMed] [Google Scholar]
- 28.Samuel S, Kundel HL, Nodine CF, Toto LC. Mechanism of satisfaction of search: eye position recordings in the reading of chest radiographs. Radiology. 1995;194(3):895-902. doi: 10.1148/radiology.194.3.7862998 [DOI] [PubMed] [Google Scholar]
- 29.Pitman AG. Perceptual error and the culture of open disclosure in Australian radiology. Australas Radiol. 2006;50(3):206-211. doi: 10.1111/j.1440-1673.2006.01563.x [DOI] [PubMed] [Google Scholar]
- 30.Kimpe T, Tuytschaever T. Increasing the number of gray shades in medical display systems: how much is enough? J Digit Imaging. 2007;20(4):422-432. doi: 10.1007/s10278-006-1052-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolla SB, Spiess PE, Sexton WJ. Interobserver reliability of the RENAL nephrometry scoring system. Urology. 2011;78(3):592-594. doi: 10.1016/j.urology.2011.05.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Computed Tomography Scan of Kidney and Mass With Corresponding 3-Dimensional, Virtual Reality Model
Data Sharing Statement