The presence of white and red colonies on red grape juice (RGJ) agar during enumeration of Oenococcus oeni in wine samples is frequently observed by stakeholders in the wine industry. Our study brings an explanation for this intriguing phenomenon and establishes a link between the white-red color switch and the lysogenic state of O. oeni. It also provides a simple and inexpensive method to distinguish between lysogenic and nonlysogenic derivatives in O. oeni with a minimum of expended time and effort. Noteworthy, the protocol could be adapted to two other species of LAB, namely, Leuconostoc citreum and Lactobacillus plantarum. It could be an effective tool to provide genetic, ecological, and functional insights into lysogeny and aid in improving biotechnological processes involving members of the lactic acid bacterium (LAB) family.
KEYWORDS: bacteriophages, lactic acid bacteria, lysogeny
ABSTRACT
Oenococcus oeni is the lactic acid bacterium (LAB) that most commonly drives malolactic fermentation in wine. Although oenococcal prophages are highly prevalent, their implications on bacterial fitness have remained unexplored and more research is required in this field. An important step toward achieving this goal is the ability to produce isogenic pairs of strains that differ only by the lysogenic presence of a given prophage, allowing further comparisons of different phenotypic traits. A novel protocol for the rapid isolation of lysogens is presented. Bacteria were first picked from the center of turbid plaques produced by temperate oenophages on a sensitive nonlysogenic host. When streaked onto an agar medium containing red grape juice (RGJ), cells segregated into white and red colonies. PCR amplifications with phage-specific primers demonstrated that only lysogens underwent white-red morphotypic switching. The method proved successful for various oenophages irrespective of their genomic content and attachment site used for site-specific recombination in the bacterial chromosome. The color switch was also observed when a sensitive nonlysogenic strain was infected with an exogenously provided lytic phage, suggesting that intracolonial lysis triggers the change. Last, lysogens also produced red colonies on white grape juice agar supplemented with polyphenolic compounds. We posit that spontaneous prophage excision produces cell lysis events in lysogenic colonies growing on RGJ agar, which, in turn, foster interactions between lysed materials and polyphenolic compounds to yield colonies easily distinguishable by their red color. Furthermore, the technique was used successfully with other species of LAB.
IMPORTANCE The presence of white and red colonies on red grape juice (RGJ) agar during enumeration of Oenococcus oeni in wine samples is frequently observed by stakeholders in the wine industry. Our study brings an explanation for this intriguing phenomenon and establishes a link between the white-red color switch and the lysogenic state of O. oeni. It also provides a simple and inexpensive method to distinguish between lysogenic and nonlysogenic derivatives in O. oeni with a minimum of expended time and effort. Noteworthy, the protocol could be adapted to two other species of LAB, namely, Leuconostoc citreum and Lactobacillus plantarum. It could be an effective tool to provide genetic, ecological, and functional insights into lysogeny and aid in improving biotechnological processes involving members of the lactic acid bacterium (LAB) family.
INTRODUCTION
Bacteriophages are obligate parasites, and their interactions with bacteria are central to the ecology and evolution of microbial communities associated with soils (1) and oceans (2, 3), as well as human and animal bodies (4–6). Phages can typically sustain two distinct life cycles, namely, lytic and lysogenic, as defined by their genetics and interactions with the bacterial host. The virulent phage life cycle consists in replicating in and then lysing their bacterial host. Unlike lytic phages, temperate phages can establish an alternative association with the host by entering into the bacterial genome and expressing only a small subset of their genes. This particular phage-host relationship is complex, and active prophages can be induced and switch back to lytic multiplication in response to a signal, such as DNA damage and subsequent SOS response.
A current and exciting field of phage research is to tackle the factors governing the lysis-lysogeny decision in natural ecosystems (1, 7) where stresses differ in their nature, intensity, possible cumulation, and temporality. The latter can affect growth rates and/or densities of both phage and hosts and create dynamics of integration and excision of prophages (7–11). Increasing the complexity of the challenge is the fact that phage infection strategies may also evolve if hosts make a shift between planktonic and sessile growth within the ecosystem considered (12). The research field is now moving toward broadening the scope of ecosystems explored, leading to conflicting theories about whether lysogeny becomes the preferred strategy when the cell density falls (13) or increases at high host density, thus leading to a decrease in the average virus yield per infection (14, 15).
Wine making is an example of a complex and fluctuating environment that is characterized by successional trajectories of distinct communities of microorganisms. In most red and dry white wines, the process starts with the selection of the fruit and its fermentation into alcohol by yeasts, followed by a malolactic fermentation (MLF). The latter process, which reduces acidity and increases microbial stability, creates good-quality grape wine. Malolactic fermentation is largely driven by the lactic acid bacterium (LAB) Oenococcus oeni, which is the species best adapted to the combined inhibitory effects of low pH, low oxygen, and high alcohol, tannin, and sulfur dioxide contents (16). MLF can obviously occur spontaneously, relying on endogenous O. oeni strains, or winemakers may decide to inoculate wine with MLF cultures. Commercial malolactic bacterium starter cultures have been selected from spontaneous MLFs because of their good fermentation kinetics, their performance under limiting wine conditions, and their desired sensory properties, while avoiding the production of negative metabolites. However, the use of MLF starters does not ensure a problem-free fermentation, and cases of stuck and sluggish fermentations are annually reported worldwide.
The pervasiveness of prophages has been long recognized in the O. oeni species (17–22). Prophages belong to the Siphoviridae family and are typically mosaic (19). They are inserted at tRNA sites and are so far clustered into four integrase gene sequence groups (IntA to IntD), which are also related to the chromosomal integration site. Members of the IntA group are the most prevalent prophages identified in oenococcal genomes (19, 20) and also as replicating phages associated with wines and wine making production facilities, as reported in recent surveys (20, 22). Lysogeny is frequent among commercial strains. This is intriguing since the use of a lysogenic strain is usually seldom warranted in industrial processes because of possible instability during inoculation due to phage release (23). There is a growing interest in understanding whether temperate oenophages have a parasitic or a mutualistic interaction with their host during wine making, as this could help elucidate problem fermentations and also provide a rationale for starter selection. From the current literature, a key requirement to explore the consequences of lysogeny on life traits is the construction of isogenic pairs of bacterial strains that differ only by the lysogenic presence of a given prophage. Such pairs can be further used to compare different phenotypic traits (kinetics of growth, metabolic profiles, antibiotics susceptibility, or biofilm formation) and possibly perform competition experiments between lysogenic and nonlysogenic derivatives. Isogenic pairs can be obtained through the curing of prophages from lysogenic cells (24). However, this strategy is often difficult and sometimes unattainable, especially for strains not amenable to genetic manipulations, which is the case for O. oeni (25). An alternative is to establish lysogens for a purified phage using a phage-sensitive bacterium devoid of phages (26). Regardless of the strategy used, the screening step requires different time-consuming tests to be carried out on a number of randomly picked colonies, including superinfection immunity tests and PCR assays targeting phage sequences.
In this work, we designed an inexpensive method to distinguish between lysogenic and nonlysogenic derivatives in O. oeni with a minimum of expended time and effort. Interestingly, the reported technique could be applied to other species of LAB.
RESULTS
Lysogenization of strain IOEBS277 by phage CiNeMCA produces two morphotypes of colonies on RGJ agar.
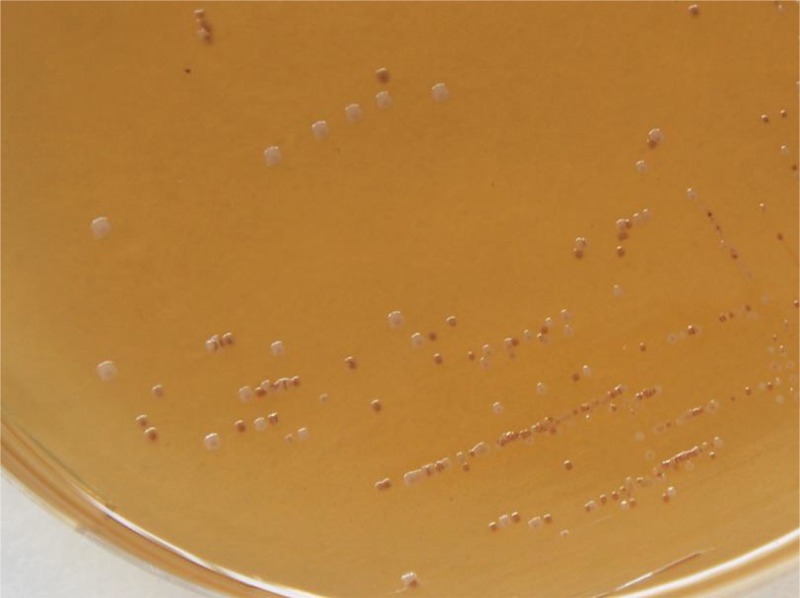
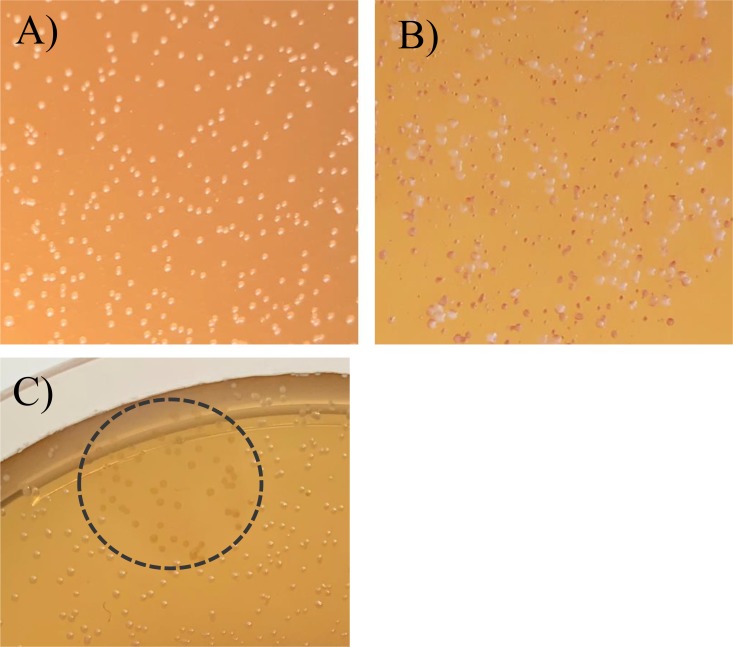
The first candidate for the design of a rapid lysogenization test was the IntA temperate oenophage CiNeMCA, which was harbored in the chromosome of the commercial Viniflora CiNe starter (Chr. Hansen). The phage lysate of a mitomycin C (MC)-treated culture was enumerated on Man Rogosa and Sharpe (MRS) agar supplemented with cations (MRSϕ) using the sensitive host O. oeni IOEBS277. Putative lysogens were recovered from the turbid plaques obtained and streaked onto agar plates. MRS is known to be the standard medium for LAB in terms of cultivation. However, the slow-growing bacterium O. oeni often requires up to 10 days of incubation before small white colonies become evident on this medium. A key criterion in the design of our protocol of lysogenization was to achieve the fastest colony growth. As a first step to meet this requirement, red grape juice (RGJ) agar was preferred to MRS agar to streak bacteria, as it allows the development of O. oeni IOEBS277 as smooth white colonies in 5 to 6 days. Surprisingly, bacteria contained in turbid plaques consistently segregated into white and red-brown colonies, with the latter type being dominant (Fig. 1). It was observed that the brown-red color appeared in the center of the colony and then progressed to the edge and that color deepened with incubation time. Segregation was not influenced by the incubation conditions and was observed when RGJ plates were incubated in unsealed plastic bags or under strict anaerobiosis in a jar. As other distinctive colony characteristics, red colonies had a slightly granular appearance and were hard to pick off the plate compared with white smooth colonies. Last, the observed red phenotype was found to be stable after repeated subculturing of red colonies on RGJ plates (data not shown).
FIG 1.
An individual plaque produced by the temperate phage CiNeA on its host O. oeni IOEBS277 on MRSϕ agar was picked and streaked on red grape juice agar. Both red and white colonies were observed after 5 days of incubation at 25°C.
Red colonies correspond to lysogenic derivatives of strain IOEBS277.
Five colonies of each morphotype (red and white) obtained during the lysogenization of strain IOEBS277 by phage CiNeMCA were randomly selected from an RGJ agar plate and characterized. All multilocus variable-number tandem-repeat analysis profiles were identical and corresponded to that of the parental nonlysogenic strain IOEBS277. Superinfection immunity was checked, and all red colonies were resistant to phage CiNeMCA, while white colonies were sensitive (n = 4) or resistant (n = 1). Next, red and white colonies were PCR tested for the presence of phage-related sequences. Red colonies showed PCR signals for the IntA-type integrase sequence and for the cognate left junction attachment site (attLA), demonstrating that the integration of an IntA-type prophage had occurred at the expected attachment site (attBA) in the bacterial chromosome. All five white colonies (sensitive or resistant to phage CiNeMCA) were negative for prophage presence and, therefore, mostly corresponded to surviving noninfected host cells following temperate phage infection.
The possibility that the red colonies obtained during lysogenization may have arisen through accidental contamination and lysogenization events by a distinct temperate oenophage (IntB, IntC, or IntD type) was considered at this stage. We ruled out this possibility experimentally by performing additional PCR amplifications on the 10 selected white/red colonies. We used six couples of primers targeting the integrase gene present in IntB, IntC, and IntD phages and the corresponding empty bacterial attachment sites in the bacterial chromosome (20). Experiments showed that all three chromosomal sites used for site-specific recombination of IntB, IntC, or IntD types were prophage free in all colonies tested, while none of the integrase signals for IntB, IntC, and IntD type was amplified (data not shown). We concluded that no lysogenization or pseudolysogenization of colonies by any IntB-, IntC-, or IntD-type phage occurred during our experiments. To definitively rule out the possibility of a lysogenization event by a putative and so far undescribed temperate oenophage, one red colony was further selected and its genome fully sequenced. Comparison with the genome data of strain IOEBS277 confirmed the integration of the CiNeA phage and the absence of an additional phage-related sequence and any recombination event in the chromosome of the lysogenic derivative.
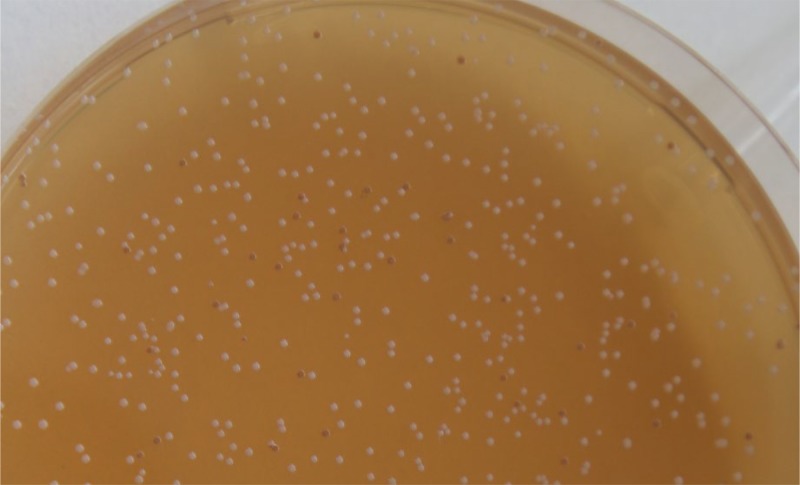
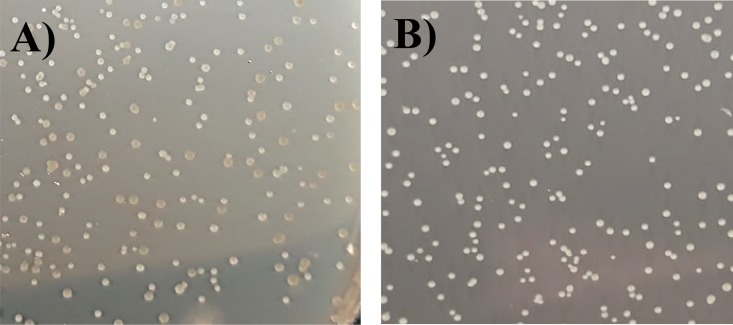
A blind test was carried out among uninformed investigators in our laboratory, in order to assess their capacity to visually differentiate red and white colonies. Strain IOEBS277 and the sequenced lysogenic derivative harboring the CiNeA prophage were grown separately to exponential phase in MRS broth and mixed to create different initial CFU ratios (1/1, 2/1, and 1/2). Samples were enumerated on RGJ agar plates and counting was assigned to three candid collaborators. The ratios of white to red colonies measured were in agreement with the initial values in the original samples, suggesting that the test is reliable (Fig. 2; see Table S1 in the supplemental material).
FIG 2.
Enumeration of a mixture 1:10 of O. oeni IOEBS277 and its lysogenic derivative IOEBS277 (CiNeA) on RGJ plates, showing different colony morphotypes (red/white).
The method proved successful with another phage-sensitive strain.
We assessed whether our method for the rapid isolation of lysogenic derivatives also proved successful when replacing strain IOEBS277 with another phage-sensitive strain. We screened our collection (Centre de Ressources Biologiques Oenologiques [CRB Oeno]) for a strain that was sensitive to phage CiNeMCA and genetically distinct from IOEBS277 (27). The nonlysogenic strain S25 was found to meet both criteria and was submitted to the protocol of lysogenization. The streaking of the bacteria recovered from plaques formed by CiNeMCA also resulted in the formation of white and red colonies on RGJ agar. Multilocus variable-number tandem-repeat analysis (MLVA) and PCR experiments confirmed that red colonies, and not white ones, corresponded to lysogenic derivatives. Enumeration of a culture containing both O. oeni S25 and a confirmed lysogenic derivative (1:1 ratio) was carried out on RGJ agar, and counts of red and white colonies corroborated our experimental results obtained with strain IOEBS277 (Table S1). We concluded that CiNeMCA lysogens of O. oeni undergo white-red morphotypic switching when grown on agar medium containing red grape juice.
Is the red morphotype a consequence of prophage integration?
We next assessed whether the red morphotype was a consequence of prophage integration in the bacterial chromosome as part of a process called lysogenic conversion. This could possibly result from the disruption of a bacterial gene (or sequence) upon site-specific integration at the attBA site or from the expression of prophage genes, providing novel traits to the host.
The first hypothesis was ruled out by performing the lysogenization test in strain IOEBS277 using oenophages that integrate at other loci in the bacterial chromosome, such as attBB and attBD. Phage 10MC (IntB type) (28) and the IntD-type phages OE33SAG and 9805MC (20, 22) were chosen. It should be pointed out that no IntC-type oenophage was available in our laboratory for this experiment. As a matter of fact, we were unsuccessful in isolating any IntC oenophage during our two past sampling campaigns targeting musts and wines (20, 22). Moreover, no monolysogenic strain containing an IntC prophage was identified in the CRB Oeno collection. In contrast, IntC prophages were systematically associated with other types (IntA, IntB, and IntD) in double or triple lysogens. The latter strains were MC induced. However, no plaques corresponding to IntC phages could be detected in the supernatant, and no PCR signal was detected using primers specific for the IntC integrase (20). This suggests that IntC prophages in lysogenic strains contained in the CRB Oeno collection are defective. Despite the lack of IntC phages, lysogenization assays of strain IOEBS277 were carried out with the IntB- or IntD-type temperate oenophages and produced white and red colonies on RGJ agar. Only the latter colonies corresponded to lysogenic derivatives, as shown by PCR amplification of the integrase and left attachment sequences, which demonstrated that the bacterial chromosome harbored a phage belonging to the expected group (i.e., the one used for lysogenization). We concluded that lysogenization of O. oeni with a member of phage group IntA, IntB, or IntD confers a distinctive colony appearance on RGJ agar. Since the tested temperate oenophages use 3 distinct host tRNA genes as the target sites for integration in O. oeni (20), it is reasonable to assume that the transition from white to red colonies does not result from a putative disruption of a bacterial gene (or a regulatory region) during site-specific recombination of temperate oenophages in the bacterial chromosome.
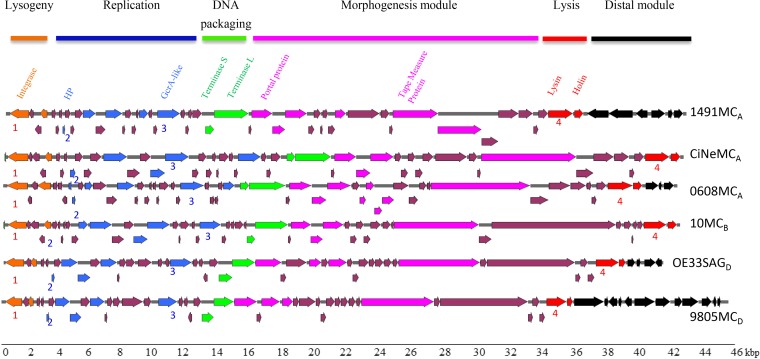
Another tempting hypothesis is that the expression of genes carried by prophages during lysogeny could impact colonial development of lysogens on RGJ agar. This would imply that the different prophages tested in our study share common genes. This scenario was, therefore, tested through a genomic comparison of prophages CiNeMCA, 10MCB, OE33SAGD, and 9805MCD. Two additional IntA-type oenophages, namely, 0608MCA and 1491MCA, were also included as they were also shown to yield red colonies on RGJ agar after lysogenization of strain IOEBS277 (29). Both phages share a genome organization with CiNeMCA but exhibit slight differences in their gene contents, especially in the rightward module containing putative moron genes (beneficial phage genes, which are not required for the life cycle of the phage itself, and add “more on” the phage genome in which they are found) (29) (Fig. 3). The Anvi’o program (30) was used to visualize the core genome of the 6 selected phages. A set of 4 shared genes (1 to 4) was clearly shown across the 6 genomes. Their functional homogeneity indexes were 0.76, 0.86, 0.82, and 0.76, respectively. None of the corresponding genes were located in the rightward module containing moron genes (19, 20, 31) (Fig. 3). Open reading frames 2 (ORF2) and 3 (ORF3) were both located in the replication module (ORF8 and ORF22 on the genome of 1491MCA). ORF2 codes for a small hypothetical protein, while ORF3 specifies a 407-amino-acid (aa) protein that contains a helix-turn-helix (HTH) motif and is related to the Caulobacter crescentus transcription factor GcrA. ORF1 and ORF4 correspond to the integrase and endolysin sequences, respectively. Both sequences have been previously reported to share significant overall nucleotide and deduced amino acid similarities, even though polymorphic zones allowed the design of specific primer couples (20, 21).
FIG 3.
Characteristics of the oenophages used in this study. Schematic representation of the organization of the 6 distinct oenophages used in the study, namely, 1491MCA, CiNeMCA, O608MCA, 10MCB, O33SAGD, and 9805MCD. Modules are indicated with the following color code: orange, lysogeny module; blue, replication-associated genes; green, DNA packaging; pink, morphogenesis module; red, lysis; black, distal modules; and purple, hypothetical genes. Information is taken from references 20, 27, 28, and 32. Conserved ORFs are numbered from 1 to 4.
With regard to our initial question, the genomic location and putative functions (replication associated-genes, lysin, and integrase) of the four conserved genes are not consistent with their expression during lysogeny. Considering basic phage biology, it is a striking fact that such genes are expressed in the course of induction, as the prophage excises from the host, enters the lytic cycle, and releases new virions. We, therefore, explored the alternative hypothesis that the red morphotype might be a consequence of prophage maintenance instability on RGJ agar.
The red morphotype requires intracolonial lysis events.
We hypothesized that lysogenic cells, when grown as colonies on RGJ agar, could experience prophage excision leading to cell lysis. In this scenario, the white to red color switch would next be triggered by some interactions between lysed cells and so far uncharacterized pigments present in RGJ.
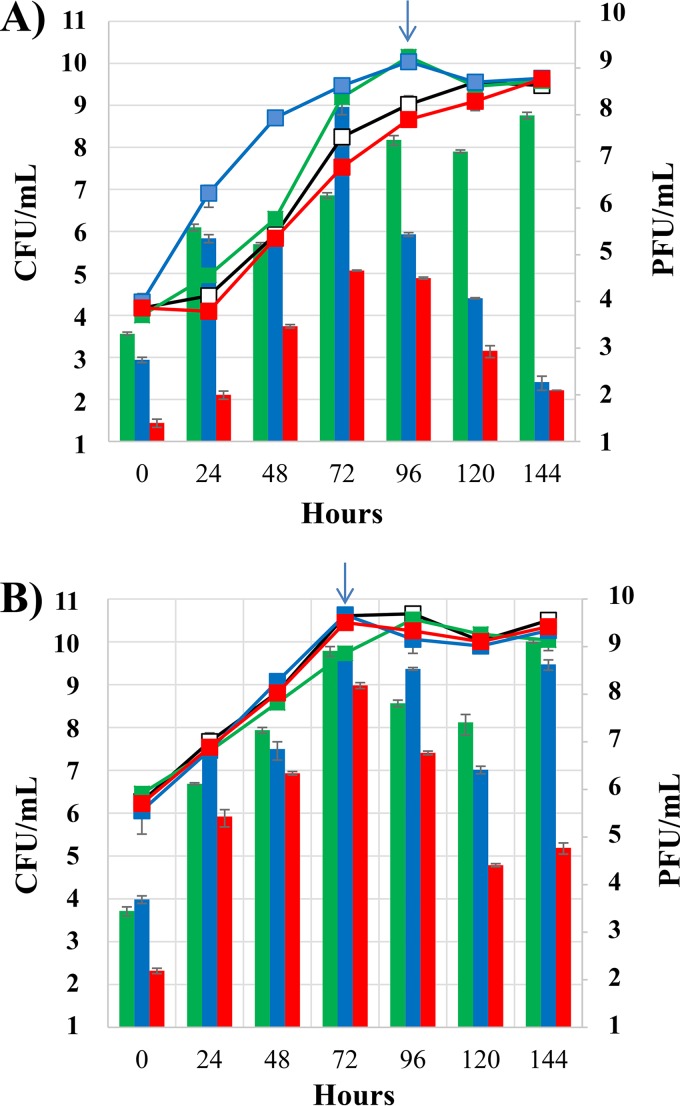
To confirm our hypothesis, we first verified that lysogenic cells containing IntA, IntB, or IntD phages could indeed spontaneously release phage in RGJ broth. The growth profiles and quantification of phage particle release of the three derivatives harboring CiNeMCA, OE33SAGD, and 10MCB were monitored simultaneously (Fig. 4). Strains had slightly different growth patterns in RGJ broth (Fig. 4A), and the lag time duration was significantly lower for the derivative harboring 10MCB. All strains produced phage particles and titers increased during exponential growth. Spontaneous induction dynamics performed for strains lysogenized with 10MCB and CiNeMCA showed maximum yields of phage crop of 108 PFU/ml as the cells reached the stationary phase. The titer was reduced to 4 × 104 PFU/ml for the third lysogen harboring OE33SAGD. The dynamics of release were also different between phages. While both OE33SAGD and 10MCB were transiently detected and showed a strong decrease in titer during stationary phase, phage titer remained the same for phage CiNeMCA throughout the stationary phase. This suggests that its production and/or persistence in the medium is different compared with the D- and B-type phages tested. Interestingly, we observed that prophage induction is phage and also medium specific, as the dynamics differed between RGJ and MRS broth (Fig. 4B). As an example, the spontaneous release of 10MCB was observed to be significantly higher in MRS than in RGJ.
FIG 4.
Growth curves and spontaneous phage release of lysogenic derivatives of O. oeni IOES277 in red grape juice (RGJ) (A) or in MRS (B). Precultures were prepared in RGJ broth and MRS broth. Strains were IOEBS277 (open box), IOEBS277 (CiNeA) (green box), IOEBS277 (10MCB) (blue box), and IOEBS277 (OE33SAGD) (red box). The arrow represents the end of exponential growth.
We next searched for additional evidence that the red morphotype was due to intracolonial lysis of lysogens on RGJ agar. We reasoned that this hypothesis would predict that white colonies formed by a phage-sensitive strain on RGJ agar would also experience lysis and then turn to red following infection by an exogenously provided lytic phage. To test this, RGJ agar plates were spread in duplicate with serial dilutions of a culture of the sensitive strain IOEBS277. One plate was further air sprayed with the virulent oenophage OE33PA (31, 32), while the control was kept uninfected. After incubation, we observed a transition from white to red morphotype for most colonies (Fig. 5A and B). Additional experiments showed that no red color was observed when a drop of the phage lysate was used or when sterile MRS (pH 4.8) was spotted onto an RGJ plate (see Fig. S1 in the supplemental material). Accordingly, the transition was also observed when the phage lysate was replaced with lysozyme (Fig. 5C). Since the observed red morphotype on RGJ agar occurs in a lysogen and also in a sensitive strain following the attack by a true lytic phage or treatment with lysozyme, we concluded that the white-red morphotypic switching is, therefore, most likely due to intracolonial lysis events. Also of note is that faint traces of red appeared in the whole agar zone sprayed with lysozyme (Fig. 5C). Accordingly, a drop of lysozyme or bovine serum albumin produced a red color on RGJ agar in the absence of bacteria after 24 h of incubation (Fig. S1). This suggests the formation of a chromogenic-complexing reaction between polyphenolic compounds (PC) and proteins. The deep red color associated with lysed colonies may, thus, result from a local increase in the protein content and/or in other macromolecules also able to form a complex with PC.
FIG 5.
Colonies produced by O. oeni IOEBS277 are white on RGJ agar (A) and turn to red following a lytic phage (B) or lysozyme spray application (C). A MRS culture was enumerated on RGJ agar in duplicate (control and assay) and plates were incubated at 25°C. After 3 days, the plate was air sprayed with the lytic phage OE33PA (lysate containing ∼109 PFU/ml) and further incubated for 3 days. Both control (A) and assay (B) were observed after 6 days of incubation. Alternately, a zone of the plate (represented by the circle) was sprayed with a freshly prepared lysozyme solution (10 mg/ml) in 10 mM Tris-HCl (pH 8.0) and 2 mM EDTA and observed after 15 min of incubation (C).
The growth of a bacterial colony on an agar plate proceeds in the following distinct stages: lag phase, exponential-growth phase, and, finally, a stationary phase where cell death occurs and gives rise to an accumulation of dead and disintegrated cells in the interior of the growing colony (33). Therefore, cell death also naturally occurs in nonlysogens in response to the limitation of local nutrients or as a consequence of an active death process, known as programmed cell death (34). A nonlysogen might, therefore, produce red colonies on RGJ agar after prolonged incubation. To check this, a culture of O. oeni IOEB277 was enumerated on RGJ agar and plates were incubated for more than 7 days at 25°C. After 10 days, we observed that colonies progressively switched from a white to a light pink color in the absence of phages. The same experiment was next carried out with a mixture of lysogens/nonlysogens. Both populations were readily distinguished as white and red colonies after 7 days. After 10 to 12 days, white colonies gradually turned to pink. However, a deeper tone was still exhibited by lysogens and a clear distinction between the two populations (lysogens/nonlysogens) was still observed (data not shown). We conclude that phage excision in colonies formed by lysogens causes intracolonial lysis mechanisms in O. oeni IOEBS277 that can be captured on RGJ agar and easily distinguished from autolysis.
Polyphenol-rich extracts from grape are involved in the color switch exhibited by lysogenic cells.
The final piece of the puzzle was to identify the components in the RGJ medium that could interact with lysed cells, resulting in a change in colony color phenotype from white to red. We first observed that filter sterilization of RGJ medium produced red colonies similar to those obtained after sterilization by autoclaving (data not shown). This shows that the presumptive compounds were not formed during heat treatment and were natural constituents of the RGJ medium. Polyphenolic compounds (PC) retained our attention. They can be classified into two groups, namely, nonflavonoids and flavonoids. A most significant function of the flavonoids, especially the anthocyanins, is their contribution to flower and fruit colors (35, 36). In particular, anthocyanins appear red or purple at low pH. Our first strategy was to implement methods to release putative PC bound to the cell wall matrix of bacteria forming red colonies on RGJ agar plates. Standard extractions were performed with methanol and formic acid or with acetone. The compounds contained in the extracts were separated and identified using liquid chromatography-mass spectrometry (LC-MS) using a high-tech detection method (see Materials and Methods). Despite many attempts, only quercetin glucoside, a colorless flavonoid molecule, was detected in trace quantities in red and not in white colonies (see Fig. S2 in the supplemental material). This may suggest that there are irreversible covalent bonds between PC and macromolecules within the cell wall matrix, as already reported with yeasts (37), and that the methods used failed to release PC from their environment. An alternative is that the red color does not result from a reaction between PC and the cell wall matrix but between PC and other macromolecules released after cell lysis.
Another approach was, therefore, followed. White grape juice (WGJ) agar was prepared and enriched with an extract containing anthocyanins and tannins (38). Plates were used to enumerate a 1:1 mixture of O. oeni IOEBS277 and its lysogenic derivative harboring CiNeMCA, and 50% white and 50% red colonies were observed (Fig. 6A). All colonies were white in the absence of PC (Fig. 6B). We conclude that PC from red grape juice are the compounds that interact with damaged cells produced during prophage release in a colony formed by a lysogen. This yields a red color in the colony, allowing for an easy differentiation between lysogens and nonlysogens.
FIG 6.
The white-to-red switch of lysogens is observed on white grape juice agar supplemented with an extract containing PC. A mixture of O. oeni IOEBS277 and its lysogenic derivative IOEB277 (CiNeA) (1:1) was enumerated on WGJ agar containing an extract containing PC (50 mg/ml) (A) or without PC (B). Red and white colonies were observed in the presence of PC only. PCR tests targeting phage sequences showed that they corresponded to lysogens and nonlysogens.
The test is applicable to other LAB species.
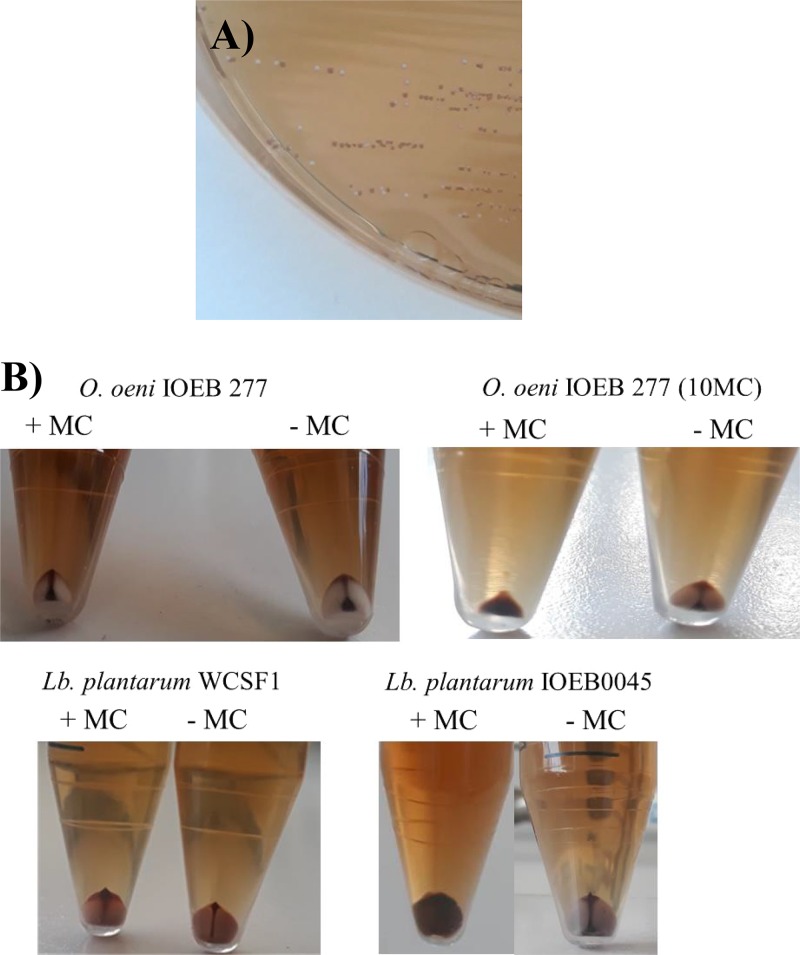
From our observations, we hypothesized that our test could be adapted to other LAB species, as many can grow at pH 4.8 to 5. We took advantage of an ongoing project focusing on prophage diversity among LAB in sourdough fermentation, as previously described (39). These studies showed that strain Leuconostoc citreum A7 is a lysogen. The strain was indeed observed to lyse when treated with MC, while the MC supernatant yielded plaques on Lc. citreum NRRL B-742. This was a unique opportunity to assess whether the proposed protocol could be successful in selecting an isogenic pair (wild type [wt]/lysogenic derivative) in Lc. citreum NRRL B-742. Plaques formed by phage A7MC were picked and isolated on RGJ agar. As observed with O. oeni, cells segregated into white and red colonies (Fig. 7A). Randomly picked red colonies (n = 3) were resistant to phages, while white colonies (n = 3) were sensitive. Red and white colonies of Lc. citreum were subsequently grown in MRS and induced with MC. Only cultures from red colonies lysed. Accordingly, DNA was successfully extracted from the cell-free lysates obtained from red colonies only. Their restriction profiles were in agreement with the presence of a prophage (∼40 kb) in the bacterial chromosome (data not shown).
FIG 7.
The test is applicable to other species of LAB. (A) A turbid plaque produced by phage A7MC on Leuconostoc citreum NRRL B-742 was streaked onto RGJ agar. Bacterial cells segregated into white (strain NRRL B-742) and red (lysogenized derivatives) colonies. (B) The method was adapted to RGJ broth. Cells were grown to stationary phase in RGJ broth with (+MC) or without mitomycin C (−MC). After 24 h (Lb. plantarum) or 72 h (O. oeni), the culture was centrifuged (2,000 × g for 2 min) and the color of the pellet was observed. The volumes of the cultures were 10 ml, except for those of IOEB277 (10MC), which were 5 ml.
Lactobacillus plantarum was also an interesting candidate. This ubiquitous LAB is able to colonize several ecological niches, including vegetables, meat, dairy substrates, and the gastrointestinal tract. In addition, Lb. plantarum strains commonly exhibit probiotic benefits and are an alternative to perform MLF. Three out of the five strains tested (ATCC 8014, ATCC 14917, and WSCF1) did not lyse upon treatment with MC, and this result was in agreement with previous reports showing that the strains are nonlysogens and/or contain noninducible prophages under similar induction conditions (40, 41). Strains IOEB1647 and IOEB0045, both isolated from wine, lysed upon treatment with MC. Unfortunately, the corresponding lysates did not form any plaques on the other strains tested. No isogenic pair (wt/lysogenic derivative) could, therefore, be obtained in Lb. plantarum. To circumvent this problem, we decided to grow each strain in RGJ broth in the presence or absence of MC and to observe the color of the cell pellet upon centrifugation. We assumed that cell lysis would occur in lysogens in the presence of MC, increasing the interactions between damaged cells and PC, and then triggering a color switch from white to red in the pellet. The test was first assessed with two isogenic pairs selected in the present study in O. oeni (Fig. 7B) and Lc. citreum (data not shown). For the nonlysogenic strains, cell pellets obtained with or without MC were white, with small faint traces of red materials. In agreement with our hypothesis, the cell pellet obtained with the lysogen derivatives completely turned to red in the presence of MC. Next, Lb. plantarum strains were tested. Results obtained with strain WSCF1 are shown in Fig. 7B. The introduction of the inducing agent to the cultures did not trigger the white-red color switch. In contrast, both wine isolates yielded red pellets in the presence of MC. Results for strain IOEB0045 are shown. The presence of phage DNA was observed in the supernatant of both MC-induced cultures (data not shown).
These data support our hypothesis that our technique will be a valuable tool in bacteriophage research in the LAB family.
DISCUSSION
The recent finding that temperate phages are an integral part of many bacterial genomes and appear to be responsible for interstrain genetic variation has led to a resurgence of interest in phage research in the LAB family and O. oeni (19–22, 31, 32, 42). However, we still know relatively little about the impact of lysogeny on MLF, irrespective of whether the fermentation process relies on indigenous strains or on inoculated commercial MLF starter cultures. In this work, we designed a quick and reliable method to establish bacterial lysogens for temperate phages in O. oeni. We observed that lysogens undergo white-red morphotypic switching when grown on agar medium containing red grape juice, providing a simple and inexpensive method to distinguish between lysogenic and nonlysogenic derivatives with a minimum of expended time and effort. We established a link between the occurrence of red pigmentation of colonies and intracolonial lysis on RGJ agar and posit that lysogens produce red colonies on RGJ agar as a consequence of phage-mediated lysis due to prophage release. This assumption is consistent with our earlier findings about the peculiar colonial morphology associated with red colonies. This finding was also borne out by the detection of spontaneous release of phage particles by all tested lysogens in RGJ broth. Even though variations in titers were observed among tested phages, the minimum level of spontaneous excision (104 PFU/ml) was sufficient to trigger the switch to red color in 7 days on RGJ agar, irrespectively of the prophage type. The reason why a given strain when lysogenized by different phages produces such different amounts of particles is not known. However, this is most probably dependent on the phage repressor system in each phage and/or the persistence capacities of the particles following their release in the RGJ medium. Differences in the capacity of adsorption of the released phages to cell debris are an alternative explanation.
The other key factor required for the observation of red colonies is the interaction of lysed cells with PC and especially flavonoids. The growth of lysogens as colonies on agar medium is proposed to foster the development of the red color, as the colonies formed by lysogens are likely to create microzones where a local release of cytoplasmic content and cell wall fragments occurs. An increase in the concentration of and/or a better access to macromolecules may then facilitate binding to PC. This is consistent with the fact that anthocyanins appear red or purple at low pH and are prone to form noncovalent as well as covalent associations with macromolecules in foods and beverage (35, 36, 43). Mechanisms of complexation between PC and biopolymers in cell walls have also been studied in yeasts (37, 44) and bacteria (45). Further studies are under way to better characterize the molecular mechanisms underlying these interactions between PC and lysed cells. Proteins may be good candidates (46, 47). Accordingly, spots of concentrated solutions of lysozyme and bovine serum albumin produced a red pigmentation on RGJ agar (Fig. S1). Polysaccharides can also be proposed (48), and this hypothesis is all the more attractive as Oenococcus oeni synthesizes both homo- and heteropolysaccharides (49) which can be found associated with the cell wall or as precursors form in the cytoplasm.
It would also be worthwhile to consider that PC may play more than a role of marker for cell lysis in lysogens and could also regulate prophage excision. The existence of an interplay between prophage induction and the synthesis of polyphenolic pigments has been proposed in Marinomonas mediterranea (50). In our study, patterns of phage release, such as for 10MC, were slightly different in RGJ and MRS. This aspect deserves more attention as it may have implications for environmental control of the lysogenic switch in natural populations of O. oeni during wine making and influence bacterial diversity. Accordingly, changes in the diversity of O. oeni strains were recently observed when grape phenolic compounds were added to red wines from the Douro demarcated region in Portugal (51).
In the course of conversations with wine microbiologists from academic institutes and industries, it appears that many of our colleagues have repeatedly observed the presence of both red and white colonies on RGJ agar when enumerating LAB during wine fermentation, and this was often attributed to contamination. Our study brings an explanation for these intriguing field observations. Interestingly, our preliminary data also suggest that the test can be used in two other industrially important species of LAB (Lc. citreum and Lb. plantarum), even though a limited number of strains have been tested in the present study. The information that the red phenotype reflects spontaneous induction of prophage (and its commitment to lytic cycle) is attractive, as it makes the test a marker for the presence of complete and inducible prophages among the tested LAB. Supporting evidence was provided with strains Lb. plantarum WSCF1 and Lc. citreum NRRL B-742 which both contain remnants/noninducible prophages and give white colonies/cell pellets in RGJ (41, 52). The test is also expected not to be applicable to the detection of pseudolysogeny, which exists in O. oeni (53), as the mechanism does not produce virions or induce cell lysis.
In the wine making context, our study brings an inexpensive and simple way to isolate lysogens to better characterize the lysogenic status of future commercial FML starters and understand the impact of lysogeny in O. oeni. A relevant question is whether the test will be applicable to wine samples in order to monitor the extent of lysogeny in the autochthonous population of O. oeni over the wine making process. This is not so straightforward since prolonged incubation of RGJ plates revealed a weak color switch in nonlysogens that we interpret as a result of autolysis. Wines are known to contain a diversity of indigenous O. oeni strains, as well as other LAB, which exhibit different growth and autolysis rates (54) and prophage contents (19, 20, 42). Diversity may possibly hamper the clear distinction between white and red colonies. Future experiments will be performed to test the theory on samples collected in the course of the 2019 vintage.
Moving forward, the simplicity of the test also opens up a new perspective of testing other bacterial models, with the aim of providing the phage community a new tool.
MATERIALS AND METHODS
Bacterial and culture conditions.
Strains of O. oeni and Lb. plantarum were obtained from the Centre de Ressources Biologiques Oenologiques (CRB Oeno; ISVV, Villenave d’Ornon, France). The genome sequences of strains of O. oeni are publicly available (27, 28, 49, 55, 56). Leuconostoc citreum LBAE-A7 and NRRL B-742 were obtained from the Laboratoire de Biotechnologie Agroalimentaire et Environnementale (LBAE) collection (Université Paul Sabatier, Auch, France). All bacterial strains were grown in MRS (Difco, Fischer Bioblock Scientific, Illkirch, France) or in red grape juice (RGJ) broth. RGJ contains 25% (vol/vol) commercial red grape juice (Reflets de France), 0.5% (wt/vol) yeast extract, and 0.1% (vol/vol) Tween 80. White grape juice (WGJ) medium was obtained by replacing commercial red grape juice with clear white grape juice (Reflets de France). Reconstituted red grape juice (RRGJ) medium was obtained by adding a grape extract containing polyphenolic compounds (38) to WGJ broth to a final concentration of 50 mg/liter. Media were adjusted to pH 4.8 at 25°C. When requested, 1.6% (wt/vol) agar was added. All media were heat or filter sterilized. Plates were placed in nonsealed plastic bags to avoid desiccation and incubated at 25°C. Alternately, plates were incubated anaerobically (AnaeroGen; Oxoid).
Phage lysates.
Lysates of the OESAG and OE33PA oenophages were used (Table 1). Tested phages belong to the IntD and IntB groups, respectively, and have been previously isolated from red wines (20). We also obtained phage lysates after mitomycin C (MC) induction of lysogenic strains of O. oeni (Table 1), Lc. citreum LBAE-A7, and all Lb. plantarum strains tested. Briefly, strains were grown in MRS broth to reach an optical density at 600 nm (OD600) of 0.2 before adding MC (1 μg/ml) and incubated for 24 h at 25°C. OD600 was monitored until a decrease was observed. Supernatants were filtered through a 0.2-μm polyethersulfone (PES) membrane filter (20).
TABLE 1.
Phages used in this study
| Phage lysate | Origin | Reference(s) |
|---|---|---|
| 10MCB | O. oeni B10 (CRB Oeno); MC-induced | 49 |
| CiNeMCA | O. oeni Viniflora CiNe (CHR Hansen); MC-induced | 49 |
| 1491MCA | O. oeni IOEB1491 (CRB Oeno); MC-induced | 49 |
| O608MCA | O. oeni IOEBO608 (CRB Oeno); MC-induced | 49 |
| 9805MCD | O. oeni IOEB9805 (CRB Oeno); MC-induced | 49 |
| OE33SAGD | Isolated from sweet wine (must) (Bordeaux area, France; Sauvignon) | 49 |
| OE33PAB | Extemperate phage isolated from red wine (Bordeaux) | 31, 32 |
| A7MC | Lc. citreum LBAE-A7 (wheat sourdough); MC-induced | This study |
Oenophages were propagated on strain IOEBS277, which does not contain endogenous phage and is sensitive to all oenophages so far isolated in our laboratory (20, 22, 31, 32). The Lc. citreum phage A7MC was propagated on strain NRRL B-742, which does not harbor any inducible prophage (52).
Plaques were obtained using the classical double-layer plating technique, using MRS agar supplemented with MgSO4 (3.75 g/liter) and CaCl2 (2.375 g/liter) (MRSϕ). Plates were placed in unsealed plastic bags and incubation was carried out at 25°C for 4 to 7 days to allow plaque formation. Single plaques were reisolated three times to ensure the purity of the phage lysates. The absence of contaminating oenophage in lysates was checked by PCR amplifications using primers targeting the different integrase sequences (IntAf, IntAr, IntBf, IntBr, IntDf, and IntDr) (20).
Construction and characterization of isogenic strain pairs.
Isogenic pairs (wt/lysogenic derivative) were selected in O. oeni and Lc. citreum as follows. Each phage lysate was enumerated on a sensitive host, namely, Lc. citreum NRRL B-742 for phage A7 and O. oeni IOEB277 for oenophages. Alternately, we also used the nonlysogenic host strain O. oeni S25, which was also found to be phage sensitive in earlier studies (20). Previous population structure analyses based on multilocus sequence typing (MLST) of strains from diverse products (wines, ciders, and kombucha) and geographic origins have revealed that O. oeni consists of four genetic groups of strains (A to D) (27, 56, 57). Group D comprises all five strains recently isolated from kombucha. Group B and C strains have been isolated from wine and cider, while group A contains only wine strains, suggesting they are the best adapted to wine. IOEBS277 and S25 were shown to belong to different subgroups in the phylogroup A (27, 57).
Phages were observed to produce turbid plaques on MRSϕ plates after 5 to 6 days of incubation. Presumptive lysogenic colonies were obtained by picking viable cells from the center of a turbid plaque and further streaking onto RGJ plates to obtain single colonies. Ten colonies were recovered and 3 serial-streak isolations were made. Immunity to phage infection was tested by spotting serial dilutions of phage lysates onto lawns of the obtained colonies. For O. oeni, bacterial colonies were genotyped by multilocus variable-number tandem-repeat analysis (MLVA) specific to the species (58). Colonies were then submitted to PCR tests to confirm their lysogenization by a single phage belonging to the IntA, IntB, IntC, or IntD group. Last, the attL phage insertion sites present in lysogenic strains were amplified by PCR in order to confirm site-specific integration in the bacterial chromosome. The protocol has been previously described (20). For Lc. citreum, putative lysogens were grown in MRS broth and MC induced. The supernatant was used to extract phage DNA as previously reported (22). The DNA was digested with restriction endonucleases under the conditions recommended by the manufacturer.
Rapid test for assessment of lysogeny in RGJ broth.
Cells (lysogens or nonlysogens) were grown to stationary phase in RGJ broth (10 ml) with (+MC) or without mitomycin C (−MC). After 24 h (Lb. plantarum) or 72 h (O. oeni and Lc. citreum), the cultures were centrifuged (2,000 × g for 2 min), and the colors of the pellets were observed.
Spraying of seeded plates with phage lysate.
A culture of strain O. oeni IOEBS277 was grown in MRS and enumerated in duplicate using serial dilutions and the spread plate method on RGJ agar. Incubation of both series was performed at 25°C. After 3 days of incubation, one of the series was removed and air sprayed with a suspension of phage OE33PA (109 PFU/ml) using a commercial 30-ml spray bottle (Sephora collection). Plates were placed back into the incubator for an additional 4-day period. The aspects of the colonies were compared in the control and the phage-treated plates after 7 days.
Genomic analyses.
The 6 prophage genomes analyzed have been previously published (27, 28). The anvi’o pangenomic workflow (version 5.3) was used to analyze the data as previously reported (30, 59). In particular, the flags “–use-ncbi-blast,” and parameters “–minbit 0.2,” and “–mcl-inflation 2” were used to calculate similarities of each amino acid sequence encoded by every genome against every other amino acid sequence using blastp. A total of 318 genes were identified and classified in 143 gene clusters (GCs). Each GC contains one or more genes contributed by one or more isolated phage genomes. Functional homogeneity indexes (FHIs) were also calculated for each GC. The FHI considers aligned residues (by ignoring gaps) and attempts to quantify differences across residues in a site by considering the biochemical properties of differing residues (which could affect the functional conservation of genes at the protein level).
The chromosome of the lysogenic derivative IOEB277 (CiNeMCA) was sequenced at the Centre Génomique Fonctionnelle de Bordeaux (CGFB) platform using the TruSeq kit (Illumina, San Diego, CA, USA) and an Illumina MiSeq system with 2 × 250-bp paired-end reads. Assembly of the resulting reads was performed using SPAdes version 3.10.1 (60).
Extraction and analysis of polyphenolic compounds.
Two sets of 60 red colonies of lysogens were recovered from RGJ agar plates. The same protocol was used for white colonies of nonlysogen O. oeni IOEBS277. One set of red or white colonies was added with 150 μl of methanol (MeOH) and formic acid (1%). The second set was added with 150 μl of acetone. The four samples were vortexed and cells were disrupted by sonication for 20 min with regular vortexing. They were centrifuged at 3,000 rpm for 5 min. The acetone supernatants were evaporated overnight and resuspended with 150 μl of MeOH. The four samples were filtered (0.45 μm). They were analyzed on a high-pressure liquid chromatography (HPLC) instrument with a diode array detector, coupled to mass spectrometry (MS) using the electrospray ionization interface (ESI), and chromatographed using an HPLC-MS system. The chromatography apparatus was Agilent 1200 from Agilent Technologies (Santa Clara, CA). The fractions were analyzed using a Prontosil 120-5-C18-AQ reverse phase column (Bischoff, Leonberg, Germany) as previously described (61).
Supplementary Material
ACKNOWLEDGMENTS
Support for this project was provided by the French ANR (grant ANR‐14‐CE20‐0007) to the Enology group.
C.L.M. devised the study concept. F.J., P.L., and O.C. annotated the genomes and performed genomic comparisons. A.C., C.P., and F.J performed the experiments. S.C. and M.J. provided the extracts containing polyphenolic compounds. All authors contributed to the data. C.L.M., O.C., and A.C. wrote the initial manuscript draft.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00997-19.
REFERENCES
- 1.Pratama AA, van Elsas JD. 2018. The “neglected” soil virome—potential role and impact. Trends Microbiol 26:649–662. doi: 10.1016/j.tim.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Motegi C, Nagata T, Miki T, Weinbauer MG, Legendre L, Rassoulzadegan F. 2013. Interactive effects of viral and bacterial production on marine bacterial diversity. PLoS One 8:e76800. doi: 10.1371/journal.pone.0076800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitbart M, Bonnain C, Malki K, Sawaya NA. 2018. Phage puppet masters of the marine microbial realm. Nat Microbiol 3:754–766. doi: 10.1038/s41564-018-0166-y. [DOI] [PubMed] [Google Scholar]
- 4.Mills S, Shanahan F, Stanton C, Hill C, Coffey A, Ross RP. 2013. Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes 4: 4–16. doi: 10.4161/gmic.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr J. 2017. A bacteriophages journey through the human body. Immunol Rev 279:106–122. doi: 10.1111/imr.12565. [DOI] [PubMed] [Google Scholar]
- 6.De Sordi L, Lourenço M, Debarbieux L. 2018. “I will survive”: a tale of bacteriophage-bacteria coevolution in the gut. Gut Microbes 10:1–8. doi: 10.1080/19490976.2018.1474322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard-Varona C, Hargreaves KR, Abedon ST, Sullivan MB. 2017. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J 11:1511–1520. doi: 10.1038/ismej.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feiner R, Argov T, Rabinovich L, Sigal N, Borovok I, Herskovits AA. 2015. A new perspective on lysogeny: prophages as active regulatory switches of bacteria. Nat Rev Microbiol 13:641–650. doi: 10.1038/nrmicro3527. [DOI] [PubMed] [Google Scholar]
- 9.Luo E, Aylward FO, Mende DR, DeLong EF. 2017. Bacteriophage distributions and temporal variability in the ocean’s interior. mBio 8:e01903-17. doi: 10.1128/mBio.01903-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ofir G, Sorek R. 2018. Contemporary phage biology: from classic models to new insights. Cell 172:1260–1270. doi: 10.1016/j.cell.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Choua M, Bonachela J. 2018. Ecological and evolutionary consequences of viral plasticity. bioRxiv 10.1101/289116. [DOI] [PubMed]
- 12.Obeng N, Pratama AA, van Elsas JD. 2016. The significance of mutualistic phages for bacterial ecology and evolution. Trends Microbiol 24:440–449. doi: 10.1016/j.tim.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Thingstad TF, Lignell R. 1997. Theoretical models for the control of bacterial growth rate, abundance, diversity, and carbon demand. Aquat Microb Ecol 13:19–27. doi: 10.3354/ame013019. [DOI] [Google Scholar]
- 14.Knowles B, Silveira CB, Bailey BA, Barott K, Cantu VA, Cobián-Güemes AG, Coutinho FH, Dinsdale EA, Felts B, Furby KA, George EE, Green KT, Gregoracci GB, Haas AF, Haggerty JM, Hester ER, Hisakawa N, Kelly LW, Lim YW, Little M, Luque A, McDole-Somera T, McNair K, de Oliveira LS, Quistad SD, Robinett NL, Sala E, Salamon P, Sanchez SE, Sandin S, Silva GG, Smith J, Sullivan C, Thompson C, Vermeij MJ, Youle M, Young C, Zgliczynski B, Brainard R, Edwards RA, Nulton J, Thompson F, Rohwer F. 2016. Lytic to temperate switching of viral communities. Nature 531:466–470. doi: 10.1038/nature17193. [DOI] [PubMed] [Google Scholar]
- 15.Silveira C, Rohwer FL. 2016. Piggyback-the-winner in host-associated microbial communities. NPJ Biofilms Microbiomes 2:16010. doi: 10.1038/npjbiofilms.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonvaud-Funel A. 1999. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Van Leeuwenhoek 76:317–331. doi: 10.1023/A:1002088931106. [DOI] [PubMed] [Google Scholar]
- 17.Arendt EK, Lonvaud A, Hammes WP. 1991. Lysogeny in Leuconostoc oenos. J Gen Microbiol 137:2135–2139. doi: 10.1099/00221287-137-9-2135. [DOI] [PubMed] [Google Scholar]
- 18.Tenreiro R, Santos R, Brito L, Paveia H, Vieira G, Santos MA. 1993. Bacteriophages induced by mitomycin C treatment of Leuconostoc oenos strains from Portuguese wines. Lett Appl Microbiol 16:207–209. doi: 10.1111/j.1472-765X.1993.tb01398.x. [DOI] [Google Scholar]
- 19.Borneman AR, McCarthy JM, Chambers PJ, Bartowsky EJ. 2012. Comparative analysis of the Oenococcus oeni pan genome reveals genetic diversity in industrially-relevant pathways. BMC Genomics 13:373. doi: 10.1186/1471-2164-13-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaomanjaka F, Ballestra P, Dols-Lafargue M, Le Marrec C. 2013. Expanding the diversity of oenococcal bacteriophages: insights into a novel group based on the integrase sequence. Int J Food Microbiol 166:331–340. doi: 10.1016/j.ijfoodmicro.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 21.Doria F, Napoli C, Costantini A, Berta G, Saiz JC, Garcia-Moruno E. 2013. Development of a new method for detection and identification of Oenococcus oeni bacteriophages based on endolysin gene sequence and randomly amplified polymorphic DNA. Appl Environ Microbiol 79:4799–4805. doi: 10.1128/AEM.01307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philippe C, Jaomanjaka F, Claisse O, Laforgue R, Maupeu J, Petrel M, Le Marrec C. 2017. A survey of oenophages during wine making reveals a novel group with unusual genomic characteristics. Int J Food Microbiol 257:138–147. doi: 10.1016/j.ijfoodmicro.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Torriani S, Felis GE, Fracchetti F. 2011. Selection criteria and tools for malolactic starters development: an update. Ann Microbiol 61:33–39. doi: 10.1007/s13213-010-0072-x. [DOI] [Google Scholar]
- 24.Matos RC, Lapaque N, Rigottier-Gois L, Debarbieux L, Meylheuc T, Gonzalez-Zorn B, Repoila F, de Fatima Lopes M, Serror P. 2013. Enterococcus faecalis prophage dynamics and contributions to pathogenic traits. PLoS Genet 9:e1003539. doi: 10.1371/journal.pgen.1003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandvalet C. 2017. Oenococcus oeni: queen of the cellar, nightmare of geneticists. Microbiology 163:297–299. doi: 10.1099/mic.0.000456. [DOI] [PubMed] [Google Scholar]
- 26.Gillis A, Mahillon J. 2014. Influence of lysogeny of tectiviruses GIL01 and GIL16 on Bacillus thuringiensis growth, biofilm formation, and swarming motility. Appl Environ Microbiol 80:7620–7630. doi: 10.1128/AEM.01869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell-Sills H, Khoury ME, Favier M, Romano A, Biasioli F, Spano G, Sherman DJ, Bouchez O, Coton E, Coton M, Okada S, Tanaka N, Dols-Lafargue M, Lucas PM. 2015. Phylogenomic analysis of Oenococcus oeni reveals specific domestication of strains to cider and wines. Genome Biol Evol 7:1506–1518. doi: 10.1093/gbe/evv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gindreau E, Torlois S, Lonvaud-Funel A. 1997. Identification and sequence analysis of the region encoding the site-specific integration system from Leuconostoc oenos (OEnococcus oeni) temperate bacteriophage phi 10MC. FEMS Microbiol Lett 147:279–285. doi: 10.1016/S0378-1097(96)00540-X. [DOI] [PubMed] [Google Scholar]
- 29.Taylor VL, Fitzpatrick AD, Islam Z, Maxwell KL. 2019. The diverse impacts of phage morons on bacterial fitness and virulence. Adv Virus Res 103:1–31. doi: 10.1016/bs.aivir.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Eren AM, Esen ÖC, Quince C, Vineis JH, Morrison HG, Sogin ML, Delmont TO. 2015. Anvi’o: an advanced analysis and visualization platform for ‘omics data. PeerJ 3:e1319. doi: 10.7717/peerj.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaomanjaka F, Claisse O, Blanche-Barbat M, Petrel M, Ballestra P, Le Marrec C. 2016. Characterization of a new virulent phage infecting the lactic acid bacterium Oenococcus oeni. Food Microbiol 54:167–177. doi: 10.1016/j.fm.2015.09.016. [DOI] [Google Scholar]
- 32.Jaomanjaka F, Claisse O, Philippe C, Le Marrec C. 2018. Complete genome sequence of lytic Oenococcus oeni bacteriophage OE33PA. Microbiol Resour Announc 7:e00818-18. doi: 10.1128/MRA.00818-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh P, Levine H. 2017. Morphodynamics of a growing microbial colony driven by cell death. Phys Rev E 96:052404. doi: 10.1103/PhysRevE.96.052404. [DOI] [PubMed] [Google Scholar]
- 34.Allocati N, Masulli M, Di Ilio C, De Laurenzi V. 2015. Die for the community: an overview of programmed cell death in bacteria. Cell Death Dis 6:e1609. doi: 10.1038/cddis.2014.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorrain B, Ky I, Pechamat L, Teissedre PL. 2013. Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules 18:1076–1100. doi: 10.3390/molecules18011076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dangles O, Fenger JA. 2018. Chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 23:E1970. doi: 10.3390/molecules23081970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mekoue Nguela J, Sieczkowski N, Roi S, Vernhet A. 2015. Sorption of grape proanthocyanidins and wine polyphenols by yeasts, inactivated yeasts, and yeast cell walls. J Agric Food Chem 63:660–670. doi: 10.1021/jf504494m. [DOI] [PubMed] [Google Scholar]
- 38.Breniaux M, Dutilh L, Petrel M, Gontier E, Campbell-Sills H, Deleris-Bou M, Krieger S, Teissedre PL, Jourdes M, Reguant C, Lucas P. 2018. Adaptation of two groups of Oenococcus oeni strains to red and white wines: the role of acidity and phenolic compounds. J Appl Microbiol 125:1117–1127. doi: 10.1111/jam.13946. [DOI] [PubMed] [Google Scholar]
- 39.Coudray-Meunier C, Gabriel V, Le Marrec C, Fontagné-Faucher C. 2018. 7th Int Symp Sourdough, Cork, Ireland, 6 to 8 June 2018. [Google Scholar]
- 40.Caso JL, De Los Reyes-Gavilan CG, Herrero M, Montilla A, Rodriguez A, Suarez JE. 1995. Isolation and characterization of temperate and virulent bacteriophages of Lactobacillus plantarum. J Dairy Sci 78:741–750. doi: 10.3168/jds.S0022-0302(95)76685-1. [DOI] [Google Scholar]
- 41.Ventura M, Canchaya C, Kleerebezem M, de Vos WM, Siezen RJ, Brüssow H. 2003. The prophage sequences of Lactobacillus plantarum strain WCFS1. Virology 316:245–255. doi: 10.1016/j.virol.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Bon E, Delaherche A, Bilhère E, De Daruvar A, Lonvaud-Funel A, Le Marrec C. 2009. Oenococcus oeni genome plasticity is associated with fitness. Appl Environ Microbiol 75:2079–2090. doi: 10.1128/AEM.02194-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golasz LB, da Silva J, da Silva SB. 2013. Film with anthocyanins as an indicator of chilled pork deterioration. Ciênc Tecnol Aliment Campinas 33:155–162. doi: 10.1590/S0101-20612013000500023. [DOI] [Google Scholar]
- 44.Medina K, Boido E, Dellacassa E, Carrau F. 2005. Yeast interactions with anthocyanins during red wine fermentation. Am J Enol Vitic 56:104–109. [Google Scholar]
- 45.Kudoh Y, Matsuda S. 2000. Effect of lactic acid bacteria on color tone and anthocyanin content of sweet potato yoghurt. Jpn Soc Food Sci Technol J 7:619–625. doi: 10.3136/nskkk.47.619. [DOI] [Google Scholar]
- 46.Hagerman AE, Butler LG. 1981. The specificity of proanthocyanidin-protein interactions. J Biol Chem 256:4494–4497. [PubMed] [Google Scholar]
- 47.Prigent SV, Voragen AG, van Koningsveld GA, Baron A, Renard CM, Gruppen H. 2009. Interactions between globular proteins and procyanidins of different degrees of polymerization. J Dairy Sci 92:5843–5853. doi: 10.3168/jds.2009-2261. [DOI] [PubMed] [Google Scholar]
- 48.Renard CMGC, Watrelot AA, Le Bourvellec C. 2017. Interactions between polyphenols and polysaccharides: mechanisms and consequences in food processing and digestion. Trends Food Sci Technol 60:43–51. doi: 10.1016/j.tifs.2016.10.022. [DOI] [Google Scholar]
- 49.Dimopoulou M, Vuillemin M, Campbell-Sills H, Lucas PM, Ballestra P, Miot-Sertier C, Favier M, Coulon J, Moine V, Doco T, Roques M, Williams P, Petrel M, Gontier E, Moulis C, Remaud-Simeon M, Dols-Lafargue M. 2014. Exopolysaccharide (EPS) synthesis by Oenococcus oeni: from genes to phenotypes. PLoS One 9:e98898. doi: 10.1371/journal.pone.0098898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernández-Romero D, Lucas-Elío P, López-Serrano D, Solano F, Sanchez-Amat A. 2003. Marinomonas mediterranea is a lysogenic bacterium that synthesizes R-bodies. Microbiology 149:2679–2686. doi: 10.1099/mic.0.26524-0. [DOI] [PubMed] [Google Scholar]
- 51.Collombel I, Campos FM, Hogg T. 2018. Changes in the composition of the lactic acid bacteria behavior and the diversity of Oenococcus oeni isolated from red wines supplemented with selected grape phenolic compounds. Fermentation 5:1. doi: 10.3390/fermentation5010001. [DOI] [Google Scholar]
- 52.Passerini D, Vuillemin M, Laguerre S, Amari M, Loux V, Gabriel V, Robert H, Morel S, Monsan P, Gabriel B, Fontagné-Faucher C, Remaud-Siméon M, Moulis C. 2014. Complete genome sequence of Leuconostoc citreum strain NRRL B-742. Genome Announc 2:e01179-14. doi: 10.1128/genomeA.01179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arendt EK, Neve H, Hammes WP. 1990. Characterization of phage isolates from a phage-carrying culture of Leuconostoc oenos 58N. Appl Microbiol Biotechnol 34:220–224. doi: 10.1007/BF00166784. [DOI] [Google Scholar]
- 54.Crouigneau A, Feuillat M, Guilloux-Benatier M. 2000. Influence of some factor on autolysis of Oenococcus oeni. Vitis 39:167–171. [Google Scholar]
- 55.El Khoury M, Campbell-Sills H, Salin F, Guichoux E, Claisse O, Lucas PM. 2017. Biogeography of Oenococcus oeni reveals distinctive but nonspecific populations in wine-producing regions. Appl Environ Microbiol 83:e02322-16. doi: 10.1128/AEM.02322-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell-Sills H, El Khoury M, Gammacurta M, Miot-Sertier C, Dutilh L, Vestner J, Capozzi V, Sherman D, Hubert C, Claisse O, Spano G, de Revel G, Lucas P. 2017. Two different Oenococcus oeni lineages are associated to either red or white wines in Burgundy: genomics and metabolomics insights. OENO One 51:309. doi: 10.20870/oeno-one.2017.51.4.1861. [DOI] [Google Scholar]
- 57.Lorentzen MPG, Lucas PM. 2019. Distribution of Oenococcus oeni populations in natural habitats. Appl Microbiol Biotechnol 103:2937–2945. doi: 10.1007/s00253-019-09689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Claisse O, Lonvaud-Funel A. 2014. Multiplex variable number of tandem repeats for Oenococcus oeni and applications. Food Microbiol 38:80–86. doi: 10.1016/j.fm.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Delmont TO, Eren AM. 2018. Linking pangenomes and metagenomes: the Prochlorococcus metapangenome. PeerJ 6:e4320. doi: 10.7717/peerj.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansour RB, Wided MK, Cluzet S, Krisa S, Richard T, Ksouri R. 2017. LC-MS identification and preparative HPLC isolation of Frankenia pulverulenta phenolics with antioxidant and neuroprotective capacities in PC12 cell line. Pharma Biol 55:880–887. doi: 10.1080/13880209.2016.1278452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.