Abstract
This case series study provides a national estimate of opioid and benzodiazepine coprescribing before and after the US Food and Drug Administration (FDA) boxed warning.
Overdose deaths involving prescription opioids have increased 5-fold in the United States since 1999.1 Benzodiazepines are frequently involved in opioid-related overdoses. Concomitant use of benzodiazepines and opioids increases the risk of overdose and death.2 In response to rising coprescriptions,3 the US Food and Drug Administration issued a boxed warning on August 31, 2016, highlighting the risks of coprescribing opioids and benzodiazepines. Prior research examined trends before the warning3 and found a decrease in opioid prescribing after the March 2016 Centers for Disease Control and Prevention guideline.4 However, it is not known whether coprescriptions further declined significantly after the boxed warning. We provide the first national estimate of opioid and benzodiazepine coprescribing before and after the boxed warning, to our knowledge.
Methods
We analyzed all benzodiazepine and opioid analgesic prescriptions in IQVIA LRx from January 2015 to December 2017. Buprenorphine formulations for opioid addiction were not available. The data contain approximately 90% of retail prescriptions in the United States and are representative of age, sex, and insurance coverage. All data are deidentified and exempt from consent by the institutional review board of Yale University. We adjusted patient counts to reflect the US population based on the estimated coverage of IQVIA LRx, calculated as the volume of opioid prescriptions in IQVIA LRx divided by the total opioid volume reported by Centers for Disease Control and Prevention.5 For each patient, we constructed prescription episodes as starting from the prescription dispense date lasting up to the number of days’ supply. A patient had coprescriptions if there was an overlap of 1 day or longer between their opioid and benzodiazepine prescription episodes within a given month.
We used interrupted time series to estimate the association of the boxed warning with coprescribing. This quasi-experimental design controls for secular trends and tests whether an intervention was associated with a change in the slope. Prais-Winsten regression with the Cochrane-Orcutt transformations was used to account for first-order autocorrelation. To assess the long-term association of the boxed warning with coprescribing, we derived a counterfactual estimate of the expected number of patients with coprescriptions based on the prewarning trend. The counterfactual estimates what would have occurred at the end of the period had the prewarning trend continued unchanged.
Results
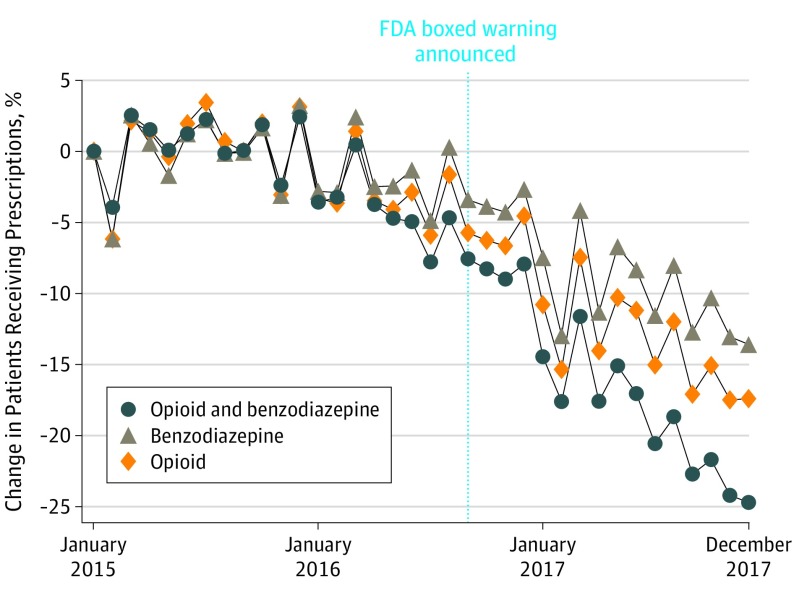
Approximately 67.3 million patients in the United States filled either an opioid or benzodiazepine prescription in 2015. A statistically significant change in the slope occurred after the warning (−18.8; 95% CI, −25.1 to −12.4) (Table). This translates to an absolute drop of approximately 315 900 patients with coprescriptions from September 2016 to December 2017 (95% CI, −419 200 to −212 500), a 17.9% relative reduction (95% CI, −23.7% to −12.1%). The percentage change in patients receiving opioids, patients receiving benzodiazepines, and patients with coprescriptions are presented in the Figure. The monthly means in the percentages of patients with coprescriptions among patients receiving opioids and among patients receiving benzodiazepines both decreased (0.8%; 95% CI, 0.6%-1.0% and 2.95%; 95% CI, 2.3%-3.5%, respectively). Women accounted for a higher absolute decline than men. Stratified analysis showed that the change in slopes was statistically different for men and women. Patients aged 50 to 65 years had the highest absolute declines of approximately −47 000 (95% CI, −62 500 to −31 500) among men and −82 900 (95% CI, −113 200 to −52 500) among women. All findings were robust to tests for higher-order autocorrelations and to analysis excluding the month of the warning announcement.
Table. Absolute and Relative Changes in the Number of Patients With Coprescription of Opioids and Benzodiazepines Before and After the US Food and Drug Administration Boxed Warninga.
| Group | Mean in Thousands (95% CI)b | Slope (95% CI) | Absolute Change in Thousands (95% CI)e | Relative Change (95% CI), %e | |||
|---|---|---|---|---|---|---|---|
| Prewarningc | Postwarningd | Prewarningc | Postwarningd | Change | |||
| Total | 2295.3 (2262.1 to 2328.5) | 1950.3 (1877.0 to 2023.5) | −8.5 (−12.4 to −4.7) | −27.3 (−32.4 to −22.2) | −18.8 (−25.1 to −12.4) | −315.9 (−419.2 to −212.5) | −17.9 (−23.7 to −12.1) |
| Men | 785.4 (773.7 to 797.0) | 661.6 (636.1 to 687.1) | −3.2 (−4.4 to −2.0) | −9.6 (−11.2 to −8.0) | −6.4 (−8.4 to −4.4) | −108.1 (−140.2 to −759.1) | −18.1 (−23.4 to −12.8) |
| Age, y | |||||||
| <18 | 6.3 (6.0 to 6.7) | 4.8 (4.5 to 5.2) | −0.01 (−0.1 to 0) | −0.01 (−0.2 to 0) | −0.01 (−0.01 to 0.1) | −0.9 (−3.0 to 1.2) | −20.7 (−68.0 to 26.5) |
| 18-34 | 60.3 (58.1 to 62.5) | 42.9 (39.9 to 45.8) | −0.01 (−0.2 to 0.1) | −0.01 (−0.2 to 0.1) | −0.01 (−0.2 to 0.2) | −7.0 (−15.2 to 1.1) | −19.9 (−42.4 to 2.7) |
| 35-49 | 175.0 (170.9 to 179.1) | 136.5 (129.3 to 143.8) | −0.7 (−1.0 to −0.4) | −1.0 (−1.4 to −0.7) | −0.3 (−0.8 to 0.2) | −23.9 (−36.8 to −11.0) | −20.3 (−31.1 to −9.5) |
| 50-65 | 338.0 (333.0 to 343.0) | 284.2 (272.9 to 295.5) | −1.2 (−1.7 to −0.6) | −1.9 (−2.5 to −1.2) | −0.7 (−1.6 to −0.2) | −47.0 (−62.5 to −31.5) | −18.4 (−24.4 to −12.4) |
| >65 | 205.7 (203.1 to 208.3) | 193.2 (188.3 to 198.1) | −1.3 (−1.8 to −0.8) | −2.7 (−3.3 to −2.1) | −1.4 (−2.2 to −0.6) | −28.0 (−43.1 to −12.9) | −15.3 (−23.3 to −7.2) |
| Women | 1510.0 (1488.3 to 1531.7) | 1288.7 (1240.9 to 1336.4) | −5.3 (−9.0 to −2.6) | −17.7 (−21.3 −14.1) | −12.4 (−16.8 to −7.9) | −207.7 (−279.9 to −135.5) | −17.8 (−23.9 to −11.7) |
| Age, y | |||||||
| <18 | 7.0 (6.5 to 7.4) | 5.3 (4.9 to 5.8) | −2.1 (−3.1 to −1.1) | −4.7 (−5.9 to −3.5) | −2.6 (−4.3 to −1.0) | −0.9 (−3.6 to 1.8) | −18.0 (−72.2 to 36.3) |
| 18-34 | 111.9 (108.2 to 115.7) | 81.7 (76.4 to 86.9) | −1.4 (−2.0 to −0.8) | −4.2 (−5.0 − 3.5) | −2.8 (−3.8 to −1.9) | −14.2 (−28.9 to 0.4) | −20.7 (−41.8 to 0.3) |
| 35-49 | 329.8 (323.0 to 336.7) | 265.4 (252.6 to 278.2) | −1.9 (−3.0 to −0.7) | −6.9 (−8.3 to −5.4) | −5.0 (−6.9 to −3.1) | −45.2 (−71.7 to −18.8) | −19.4 (−30.6 to −8.2) |
| 50-65 | 586.3 (578.4 to 594.2) | 503.3 (484.7 to 522.0) | 0.2 (−0.3 to 0.8) | −1.5 (−2.2 to −0.8) | −1.8 (−2.7 to −0.8) | −82.9 (−113.2 to −52.5) | −18.1 (−24.7 to −11.6) |
| >65 | 475.0 (470.0 to 480.1) | 432.9 (421.0 to 444.9) | −3.7 (−1.1 to −0.4) | −4.1 (−5.2 to −0.1) | −3.9 (−5.1 to −2.5) | −59.5 (−80.3 to −38.7) | −14.7 (−19.7 to −9.7) |
Total number (in thousands) of patients receiving opioids dropped 1568.9 (95% CI, 1199.1-1937.7), from 14493.7 (95% CI, 14 290.7-14 696.7) to 12 924.8 (95% CI, 12 575.9-13 273.7). Total number (in thousands) of patients receiving benzodiazepines dropped 490.8 (95% CI, 350.4-631.1), from 6317.6 (95% CI, 5240.4-6394.7) to 5826.8 (95% CI, 5694.5-5959.2). The monthly mean of the percentage of patients receiving opioids who were coprescribed benzodiazepines decreased from 15.8% (95% CI, 15.8%-15.9%) before the warning to 15.1% (95% CI, 14.9%-15.3%) after the warning (difference, 0.8%; 95% CI, 0.6%-1.0%). The monthly mean of the percentage of patients receiving benzodiazepines who were coprescribed opioids decreased from 36.3% (95% CI, 36.0%-36.6%) before the warning to 33.4% (95% CI, 32.8%-34.1%) after the warning (difference, 2.95%; 95% CI, 2.3%-3.5%). Data are from IQVIA LRx.
The first 2 columns report monthly means of the number of patients with coprescriptions in the prewarning period and the postwarning period, respectively.
Prewarning period is from January 2015 to August 2016.
Postwarning period is from September 2016 to December 2017.
Absolute and relative changes were calculated in December 2017 based on the difference between the counterfactual estimate derived using the prewarning trend and the linear estimate derived using the observed postwarning trend.
Figure. Percentage Change in US Patients Prescribed Concurrent Opioids and Benzodiazepines, Opioids, and Benzodiazepines.
The vertical dotted line represents the time when the US Food and Drug Administration (FDA) boxed warning was announced. The y-axis reports percentage changes, calculated as Δt = (nt − n1)/n1, where n1 is the count in the first month (January 2016), and nt is the count in month t. Data are from IQVIA LRx. Patient counts were projected to reflect the overall US population. Adjustments implemented with the following steps: (1) We estimated the annual percentage coverages of IQVIA LRx. This was done by dividing the number of opioid prescriptions in IQVIA LRx by the total number of opioid prescriptions reported by Centers for Disease Control and Prevention. The coverages for IQVIA were 86.3%, 86.8%, and 90.5% for 2015, 2016, and 2017, respectively. (2) We also calculated the number of patients with coprescriptions from IQVIA LRx. (3) We projected counts from IQVIA LRx to national estimates by dividing by the percentage coverages.
Discussion
The Food and Drug Administration boxed warning was associated with a modest decline in the number of patients with coprescriptions. The study has several limitations. First, although the March 2016 Centers for Disease Control and Prevention guideline was primarily targeted at opioid prescribing,6 it may have also contributed to some decline in coprescriptions during this period. Second, the accuracy of the national projection cannot be assessed. Third, the IQVIA LRx records dispensed medications rather than medication use. Despite declining rates, a substantial number of US adults, especially women 50 years and older, continue to be coprescribed opioids and benzodiazepines, underscoring the importance of closely monitoring specific patient populations, tapering either or both medications as clinically appropriate, and considering alternative interventions.
References
- 1.HealthData.gov Wide-ranging Online Data for Epidemiologic Research (WONDER). https://healthdata.gov/dataset/wide-ranging-online-data-epidemiologic-research-wonder. Accessed August 7, 2019.
- 2.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert ASB. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002-2014. Am J Prev Med. 2016;51(2):151-160. doi: 10.1016/j.amepre.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 4.Bohnert ASB, Guy GP Jr, Losby JL. Opioid prescribing in the United States before and after the centers for disease control and prevention’s 2016 opioid guideline. Ann Intern Med. 2018;169(6):367-375. doi: 10.7326/M18-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention U.S. Opioid Prescribing Rate Maps. https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html Updated October 3, 2018. Accessed August 7, 2019.
- 6.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain: United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]