Key Points
Question
Is the expression of 18-kDa translocator protein altered in long-term cannabis users?
Findings
In this case-control study of 24 long-term cannabis users and 27 non–cannabis-using controls, cannabis users showed higher neuroimmune activation or translocator protein levels compared with controls, with a more prominent implication for those with cannabis use disorder. Greater brain translocator protein levels were associated with chronic stress and anxiety as well as higher circulating C-reactive protein levels.
Meaning
The finding of higher translocator protein levels in cannabis users is an important step forward in understanding the role of cannabis in vivo in the brain; more complementary preclinical systems are needed to explain the role of cannabinoids and translocator protein in neuroimmune signaling.
This study conducts in vivo data imaging of the brain of long-term cannabis users and nonusers to examine the association between translocator protein levels, stress and anxiety, and cannabis use behaviors among young adults.
Abstract
Importance
Cannabis is the most commonly used illicit drug in the world. Cannabinoids have been shown to modulate immune responses; however, the association of cannabis with neuroimmune function has never been investigated in vivo in the human brain.
Objective
To investigate neuroimmune activation or 18-kDa translocator protein (TSPO) levels in long-term cannabis users, and to evaluate the association of brain TSPO levels with behavioral measures and inflammatory blood biomarkers.
Design, Setting, and Participants
This cross-sectional study based in Toronto, Ontario, recruited individuals from January 1, 2015, to October 30, 2018. Participants included long-term cannabis users (n = 24) and non–cannabis-using controls (n = 27). Cannabis users were included if they had a positive urine drug screen for only cannabis and if they used cannabis at least 4 times per week for the past 12 months and/or met the criteria for cannabis use disorder. All participants underwent a positron emission tomography scan with [18F]FEPPA, or fluorine F 18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide.
Main Outcomes and Measures
Total distribution volume was quantified across regions of interest. Stress and anxiety as well as peripheral measures of inflammatory cytokines and C-reactive protein levels were also measured.
Results
In total, 24 long-term cannabis users (mean [SD] age, 23.1 [3.8] years; 15 men [63%]) and 27 non–cannabis-using controls (mean [SD] age, 23.6 [4.2] years; 18 women [67%]) were included and completed all study procedures. Compared with the controls, cannabis users had higher [18F]FEPPA total distribution volume (main group effect: F1,48 = 6.5 [P = .01]; ROI effect: F1,200 = 28.4 [P < .001]; Cohen d = 0.6; 23.3% higher), with a more prominent implication for the cannabis use disorder subgroup (n = 15; main group effect: F1,39 = 8.5 [P = .006]; ROI effect: F1,164 = 19.3 [P < .001]; Cohen d = 0.8; 31.5% higher). Greater TSPO levels in the brain were associated with stress and anxiety and with higher circulating C-reactive protein levels in cannabis users.
Conclusions and Relevance
The results of this study suggest that TSPO levels in cannabis users, particularly in those with cannabis use disorder, are higher than those in non–cannabis-using controls. The findings emphasize the need for more complementary preclinical systems for a better understanding of the role of cannabinoids and TSPO in neuroimmune signaling.
Introduction
Almost 4% of the global population used cannabis in the past year,1 consistent with higher rates of cannabis use and cannabis use disorder (CUD), along with a decline in its perceived risk.2,3 Habitual cannabis use is associated with long-term changes in the brain,2 making it a growing public health concern in youth, particularly given the legalization trend across the world.
Adolescence is a critical period for brain development, including synaptic remodeling and maturation, and is a time when the brain is susceptible to psychosocial and physiological changes. Initiation of cannabis use during adolescence coincides with this critical period, highlighting the importance of the timing of cannabis exposure and subsequent vulnerability to CUD and other psychiatric disorders. Cannabinoids, including Δ9-tetrahydrocannabinol (THC), the main psychoactive component of cannabis, act as partial agonists on endogenous cannabinoid receptors: cannabinoid type 1 receptor (CB1R), found mainly on neural tissues, and cannabinoid type 2 receptor (CB2R), primarily located on central and peripheral immune tissue,4 including glial cells.
Increasing evidence suggests that cannabinoid signaling plays a critical role in the modulation of inflammatory responses. Microglia are key players in the immune surveillance system of the central nervous system, in which they act as brain-resident macrophages5 and are first responders to brain insults.6 In response to brain insults, microglia are transformed from a sentry state into an active state and increase the expression of a mitochondrial protein, the 18-kDa translocator protein (TSPO). Thus, in response to microglial activation, TSPO is overexpressed compared with its expression in normal tissues, making it a key marker of immune activation in the brain. In addition to their role in inflammation, microglia also play a critical role in neurodevelopmental processes such as synaptogenesis (ie, synaptic remodeling) and in the maintenance of synaptic plasticity.
Although preclinical studies have investigated the association between neuroimmune function and cannabis use,7,8 clinical evidence remains sparse. For example, several studies have reported the anti-inflammatory properties of cannabinoids through several mechanisms, including limiting infiltration of immune cells into the brain, preventing blood-brain barrier dysfunction, and reducing brain immunoreactivity.8,9,10,11,12,13,14,15 Cannabinoids may act as an immunosuppressant by inhibiting microglial activation,9,11,16 inhibiting the release of free radicals and reactive oxygen species from microglia,16 decreasing proinflammatory cytokine secretion from microglia,17 and increasing anti-inflammatory cytokine release.18 Moreover, 2 cannabis components, THC and cannabidiol, are currently being investigated as potential therapeutic agents for several inflammatory or immune diseases; however, to date, it remains unknown whether cannabis plays a role in an anti-inflammatory or proinflammatory state in the living human brain.
In this study, we examined in vivo data that imaged neuroimmune activation or TSPO levels in long-term cannabis users after overnight abstinence by using fluorine F 18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide ([18F]FEPPA) positron emission tomography (PET) scan. On the basis of preclinical evidence suggesting the potential anti-inflammatory properties of cannabinoids, we hypothesized that long-term cannabis users would have lower TSPO levels in the brain compared with non–cannabis-using controls. We also explored the association between brain TSPO levels and behavioral measures and inflammatory blood biomarkers.
Methods
Participants
Potential participants were recruited from the Toronto (Ontario, Canada) area at the Centre for Addiction and Mental Health from January 1, 2015, to October 20, 2018. This study was approved by the Research Ethics Board at the Centre for Addiction and Mental Health. All participants provided written informed consent after the study procedures were explained to them thoroughly.
Cannabis users were included in the study if they had a positive urine drug screen for only cannabis and if they used cannabis at least 4 times per week for the past 12 months and/or met the criteria for CUD. Controls were excluded if they had a past history of or current psychoactive drug use.
All participants were screened with the Structured Clinical Interview19 for DSM-IV Axis I disorders and were excluded for any of the following: past or current Axis I disorder, including but not limited to major depressive disorder and/or any anxiety disorders; current or past substance use disorder (except for CUD in cannabis users); pregnancy or current breastfeeding; unstable medical or neurological illness; history of severe head trauma; and the presence of metal implants precluding a magnetic resonance imaging scan. Chronic stress was evaluated with the Trier Inventory for Chronic Stress (score range: 0-120, with higher scores indicating greater self-reported ratings of chronic stress),20 and anxiety was assessed with the Beck Anxiety Inventory (score range: 0-63, with higher scores indicating greater self-reported ratings of anxiety).21
A detailed history of cannabis use was identified for each participant with an in-house assessment called the Drug History Questionnaire. On the basis of the information from the Drug History Questionnaire and the Structured Clinical Interview for DSM-IV Axis I disorders, we established whether the cannabis users among the participants met the DSM-5 criteria for CUD. In this cohort, 15 cannabis users met the DSM-5 criteria for CUD. In addition, lifetime use and past-year cannabis use were estimated for each participant. Cannabis craving was assessed with the Marijuana Craving Questionnaire (score range: 12-84, with higher scores indicating greater levels of cannabis craving),22 and the severity of dependence was evaluated with the Severity of Dependence Scale (score range: 0-15, with higher scores indicating greater severity of cannabis dependence).23 Cannabis users were instructed to abstain from cannabis for at least 12 hours (ie, overnight) before the scheduled PET scan.
PET and Structural MRI Data
Acquisition of PET data has been described in detail elsewhere24,25 and is included in the eAppendix in the Supplement. Briefly, each participant was scanned with [18F]FEPPA for 125 minutes on a high-resolution PET scanner (CPS HRRT; Siemens). All participants were grouped according to their TSPO rs6971 polymorphism as high-affinity binders (C/C), mixed-affinity binders (C/T), or low-affinity binders (T/T), as previously described.26
Blood Serum Levels
Blood serum levels of cytokines and high-sensitivity C-reactive protein (hsCRP) levels were available in 16 cannabis users. Cytokines (IFN-γ, TNF-α, IL-1β, IL-2, IL-6, IL-8, IL-10, and IL-12 from a human high-sensitivity T-cell panel (HST-CYTOMAG60SK; Merck Millipore) were assayed using a multiplex immunoassay (Luminex MAGPIX; Luminex Corporation). The hsCRP levels were measured in serum using a high-sensitivity enzyme-linked immunosorbent assay according to the manufacturer's instructions (IBL International) (eAppendix in the Supplement).
Blood serum levels of THC, OH-THC (11-hydroxy-THC), COOH-THC (11-nor-9-carboxy-THC), and cannabidiol metabolites were available in 14 long-term cannabis users. The cannabinoids multiplex assay was performed at the Centre for Addiction and Mental Health Clinical Laboratory by gas chromatography coupled with mass spectrometry, as described in the software (Varian; Agilent Technologies) application note with slight analytical modifications and cannabidiol addition to the assay (eAppendix in the Supplement).
Statistical Analysis
Demographic measures were compared using χ2 tests for categorical variables, and independent-sample, unpaired, 2-tailed t tests were used for continuous variables. Group differences in [18F]FEPPA total distribution volume (VT) were analyzed using a linear mixed model analysis, with group and region of interest (ROI) as fixed factors, TSPO genotype as a covariate, and [18F]FEPPA VT as the dependent variable. The ROIs included in the main model were dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), temporal cortex, and cerebellum (eAppendix in the Supplement). Gray matter as a whole (GM) was analyzed separately using analysis of variance, controlling for TSPO genotype. To assess whether a difference in [18F]FEPPA VT exists between cannabis users and non–cannabis-using controls, we ran a separate linear mixed model analysis, including all gray matter regions sampled (eAppendix in the Supplement). Effect size (Cohen d) was calculated as the difference between the estimated marginal means between groups divided by the mean SD across all prioritized brain regions.
As secondary analyses, we explored the associations between [18F]FEPPA VT and stress and anxiety as well as cannabis use behaviors (use, craving, and dependence) in cannabis users by using Pearson partial correlations, controlling for TSPO genotype. Similarly, we assessed the associations between [18F]FEPPA VT and peripheral inflammatory biomarkers and blood serum cannabinoid levels using Pearson partial correlations, controlling for TSPO genotype. Associations were exploratory in nature and, as such, P values were not corrected for multiple comparisons. All statistical analyses were performed using SPSS, version 22.0 (IBM), with 2-sided P < .05 considered to be statistically significant.
Results
In total, 24 long-term cannabis users and 27 controls aged 18 to 35 years completed all study procedures and had usable data (eAppendix in the Supplement). Of the cannabis users, the mean (SD) age was 23.1 (3.8) years and 15 (63%) were male. Of the controls, the mean (SD) age was 23.6 (4.2) years and 18 (67%) were female. Demographic and clinical measures are presented in the Table.
Table. Demographic and Clinical Measures of the Participants.
| Variable | Mean (SD) | t Test or χ2 Test Value | P Value | |
|---|---|---|---|---|
| Non–Cannabis-Using Controls (n = 27) | Long-Term Cannabis Users (n = 24) | |||
| Age, y | 23.6 (4.2) | 23.1 (3.8) | t: 0.4 | .68 |
| Sex | χ2: 4.3 | .04 | ||
| Male | 9 | 15 | ||
| Female | 18 | 9 | ||
| Current drug usea | ||||
| Tobacco | 0 | 7 | NA | NA |
| Other drugs of abuse | 0 | 0 | NA | NA |
| Cannabis | 0 | 24 | NA | NA |
| Cannabis use and behavior | ||||
| Age at first use, y | NA | 16.4 (3.4) | NA | NA |
| Estimated cannabis use, g | ||||
| Lifetime | NA | 2163.6 (1641.9) | NA | NA |
| Past year | NA | 448.5 (262.0) | NA | NA |
| Current dose, g | NA | 1.4 (0.8) | NA | NA |
| CUD | 0 | 15 | NA | NA |
| TSPO (rs6971) genotype | χ2: 0.1 | .71 | ||
| HAB | 19 | 18 | ||
| MAB | 8 | 6 | ||
| [18F]FEPPA PET parameters | ||||
| Amount injected, mCi | 4.9 (0.4) | 5.0 (0.3) | t: −0.7 | .49 |
| Specific activity, mCi/μmol | 3281.0 (3672.0) | 2082.5 (2194.1) | t: 1.4 | .16 |
| Mass injected, μg | 1.4 (1.3) | 1.4 (0.7) | t: 0.1 | .93 |
| BAI scoreb | 5.7 (6.1) | 9.6 (11.4) | ||
| TICS scoreb | 34.0 (17.5) | 43.6 (15.8) | ||
| MCQ score | NA | 41.5 (10.9) | ||
| SDS score | NA | 2.8 (2.2) | ||
Abbreviations: [18F]FEPPA, fluorine F 18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide; BAI, Beck Anxiety Inventory; CUD, cannabis use disorder; HAB, high-affinity binder; MAB, mixed-affinity binder; MCQ, Marijuana Craving Questionnaire; NA, not applicable; PET, positron emission tomography; SDS, Severity of Dependence Scale; TICS, Trier Inventory for Chronic Stress; TSPO, translocator protein.
All participants had a negative urine drug screen for ethanol, methadone, benzodiazepines, and cocaine at baseline. All cannabis users had a positive urine drug screen for cannabis.
BAI was not available for 2 cannabis users, TICS was not available for 1 cannabis user, and TICS and BAI were not available for 12 non–cannabis-using controls.
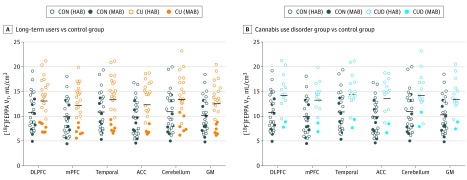
The mean [18F]FEPPA VT was significantly higher in long-term cannabis users (12.9 mL/cm3; 95% CI, 11.5-14.3 mL/cm3) compared with the controls (10.4 mL/cm3; 95% CI, 9.1-11.8 mL/cm3; main group effect: F1,48 = 6.5 [P = .01]; ROI effect: F1,200 = 28.4 [P < .001]; Cohen d = 0.6; 23.3% higher) (Figure 1A). Differences across prioritized brain regions were robust (DLPFC, 22.2%; mPFC, 23.5%; temporal cortex, 25.1%; ACC, 25.4%; and cerebellum, 24.8%). Similar elevations were also present in GM (main group effect: F1,48 = 6.1; P = .02; 22.3% higher) and across all gray matter regions sampled (eAppendix in the Supplement). Results remained unchanged after controlling for tobacco use (main group effect: F1,47 = 7.2; P = .01) and sex (main group effect: F1,47 = 11.5; P = .001). However, a significant association was found between sex and [18F]FEPPA VT, such that female participants had higher TSPO levels than male participants (main sex effect: F1,47 = 7.5; P = .009). A subgroup analysis revealed that this association was primarily driven by the cannabis user group (eAppendix in the Supplement). In addition, the mean [18F]FEPPA VT was significantly higher in the 15 cannabis users with CUD (13.8 mL/cm3; 95% CI, 12.0-15.7 mL/cm3) compared with controls (10.5 mL/cm3; 95% CI, 9.2-11.9 mL/cm3; main group effect: F1,39 = 8.5 [P = .006]; ROI effect: F1,164 = 19.3 [P < .001]; Cohen d = 0.8; 31.5% higher; DLPFC, 30.2%; mPFC, 32.6%; temporal cortex, 30.9%; ACC, 36.0%; and cerebellum, 28.2%), suggesting a more prominent association in the CUD subgroup (Figure 1B). Similar elevations were also present in GM (main group effect: F1,39 = 7.7; P = .008; 29.7% higher in CUD).
Figure 1. Higher Fluorine F 18–Labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide ([18F]FEPPA) Distribution Volume in Long-term Cannabis Users.
In the dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (mPFC), temporal cortex, anterior cingulate cortex (ACC), cerebellum, and gray matter as a whole (GM), the total distribution volume (VT) of [18F]FEPPA was statistically significantly higher in long-term cannabis users (CU) compared with the non–cannabis-using control (CON) group (A) and in the cannabis use disorder (CUD) group (n = 15) compared with the control group (B). Participants were grouped based on their translocator protein rs6971 polymorphism as high-affinity binders (HAB) or mixed-affinity binders (MAB). [18F]FEPPA VT values represent raw values unadjusted for genotype. Horizontal bar indicates group mean adjusted for genotype using the estimated marginal means of each region.
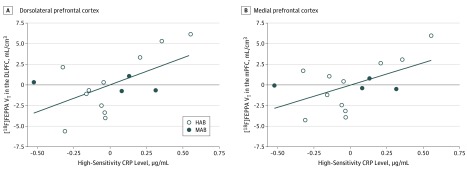
In the cannabis user group, none of the peripheral inflammatory markers (IFN-γ, IL-10, IL-12, IL-1β, IL-2, IL-6, IL-8, and TNF-α) had an association with [18F]FEPPA VT (eTable 1 in the Supplement). One cannabis user had high hsCRP levels (10.2 μg/mL; mean [SD] CRP level in this cohort, 0.5 [0.3] μg/mL). Reanalysis of the data excluding this outlier revealed a significant positive association between [18F]FEPPA VT and hsCRP levels across all prioritized brain regions (DLPFC: r = 0.6 [P = .03]; mPFC: r = 0.6 [P = .04]; temporal cortex: r = 0.6 [P = .03]; ACC: r = 0.5 [P = .05]; cerebellum: r = 0.6 [P = .02]; GM: r = 0.6 [P = .03]) (Figure 2 and eTable 2 in the Supplement).
Figure 2. Association of Higher Fluorine F 18–Labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide ([18F]FEPPA) Distribution Volume With Higher Circulating High-Sensitivity C-reactive Protein (CRP) Levels in Blood Serum of Long-term Cannabis Users.
The associations, adjusted for translocator protein rs6971 genotype (high-affinity binders [HAB] or mixed-affinity binders [MAB]), are shown for the dorsolateral prefrontal cortex (DLPFC: r = 0.6; P = .03) (A) and the medial prefrontal cortex (mPFC: r = 0.6; P = .04) (B). Similar correlations were present in the temporal cortex (r = 0.6; P = .03), anterior cingulate cortex (r = 0.5; P = .05), cerebellum (r = 0.6; P = .02), and gray matter as a whole (r = 0.6; P = .03).
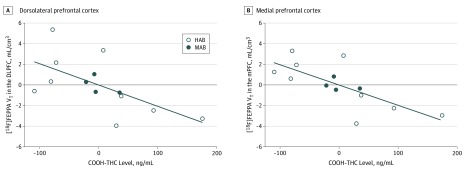
Higher [18F]FEPPA VT in the DLPFC (r = –0.6; P = .03), mPFC (r = –0.7; P = .007), temporal cortex (r = –0.6; P = .02), and ACC (r = –0.6; P = .02) had a significant correlation with lower COOH-THC (Figure 3). No significant associations between [18F]FEPPA VT and THC or OH-THC metabolite levels were found (eTable 3 in the Supplement).
Figure 3. Association of Higher Fluorine F 18–Labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide ([18F]FEPPA) Distribution Volume With Lower 11-Nor-9-carboxy-Δ9-tetrahydrocannabinol (COOH-THC) Metabolite Levels in Blood Serum of Long-term Cannabis Users.
The associations, adjusted for translocator protein rs6971 genotype (high-affinity binders [HAB] or mixed-affinity binders [MAB]), are shown for the dorsolateral prefrontal cortex (DLPFC: r = –0.6; P = .03) (A) and medial prefrontal cortex (mPFC: r = –0.7; P = .007) (B). Similar correlations were present in the temporal cortex (r = –0.6; P = .02) and anterior cingulate cortex (r = –0.6; P = .02).
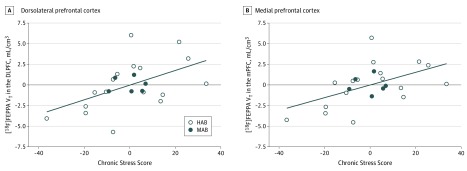
In cannabis users, higher [18F]FEPPA VT in the DLPFC (r = 0.5; P = .02), mPFC (r = 0.5; P = .01), temporal cortex (r = 0.5; P = .009), ACC (r = 0.6; P = .002), and GM (r = 0.5; P = .02) had a significant correlation with higher chronic stress scores as reported on the Trier Inventory for Chronic Stress scale (Figure 4 and eTable 4 in the Supplement). In addition, higher [18F]FEPPA VT in the ACC (r = 0.4; P = .04) and GM (r = 0.5; P = .04) was associated with higher anxiety scores as reported on the Beck Anxiety Inventory scale (eTable 4 in the Supplement). No significant associations between [18F]FEPPA VT and stress and anxiety scores were observed in the control group (n = 15; eTable 5 in the Supplement). A significant negative correlation between [18F]FEPPA VT and estimated lifetime cannabis use, but not past-year cannabis use, was found in all prioritized brain regions except for a trend-level association in the cerebellum (eTable 5 in Supplement). However, this association was driven by sex, given that male cannabis users had significantly more cumulative cannabis exposure compared with female cannabis users; after controlling for sex, this correlation was no longer significant, although it remained trend-level in some regions (eTable 6 in the Supplement). No significant associations between [18F]FEPPA VT and cannabis craving and severity of dependence (eTable 7 in the Supplement) were found.
Figure 4. Association of Higher Fluorine F 18–Labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide ([18F]FEPPA) Distribution Volume With Higher Stress Scores in Long-term Cannabis Users.
The associations, as measured by the Trier Inventory for Chronic Stress after adjustment for translocator protein rs6971 genotype (high-affinity binders [HAB] or mixed-affinity binders [MAB]), are shown for the dorsolateral prefrontal cortex (DLPFC: r = 0.5; P = .02) (A) and the medial prefrontal cortex (mPFC: r = 0.5; P = .01) (B). Similar correlations were present in the temporal cortex (r = 0.5; P = .009), anterior cingulate cortex (r = 0.6; P = .002), and gray matter as a whole (r = 0.5; P = .02).
Discussion
To our knowledge, this PET study is the first to investigate neuroimmune activation or TSPO levels in long-term cannabis users. Contrary to our hypothesis, long-term cannabis users had significantly higher brain TSPO levels compared with non–cannabis-using individuals, with a more prominent implication for cannabis users with CUD.
Many studies support the immunosuppressive properties of cannabinoids under inflammatory conditions,7,8 but several reports are also consistent with the proinflammatory properties of cannabinoids. For example, Bayazit et al27 showed that individuals with CUD had higher serum levels of proinflammatory cytokines compared with healthy controls. Furthermore, long-term THC exposure in mice increased cerebellar microglial activation and the expression of proinflammatory markers as well as produced deficits in cerebellar learning, which were prevented by the administration of minocycline hydrochloride, an inhibitor of microglial activation.28 These neuroinflammatory and behavioral alterations were mediated by the downregulation of CB1R,28 consistent with other PET studies that reported reduced CB1R expression with long-term cannabis use.29,30,31 Similarly, THC exposure during adolescence also produced a persistent neuroinflammatory state in adult female rats and mice, characterized by altered microglia morphologic structure, increased proinflammatory mediators, reduced CB1Rs, and increased CB2Rs.32,33 Inhibition of microglial activation during adolescent THC exposure prevented the development of a prefrontal neuroinflammatory phenotype and attenuated the short-term memory impairments present in adult rats, providing a direct association between THC and microglial activation.33 Thus, these findings suggest that long-term cannabinoid exposure may be correlated with the cognitive and behavioral impairments associated with CUD, perhaps through neuroimmune contributions.34,35
In addition to mediating immune responses in the brain, microglia also have a role in neuronal development and synaptic plasticity.36 Cannabis use, especially in early adolescence, can alter brain structure and neural activity in prefrontal and temporal cortical regions,37,38 the same regions associated with long-term THC exposure–induced microglia-mediated neuroinflammation in rats.33 Consistent with this finding, exposure to THC in adolescent rats altered neuronal and synaptic morphologic structure across cortical regions39,40 and produced persistent changes in synaptic plasticity.39 Furthermore, a study that used models of synaptic pruning derived from patients with schizophrenia reported excessive synaptic elimination that reflected abnormalities in microglia-like cells.41 These findings suggest that cannabis-induced alterations in the brain may be mediated at least in part by increased or excessive neuroimmune or microglial activation in cannabis users.28,32,33,34,42 However, whether TSPO can be used as a proxy for microglia-mediated synaptic pruning remains unknown.
Results of this study may seem to contradict several studies reporting the neuroprotective properties of cannabinoids7,8; however, these properties are often observed in experimental models of disease, with an underlying inflammatory or neurodegenerative condition. It is possible that cannabinoids can induce either a proinflammatory or anti-inflammatory picture depending on the status of the brain. Furthermore, reduced TSPO levels in an infection-mediated mouse model were recently associated with increased cytokine expression, challenging the simple assumption that proinflammatory immune activation is mirrored by increased TSPO expression.43
In cannabis users, no significant associations between [18F]FEPPA VT and any of the peripheral cytokine levels were observed. These results are consistent with previous reports, which found a lack of correlation between central and peripheral markers of inflammation in other disorders.44,45 However, a significant association between [18F]FEPPA VT and hsCRP levels was observed, suggesting that higher brain TSPO levels may be associated with higher levels of CRP in cannabis users. In addition, [18F]FEPPA VT was inversely associated with COOH-THC blood serum metabolite levels but not with THC or OH-THC levels in cannabis users. However, in the only study that paired blood and postmortem brain samples, no correlation between THC metabolite concentrations in brain and peripheral blood was found, suggesting that peripheral THC metabolite levels may not necessarily reflect levels in the brain.46 The interpretation of these associations, however, is limited by different potencies, doses, and routes of administration, which may affect the outcome variable.
In addition, we observed a significant correlation between [18F]FEPPA VT and chronic stress and anxiety scores in cannabis users, suggesting that higher brain TSPO levels may be associated with higher stress and anxiety. This finding is consistent with previous reports of higher TSPO in illnesses with dysregulated stress responses, including major depressive disorder45,47,48 and obsessive-compulsive disorder.49 Furthermore, preclinical evidence suggests that acute and chronic psychological stress can directly induce microglial activation and cytokine release.50,51,52,53,54,55 Similarly, studies have shown an association between activated microglia and inflammatory mediators in anxiety-like behaviors.55,56 These associations between TSPO and stress and anxiety may be secondary to cannabis withdrawal.
All of these correlations were exploratory in nature and thus need to be confirmed in larger samples. In addition, because correlational analyses cannot be used to infer causation, caution should be taken when interpreting these results until preclinical studies can explain the mechanisms underlying these associations.
Limitations
The limitations of this study should be considered when interpreting the results. First, although an increase in [18F]FEPPA VT is mostly attributed to microglial activation, studies show that astrocytes and vascular endothelial cells also express TSPO.57 However, both astrocytes and endothelial cells are known to be key factors in brain immunity, and the potential role of these cells in the [18F]FEPPA VT signal does not undermine our conclusion. Furthermore, it has been suggested that endogenous ligands, such as cholesterol, may also be factors in altered TSPO levels.58 Second, the cannabis user group comprised more tobacco users compared with the control group. However, a [11C]DAA1106 PET study reported decreased TSPO levels in cigarette smokers compared with controls,59 a finding that makes it less likely that tobacco use in some of our study participants might account for the detected difference. Third, the cannabis user group had more male than female participants compared with the control group. In this sample, female cannabis users had higher TSPO levels compared with male cannabis users. Several studies have reported sex differences in cannabinoid sensitivity: in particular, female cannabis users were more susceptible to the deleterious effects of cannabinoid exposure compared with males.60 This study may provide preliminary evidence suggesting sex-dependent alterations in TSPO levels in cannabis users.61,62
Fourth, although participants were instructed to abstain from smoking cannabis 12 hours before the PET scan, the levels of cannabinoids in the blood, as well as inflammatory biomarkers, were not available for all participants; thus, these subsets of individuals may not be representative of the larger study population. Fifth, a high-sensitivity enzyme-linked immunosorbent assay was used to measure peripheral CRP levels, whereas a multiplex assay was used to measure the levels of inflammatory cytokines, which may account for the discrepancy observed in the association of TSPO with CRP but not with other immune markers.
Conclusions
Findings from this study suggest higher TSPO levels in cannabis users compared with non–cannabis-using controls, with a more prominent implication for cannabis users with CUD. Greater brain TSPO was associated with increased blood CRP measures as well as stress and anxiety. This study emphasizes the need for more complementary preclinical systems to inform the role of cannabinoids and TSPO in neuroimmune signaling.
eAppendix. Methods and Results
eTable 1. Associations Between [18F]FEPPA VT and Peripheral Cytokine Serum Levels (pg/mL) in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
eTable 2. Associations Between [18F]FEPPA VT and High-Sensitivity CRP Blood Serum Levels (μg/mL) in Long-Term Cannabis Users (n=15, Removing Cannabis User With High CRP Levels), Adjusted for rs6971 TSPO Genotype
eTable 3. Associations Between [18F]FEPPA VT and THC, COOH-THC and OH-THC Blood Serum Levels (ng/mL) in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
eTable 4. Association Between [18F]FEPPA VT and Chronic Stress and Anxiety as Measured by TICS and BAI, Respectively, in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
eTable 5. Association Between [18F]FEPPA VT and Chronic Stress and Anxiety as Measured by TICS and BAI, Respectively, in Non–Cannabis-Using Controls, Adjusted for rs6971 TSPO Genotype
eTable 6. Association Between [18F]FEPPA VT and Estimated Lifetime and Past-Year Cannabis Use (Grams) in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
eTable 7. Association Between [18F]FEPPA VT and Cannabis Craving and Severity of Dependence as Measured by MCQ and SDS Scores, Respectively, in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
References
- 1.United Nations Office on Drugs and Crime World Drug Report 2018. https://www.unodc.org/wdr2018/prelaunch/WDR18_Booklet_1_EXSUM.pdf. Published June 2018. Accessed February 19, 2019.
- 2.Carliner H, Brown QL, Sarvet AL, Hasin DS. Cannabis use, attitudes, and legal status in the U.S.: a review. Prev Med. 2017;104:13-23. doi: 10.1016/j.ypmed.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212. doi: 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129-180. doi: 10.1016/S0163-7258(97)82001-3 [DOI] [PubMed] [Google Scholar]
- 5.Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20(3):269-287. doi: 10.1016/0165-0173(94)00015-H [DOI] [PubMed] [Google Scholar]
- 6.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312-318. doi: 10.1016/0166-2236(96)10049-7 [DOI] [PubMed] [Google Scholar]
- 7.Mecha M, Carrillo-Salinas FJ, Feliú A, Mestre L, Guaza C. Microglia activation states and cannabinoid system: therapeutic implications. Pharmacol Ther. 2016;166:40-55. doi: 10.1016/j.pharmthera.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 8.Suárez-Pinilla P, López-Gil J, Crespo-Facorro B. Immune system: a possible nexus between cannabinoids and psychosis. Brain Behav Immun. 2014;40:269-282. doi: 10.1016/j.bbi.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 9.Marchalant Y, Rosi S, Wenk GL. Anti-inflammatory property of the cannabinoid agonist WIN-55212-2 in a rodent model of chronic brain inflammation. Neuroscience. 2007;144(4):1516-1522. doi: 10.1016/j.neuroscience.2006.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mecha M, Torrao AS, Mestre L, Carrillo-Salinas FJ, Mechoulam R, Guaza C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012;3(6):e331. doi: 10.1038/cddis.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mecha M, Feliú A, Iñigo PM, Mestre L, Carrillo-Salinas FJ, Guaza C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013;59:141-150. doi: 10.1016/j.nbd.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 12.Baker D, Jackson SJ, Pryce G. Cannabinoid control of neuroinflammation related to multiple sclerosis. Br J Pharmacol. 2007;152(5):649-654. doi: 10.1038/sj.bjp.0707458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jhaveri MD, Richardson D, Chapman V. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Br J Pharmacol. 2007;152(5):624-632. doi: 10.1038/sj.bjp.0707433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zajicek JP, Apostu VI. Role of cannabinoids in multiple sclerosis. CNS Drugs. 2011;25(3):187-201. doi: 10.2165/11539000-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 15.Bolognini D, Costa B, Maione S, et al. The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol. 2010;160(3):677-687. doi: 10.1111/j.1476-5381.2010.00756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merighi S, Gessi S, Varani K, et al. Cannabinoid CB(2) receptors modulate ERK-1/2 kinase signalling and NO release in microglial cells stimulated with bacterial lipopolysaccharide [retracted in Br J Pharmacol. 2017 Aug;174(15):2609]. Br J Pharmacol. 2012;165(6):1773-1788. doi: 10.1111/j.1476-5381.2011.01673.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29(1):58-69. doi: [DOI] [PubMed] [Google Scholar]
- 18.Molina-Holgado F, Pinteaux E, Moore JD, et al. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci. 2003;23(16):6470-6474. doi: 10.1523/JNEUROSCI.23-16-06470.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Research Version; Patient Edition; SCID-I/P; Washington, DC: American Psychiatric Publishing Inc; 2002. [Google Scholar]
- 20.Schulz P, Schlotz W. The Trier Inventory for the Assessment of Chronic Stress (TICS): scale construction, statistical testing, and validation of the scale work overload. Diagnostica. 1999;45(1):8-19. doi: 10.1026//0012-1924.45.1.8 [DOI] [Google Scholar]
- 21.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893-897. doi: 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- 22.Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009;102(1-3):35-40. doi: 10.1016/j.drugalcdep.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin G, Copeland J, Gates P, Gilmour S. The Severity of Dependence Scale (SDS) in an adolescent population of cannabis users: reliability, validity and diagnostic cut-off. Drug Alcohol Depend. 2006;83(1):90-93. doi: 10.1016/j.drugalcdep.2005.10.014 [DOI] [PubMed] [Google Scholar]
- 24.Hafizi S, Tseng H-H, Rao N, et al. Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F] FEPPA. Am J Psychiatry. 2017;174(2):118-124. doi: 10.1176/appi.ajp.2016.16020171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenk M, Selvanathan T, Rao N, et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull. 2015;41(1):85-93. doi: 10.1093/schbul/sbu157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizrahi R, Rusjan PM, Kennedy J, et al. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab. 2012;32(6):968-972. doi: 10.1038/jcbfm.2012.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayazit H, Selek S, Karababa IF, Cicek E, Aksoy N. Evaluation of oxidant/antioxidant status and cytokine levels in patients with cannabis use disorder. Clin Psychopharmacol Neurosci. 2017;15(3):237-242. doi: 10.9758/cpn.2017.15.3.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutando L, Busquets-Garcia A, Puighermanal E, et al. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Invest. 2013;123(7):2816-2831. doi: 10.1172/JCI67569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol. 2015;20(2):357-367. doi: 10.1111/adb.12116 [DOI] [PubMed] [Google Scholar]
- 30.D’Souza D, Cortes-Briones J, Ranganathan M, et al. Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(1):60-67. doi: 10.1016/j.bpsc.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirvonen J, Goodwin RS, Li C-T, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17(6):642-649. doi: 10.1038/mp.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moretti S, Franchi S, Castelli M, et al. Exposure of adolescent mice to delta-9-tetrahydrocannabinol induces long-lasting modulation of pro-and anti-inflammatory cytokines in hypothalamus and hippocampus similar to that observed for peripheral macrophages. J Neuroimmune Pharmacol. 2015;10(2):371-379. doi: 10.1007/s11481-015-9592-2 [DOI] [PubMed] [Google Scholar]
- 33.Zamberletti E, Gabaglio M, Prini P, Rubino T, Parolaro D. Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats. Eur Neuropsychopharmacol. 2015;25(12):2404-2415. doi: 10.1016/j.euroneuro.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 34.Melis M, Frau R, Kalivas PW, et al. New vistas on cannabis use disorder. Neuropharmacology. 2017;124:62-72. doi: 10.1016/j.neuropharm.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlienz NJ, Budney AJ, Lee DC, Vandrey R. Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr Addict Rep. 2017;4(2):75-81. doi: 10.1007/s40429-017-0143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018-1027. doi: 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- 37.Bossong MG, Jansma JM, Bhattacharyya S, Ramsey NF. Role of the endocannabinoid system in brain functions relevant for schizophrenia: an overview of human challenge studies with cannabis or ∆9-tetrahydrocannabinol (THC). Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:53-69. doi: 10.1016/j.pnpbp.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 38.Mizrahi R, Watts JJ, Tseng KY. Mechanisms contributing to cognitive deficits in cannabis users. Neuropharmacology. 2017;124:84-88. doi: 10.1016/j.neuropharm.2017.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolb B, Li Y, Robinson T, Parker LA. THC alters morphology of neurons in medial prefrontal cortex, orbital prefrontal cortex, and nucleus accumbens and alters the ability of later experience to promote structural plasticity. Synapse. 2018;72(3):e22020. doi: 10.1002/syn.22020 [DOI] [PubMed] [Google Scholar]
- 40.Miller ML, Chadwick B, Dickstein DL, et al. Adolescent exposure to Δ9-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol Psychiatry. 2019;24(4):588-600. doi: 10.1038/s41380-018-0243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sellgren CM, Gracias J, Watmuff B, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22(3):374-385. doi: 10.1038/s41593-018-0334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691-705. doi: 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Notter T, Coughlin JM, Gschwind T, et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry. 2018;23(2):323-334. doi: 10.1038/mp.2016.248 [DOI] [PubMed] [Google Scholar]
- 44.Coughlin JM, Wang Y, Ambinder EB, et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry. 2016;6(4):e777. doi: 10.1038/tp.2016.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Setiawan E, Wilson AA, Mizrahi R, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72(3):268-275. doi: 10.1001/jamapsychiatry.2014.2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mura P, Kintz P, Dumestre V, Raul S, Hauet T. THC can be detected in brain while absent in blood. J Anal Toxicol. 2005;29(8):842-843. doi: 10.1093/jat/29.8.842 [DOI] [PubMed] [Google Scholar]
- 47.Li L, Wang W, Zhang L-M, et al. Overexpression of the 18 kDa translocator protein (TSPO) in the hippocampal dentate gyrus produced anxiolytic and antidepressant-like behavioural effects. Neuropharmacology. 2017;125:117-128. doi: 10.1016/j.neuropharm.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 48.Richards EM, Zanotti-Fregonara P, Fujita M, et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018;8(1):57. doi: 10.1186/s13550-018-0401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attwells S, Setiawan E, Wilson AA, et al. Inflammation in the neurocircuitry of obsessive-compulsive disorder. JAMA Psychiatry. 2017;74(8):833-840. doi: 10.1001/jamapsychiatry.2017.1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21(1):47-59. doi: 10.1016/j.bbi.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 51.Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171(1-2):72-85. doi: 10.1016/j.jneuroim.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 52.O’Connor KA, Johnson JD, Hansen MK, et al. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991(1-2):123-132. doi: 10.1016/j.brainres.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 53.Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007;146(3):1388-1399. doi: 10.1016/j.neuroscience.2007.02.043 [DOI] [PubMed] [Google Scholar]
- 54.Tynan RJ, Naicker S, Hinwood M, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24(7):1058-1068. doi: 10.1016/j.bbi.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 55.Wohleb ES, Hanke ML, Corona AW, et al. β-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277-6288. doi: 10.1523/JNEUROSCI.0450-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawada A, Niiyama Y, Ataka K, Nagaishi K, Yamakage M, Fujimiya M. Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain. 2014;155(9):1762-1772. doi: 10.1016/j.pain.2014.05.031 [DOI] [PubMed] [Google Scholar]
- 57.Veronese M, Reis Marques T, Bloomfield PS, et al. Kinetic modelling of [11C] PBR28 for 18 kDa translocator protein PET data: a validation study of vascular modelling in the brain using XBD173 and tissue analysis. J Cereb Blood Flow Metab. 2018;38(7):1227-1242. doi: 10.1177/0271678X17712388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim SW, Wiers CE, Tyler R, et al. Influence of alcoholism and cholesterol on TSPO binding in brain: PET [11C]PBR28 studies in humans and rodents. Neuropsychopharmacology. 2018;43(9):1832-1839. doi: 10.1038/s41386-018-0085-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brody AL, Hubert R, Enoki R, et al. Effect of cigarette smoking on a marker for neuroinflammation: a [11C]DAA1106 positron emission tomography study. Neuropsychopharmacology. 2017;42(8):1630-1639. doi: 10.1038/npp.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 2013;92(8-9):476-481. doi: 10.1016/j.lfs.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nia AB, Mann C, Kaur H, Ranganathan M. Cannabis use: neurobiological, behavioral, and sex/gender considerations. Curr Behav Neurosci Rep. 2018;5(4):271-280. doi: 10.1007/s40473-018-0167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Struik D, Sanna F, Fattore L. The modulating role of sex and anabolic-androgenic steroid hormones in cannabinoid sensitivity. Front Behav Neurosci. 2018;12:249. doi: 10.3389/fnbeh.2018.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods and Results
eTable 1. Associations Between [18F]FEPPA VT and Peripheral Cytokine Serum Levels (pg/mL) in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
eTable 2. Associations Between [18F]FEPPA VT and High-Sensitivity CRP Blood Serum Levels (μg/mL) in Long-Term Cannabis Users (n=15, Removing Cannabis User With High CRP Levels), Adjusted for rs6971 TSPO Genotype
eTable 3. Associations Between [18F]FEPPA VT and THC, COOH-THC and OH-THC Blood Serum Levels (ng/mL) in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
eTable 4. Association Between [18F]FEPPA VT and Chronic Stress and Anxiety as Measured by TICS and BAI, Respectively, in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
eTable 5. Association Between [18F]FEPPA VT and Chronic Stress and Anxiety as Measured by TICS and BAI, Respectively, in Non–Cannabis-Using Controls, Adjusted for rs6971 TSPO Genotype
eTable 6. Association Between [18F]FEPPA VT and Estimated Lifetime and Past-Year Cannabis Use (Grams) in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype
eTable 7. Association Between [18F]FEPPA VT and Cannabis Craving and Severity of Dependence as Measured by MCQ and SDS Scores, Respectively, in Long-Term Cannabis Users, Adjusted for rs6971 TSPO Genotype