Key Points
Question
Do patients with mild echocardiographic pulmonary hypertension have worse right ventricular function and mortality than patients with pulmonary pressures in the normal range?
Findings
In this cohort study of 47 784 patients, those with mild echocardiographic pulmonary hypertension (right ventricular systolic pressure of 33 to 39 mm Hg) had higher mortality, reduced right ventricular function, and impaired right ventricular–pulmonary arterial coupling compared with patients with right ventricular systolic pressure less than 33 mm Hg.
Meaning
In a clinical referral population, mildly elevated pulmonary pressures were associated with adverse right ventricular compensation and increased adjusted mortality.
This cohort study evaluates if mild echocardiographic pulmonary hypertension is associated with reduced right ventricular function and increased risk of mortality.
Abstract
Importance
Current guidelines recommend evaluation for echocardiographically estimated right ventricular systolic pressure (RVSP) greater than 40 mm Hg; however, this threshold does not capture all patients at risk.
Objectives
To determine if mild echocardiographic pulmonary hypertension (ePH) is associated with reduced right ventricular (RV) function and increased risk of mortality.
Design, Setting, and Participants
In this cohort study, electronic health record data of patients who were referred for echocardiography at Vanderbilt University Medical Center, Nashville, Tennessee, from March 1997 to February 2014 and had recorded estimates of RVSP values were studied. Data were analyzed from February 2017 to May 2019.
Exposures
Mild ePH was defined as an RVSP value of 33 to 39 mm Hg. Right ventricular function was assessed using tricuspid annular plane systolic excursion (TAPSE), and RV–pulmonary arterial coupling was measured using the ratio of TAPSE to RVSP.
Main Outcomes and Measures
Associations of mild ePH with mortality adjusted for relevant covariates were examined using Cox proportional hazard models with restricted cubic splines.
Results
Of the 47 784 included patients, 26 758 of 47 771 (56.0%) were female and 6040 of 44 763 (13.5%) were black, and the mean (SD) age was 59 (18) years. Patients with mild ePH had worse RV function compared with those with no ePH (mean [SD] TAPSE, 2.0 [0.6] cm vs 2.2 [0.5] cm; P < .001) and nearly double the prevalence of RV dysfunction (32.6% [92 of 282] vs 16.7% [170 of 1015]; P < .001). Compared with patients with RVSP less than 33 mm Hg, those with mild ePH also had reduced RV–pulmonary arterial coupling (mean [SD] ratio of TAPSE to RVSP, 0.55 [0.18] mm/mm Hg vs 0.93 [0.39] mm/mm Hg; P < .001). An increase in adjusted mortality began at an RVSP value of 27 mm Hg (hazard ratio, 1.32; 95% CI, 1.02-1.70). Female sex was associated with increased mortality risk at any given RVSP value.
Conclusions and Relevance
Mild ePH was associated with RV dysfunction and worse RV–pulmonary arterial coupling in a clinical population seeking care. Future studies are needed to identify patients with mild ePH who are susceptible to adverse outcomes.
Introduction
Pulmonary hypertension (PH) is associated with adverse clinical outcomes regardless of the underlying etiology.1,2,3 Echocardiography permits noninvasive estimation of right ventricular systolic pressure (RVSP) based on the velocity of the tricuspid regurgitant jet (TRV) and is the screening test of choice for PH.4 Consensus guidelines based on expert opinion, not clinical risk, recommend consideration of further evaluation if RVSP is greater than 40 mm Hg or TRV is greater than 2.8 m/s in the setting of unexplained dyspnea or right ventricular (RV) dysfunction.5,6,7
Emerging epidemiologic data suggest that invasively measured mean pulmonary arterial pressures (mPAPs) from 20 to 24 mm Hg are associated with increased risk of clinical events.8,9,10 Existing data of echocardiographic cohorts, although from relatively small or disease-specific cohorts, also suggest that clinical risk is increased at RVSP estimates less than 40 mm Hg.1,8,9,11 A missing piece in the epidemiology of mild or borderline PH is whether modestly elevated pressures are sufficient to cause RV dysfunction or whether the increase in adverse outcomes is solely driven by concomitant comorbid conditions. Understanding the effect of mild PH on RV function is important to clinicians because such knowledge may influence management strategies and identify individuals who may benefit from more aggressive therapy.
We examined the association of all-cause mortality with RVSP estimates in more than 43 000 individuals referred for echocardiography at a tertiary care center. We also examined the association of mildly elevated RVSP values with RV function and noninvasive RV–pulmonary artery (PA) coupling, a measurement that informs how the RV compensates for a given afterload.12,13,14,15,16,17,18 We hypothesized that clinical risk and RV dysfunction develop at RVSP values lower than 40 mm Hg.
Methods
Study Population
The Vanderbilt Institutional Review Board approved this study (IRB No. 140544). We developed a cohort of patients who were referred for echocardiography at Vanderbilt University Medical Center, Nashville, Tennessee, from March 1997 to February 2014 using Synthetic Derivative, Vanderbilt’s deidentified electronic medical record database. Informed consent was waived per institutional policy, as all patients consented to participate in the Synthetic Derivative at the time of consent to treatment. The design, implementation, and content of the Synthetic Derivative have been described previously.19,20,21
Clinical Data
We identified patients referred for transthoracic echocardiography from March 1997 to February 2014 with a recorded RVSP or TRV value. If more than 1 qualifying echocardiogram was available, we analyzed the echocardiogram with the highest RVSP or TRV value to best capture a given patient’s potential for developing PH. Further details of clinical data collection and comorbidity phenotyping are given in the eMethods in the Supplement.
Echocardiographic Data
Echocardiographic data were extracted directly from reports in the Synthetic Derivative, as previously described.21 Study images are not available for review in the Synthetic Derivative; however, echocardiograms were interpreted clinically and reflect the expert opinion of a board-certified cardiologist and echocardiographer based on contemporary guidelines for interpretation. We used the reported RVSP value when available. When only TRV and right atrial pressure (RAP) values were reported, we calculated RVSP using the modified Bernoulli equation (RVSP = 4(TRV)2 + RAP).22 Further details of echocardiographic data collection are given in the eMethods in the Supplement.
Outcomes
Follow-up time for all-cause mortality was calculated from the date of echocardiography. The Synthetic Derivative is linked to the Social Security Administration Death Master File and is updated monthly to ascertain vital status. Further details of outcome are given in the eMethods in the Supplement.
Statistical Analysis
Descriptive statistics of demographic and clinical characteristics were analyzed. Unless otherwise stated, data are expressed as means and standard deviations for continuous variables and as counts and percentages for categorical variables. Comparisons of demographic and clinical characteristics between PH groups were performed using nonparametric Wilcoxon tests for continuous variables and χ2 tests for categorical variables. The reference threshold for group comparisons was an RVSP value of less than 33 mm Hg or a TRV value of less than 2.6 m/s. Cox proportional hazard models were used to examine the associations of RVSP or TRV with mortality. In Cox models, we adjusted for the following potential confounders a priori based on clinical knowledge: age, race, sex, body mass index, hypertension, heart failure (HF) with reduced ejection fraction (EF), HF with preserved EF, left-sided valve disease, left atrial dilation, interstitial lung disease, chronic obstructive pulmonary disease (COPD), connective tissue disease, coronary disease, atrial fibrillation, sleep apnea, and diabetes. We also adjusted by cohort of time in which echocardiography was performed. The nonlinear associations of RVSP or TRV with mortality hazard was assessed by restricted cubic spline with 4 knots. Hazard ratios (HRs) were determined by comparing RVSP or TRV values with reference values of 15 mm Hg for RVSP and 1.9 m/s for TRV based on prior studies examining pulmonary pressure as a continuous variable.8,9 In additional analyses of mortality and RV function, we divided RVSP values into the following groups to correspond with invasive hemodynamic cutoffs: normal (reference group; RVSP value less than 33 mm Hg), mild echocardiographic PH (ePH; RVSP value of 33 to 39 mm Hg), and ePH (RVSP value of 40 mm Hg or greater).8,9,23 These group definitions were influenced by the 2019 sixth World Symposium on Pulmonary Hypertension consensus document24 acknowledging an mPAP of greater than 20 mm Hg as abnormal. An mPAP of 21 to 24 mm Hg on right heart catheterization has been called borderline or mild PH. Using the conversion equation of mPAP = systolic blood pressure × 0.62, these values correspond to an RVSP range of 33 to 39 mm Hg.22,25,26,27 Further details of statistical methods can be found in the eMethods of the Supplement. A 2-sided P value less than .05 was considered statistically significant. All analyses were conducted using R version 3.3.1 (The R Foundation).
Results
Cohort Description and Clinical Characteristics
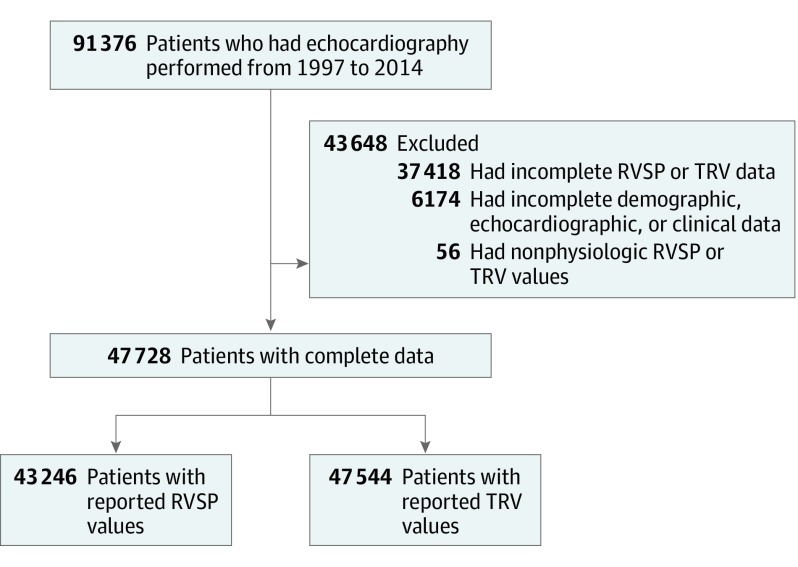
We identified a total of 91 376 patients who had echocardiography performed from March 1997 to February 2004. We excluded 37 418 patients who did not have RVSP or TRV measurements and 56 patients whose values were nonphysiologic. Of the resultant 47 784 patients, there were 43 246 patients who had reported RVSP values and 47 544 who had reported TRV values (Figure 1). There were 7116 of 47 784 patients (14.9%) who had multiple echocardiograms in which the study with the highest RVSP or TRV values was used in lieu of the incident study. The mean (SD) age at the time of echocardiography was 59 (18) years, and 26 758 of 47 771 (56.0%) were female and 6040 of 44 763 (13.5%) were black. The 5 most common indications for echocardiography were coronary artery disease (13.0% [3337 of 25 664]), dyspnea (13.0% [3346 of 25 664]), arrhythmia (11.9% [3046 of 25 664]), HF (9.9% [2535 of 25 664]), and aortic valvular disease (7.4% [1920 of 25 664]) (eTable 1 in the Supplement). The mean (SD) RVSP and TRV values were 35 (15) mm Hg and 2.6 (0.6) m/s, respectively. In total, 8219 patients (19.0%) had RVSP values between 33 and 39 mm Hg, and 11 729 (27.1%) had values of 40 mm Hg or greater (eFigure 1 in the Supplement). When reported by sex, 3634 of 18 796 men (19.3%) and 4585 of 24 438 women (18.8%) had RVSP values between 33 and 39 mm Hg. Compared with the reference group, those with mild ePH were older (mean [SD] age, 63 [16] years vs 55 [18] years; P < .001) and had a higher prevalence of cardiometabolic and pulmonary comorbidities (Table 1). Patients with mild ePH also had evidence of myocardial remodeling on echocardiography (lower left ventricular EF, larger left atrial size), higher prevalence of any grade of diastolic dysfunction, and higher brain-type natriuretic peptide levels. The risk of developing mild ePH was increased in the presence of left atrial enlargement (4.2 cm vs 3.3 cm: odds ratio, 1.94; 95% CI, 1.78-2.11), left-sided valvular disease (odds ratio, 1.29; 95% CI, 1.17-1.41), and diastolic dysfunction (odds ratio, 1.25; 95% CI, 1.05-1.49). The distribution of demographic and clinical characteristics were similar when groups were defined on the basis of TRV values (eTable 2 in the Supplement).
Figure 1. Flowchart of Patients With Echocardiography Measurements of Right Ventricular Systolic Pressure (RVSP) or Tricuspid Regurgitant Velocity (TRV) Included in the Analysis.
Table 1. Demographic, Echocardiographic, and Laboratory Characteristics of Patients With Normal Right Ventricular Systolic Pressure (RVSP), Mild Pulmonary Hypertension, and Pulmonary Hypertension by Echocardiography.
| Characteristic | RVSP, No./Total No. (%) | ||
|---|---|---|---|
| <33 mm Hg (n = 23 299) | 33-39 mm Hg (n = 8219) | ≥40 mm Hg (n = 11 728) | |
| Demographic Characteristics | |||
| Deceased | 901/23 229 (3.9) | 617/8219 (7.5)a | 1582/11 728 (13.5) |
| Age, mean (SD), y | 55 (18) | 63 (16)a | 65 (16) |
| Sexa | |||
| Male | 9960/23 291 (42.7) | 3634/8219 (44.3) | 5202/11 724 (44.3) |
| Female | 13 331/23 291 (57.2) | 4585/8219 (55.8) | 6522/11 724 (55.6) |
| Racea | |||
| White | 18 298/21 791 (83.9) | 6526/7727 (84.5) | 8934/11 724 (76.2) |
| Black | 2708/21 791 (12.4) | 998/7727 (12.9) | 1822/11 724 (15.5) |
| Other | 785/21 791 (3.6) | 203/7727 (2.6) | 220/11 724 (1.9) |
| BMI, mean (SD)b | 28.5 (6.9) | 29.1 (7.6)a | 28.9 (7.9) |
| RVSP, mean (SD), mm Hg | 26 (5) | 36 (2)a | 54 (15) |
| TRV, mean (SD), m/s | 2.2 (0.3) | 2.7 (0.2)a | 3.3 (0.5) |
| Comorbidity | |||
| Hypertension | 15 688/23 106 (67.9) | 6436/8123 (79.2)a | 9262/11 425 (81.1) |
| COPD | 2326/23 299 (9.9) | 1365/8219 (16.6)a | 2591/11 728 (22.1) |
| CAD | 8153/23 299 (34.9) | 3879/8219 (47.2)a | 6193/11 728 (52.8) |
| Atrial fibrillation | 4978/23 299 (21.4) | 2692/8219 (32.8)a | 4792/11 728 (40.9) |
| Sleep apnea | 2708/23 299 (11.6) | 1053/8219 (12.8)a | 1462/11 728 (12.5) |
| HF | 3182/23 299 (13.6) | 1992/8219 (24.2)a | 4500/11 728 (38.4) |
| LVEF, %a | |||
| <40 | 884/3089 (28.6) | 573/1930 (29.7) | 1440/4321 (33.3) |
| 40-50 | 443/3089 (14.3) | 324/1930 (16.8) | 616/4321 (14.3) |
| >50 | 1762/3089 (57.0) | 1033/1930 (53.5) | 2265/4321 (52.4) |
| HF with reduced EF | 1208/23 206 (5.2) | 810/8157 (9.9)a | 1903/11 549 (16.5) |
| HF with preserved EF | 506/23 279 (2.2) | 271/8200 (3.3)a | 567/11 691 (4.8) |
| Left-sided valvular disease | 1942/22 448 (8.6) | 1253/7774 (16.1)a | 3087/11 728 (26.3) |
| Diabetes | 7304/23 299 (31.3) | 3047/8219 (37.1)a | 4769/11 728 (40.7) |
| Systemic sclerosis | 151/23 299 (0.6) | 62/8219 (0.8) | 212/11 728 (1.8) |
| Echocardiographic Parameters | |||
| LVEF, mean (SD), % | 55 (9) | 53 (12)a | 49 (15) |
| Mitral inflow E/a ratio, mean (SD) | 1.2 (0.6) | 1.2 (0.9) | 1.4 (0.8) |
| Left atrial diameter, mean (SD), mm | 37 (7) | 40 (8)a | 43 (9) |
| Left atrial volume index, mean (SD), mL/m2 | 25 (10) | 32 (19)a | 41 (18) |
| Diameter, mean (SD), mm | |||
| Interventricular septal | 12 (7) | 13 (8)a | 13 (7) |
| Left ventricular posterior wall | 10 (2) | 11 (2)a | 11 (2) |
| RV end diastolic diameter, mean (SD), cm | 2.9 (0.8) | 3.0 (0.6)a | 3.2 (0.7) |
| TAPSE, mean (SD), cm | 2.2 (0.5) | 2.0 (0.6)a | 1.8 (0.6) |
| Diastolic dysfunctiona | |||
| Grade 1 | 3682/4280 (86.0) | 1199/1606 (74.7) | 1035/2134 (48.5) |
| Grade 2 | 520/4280 (12.1) | 302/1606 (18.8) | 604/2134 (28.3) |
| Grade 3 | 78/4280 (1.8) | 105/1606 (6.5) | 495/2134 (23.2) |
| RV size (qualitative)a | |||
| Normal | 12 686/13 415 (94.6) | 3958/4408 (89.8) | 4410/5913 (74.6) |
| Mildly dilated | 602/13 415 (4.5) | 367/4408 (8.3) | 980/5913 (16.6) |
| Moderately dilated | 107/13 415 (0.8) | 77/4408 (1.7) | 404/5913 (6.8) |
| Severely dilated | 20/13 415 (0.1) | 6/4408 (0.1) | 119/5913 (2.0) |
| RV function (qualitative)a | |||
| Normal | 12 798/13 287 (96.3) | 4053/4335 (93.4) | 4635/5842 (79.3) |
| Mildly reduced | 283/13 287 (2.1) | 154/4335 (3.6) | 616/5842 (10.5) |
| Moderately reduced | 167/13 287 (1.3) | 104/4335 (2.4) | 423/5842 (7.2) |
| Severely reduced | 39/13 287 (0.3) | 25/4335 (0.6) | 168/5842 (2.9) |
| Laboratory Values | |||
| BNP, median (IQR), pg/mL (n = 15 082) | 140 (49-421) | 234 (87-633)a | 386 (158-934) |
| Creatinine, mean (SD), mg/dL (n = 40 846) | 1.1 (1.1) | 1.3 (1.2)a | 1.6 (1.6) |
| CRP, median (IQR), mg/L (n = 10 352) | 8 (2-51) | 17 (4-88)a | 25 (5-101) |
| eGFR, mean (SD), mL/min/1.73 m2 (n = 40 784) | 84 (39) | 75 (38)a | 68 (39) |
| Hemoglobin A1c, mean (SD), % (n = 18 148) | 6.2 (1.5) | 6.3 (1.4)a | 6.4 (1.5) |
| HDL, mean (SD), mg/dL (n = 24 542) | 50 (18) | 48 (19)a | 45 (18) |
| LDL, mean (SD), mg/dL (n = 23 988) | 101 (37) | 95 (38)a | 89 (39) |
| Triglycerides, mean (SD), mg/dL (n = 25 366) | 144 (116) | 146 (138) | 145 (117) |
| Hemoglobin, mean (SD), g/dL (n = 40 002) | 12.7 (2) | 12.1 (2)a | 11.7 (2) |
| Medications | |||
| β-Blocker | 13 999/23 299 (60.0) | 5966/8219 (72.5)a | 8580/11 728 (73.2) |
| ACE inhibitor | 11 022/23 299 (47.3) | 4685/8219 (57.0)a | 7010/11 728 (59.8) |
| CCB | 10 386/23 299 (44.6) | 4508/8219 (54.8)a | 6540/11 728 (55.8) |
| ARB | 6097/23 299 (26.2) | 2533/8219 (30.8)a | 3370/11 728 (28.7) |
| Loop diuretic | 10 476/23 299 (44.9) | 5257/8219 (63.9)a | 8876/11 728 (75.7) |
| Nonloop diuretic | 8611/23 299 (36.9) | 3651/8219 (44.4)a | 5184/11 728 (44.2) |
| Mineralocorticoid antagonist | 3317/23 299 (14.2) | 1611/8219 (19.6)a | 2993/11 728 (25.5) |
| α-Blocker | 1074/23 299 (4.6) | 519/8219 (6.3)a | 697/11 728 (5.9) |
| α2 Agonist | 3066/23 299 (13.2) | 1439/8219 (17.5)a | 2135/11 728 (18.2) |
| Nitrates | 7175/23 299 (30.8) | 3171/8219 (38.5)a | 5114/11 728 (43.6) |
| Statins | 11 830/23 299 (50.8) | 4818/8219 (58.7)a | 6413/11 728 (54.7) |
| Nicotinic acid | 1080/23 299 (4.6) | 412/8219 (5.0)a | 452/11 728 (3.9) |
| Fibrates | 4988/23 299 (21.4) | 2199/8219 (26.8)a | 3070/11 728 (26.2) |
| Oral anticoagulant | 6229/23 299 (26.7) | 3032/8219 (36.9)a | 5335/11 728 (45.5) |
| Subcutaneous anticoagulant | 5056/23 299 (21.7) | 2277/8219 (27.7)a | 3560/11 728 (30.3) |
| Any PAH medication | 10 271/23 299 (44.1) | 4458/8219 (54.2)a | 6791/11 728 (57.9) |
| PAH CCB | 9966/23 299 (42.8) | 4343/8219 (52.8)a | 6317/11 728 (53.9) |
| Prostacyclin | 293/23 299 (1.3) | 175/8219 (2.1)a | 825/11 728 (7.0) |
| Endothelin receptor blocker | 48/23 299 (0.2) | 23/8219 (0.3)a | 337/11 728 (2.8) |
| Phosphodiesterase 5 inhibitor | 644/23 299 (2.8) | 247/8219 (3.0) | 734/11 728 (6.3) |
| Guanylate cyclase stimulator | 0 | 0 | 5/11 728 (<0.1) |
| Metformin | 3590/23 299 (15.4) | 1403/8219 (17.1)a | 1789/11 728 (15.3) |
| Insulin | 7631/23 299 (32.8) | 3533/8219 (42.9)a | 5716/11 728 (48.7) |
| Sulfonylurea | 2140/23 299 (9.2) | 1031/8219 (12.5)a | 1587/11 728 (13.5) |
| Thiazolidinedione | 885/23 299 (3.8) | 465/8219 (5.7)a | 697/11 728 (5.9) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain-type natriuretic peptide; CAD, coronary artery disease; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HF, heart failure; IQR, interquartile range; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; PAH, pulmonary arterial hypertension; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TRV, tricuspid regurgitant velocity.
SI conversion factor: To convert to BNP to nanograms per liter, multiply by 1; creatinine to micromoles per liter, multiply by 88.4; CRP to nanomoles per liter, multiply by 9.524; HDL to millimoles per liter, multiply by 0.0259; LDL to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113; hemoglobin to grams per liter, multiply by 10.
P < .05 comparing RVSP of 33 to 39 mm Hg with RVSP less than 33 mm Hg.
Calculated as weight in kilograms divided by height in meters squared.
RV Function
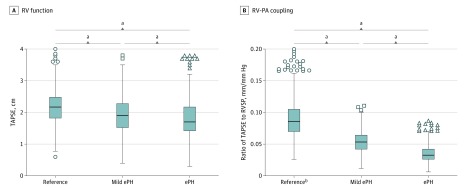
Among 1994 patients with a tricuspid annular plane systolic excursion (TAPSE) measurement, mild ePH was associated with significantly reduced RV function compared with the reference group as a continuous measure (mean [SD] TAPSE, 2.0 [0.6] cm vs 2.2 [0.5] cm; P < .001) (Figure 2A; eFigure 2 in the Supplement). When dichotomized into normal and abnormal function, the prevalence of RV dysfunction (defined as TAPSE <1.7 cm) was nearly double in the mild ePH group vs the reference group (32.6% [92 of 282] vs 16.7% [170 of 1015]; P < .001). In the total cohort, we found that higher RVSP values were significantly associated with lower TAPSE values (χ2 = 160; P < .001) (eFigure 3 in the Supplement). Compared with the reference group, patients with mild ePH had a higher prevalence of RV dilation both qualitatively (mild or greater dilation, 10.2% [450 of 4408] vs 5.4% [729 of 13 415]; P < .001) and quantitatively (mean [SD] RV end diastolic diameter, 3.0 [0.6] vs 2.9 [0.8]; P < .001). Right ventricular end diastolic diameter was largest (mean [SD] diameter, 3.2 [0.7] cm) and TAPSE values were lowest (mean [SD] excursion, 1.8 [0.6] cm) in those with RVSP values of 40 mm Hg or greater. Patients with mild ePH exhibited evidence of impaired RV-PA coupling compared with the reference group (mean [SD] ratio of TAPSE to RVSP, 0.55 [0.18] mm/mm Hg vs 0.93 [0.39] mm/mm Hg; P < .001) (Figure 2B; eFigure 2 in the Supplement). Using a previously published cutoff value of 0.36 mm/mm Hg for an abnormal ratio of TAPSE to RVSP,28 the prevalence of impaired RV-PA coupling in the mild ePH group was 12.8% (36 of 282) compared with 1.7% (17 of 1015) in the reference group (P < .001). Coupling was most impaired in patients with an RVSP value of 40 mm Hg or greater (mean [SD] ratio of TAPSE to RVSP, 0.35 [0.14] mm/mm Hg).
Figure 2. Whisker Plots of Right Ventricular (RV) Function and Right Ventricular–Pulmonary Artery (RV-PA) Coupling Stratified by Degree of Echocardiographic Pulmonary Hypertension (ePH) Estimated by Right Ventricular Systolic Pressure (RVSP).
A, Right ventricular function as measured by tricuspid annular plane systolic excursion (TAPSE) was significantly lower in patients with mild ePH and ePH compared with the reference group. B, Right ventricular–pulmonary artery coupling as measured by the ratio of TAPSE to RVSP worsened with increasing pulmonary pressure. Boxes indicate the interquartile range; bisecting line, median; and error bars, 76.5th and 23.5th percentiles.
aP < .001.
bTwenty-one values fall outside the 76.5th percentile in the reference group and are not included.
Outcomes
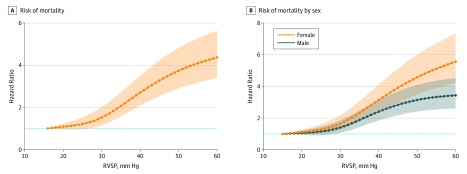
A total of 3492 deaths occurred over a median (interquartile range) duration of 6.8 (4.7-11.6) years. Adjusted risk of all-cause mortality was more than 50% higher in the mild ePH group than the reference group when the model was not adjusted for HF with reduced EF, HF with preserved EF, and left-sided valve disease (HR, 1.50; 95% CI, 1.39-1.74; P < .001). After further adjusting for prevalent HF with reduced EF, HF with preserved EF, and left-sided valvular disease, the association of mild ePH with all-cause mortality persisted (HR, 1.65; 95% CI, 1.46-1.86; P < .001). Compared with an RVSP value of 15 mm Hg and a TRV value of 1.9 m/s, increased risk of mortality began at an RVSP value of 27 mm Hg (HR, 1.32; 95% CI, 1.02-1.70) and a TRV value of 2.3 m/s (HR, 1.14; 95% CI, 1.02-1.27), respectively, after adjusting for relevant clinical covariates (Figure 3A; eFigure 4 and eTable 3 in the Supplement). Additionally, mortality risk was doubled at an RVSP value of 35 mm Hg (HR, 2.08; 95% CI, 1.59-2.72) and a TRV value of 2.8 m/s (HR, 2.08; 95% CI, 1.83-2.37). There was no significant difference in mortality in patients with mild ePH stratified by normal vs impaired RV function by TAPSE (χ2 = 2; P = .15).
Figure 3. Adjusted Risk of Mortality by Right Ventricular Systolic Pressure (RVSP).
A, Mortality increased significantly as RVSP increased. B, Women had a higher risk of mortality compared with men at any given RVSP value (P < .001). The reference value was an RVSP value of 15 mm Hg. See eTables 1 and 2 in the Supplement for hazard ratios. Hazard ratios were adjusted for the following potential confounders a priori based on clinical knowledge: age, race, sex, body mass index, hypertension, heart failure with reduced ejection fraction, heart failure with preserved ejection fraction, left-sided valve disease, left atrial dilation, interstitial lung disease, chronic obstructive pulmonary disease, connective tissue disease, coronary disease, atrial fibrillation, sleep apnea, and diabetes as well as by cohort of time in which the echocardiography was performed. The shaded regions indicate 95% CIs.
In prespecified subgroup analyses, we compared patients with known or suspected PH risk factors and mild ePH with those with RVSP values less than 33 mm Hg. We observed a significant increase in mortality among patients with HF or COPD with mild ePH vs those with normal RVSP values (Table 2). Black patients with mild ePH had higher adjusted mortality compared with those with normal RVSP values (HR, 1.81; 95% CI, 1.29-2.54). Female sex was associated with higher adjusted mortality at any given RVSP or TRV value (Figure 3B; eFigure 4 and eTable 4 in the Supplement). When analyzed as a group, female patients with mild ePH had higher adjusted all-cause mortality compared with male patients (female: HR, 1.78; 95% CI, 1.49-2.13; male: HR, 1.55; 95% CI, 1.32-1.82; P < .001).
Table 2. Association of Mild Echocardiographic Pulmonary Hypertension With Mortality in Patients With Specific Pulmonary Hypertension Risk Factors.
| Variable | RVSP, HR (95% CI) | |
|---|---|---|
| 33-39 mm Hg | ≥40 mm Hg | |
| Heart failure | 1.27 (1.06-1.54)a | 1.91 (1.64-2.23)a |
| COPD | 1.47 (1.11-1.95)a | 2.12 (1.66-2.71)a |
| OSA | 1.17 (0.84-1.64) | 1.87 (1.39-2.51)a |
| Systemic sclerosis | 0.73 (0.06-8.66) | 11.79 (2.67-61.37) |
| Black race | 1.81 (1.29-2.54)a | 2.87 (2.14-3.84)a |
Abbreviations: COPD, chronic obstructive pulmonary disease; HR, hazard ratio; OSA, obstructive sleep apnea; RVSP, right ventricular systolic pressure.
P < .05.
Discussion
In this clinical cohort study of more than 43 000 patients referred for echocardiography, we found that the adjusted risk of mortality began at RVSP values less than 40 mm Hg. The adjusted hazard for mortality was increased by 65% in patients with RVSP values between 33 and 39 mm Hg and nearly doubled at RVSP values of 35 mm Hg compared with a reference value of 15 mm Hg. Parallel analyses in a cohort with TRV values yielded similar results. By convention, the upper limit of normal has been defined as 2 SDs above the mean (or greater than the 95th percentile); in our study, this equates to an RVSP value of 65 mm Hg and a TRV value of 3.8 m/s, missing an at-risk population. Moreover, RVSP values that are currently considered normal (those between 33 and 39 mm Hg) are associated with RV dysfunction and dilation on a population level. These findings are important for patients and their clinicians because they suggest that the current echocardiographic thresholds for defining PH and triggering further evaluation do not adequately capture clinical risk related to rising RVSP values. To our knowledge, no prior studies have examined the association of mild PH with quantitative measures of RV function or RV-PA coupling in a racially mixed referral population.
The current recommended threshold for prompting further evaluation for PH is an RVSP value of 40 mm Hg, which approximates an mPAP of 25 mm Hg. This recommendation is based on expert consensus opinion. Emerging epidemiologic data consistently demonstrate a strong association of mortality with invasively measured pulmonary pressure values less than 25 mm Hg.8,9,10,29 Given the weight of this evidence from invasive studies, PH was recently suggested to be defined as an mPAP greater than 20 mm Hg.24 The goal of our study was to understand the association of corresponding noninvasive estimates with outcomes because most patients at risk of PH (eg, with HF and COPD) are never referred for invasive measurements. Prior studies have made similar observations using echocardiographic estimates of pulmonary pressure in populations at high risk of PH.23,30,31,32 Important strengths of our study include the large sample size, racially diverse cohort representative of the United States, unselected population, and density of clinical and laboratory data, which are lacking from similar published cohorts.33,34,35 Unlike prior studies, we specifically analyzed RVSP as a continuous variable to determine at what pressure clinical risk emerges.31 When patients had multiple studies with RVSP or TRV values, we chose the highest reported value under the presumption that this would be the point in time that represented a given patient’s highest risk. In 2018, Marra et al36 found a 95% upper limit of 2.55 m/s for TRV in healthy participants. This corresponds to an RVSP value of approximately 29 mm Hg in the setting of normal RAP, which is very similar to the threshold we identified at which adjusted mortality risk begins (27 mm Hg). Thus, the threshold we identified is consistent with normative data on the upper limit of normal TRV values identified by Marra et al.36 More recently, Strange et al37 found that an RVSP threshold of 30 mm Hg is associated with increased risk of mortality, which is also largely consistent with our results. Our work adds to these findings by examining quantitative RV function and RV-PA coupling, including additional adjustments for established PH risk factors, and by examining risk in a racially diverse population. The latter point is particularly important given our finding of increased risk of mortality among black individuals with mild ePH. We have identified substantial risk at RVSP values that have historically been considered normal or not of clinical consequence.
We observed an increased risk of mortality among both women and black individuals with mild ePH. The findings with respect to sex are consistent with our prior report of an unselected population referred for invasive catheterization but contrasts with the epidemiology of pulmonary arterial hypertension in which men have increased mortality.8,38 Our population differs substantially from the pulmonary arterial hypertension population in which men present later and with poor RV compensation and women respond better to vasodilator therapy. We have previously observed an increased risk of both PH and PH-related mortality among black individuals.8,39,40 These findings do not appear to be fully driven by differences in comorbidity burden or treatment intensity, which raises the possibility of a molecular or genetic predisposition to develop pulmonary vascular disease.8,39
Echocardiographic estimation of pulmonary pressure is recommended as a screening tool for patients at risk of PH but correlates imperfectly with invasive measurements.22,41 Despite the relative imprecision of echocardiographic estimates, clinicians use these values to inform management decisions in clinical practice and help to guide therapy.42,43 We urge that clinicians continue to interpret echocardiographic estimates with appropriate caution. However, based on our results, we emphasize that values greater than 27 mm Hg may not be benign and, at minimum, warrant consideration for further evaluation.
Prior population-based studies of echocardiographic pulmonary pressure estimates have not examined the association of mildly elevated RVSP values with RV function, to our knowledge. Our findings suggest that even modest elevations in pulmonary pressure are sufficient to adversely affect RV size and function. We found reduced RV function, near doubling in the prevalence of RV dysfunction, and evidence of impaired RV-PA coupling among patients with mild ePH compared with those with lower values. Although the definitive assessment of RV-PA coupling generally uses invasive measurements,44 the noninvasive metric of TAPSE to RVSP ratio approximates the relationship between function and pressure and has strong prognostic value in a variety of clinical populations.13,28,45 Our findings are consistent with a report from Lamia et al46 in which RV dyssynchrony was prolonged among 13 patients with mPAP values between 20 and 25 mm Hg, suggesting early RV-PA uncoupling. These observations suggest that the increased risk of clinical events among patients with mild PH is not driven solely by an increased burden of comorbidities but rather a pathologic response of the RV to increase pulmonary pressures.
One inference from these data is that using an RVSP threshold of 40 mm Hg to prompt further clinical evaluation may identify patients with relatively advanced disease. The value of any screening test is the detection of incipient disease at a stage where early treatment could be more effective.47,48 Our data suggest that mild ePH is not benign and may be a reasonable therapeutic target, particularly among some high-risk groups. Such a finding should prompt clinicians to seek an underlying cause, which most commonly would involve investigation for HF with preserved EF, obstructive sleep apnea, or COPD. Importantly, our findings do not support initiation of pulmonary vasodilators in patients with mild ePH. Future studies should focus on identifying patient subgroups who may benefit from invasive hemodynamic measurements and aggressive risk factor management. Future studies need to examine whether behavioral interventions or tight management of underlying comorbidities have an effect on outcomes among patients with mild ePH. We do not interpret our findings to suggest that all patients with mild ePH should be referred for invasive catheterization; we simply wish to raise awareness among clinicians that identification of mild ePH may warrant more aggressive management. Regardless, the finding that patients have increased mortality starting at an RVSP value of 27 mm Hg is striking. Although we do not have cause of death data, it is unlikely that patients die specifically from mildly elevated pulmonary pressures. Rather, mild ePH is a marker of prognosis that is likely driven by a higher burden of comorbidities.
Limitations
Our study has limitations. Our study reports outcomes in a population of patients referred for echocardiography, which may not be generalizable to the community. By virtue of our deidentified electronic health record interface, we were unable to directly review echocardiographic images related to pressure estimates or RV function. As such, we relied on accurate data input by physicians into echocardiographic reports and accurate coding of diagnoses. However, we used the same definitions to capture comorbidities that are used by other electronic health record–based cohorts (eg, the Precision Medicine Initiative and the Million Veterans Project). Another limitation of electronic health record cohorts is nonrandom missing data because the available clinical data reflect information specific to an individual’s medical history. Moreover, the use of International Classification of Diseases–based comorbidity definitions did not allow us to account for comorbidity severity. Echocardiographic estimates of pulmonary pressure correlate imperfectly with invasive measurements, particularly at very high pressures.49,50 As a result, some patients in our cohort will have overestimated or underestimated pressure estimates, resulting in misclassification. However, the size of our cohort likely reduces noise related to imprecise estimates. Additionally, given we are using echocardiographic data, we cannot confidently differentiate between precapillary, postcapillary, and mixed PH. Although imperfect, echocardiographic RVSP estimates are clinically valuable because they are used as a screening tool, and only a minority of individuals with ePH undergo criterion-standard assessment by right heart catheterization. Despite these limitations, clinicians routinely make management decisions based on echocardiographic estimates of RVSP. Our assessment of RV function was limited to a single metric (TAPSE). Tricuspid annular plane systolic excursion is easy to measure and highly reproducible but only reports on RV function at the base, which may overestimate or underestimate global RV function. We determined vital status using the Social Security Administration Death Master File, which does not report the cause of death and may substantially underestimate the number of deaths owing to reporting delays and underreporting.51
Conclusions
The results of this study suggest that the current thresholds for defining ePH do not adequately capture risk of mortality and RV dysfunction related to RVSP. These findings should prompt reconsideration of RVSP values at which clinicians should consider clinical action and motivate future studies to determine whether more aggressive management of risk factors in individuals with mildly elevated RVSP values (and corresponding TRV values) reduces risk of clinical outcomes.
eMethods.
eTable 1. Most frequent indications for echocardiography.
eTable 2. Demographic, echocardiographic, and laboratory differences between patients with normal, mild ePH, and ePH by TRV.
eTable 3. Adjusted risk of mortality according to RVSP and TRV extracted from Figure 4A and eFigure 3A in the Supplement.
eTable 4. Adjusted risk of mortality according to RVSP and TRV stratified by sex extracted from Figure 4B and eFigure 3B in the Supplement.
eFigure 1. Frequency distribution of RVSP and TRV values reported.
eFigure 2. Whisker plots of RV function by TAPSE and RV-PA coupling by TAPSE/RVSP ratio stratified by degree of PH by TRV.
eFigure 3. Relationship between TAPSE and RVSP.
eFigure 4. Adjusted risk of Mortality by TRV and stratified by sex.
eReferences.
References
- 1.Abramson SV, Burke JF, Kelly JJ Jr, et al. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med. 1992;116(11):888-895. doi: 10.7326/0003-4819-116-11-888 [DOI] [PubMed] [Google Scholar]
- 2.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy: importance of pulmonary artery pressure. Chest. 1995;107(5):1193-1198. doi: 10.1378/chest.107.5.1193 [DOI] [PubMed] [Google Scholar]
- 3.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31(4):373-380. doi: 10.1016/j.healun.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Archer SL, Badesch DB, et al. ; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573-1619. doi: 10.1016/j.jacc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Hoeper MM, Humbert M, et al. ; ESC Committee for Practice Guidelines (CPG) . Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537. doi: 10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903-975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Archer SL, Badesch DB, et al. ; ACCF/AHA . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294. doi: 10.1161/CIRCULATIONAHA.109.192230 [DOI] [PubMed] [Google Scholar]
- 8.Assad TR, Maron BA, Robbins IM, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol. 2017;2(12):1361-1368. doi: 10.1001/jamacardio.2017.3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking program. Circulation. 2016;133(13):1240-1248. doi: 10.1161/CIRCULATIONAHA.115.020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197(4):509-516. doi: 10.1164/rccm.201706-1215OC [DOI] [PubMed] [Google Scholar]
- 11.Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99(8):1146-1150. doi: 10.1016/j.amjcard.2006.11.052 [DOI] [PubMed] [Google Scholar]
- 12.Tello K, Axmann J, Ghofrani HA, et al. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol. 2018;266:229-235. doi: 10.1016/j.ijcard.2018.01.053 [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M, Dixon D, Labate V, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10(10, pt B):1211-1221. doi: 10.1016/j.jcmg.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 14.Hussain I, Mohammed SF, Forfia PR, et al. Impaired right ventricular-pulmonary arterial coupling and effect of sildenafil in heart failure with preserved ejection fraction: an ancillary analysis from the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX) trial. Circ Heart Fail. 2016;9(4):e002729. doi: 10.1161/CIRCHEARTFAILURE.115.002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sultan I, Cardounel A, Abdelkarim I, et al. Right ventricle to pulmonary artery coupling in patients undergoing transcatheter aortic valve implantation. Heart. 2019;105(2):117-121. doi: 10.1136/heartjnl-2018-313385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prins KW, Archer SL, Pritzker M, et al. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37(3):376-384. doi: 10.1016/j.healun.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerges M, Gerges C, Pistritto AM, et al. Pulmonary hypertension in heart failure. epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192(10):1234-1246. doi: 10.1164/rccm.201503-0529OC [DOI] [PubMed] [Google Scholar]
- 18.Guazzi M, Naeije R, Arena R, et al. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest. 2015;148(1):226-234. doi: 10.1378/chest.14-2065 [DOI] [PubMed] [Google Scholar]
- 19.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362-369. doi: 10.1038/clpt.2008.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulley J, Clayton E, Bernard GR, Roden DM, Masys DR. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3(1):42-48. doi: 10.1111/j.1752-8062.2010.00175.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells QS, Farber-Eger E, Crawford DC. Extraction of echocardiographic data from the electronic medical record is a rapid and efficient method for study of cardiac structure and function. J Clin Bioinforma. 2014;4:12. doi: 10.1186/2043-9113-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685-713, 786-788. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 23.Brittain EL, Duncan MS, Chang J, et al. Increased echocardiographic pulmonary pressure in HIV-infected and -uninfected individuals in the Veterans Aging Cohort study. Am J Respir Crit Care Med. 2018;197(7):923-932. doi: 10.1164/rccm.201708-1555OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemla D, Castelain V, Humbert M, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126(4):1313-1317. doi: 10.1378/chest.126.4.1313 [DOI] [PubMed] [Google Scholar]
- 26.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1, suppl):S55-S66. doi: 10.1016/j.jacc.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 27.Heresi GA, Minai OA, Tonelli AR, et al. Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ. 2013;3(4):916-925. doi: 10.1086/674756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305(9):H1373-H1381. doi: 10.1152/ajpheart.00157.2013 [DOI] [PubMed] [Google Scholar]
- 29.Kolte D, Lakshmanan S, Jankowich MD, Brittain EL, Maron BA, Choudhary G. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(18):e009729. doi: 10.1161/JAHA.118.009729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886-895. doi: 10.1056/NEJMoa035477 [DOI] [PubMed] [Google Scholar]
- 31.Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222-231. doi: 10.1016/j.jacc.2011.06.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szwejkowski BR, Elder DH, Shearer F, et al. Pulmonary hypertension predicts all-cause mortality in patients with heart failure: a retrospective cohort study. Eur J Heart Fail. 2012;14(2):162-167. doi: 10.1093/eurjhf/hfr159 [DOI] [PubMed] [Google Scholar]
- 33.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119(20):2663-2670. doi: 10.1161/CIRCULATIONAHA.108.838698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119-1126. doi: 10.1016/j.jacc.2008.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.United States Census Bureau Quick facts: race and Hispanic origin. https://www.census.gov/quickfacts/fact/table/US/PST045217. Accessed May 13, 2019.
- 36.Marra AM, Halank M, Benjamin N, et al. Right ventricular size and function under riociguat in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (the RIVER study). Respir Res. 2018;19(1):258. doi: 10.1186/s12931-018-0957-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strange G, Stewart S, Celermajer DS, et al. ; NEDA Contributing Sites . Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol. 2019;73(21):2660-2672. doi: 10.1016/j.jacc.2019.03.482 [DOI] [PubMed] [Google Scholar]
- 38.Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest. 2012;141(2):363-373. doi: 10.1378/chest.10-3114 [DOI] [PubMed] [Google Scholar]
- 39.Assad TR, Hemnes AR, Larkin EK, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68(23):2525-2536. doi: 10.1016/j.jacc.2016.09.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang BQ, Assad TR, O’Leary JM, et al. Racial differences in patients referred for right heart catheterization and risk of pulmonary hypertension. Pulm Circ. 2018;8(2):2045894018764273. doi: 10.1177/2045894018764273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farber HW, Foreman AJ, Miller DP, McGoon MD. REVEAL registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17(2):56-64. doi: 10.1111/j.1751-7133.2010.00202.x [DOI] [PubMed] [Google Scholar]
- 42.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615-621. doi: 10.1164/rccm.200811-1691OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139(5):988-993. doi: 10.1378/chest.10-1269 [DOI] [PubMed] [Google Scholar]
- 44.Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101(1):37-43. doi: 10.1136/heartjnl-2014-306142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.French S, Amsallem M, Ouazani N, et al. Non-invasive right ventricular load adaptability indices in patients with scleroderma-associated pulmonary arterial hypertension. Pulm Circ. 2018;8(3):2045894018788268. doi: 10.1177/2045894018788268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamia B, Muir JF, Molano LC, et al. Altered synchrony of right ventricular contraction in borderline pulmonary hypertension. Int J Cardiovasc Imaging. 2017;33(9):1331-1339. doi: 10.1007/s10554-017-1110-6 [DOI] [PubMed] [Google Scholar]
- 47.Herman C. What makes a screening exam “good”? Virtual Mentor. 2006;8(1):34-37. [DOI] [PubMed] [Google Scholar]
- 48.Herman CR, Gill HK, Eng J, Fajardo LL. Screening for preclinical disease: test and disease characteristics. AJR Am J Roentgenol. 2002;179(4):825-831. doi: 10.2214/ajr.179.4.1790825 [DOI] [PubMed] [Google Scholar]
- 49.Greiner S, Jud A, Aurich M, et al. Reliability of noninvasive assessment of systolic pulmonary artery pressure by Doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J Am Heart Assoc. 2014;3(4):e001103. doi: 10.1161/JAHA.114.001103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97(8):612-622. doi: 10.1136/hrt.2010.212084 [DOI] [PubMed] [Google Scholar]
- 51.Navar AM, Peterson ED, Steen DL, et al. Evaluation of mortality data from the Social Security Administration Death Master File for clinical research. JAMA Cardiol. 2019;4(4):375-379. doi: 10.1001/jamacardio.2019.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Most frequent indications for echocardiography.
eTable 2. Demographic, echocardiographic, and laboratory differences between patients with normal, mild ePH, and ePH by TRV.
eTable 3. Adjusted risk of mortality according to RVSP and TRV extracted from Figure 4A and eFigure 3A in the Supplement.
eTable 4. Adjusted risk of mortality according to RVSP and TRV stratified by sex extracted from Figure 4B and eFigure 3B in the Supplement.
eFigure 1. Frequency distribution of RVSP and TRV values reported.
eFigure 2. Whisker plots of RV function by TAPSE and RV-PA coupling by TAPSE/RVSP ratio stratified by degree of PH by TRV.
eFigure 3. Relationship between TAPSE and RVSP.
eFigure 4. Adjusted risk of Mortality by TRV and stratified by sex.
eReferences.