Abstract
Irradiation of CCl4, CFCl3, and CF2Cl2 in the presence of C2H6 in vessels containing silica sand or fused quartz tubing results in the formation of chlorine-containing products. The formation of these compounds occurs at wavelengths extending up to approximately 400 nm, that is, at wavelengths well beyond the absorption threshold of the chloromethanes in the gas phase. It is suggested that CCl4 adsorbed on silica surfaces photodissociates to yield CCl3 and CCl2 species. The poor material balance obtained in these experiments indicates that several of the chlorine-containing fragments are strongly adsorbed on the surface. At a CCl4 pressure of 13 Pa (0.1 torr), photolysis with 366 nm light in the presence of sand results in the decomposition of one molecule for every 104 photons striking the surface. Under otherwise identical conditions, the photon-induced breakdown of CFCl3 and CF2Cl2 is respectively only 10 percent or 3 percent as efficient.
Keywords: Chloromethanes, photochemistry, quantum yields, quartz, sand, surface reactions, tropospheric sink
1. Introduction
In a recent study [1]1 from this laboratory, it was shown that in the gas phase, the photodecomposition cross section of CCl4 at 313 nm was ⩽ 3.7 ± 0.4 × 10−26 cm2 molecule−1 at 300 K. Because sunlight reaching sea level consists mainly of wavelengths above 320 nm, this laboratory result suggests that in the troposphere gaseous CCl4 will not be dissociated by light. The photodissociation cross section of fluorine-substituted methanes at the wavelengths which reach the earth’s surface should be even smaller than that for CCl4, since the absorption spectra of the fluorine-substituted compounds are shifted to shorter wavelengths [2]. Therefore, chloromethanes and fluorochloromethanes released to the atmosphere would be expected to diffuse to the stratosphere, unless some mechanism for their removal other than photodecomposition exists. As Molina and Rowland [2] suggested, these compounds in the stratosphere would absorb high energy photons and undergo photodissociation to produce chlorine atoms, which in turn would be expected to undergo a chain reaction resulting in the removal of ozone molecules.
However, there were available two pieces of evidence which indicated that possibly CCl4 when adsorbed on certain surfaces does undergo photodecomposition at wavelengths as long as those which reach the earth’s surface. The first such observation was that the cross section for photodecomposition of CCl4 showed an apparent increase by a factor of five when the measurements were made in quartz rather than Pyrex2 vessels. Secondly, measurements of the concentration of CCl4 in the troposphere in the eastern hemisphere as a function of latitude from 50 N to 30 S have shown that there is a pronounced minimum in the CCl4 concentration in the vicinity of the Sahara desert (latitudes 20 to 30 N) [3].
It has been reported before [4, 5] that organic materials may undergo photodissociation at wavelengths well beyond the gas phase absorption region when they are adsorbed on surfaces. Most of these earlier studies dealt with materials adsorbed on metallic oxides. However, in one recent study [5] it was shown that irradiation of cis- or trans-1,3-pentadiene in Pyrex or quartz vessels leads to isomerization at wavelengths for which light absorption does not occur in the gas phase.
In the present laboratory study, the photodecomposition of CCl4, CFCl3, and CF2Cl2 adsorbed on fused quartz and different types of sand is examined. Most of the experiments were carried out using light of 366 nm, which is well beyond the gas phase absorption threshold of these substances [2, 6]. The majority of the experimental results have been obtained with CCl4, which has a higher photodecomposition rate than the fluorine-substituted compounds, but from the few experiments carried out with CFCl3 and CF2Cl2 it is possible to estimate the importance of their photodecomposition relative to that of CCl4.
Although, at this writing, no significant trophospheric removal mechanisms of these chloromethanes have been demonstrated, it has been suggested that the existence of a trophospheric sink cannot be rule out at the present time [7, 8]. The results reported here do not prove the existence of such a sink, but do suggest that removal mechanisms not previously considered may be operative.
2. Experimental Detail
Vessels.
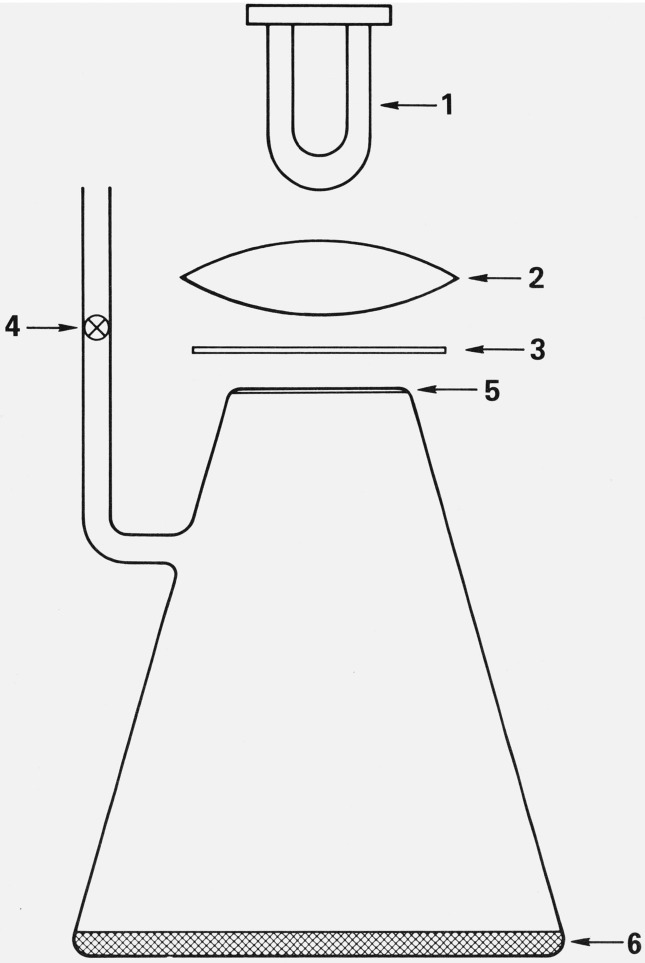
Two types of reaction vessels were used for the experiments reported here: (1) cylindrical vessels (10 cm long, 5 cm diameter) made of Pyrex,2 and with one half of the volume filled with Pyrex or fused quartz tubing (0.2 mm inside diameter, 0.4 mm outer diameter, and 5 cm length); (2) 1000 mL Erlenmeyer flasks, the neck of which has been cut off and sealed with 0.3 cm thick Pyrex window (fig. 1). The bottom of the Erlenmeyer flasks is covered with a layer of sand 0.2 to 0.5 cm thick. For each kind of sand, and for each kind of halomethane (CF2Cl2, CFCl3, and CCl4) a separate reaction vessel was constructed. As a further precaution to avoid contamination of one halomethane by traces of another, separate vacuum lines were used for handling each of the halomethanes.
Figure 1. The one-liter Erlenmeyer vessel used for photolyzing samples in the presence of sand.
(1) Medium pressure mercury lamp. (2) Quartz lens. (3). Light filter. (4) Teflon needle valve. (5) 0.3 cm thick Pyrex window. (6) Layer of sand, 0.2 to 0.5 cm thick.
Irradiation.
Medium pressure mercury arcs were used in conjunction with various filters. In most experiments, Corning 0–52 filters were used to eliminate the 313 and 334.1 nm mercury resonance lines and to transmit 65 percent of the 366 nm mercury resonance line. A Corning 3–75 filter was used to obtain 404.5 nm radiation in the absence of shorter wavelengths. In the experiments with the Erlenmeyer vessels, the light beam was focused by means of a quartz lens so that it was slightly diverging; the entire surface of the sand at the bottom of the flask was thus exposed to the light beam.
The number of 366 nm quanta (~ 1015 quanta/second) impacting on the sand layer was determined by measuring the yield of C2H6 in the photolysis of azomethane at a pressure of 60 torr3 (Φ C2H6 = 1 at 366 nm).9 The number of quanta at 313 nm (1 to 8 × 1015 quanta/seeond) was determined using diethyl ketone as an actinometer [1]. The actinometry experiments were carried out in vessels of the same dimensions as those described above, but with no sand or tubing present.
Materials.
Sea sand (washed and ignited) was obtained from Fisher Scientific Company. Surface sand collected from Mauritania was obtained through the courtesy of Dr. J. E. Lovelock. This sand which we will henceforth designate “desert sand,” consisted mainly of SiO2 with an iron oxide coating and traces of organic matter. The sea sand samples contain Al2O3 and other traces of unidentified mineral oxides. The diameter of the sea sand grains was between 0.05 and 0.03 cm. The diameter of the desert sand grains was less than 0.01 cm.
Analysis.
After photolysis, an aliquot of the irradiated material was injected into a gas chromatograph equipped with a flame detector. A 30-foot squalane column operated at room temperature was used for analysis of low boiling substances (boiling point < 350 K). For analysis of high boiling products, a 20-foot temperature-programmed silicone oil column was employed.
3. Results
Effect on the Photolysis of the Condition of the Surface.
All product yields are expressed in terms of the number of molecules formed per photon entering the reaction vessel.
Table 1 lists the yields of products which were formed in the 366 nm photolysis of CCl4 − C2H6 (10:1) mixtures in the presence of quartz, sea sand, and desert sand. For a particular surface, the reproducibility of product yields was generally better than 20 percent. However, large quantitative and qualitative variations are observed when there are slight differences in the treatment of a surface prior to a series of photolysis experiments. Therefore, the product yields given here relate only to the surfaces treated in the manner described in this article. Photolysis of this mixture in vessels which did not contain sand or fused quartz did not result in the formation of products. It is estimated that in the absence of sand or quartz, the number of molecules formed per incident photon is at least three orders of magnitude lower than that measured in sand-filled vessels.
Table 1.
Photodecomposition of C2H6-CCl4 on sand and fused quartz
| Products | Fused Quartz | Sand | ||
|---|---|---|---|---|
| + H2O* | dry | (Fisher) | (Mauritania) | |
| Molecules × 105/Incident Photon | ||||
| CCl3H | ~0.1 | <0.05 | <0.5 | 16 |
| C2H5Cl | .20 | 5 | 21 | 0.05 |
| CCl2 = CCl2 | n.d. | 0.05 | 0.3 | .3 |
| C2H5CCl3 | 1.1 | .2 | .9 | <.1 |
| C2Cl6 | .5 | .05 | .2 | <.1 |
| n-C4H10 | .1 | .01 | .05 | .015 |
| C2H4 | ~.02 | .06 | .2 | ~.01 |
| CH4 | <.01 | .04 | .009 | .1 |
| Pressure: CCl4 – 10 torr; C2H6 – 1 torr. | ||||
| T: 300 K λ: 366 nm. | ||||
H2O – 5 torr.
The results given in table 1 show that in the photolysis of CCl4 − C2H6 mixtures in this study, C2H5Cl accounts for more than 90 percent of the observed products except in the presence of desert sand. The same observation was also made concerning the products formed in the photolysis of CFCl3 and CF2Cl2, although the yields in these cases were lower, as shown in table 2. In all these experiments, the irradiation time (102 − 4 × 103 min) was adjusted in such a manner that the yield of C2H5Cl could be measured with a precision of 5 percent or better. It was found that the concentration of C2H6 did not influence the quantum yield of C2H5Cl to any great extent. For instance, a fifty-fold increase in the ethane pressure (from 0.2 to 10 torr) increased the yield of C2H5Cl formed in the 366 nm photolysis of CFCl3 or CCl4 (at a pressure of 1 torr) in the presence of sea sand by 20 ± 10 percent. On the other hand, the previous history of the photolysis vessel has a pronounced effect on the observed product yields. For instance, while no products were observed when pure ethane in a previously unused vessel is irradiated at 313 or 366 nm in the presence of sand or quartz, C2H5Cl shows up as a product if the vessel had previously been used in chloromethane photolysis experiments. Extensive degassing and heating under vacuum at temperatures up to 500 K prior to filling the vessel with pure C2H6, reduced, but did not prevent, the formation of C2H5Cl upon irradiation. Because of this effect, it was necessary to renew the sand in the vessel after every 5 to 10 experiments.
Table 2.
Photodecomposition of chloromethanes-C2H6 mixtures on sand*
| C2H5 Cl | |
|---|---|
| Molecules × 105/Incident Photon | |
| CCl4 | 21.8 |
| CFCl3 | 2.4 |
| CF2Cl2 | 0.6 |
| λ: 366 nm | |
| Pressure: 10 torr (10% C2H6) | |
| T: 300 K |
Sea sand degassed for two hours (at 300 K).
Replacement of the quartz tubing by Pyrex tubing in the cylindrical vessel reduced the yield of C2H5Cl observed in the 366 nm photolysis of a CCl4 − C2H6 (1:1) mixture (pressure, 2 torr) by a factor of five. A similar reduction is observed when the quartz tubing is treated with concentrated nitric acid, washed with distilled water, and heated to 800 K prior to its use in a photolysis vessel.
As shown in table 3, a trace of bromine inhibits the formation of C2H5Cl in CCl4 − C2H6 mixtures, but CCl3Br and CCl2Br2 appear as products. The actual amount of Br2 present in the experiment given in table 3 is unknown. Prior to the introduction of the CCl4 − C2H6 mixture into the reaction vessel, 0.1 torr of bromine was introduced. Thereafter, the vessel was evacuated and heated to 500 K for approximately one hour. Apparently, after this procedure enough bromine remained adsorbed on the surface to cause the formation of the CCl3Br and CCl2Br2 products listed in table 3.
Table 3.
Photodecomposition of CCl4-C2H6 in the absence and presence of Br2
| Molecules × 105/lncident Photon | ||
|---|---|---|
| Br2 | none | trace* |
| C2H5Cl | 8 | <0.1 |
| CCl3Br | – | 9.1 |
| CCl2Br2 | – | 10.0 |
| λ: 366 nm | ||
| Pressure: CCl4 – 10 torr | ||
| C2H6 – l torr | ||
Cylindrical vessel with fused quartz tubing degassed for two hours at 450 K. See Results.
Because the yields of the products other than C2H5Cl were so low, the precision with which these could be measured was only about 25 percent. In most experiments, no attempt was made to determine minor products, most of which were below the detection limit.
In the photolysis of CFCl3 − C2H6 mixtures in the presence of desert sand, CHFCl2 was formed as a product. In some experiments in the presence of sea sand, this product was also observed. The yield of CHFCl2 was highly irreproducible, but was always less than 5 percent of the yield of C2H5Cl.
Methane is formed as a product in some experiments. No systematic investigation was made of the conditions under which this product is formed, but the following observations seem relevant. The CH4 yield is strongly dependent on the nature and prior treatment of the surface, as well as on the wavelength (table 4). No methane was observed in the series of sea sand experiments, the results of which are represented in figure 2.
Table 4.
Photodecomposition of CCl4-C2H6 on fused quartz* Effect of wavelength
| λ nm |
Photon energy (kcal) | CH4 | C2H4 | CH3Cl | C2H5Cl |
|---|---|---|---|---|---|
| Molecules × 106/Incident Photon | |||||
| 313 | 91.3 | 13 | 2.1 | 0.7 | 170 |
| 366 | 78.1 | 0.4 | 0.6 | <0.01 | 60 |
| 404.5 | 70.2 | <0.01 | 0.04 | <0.001 | 2 |
| ≥ 435.8 | ≤ 65.6 | <0.01 | |||
| Pressure: CCl4 – 10 torr | |||||
| C2H6 – 1 torr | |||||
Cylindrical vessel with quartz tubing degassed for two hours at 450 K.
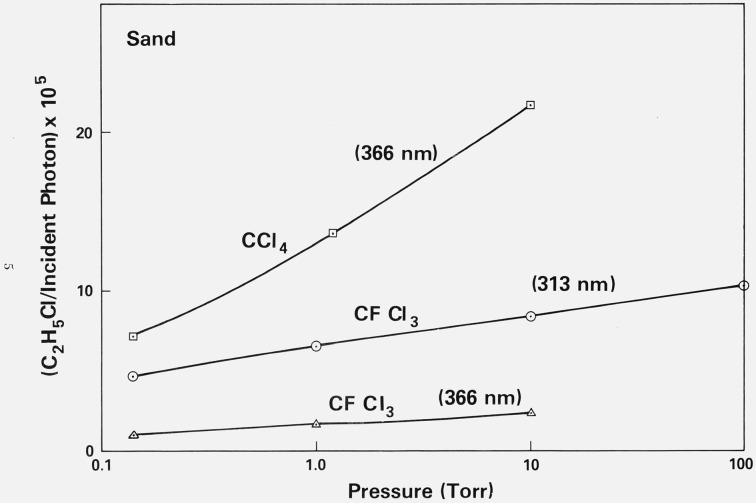
Figure 2. Variation of the yield of C2H5Cl as a function of the pressure of CCl4 at 366 nm, and as a function of the pressure of CFCl3 at 366 nm and 313 nm.
All experiments were performed in the presence of sea sand and a constant pressure (1 torr) of ethane. The sea sand was heated under vacuum for 2 hours at 450 K prior to each experiment.
Photolysis of a CCl4 − C2H6 mixture (10 torr) in the presence of H2O (5 torr) reduced the yield of C2H5Cl by a factor of twenty (table 1). However, other products increased in yield. As many as seven unidentified products were formed with boiling points around 500 K. The combined yield of these products is around 5 ± 3 molecules per 105 incident photons at 366 nm.
4. Discussion
Evidence for Surface-Sensitized Photolysis.
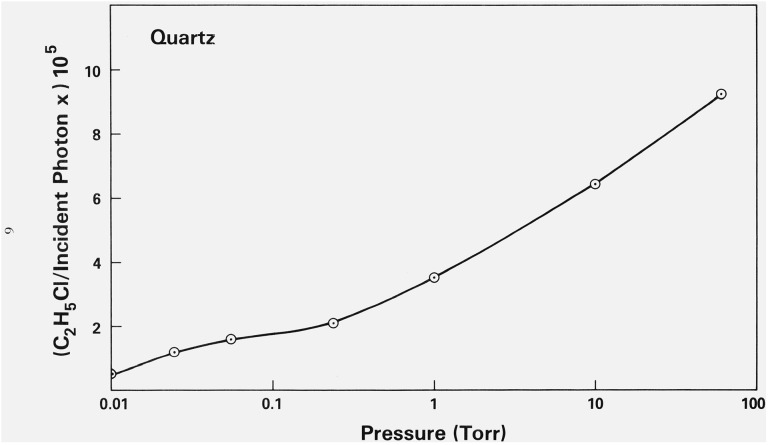
Two important observations demonstrate that the products observed in the experiments reported in this paper can be ascribed to the photolysis of the chloromethanes adsorbed on the surface of the sand or fused quartz. First, at wavelengths in the visible or near-ultraviolet region (λ: 366 to 404.5 nm) the formation of photochemical products could only be detected in the presence of sand or quartz, indicating that the presence of the surfaces of these materials must be necessary for product formation to occur. The second relevant observation is that when the pressures of the mixtures in the vessels are increased from 0.01 to 0.1 torr to approximately 100 torr, the observed yields of products show only slight increases. For instance, the results given in figure 2 show that in vessels containing sand, the yield of C2H5Cl in CFCl3 − C2H6 mixtures is nearly invariant with pressure between 0.1 and 100 torr. (As will be shown below, C2H5Cl is the major photolytic product in such experiments.) A somewhat greater pressure dependence is observed for the formation of C2H5Cl in CCl4 − C2H6 mixtures photolyzed in the presence of sand or fused quartz (figs. 2 and 3), but even in this case, a hundredfold variation in pressure brings about only a three-fold change in the yield. If these compounds were actually being photolyzed in the gas phase above the sand, it would be expected that because of the very low absorption cross sections of these compounds at these wavelengths, the number of photons absorbed should vary linearly with the pressure of the compound. That is, a 1000- or 10,000-fold variation in the pressure of the compound (figs. 2 and 3) would result in a proportional change in the number of molecules of a product formed per incident photon. The fact that this change is not observed indicates that photolysis must occur on the surface. It is of interest that in the photolysis of CCl4 − C2H6 mixtures in the presence of fused quartz tubing, when the pressure of the mixture in the vessel is decreased below 0.01 torr, the yield of C2H5Cl decreases linearly with the pressure. This suggests that in the pressure region below 0.01 torr the surface is no longer entirely covered with the molecules; characteristic adsorption isotherms show a linear fall-off in the surface area covered with decreasing pressure in the low pressure region. Plots of the amount of a gas physically adsorbed on a surface as a function of the pressure of a gas usually show a shape similar to that of the curve given in figure 3; the shape of this curve, thus, suggests that in the experiments reported here, the number of molecules photolyzed is simply proportional to the number of molecules adsorbed on the surface, as one might expect. That the gas in these systems is physically, rather than chemically, adsorbed onto the surfaces is indicated not only by the relatively small heat of adsorption (approximately 7 kcal/mol),4 [10] but also by the fact that essentially all the gas can be readily desorbed from the surface without heating.
Figure 3.
The variation of the yield of C2H5Cl as a function of the pressure of CCl4 at 366 nm in the presence of fused quartz tubing and 1 torr of ethane.
Photodecomposition Processes.
From a mechanistic point of view, the most revealing experiments are those whose results are presented in table 3. The formation of CCl3Br and CCl2Br2 in the 366 nm photolysis of CCl4 − C2H6 mixtures on a surface treated with bromine clearly points to the formation of CCl3 and CCl2 radicals [11, 12]. The formation of these radicals is also evidenced by the formation of C2Cl4 and C2Cl6 in the absence of bromine (see table 1). These products can be accounted for by the recombination reactions involving CCl2 and CCl3 radicals [1].
The following two dissociative processes can account for the formation of CCl2 and CCl3:
| (1) |
| (2) |
In the gas phase, processes 1 and 2 require 73 and 69 kcal/mol, respectively [13]. Therefore, both processes are energetically allowed at 366 nm (78.1 kcal). The heat of adsorption of CCl4 on silica is not accurately known, but appears to lie in the vicinity of 7 kcal/mol [10]. It is of interest that process 2 does not occur in the gas phase at 253.7 nm, which is close to the gas phase absorption threshold of CCl4 [2]. More recent gas phase CCl4 − Br2 photolysis experiments carried out in this laboratory [12] showed that at 213.9 nm, the quantum yield of CCl3Br is 1 ± 0.05, while the quantum yield of CCl2Br2 is less than 0.004. At short wavelengths, CCl2Br2 appears as a major product [12] in the gas phase photolysis (ϕ CCl2Br2 = 0.25 ± 0.05 at 163.3 nm). However, at these wavelengths, the formation of CCl2 is accompanied by the release of 2 chlorine atoms: [1]
| (3) |
Such a process, which probably takes place via the decomposition of a vibrationally excited CCl3 radical, requires as much as 127 kcal/mol and is therefore excluded as a mechanism for CCl2 formation at 366 nm in the surface-sensitized experiments reported here.
Formation of C2H5Cl and Material Balance.
Ethane was added to the chloromethanes in the expectation that, as shown in previous studies [1, 14], it would intercept Cl-atoms formed in process 1 by the H-atom abstraction reaction:
| (4) |
This reaction probably does occur. However, in contrast with the earlier gas phase studies [1, 14], no material balance (table 1) is achieved. For instance, if one assumes that each C2H5 radical formed in reaction 4 abstracts a Cl-atom from CCl4, the CCl3 radical yield should be twice that of C2H5Cl. This is definitely not reflected in the yields of the products measured by gas chromatography (table 1). In the sea sand experiments, the yields of CCl3, as evidenced by the yields of the recombination products C2Cl6 and C2H5CCl3, is no more than 10 percent of that of C2H5Cl. Even if the yield of CCl3Br formed in the presence of traces of Br2 is taken to represent the CCl3 yield (table 3) the yields of CCl3 and C2H5Cl would seem to be approximately equal. It follows that no matter which mechanism is proposed for the formation of C2H5Cl, there is a gross deficiency in the material balance. Apparently, CCl3 radicals or some unidentified product molecules incorporating the CCl3 radical, are strongly adsorbed on the surface. The observation of C2H5Cl when pure ethane is irradiated in a sea sand-containing vessel previously used in chloromethane experiments (see Results) points to the presence of Cl-containing species adsorbed on the surface.
The fact that in the desert sand experiments C2H5Cl is a minor product as compared to CCl3H might be interpreted to indicate that on this surface process 1 is of minor importance. However, CCl3H might be formed via the insertion reaction:
| (5) |
which was shown to occur in the presence of trace amounts of HCl in a recent gas phase study [1]. Thus, since HCl must originate in reaction 4, this would mean that with desert sand both CCl3 and C2H5 radicals remain on the surface or eventually form products which remain on the surface. That is, it may be that the products from the occurrence of process 1 in the presence of the desert sand are simply not observed.
Further evidence that many products do indeed remain adsorbed to the surface under some experimental conditions is obtained by comparing the yield of CCl2Br2 (table 3), which can be ascribed to a reaction of the CCl2 radicals, to the yield of C2Cl4 (table 1) which presumably results from the recombination of these same radicals. Since the yield of CCl2Br2 formed in the presence of Br2 is approximately 10 times greater than the combined yields of the recombination products it seems that only a small fraction of the recombination product can be detected under these experimental conditions.
Addition of water to the vessel containing fused quartz (table 1) causes a considerable improvement in the material balance. On the basis of the yields of C2H5Cl, C2H5CCl3, C2Cl6, and n-C4H10 in this experiment, one obtains CCl3/C2H5 = 1.4. It would seem, therefore, that treatment of the fused silica with water prevents the adsorption of the CCl3 and C2H5 radicals or their reaction products on the surface. In view of the large number of unidentified reaction products (see Results) no firm interpretation of the reaction mechanism occurring in the presence of H2O can be put forward at the present time.
Effect of Wavelength and Fluorine Substitution.
The results presented in table 4 demonstrate that in the surface-sensitized photolysis of CCl4 − C2H6 mixtures, the number of molecules of a given product formed per photon diminishes with an increase in wavelength. An analogous effect was observed for CFCl3 and CF2Cl2 photolysis experiments. It is of interest that when the energy of the photon roughly corresponds to the C − Cl bond strength (74 ± 2 kcal/mol) [13], the yields of the products become negligibly small. Photode-composition products are not observed when the energy of the photon is 8 kcal or more lower than the C − Cl bond strength.
The decrease in the yield of C2H5Cl with fluorine substitution (table 2) can perhaps be rationalized on the basis of a slight increase in the C − Cl bond strengths with increased fluorine substitution. It should also be pointed out that in the gas phase the absorption cross sections of these compounds in the vicinities of their absorption thresholds also decrease with increasing fluorine substitution, and the observed trends in product yields (table 2) could plausibly be attributed to a similar decrease for the absorbed materials. Unfortunately, the results reported here do not provide information relating the gas phase absorption characteristics and the surface photodecomposition efficiencies.
5. Conclusions
Although the data presented here do demonstrate that chloromethanes adsorbed on certain surfaces can be broken down by photons which these molecules could not absorb in the gas phase, the importance of such surface-induced processes to the chemistry of the earth’s atmosphere cannot be assessed from these results alone. For instance, the effect of oxygen on the photodecomposition processes remains to be explored. Because, as shown here there are problems in detecting some of the photolytic products from these surface-induced processes, a comprehensive investigation of such processes under simulated trophospheric conditions will be difficult. On the other hand, atmospheric measurements have indeed demonstrated a diminution in the concentration of CCl4 in the atmosphere in the vicinity of large bodies of windblown sand [3], a fact which does suggest that surface-induced photodecomposition may occur in the atmosphere.
Photodecomposition of molecules adsorbed on surfaces at wavelengths beyond the gas phase absorption threshold is no doubt of general occurrence. For instance, preliminary experiments carried out in this laboratory show that N2O adsorbed on desert sand photodissociates at wavelengths up to 600 nm even though the gas phase absorption extends only up to ~230 nm.
Acknowledgments
This work was supported in part by the Office of Air and Water Measurement, National Bureau of Standards, and by the Upper Atmospheric Research Office of the National Aeronautics and Space Administration. One of the authors (L. G) gratefully acknowledges the support of the Manufacturing Chemists Association and DuPont.
Footnotes
Figures in brackets indicate the literature references at the end of this paper.
In order to adequately describe materials and experimental procedures, it was occasionally necessary to identify commercial products by manufacturer’s name or label. In no instances does such identification imply endorsement by the National Bureau of Standards, nor does it imply that the particular product or equipment is necessarily the best available for that purpose.
1 torr = 133 pascals.
1 kilocalorie = 4.184 kilojoules.
6. References
- [1].Rebbert R. E., and Ausloos P. J., J. Photochem. 6(1976/77) 265. [Google Scholar]
- [2].Molina M. J., and Rowland F. S., Nature 249 (1974) 810. [Google Scholar]
- [3].Lovelock J., presented at the Third European Geophysical Society Meeting, Amsterdam, Sept. 10–12, 1976. [Google Scholar]
- [4].See for instance,; Leighton P. A., “Photochemistry of Air Pollution,” p. 96 (Academic Press, NY, NY: 1961). [Google Scholar]
- [5].Daubendiek R. L., and McMillan G. R., J. Am. Chem. Soc. 95 (1973) 1374. [Google Scholar]
- [6].Green R. G., and Wayne R. P., J. Photochem. 6 (1976/77) 375, and references therein. [Google Scholar]
- [7].Gelinas R. J., Hall D. K., and Nelson R. G., Nature 266 (1977) 229. [Google Scholar]
- [8].Sre N. D., and Wu M. F., Atmospheric Environment 10 (1976) 1117. [Google Scholar]
- [9].Calvert J. G., and Pitts J. N. Jr., Photochemistry (J. Wiley & Sons, Inc., 1966). [Google Scholar]
- [10].Tulʹbovich B. I., and Priimak E. I., Russian J. of Phys. Chem. 43 (1969) 195. [Google Scholar]
- [11].Davis D. D., Schmidt J. F., Neeley C. M., and Hanrahan R. J., J. Phys. Chem. 79 (1975) 11. [Google Scholar]
- [12].Rebbert R. E., and Ausloos P., unpublished results.
- [13].JANAF Thermochemical Tables Nat. Stand. Ref. Data Ser., Nat. Bur. Stand. (U.S.), 37, 1141 pages (June 1971). [Google Scholar]
- [14].Rebbert R. E., and Ausloos P., J. Photochem. 4 (1975) 419. [Google Scholar]