This case-control study analyzes the association of long-term antibody response to cytomegalovirus, Toxoplasma gondii, and measles and of C-reactive protein level with bipolar disorder.
Key Points
Question
Is bipolar disorder associated with exposure to infectious agents with immune activation?
Findings
In this case-control study of 1952 patient cases, cytomegalovirus IgG was significantly increased and toxoplasma IgG was significantly decreased among patients with bipolar disorder compared with control individuals. Patients with bipolar disorder who received drug treatment with antitoxoplasma activity had significantly lower toxoplasma IgM titers compared with those not receiving this treatment.
Meaning
Increased and decreased long-term antibody response to cytomegalovirus and toxoplasma, respectively, were associated with bipolar disorder in this sample; further work appears to be needed to better understand genetic vs environmental risk of the disease and how infection or immune activation contributes to overall disease pathogenesis with particular reference to disease onset.
Abstract
Importance
Infection-associated immune activation and inflammation are increasingly recognized in the pathophysiology of bipolar disorder.
Objective
To determine whether antibodies to common infectious agents, including cytomegalovirus (CMV), Toxoplasma gondii, and measles, as well as the inflammatory marker C-reactive protein, in serum samples differ between patients with bipolar disorder and control individuals without bipolar disorder.
Design, Setting, and Participants
In this case-control study, antibody titers were measured in serum samples from 1207 patients with bipolar disorder and 745 controls that were obtained from biobanks with participating sites in Rochester and Minneapolis, Minnesota (n = 1537), and Cincinnati, Ohio (n = 415), from January 5, 2009, through May 12, 2014. A subset of case patients and controls from Minnesota were matched by age, sex, and educational level. Bipolar type, age at onset, and history of psychosis were assessed for case patients as well as current drug treatment at the time of blood sample obtainment from the biobank. Data were analyzed from February 5, 2018, to January 4, 2019.
Exposures
The CMV and T gondii antibodies with IgM titers were expressed as z scores and IgG titers dichotomized into seropositive and seronegative based on expected prevalence in the US population and further classified based on the joint CMV-positive/T gondii–negative IgG status, C-reactive protein z score, and drug treatments with antitoxoplasma activity.
Main Outcomes and Measures
Patients were stratified by bipolar disorder type I or type II, nonearly (>19 years of age) and early (≤19 years of age) onset, and history of psychosis during mania or no psychosis.
Results
Of 1207 patients with bipolar disorder (mean [SD] age, 43.2 [15.1] years; 742 [61.5%] female), the CMV-positive/T gondii–negative IgG status was significantly higher (odds ratio [OR], 1.33; 95% CI, 1.09-1.62; P = .004) compared with that in the 745 controls (mean [SD] age, 44.5 [15.5] years; 444 [59.6%] female). The CMV-positive/T gondii–negative IgG status was associated with bipolar cases type I (OR, 1.41; 95% CI, 1.14-1.75; P = .001), nonearly age at onset (OR, 1.41; 95% CI, 1.16-1.72; P = .001), and history of manic psychosis (OR, 1.46; 95% CI, 1.13-1.88; P = .004). Patients with bipolar disorder who received drug treatment with antitoxoplasma activity (n = 272) had significantly lower T gondii IgM titers (median, 1.59; interquartile range, 1.30-2.07) compared with those (n = 900) who did not receive this treatment (median, 1.69; interquartile range, 1.35-2.25) (P = .03).
Conclusions and Relevance
In this sample, increased long-term antibody response to CMV and decreased long-term antibody response to T gondii were associated with bipolar disorder and the subphenotypes of bipolar type I, nonearly disease onset, and manic psychosis. Further work appears to be needed to better understand genetic vs environmental disease risk and infection or immune activation contribution to overall disease pathogenesis with particular reference to disease onset.
Introduction
The stress diathesis model of disease underscores the role of environmental factors or stress contributing to risk of major mental illness.1 Although direct causality has not been established, environmental factors, such as psychological stress and exposure to environmental substances, have been associated with mental illness (ie, substance abuse).2 Environmental exposure–related infections are ubiquitous, and the subsequent immune activation has been implicated in the etiology of major mood disorders and schizophrenia.2
In a landmark, 10-country, cross-national, population-based, epidemiology study, rates of major depressive disorder were variable by site and sex, whereas rates of bipolar disorder were similar across sites and sex.3 These data suggest that cultural or variable risk factors may contribute more to the diagnosis of major depression, whereas genetic risk or biologic factors may contribute more to the diagnosis of bipolar disorder. Case-controlled investigations of environmental infection and associated risk of bipolar disorder have been reported in diverse patient populations in the United States, France, Germany, Saudi Arabia, Denmark (mood disorders), and Ethiopia.4,5,6,7,8,9,10,11,12
Exposure to infectious agents and the associated immune activation underscore conceptualizing bipolar disorder as a multisystem inflammatory disease of the brain and the body.13 The disease risk or biologic mechanism of infectious environmental exposure, early immune activation, acute inflammation, dormant vs viral reactivation, and the consequences of longer-term immunity have yet to be systematically studied to our knowledge. The goal of this investigation was to analyze antibodies to common infectious agents, including cytomegalovirus (CMV), Toxoplasma gondii, and measles, as well as the inflammatory marker C-reactive protein (CRP), in serum samples from patients with bipolar disorder and control individuals.
Methods
Patients
This case-control study used samples from the Mayo Clinic Bipolar Biobank14 and the Mayo Clinic Biobank,15 which were established in 2009. Written informed consent was obtained from study participants for the Mayo Clinic Biobank and the Mayo Clinic Bipolar Bank. Each participating site in the Mayo Clinic Bipolar Biobank (ie, Mayo Clinic, Rochester, Minnesota; Lindner Center of HOPE, University of Cincinnati, Cincinnati, Ohio; and University of Minnesota, Minneapolis) obtained approval from their local institutional review boards. Adults aged 18 to 80 years with bipolar disorder underwent diagnostic confirmation using the Structured Clinical Interview for the DSM-IV-TR.16 Patients who were actively psychotic or actively suicidal were not enrolled. A patient questionnaire was used to obtain demographic characteristics.14 A clinical questionnaire was used to obtain data on illness variables (ie, current medications, additional psychiatric illness, or medical comorbidity). Age at diagnosis was collected using the following ranges: 19 years or younger, 20 to 49 years, 50 to 64 years, and 65 years or older. Only non-Hispanic white patients were included in this study because of the low level of minority population participation in the biobanks.17
Because bipolar disorder is highly heterogeneous, there is interest in developing operationalized criteria of a narrower phenotype that may have greater power to identify disease risk factors.18,19 Based on earlier work, age at illness onset was defined as early onset if the first episode of mania or depression occurred when the patient was 19 years or younger.20,21 For this analysis, age at diagnosis was dichotomized into early onset (≤19 years of age) vs nonearly onset (>19 years of age). This classification was used to examine the association between genetic risk score and early-onset bipolar illness.22 In addition, although history of psychotic mania may be more of a measure of illness severity,23 history of psychotic mania, obtained from the Structured Clinical Interview for DSM-IV or clinical questionnaire, has been used as a phenotype, and bipolar disorder with history of psychotic mania has been reported to be genetically more similar to schizophrenia than to nonpsychotic bipolar type I and type II illness.24
Whole blood samples from patients with bipolar disorder and controls were transported at ambient temperature to the Mayo Clinic Biospecimens Accessioning and Processing Laboratory to obtain serum samples, which were stored at –80 °C. The 749 case samples from Mayo Clinic, 46 case samples from the University of Minnesota, and 415 case samples from Lindner were selected from the Bipolar Disorder Biobank for the study. The 745 control samples from individuals with no International Classification of Diseases, Ninth Revision (ICD-9) or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis codes for bipolar disorder or schizophrenia were selected from the Mayo Clinic Biobank.15 Because viral exposures can depend on age and place of residence, the 745 controls (enrolled in Minnesota) were matched 1:1 by sex, age (within 5 years), and educational level with the subset of case patients recruited in Minnesota. The 37 case patients from Minnesota could not be matched for educational level because of missing educational information. No controls were available from Ohio, and thus, the case patients enrolled in Ohio were not matched with controls.
Serologic Measurements
Immunoassay measurements were performed on aliquots of 200 μL of serum that were shipped to Johns Hopkins University, Baltimore, Maryland, in 2 batches. Case and control serum samples were randomly distributed on twelve 96-well plates. Antibody in the plasma specimen was quantified by the measurement of colorimetric enzyme substrate by using a microplate colorimeter and was converted into a ratio by dividing the amount of color generated in the sample wells by the amount of color generated from reaction with a weakly positive sample provided by the manufacturer (IBL America).
The IgM and IgG class antibodies to CMV and T gondii, the IgG class antibodies to measles, and the level of the inflammatory marker CRP were quantified as a plate-adjusted z score, by which the mean (SD) value of each plate was 2 (1), as previously described.25 A failed antibody assay was encountered for 3 case samples, leaving 1207 case samples for analysis. Density plots of the 2 measurements are shown in the eFigure in the Supplement. The CMV and T gondii IgG measurements showed a bimodal distribution. Therefore, for each plate, a threshold corresponding to quantile of expected seropositive rate in the US population was chosen to dichotomize the variable.26,27 A joint measure of CMV IgG and T gondii IgG titers was created by categorizing patients as either CMV IgG positive and T gondii IgG negative or CMV IgG negative or T gondii IgG positive. The CMV IgM and T gondii IgM titers had a slightly skewed distribution (eFigure in the Supplement), and after a log transformation, the standardized score was approximately normal.
Statistical Analysis
Binary outcomes (CMV IgG, T gondii IgG, and CMV-positive/T gondii-negative titers) were analyzed using logistic regression, and continuous outcomes (log CMV IgM z score, log T gondii IgM z score, measles IgG z score, and CRP z score) were analyzed using linear regression. Because of skewed distributions, CMV IgM and T gondii IgM z scores were log transformed. Comparisons were made between case patients and controls as well as between subsets of case patients defined by subphenotypes (eg, bipolar type, age at onset, and history of psychosis) and controls. Because not all case patients had matching controls, case-control analyses controlled for age, sex, and educational level (dichotomized as a higher-level degree vs high school degree or less) by including these variables as covariates in the models. Because the serology measures were modeled as the outcomes, inclusion of the covariates may improve power by accounting for some of the variation in the outcome variable. Sensitivity analyses were performed by restricting the sample to the matched set (including only case patients and controls from Minnesota) to investigate any potential bias because of geographical differences. In addition, analyses of CRP controlled for body mass index and current smoking status because it has been shown that CRP can be a biomarker for increased adiposity28 and higher CRP levels have also been observed among current smokers.29
Except for CRP, serologic measurements were not significantly different between Ohio and Minnesota, and thus the unmatched and matched analyses produced similar results. Therefore, we present the results based on analyses of all cases (Minnesota and Ohio) in the Results section with the matched analyses in the eTable in the Supplement. Because CRP levels differed between the case patients from Ohio and Minnesota (Table), which was likely because of differences in body mass index between sites, all CRP analyses were restricted to the Minnesota subset to avoid confounding by site.
Table. Demographics and Antibody Quantificationa.
| Characteristic | Controls From Minnesota (n = 745) | Patients From Minnesota and Ohio (n = 1207) | P Value | Patients From Minnesota (n = 792) | Patients From Ohio (n = 415) | P Valueb |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 44.5 (15.5) | 43.2 (15.1) | .09 | 43.9 (15.5) | 42.0 (14.2) | .05 |
| Body mass index, mean (SD)c | 28.9 (6.4) | 30.1 (7.1) | .001 | 29.7 (6.8) | 30.9 (7.6) | .01 |
| Female | 444 (59.6) | 742 (61.5) | .44 | 468 (59.1) | 274 (66.0) | .02 |
| Current smoker | 44 (5.9) | 289 (23.9) | <.001 | 173 (21.8) | 116 (27.9) | .04 |
| Educational level | ||||||
| Missing or other | 7 (0.9) | 42 (3.5) | .02 | 37 (4.7) | 5 (1.2) | .01 |
| Some high school or less | 6 (0.8) | 26 (2.2) | 16 (2.0) | 10 (2.4) | ||
| High school graduate or GED | 86 (11.5) | 146 (12.1) | 94 (11.9) | 52 (12.5) | ||
| Some college or associate degree | 227 (30.5) | 424 (35.1) | 261 (33.0) | 163 (39.3) | ||
| Vocational, technical, or business school | 72 (9.7) | 93 (7.7) | 70 (8.8) | 23 (5.5) | ||
| 4-y College graduate (bachelor's degree) | 191 (25.6) | 274 (22.7) | 167 (21.1) | 107 (25.8) | ||
| Graduate or professional school | 156 (20.9) | 202 (16.7) | 147 (18.6) | 55 (13.2) | ||
| Diagnosis | ||||||
| Bipolar disorder type I or SZA | NA | 843 (69.8) | NA | 532 (67.2) | 311 (74.9) | .02 |
| Bipolar disorder type II | NA | 364 (30.2) | 260 (32.8) | 104 (25.1) | ||
| Early onset | NA | 237 (19.6) | NA | 144 (18.2) | 93 (22.4) | .16 |
| Nonearly onset | NA | 911 (75.5) | 600 (75.8) | 311 (74.9) | ||
| Manic psychosis | NA | 401 (33.2) | NA | 273 (34.5) | 128 (30.8) | .006 |
| No psychosis | NA | 366 (30.3) | 213 (26.9) | 153 (36.9) | ||
| TATA-positive medicationsd | NA | 272 (22.5) | NA | 200 (25.3) | 72 (17.3) | .001 |
| Serologic test resultse | ||||||
| Toxoplasma gondii IgG positive | 100 (13.4) | 113 (9.4) | .01 | 80 (10.1) | 33 (8.0) | .26 |
| CMV IgG positive | 293 (39.3) | 536 (44.4) | .03 | 347 (43.8) | 189 (45.5) | .61 |
| IgM log z score, mean (SD) | ||||||
| T gondii | 0.63 (0.42) | 0.58 (0.39) | .01 | 0.57 (0.40) | 0.60 (0.39) | .30 |
| CMV | 0.62 (0.38) | 0.60 (0.38) | .23 | 0.60 (0.38) | 0.61 (0.38) | .86 |
| Measles IgG z score, mean (SD) | 1.96 (0.99) | 2.02 (1.00) | .16 | 2.0 (0.97) | 2.07 (1.04) | .29 |
| CRP z score, mean (SD) | 1.92 (0.96) | 2.05 (1.01) | .01 | 2.0 (1.02) | 2.14 (1.00) | .02 |
Abbreviations: CMV, cytomegalovirus; CRP, C-reactive protein; GED, general education development; NA, not applicable; SZA, schizoaffective disorder; TATA, treatment with antitoxoplasmic activity.
Data are presented as number (percentage) of patients unless otherwise indicated.
P values were calculated using a t test for continuous and a χ2 test for categorical variables.
Calculated as weight in kilograms divided by height in meters squared.
TATA-positive medications included valproate, haloperidol, risperidone, and paliperidone.
Unadjusted comparison of serologic measurements.
Data on medications at the time of biobank blood sample obtainment were used to conduct an exploratory analysis of mood stabilizers (ie, valproate sodium) or antipsychotics (ie, haloperidol, risperidone, and paliperidone) with known antitoxoplasma activity.30 Because of the skewed distribution of T gondii IgG and IgM, Wilcoxon tests were used to compare the measurements for both groups.
Analyses were performed using R, version 3.2.3 (R Core Team). To control for multiple testing, the significance threshold for case-control comparisons was set to 2-sided P < .01. Listwise deletion was used to address missing data in all analyses. Subphenotype analyses were considered to be exploratory and thus were not further adjusted for multiple testing.
Results
Of 1207 patients with bipolar disorder (mean [SD] age, 43.2 [15.1] years; 742 [61.5%] female), the CMV-positive/T gondii negative IgG status was significantly higher (odds ratio [OR], 1.33; 95% CI, 1.09-1.62; P = .004 compared with that in the 745 controls (mean [SD] age, 44.5 [15.5] years; 444 [59.6%] female). As presented in the Table, patients with bipolar disorder had a significantly higher mean body mass index and a higher rate of smoking. In our sample, 843 case patients (69.8%) had bipolar disorder type 1 and 364 case patients (30.2%) had bipolar disorder type II; 237 case patients (19.6%) met the criteria for the phenotype of early-onset bipolar disorder (≤19 years of age), whereas 911 case patients (75.5%) had non–early-onset bipolar disorder (>19 years of age); 59 case patients were unable to identify their age at illness onset. In the sample, 401 case patients (33.2%) had a history of psychosis during manic episodes and 366 case patients (30.3%) had no history of psychosis; 440 case patients (36.4) had either psychosis not during mania or undetermined history of psychosis.
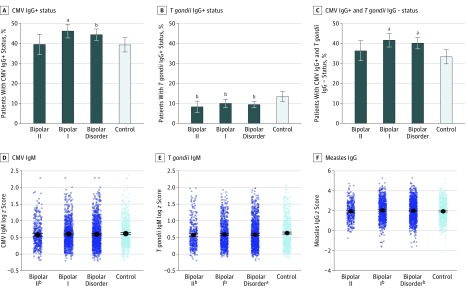
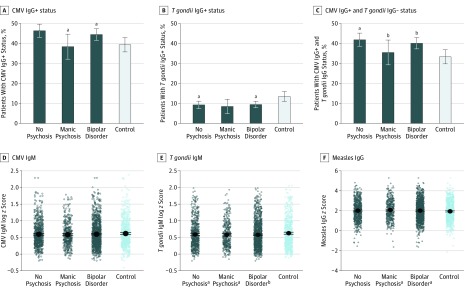
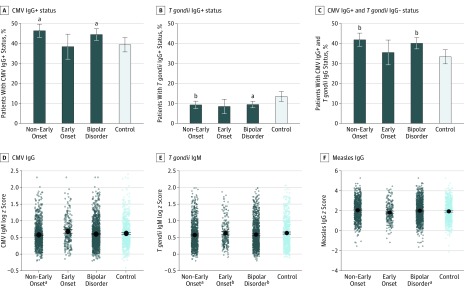
As shown in Figure 1, the seropositive rate among patients with bipolar disorder compared with controls was higher for CMV IgG titers (OR, 1.24; 95% CI, 1.02-1.51; P = .03) and lower for T gondii IgG titers (OR, 0.69; 95% CI, 0.51-0.93; P = .01) after adjusting for age, sex, and educational level. Analyzed jointly, case patients had a significantly higher rate of CMV-positive/T gondii–negative titers compared with controls (OR, 1.33; 95% CI, 1.09-1.62; P = .004). Case patients also had significantly lower T gondii IgM titers compared with controls (estimation, –0.06; SE, 0.02; P = .002) and higher titers of measles IgG (estimation, 0.09; SE, 0.04; P = .05). Similar results were obtained in the matched case-control sample (ie, Minnesota only) (eTable 1 in the Supplement). As presented in Figure 1, Figure 2, and Figure 3, the difference between case patients and controls in the rate of CMV-positive/T gondii–negative titers was associated with the subphenotypes of bipolar disorder type I (OR, 1.41; 95% CI, 1.14-1.75; P = .001), nonearly onset (OR, 1.41; 95% CI, 1.16-1.72; P = .001), and history of manic psychosis (OR, 1.46; 95% CI, 1.13-1.88; P = .004). As presented in eTable 2 in the Supplement, CRP level was not significantly different between patients with bipolar disorder and controls after controlling for potential confounders (estimation, 0.02; SE, 0.05; P = .73).
Figure 1. Adjusted Analysis of Each Serologic Measurement Stratified by Bipolar Disorder Type.
CMV indicates cytomegalovirus.
aP < .008.
bP < .05.
Figure 2. Adjusted Analysis of Each Serologic Measurement Stratified by Age at Onset of Bipolar Disorder.
CMV indicates cytomegalovirus.
aP < .05.
bP < .008.
Figure 3. Adjusted Analysis of Each Serologic Measurement Stratified by Psychosis .
CMV indicates cytomegalovirus.
aP < .05.
bP < .008.
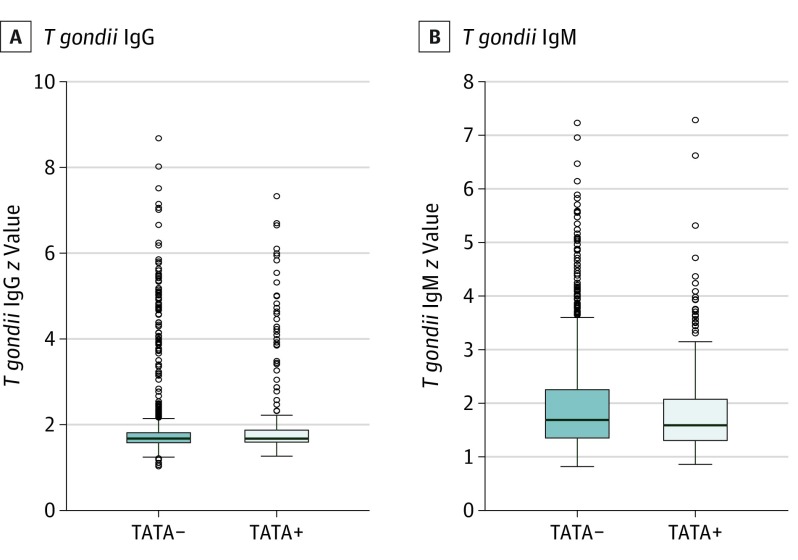
As presented in Figure 4, patients with bipolar disorder who received medications with antitoxoplasma activity had significantly lower T gondii IgM titers (median, 1.59; interquartile range, 1.30-2.07) compared with those not receiving these medications (median, 1.69; interquartile range, 1.35-2.25) (P = .02). There was no significant difference for T gondii IgG titers between the 2 groups (median, 1.67; interquartile range, 1.59-1.87 vs median, 1.67; interquartile range, 1.58-1.81; P = .45). Controlling for use of medication with antitoxoplasma activity in the above analyses did not change the results shown in Figure 1 and Figure 2 (eTable 3 in the Supplement).
Figure 4. Comparison of Toxoplasma gondii IgG and IgM Distributions Stratified by Use of Treatment With Antitoxoplasmic Activity (TATA).
Case patients receiving TATA-positive medications had significantly lower T gondii IgM titers than did those not receiving TATA-positive medications (P = .02). There was no significant difference in T gondii IgG titers between the 2 groups (P = .45).
Discussion
These data suggest that increased long-term antibody response to CMV and decreased long-term antibody response to T gondii are associated with bipolar disorder and the subphenotypes of bipolar type I, nonearly disease onset, and history of manic psychosis. To our knowledge, this cohort of more than 1200 participants with bipolar disorder was the largest to be evaluated for environmental exposure to infection with quantification of immune activation and inflammation. The sample size and clinical phenotyping of illness may provide potential new insights into pathophysiologic mechanisms warranting further study. In contrast to other studies that identified an increase in T gondii IgG titers in patients with bipolar disorder,4,11 recent-onset psychotic illness,31 schizophrenia and related disorders,12 or attempted suicide,32 we did not observe an increase in IgG titers, but a decrease (ie, less seropositive) compared with controls. Although T gondii is widespread, there is variability in US age-adjusted seroprevalence rates, with higher rates in the Northeast compared with the Midwest, with risk factors including lower educational level and crowded living conditions.27 Furthermore, because current psychosis and active suicidal ideation are common exclusion criteria in mood disorder research, participants with bipolar disorder and greater symptom severity (not included in our study) may have yielded different immune activation patterns.
Although we controlled for potential confounders (ie, sex, age, and educational level), the biobank was not originally designed for infectious diseases analyses, and as such, data on risk factors such as immunodeficiency, presence of cats in the house at birth or young age, and maternal educational level were not collected. Furthermore, causality could not be determined. In addition, we do not know the time of exposure to the pathogen and subsequent short-term (ie, IgM) or long-term (ie, IgG) immune response or whether the infection was recent or a latent infection reactivated. Aligning with our CMV finding, however, a Danish registry nested case-control study12 recently reported a significantly increased OR of CMV among individuals with onset of mood disorders (not specifically bipolar disorder) after collection of blood samples following initial exposure); this significant association was not identified for schizophrenia; neurotic, stress-related, or somatoform disorders; or suicide or suicide attempt. We did not observe sex differences, but the National Health and Nutrition Examination Survey, a population-based survey of more than 6800 young Americans (ages 15-39 years), reported that the presence of CMV seropositivity was associated with lower odds (ie, protective) of major depression among men whereas women were noted to have marginally associated increased odds of major depression.10
Despite recent investigations that have identified reduced immunity to measles among adults with major depression (ie, current, remitted, and medicated),33 we did not find that participants with bipolar disorder were less likely to test seropositive for measles in comparison with controls. The absence of antimeasles antibodies may represent either failure to receive initial immunization, failure to seroconvert after vaccination, or loss of immunity after seroconversion, and our study was not designed to critically examine these variables. Furthermore, Ford et al33 recognized the smaller sample size of the cohort with bipolar disorder (n = 64) compared with the cohort with depression (present [n = 85] and remitted [n = 82]) in their study, the associated limited power to detect differences compared with healthy controls (25% power for bipolar disorder compared with controls vs >70% for depression compared with controls), and the possibility of immunosuppression associated with selective serotonin reuptake inhibitors.
C-reactive protein has been extensively investigated as a marker of acute and low-level chronic inflammation in patients with bipolar disorder. In a 2016 meta-analysis (27 studies, 2161 patients with bipolar disorder, and 81 932 controls), after controlling for age and body mass index, CRP level was elevated in patients with bipolar disorder regardless of mood state.34 Although our study did not record current mood state, we found no significant difference in CRP levels between patients with bipolar disorder and controls; however, in contrast to the meta-analysis, we additionally controlled for current smoking status, which has been shown to be associated with higher CRP levels.29 In our study, there was no exclusion criterion based on CRP levels. A secondary analysis comparing CRP levels in patients with acute inflammation and patients with nonacute inflammation would further assess the association between CRP and our CMV and T gondii findings. However, we did not systematically assess at the time of study enrollment the presence or absence of current episode (ie, manic, depressive, and euthymic); length, severity, and time from last episode; or quantification of additional acute systemic illness at the time of study participation, all of which may affect CRP results.
Cytomegalovirus is a human herpes virus present worldwide and viewed as a lifelong infection with recurrent viral latency and reactivation.35 Expression of the CMV immediate–early 2 protein has been shown to impede the proliferation and regenerative capacity of neural progenitor cells in vitro and induces nonfunctional immature neurons during brain development in vivo.36 Translational work possibly highlighting the association of these neurodevelopmental aberrancies include CMV seropositivity and serointensity being associated with smaller right hippocampal volume in bipolar disorder8; cumulative exposure to herpes simplex virus types 1 and 2, CMV, and T gondii associated with neurocognitive impairment5; shorter telomeres among patients with bipolar disorder associated with higher CMV IgG titers7; and increased titers of human herpesvirus–6 DNA in patients with bipolar disorder compared with controls with viral concentration in Purkinje cells or GABAergic (gamma aminobutyric acid) cerebellar neurons.37 These translational data show associations and not cause and effect or specific viral pathological mechanistic contributions to the neuroanatomic or neurocognitive deficit. Further preclinical research is encouraged to better guide research into CMV-associated bipolar disease pathogenesis, which may provide a road map for novel drug development.
As a treatment target, letermovir is a viral terminase inhibitor newly approved by the US Food and Drug Administration for CMV prophylaxis among patients with seropositive allogeneic hematopoietic stem cell transplants.38 It is less recognized that both lithium and valproate sodium have antiviral and anti–T gondii properties,39,40 findings that are consistent with our data. Furthermore, there are clinical trials under way to investigate the efficacy of monoclonal antibody treatments for schizophrenia.41
During the past 20 years, drug development in bipolar disorder has been significantly less than that for schizophrenia and depression, and thus, we believe that further focused efforts in immune modulators and anti-inflammation interventions in patients with bipolar disorder should be encouraged. An earlier study14 found that growth differentiation factor 15, an immune modulator, and hemopexin, an antioxidant facilitator, were significantly increased in a cohort of patients with mood disorder, particularly patients with bipolar disorder type I, compared with healthy controls. These and other immune targets should be considered for future drug development.
Limitations
The limitations of this study are primarily related to the absence of symptomatic rating scales and the cross-sectional nature of the analysis without quantification of time from the last manic, hypomanic, or depressive mood episode. This is relevant because at least 1 investigation identified significantly increased CMV IgG antibody titers among patients with bipolar disorder type I compared with controls, with secondary analyses reporting the antibody titer elevation only among patients with hypomania.9 In another study31 of patients with schizophrenia, bipolar disorder, and major depression, there was a significantly increased odds of T gondii exposure in patients with recent-onset psychosis, defined as first onset of positive psychotic symptoms within the past 2 years, but not in the group with established disease. These studies would suggest that recent infection and greater symptom severity, such as with our finding of manic psychosis, may be key determinants of increased antibody expression. The exact bidirectional neurobiologic association between symptom severity and viral exposure, both latent and reactivated, and secondary immune activation remains to be clarified. These environmental exposure–related infections are ubiquitous, and it is likely that exposure is common in early life. Our study, however, found that non–early-onset disease was associated with higher IgG antibody titers. Given earlier work22 that identified an association between genetic risk score and early-onset bipolar illness, in the present cohort, later-onset disease may have been associated with less genetic risk. Furthermore, later-onset disease may be associated with higher rates of environmental exposure that could alone or in a genetic and environmental interaction facilitate disease onset. However, our study had limited environmental data collected including environmental data during adolescence. Furthermore, we did not systematically quantify cumulative early trauma, which has recently been associated with increased odds of CMV-positive status.42 To our knowledge, the overall temporal association among exposure, latency, and reactivation of virus and the association with mood state or episode recurrence has not been investigated longitudinally among patients with bipolar disorder.
Mood stabilization drug treatment with known antitoxoplasma activity was associated with significantly lower T gondii IgM titers. Again, time of exposure to infection was not known. Although IgM classification has been conceptualized as the antibody response of the acute primary infection, the IgM class can similarly arise with reactivation of latent infection.43
Conclusions
In this sample, increased long-term antibody response to CMV and decreased long-term antibody response to T gondii were associated with bipolar disorder and the subphenotypes of bipolar type I, nonearly disease onset, and manic psychosis. Further work appears to be needed to better understand genetic vs environmental disease risk and infection or immune activation contribution to overall disease pathogenesis with particular reference to disease onset.
eTable 1. Case-control Analyses.
eTable 2. CRP analysis
eTable 3. TATA+ Sensitivity Analysis Results
eFigure. Distributions of Each Serology Measurement.
References
- 1.Lazarus RS. From psychological stress to the emotions: a history of changing outlooks. Annu Rev Psychol. 1993;44(1):1-21. doi: 10.1146/annurev.ps.44.020193.000245 [DOI] [PubMed] [Google Scholar]
- 2.Leboyer M, Berk M, Yolken RH, Tamouza R, Kupfer D, Groc L. Immuno-psychiatry: an agenda for clinical practice and innovative research. BMC Med. 2016;14(1):173. doi: 10.1186/s12916-016-0712-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weissman MM, Bland RC, Canino GJ, et al. . Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276(4):293-299. doi: 10.1001/jama.1996.03540040037030 [DOI] [PubMed] [Google Scholar]
- 4.Tedla Y, Shibre T, Ali O, et al. . Serum antibodies to Toxoplasma gondii and herpesvidae family viruses in individuals with schizophrenia and bipolar disorder: a case-control study. Ethiop Med J. 2011;49(3):211-220. [PubMed] [Google Scholar]
- 5.Hamdani N, Daban-Huard C, Godin O, et al. . Effects of cumulative herpesviridae and Toxoplasma gondii infections on cognitive function in healthy, bipolar, and schizophrenia subjects. J Clin Psychiatry. 2017;78(1):e18-e27. doi: 10.4088/JCP.15m10133 [DOI] [PubMed] [Google Scholar]
- 6.Stich O, Andres TA, Gross CM, Gerber SI, Rauer S, Langosch JM. An observational study of inflammation in the central nervous system in patients with bipolar disorder. Bipolar Disord. 2015;17(3):291-302. doi: 10.1111/bdi.12244 [DOI] [PubMed] [Google Scholar]
- 7.Rizzo LB, Do Prado CH, Grassi-Oliveira R, et al. . Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disord. 2013;15(8):832-838. doi: 10.1111/bdi.12121 [DOI] [PubMed] [Google Scholar]
- 8.Houenou J, d’Albis M-A, Daban C, et al. . Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:142-148. doi: 10.1016/j.pnpbp.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 9.Prossin AR, Yolken RH, Kamali M, et al. . Cytomegalovirus antibody elevation in bipolar disorder: relation to elevated mood states. Neural Plast. 2015;2015:939780. doi: 10.1155/2015/939780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simanek AM, Parry A, Dowd JB. Differences in the association between persistent pathogens and mood disorders among young- to middle-aged women and men in the US. Brain Behav Immun. 2018;68:56-65. doi: 10.1016/j.bbi.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 11.Afifi MA, Jiman-Fatani AA, Al-Rabia MW, Al-Hussainy NH, El Saadany S, Mayah W. More than an association: latent Toxoplasmosis might provoke a local oxidative stress that triggers the development of bipolar disorder. J Microsc Ultrastruct. 2018;6(3):139-144. doi: 10.4103/JMAU.JMAU_22_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgdorf KS, Trabjerg BB, Pedersen MG, et al. . Large-scale study of toxoplasma and cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019;79(January):152-158. doi: 10.1016/j.bbi.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 13.Leboyer M, Soreca I, Scott J, et al. . Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012;141(1):1-10. doi: 10.1016/j.jad.2011.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye MA, McElroy SL, Fuentes M, et al. . Development of a bipolar disorder biobank: differential phenotyping for subsequent biomarker analyses. Int J Bipolar Disord. 2015;3(1):30. doi: 10.1186/s40345-015-0030-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson JE, Ryu E, Johnson KJ, et al. . The Mayo Clinic Biobank: a building block for individualized medicine. Mayo Clin Proc. 2013;88(9):952-962. doi: 10.1016/j.mayocp.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 17.Akinhanmi MO, Biernacka JM, Strakowski SM, et al. . Racial disparities in bipolar disorder treatment and research: a call to action. Bipolar Disord. 2018;20(6):506-514. doi: 10.1111/bdi.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winham SJ, Cuellar-Barboza AB, Oliveros A, et al. . Genome-wide association study of bipolar disorder accounting for effect of body mass index identifies a new risk allele in TCF7L2. Mol Psychiatry. 2014;19(9):1010-1016. doi: 10.1038/mp.2013.159 [DOI] [PubMed] [Google Scholar]
- 19.Cuellar-Barboza AB, Winham SJ, McElroy SL, et al. . Accumulating evidence for a role of TCF7L2 variants in bipolar disorder with elevated body mass index. Bipolar Disord. 2016;18(2):124-135. doi: 10.1111/bdi.12368 [DOI] [PubMed] [Google Scholar]
- 20.Bellivier F, Golmard J-L, Rietschel M, et al. . Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160(5):999-1001. doi: 10.1176/appi.ajp.160.5.999 [DOI] [PubMed] [Google Scholar]
- 21.Bellivier F, Etain B, Malafosse A, et al. . Age at onset in bipolar I affective disorder in the USA and Europe. World J Biol Psychiatry. 2014;15(5):369-376. doi: 10.3109/15622975.2011.639801 [DOI] [PubMed] [Google Scholar]
- 22.Croarkin PE, Luby JL, Cercy K, et al. . Genetic risk score analysis in early-onset bipolar disorder. J Clin Psychiatry. 2017;78(9):1337-1343. doi: 10.4088/JCP.15m10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2nd ed New York: Oxford University Press; 2007. [Google Scholar]
- 24.Markota M, Coombes BJ, Larrabee BR, et al. . Association of schizophrenia polygenic risk score with manic and depressive psychosis in bipolar disorder. Transl Psychiatry. 2018;8(1):188. doi: 10.1038/s41398-018-0242-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickerson F, Stallings C, Origoni A, et al. . Antibodies to Toxoplasma gondii in individuals with mania. Bipolar Disord. 2014;16(2):129-136. doi: 10.1111/bdi.12123 [DOI] [PubMed] [Google Scholar]
- 26.Dollard SC, Staras SAS, Amin MM, Schmid DS, Cannon MJ. National prevalence estimates for cytomegalovirus IgM and IgG avidity and association between high IgM antibody titer and low IgG avidity. Clin Vaccine Immunol. 2011;18(11):1895-1899. doi: 10.1128/CVI.05228-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones JL, Kruszon-Moran D, Elder S, et al. . Toxoplasma gondii infection in the United States, 2011-2014. Am J Trop Med Hyg. 2018;98(2):551-557. doi: 10.4269/ajtmh.17-0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timpson NJ, Nordestgaard BG, Harbord RM, et al. . C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes (Lond). 2011;35(2):300-308. doi: 10.1038/ijo.2010.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohsawa M, Okayama A, Nakamura M, et al. . CRP levels are elevated in smokers but unrelated to the number of cigarettes and are decreased by long-term smoking cessation in male smokers. Prev Med. 2005;41(2):651-656. doi: 10.1016/j.ypmed.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 30.Fond G, Boyer L, Gaman A, et al. . Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: a cross-sectional study. J Psychiatr Res. 2015;63:58-64. doi: 10.1016/j.jpsychires.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 31.Yolken R, Torrey EF, Dickerson F Evidence of increased exposure to Toxoplasma gondii in individuals with recent onset psychosis but not with established schizophrenia. PLoS Negl Trop Dis 2017;11(11):e0006040. doi: 10.1371/journal.pntd.0006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickerson F, Origoni A, Schweinfurth LAB, et al. . Clinical and serological predictors of suicide in schizophrenia and major mood disorders. J Nerv Ment Dis. 2018;206(3):173-178. doi: 10.1097/NMD.0000000000000772 [DOI] [PubMed] [Google Scholar]
- 33.Ford BN, Yolken RH, Dickerson FB, et al. . Reduced immunity to measles in adults with major depressive disorder. Psychol Med. 2019;49(2):243-249. doi: 10.1017/S0033291718000661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes BS, Steiner J, Molendijk ML, et al. . C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3(12):1147-1156. doi: 10.1016/S2215-0366(16)30370-4 [DOI] [PubMed] [Google Scholar]
- 35.Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235(2):288-297. doi: 10.1002/path.4437 [DOI] [PubMed] [Google Scholar]
- 36.Han D, Byun S-H, Kim J, et al. Human cytomegalovirus IE2 protein disturbs brain development by the dysregulation of neural stem cell maintenance and the polarization of migrating neurons. J Virol 2017;91:e00799-17. doi: 10.1128/JVI.00799-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prusty BK, Gulve N, Govind S, et al. . Active HHV-6 infection of cerebellar purkinje cells in mood disorders. Front Microbiol. 2018;9:1955. doi: 10.3389/fmicb.2018.01955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meesing A, Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs. 2018;78(11):1085-1103. doi: 10.1007/s40265-018-0943-1 [DOI] [PubMed] [Google Scholar]
- 39.Amsterdam JD, Maislin G, Rybakowski J. A possible antiviral action of lithium carbonate in herpes simplex virus infections. Biol Psychiatry. 1990;27(4):447-453. doi: 10.1016/0006-3223(90)90555-G [DOI] [PubMed] [Google Scholar]
- 40.Michaelis M, Ha TAT, Doerr HW, Cinatl J Jr. Valproic acid interferes with antiviral treatment in human cytomegalovirus-infected endothelial cells. Cardiovasc Res. 2008;77(3):544-550. doi: 10.1093/cvr/cvm061 [DOI] [PubMed] [Google Scholar]
- 41.Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry–novel perspectives on brain disorders. Nat Rev Neurol. 2019;15(6):317-328. doi: 10.1038/s41582-019-0174-4 [DOI] [PubMed] [Google Scholar]
- 42.Ford BN, Yolken RH, Aupperle RL, et al. . Association of early-life stress with cytomegalovirus infection in adults with major depressive disorder. JAMA Psychiatry. 2019;76(5):545-547. doi: 10.1001/jamapsychiatry.2018.4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villard O, Breit L, Cimon B, et al. ; French National Reference Center for Toxoplasmosis Network . Comparison of four commercially available avidity tests for Toxoplasma gondii–specific IgG antibodies. Clin Vaccine Immunol. 2013;20(2):197-204. doi: 10.1128/CVI.00356-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Case-control Analyses.
eTable 2. CRP analysis
eTable 3. TATA+ Sensitivity Analysis Results
eFigure. Distributions of Each Serology Measurement.