Archaea, the third domain of life, have evolved diversified metabolic pathways to cope with their extreme habitats. Phosphoenol pyruvate (PEP)/pyruvate interconversion during carbohydrate metabolism is one such important metabolic process that is highly differentiated among Archaea. However, this process is still uncharacterized in the haloarchaeal group. Haloferax mediterranei is a well-studied haloarchaeon that has the ability to produce polyhydroxyalkanoates (PHAs) under unbalanced nutritional conditions. In this study, we identified the key enzymes involved in this interconversion and discussed their differences with their counterparts from other members of the Archaea and Bacteria domains. Notably, we found a novel protein, phosphoenolpyruvate synthetase-like (PPS-like), which exhibited high homology to PPS enzyme. However, PPS-like protein has evolved some distinct sequence features and functions, and strikingly the corresponding gene deletion helped to enhance poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) synthesis significantly. Overall, we have filled the gap in knowledge about PEP/pyruvate interconversion in haloarchaea and reported an efficient strategy for improving PHBV production in H. mediterranei.
KEYWORDS: central carbon metabolism, Haloarchaea, phosphoenolpyruvate synthetase, poly(3-hydroxybutyrate-co-3-hydroxyvalerate), polyhydroxyalkanoates, pyruvate kinase
ABSTRACT
Phosphoenolpyruvate (PEP)/pyruvate interconversion is a major metabolic point in glycolysis and gluconeogenesis and is catalyzed by various sets of enzymes in different Archaea groups. In this study, we report the key enzymes that catalyze the anabolic and catabolic directions of the PEP/pyruvate interconversion in Haloferax mediterranei. The in silico analysis showed the presence of a potassium-dependent pyruvate kinase (PYKHm [HFX_0773]) and two phosphoenol pyruvate synthetase (PPS) candidates (PPSHm [HFX_0782] and a PPS homolog protein named PPS-like [HFX_2676]) in this strain. Expression of the pykHm gene and ppsHm was induced by glycerol and pyruvate, respectively; whereas the pps-like gene was not induced at all. Similarly, genetic analysis and enzyme activities of purified proteins showed that PYKHm catalyzed the conversion from PEP to pyruvate and that PPSHm catalyzed the reverse reaction, while PPS-like protein displayed no function in PEP/pyruvate interconversion. Interestingly, knockout of the pps-like gene led to a 70.46% increase in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) production. The transcriptome sequencing (RNA-Seq) and quantitative reverse transcription-PCR (qRT-PCR) results showed that many genes responsible for PHBV monomer supply and for PHBV synthesis were upregulated in a pps-like gene deletion strain and thereby improved PHBV accumulation. Additionally, our phylogenetic evidence suggested that PPS-like protein diverged from PPS enzyme and evolved as a distinct protein with novel function in haloarchaea. Our findings attempt to fill the gaps in central metabolism of Archaea by providing comprehensive information about key enzymes involved in the haloarchaeal PEP/pyruvate interconversion, and we also report a high-yielding PHBV strain with great future potentials.
IMPORTANCE Archaea, the third domain of life, have evolved diversified metabolic pathways to cope with their extreme habitats. Phosphoenol pyruvate (PEP)/pyruvate interconversion during carbohydrate metabolism is one such important metabolic process that is highly differentiated among Archaea. However, this process is still uncharacterized in the haloarchaeal group. Haloferax mediterranei is a well-studied haloarchaeon that has the ability to produce polyhydroxyalkanoates (PHAs) under unbalanced nutritional conditions. In this study, we identified the key enzymes involved in this interconversion and discussed their differences with their counterparts from other members of the Archaea and Bacteria domains. Notably, we found a novel protein, phosphoenolpyruvate synthetase-like (PPS-like), which exhibited high homology to PPS enzyme. However, PPS-like protein has evolved some distinct sequence features and functions, and strikingly the corresponding gene deletion helped to enhance poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) synthesis significantly. Overall, we have filled the gap in knowledge about PEP/pyruvate interconversion in haloarchaea and reported an efficient strategy for improving PHBV production in H. mediterranei.
INTRODUCTION
Archaea are known for their prevalence in environments characterized by high temperature, high salt concentrations, high/low pH, low oxygen, or a combination thereof (1). In order to survive and thrive under such harsh environmental conditions, Archaea have evolved unique characteristics during the course of evolution (2). One of their striking features is that they have versatile nutritional requirements (i.e., they can utilize various types of substrates as their carbon source). Most extremophiles can utilize amino acids or organic acids as carbon sources, and a few even have the ability to use carbohydrates, including glucose, fructose, arabinose, xylulose, sucrose, and lactose, as well as starch and chitin (3–5). In the last few decades, numerous oddities of carbohydrate metabolism have been observed in different members of Archaea domain (4, 6–8).
Glycolysis and gluconeogenesis are the two critical pathways in glucose metabolism. The Embden-Meyerhof pathway is the common route for glucose degradation although some microorganisms use the Entner-Doudoroff (ED) pathway and oxidative pentose phosphate (PP) pathway (9). The metabolic conversions and intermediates involved in these pathways are mostly conserved in all three domains of life (10). However, in Archaea, the enzymes involved exhibit vast differences from their bacterial and eukaryotic counterparts, leading to emergence of modified versions of the classical metabolic pathways (11, 12). Glucose degradation to pyruvate in the thermoacidophilic Euryarchaeota Thermoplasma acidophilum and Picrophilus torridus is mediated by a nonphosphorylative version of the ED pathway (13), whereas in various haloarchaea, such as Haloferax volcanii, Haloarcula marismortui, and Halococcus saccharolyticus, glucose degradation is mediated by the semiphosphorylated ED (spED) pathway (14). Recently, Haloferax volcanii has been reported to contain two functionally distinct glyceraldehyde-3-phosphate dehydrogenases that regulate glycolysis and gluconeogenesis (15). Thus, it is well evident that there exists significant diversity in glucose metabolic pathways among Archaea.
Phosphoenolpyruvate (PEP)/pyruvate interconversion is an important control point in glucose metabolism that has been intensively researched in recent years. Pyruvate kinase (PYK) is the key enzyme involved in the PEP-to-pyruvate conversion (16). PYK is commonly a homotetrameric protein and is conserved in all three domains of life, with the critical function of phosphoryl transfer from PEP to ADP yielding pyruvate and ATP in all glucose-degrading organisms (17). Based on the phylogenetic analysis, PYKs can be broadly classified into K+ dependent and K+ independent, depending on the presence of conserved glutamate in the K+ binding site of PYKs (16, 18–20). PYKs have been identified and characterized from several Crenarchaeota, e.g., Thermoproteus tenax, Aeropyrum pernix, Pyrobaculum aerophilum, and Sulfolobus solfataricus and thermophilic Euryarchaeota, for example, T. acidophilum, Thermococcus kodakarensis, and Archaeoglobus fulgidus (18, 19, 21–23). In the case of the pyruvate-to-PEP conversion, the reaction proceeds by two steps, catalyzed by pyruvate carboxylase and PEP carboxykinase in most mammals, plants, and microorganisms (17). However, in the Archaea, phosphoenolpyruvate synthetase (PPS) is the primary enzyme that directly converts pyruvate to PEP in one step. Significant functional variance of PPSs in different strains has been reported so far. For instance, members of Thermococcales in the phylum Euryarchaeota use PPS for catalyzing the conversion of PEP to pyruvate. In T. kodakarensis, Pyrococcus furiosus, and Methanobacterium thermoautotrophicum, PPS catalyzes both glycolytic and gluconeogenic directions in PEP/pyruvate interconversion (22, 24, 25). Some Archaea, such as Thermoplasma spp. and T. tenax, have a second enzyme, pyruvate:phosphate dikinase (PPDK), that is responsible for PEP synthesis from pyruvate. Additionally, T. tenax PPDK can also catalyze the conversion of PEP to pyruvate (17, 26).
Thus, from the above investigations, it is evident that members of the Archaea have highly differentiated glucose metabolic pathways. To date, most of the research on PEP/pyruvate interconversion is focused on thermophiles and methanogens. However, the PEP/pyruvate interconversion is poorly understood in haloarchaea although its pyruvate formation from PEP is speculated to be catalyzed by PYK and PPDK (5). Haloferax mediterranei is a model haloarchaeon for physiological and metabolic study and is capable of utilizing glucose as the sole carbon source (27). The most interesting feature of H. mediterranei is its inherent ability to produce polyhydroxyalkanoates (PHAs) (28). PHAs are a kind of biodegradable polyesters that are produced by various bacteria and many haloarchaeal species under unbalanced growth conditions (29–32). To date, much effort has been made to obtain ultrahigh PHA accumulations by improving carbon metabolic flux to PHA synthesis. However, the existing polymer production processes are still uneconomical at a large scale. H. mediterranei has several unique features, such as easy lysis in water because of high intracellular osmotic pressure, undemanding strict sterilization due to the high salt concentrations in growth media, a low endotoxin level, and ability to accumulate poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) naturally, under appropriate culture conditions (28, 33). These characteristics make H. mediterranei a potential industrial strain for PHA production. In previous years, our research group has reported PHBV synthesis and its regulation in H. mediterranei (28, 34–36). In addition, an improved PHBV-producing variant, ES1 with a deletion of genes for exopolysaccharide (EPS) production, was constructed (37). Some research groups have also proposed that genetic engineering of central metabolic pathways can be a promising approach toward improved PHA synthesis. In Pseudomonas putida, an engineered glycolysis pathway channeled more of the carbon source toward PHA synthesis and led to a 20-fold increase in PHA production (38). Thus, it is likely that a thorough understanding of the central carbohydrate metabolism, e.g., PEP/pyruvate interconversion, in haloarchaea might also help to optimize its PHA synthesis.
The present study is an initial endeavor to understand the PEP/pyruvate interconversion in H. mediterranei from genetic and biochemical points of interest. As inferred from our genome sequencing, the key enzymes involved in this interconversion could be H. mediterranei PYK (PYKHm), PPSHm, or PPSHm/PPDKHm. However, their specific functions need experimental characterization and confirmation. Thus, we designed and developed mutant strains of H. mediterranei and checked the corresponding effect on glycolysis and gluconeogenesis. In addition, we performed enzymatic analysis to verify the function of these candidate enzymes. We further investigated the impact of gene knockout on the overall cell metabolism. In a nutshell, this study is also an attempt to develop an engineered H. mediterranei strain for improved PHBV synthesis.
RESULTS
Bioinformatic analysis of potential genes encoding PYK and PPS.
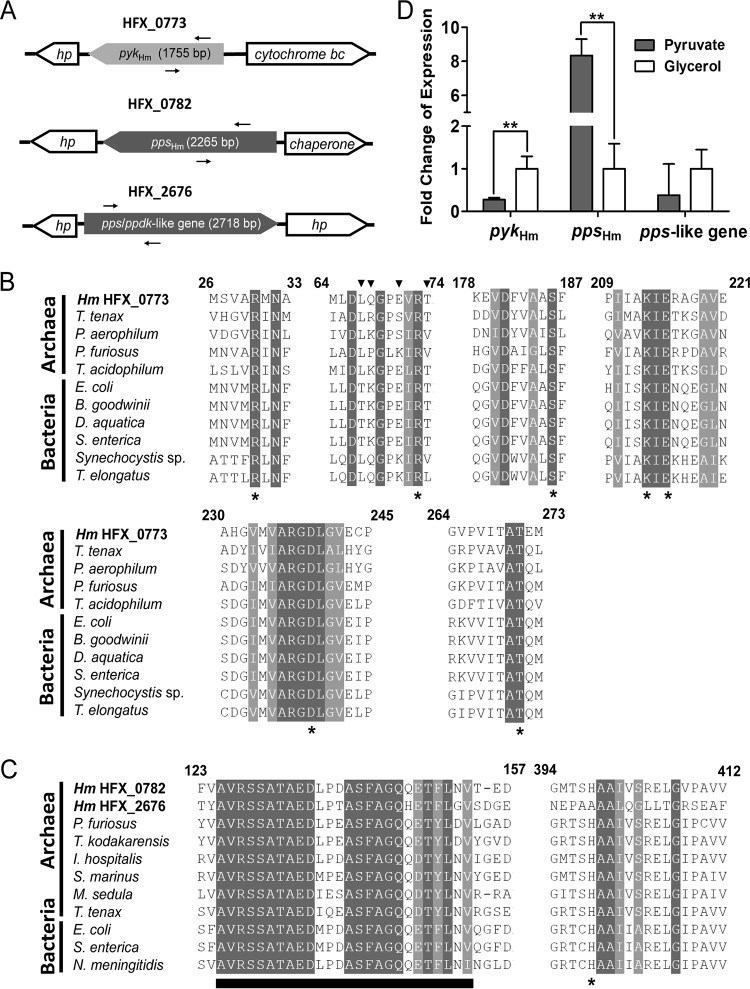
Our in silico analysis of the genome sequence of H. mediterranei revealed the presence of genes homologous to the PYK gene and pps (Fig. 1A). HFX_0773 was annotated as the putative pykHm gene encoding a 585-amino-acid polypeptide with a calculated molecular mass of 62.0 kDa. PYKHm showed overall amino acid identity ranging approximately from 30% to 45% to the PYKs from Bacteria, Eucarya, and thermophilic archaea. The conserved residues indispensable for catalytic activity, Arg30, Arg73, Ser186, Lys213, Glu215, Asp239, and Thr271, were detected in PYKHm (Fig. 1B). All characterized members of PYKs with a Glu residue at the equivalent position exhibit K+-dependent activity (39). Similar to K+-dependent PYKs from Bacteria and Eukarya, PYKHm had a conserved Glu at position 71. Interestingly, unlike the PYKs in Bacteria and Eukarya, coevolution between Glu71 and its neighboring residues (Leu67, Gln68, and Thr74) was not observed. Hence, although the amino acid sequence of PYKHm was highly suggestive that its enzyme activity was likely dependent on potassium, it still possessed some distinctive features. HFX_0782 was annotated as a putative ppsHm homologous gene coding for a 755-amino-acid polypeptide with a calculated molecular mass of 82.4 kDa. The level of amino acid identity between PPSHm and representative PPSs ranges from about 33% to 52%. In addition to ppsHm, a further gene, HFX_2676, putatively encoded a 906-amino-acid polypeptide with significant similarities to PPS and PPDK. PPS/PPDKHm showed 41% amino acid sequence identity to PPSHm. The theoretical molecular mass of the PPS/PPDKHm protein was 98.2 kDa, and it was about 19% larger than PPSHm. The additional sequences in the C terminus of the PPS/PPDKHm protein were absent in the PPSHm sequence. Multiple sequence alignments were performed to distinguish PPSHm and PPS/PPDKHm at the sequence level. Both PPSHm and PPS/PPDKHm contain a common sequence motif (AVRSSATAED-ASFAGQ-T-L) (26), which is specific for all known PPSs (Fig. 1C), except that the conserved histidine (His398) of PPSHm is replaced by alanine at the equivalent position in PPS/PPDKHm. In addition, no conserved motif for PPDK proteins (PLLVSVRSGA-SMPGMMD) (26) is present in PPS/PPDKHm. Thus, we assumed that PPS/PPDKHm is a PPS-like protein rather than a PPDK protein.
FIG 1.
(A) Genetic organization of pykHm, ppsHm, and the pps-like gene in H. mediterranei. The locations of primers used in for the panel D are represented by arrows. Hp, hypothetical protein-encoding gene. (B) Alignment of partial amino acid sequences of PYKs from Archaea and Bacteria. NCBI accession numbers are as follows: H. mediterranei (HmHFX_0773), AFK18496.2; P. furiosus, AAL81312.1; T. tenax, AAF06820.1; P. aerophilum, AAL63053.1; E. coli, KEN69166.1; B. goodwinii, CPR19480.1; D. aquatica, SLM63613.1; Synechocystis sp. strain PCC 6803, BAA17574.1; T. elongatus, WP_011058108.1; S. enterica, GAR62769.1. The triangles indicate conserved Glu residues and the coevolving amino acids essential for K+ dependence; the star indicates conserved residues that have been proposed to be indispensable for catalytic activity. (C) Alignment of partial amino acid sequences of PPSHm and PPS-like proteins with PPSs from archaea and bacteria. NCBI accession numbers are as follows: H. mediterranei (HmHFX_2676), AFK20354.1; H. mediterranei (HmHFX_0782), AFK18505.1; P. furiosus, WP_011011155.1; T. kodakarensis, WP_011250243.1; Ignicoccus hospitalis, WP_012123254.1; Staphylothermus marinus, WP_011838445.1; Metallosphaera sedula, AKV83648.1; T. tenax, CAD56491.1; E. coli, AAN80558.1; S. enterica, NP_460315.1; Neisseria meningitidis, NP_273662.1. The star indicates the conserved histidine residues that have been proposed to be indispensable for catalytic activity; the black bar indicates the common sequence motif in both PPSs and PPS-like protein. (D) qRT-PCR result for the fold change expression of pykHm, ppsHm, and pps-like gene under gluconeogenic (pyruvate) and glycolytic (glycerol) culture conditions. Significance analysis was conducted by ANOVA (**, P < 0.01).
Inducible expression of the pyk and pps genes.
To analyze the transcription level of pykHm, ppsHm, and the pps-like gene in glycolysis and gluconeogenesis, H. mediterranei cells were cultured in medium with glycerol or pyruvate as the sole carbon source. Since pyruvate is converted to PEP during gluconeogenesis, use of pyruvate as the sole carbon source represents a gluconeogenic growth mode. Instead, glycerol represents a glycolytic growth mode as it is catabolized via glycolysis. Quantitative reverse transcription-PCR (qRT-PCR) was performed with the total RNA extracted from cells harvested at the exponential growth phase and the specific primers indicated in Fig. 1A and Table 1. As shown in Fig. 1D, the transcription level of pykHm was 3.7-fold higher in glycerol-grown cells than in pyruvate-grown cells. In contrast, the transcription level of ppsHm was 8.3-fold higher in pyruvate-grown cells than in glycerol-grown cells. Unlike ppsHm, the transcription levels of the pps-like gene exhibited no significant difference in pyruvate-grown and glycerol-grown cells. These observations indicated that pykHm expression was upregulated by glycerol but not by pyruvate, whereas ppsHm was upregulated by pyruvate but not by glycerol. Thus, we can infer that PYKHm is involved in glycolysis while PPSHm participates in gluconeogenesis. However, the PPS-like protein is probably nonfunctional in the two processes.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′–3′)a | Use |
|---|---|---|
| pykNF | CAACTGCAGATTGCGGGCGTCGGAACC | Amplification of upstream and downstream flanking regions of the pyk gene inserted into pHFX to construct pHFX-pyk |
| pykNR | CGAGGATCCGCCGAGACGCACGCGACG | |
| pykCF | CTACCATGGATACGTAGTCTATGTAGA | |
| pykCR | CGGGGTACCCCGAACGACCAGTAGAGA | |
| ppsNF | GAACTGCAGCGAGGATTGATAGGACCG | Amplification of upstream and downstream flanking regions of pps gene inserted into pHFX to construct pHFX-pps |
| ppsNR | CGAGGATCCGCAGCGTCTCATCCTCGA | |
| ppsCF | ATACCATGGGTGGTATTCGTATGGTCC | |
| ppsCR | CGGGGTACCCGTGAATTGGTCGGTCTT | |
| 2676NF | CGTCTGCAGATCTGATGGTGACAAACT | Amplification of upstream and downstream flanking regions of pps-like gene inserted into pHFX to construct pHFX-pps-like gene |
| 2676NR | CATCCATGGCCACACCTTACTCTCGCA | |
| 2676CF | CATCCATGGTGTTAACTTATGGTTCTC | |
| 2676CR | AGAGGATCCGTTTGACGCTGAATATCC | |
| pWpykF | CGCGGATCCCTCGGCGTGAACCTCTTACTCG | Amplification of the pyk gene inserted into pWL502 to construct pPYK |
| pWpykR | CGGGGTACCTCAGCGACTGCCGCGGTTGTAGC | |
| pWppsF | CGCGGATCCGGCTAGCGGTCTTCTTTCGG | Amplification of pps gene inserted into pWL502 to construct pPPS |
| pWppsR | TGCTCTAGACTAGCGAACCGAGTCGAGGATG | |
| pW2676F | CGCCATATGGCACTGGTAAACAGCGCTCTGCA | Amplification of pps-like gene inserted into pWL502 to construct pPPS-like |
| pW2676R | CGCGGATCCTCAGTACCGTTCGGACCGGTCGA | |
| pykF | CGGGGTACCCGAAACGCAAAAATCGTCTG | Amplification of pyk gene inserted into pTA03 to construct pTA03-PYK |
| pykR | CGCGGATCCGCGACTGCCGCGGTTGATG | |
| ppsF | CCGGAATTCGCTGTAGTCTGGCTGGAC | Amplification of pps gene inserted into pTA03 to construct pTA03-PPS |
| ppsR | CGCGGATCCGCGAACCGAGTCGAGGTAG | |
| 2676F | CGCCATATGAACGAGAGGGGAGACACGCCAG | Amplification of pps-like gene inserted into pTA06 to construct pTA06-PPS-like |
| 2676R | CGCGGATCCGTACCGTTCGGACCGGTCGAGG | |
| ppsmF | GGCGGCATGACTTCGGCCGCAGCCATCGTCTC | Amplification of mutant pps gene inserted into pTA03 to construct pTA03-mutant PPS |
| ppsmR | GAGACGATGGCTGCGGCCGAAGTCATGCCGCC | |
| pykmF1 | GATTCCCATCATCGCCGCAATCGAGCGCGCCGGTG | Amplification of mutant pyk gene inserted into pTA03 to construct pTA03-mutant PYK |
| pykmR1 | CACCGGCGCGCTCGATTGCGGCGATGATGGGAATC | |
| pykmF2 | GATGGTCGCTCGCGGCGCACTCGGTGTCGAGTGCC | |
| pykmR2 | GGCACTCGACACCGAGTGCGCCGCGAGCGACCATC | |
| qRTpykF | CGACACCGAGTTCATCATCGG | qRT-PCR analysis of pyk gene |
| qRTpykR | CGCGGATGCTATCGATGACCT | |
| qRTppsF | TGGCTGTAGTCTGGCTGGACG | qRT-PCR analysis of pps gene |
| qRTppsR | GCGGAATCCTCGTGGTCGACT | |
| qRT2676F | CGAGAGGGGAGACACGCCAGAG | qRT-PCR analysis of pps-like gene |
| qRT2676R | GCGCAAGTGCTTCCAACGATTC | |
| qRTphaRF | GACGAACGACTCAAACGATGC | qRT-PCR analysis of phaR gene |
| qRTphaRR | GATGAGTCTCCAAACCGCGGT | |
| qRTphaPF | GGACGCGATGGACGAGCAGTT | qRT-PCR analysis of phaP gene |
| qRTphaPR | TCACACCAGACACGCCGGAGT | |
| qRTphaECF | CTCGTCGAACTCGAACGGAGA | qRT-PCR analysis of phaEC genes |
| qRTphaECR | CCGACCGATACCTCCGACACG | |
| 7SF | CCAACGTAGAAACCTCGTC | Quantification of 7S rRNA |
| 7SR | GATGGTCCGCTGCTCGCTTC |
Sequences representing restriction sites are underlined.
Genetic determination of the involvement of PYKHm and PPSHm in the interconversion of pyruvate and PEP.
To study the in vivo physiological functions of PYKHm, PPSHm, and PPS-like protein, the corresponding gene deletion mutants (ΔpykHm, Δpps, Δpps-like gene, and Δpps Δpps-like gene strains) and their complementation strains were constructed. The plasmid pWL502 was transformed into the relevant gene knockout strains to avoid the effect of uracil auxotrophy. Then, these strains were cultured in medium with glycerol or pyruvate as a sole carbon source. Drastic changes in phenotype were observed in ΔpykHm, Δpps, and Δpps Δpps-like gene strains. The pykHm mutant strain was not able to grow in medium with glycerol as the sole carbon source (Fig. 2A), whereas its growth on pyruvate was not affected (Fig. 2D). As expected, the complementation of pykHm enabled the Δpyk strain to grow on glycerol, and the growth was even better than that of the control strain DF50 (pWL502) (Fig. 2A). These data suggested that PYKHm is the only enzyme involved in the glycolysis pathway by catalyzing the conversion of PEP to pyruvate. The growth of Δpps and Δpps Δpps-like gene strains in medium with pyruvate as the sole carbon source was totally inhibited, whereas the growth of the Δpps-like gene strain was not influenced (Fig. 2B). The complementation of ppsHm restored the ability of Δpps and Δpps Δpps-like gene strains to grow on pyruvate. In contrast, Δpps, Δpps-like gene, and Δpps Δpps-like gene strains grew well on glycerol (Fig. 2C). These results indicated the involvement of PPSHm instead of PPS-like protein in gluconeogenesis pathway, which is in agreement with their proposed roles in the conversion of pyruvate to PEP. These data also implied that PPSHm was the only enzyme responsible for catalyzing pyruvate to PEP in H. mediterranei.
FIG 2.
Growth curves of H. mediterranei DF50 and its mutant strains under a glycolytic (A and C; glycerol as carbon source) or gluconeogenic (B and D; pyruvate as carbon source) growth condition. The plasmid pWL502 was transformed into DF50, ΔpykHm, Δpps, Δpps Δpps-like gene, and Δpps-like gene strains to get rid of the effect of uracil auxotrophy. Cell growth was quantified by DNA content, which was measured by diphenylamine monitored at 595 nm.
Enzyme activity of PYKHm, PPSHm, and PPS-like protein.
To determine the biochemical activity of PYKHm, PPSHm, and PPS-like proteins, we expressed these proteins with a His6 tag at their N or C terminus in the haloarchaeon H. volcanii H1424. The molecular weight of purified PYKHm, PPSHm, and PPS-like with the terminal His6 tag was greater than their theoretical size, as judged by SDS-PAGE, because of their high acidic amino acid content (Fig. 3A). The purity of PYKHm, PPSHm, and PPS-like proteins was found to be 92.16%, 90.85%, and 57.72%, respectively. These purified proteins were further verified by matrix-assisted laser desorption ionization–two-stage time of flight mass spectrometry (MALDI-TOF/TOF MS) with high matching scores (see Table S1 in the supplemental material) and subsequently subjected to enzyme activity analysis.
FIG 3.
Enzyme activity analysis of PYKHm, PPSHm, and PPS-like proteins. (A) SDS-PAGE analysis of purified PYKHm, PPSHm, and PPS-like proteins. The red triangles indicate the target proteins. Lanes M, protein marker. (B) Conversion of PEP to pyruvate catalyzed by PYKHm and its mutant PYK_A213A239 (negative control), with ADP as a cosubstrate. (C) Interconversion of pyruvate and PEP catalyzed by PPS and its mutant PPS_A398 (negative control), with ATP and with AMP plus Pi as cosubstrates for gluconeogenic and glycolytic directions, respectively. (D) Interconversion of PEP and pyruvate catalyzed by PPS-like protein in PPS and PPDK reaction systems. For the glycolytic or gluconeogenic direction of PPDK, AMP plus PPi or ATP plus Pi was used as cosubstrates. glu, conversion of pyruvate to PEP (gluconeogenesis direction); gly, conversion of PEP to pyruvate (glycolysis direction).
The enzymatic activities of these proteins were qualitatively analyzed by detecting PEP or pyruvate in the reaction system using ion chromatography. The retention time of pyruvate was about 3.8 min, and for PEP it was about 11.8 min. The activity of PYKHm was measured by the formation of pyruvate from PEP. Compared to results for the negative control without cosubstrate, ADP (Fig. S1A), the chromatogram of the PYKHm assay exhibited a significant reduction in the peak representing PEP with simultaneous appearance of a pyruvate peak after completion of the reaction. However, a mutant enzyme with the replacement of the putative catalytic Lys213 and Asp239 by Ala residues (PYK_A213A239) lost catalytic activity completely (Fig. 3B). Therefore, PYKHm catalyzed the conversion of PEP and ADP to pyruvate and ATP. With respect to PPSHm, its activity was measured by either the formation of pyruvate from PEP (called the gluconeogenesis direction) or the reverse reaction (called the glycolysis direction). Obviously, PPSHm led to depletion of pyruvate with formation of PEP after the completion of the reaction compared to results with its mutant enzyme (PPS_A398) and the negative control without cosubstrate, ATP (Fig. S1B). In contrast, the substrates remained unchanged in the reaction system during the glycolysis reaction (Fig. 3C). These results illustrated that PPSHm catalyzed the unidirectional conversion of pyruvate and ATP to PEP, AMP, and Pi. For the activity of PPS-like protein, we analyzed the interconversion of pyruvate and PEP catalyzed by PPS or PPDK. Notably, as indicated in Fig. 3D, the PPS-like protein did not change the peak representing pyruvate or PEP and, thus, exhibited no activity in either the PPS or PPDK reaction system. Therefore, these results, in accordance with growth curve analysis (Fig. 2), confirmed the unidirectional function of PYKHm and PPSHm in PEP/pyruvate interconversion during glycolysis and gluconeogenesis, respectively. In addition, it also confirmed the nonfunctionality of PPS-like protein in these metabolic reactions.
pps-like gene deletion strain shows enhanced PHBV accumulation capability.
Acetyl-coenzyme A (CoA) is an important precursor for generating 3-hydroxybutyrate (3HB)-CoA and 3-hydroxyvalerate (3HV)-CoA as PHBV monomers. As PPS participated in gluconeogenesis by converting pyruvate to PEP, PPS deficiency was presumed to influence PHBV accumulation. Thus, we knocked out the pps and pps-like genes in an EPS-deficient (ΔEPS) H. mediterranei strain and obtained the mutants ΔEPS Δpps, ΔEPS Δpps-like gene, and ΔEPS Δpps Δpps-like gene and then investigated their ability to synthesize PHBV by using glucose as carbon source. After cultivation in shake flasks containing 50 ml of polyhydroxyalkanoate (PHA) accumulation medium for 5 days, PHBV production by the H. mediterranei ΔEPS gene deletion strains was determined by GC analysis (Table 2). Cell dry weight increased from 4.47 g/liter for the ΔEPS control strain to 5.49 g/liter for the ΔEPS Δpps-like gene deletion strain. PHBV content and PHBV concentration also increased to 65.63% and 3.22 g/liter, 73.63% and 4.04 g/liter, and 67.39% and 3.22 g/liter in the ΔEPS Δpps, ΔEPS Δpps-like gene, and ΔEPS Δpps Δpps-like gene mutants, respectively, in contrast to 53.37% and 2.37 g/liter for the control strain. These results showed that both pps and pps-like gene knockouts enhanced cell dry weight and PHBV accumulation. Notably, ΔEPS Δpps-like gene had the most significant effect, yielding a 70.46% increase in PHBV concentration over that of H. mediterranei ΔEPS.
TABLE 2.
PHBV accumulation in H. mediterranei strainsa
| Strain | CDW (g/liter)b | PHBV content (wt%) | 3HV fraction (mol%) | PHBV concn (g/liter) |
|---|---|---|---|---|
| ΔEPS | 4.47 ± 0.28 | 53.37 ± 6.81 | 12.75 ± 0.68 | 2.37 ± 0.15 |
| ΔEPS Δpps | 4.90 ± 0.08 | 65.63 ± 11.18 | 14.23 ± 1.75 | 3.22 ± 0.61 |
| ΔEPS Δpps-like gene | 5.49 ± 0.24 | 73.63 ± 4.10 | 8.55 ± 0.26 | 4.04 ± 0.29 |
| ΔEPS Δpps Δpps-like gene strain | 4.77 ± 0.13 | 67.39 ± 3.45 | 13.52 ± 0.40 | 3.22 ± 0.23 |
All data are expressed as means ± standard deviations from three independent experiments.
CDW, cell dry weight.
Subsequently, we scaled up the PHBV fermentation process from 250-ml shake flasks to a 7-liter fermentor for a detailed investigation of cell growth, glucose consumption, and PHBV synthesis capability of the ΔEPS Δpps-like gene and ΔEPS strains. The plots of the optical density at 595 nm (OD595) showed that ΔEPS Δpps-like gene exhibited a higher growth rate than the ΔEPS strain during the entire growth phase (Fig. 4A). The residual glucose concentration in the medium of ΔEPS Δpps-like gene was always lower than that of ΔEPS. Consequently, glucose was consumed completely by ΔEPS Δpps-like gene after a 42-h fermentation, whereas ΔEPS used up the glucose in medium only after a 72-h fermentation (Fig. 4A). This result implied that ΔEPS Δpps-like gene utilized glucose faster than ΔEPS during the whole fermentation. The PHBV concentration curves showed that ΔEPS Δpps-like gene synthesized much more PHBV than ΔEPS in the whole process. The ΔEPS Δpps-like gene strain gave a final PHA production of 5.71 g/liter, which was 52.3% higher than that of ΔEPS (Fig. 4A). In addition, we further examined the intracellular PHBV granules in the two strains. Consistently, many PHBV granules were observed in ΔEPS Δpps-like gene cells, but only a few were present in ΔEPS cells at the exponential growth phase. At the stationary growth phase, the number of PHBV granules increased more rapidly, and also their sizes were much more uniform in ΔEPS Δpps-like gene cells than in ΔEPS cells (Fig. 4B). Moreover, the PHBV granules occupied a significant portion in ΔEPS Δpps-like gene cells compared to that in ΔEPS cells. From these observations, it can be concluded that, as was expected, pps gene deletion could enhance PHBV accumulation, probably because cutting off gluconeogenesis channeled more pyruvate to acetyl-CoA and thus into PHBV accumulation. Contrary to expectations, the PPS-like protein does not appear to be a PPS enzyme; however, its inactivation led to higher PHBV production.
FIG 4.
Effect of pps-like gene deletion on cell growth, glucose consumption, and PHBV accumulation in H. mediterranei. (A) Time course of cell growth, glucose consumption, and PHBV accumulation. open patterns, ΔEPS; closed patterns, ΔEPS Δpps-like gene; purple circles, PHBV concentration; green triangles, residual sugar concentration in medium; black rhombuses, cell growth. (B) TEM images of ΔEPS and ΔEPS Δpps-like gene at exponential growth phase (EP) and stationary growth phase (SP).
Impact of the pps-like gene knockout on cell metabolism.
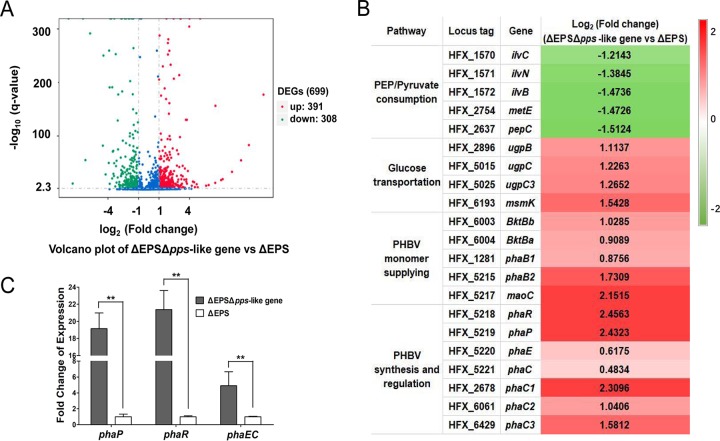
To investigate the unexpected function of PPS-like protein in PHBV accumulation, we studied the influence of pps-like gene deletion on the whole-cell metabolism at the transcript level by RNA sequencing of ΔEPS Δpps-like gene. The total RNA was extracted from the ΔEPS Δpps-like gene and ΔEPS strains at exponential growth phase. The genome-wide transcriptome analysis was performed using next-generation RNA sequencing (RNA-Seq). A volcano plot illustrates the distribution of differentially expressed genes (DEGs) in ΔEPS Δpps-like gene and ΔEPS (Fig. 5A). Overall, expression of 699 genes in ΔEPS Δpps-like gene changed significantly [P value of 0.005; log2(fold change) of 1] compared to levels in ΔEPS, including 391 upregulated and 308 downregulated genes when the pps-like gene was knocked out (Fig. 5A). The DEGs were presumed to be involved in various processes, including glycolysis and gluconeogenesis, the tricarboxylic acid (TCA) cycle, fatty acid metabolism, amino acid metabolism, biosynthesis of secondary metabolites, energy metabolism, transport system, gas vesicle formation, reactive oxygen species scavenging, and stress response. This observation implied that deletion of the pps-like gene led to a noticeable change in the overall cell metabolism.
FIG 5.
Influence of pps-like gene deletion on cell metabolism at the transcriptional level in H. mediterranei. (A) Volcano plot of differentially expressed gene (DEG) distribution. Red, upregulated genes; green, downregulated genes; blue, nonsignificantly differently expressed genes. (B) Fold change of gene expression when the pps-like gene is knocked out. A corrected P value of 0.005 and log2(fold change) of 1 were set as the thresholds for significantly differential expression. Red, upregulation; green, downregulation. (C) qRT-PCR analysis of phaR, phaP, and phaEC expression levels in the ΔEPS Δpps-like gene compared to that in ΔEPS. Significance analysis was conducted by ANOVA (**, P <0.01).
Four glucose transporter candidates were significantly upregulated in the ΔEPS Δpps-like gene mutant (Fig. 5B). Probably this led to faster glucose consumption in the ΔEPS Δpps-like gene. Acetyl-CoA and propionyl-CoA are two important precursors for monomer generation during PHA synthesis. Generally, it is considered that monomer-supplying pathways and the proteins required for PHA synthesis play crucial roles during PHBV accumulation. Therefore, we summarized the DEGs participating in the precursor metabolism and monomer supply and PHA synthesis in Fig. 5B. Several genes (ketol-acid reductoisomerase ilvC, HFX_1570; acetolactate synthase ilvN, HFX_1571; acetolactate synthase I/II/III large subunit ilvB, HFX_1572; 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase metE, HFX_2754; phosphoenolpyruvate carboxylase pepC, HFX_2637) were significantly downregulated in ΔEPS Δpps-like gene. Downregulation of these genes probably restricted the conversion of PEP/pyruvate to other intermediates such as hydroxy-2-methyl-3-oxobutanoate, acetolactate, and oxaloacetate for biosynthesis of amino acids or other metabolic pathways. This enhanced acetyl-CoA flux to PHBV synthesis. Usually, two PHBV monomers, 3HB-CoA and 3HV-CoA, are supplied via a two-step process, catalyzed sequentially by β-ketothiolase and β-ketoacyl-CoA reductase, from acetyl-CoA and propionyl-CoA. Interestingly, both β-ketothiolase genes (bktBβ, HFX_6003; and bktBα, HFX_6004) and β-ketoacyl-CoA reductase genes (phaB1, HFX_1281; phaB2, HFX_5215) were significantly upregulated in ΔEPS Δpps-like gene. In addition, enoyl-CoA hydratase (maoC, HFX_5217), which can supply monomers from fatty acids, was also upregulated. Thus, the higher-level expression of monomer-supplying genes might have generated more monomers and led to an enhanced PHBV yield. Meanwhile, expression of the gene cluster for PHBV biosynthesis and regulation (phaR, PHBV synthesis regulator, HFX_5218; phaP, PHA granule structure protein, HFX_5219; phaEC, PHA synthase, HFX_5220 and HFX_5221) were also elevated to different levels. Among them, phaP and phaR have been shown to promote PHBV synthesis independently, and PHA synthase (PhaEC) is the key enzyme for PHA polymerization. In addition, three cryptic genes encoding a PhaC subunit in the wild-type strain, phaC1, phaC2, and phaC3 (HFX_2678, HFX_6061, and HFX_6429, respectively), were upregulated as well. These results indicated that their heightened expression might be closely associated with the high PHBV production in ΔEPS Δpps-like gene.
To further verify the expression level of PHA synthesis cluster genes, we conducted qRT-PCR with the same RNA used for RNA-Seq and with the primers listed in Table 1. Consistent with the expression tendency determined by RNA-Seq, qRT-PCR revealed that the phaEC genes were upregulated by 4.9-fold along with phaR and phaP, which were upregulated by 21- and 19-fold, respectively (Fig. 5C). Thus, from the RNA-Seq and qRT-PCR results, it can be concluded that considerable disturbance in cell status occurred due to the knockout of the pps-like gene. In particular, deletion of the pps-like gene reduced the expression levels of genes involved in PEP or pyruvate conversion and promoted the expression of the PHA monomer supply and PHA synthesis-related genes, thereby leading to more PHBV accumulation in H. mediterranei.
DISCUSSION
In the present study, we identified and characterized the key enzymes PYKHm and PPSHm involved in PEP/pyruvate interconversion in H. mediterranei. Similar to findings in most members of the Bacteria and Eukarya, PYKHm catalyzed the conversion of PEP to pyruvate during glycolysis, and PPSHm catalyzed the reverse reaction during gluconeogenesis. Depending upon the requirement of K+ for enzyme activity, PYKs can be classified into two distinct groups of K+-dependent and K+-independent PYKs (18). The presence of a conserved glutamate residue at position 71 in PYKHm renders it possibly dependent on potassium. However, the coevolving amino acids with Glu71 are not conserved in PYKHm, except for threonine at position 74. Oria-Hernández et al. reported that in 99% of K+-dependent PYKs, position 67 (or an equivalent position) was occupied by threonine, and in 94%, position 68 (or an equivalent position) was occupied by lysine (16). Instead, PYKHm has two other residues of Leu67 and Gln68. This variation in PYKHm is also comparable to that of other Archaea (see Fig. S2A in the supplemental material). For instance, in K+-dependent PYK from the haloarchaeon Haloarcula marismortui, position 74 is conserved, but positions 67 and 68 are methionine and proline, respectively. In T. acidophilum, K+-dependent PYK contains conserved Lys68 and Thr74 but, like PYKHm, has leucine at position 67 (21, 40). Obviously, these kinds of deviations in conserved residues are more common among K+-dependent PYKs from Archaea (Fig. S2A) than in those of Bacteria (Fig. S2B). For instance, K+-dependent PYKs from Bacteria, including those of Escherichia coli, Brenneria goodwinii, Dickeya aquatica, and Salmonella enterica, share conserved residues such as Thr67, Lys68, Glu71, and Thr74. Likewise, in the case of K+-independent PYKs, Glu71 is replaced by Lys71, and the coevolving residues change to leucine, glutamine, and leucine, valine, or isoleucine at positions 67, 68, and 74 (or equivalent positions), respectively. Bacteria such as Synechocystis sp. and T. elongatus have conserved Leu67, Gln68, Lys71, and Val74/Leu74. In contrast, in the archaeon P. furiosus, K+-independent PYK has proline at position 68 although Lys71 and other coevolving residues, Leu67 and Val74, are conserved. More strikingly, in T. tenax and P. aerophilum, K+-independent PYKs have a conserved Ser71 instead of Lys71. In addition, they have different amino acids at positions 68 and 74 although Leu67 is conserved. T. tenax possesses Arg68 and Thr74, whereas P. aerophilum has Lys68 and Val74 (20). Thus, such a variance in conserved residues explains the high-level sequence diversity of PYKs from Archaea compared to those from Bacteria.
PPS belongs to the family of PEP-utilizing enzymes. One obvious difference of reported PPS enzymes is size. PPSHm has a subunit size of 82.4 kDa. However, the PPSs of E. coli and some archaea including Staphylothermus marinus, P. furiosus, M. thermoautotrophicum, and T. tenax have masses of 87, 93, 90.4, 75.6, and 90.5 kDa, respectively (22, 25). Commonly, presence of a conserved histidine residue is a sequence signature of PPS that is indispensable for its catalytic activity. PPS phosphorylates pyruvate to PEP, and this step proceeds via formation of a phospho-histidine intermediate (41). Our results show that, as in most bacteria and other archaea, PPSHm has the conserved histidine residue at position 398, and its mutant enzyme PPS_A398 has no PPS function (Fig. 3C). Unlike the uniform conversion of PEP to pyruvate, catalyzed by PYKs, the reverse reaction is often complicated due to involvement of different enzyme variants during the conversion of pyruvate to PEP (Fig. S3). Our gene knockout results show that PPSHm specifically facilitates the pyruvate conversion to PEP under gluconeogenic conditions, with absolutely no effect under glycolytic conditions. Contradictorily, in many archaea including P. furiosus and M. thermoautotrophicum, PPS is known to be bifunctional, catalyzing both anabolic and catabolic directions of PEP/pyruvate interconversion, although the catalytic efficiency in glycolysis is very low (Fig. S3). The PPS of T. kodakarensis (PPSTk) actively participates in gluconeogenesis as well as glycolysis, and, strikingly, its efficiency in converting PEP to pyruvate is significantly higher than that of PYKTk (22). Additionally, Thermoplasma sp. lacks the PPS gene and uses PPDK for the conversion of pyruvate to PEP (17). PPDK is another enzyme belonging to PEP-utilizing family. PPS and PPDK have similar structures and functions, but the latter requires an additional monophosphate (Pi), along with the common substrates of ATP, for catalyzing the anabolic reaction. Strikingly, in T. tenax, PPDK has bifunctional activity in PEP/pyruvate interconversion, with a preference for the glycolytic direction and with AMP and diphosphate (PPi) as cosubstrates (Fig. S3) (26). This shows that the anabolic direction in PEP/pyruvate interconversion in Archaea is quite diverse. Similarly, in Bacteria pyruvate-to-PEP conversion proceeds through diversified metabolic reactions. For instance, in E. coli and Rhodopseudomonas palustris, PPS can directly convert pyruvate to PEP. However, they have an alternative pathway whereby pyruvate is first converted to oxaloacetate via the glyoxylate cycle, and then PEP carboxykinase converts oxaloacetate to PEP (42, 43). Moreover, in propionic acid bacteria, PPDK serves as the major enzyme catalyzing the conversion of pyruvate to PEP (44). However, our results clearly defined the function of PPS in H. mediterranei. PPSHm exhibited no functional variation, and the conversion occurred through a conventional mechanism. It did not catalyze PPDK or PYK reactions to any extent. Thus, from these obvious differences, it can be deduced that PPS enzyme in H. mediterranei is a unique and much simpler enzyme than that in bacteria and other archaea.
Our study further identified another protein, named PPS-like, which was initially designated PPS/PPDKHm due to its significant similarity with PPSHm and PPDKHm. Multiple-sequence alignment showed the presence of a conserved sequence motif, AVRSSATAED-ASFAGQ-T-L (26), in PPSHm and PPS/PPDKHm of H. mediterranei as well as PPS enzymes of other bacteria and archaea. However, the conserved motif for PPDK, PLLVSVRSGA-SMPGMMD (26), is absent in PPS/PPDKHm. This led us to an assumption that PPS/PPDKHm is more of a PPS protein than a PPDK protein. Interestingly, in all of the 25 haloarchaeal PPS-like proteins distributed in 14 haloarchaeal genera, the conserved His of PPS is replaced by other residues such as Ala, Ser, Thr, and Gly. This major difference in the signature residue alters the conventional PPS function, and, hence, we named the protein “PPS-like.” To analyze the pattern of evolutionary relationships of PPS-like proteins, PPS, and PPDK, we compiled a data set of 56 protein sequences from some representative bacteria and archaea (Table S2). Our unrooted phylogenetic tree has three distinct branches (Fig. 6). Branch I is divided into two subbranches, PPS and PPS-like proteins. The PPS subbranch is comprised of bacteria and two methanogenic species of archaea, while the PPS-like subbranch consists only of haloarchaea. Branch II represents PPS proteins from a few bacteria but mostly from archaea, including haloarchaea, methanogens, and thermophiles. The PPS lineage within Methanothermobacter thermautotrophicus, present in this branch, might be an ancestral lineage to the Methanococcus, Methanocaldococcus, and haloarchaeal sequences. Hence, the presented analyses identified two different PPS isoforms, PPS enzyme (branch II) and PPS-like protein (branch I), in haloarchaea not previously reported in Archaea. However, the PPS-like protein lacks PPS enzyme activity, probably due to the mutation of the catalytic His residue, conserved in PPS enzymes. Moreover, PPS-like protein is specific for haloarchaea and is more closely related to the PPS proteins of a few methanogenic archaea and most bacteria (Branch I) instead of most of the archaeal PPSs (branch II). Branch III comprises PPDK proteins from several different species of Archaea and Thermotoga maritima, a hyperthermophilic bacterium. The PPS-like protein in haloarchaea is distantly related to PPDK (branch III). This reconfirmed that the PPS/PPDKHm of H. mediterranei is more of a PPS protein than a PPDK protein. However, the question arises as to how the PPS-like gene evolved. Based on the phylogenetic analysis, we assumed that the pps gene underwent duplication in haloarchaeal species, thus providing the possibility for one copy to diverge and evolve as the PPS-like gene. This reconfirmed that PPS/PPDKHm is more like a PPS protein than a PPDK protein and justified the name PPS-like protein. Our bioinformatic structural analysis (HHPRED) of the PPS-like protein showed that the protein possesses highest structural similarity with the crystal structure of rifampin phosphotransferase (RPH) from Listeria monocytogenes. Interestingly, RPHLm confers antibiotic resistance by phosphorylating rifampin to its inactive form (45). Therefore, emergence of a protein with a new function through the process of gene combination, duplication. and sequence divergence is a common phenomenon during evolution.
FIG 6.
An unrooted phylogenetic tree of PPS, PPDK, and PPS-like proteins. The phylogenetic tree indicates the distinct lineages, conducted in MEGA X and enhanced using the iTOL server (https://itol.embl.de/). The evolutionary relationship was inferred by using the maximum likelihood method and a Jones-Taylor-Thornton (JTT) matrix-based model. One thousand bootstrap replicates were performed. The percentage of trees in which the associated taxa clustered together, shown as black circles on the branches, represents bootstrap values ranging from 0.75 to 1. The accession numbers of the PPS-like, PPS, and PPDK sequences used for the construction of the tree are listed in Table S2 in the supplemental material.
Furthermore, our enzyme activity analysis and gene knockout study clearly demonstrated that PPS-like protein had no function in PEP/pyruvate interconversion under either glycolytic or gluconeogenic conditions. Surprisingly, pps-like gene knockout led to an accumulation of higher levels of uniformly sized PHBV granules in H. mediterranei. To explain why pps-like gene knockout increased PHBV synthesis, the impact of the knockout on whole-cell metabolism was studied. The PPS-like gene knockout downregulated the genes involved in PEP/pyruvate conversion to other metabolites and upregulated the genes responsible for glucose transportation and monomer generation for PHBV synthesis. Furthermore, significant overexpression of PHA synthesis and regulation gene cluster in pps-like gene knockout improved PHA polymerization and PHA granule formation. Until now, the only successful case for enhancing PHBV accumulation in H. mediterranei was realized by deletion of genes for EPS production. Now, in the present research work, we found serendipitously that deletion of PPS-like gene in an EPS-deficient strain had an added effect on the PHBV accumulation. However, the exact mechanism by which PPS-like protein improves PHBV yield remains unclear and needs our further research.
Taken together, the results of our work filled the major gaps in the domain Archaea by identifying the conventional enzymes, PYKHm and PPSHm, participating in PEP/pyruvate interconversion in haloarchaea. Moreover, we developed a high-yielding PHBV strain which can serve as a potential candidate for biopolymer production at a commercial scale.
MATERIALS AND METHODS
Strains, medium, and culture conditions.
The strains used in this study are listed in Table 3. Escherichia coli JM109 and E. coli JM 110 were used to construct plasmids and eliminate methylation of plasmids, respectively. They were cultivated in Luria-Bertani medium (46) at 37°C. When required, ampicillin was added at a concentration of 100 μg/ml. H. mediterranei DF50, a uracil-auxotrophic strain with pyrF gene knockout (47), and its gene deletion mutants were grown in AS-168 medium (35) at 37°C and were used as seed culture. The seed culture was then inoculated in a chemically defined medium (48) with 2 g/liter glycerol or pyruvate as a sole carbon source. H. mediterranei ΔEPS and its mutant strains were grown at 37°C in a 250-ml shake flask for 120 h or in a 7-liter fermentor for 72 h in PHA production medium with 10 g/liter glucose as a carbon source. For the cultivation of pyrF-deleted strains, uracil was added at a concentration of 50 μg/ml. Haloferax volcanii H1424 was used for protein expression and was grown in Hv-YPC (H. volcanii medium consisting of yeast extract, peptone, and Casamino Acids) medium (49) at 45°C, supplemented with 50 μg/ml uracil and 20 μg/ml thymidine.
TABLE 3.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi | 46 |
| E. coli JM110 | dam dcm mutant of JM109 | Novagen |
| H. mediterranei strains | ||
| DF50 | pyrF-deleted mutant of wild-type strain H. mediterranei ATCC 33500 | 47 |
| ΔEPS | EPS synthesis gene cluster deletion mutant of DF50 | 37 |
| Δpyk | pyk deletion mutant of DF50 | This study |
| Δpps strain | pps deletion mutant of DF50 | This study |
| Δpps-like gene strain | pps-like gene deletion mutant of DF50 | This study |
| Δpps Δpps-like gene strain | pps and pps-like gene deletion mutant of DF50 | This study |
| ΔEPS Δpps | pps deletion mutant of ΔEPS | This study |
| ΔEPS Δpps-like gene | pps-like gene deletion mutant of ΔEPS | This study |
| ΔEPS Δpps Δpps-like gene | pps and pps-like gene deletion mutant of ΔEPS | This study |
| H. volcanii H1424 | ΔpyrE2 ΔhdrB pitANph Δmrr cdc48d-Ct | 54 |
| Plasmids | ||
| pHFX | 4.0 kb; integration vector containing pyrF and its native promoter, Ampr | 47 |
| pHFX-pyk | 5.2 kb; integration vector of pHFX for pyk deletion | This study |
| pHFX-pps | 5.2 kb; integration vector of pHFX for pps deletion | This study |
| pHFX-pps-like gene | 5.2 kb; integration vector of pHFX for pps-like gene deletion | This study |
| pWL502 | 7.9 kb; shuttle vector with pyrF marker, Ampr | |
| pPYK | 9.6 kb; expression vector of pWL502 containing pyk | This study |
| pPPS | 10.1 kb; expression vector of pWL502 containing pps | This study |
| pPPS-like | 10.5 kb; expression vector of pWL502 containing pps-like gene | This study |
| pTA03 | 8.0 kb; expression vector with C-terminal His6 tag and promoter PphaR | 49 |
| pTA03-PYK | 9.7 kb; expression plasmid of pTA03 containing pyk | This study |
| pTA03-PPS | 10.2 kb; expression plasmid of pTA03 containing pps | This study |
| pTA03-mutant PYK | 9.7 kb; expression plasmid of pTA03 containing mutant pyk | This study |
| pTA03-mutant PPS | 10.2 kb; expression plasmid of pTA03 containing mutant pps | This study |
| pTA06 | 8.0 kb; expression vector with N-terminal His6 tag and promoter PphaR | 49 |
| pTA06-PPS-like | 10.6 kb; expression plasmid of pTA06 containing pps-like gene | This study |
Construction of plasmids and mutants.
The primers and plasmids used for gene knockout, complementation, and point mutation in this study are summarized in Tables 1 and 3. All plasmids for gene deletion were constructed based on the suicide plasmid pHFX. All plasmids for gene complementation were constructed based on the shuttle plasmid pWL502. The plasmids for protein expression in H. volcanii H1424 were constructed based on the expression plasmid pTA03 or pTA06. The transformations of H. mediterranei and H. volcanii were performed by the polyethylene glycol (PEG)-mediated method (50). Gene mutant construction and verification were performed by a pop-in/pop-out method and PCR respectively, as described previously. (47).
RNA extraction and qRT-PCR.
H. mediterranei cells were grown in a chemically defined medium with glycerol or pyruvate as a sole carbon source until exponential phase and stationary growth phase. The harvested cells were used for total RNA extraction using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. A total of 1 μg of DNA-free RNA was used to synthesize cDNA using random hexamers and murine leukemia virus (MLV) reverse transcriptase (Promega, USA). A fluorogenic quantitative PCR was performed and analyzed by a ViiA 7 real-time PCR system (Applied Biosystems, Inc., USA). The relative fold change of target gene expression was calculated by the 2−ΔΔCT (where CT is threshold cycle) mathematical model (51), using 7S RNA as an endogenous control. The primers used are listed in Table 1.
Cell growth measurement and residual glucose determination.
The growth of H. mediterranei strains was monitored by the diphenylamine colorimetric method as described previously (36). The DNA content of cells was quantified by the absorbance at 595 nm using a Beckman Coulter DU800 spectrophotometer (Jersey City, NJ, USA). The residual sugar concentration in the supernatant was determined by a biosensor analyzer (SBA-40C; Shandong, China) with a 20-times dilution using distilled water every 12 h during the cultivation process.
PHBV accumulation analysis.
When glucose was consumed completely, the cells were harvested and then lyophilized overnight. A total of 50 mg of lyophilized cells was used for methanolysis treatment, and the resulting hydroxyacyl methyl esters were analyzed by gas chromatography (GC 6820; Agilent, USA) as previously described (34).
Protein expression and purification.
The plasmids constructed based on pTA03 or pTA06 used for target protein expression (52) in H. volcanii H1424 are listed in Table 3. H. volcanii H1424 strains were cultured in Hv-YPC medium at 42°C for 72 h and then collected for ultrasonication. His-tagged target protein in the supernatant was purified by AKTA purifier UPC10 (GE, USA) with a HisTrap high-performance (HP) column (GE, USA) according to the manufacturer’s instructions. The elution buffer used for protein purification contained 20 mM Tris-HCl, 2 M NaCl (pH 8.0) and 200 mM imidazole. The proteins were analyzed by SDS-PAGE, and their purity was determined by using ImageJ software. Purified proteins were further identified by MALDI-TOF/TOF MS (ABI 4700; Bruker, USA). Protein concentration was determined by bicinchoninic acid (BCA) protein assay (Sangon Biotech, Shanghai) according to the manufacturer’s instruction.
Enzyme assays.
Three enzyme activities of PYK, PPS, and PPDK were measured. Mutant proteins of PYK (PYK_A213A239) or PPS (PPS_A398) were used as negative controls in the enzyme assay. Briefly, the reaction was started by the addition of purified protein. After overnight incubation at 37°C, the reaction was stopped by the addition of 10 μl of 0.01 M HCl and transferred to ice. Then, the reaction mixture was desalted twice using ethanol. After proteins and salts were removed by centrifugation, the supernatant was analyzed by ion chromatography (IonPac AS11-HC; Dionex, USA). NaOH (30 mM or 100 mM) was used as a mobile phase, and its flow rate was maintained at 1.0 ml/min.
Pyruvate kinase activity was measured by the formation of pyruvate from PEP. The 200-μl reaction mixture contained 100 mM Tris-HCl (pH 7.5), 2 M KCl, 20 mM MgCl2, 10 mM ADP, 10 mM PEP, and 40 μg of purified PYK protein.
Phosphoenolpyruvate synthetase activity was measured either by the formation of pyruvate from PEP or by the formation of PEP from pyruvate. For the direction of pyruvate formation, the 200-μl reaction mixture contained 100 mM Tris-HCl (pH 7.5), 10 mM KH2PO4-NaOH (pH 7.5), 2 M KCl, 20 mM MgCl2, 10 mM AMP, 10 mM PEP, and 40 μg of purified PPS or PPS-like protein. For the measurement in the direction of PEP formation, the 200-μl reaction mixture contained 100 mM Tris-HCl (pH 7.5), 2 M KCl, 20 mM MgCl2, 10 mM ATP, 10 mM pyruvate, and 40 μg of purified PPS or PPS-like protein.
Pyruvate:phosphate dikinase activity was also measured in the same manner as described above for phosphoenolpyruvate synthetase activity, with minor modifications. For measurement in the direction of pyruvate formation, the 200-μl reaction mixture contained 100 mM Tris-HCl (pH 7.5), 2 M KCl, 20 mM MgCl2, 10 mM AMP, 10 mM PEP, 20 mM PPi, and 80 μg of purified PPS-like protein. For the measurement in the direction of PEP formation, the 200-μl reaction mixture contained 100 mM Tris-HCl (pH 7.5), 10 mM KH2PO4-NaOH (pH 7.5), 2 M KCl, 20 mM MgCl2, 10 mM ATP, 10 mM pyruvate, and 80 μg of purified PPS-like protein.
TEM analysis.
H. mediterranei ΔEPS and ΔEPS Δpps-like gene, grown in PHA accumulation medium to their exponential phase and stationary growth phase, were harvested and used for transmission electron microscopy (TEM) analysis. The TEM samples were prepared according to our previous description (53) and then observed using a JEM-1400 electron microscope (JEOL, Japan).
Transcriptome analysis.
H. mediterranei ΔEPS and ΔEPS Δpps-like gene cells were grown in PHA accumulation medium until their exponential growth phase. The total RNA was extracted from the collected cells. After quantification and qualification, 3 μg of RNA was used to construct a library for strand-specific transcriptome sequencing. After clustering and sequencing using a HiSeq T2500 (Illumina, USA) by Novogene Co., Ltd. (Beijing, China), clean data were obtained by removing reads containing adapter or poly(N) and low-quality reads. The Q20 and Q30 values (percentages of bases with Phred values greater than 20 and 30, respectively) and GC content of our clean data were calculated. Then, the read numbers mapped to each gene were counted by HTSeq, version 0.6.1 (50). The expected number of fragments per kilobase of transcript per million mapped reads (FPKM) of each gene was further calculated and used to present the change in expression of each gene. Differential expression analysis of two samples was performed using the DEGSeq R package (1.20.0) (51). A P value of 0.005 and log2(fold change) of 1 were set as the thresholds for significantly differential expression.
Sequence analysis and database.
Query sequences were accessed from the National Center for Biotechnology Information (NCBI) Protein Database. Sequence homology was analyzed via the Uniprot BLAST server (https://www.uniprot.org/). An unrooted phylogenetic tree was constructed using the maximum likelihood method and MEGA X software (52) and enhanced using the iTOL server (https://itol.embl.de/). The confidence for the internal branches of the tree was determined through bootstrap analysis with 1,000 replicates. Logos of the conserved amino acid sites were created via the WebLogo server (http://weblogo.threeplusone.com/).
Statistical analysis.
The results are presented as the means ± standard errors of three independent replicates. Significant differences among groups were identified by one-way analysis of variance (ANOVA), with statistical significance defined at a P value of <0.01.
Data availability.
Our RNA-seq data were deposited in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/Traces/sra/) under accession number PRJNA523064.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by the National Key Research and Development Program (no. 2018YFA0900200), the National Natural Science Foundation of China (NSFC-91751201), the Youth Innovation Promotion Association of CAS (no. 2015070), and the National Natural Science Foundation of China (no. 91751201).
J.H., J.C., and H.X. designed and conceived the project. J.C. performed the experiments; J.H., J.C., R.M., S.Z., Z.Z., L.L., and D.Z. analyzed the data. J.H., R.M., J.C., and H.X. drafted the manuscript. All authors contributed to the revision of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00984-19.
REFERENCES
- 1.Eme L, Doolittle WF. 2015. Archaea. Curr Biol 25:R851–R855. doi: 10.1016/j.cub.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 2.Allers T, Mevarech M. 2005. Archaeal genetics—the third way. Nat Rev Genet 6:58–73. doi: 10.1038/nrg1504. [DOI] [PubMed] [Google Scholar]
- 3.Hou J, Han J, Cai L, Zhou J, Lu Y, Jin C, Liu J, Xiang H. 2014. Characterization of genes for chitin catabolism in Haloferax mediterranei. Appl Microbiol Biotechnol 98:1185–1194. doi: 10.1007/s00253-013-4969-8. [DOI] [PubMed] [Google Scholar]
- 4.Johnsen U, Dambeck M, Zaiss H, Fuhrer T, Soppa J, Sauer U, Schonheit P. 2009. d-Xylose degradation pathway in the halophilic archaeon Haloferax volcanii. J Biol Chem 284:27290–27303. doi: 10.1074/jbc.M109.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falb M, Muller K, Konigsmaier L, Oberwinkler T, Horn P, von Gronau S, Gonzalez O, Pfeiffer F, Bornberg-Bauer E, Oesterhelt D. 2008. Metabolism of halophilic archaea. Extremophiles 12:177–196. doi: 10.1007/s00792-008-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnsen U, Schönheit P. 2004. Novel xylose dehydrogenase in the halophilic archaeon Haloarcula marismortui. J Bacteriol 186:6198–6207. doi: 10.1128/JB.186.18.6198-6207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siebers B, Wendisch VF, Hensel R. 1997. Carbohydrate metabolism in Thermoproteus tenax: in vivo utilization of the non-phosphorylative Entner-Doudoroff pathway and characterization of its first enzyme, glucose dehydrogenase. Arch Microbiol 168:120–127. doi: 10.1007/s002030050477. [DOI] [PubMed] [Google Scholar]
- 8.Reher M, Gebhard S, Schönheit P. 2007. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) and nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPN), key enzymes of the respective modified Embden-Meyerhof pathways in the hyperthermophilic crenarchaeota Pyrobaculum aerophilum and Aeropyrum pernix. FEMS Microbiol Lett 273:196–205. doi: 10.1111/j.1574-6968.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 9.Verhees CH, Kengen SWM, Tuininga JE, Schut GJ, Adams MWW, De Vos WM, Van Der Oost J. 2003. The unique features of glycolytic pathways in Archaea. Biochem J 375:231–246. doi: 10.1042/BJ20021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meléndez-Hevia E, Waddell TG, Heinrich R, Montero F. 1997. Theoretical approaches to the evolutionary optimization of glycolysis: chemical analysis. Eur J Biochem 244:527–543. doi: 10.1111/j.1432-1033.1997.t01-1-00527.x. [DOI] [PubMed] [Google Scholar]
- 11.Galperin MY, Koonin EV. 1999. Functional genomics and enzyme evolution, p 265–283. In Morton BE, Pongor S (ed), Structural biology and functional genomics. Springer, New York, NY. [Google Scholar]
- 12.Makarova KS, Aravind L, Galperin MY, Grishin NV, Tatusov RL, Wolf YI, Koonin EV. 1999. Comparative genomics of the Archaea (Euryarchaeota): evolution of conserved protein families, the stable core, and the variable shell. Genome Res 9:608–628. [PubMed] [Google Scholar]
- 13.Reher M, Schönheit P. 2006. Glyceraldehyde dehydrogenases from the thermoacidophilic euryarchaeota Picrophilus torridus and Thermoplasma acidophilum, key enzymes of the non-phosphorylative Entner-Doudoroff pathway, constitute a novel enzyme family within the aldehyde dehydrogenase superfamily. FEBS Lett 580:1198–1204. doi: 10.1016/j.febslet.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Johnsen U, Selig M, Xavier KB, Santos H, Schönheit P. 2001. Different glycolytic pathways for glucose and fructose in the halophilic archaeon Halococcus saccharolyticus. Arch Microbiol 175:52–61. doi: 10.1007/s002030000237. [DOI] [PubMed] [Google Scholar]
- 15.Tästensen JB, Schönheit P. 2018. Two distinct glyceraldehyde-3-phosphate dehydrogenases in glycolysis and gluconeogenesis in the archaeon Haloferax volcanii. FEBS Lett 592:1524–1534. doi: 10.1002/1873-3468.13037. [DOI] [PubMed] [Google Scholar]
- 16.Oria-Hernández J, Riveros-Rosas H, Ramírez-Sílva L. 2006. Dichotomic phylogenetic tree of the pyruvate kinase family: K+-dependent and -independent enzymes. J Biol Chem 281:30717–30724. doi: 10.1074/jbc.M605310200. [DOI] [PubMed] [Google Scholar]
- 17.Bräsen C, Esser D, Rauch B, Siebers B. 2014. Carbohydrate metabolism in Archaea: current insights into unusual enzymes and pathways and their regulation. Microbiol Mol Biol Rev 78:89–175. doi: 10.1128/MMBR.00041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schramm A, Siebers B, Tjaden B, Brinkmann H, Hensel R. 2000. Pyruvate kinase of the hyperthermophilic crenarchaeote Thermoproteus tenax: physiological role and phylogenetic aspects. J Bacteriol 182:2001–2009. doi: 10.1128/jb.182.7.2001-2009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnsen U, Hansen T, Schonheit P. 2003. Comparative analysis of pyruvate kinases from the hyperthermophilic archaea Archaeoglobus fulgidus, Aeropyrum pernix, and Pyrobaculum aerophilum and the hyperthermophilic bacterium Thermotoga maritima: unusual regulatory properties in hyperthermophilic archaea. J Biol Chem 278:25417–25427. doi: 10.1074/jbc.M210288200. [DOI] [PubMed] [Google Scholar]
- 20.De la Vega-Ruíz G, Domínguez-Ramírez L, Riveros-Rosas H, Guerrero-Mendiola C, Torres-Larios A, Hernández-Alcántara G, García-Trejo JJ, Ramírez-Silva L. 2015. New insights on the mechanism of the K+-independent activity of Crenarchaeota pyruvate kinases. PLoS One 10:e0119233. doi: 10.1371/journal.pone.0119233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter S, Fothergill-Gilmore LA. 1992. The pyruvate kinase of Thermoplasma acidophilum: purification, kinetic characterisation & use as a phylogenetic marker. Biochem Soc Trans 20:11S. doi: 10.1042/bst020011s. [DOI] [PubMed] [Google Scholar]
- 22.Imanaka H, Yamatsu A, Fukui T, Atomi H, Imanaka T. 2006. Phosphoenolpyruvate synthase plays an essential role for glycolysis in the modified Embden-Meyerhof pathway in Thermococcus kodakarensis. Mol Microbiol 61:898–909. doi: 10.1111/j.1365-2958.2006.05287.x. [DOI] [PubMed] [Google Scholar]
- 23.Haferkamp P, Tjaden B, Shen L, Bräsen C, Kouril T, Siebers B. 2019. The carbon switch at the level of pyruvate and phosphoenolpyruvate in Sulfolobus solfataricus P2. Front Microbiol 10:757. doi: 10.3389/fmicb.2019.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans J, Raleigh DP, Tolman CJ, Roberts MF. 1986. 13C NMR spectroscopy of Methanobacterium thermoautotrophicum. Carbon fluxes and primary metabolic pathways. J Biol Chem 261:16323–16331. [PubMed] [Google Scholar]
- 25.Hutchins AM, Holden JF, Adams MW. 2001. Phosphoenolpyruvate synthetase from the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol 183:709–715. doi: 10.1128/JB.183.2.709-715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjaden B, Plagens A, Dorr C, Siebers B, Hensel R. 2006. Phosphoenolpyruvate synthetase and pyruvate, phosphate dikinase of Thermoproteus tenax: key pieces in the puzzle of archaeal carbohydrate metabolism. Mol Microbiol 60:287–298. doi: 10.1111/j.1365-2958.2006.05098.x. [DOI] [PubMed] [Google Scholar]
- 27.Zuo ZQ, Xue Q, Zhou J, Zhao DH, Han J, Xiang H. 2018. Engineering Haloferax mediterranei as an efficient platform for high level production of lycopene. Front Microbiol 9:2893. doi: 10.3389/fmicb.2018.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Hou J, Liu H, Cai S, Feng B, Zhou J, Xiang H. 2010. Wide distribution among halophilic archaea of a novel polyhydroxyalkanoate synthase subtype with homology to bacterial type III synthases. Appl Environ Microbiol 76:7811–7819. doi: 10.1128/AEM.01117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen GQ, Jiang XR. 2018. Engineering microorganisms for improving polyhydroxyalkanoate biosynthesis. Curr Opin Biotechnol 53:20–25. doi: 10.1016/j.copbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Zhao YX, Rao ZM, Xue YF, Gong P, Ji YZ, Ma YH. 2015. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Haloarchaeon Halogranum amylolyticum. Appl Microbiol Biotechnol 99:7639–7649. doi: 10.1007/s00253-015-6609-y. [DOI] [PubMed] [Google Scholar]
- 31.Koller M. 2018. Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 23:362. doi: 10.3390/molecules23020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jendrossek D, Pfeiffer D. 2014. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ Microbiol 16:2357–2373. doi: 10.1111/1462-2920.12356. [DOI] [PubMed] [Google Scholar]
- 33.Xue Q, Liu XB, Lao YH, Wu LP, Wang D, Zuo ZQ, Chen JY, Hou J, Bei YY, Wu XF, Leong KW, Xiang H, Han J. 2018. Anti-infective biomaterials with surface-decorated tachyplesin I. Biomaterials 178:351–362. doi: 10.1016/j.biomaterials.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Lu Q, Han J, Zhou L, Zhou J, Xiang H. 2008. Genetic and biochemical characterization of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranei. J Bacteriol 190:4173–4180. doi: 10.1128/JB.00134-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han J, Hou J, Zhang F, Ai G, Li M, Cai S, Liu H, Wang L, Wang Z, Zhang S, Cai L, Zhao D, Zhou J, Xiang H. 2013. Multiple propionyl coenzyme A-supplying pathways for production of the bioplastic poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Haloferax mediterranei. Appl Environ Microbiol 79:2922–2931. doi: 10.1128/AEM.03915-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou J, Xiang H, Han J. 2015. Propionyl coenzyme A (propionyl-CoA) carboxylase in Haloferax mediterranei: Indispensability for propionyl-CoA assimilation and impacts on global metabolism. Appl Environ Microbiol 81:794–804. doi: 10.1128/AEM.03167-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao D, Cai L, Wu J, Li M, Liu H, Han J, Zhou J, Xiang H. 2013. Improving polyhydroxyalkanoate production by knocking out the genes involved in exopolysaccharide biosynthesis in Haloferax mediterranei. Appl Microbiol Biotechnol 97:3027–3036. doi: 10.1007/s00253-012-4415-3. [DOI] [PubMed] [Google Scholar]
- 38.Beckers V, Poblete-Castro I, Tomasch J, Wittmann C. 2016. Integrated analysis of gene expression and metabolic fluxes in PHA-producing Pseudomonas putida grown on glycerol. Microb Cell Fact 15:73. doi: 10.1186/s12934-016-0470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnsen U, Reinhardt A, Landan G, Tria FDK, Turner JM, Davies C, Schönheit P. 2019. New views on an old enzyme: allosteric regulation and evolution of archaeal pyruvate kinases. FEBS J 10:1111. doi: 10.1111/febs.14837. [DOI] [PubMed] [Google Scholar]
- 40.Potter S, Fothergill-Gilmore LA. 1992. Purification and properties of pyruvate kinase from Thermoplasma acidophilum. FEMS Microbiol Lett 73:235–239. doi: 10.1016/0378-1097(92)90636-3. [DOI] [PubMed] [Google Scholar]
- 41.Cicicopol C, Peters J, Lupas A, Cejka Z, Muller SA, Golbik R, Pfeifer G, Lilie H, Engel A, Baumeister W. 1999. Novel molecular architecture of the multimeric archaeal PEP-synthase homologue (MAPS) from Staphylothermus marinus. J Mol Biol 290:347–361. doi: 10.1006/jmbi.1999.2878. [DOI] [PubMed] [Google Scholar]
- 42.Inui M, Nakata KO, Roh JH, Zahn K, Yukawa H. 1999. Molecular and functional characterization of the Rhodopseudomonas palustris No. 7 phosphoenolpyruvate carboxykinase gene. J Bacteriol 181:2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long CP, Au J, Sandoval NR, Gebreselassie NA, Antoniewicz MR. 2017. Enzyme I facilitates reverse flux from pyruvate to phosphoenolpyruvate in Escherichia coli. Nat Commun 8:14316. doi: 10.1038/ncomms14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans HJ, Wood HG. 1971. Purification and properties of pyruvate phosphate dikinase from propionic acid bacteria. Biochemistry 10:721–729. doi: 10.1021/bi00781a001. [DOI] [PubMed] [Google Scholar]
- 45.Stogios PJ, Cox G, Spanogiannopoulos P, Pillon MC, Waglechner N, Skarina T, Koteva K, Guarné A, Savchenko A, Wright GD. 2016. Rifampin phosphotransferase is an unusual antibiotic resistance kinase. Nat Commun 7:11343. doi: 10.1038/ncomms11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 47.Liu H, Han J, Liu X, Zhou J, Xiang H. 2011. Development of pyrF-based gene knockout systems for genome-wide manipulation of the archaea Haloferax mediterranei and Haloarcula hispanica. J Genet Genomics 38:261–269. doi: 10.1016/j.jgg.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Han J, Wu LP, Hou J, Zhao D, Xiang H. 2015. Biosynthesis, characterization, and hemostasis potential of tailor-made poly(3-hydroxybutyrate-co-3-hydroxyvalerate) produced by Haloferax mediterranei. Biomacromolecules 16:578–588. doi: 10.1021/bm5016267. [DOI] [PubMed] [Google Scholar]
- 49.Liu G, Hou J, Cai S, Zhao D, Cai L, Han J, Zhou J, Xiang H. 2015. A patatin-like protein associated with the polyhydroxyalkanoate (PHA) granules of Haloferax mediterranei acts as an efficient depolymerase in the degradation of native PHA. Appl Environ Microbiol 81:3029–3038. doi: 10.1128/AEM.04269-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cline SW, Lam WL, Charlebois RL, Schalkwyk LC, Doolittle WF. 1989. Transformation methods for halophilic archaebacteria. Can J Microbiol 35:148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- 51.Schmittgen TD, Livak K. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 52.Liu G, Cai S, Hou J, Zhao D, Han J, Zhou J, Xiang H. 2016. Enoyl-CoA hydratase mediates polyhydroxyalkanoate mobilization in Haloferax mediterranei. Sci Rep 6:24015. doi: 10.1038/srep24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai S, Cai L, Liu H, Liu X, Han J, Zhou J, Xiang H. 2012. Identification of the haloarchaeal phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl Environ Microbiol 78:1946–1952. doi: 10.1128/AEM.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stroud A, Liddell S, Allers T. 2012. Genetic and biochemical identification of a novel single-stranded DNA-binding complex in Haloferax volcanii. Front Microbiol 3:224. doi: 10.3389/fmicb.2012.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our RNA-seq data were deposited in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/Traces/sra/) under accession number PRJNA523064.