Abstract
Peak exercise performance in healthy man is limited not only by pulmonary or skeletal muscle function but also by cardiac function. Thus, abnormalities in cardiac function will have a major impact on exercise performance. Many cardiac diseases affect exercise performance and indeed for some cardiac conditions such as atherosclerotic heart disease, exercise testing is frequently used not only to measure functional capacity but also to make a diagnosis of heart disease, evaluate the efficacy of treatment, and predict prognosis. Early in the course of cardiac diseases, exercise performance will be minimally affected but with disease progression impairment in exercise capacity will become apparent. Ejection fraction, that is, the percent of blood volume ejected with each cardiac cycle is often used as a measure of cardiac performance but frequently there is a dissociation between the ejection fraction and exercise capacity in patients with heart disease. How abnormalities in cardiac function impacts the muscles, vasculature, and lungs to impact exercise performance will here be reviewed. The focus of this work will be on patients with systolic heart failure as the incidence and prevalence of heart failure is reaching epidemic proportions and heart failure is the end result of many other chronic cardiac diseases. The prognostic role of exercise and benefits of exercise training will also be discussed.
Introduction
Cardiac function is a major determinant of peak exercise performance in normal man in addition to pulmonary and skeletal muscle function. Abnormal cardiac function has, therefore, a major impact on exercise performance. Exercise performance is affected by many cardiac disease states and for some cardiac conditions such as atherosclerotic heart disease, exercise testing is frequently used both as a prognostic and diagnostic test to assess both functional capacity and diagnose heart disease. The results of these assessments have impact on the efficacy of treatment and predict prognosis. Early in cardiac diseases, exercise performance will be minimally affected but with disease progression impairment in exercise capacity will become apparent. Ejection fraction, that is, the percent of blood volume ejected with each cardiac cycle is often used as a measure of cardiac performance but frequently there is a dissociation between the ejection fraction and exercise capacity in patients with heart disease.
The cardiovascular system is a continuous vascular circuit that includes a muscular pump and the vasculature to deliver oxygen and nutrients to all organs. As such, the ability of the heart to augment its output in response to the energy demands of exercise makes it an integral part of any discussion of exercise physiology. Maximum oxygen uptake during exercise is derived from the Fick equation, that is, the product of maximum cardiac output times the arteriovenous oxygen difference. Resting cardiac output averages 4 to 6 L/min and can increase by a factor of 4 to 6 during maximal upright exercise. The distribution of blood secondary to the increased cardiac output during exercise is primarily to the working muscle, that is, skeletal and cardiac muscle with the vast majority of the blood flow redirected to the skeletal muscle. The heart generates this response primarily by not only an increase in heart rate, but also by augmentation of stroke volume. This increase of stroke volume results from increased venous return and the Frank Starling principle as well as increased contractility from sympathetic stimulation and peripheral vasodilation. End-diastolic volume increases with exercise and end-systolic volume decreases during upright but not supine exercise as a consequence of the difference in venous return affected by gravity. In patients with cardiac disease, failure to increase cardiac output can be derived from a variety of mechanisms including cardiomyopathies, coronary artery disease with active ischemia, valvular heart disease, and pulmonary hypertension. Additionally, the reduced cardiac output can over time affect the peripheral vasculature as well as the skeletal muscle. In this article, we will review the effect of chronic heart failure (CHF) on exercise performance. The use of exercise to risk-stratify patients with heart failure (HF) will be discussed as well as the use of exercise as therapy for cardiac diseases.
Abnormalities in Exercise Performance in Chronic HF
The exercise response in patients with HF
HF is defined as the inability of the heart to adequately perfuse the metabolizing tissue. The clinical syndrome of congestive HF (HF) involves a complex interplay of skeletal muscle and peripheral vascular adaptations resulting from decreased myocardial performance. The classic symptoms of HF are exertional fatigue and dyspnea. Traditionally, it has been hypothesized that the major limitation to exercise performance in these patients results from a reduced cardiac output response to exercise leading to skeletal muscle hypoperfusion and lactic acidosis (120). However, secondary changes in other organ systems such as skeletal muscle, the vasculature, and the lungs play an important role in the genesis of both fatigue and dyspnea (33). Later in this review, we will review in detail the numerous peripheral changes that are observed in the vasculature, respiratory tract, and skeletal muscles of HF patients and discuss how these changes impact exercise performance.
In the presence of a reduced cardiac output, the heart is dependent on three principle compensatory mechanisms to maintain normal function. First, the Frank-Starling mechanism which increases preload to sustain cardiac stroke volume. Second, myocardial hypertrophy occurs, to increase the mass of contractile tissue. Third, the sympathetic nervous system is activated to augment myocardial contractility. These compensatory mechanisms are limited and with persistent stimulation ultimately become detrimental, contributing to the progression of the disease process. The heart rate response which is critical in increasing cardiac output frequently is blunted in HF patients due to both chronotropic incompetence as well as the widespread use of beta blockers which have become a cornerstone of treatment for this disease (151).
In the short term, these compensatory mechanisms serve to preserve cardiac output which is the primary limitation to maximal physical performance in normal subjects. In HF patients, decreased exercise capacity has similarly been attributed to a decreased cardiac output response which leads to skeletal muscle underperfusion and intramuscular lactic acidosis (48, 64). This is based on observations that patients with HF exhibit reduced cardiac output responses to exercise compared to normal subjects. Additionally, there is a pronounced increase in filling pressures with the development of marked pulmonary hypertension with pulmonary capillary wedge pressures reaching as high as 50 to 60 mmHg.
Exercise hemodynamic measurements and ventilatory gas measurements during progressive treadmill exercise in patients with HF have first been described by Karl Weber in 1981 (204). This report demonstrated the usefulness of this technique as a noninvasive method for characterizing cardiac reserve and functional status. Weber demonstrated a significant correlation between cardiac output response and oxygen consumption and was able to classify patients into groups of worsening severity on the basis of this noninvasive technique. He found that with worsening HF the cardiac output response is markedly diminished. Several other studies have shown a significant correlation between the peak VO2 and cardiac output (99,188). It is this correlation between peak VO2 and cardiac output which underlies the prognostic value of exercise variables in HF leading to the widespread use of cardiopulmonary exercise testing in the evaluation of patients with HF. Use of metabolic carts equipped with rapidly responding carbon dioxide and oxygen sensors have not only provided a means of better understanding the exercise response of patients but also in providing important clinical information on prognosis and how to direct medical therapy.
Peripheral Factors Limiting Exercise Performance in HF
Skeletal muscle abnormalities
Though peak exercise capacity is clearly dependent on the cardiac output response to exercise, this is not the sole determinant of exercise performance in patients with HF. Patients with similar reductions in left ventricular function have a wide range of exercise capacity. Furthermore, therapeutic interventions aimed at acutely increasing cardiac output such as inotropic drugs like dobutamine and milrinone, do not significantly increase exercise capacity or peak VO2 (130,134,210). This discrepancy between enhanced cardiac output and fixed peak VO2 can be explained on the basis of the peripheral vascular and skeletal muscle derangements in CHF. Due to regional vascular or skeletal abnormalities, the augmented cardiac output cannot be utilized by the exercising muscle beds and therefore peak VO2 is not altered. Evidence for a peripheral abnormality in CHF was first described in the 1970s by Zelis (214,215).
Skeletal muscles are not simply a mechanical system, but sensory organs that sense effort, tension, displacement, and fatigue via tendon organs, muscle spindles, joint receptors, and small nerve endings. It is the sensory aspect of skeletal muscles that mediates the symptoms of exercise intolerance in both healthy and disease states (111,142,164,180).
Alterations of skeletal muscle metabolism and mass also play an important role in limiting peak functional capacity in patients with HF (82,133,200). Metabolic and atrophic alterations have been shown in skeletal and diaphragmatic muscle of patients with HF (45,108,131,145,184). It is well established that muscle wasting is a strong independent risk factor for mortality in HF (4). However, the etiology underlying the muscle changes remain unclear. Most investigators agree that reduced physical activity (disuse and immobilization in advanced stages) plays some part in the muscle alterations in HF but cannot explain the full extent of changes in muscle structure, function, and metabolism (28,29,139,179). Of note, HF-related muscle abnormalities are not substantially different from those observed in other chronic conditions such as chronic pulmonary or renal disease (197). Chronic low-level systemic inflammation characteristic of the HF state effect changes in skeletal muscle (132) and with the progression of HF, inflammatory mediators released into the circulation further activate systemic inflammation and promote muscle atrophy.
Skeletal muscle alterations develop and worsen as symptoms in patients with HF intensify. Severity of HF due to LV systolic dysfunction is currently categorized in four stages: Stage A includes those patients with risk factors for developing HF; Stage B includes patients with LV remodeling in the absence of symptoms (61); Stage C is defined by the occurrence and progression of symptoms; and Stage D includes patients with symptoms refractory to medical therapy.
Histologic changes of skeletal muscle in HF
Histomorphologic and metabolic changes of skeletal muscle in HF include changes in the fiber composition of muscle, fiber atrophy, fatty infiltration, and decrease in oxidative enzymes. Adult human muscle is composed various fiber types: Types I, IIa, and IIx. The fiber types are defined by their myosin heavy-chain isoforms, and can be identified by ATPase staining or immunohistochemistry (9, 175) (Table 1).
Table 1.
Skeletal Muscle Fiber Types
| Fiber type | Type I | Type IIa | Type IIX |
|---|---|---|---|
| Color | Red | Red | White |
| Contraction time | Slow | Moderate | Fast |
| Oxidative capacity | High | High | Medium |
| Mitochondrial density | High | High | Medium |
| Glycolytic capacity | Low | High | High |
| Resistance to fatigue | High | Intermediate | Lower |
| Major storage fuel | TAG | CP/glycogen | CP/glycogen |
| Capillary density | High | Intermediate | Low |
Abbreviation: CP, creatine phosphate, TAG, triglyceride.
Several investigators have reported that patients with HF develop a characteristic shift in muscle fiber distribution with an increased number of Type II fibers (anaerobic, gylcolytic) as compared to Type I (aerobic, oxidative) (174, 187). In muscle biopsies, an increase in glycolytic, fast-twitch type IIa/x fibers, type II fiber atrophy, and a reduction in lipolytic and oxidative enzymes was described (157,179). Nevertheless, although conventional wisdom suggests that fiber type is shifted by HF, this could be due to muscle disuse. Excellent work by Mettauer et al. (140), which controlled for deconditioning in the HF population, found no fiber type shift compared to controls with similar VO2 max. These findings have recently been substantiated (144). Drexler et al. further described decreased volume density of the mitochondria and surface density of the mitochondrial cristae, implying that oxidative transport coupling was compromised (45). The decreased mitochondrial volume correlated with peak aerobic capacity, suggesting a major contribution of altered skeletal muscle metabolism to exercise intolerance. The content of intramitochondrial Krebs cycle citrate synthase, total cytosolic creatine kinase (CK), skeletal muscle-specific CK (MM-CK) and lactate dehydrogenase (LDH) decreases in HF patients (139). Notably, angiotensin-converting enzyme inhibition seems to prevent the fiber switch from Type I to II in the skeletal muscle (172,176,202).
Abnormal skeletal muscle metabolism in HF
Muscle metabolism in various muscle groups has been studied in patients with HF. Abnormal skeletal muscle metabolism, that is, reduced oxidative metabolism with earlier shift to glycolytic metabolism, has been demonstrated in patients with HF using 31P magnetic resonance spectroscopy (29,55,114,123,136,155). These studies consistently show an accelerated rate of phosphocreatine utilization (PCr, a high-energy phosphate), accumulation of inorganic phosphate (Pi, a by-product of ATP utilization), early intracellular acidification (low Ph) and delayed PCr recovery after exercise. These abnormalities appear to be independent of total limb perfusion (55,114,135,206), histochemical changes (184), muscle mass (98), or severe tissue hypoxia (87). Venous plethysmography to measure limb blood flow demonstrated that metabolic changes during exercise in HF occurs in the absence of associated decrease in limb perfusion (206). Persistent metabolic abnormalities in patients with CHF compared to normal subjects was also demonstrated during ischemic exercise, that is, exercise performed during arterial occlusion. Moreover, acutely increasing cardiac output with therapeutic agents such as dobutamine did not improve the metabolic abnormalities observed in these patients (134).
Simultaneous monitoring of cellular metabolism and oxygenation during leg exercise in HF patients by coupling 31P magnetic resonance spectroscopy to near infrared spectroscopy revealed that metabolic abnormalities observed in patients with HF occurred despite what appears to be adequate muscle oxygenation. This again supports an intrinsic skeletal muscle metabolic change in these patients. Overall, patients with HF experience metabolic alterations that are similar to those reported after deconditioning. To what extent muscle atrophy, fibrosis and inflammation underlie 31P-MRS metabolic alterations remains unclear.
Adamopoulos (2) showed that physical conditioning substantially improve the muscle metabolic alterations in patients with HF but did not normalize it. After 8 weeks of home-based bicycle exercise training in a randomized-crossover trial, patients with HF exhibited less PCr depletion and faster PCr recovery. However, exercise-induced acidification was unaffected by physical conditioning.
Impaired skeletal muscle excitation contraction coupling in HF
Excitation contraction coupling of the skeletal muscle sarcomere is abnormal in HF and is in part related to abnormalities in intracellular calcium handling leading to an intracellular calcium overload state.
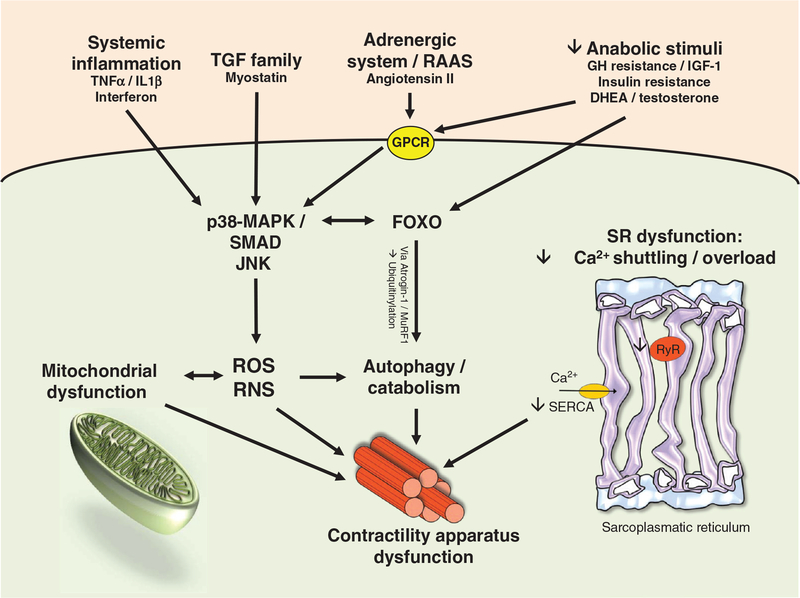
In the myocardium, calcium cycling mediated by the sarcoplasmic reticulum Ca2+ ATPase (SERCA) 2a is a critical control mechanism in regard to cardiac contraction. During the action potential, calcium enters the cell through L-type calcium channels as inward calcium current. Calcium entry triggers further calcium release from the sarcoplasmic reticulum into the cytosol by activation of the ryanodine receptor. The increase in cytoplasmic free calcium allows calcium to bind to troponin C triggering contraction. Relaxation depends on the release and decline of cytosolic calcium. This requires calcium transport out of the cytosol by multiple pathways including SERCA 2a at the sarcoplasmic reticulum, the membrane-bound sodium-calcium exchange pump, mitochondrial transport, and ryanodine receptors. SERCA 2a removes up to 90% of intracellular calcium in rodents and 70% in humans and large animals. Abnormal cellular calcium handling is observed in the failing heart (18,119,162). Increased sodium/calcium exchange mechanism, reduction in SERCA2a activity, decreased phospholamban/SERCA 2a ratios and increased phosphorylation of ryanodine receptor producing “leakiness” altogether result in decreased sarcoplasmic reticulum calcium content and a prolonged calcium transient flux (Fig. 1).
Figure 1.
Pathophysiological mechanisms impairing skeletal muscle function in heart failure.
Skeletal muscles from animals with HF exhibit increased Ca2+ spark frequency, decreased Ca2+ spark amplitude, and increased Ca2+ spark duration consistent with leaky sarcoplasmic reticulum Ca2+ release and with decreased sarcoplasmic reticulum Ca2+ content (162). Comparable to the failing myocardium, the muscle-specific Type 1 ryanodine receptor (RyR1) becomes leaky in HF as chronic adrenergic stimulation results in hyperphosphorylation of the channel. This change dissociates calstabin-1, the stabilizing protein that keeps the RyR1 channel in a closed state (18). Data regarding sarcoplasmic reticulum Ca2+ pumping in HF differ in fast and slow twitch muscles and according the level of fatigue (119). In agreement with data in aging, sarcoplasmic reticulum Ca2+ pumping appears to be most impaired in slow twitch muscles of animals with HF. Data regarding skeletal muscle protein and mRNA expression of SERCA 1a, the skeletal muscle specific isoform of SERCA, are divergent in HF (120,162) Predominant muscle fiber composition, degree functional impairment, and nature of the HF model may in part account for the disparate findings that have been occasionally correlated with early muscle fatigue.
Another recently investigated target in Ca2+ signaling pathomechanisms in HF are calpains. These Ca2+-activated proteases appear to have pleiotropic effects on various aspects of skeletal and cardiac muscle homeostasis such as cardiac remodeling, inflammation, and fibrosis as well as skeletal muscle atrophy (16,43,110).
Muscle fiber atrophy and changes in total muscle mass
In addition to fiber atrophy noted on muscle biopsy, HF patients also exhibit generalized muscle atrophy (131). Analysis of skinfold fat thickness, arm circumference, and 24 h urine collections for creatinine to estimate total muscle mass showed that patients with HF have adequate fat stores but in 60% of patients significant muscle loss develops. In another study, calf muscle volume assessed by magnetic resonance imaging showed reduced muscle volume in patients with HF and significant water and fat infiltration was also noted in the muscle sections of these patients (126). Calf muscle mass was reported to be significantly lower in patients with severe HF as evidenced by a peak VO2 of 13 mL/min/kg when compared to age and gender matched controls. However, some investigators have reported normal muscle mass in patients with HF (98).
The assessment of muscle function in patients with HF consistently showed decreased muscle strength and increased fatigability when normalized to fiber cross-sectional area in patients with HF (48). Slowing of relaxation and steady decline in strength has been reported during low-frequency stimulation of slow twitch muscles in rats with congestive HF (119). While still controversial, reduced isometric force and calcium-activated actomyosin ATPase activity in patients with HF may be due to a decline in density of contractile proteins and the specific rate of cross-bridge attachment or in both (143,144,187). A reduction in contractile proteins contents appears to be the most likely explanation of reduced muscle strength since single fiber muscle contractile protein function has been reported to unaltered in patients with HF (156) (Fig. 1).
The impact of muscle mass on peak VO2 has been investigated by several groups and a weak but significant correlation with total skeletal muscle mass derived from equations using 24 h urinary protein measurements have been reported (131, 162). LeJemtel contrasted peak oxygen consumption during combined upper arm and maximal leg exercise (103). In normal subjects, the addition of more muscle mass via arm exercise did not increase peak exercise performance and VO2 remains unchanged suggesting that under normal conditions, cardiac output response to exercise rather than amount of exercising muscle determines peak VO2. However, in patients with severe HF, the addition of upper arm exercise significantly increases peak VO2 suggesting the importance of skeletal muscle mass in limiting peak VO2 in patients with HF. An alternative hypothesis is that the peripheral vasodila-tory abnormalities in these patients resulted in a physiologic shunt to the upper arm musculature resulting in higher peak VO2 with combined arm and leg exercise.
Mechanisms of skeletal muscle atrophy
The mechanisms that mediate skeletal muscle wasting and atrophy have only recently been studied in patients with HF and animal models of cardiac dysfunction. Generally, muscle atrophy may result from a decrease of anabolic mechanisms, increased protein degradation or both. The impact of the clinical compensatory status and acute disease exacerbations on muscle atrophy in HF is controversially discussed. The majority of studies have shown no alterations in either muscle protein synthesis or breakdown in clinically stable HF patients which indicates that the onset and progression of muscle atrophy is linked to disease exacerbation. To date, no studies investigated possible metabolic derangements in skeletal muscle during acute disease exacerbation. Below, we will review the known pivotal signaling pathways that are involved in protein metabolism.
Enhanced protein degradation.
Experimental data in a murine model of chronic HF indicate that the ubiquitinproteasome pathway plays an important role in HF induced skeletal muscle atrophy (46). Through this central pathway of protein degradation, proteins are marked by ubiquitin for rapid degradation through the 26S proteasome in an ATP-dependent pathway that yields peptides and intact ubiquitin. Two pivotal enzymes of this pathway are ubiquitin-ligases MuRF1 and Atrogin-1 (63,69). MuRF1 and Atrogin-1 are a downstream part of a signal cascade involving p38 MAPK/FoxO which is parallel to the myostatin signaling mechanism (69) (see below). Skeletal muscle from mice with left ventricular dysfunction revealed activation of the ubiquitin-proteasome pathway with increased protein ubiquitination, FoxO transcription factor activation, increased expression of muscle-specific atrogenes, and overall muscle atrophy. In the pathway mentioned above, IGF-1 is a major upstream signaling molecule. Transgenic overexpression of a local isoform of IGF-1 prevents proteasome activation, breakdown of skeletal muscle structural proteins, and atrophy (177). Further experimental data regarding the critical inhibitory role of IGF-1 in the development of skeletal muscle atrophy have been provided in a model of skeletal muscle wasting induced by chronic administration of angiotensin II (AII) (181). Chronic administration of AngII suppresses IGF-1 signaling via the Akt/mTOR/p70S6k pathway that is critical for caspase-3 activation, actin cleavage, ubiquitination, and apoptosis. Caspase-3 activation is another important mechanism in muscle protein degradation. Muscle-specific expression of IGF-1 blocks the AngII-induced muscle atrophy. Whether the beneficial effects of ACE inhibition on skeletal muscle in HF patients are mediated though the IGF-1 pathway or result from direct effect on skeletal muscle vasculature via improvement of vascular endothelial function remains to be determined. In addition to AngII, elevated cytokines can directly activate the ubiquitinproteasome pathway and thereby result in skeletal muscle protein degradation (161).
Impaired growth factor signaling and protein synthesis.
HF is a catabolic state with deficiencies in several anabolic hormones (25, 77, 173). In male HF patients, a decrease of circulating total testosterone, dehydroepiandrosterone and IGF-1 levels have been described which correlate with a poor prognosis (13). Testosterone has anabolic effects on skeletal muscle by increasing fractional muscle protein synthesis (20) and appears to stimulate IGF-expression. In a placebo-controlled trial of 70 elderly patients with moderately severe HF, testosterone application improved exercise capacity, muscle strength, glucose metabolisms, and baroreflex sensitivity (25). Despite beneficial effects on insulin resistance, testosterone had no apparent effects on myocardial performance and left ventricular function. Long-standing therapy seems to be well tolerated by elderly patients with moderately severe HF.
Growth hormone (GH) resistance and reduction in skeletal muscle IGF-1 concentration contribute to skeletal muscle atrophy in HF by directly reducing protein synthesis and by decreasing muscle satellite cell recruitment and differentiation (181). Exercise training has been shown to increase local expression of IGF-1 in normal subjects and patients with HF (178). Ghrelin, a novel GH-releasing peptide stimulates physiological release of IGF-1 through a mechanism that is independent from hypothalamic GH releasing hormone (150). Administration of Ghrelin for 3 weeks improves functional capacity and alleviates skeletal muscle atrophy in patients with chronic HF and COPD (149).
Recently, the possible role of myostatin in muscle atrophy in advanced HF has been investigated (74). Myostatin is secreted from skeletal muscle and is a local and circulating factor with antianabolic and antihypertrophic effects. It has been shown in a murine model that myostatin activation leads to profound muscle wasting (217) through a p38-MAPK/SMAD 2/3-mediated pathway (69). Furthermore, there is evidence from animal experiments that circulating myostatin released from cardiomyocytes during myocardial injury induces skeletal muscle atrophy (74). However, skeletal muscle-derived myostatin provides the abundance of systemic myostatin (24). In conclusion, Myostatin could be an attractive therapeutic target since circulating levels of myostatin (57), and other TGF receptor ligands, such as activin (212), are increased in HF patients. Consequently, it has been proposed to use these circulating members of the TGF family such as myostatin as biomarkers assessing for cachexia in HF patients (62). However, this approach is controversially discussed (67,213).
Patients with HF develop worsening insulin resistance that is related to clinical events and mortality (42). Impaired insulin signaling in skeletal muscle has been linked to muscle atrophy and impaired metabolism with accumulation of toxic metabolic molecules.
Skeletal muscle inflammation.
HF induces a chronic low level inflammatory state as evidenced by modest elevation of circulating IL-1 and −6 and TNF-a (51, 191). The initiating factors of skeletal muscle inflammation are incompletely understood. Disuse induced atrophy promotes skeletal muscle inflammation through activation of several signaling pathways.
Increased skeletal muscle inflammation relates to reduced O2 delivery and local IGF-1 concentration as well as increasing oxidative stress. In turn, muscle inflammation induces cytokines especially IL-6 and TNF-a and expression of inducible nitric oxide (NO) synthase (59). There is experimental evidence that oxidative stress causes changes in muscle fiber elasticity via titin modification and activation of Calpain-1, a protease degrading titin, as well as other mechanisms (16). Elevated IL-6 and TNF-a concentrations activate the NFκB signaling pathway that modulates immune and inflammatory skeletal muscle responses and thereby exacerbating muscle wasting (146). Increased IL-6 concentration has been associated with skeletal muscle and diaphragmatic atrophy in rats and the Janus activated kinase, activator of transcription, and cAMP-activated protein kinase signaling pathways (78). As previously mentioned, increased FoxO activity precipitates muscle protein degradation and muscle atrophy through the ubiquitin-proteasome pathway in HF. In summary, SM inflammation exacerbates SM atrophy through stimulation of multiple cytokines activated signaling pathways.
The activation of the renin-angiotensin system results in vasoconstriction and elevated skeletal muscle concentrations of AngII which increases local oxidative stress and lowers skeletal muscle concentration of IGF-1. Consequently, these mechanisms may accelerate protein degradation while decreasing protein anabolism. In HF, muscle reflex alterations have been reported (200) which are explained by the interplay of loss of skeletal muscle mass, reduction in muscle perfusion and blood flow distribution and altered fiber-type composition. Physiologically, muscle reflex activation increases systemic blood pressure and thereby maintains muscle perfusion during muscle acidosis. However in the failing heart, muscle reflex activation occurs at the onset of exercise resulting in vasoconstriction and limited skeletal muscle perfusion.
In summary, chronic disease states and particularly chronic HF are associated with reduced physical activity resulting in disuse and low level systemic inflammation. Both disuse and systemic inflammation promote loss of muscle mass and ultimately atrophy. Continuous loss of skeletal muscle mass activates multiple signaling pathways that mediate muscle inflammation. In turn, local inflammation activates signaling pathways that promote further loss of muscle mass and exacerbates local inflammation. The circulus vitiosus between atrophy and inflammation within the skeletal muscle appears to progress independently from the initial event and may be related to the presence of comorbidities including hormonal deficiencies, diabetes mellitus, and obesity/sleep disorder. An important goal of therapy in HF patients is to reverse or optimally prevent the development of skeletal muscle alterations to restore a normal functional capacity.
Vascular dysfunction
Experimental and clinical evidence suggests that the peripheral circulation undergoes substantial transformations during the progression of HF with an alteration of regional vascular control. These changes occur both at the level of the vascular endothelium, an important modulator of vascular tone, and at the level of the vascular smooth muscle. Furthermore, changes in capillary density have been reported in skeletal muscle of patients with HF.
Changes in capillary density in HF
At stage C of HF, muscle perfusion during exercise is primarily limited by an impaired vasodilatory response of lower limb skeletal muscle beds in addition to the impaired cardiac output response to exercise. At stage D of HF, skeletal muscle perfusion during exercise is limited by the cardiac output response as it is in normal subjects (174). The findings regarding muscle microcirculation are controversial in severe HF (48). Capillary density has been reported to be normal or decreased depending on normalization to muscle fibers number and size. Duscha et al. (47) measured vascular density in the skeletal muscle of patients with HF. Using cell-specific antibodies to measure vascular density, these investigators found decreased numbers of endothelial cells per fiber, which indicates a reduced capillary density. However, since capillary density varies with fiber type, and fiber-type switching is a dynamic process in patients with HF, studies on capillary density ratio should be interpreted with caution. Other investigators have reported a normal skeletal muscle capillary density in patients with HF (121). Thickness of the capillary basement membrane is increased in the pronator teres muscle of patients with HF (115). Finally, the resistance of the microvasculature is only minimally increased in the cutaneous tissue in patients with HF (115,121). Of note, although vascular endothelial dysfunction (an important determinant of capillary behavior during exercise) is impaired in HF, oxygen extraction by exercising SM is nearly complete in severely symptomatic patients (87). Similarly, reduced muscle oxidative capacity does not limit oxygen extraction by exercising muscles in HF (201). The oxygen content of the deep femoral vein that exclusively drains skeletal muscle is less than 1 to 2 mL/100 mL in patients with severe HF (87). In summary, while skeletal muscle capillary density is likely to be altered in HF and oxidative capacity is reduced, they do not limit oxygen extraction by exercising muscles.
Abnormal endothelial function in HF
The mechanisms of vascular alterations are incompletely understood in HF. A number of studies have systemically studied vascular endothelial function, and particularly NO-mediated control of vasomotor tone has been extensively studied in HF (85,86,95,190). In both animal and human models of HF, the responses to endothelium-mediated vasodilation are blunted. Endothelial dependent dilation in the aorta of rats with ischemic cardiomyopathy is reduced (44). Kubo et al. demonstrated that endothelium-dependent vasodilation is attenuated in patients with HF (94). Changes in sheer stress is an important determinate of endothelial function. In normal vasculature, changes in sheer stress on the endothelial cell that accompany alterations in blood flow serve to enhance vasodilation via release of NO and prostaglandins (15,70). Of note, aerobic training normalizes endothelial function in patients with HF probably via a mechanism of increased shear stress. In canine models of HF, the vascular responses to low-dose acetylcholine are amplified by pretreatment with indomethacin (192). These alterations in vascular reactivity in CHF have been postulated to be adaptive in that they function to maintain shear stress close to control levels (75).
In the rat model, HF induced by ligation of the left anterior descending artery causes progressive peripheral vascular endothelial dysfunction (190). The systemic vascular response to acetylcholine was not decreased until four weeks after coronary ligation and progressively worsened through week 16. Similar data are not available in human subjects. The mechanisms that mediate vascular endothelial dysfunction early in the course of HF are likely to be different than those responsible at later stages. In patients with symptomatic HF, the improvement induced by vitamin C administration and subsequent decreased extra-cellular SOD (superoxide dismutase) activity implies that increased oxidative stress may accelerate NO degradation, and may be partially responsible for vascular endothelial dysfunction (15). Additional data suggest that there is increased expression of endothelial NO synthase (eNOS) when normalized to von Wille-brand factor in the vastus lateralis muscle of patients with HF (192). Increased eNOS endothelial expression implies the development of an adaptive mechanism to compensate for accelerated NO degradation. Nevertheless, it is unclear whether uncoupling of eNOS with conversion of bioactive NO to reactive nitrate species develops in the vascular bed of patients with HF as it is known in patients with diabetes mellitus.
Using a canine model of HF produced by rapid ventricular pacing, Zelis also measured the arterial sodium content in the aorta and femoral artery in control and HF animals (214). A significant increase in the arterial sodium content in the HF animals was demonstrated. Zelis postulated that arteriolar stiffness from increased salt and water content resulted in an abnormal vasodilatory response in HF. Longhurst et al. showed a similar deficiency in the forearm vasculature responses to static exercise.
Endothelial function in patients with HF was first evaluated in the upper limb vasculature by measuring forearm blood flow in response to methacholine, an endothelium-dependent dilator, and nitroprusside, an endothelium-independent dilator (95). Blood flow was measured by venous plethysmography. The response to methacholine was significantly lower in patients with HF, when compared to age-matched controls, while the response to nitroprusside tended to be lower, although it did not reach statistical significance. The blunted response to methacholine implies that patients with HF have an impaired reserve of vascular endothelium. Endothelial function was also studied in the lower limb vasculature of patients with HF using Doppler ultrasonography of the superficial femoral artery (44). Patients did not have comorbid vascular disease that could have contributed to endothelial dysfunction. The maximal velocity of blood using a volume sampler positioned in the center of the vessel was evaluated during intra-arterial administration of acetylcholine, an endothelial-dependent dilator, and nitroglycerin, an endothelial-independent dilator. Maximal blood flow velocity did not increase in response to acetylcholine at concentrations of 10–5 M, while it increased by fivefold in healthy subjects. Maximal blood flow velocity in response to nitroglycerin at 10–7 M was substantially reduced in patients when compared to the response elicited in healthy subjects. Following administration of a dose of nitroglycerin at 10–5 M, patients with HF experienced an increase in maximal blood flow velocity similar to that produced by nitroglycerin at 10–7 M in healthy subjects.
The lack of response to acetylcholine clearly demonstrates the absence of functional reserve of the vascular endothelium in patients with HF. However, reduced vascular endothelial function in patients with HF may, in part, be due to reduced functional integrity of the cyclic GMP pathway that is evidenced by the depressed response to nitroglycerin. The mechanisms that are responsible and mediate the progression of vascular endothelial function in patients with HF are still poorly understood. Furthermore, the state of vascular function under basal conditions in patients with CHF is still debated. Administration of L-NMMA (N-monomethyl-L-arginine), a selective inhibitor of the production of NO from arginine, yielded similar forearm blood flow decreases in patients with CHF and in healthy subjects (85;86). However, others observed the expected decrease in blood flow following administration of L-NMMA in healthy subjects, but did not observe a decrease in patients with HF (70). Experimental data indicate that increases in oxidative stress associated with HF may accelerate NO degradation, and thus may impact on NO availability (15).
Endothelial dysfunction appears to be a time-dependent alteration. With increasing severity of HF, there is a deterioration of endothelial vascular function. In rats with early stages of HF, vascular endothelial function was preserved, whereas in more severe stages, an impairment was noted (190). Circulating cytokines such as TNFa are elevated in severe HF and TNFa impairs the release of NO. Plasma levels of TNFa in patients with HF are correlated with the degree of endothelial dysfunction assessed by infusion of acetylcholine (132). Chronically decreased skeletal muscle perfusion, increased tissue ACE activity, increased oxygen free radical formation, and increased endothelial vasoconstriction agents are other potential mechanisms which are involved in the development of endothelial dysfunction in these patients.
The impaired vascular response to exercise in HF
A fixed vasodilatory capacity of the skeletal bed during exercise exists in CHF. This was demonstrated in a study comparing one versus two-leg bicycle exercise. In contrast to normal controls, patients with severe HF were unable to augment maximal limb blood flow during one-leg bicycle over that reached during two-leg bicycle exercise (103). Furthermore, regional differences exist in abnormal peripheral circulatory abnormalities and a comparison of peak reactive hyperemia between the forearms and calves of HF patients demonstrated abnormal calf flow only (81). Furthermore, only calf peak reactive hyperemia correlated to peak VO2. Selective deconditioning may be responsible for these regional differences. As HF progresses, decreased use of lower extremities but upper extremity use remains relatively preserved which might explain these regional differences in blood flow.
During exercise, peripheral vasoconstriction is increased to prevent systemic hypotension in patients given the blunted rise in cardiac output (192). Tissue hypoxia and enhanced sympathetic and angiotensin activation are two proposed mechanisms mediating abnormal peripheral vasoconstriction in HF. Institution of sympathetic and renin-angiotensin blockade do not completely reverse the peripheral derangements seen in CHF though they modify it.
While lower limb blood flow may increase up to 20 times from rest to peak exercise in healthy subjects, lower limb flow can increase only two to three times in patients with advanced HF due to LV systolic dysfunction (108,215). Thus, the vasodilatory response of the lower limb skeletal muscle beds to exercise is relatively much more impaired than that of the cardiac output in severe HF. Some investigators have failed to observe a limited vasodilatory response of skeletal muscle vascular beds to exercise in patients with severe HF (10,76,210). Etiology of HF (primarily due to valvular disease rather than end stage cardiomyopathy), and technical issues in the measurement of skeletal muscle blood flow (use of single thermistor for the thermodilution technique and measurement of blood flow draining other tissues than skeletal muscles) may in part explain the disparate findings.
Changes in respiratory muscle function
General mechanisms of dyspnea
A unified mechanism for dyspnea based on respiratory muscle function is that breathlessness occurs when the activity of the respiratory muscles is increased and/or the respiratory muscles are weak (89). Varying respiratory muscle strength and workload will result in differences in perception of load and dyspnea (26,27,83,171,183). Dyspnea is a conscious sensation that results from an unusual perception of discomfort during breathing (52,203). As it is a sensation, it has a significant affective component that can be modified by cognitive and contextual influences. Dyspnea occurs in both normal and diseased states and originates from the stimulation of mechano-, chemo-, or proprioreceptors. The magnitude of a stimulus to the peripheral receptor is transduced by the firing frequency in afferent nerves to the central nervous system. The central impression constructed is interpreted based on past experience to generate a conscious sensation (52).
Respiratory muscle fatigue may also contribute to dyspnea via biochemical changes in the muscles (80). Dempsey et al. have shown fatiguing contractions of the inspiratory muscles result in an accumulation of metabolites that activate Type IV phrenic afferents resulting in an increase in sympathetic vasoconstrictor activity via a supraspinal reflex, that is, inspiratory muscle metaboreflex (80). This reflex is important during heavy sustained exercise and modulates the competition for blood flow between the respiratory and locomotor muscles. Activation of this reflex redirects blood flow from the periphery to the ventilatory muscles.
Additionally, increased respiratory drive during exercise may be due to metabolic stimuli arising from limb skeletal muscle (55, 136). To proof that metabolic changes in skeletal muscle stimulate respiration, Oelberg et al. (155) used magnetic resonance spectroscopy to demonstrate that skeletal muscle pH correlates with minute ventilation. However, it is not clear whether such stimulation occurs through central or peripheral pathways (136).
Abnormal respiratory muscle function in HF
Respiratory muscle function is assessed by measurement of respiratory muscle strength (166). Maximal inspiratory pressure is measured with the subject inhaling against resistance at functional residual volume to maximize the force length relationship of the muscles. This measurement is volitional and as such may overestimate the degree of muscle weakness but this measurement has been shown to be reproducible. Inspiratory muscle weakness is arbitrarily defined as a Pimax <70% of predicted value. A reduction in inspiratory and expiratory respiratory muscle strength in patients with HF from both systolic and diastolic dysfunction has been shown in many studies (3,72, 137, 152). Studies measuring diaphragmatic strength using esophageal and transdiaphragmatic pressure during maximal sniffs and phrenic nerve stimulation have also demonstrated reduction in diaphragmatic strength in HF patients.
When both respiratory muscle and limb muscle strength are measured in HF patients, a more marked reduction in respiratory rather than peripheral muscle strength has been observed. Chua et al. failed to find a correlation between Pimax and quadriceps strength (32). Ambrosino (3) reported reductions in maximal inspiratory and expiratory pressures that paralleled the severity of HF. HF patients with relatively preserved exercise capacity had reduced Pimax.
Pimax has been shown to have prognostic value independent of peak VO2. Meyer et al. (141) measured Pimax in 244 consecutive HF patients and found this measurement to be a strong univariate and multivariate predictor of survival. Improvement in Pimax has been found in patients with HF following initiation of angiotensin converting enzyme inhibitor therapy, CPAP and respiratory muscle training. However, serial changes in inspiratory strength was not predictive of prognosis (54).
Increased activity of the respiratory muscles and/or respiratory muscle weakness rather than fatigue may be sufficient to evoke the sensation of dyspnea. Measurement of the tension-time index and thus the work of the diaphragm per breath demonstrated dramatic increases in patients with HF at rest and during exercise (129). The tension time index is calculated for each breath and is the product of the ratio of the time in inspiration divided by the time per breath (Ti/Ttot), and the ratio of the mean transdiaphragmatic pressure to maximal transdiaphragmatic pressure. It approximates oxygen consumption of the diaphragm. Fatigue of the diaphragm is thought to occur when the tension time index reaches a ratio of 0.15 or greater (12). However, this fatiguing ratio may be lower in ischemic muscle, or with high tidal volumes, breathing frequency, and minute ventilation. In the majority of the patients studied, the tension time index at end exercise was ≥0.1 and thus approached fatiguing levels.
The relationship between parameters of respiratory muscle function and ratings of perceived dyspnea during sub-maximal exercise was also examined (129). Significant linear correlations were observed between a rating of perceived dyspnea (i.e., the Borg Scale) at a fixed exercise workload (25 watts) and parameters of respiratory muscle strength (maximal inspiratory and expiratory pressures), and work (tension-time index), but not with lung volumes (tidal volume, minute ventilation). McParland also demonstrated a strong correlation between inspiratory muscle weakness and dyspnea during daily activities in stable ambulatory HF patients, as quantitated by the Dyspnea Index (137).
The endurance of the respiratory muscles in HF patients is also diminished. Respiratory muscle endurance can be assessed by progressive isocapnic hyperpnea using a rebreathing circuit to measure maximal sustainable ventilatory capacity (128). Both maximal voluntary ventilation and maximal sustainable ventilatory capacity are significantly reduced in HF patients compared to normal subjects consistent with lowered respiratory muscle endurance.
Impaired respiratory muscle perfusion in HF
As described above, limb skeletal muscle hypoperfusion has been described in patients with HF (216). Animal and human studies suggest that with HF, respiratory muscle hypoperfusion may also occur. The diaphragm, the major respiratory muscle, has a complex and generous blood supply provided by the internal mammary, intercostal, and phrenic arteries and the costophrenic arcades. Because of its rich perfusion, the diaphragm is relatively resistant to ischemia, even during exercise, when large increases in perfusion of the diaphragm are needed. Examination of regional muscle perfusion in the dog during exercise has demonstrated that the muscle group with the largest increase in perfusion is the diaphragm (53). In animal models of HF, the increase in diaphragmatic blood flow during submaximal exercise (147) was greatest in those animals with the most severe HF. Presumably the increased blood flow was required for increased work of breathing. During exercise, dramatic increases in blood flow occur in the diaphragm and this response is accentuated in HF.
Application of near-infrared spectroscopy during maximal exercise has demonstrated accessory respiratory muscle deoxygenation in HF but not in normal subjects (125). Whether this respiratory muscle deoxygenation represents underperfusion, ischemia, and/or fatigue was investigated by measuring the development of low-frequency muscle fatigue following exercise in HF patients. Davies et al. (39) demonstrated a decrease in maximum inspiratory and expiratory pressure following bicycle exercise in HF patients consistent with respiratory muscle fatigue. However, measurements of maximal inspiratory and expiratory pressures are motivation dependent, and the observed reduction may have occurred from central mechanisms. To investigate objectively whether low frequency diaphragmatic fatigue occurs in HF patients, supramaximal bilateral transcutaneous phrenic nerve stimulation before and after maximal bicycle exercise has been performed (129). Maximal transdiaphragmatic pressure was derived before and after exercise using the twitch interpolation technique. In both normal and HF subjects, maximal inspiratory and expiratory pressures decreased significantly with peak exercise. However, the maximal transdiaphragmatic pressure derived from the twitch interpolation technique was unchanged in both normal and HF subjects. Thus, low-frequency diaphragmatic muscle fatigue did not occur in patients with HF despite accessory respiratory muscle deoxygenation during exercise.
Changes in diaphragmatic histochemistry in HF
Histochemical and metabolic changes that occur in respiratory muscles may serve to accentuate the sensation of dyspnea in HF patients. A diaphragmatic myopathy has been demonstrated in an animal model of chronic HF (105) with decreased contractile and relaxation parameters (106). Other studies have also characterized these fundamental molecular defects (165,199) and linked the molecular abnormalities to mechanisms operable in other chronic diseases in humans (159). Impaired diaphragmatic performance was correlated to total cross-bridge number and altered calcium regulation (104). Cytokine activation has been suggested to explain the diaphragmatic dysfunction (78, 112, 165) and malnutrition have been shown to affect diaphragmatic weight and histochemistry (11,193).
Various histologic abnormalities in the diaphragm of patients undergoing cardiac transplantation have been described (40,113,194). In one study, costal diaphragmatic biopsies were obtained from 7 normal subjects and 10 patients at the time of transplant or left ventricular assist placement (194). The distribution of myosin heavy chain isoforms I, IIa, and IIb (MHC) by SDS gel electrophoresis was measured along with the activities of the oxidative (citrate synthase), lipolytic (beta hydroxyacyl CoA dehydrogenase) and glycolytic (LDH) enzymes. In normal subjects the distribution of MHC isoforms I, IIa, IIb was 35%, 49%, and 17% respectively versus was 54%, 40%, and 6% in the HF subjects. Therefore in the patients with HF, slow myosin heavy chains were significantly increased and glycolytic fast twitch fibers significantly reduced compared to normal subjects. Additionally, oxidative and lipolytic enzymatic activities were greater and glycolytic enzyme activity was significantly less in HF than normal subjects. Thus in the diaphragm in HF, there is a shift from fast to slow myosin isoforms with an increase in oxidative capacity and a decrease in glycolytic capacity. These changes are consistent with those elicited by endurance training. The endurance changes described in the diaphragm probably are the consequence of the increased work of breathing that occurs in this patient population. These findings were subsequently found in animal models (105,106).
However despite the shift to more oxidative metabolism, evidence of decreased contractility and strength were described in animal models. A rat model of HF demonstrated similar histochemical changes as described in HF patients (198). However, the mitochondrial function of the rat costal diaphragmatic muscle was reduced as measured in skinned muscle fibers using an oxygen electrode. ADP sensitivity of the mitochondria was increased but returned to normal in the presence of creatine. Decreased concentration of the mitochondrial isoform of CK has been previously demonstrated in HF. Thus, though the diaphragmatic muscle had an appropriate training response to the heightened work of breathing, the performance of the muscle was still attenuated due to the altered mitochondrial function.
Myogenic regulatory factors regulate the expression of myosin heavy chains. In a rat model of HF, the expression of Myo D, myogenin, and MRF4 were examined in the diaphragm of HF and control rats. Myogenin is expressed at higher concentrations in slow twitch muscle whereas Myo D expression is increased in fast twitch fibers. In the HF rats, selective suppression of Myo D associated with a lower percentage of fast twitch fibers was observed. The trigger for the downregulation of the Myo D is unknown but also thought to be due to neurohormonal and cytokine activation (116).
In conclusion, dyspnea is an extremely common symptom in patients with HF. In these patients, an increased work of breathing results from a combination of factors, including excessive ventilatory response during exercise from increased dead space ventilation from ventilation perfusion mismatching; an increased impedance to breathing from bronchial hyperreactivity due to venous engorgement; and decreased lung compliance from elevated filling pressures and subsequent chronic fibrotic changes. This increased work of breathing is transduced into the sensation of dyspnea by stimulation of receptors in weak, atrophic, underperfused, and metabolically abnormal respiratory muscles. Furthermore, the atrophic and metabolically abnormal limb skeletal muscles may also exacerbate this sensation through an excessive muscle reflex activity.
The prognostic role of exercise testing
Exercise testing as a widely applicable form of stress-testing, the cardiovascular system is the most established form of assessing peak functional capacity, analysis of the cardiovascular reserve as well as to define margins and limitations for exercise training protocols. The following section will highlight several of the most commonly used modes of exercise testing in patients with HF and also discuss the specific advantages and limitations of these forms of exercise testing.
The 6-min-walk test in patients with HF
Reduced functional capacity is the cardinal symptom of HF. Functional capacity has been traditionally assessed by the New York Heart Association (NYHA) criteria. Such assessment is both subjective and insensitive. The 6-min-walk test, that is, the distance walked over a period of 6 min, is less subjective than the NYHA functional class, but still can be heavily influenced by the patient’s and/or tester’s motivation. Additionally, the 6-min-walk test results cannot estimate how close the patient was to his or her maximal capacity and in patients with severe HF this submaximal test approaches maximal effort (168). Despite these caveats, the 6-min-walk test has been shown to provide prognostic information by the Study of Left Ventricular Dysfunction investigators who demonstrated in a substudy of 898 HF patients in their registry that mortality risk was 3.7 times higher in those patients with a 6-min-walk distance <350 m compared to those who walked >450 m. Similarly, the risk of HF hospitalization was 1.4 times higher in those with reduced walk distance (21). Subsequently, in some cohorts, investigators have shown the prognostic value of the 6-min-walk test but the prognostic significance of this test diminishes (168,169).
Peak oxygen uptake during maximal symptom limited exercise in HF
Determination of peak oxygen uptake during a symptom limited treadmill or bicycle exercise test is the most objective method to assess maximal functional capacity in HF patients. By identifying the ventilatory threshold, the physician can determine the adequacy of the patient’s effort, and if not maximal, how close the patient was to achieving his or her maximal effort. Thus, noninvasive cardiopulmonary exercise testing has gained widespread application in the functional assessment of patients with congestive HF. It is a useful test to determine the severity of the disease, provide important prognostic information, and assess the efficacy of new drugs and devices.
Peak oxygen consumption is derived from the Fick Principle: peak oxygen consumption (VO2) is the product of peak cardiac output and maximal arteriovenous oxygen difference. As most sedentary individuals will achieve comparable maximal arteriovenous difference, peak VO2 provides an indirect assessment of cardiac output reserve and this largely underlies the effectiveness of peak VO2 in risk stratification. Moreover, several peripheral factors may also impact peak oxygen consumption, such as the metabolic activity of skeletal muscle mass and endothelial function, as well as age, gender, and conditioning status. Since skeletal muscle mass and its metabolic activity both decrease and endothelial function is progressively impaired as HF severity increases, the prognostic utility of peak VO2 is enhanced.
Analysis of the ventilatory data obtained during cardiopulmonary testing enables the clinician investigator to determine if a maximal test has been performed and thus whether an accurate peak VO2 has been measured. Identification of the anaerobic threshold at 50% to 80% of peak VO2 generally indicates a maximal effort. Peak VO2 is a continuous variable. Use of statistical methods such as stratum specific ratios to identify a clear threshold below of which the relative risk of death will precipitously increase, have yielded a linear relationship of VO2 to outcome without clear thresholds (1,138). Peak exercise VO2 can be influenced by noncardiac factors such as muscle mass and deconditioning, age, gender, and obesity. Analysis of peak VO2 normalized by a predicted maximum based on age, obesity, and gender has been performed to determine if better prognostication can be achieved by using a percentage of predicted peak VO2. Some investigators have suggested the superiority of this approach, though others have shown no clear benefit (31,170). Likely, the additional value of adjusting for sex, age, and body composition in any study cohort is a function of how these characteristics are distributed across the cohort; in studies of largely middle age men of average weight, the methods give similar results while cohorts with greater heterogeneity would likely be better served by reference to sex and age specific prediction equations with adjustment for weight extremes.
Prognosis of patients with HF based on exercise perform ance and peak VO2
The use of peak VO2 to predict prognosis of patients with HF was first described by Szlachcic (188). In 27 patients, she reported a 77% 1-year mortality rate for patients with a VO2 of less than 10 mL/kg/min and a 21% mortality rate for those with a VO2 of 10 to 18 mL/kg/min. In a prospective study of 114 ambulatory patients with CHF referred for cardiac transplantation, a VO2 of less than 14 mL/kg/min was used as a criterion for acceptance for cardiac transplantation (124). Patients were divided into three groups based on the results of their cardiopulmonary stress tests. Patients with a peak VO2 below 14 mL/kg/min were accepted as transplant candidates (group 1); transplant was deferred for patients with a peak VO2 above 14 mL/kg/min (group 2) and patients with a peak VO2 below 14 mL/kg/min who had a significant comorbidity that precluded transplant (group 3). One-year survival was 94% in patients with a VO2 above 14 mL/kg/min. Accepted transplant candidates with a VO2 below 14 mL/kg/min had a 1-year survival of 70%, whereas the patients with a significant comorbidity and reduced VO2 had a 1-year survival of 47%. This approach permitted the identification of candidates whose transplant could be safely deferred.
Use of serial measurements of peak VO2 has also been shown to effectively identify patients in a low-risk category over time (118), and conversely, a significant decline usually parallels clinical worsening and a worse prognosis. This is particularly important as the therapy for cardiac diseases continue to evolve and improve. Since the initial report of the value of peak VO2 in guiding transplant candidate selection in 1991, there have been many advances in the treatment of HF. In particular, the use of beta blockade has significant impact on the long-term survival without significantly improving peak VO2. Whether VO2 retains its predictive power in the beta-blocker era has been the subject of several reports (92, 117, 154). Consistent across the reports was the sustained utility of this parameter in predicting survival. Cohorts dichotomized by threshold values of above and below 14 mL/kg/min, or above and below 10 mL/kg/min in patients on beta blockers, demonstrated that VO2 retained its predictive value. The survival for patients on beta blocking agents shifted up but nevertheless diverged according to peak VO2. With the improved survival, a lower cut point than 14 mL/kg/min for referral or listing for cardiac transplant has generally been accepted, with the American Heart Association (AHA)/American College of Cardiology (ACC) guidelines now selecting a peak VO2 below 10 mL/kg/min with achievement of anaerobic threshold as an absolute indication for transplant (in the absence of significant contraindications). A peak VO2 of 11 to 14 mL/kg/min or 55% of predicted peak VO2 resulting in major limitation of the patient’s daily activities is considered a relative indication for transplant listing (14, 79).
VE/VCO2 ratio during maximal symptom limited exercise in HF
During cardiopulmonary exercise testing many variables are collected that also confer prognostic information. The ventilatory response to exercise, most frequently measured by the VE/VCO2 ratio or slope, has been found by several investigators to be even more predictive of outcome than peak VO2 (91,158,167). The abnormal VE/VCO2 response results from increased ventilation-perfusion mismatching and heightened chemosensitivity and ergoreflex responses. This heightened ventilatory response occurs from the onset of exercise and thus unlike peak VO2, the VE/VCO2 relation does not require a maximal effort. However, there has been no consensus on how best to derive this parameter. Both VE/VCO2 ratios and slopes have been reported (i.e., VE/VCO2 ratio at anaerobic threshold or at peak exercise) and the VE/VCO2 slope from onset of exercise to the anaerobic threshold or throughout the total exercise period. VE/VCO2 slope derived throughout exercise testing appears to have the greatest prognostic power. VE/VCO2 > 34 has been the cut-point selected in many studies but similar to peak VO2, this parameter is a continuous variable with no absolute cut-point. Published studies have shown a VE/VCO2 ratio of >30 conferring increased risk with worst prognosis associated with VE/VCO2 >40.
VE/VCO2 correlates more strongly with pulmonary pressures measured during exercise than does peak VO2. Frequently in studies, both peak VO2 and VE/VCO2 are found to have independent prognostic power. Therefore the combination of both VE/VCO2 and peak VO2 may provide the strongest way to determine risk. For example a patient with a preserved peak VO2 yet an abnormal VE/VCO2 remains at greater risk than if the ventilatory response was normal. Similarly, with the converse situation where peak VO2 is severely reduced but VE/VCO2 normal, that patient remains at increased risk despite the normal ventilatory response. Accordingly, those with severely reduced VO2 (<10 mL/kg/min) and excessive VE/VCO2 (>40) fall into the poorest survival group. Thus both peak VO2 and VE/VCO2 slope provide independent and complementary data on prognosis and should be used together to assess risk (6,8).
Other markers of prognosis related to exercise performance in HF
Exercise oscillatory breathing is associated with a poor prognosis in both patients with diastolic and systolic dysfunction. There is no uniform definition of this type of breathing but it is a periodic cycling of hyper and hypopnea with appropriate changes in PET O2 and PET CO2. This breathing pattern is observed in about 12% to 30% of HF patients during exercise and most patients with exercise oscillatory breathing will have central sleep apnea. A definition for this breathing pattern is not established but a persistence of periodic breathing for 60% of exercise with amplitude of oscillations >15% over rest has been suggested. Presence of periodic breathing can predict mortality by itself or when combined with the ventilatory slope. In 156 patients with HF, this breathing pattern was strongly correlated with sudden death (68, 186).
Other parameters measured during cardiopulmonary exercise testing that also have been shown to have prognostic power in chronic HF include: blood pressure response to exercise (i.e., blunted or failure to increase BP with exercise associated with poor prognosis), the heart rate response to exercise (i.e., chronotropic incompetence), the ventilatory threshold, circulatory power (Peak VO2 × systolic BP), oxygen kinetics, end tidal PCO2, and oxygen recovery postexercise (5,7,36,60,151).
Invasive versus noninvasive assessm ent of hemodynamic changes during exercise in HF
As the therapy of HF has advanced with time so has the technology of metabolic carts. Advances in technology now permit noninvasive measurement of cardiac output using inert gas rebreathing techniques (97, 99). Also bioreactance technology can be used at rest and during exercise to derive cardiac output measurements. The prognostic value of peak VO2 has been demonstrated as a noninvasive indicator of peak cardiac output response to exercise. Prior to the availability of the newer noninvasive methodologies, several studies suggested that hemodynamically derived variables from Swan Ganz catheters may enhance risk stratification over peak VO2 (97).
The prognostic superiority of hemodynamically derived exercise variables over peak VO2 was first shown by Griffin (66) who reported data on 49 HF patients. In this study, left ventricular stroke work index (LVSWI) at peak exercise dichotomized at 20 gm/m2, identified patients with a threefold to fivefold higher mortality compared with the remaining patients. Exercise duration and peak VO2 were not able to discriminate survivors from nonsurvivors. This was followed by the study of Roul (170) who measured hemodynamic variables during exercise in 50 patients with NYHA class III CHF. This study population had 26% mortality over 21.2 ± 1.2 months. Cardiac power output (CPO) and LVSWI measured at peak exercise were strong mortality predictors. The authors also showed that, as a survival predictor, peak VO2 was almost as powerful as CPO. Since, the measurement of CPO at their center required right heart catheterization with the patients supine, which was not always tolerated well, they recommended peak VO2 as an alternative to the hemodynamic measurements.
Wilson (211) investigated the relationship between hemodynamic data and peak VO2 in 64 patients with stable heart CHF referred for cardiac transplant. Forty-four percent of patients had only mild or moderate hemodynamic dysfunction despite a peak VO2 value less than the recommended limit for transplantation (<14 mL/kg/min). Conversely, 33% of patients with a peak VO2 > 14 had severely impaired cardiac output at peak exercise. His conclusions were that invasive hemodynamic information should be used in combination with VO2 to determine transplant eligibility, and that patients with a low VO2 who can demonstrate appropriate cardiac function at peak exercise not be listed (hypothesizing that the low peak VO2 in such patients likely resulted from deconditioning, obesity or other peripheral factors). The same researchers (31) studied a larger group of 185 patients in NYHA class II-IV HF, and found the CO response to exercise the most powerful predictor of survival by both univariable and multivariable analyses. The additional finding of a peak VO2 of ≤ 10 mL/min/kg in such patients identified a group with a particularly unfavorable prognosis (38% were alive at 1 year).
The findings by Wilson (211) required further confirmation. Other investigators (122,138) reported that LVSWI appeared to be a better prognostic indicator than peak VO2 but it was unclear whether the risk of the catheter placement was acceptable, particularly for serial assessment, given the small enhancement in risk prognostication.
In 2001, Williams et al. (208) published the first study on the correlation between survival and hemodynamic data obtained by noninvasive measurement of CO using CO2 rebreathing integrated with a standard exercise test. This modification was important because it is difficult to imagine that the complex procedure of invasive measurements could be implemented during the standard clinical exercise test. Two hundred and nineteen patients with mild HF (mean peak VO2 23 mL/min/kg) were studied. Peak CPO was a stronger predictor than peak VO2. CPO incorporates blood pressure into the exercise hemodynamic assessment. The CPO takes into account both the flow and pressure generating ability of the heart, and Tan (189) has argued that it could be viewed as a comprehensive indicator of cardiac function. The equipment for the rebreathing CO2 method of measuring CO is not widely available and there are methodological problems. CO is measured during a separate run at a work rate corresponding to the peak work rate in the preceding incremental test, and so CPO, as measured, reflects a high work load, but not the true peak CPO. Cohen-Solal (36) proposed another index, the “peak circulatory power” which is the product of the peak VO2 and the last systolic arterial pressure measurement. He argued that the information for its calculation is available from any cardiopulmonary exercise test without the need for special equipment. The value of the “circulatory power” was assessed in a study involving 175 HF patients. During a 25 ± 10 month follow-up, 16% died and 18% underwent cardiac transplantation. Multivariable analysis demonstrated that the peak “circulatory power” (chi-square = 19.9, P < 0.001) was the only variable predictive of death or need for transplant. When this was analyzed in terms of quartiles of peak VO2 or circulatory power, it appeared that prognosis was worse as peak VO2 declined, but that circulatory power aids in selecting subgroups with particularly poor prognosis—those with both reduced peak VO2 and reduced blood pressure.
Over the last 5 years, there have been advances in technology which now permit easily obtainable noninvasive measurement of cardiac output at rest and during exercise. Inert gas rebreathing is a novel, noninvasive method to measure cardiac output during exercise and is reliable, safe, and easily performed in patients with CHF (97, 99). The Innocor rebreathing system uses an oxygen enriched mixture of an inert soluble gas (0.5% nitrous oxide [N2O]) and an inert insoluble gas (0.1% sulfur hexafluoride, [SF6]) N2O concentration decreases during the rebreathing maneuver, with a rate proportional to pulmonary blood flow. Recently we applied this technology in 171 consecutive CHF patients during symptom limited bicycle exercise (96). An accurate measure of peak CO was obtained in 148 patients (85% of patients). Endpoints consisted of death, urgent heart transplant or left ventricular assist device implantation (LVAD). Duration of follow-up averaged 1 year. Univariable and multivariable analyses were performed using cardiopulmonary exercise variables (i.e., peak VO2, peak CO, peak cardiac power, VE/VCO2 slope, and VO2 at anaerobic threshold). Event-free survival for the entire cohort was 83% with 5 deaths, 4 LVAD implants, and 16 urgent transplants. In this cohort, peak VO2 was 12.9 ± 4.5 mL/kg/min and peak cardiac power was 1.7 ± 0.9 watts. Univariable predictors of adverse outcome were peak VO2, peak CO, peak cardiac power, VE/VCO2 slope, and VO2 at anaerobic threshold. By multivariable analysis, peak cardiac power and peak CO were predictive of outcome with peak cardiac power being the most powerful independent predictor of outcome (P = 0.01).
Safety of exercise testing
Exercise intolerance is one of the primary characteristics of chronic HF. Though widely utilized now to categorize the severity of HF, exercise testing was not routinely used in the diagnosis and management of patients with HF due to safety concerns. In one study cohort, low-level exercise testing has been used in 607 patients with moderately advanced HF (mean peak VO2 14.5 ± 3.9 mL/kg/min). These patients underwent two graded symptom-limited bicycle exercise tests. The initial stress test was terminated in only 10 (1.6%) of patients for arrhythmias, and in only one patient for hypotension, but no major complications occurred during testing (196). The low incidence of adverse events with exercise stress testing in HF patients demonstrates that stable patients with HF can safely exercise in a well-supervised setting.
Limitations
Like much HF research, studies of cardiopulmonary exercise have focused largely on systolic HF and have enrolled mainly middle-aged men (reflecting the central role of these studies in the evaluation of heart transplant candidates). However, recent studies have begun to investigate the prognostic value of peak VO2 in women, the elderly, and in those with diastolic HF. In a study by Elmariah (50), peak VO2 identified those women with the worse prognosis, though the overall survival of women was significantly better than of their male counterparts. These findings were confirmed by Green (65). Furthermore, exercise testing has been used rarely in the geriatric populations, but several recent studies have also demonstrated the predictive value of peak VO2 in this population (93,160). Recently, Parikh demonstrated the prognostic value of peak VO2 in 396 patients with HF over 65 years of age (160).
Benefits of exercise for the treatment of chronic HF
Exercise is a physiologic intervention with a broad variety of positive cardiovascular effects that include changes in lipid metabolism, insulin resistance, weight, arterial hypertension, inflammation, endogenous anabolism, and mood (93). It seems, in fact, justified to hypothesize that metabolic abnormalities associated with the primarily sedentary lifestyle in modern western civilizations are not only counteracted but normalized by physical activity. Therefore, regular exercise would be a physiologic correction rather than a medical treatment intervention.
Exercise programs distinguish between primarily aerobic dynamic (e.g., running and cycling) and resistance (e.g., strength training) exercises. Dynamic exercise with alternating muscle contraction and relaxation results in a steady rise of systolic blood pressure when intensity increases, while the diastolic pressure varies minimally. In contrast, resistance exercise is characterized by prolonged isometric muscle contraction before relaxation with high interstitial pressure that causes collapse of arterioles and capillaries. Blood pressure increases in relation to intensity and duration of the contraction. Although of minimal benefit with respect to cardiac adaptions (73,182), strength training has been shown to also be safe and effective at correcting muscle atrophy and weakness (182), two parameters that are generally less affected by aerobic-type training.
Effects of aerobic exercise on the myocardium have been well established. Regular dynamic exercise increases stroke volume, cardiac output and reduces beta-adrenergic stimulation (22). Exercise training increases myocardial mass, left ventricular dimensions, and stroke volume in healthy subjects (22, 93). In chronic HF, exercise has been shown to improve exercise tolerance and symptoms which is attributed to peripheral adaptations such as improved endothelial function and skeletal muscle strengthening (163). Moreover, exercise training has been associated with reversal of molecular and structural changes in the myocardium of hypertensive animals (93).
Exercise training has long been advocated as a means to delay aging, and decrease cardiovascular mortality in normal subjects. The evidence to support either one of these outcomes in normal or disease states is lacking. However, aerobic training can confer a multitude of significant hemodynamic, morphologic, and metabolic changes in man. We now will address the potentially beneficial effects of exercise in cardiac disease states with primary focus on aerobic exercise training.
Many of the central and peripheral changes induced by aerobic training may have a marked therapeutic effect in patients with HF. Potential advantages of training in HF (Table 2) include central hemodynamic changes such as an increase in stroke volume, and a potential increase in contractility, alteration of autonomic tone with a decrease in sympathetic stimulation, an improvement in endothelial and vascular function with a decrease in peripheral vascular resistance, muscle enzymatic changes with an increased oxidative capacity, and decrease in lactate production.
Table 2.
Effects of Exercise in Patients with Chronic Heart Failure
| Investigator | n | LVEF% | Duration (months) | Training effects |
|---|---|---|---|---|
| LETAC | 8 (CAD) | <45 | 2 | ↑WC, ↓HR, ↓BP |
| LEE | 18 (CAD) | 35 | 12–42 | ↑WC, ↓HR, ↔HD, ↔EF |
| COBB | 6 (CAD) | <35 | 6 | ↔EF, ↓HR |
| CONN | 10 (CAD) | <27 | 12 | ↑VO2 |
| JETTE | 39 (CAD) | <50 | 1 | ↔HD, ↑WC |
| SULLIVAN | 12 (CAD/IDC) | 24 | 4–6 | ↑VO2, ↓A-VO2, ↓HR, ↓lactate, ↑peak muscle flow |
| COATS | 11 (CAD) | 19 | 2 | ↑VO2, ↓HR |
| HF-ACTION | 2331 | <35 | 12 | ↑VO2, ↓HR, no difference in survival reduced hospitalization |
Abbreviations: CAD, coronary artery disease; IDC, idiopathic dilated cardiomyopathy; WC, work capacity; HR, heart rate; BP, blood pressure; HD, hemodynamics; EF, ejection fraction; VO2max, maximal VO2; A-VO2, arterial-venous oxygen difference.
Central cardiac effects of exercise training in HF
Animal models have been used to investigate the effects of exercise training on the failing heart. Using an ischemic rat model of HF, Musch et al. (148) studied the effects of endurance training. The training protocol consisted of 60-min sessions of treadmill exercise 5 days a week for 10 to 12 weeks. Following the training program in the rats with HF, VO2 was higher, succinate dehydrogenase activity increased and lactate levels were lower during submaximal exercise. No differences were observed in regional perfusion or in hemodynamic measurements. In this rat model of HF, all derived benefits of training were from peripheral mechanisms. Central cardiac function was not altered.
Todaka et al. (195) using a pacing-induced model of HF in dogs demonstrated both central and peripheral effects of exercise training. One group received daily treadmill exercise (4.4 km/h, 2 h/day) while the other dogs remained sedentary. At 4 weeks, in vivo hemodynamic measurements revealed relative preservation of maximum rate of pressure rise and left ventricular end-diastolic pressure in the exercised compared with the sedentary dogs. Subsequent in vitro analysis of cardiac function revealed similarly depressed systolic functions in both groups. However, whereas the diastolic myocardial stiffness constant was elevated in the sedentary group, it was normal in the exercise training group (32 ± 3 in sedentary dogs, 21 ± 3 in exercised dogs, and 20 ± 4 in otherwise normal dogs). Thus, daily exercise training preserved in vivo hemodynamics and in vitro measures of diastolic stiffness. The authors concluded that changes in heart function may contribute to the overall beneficial hemodynamic effects of exercise training in this canine model of CHF by a significant effect on diastolic properties.
In patients with CHF, a wide range of hemodynamic changes have been observed in response to exercise training also impacting on reverse remodeling (35,73,185). However, one study reported an increase in peak cardiac output (34). The same work load is achieved at a lower heart rate and rate-pressure product (34), indicating a more efficient utilization of myocardial work and oxygen consumption. Similarly, Demopoulus et al. demonstrated the diastolic wall stress significantly reduced at the lower than at the conventional training rates in patients with severe HF (41). In contrast, echocardiographic studies in patients early postmyocardial infarction involved in a 12-week exercise training program demonstrate deleterious effects of training. In this study, Jugdutt (84) showed further distortion of left ventricular shape, increased infarct size, reduced scar thickness, and ejection fraction.
Peripheral effects of exercise training in HF
Several studies have attempted to characterize the physiologic mechanisms for the clinical improvement in patients with HF. Sullivan studied 12 patients with HF with a mean VO2 of 16.3 mL/kg/min and an ejection fraction 21% (185). Aerobic training was performed 3 to 5 h a week for 6 months. With exercise training, peak VO2 rose 23% from 16.8 to 20.6 mL/kg/min. Peak cardiac output, peak A-VO2 difference and peak leg blood flow also significantly increased but ejection fraction was unchanged. Training decreased leg lactate production. Leg blood flow during submaximal exercise did not increase suggesting that the major benefit derived from training was via increased oxygen extraction by the skeletal muscles and the largest proportion of the increase in VO2 was derived from peripheral adaptation with a possible small central contribution.
Other studies using selective arm training (56,58) and aerobic exercise (209) have demonstrated an improvement but not normalization in the metabolic abnormalities observed with exercise in patients with HF. Percutaneous muscle biopsies of the vastus lateralis showed that aerobic training increased the volume density of mitochondria. Oxidative enzyme activity in skeletal muscle also increased with exercise training indicating improved oxidative function (71).
Belardinelli investigated the value of low intensity exercise training in patients with HF randomized to training versus control groups (17). The exercise prescription in this study called for three weekly training sessions of bicycle exercise at 40% of peak VO2 for 8 weeks. Peak VO2, serum catecholamines, lactate and vastus lateralis skeletal muscle biopsies were performed before and after training. Peak VO2 increased, serum lactate and catecholamine levels declined during submaximal exercise, and the volume density of mitochondria were enhanced at the conclusion of the study only in the trained group. Similarly, Demopoulus et al. demonstrated the value of low intensity training in patients with severe HF (41). Using a semirecumbent stationary bicycle, patients trained below 50% of peak VO2, 1 h/day, 4×/week, for 3 months. Peak VO2 rose from 11.5 to 15 mL/kg/min. Peak reactive hyperemia of the calf but not the forearm muscle increased with training. In this study, left ventricular diastolic wall stress was measured during bicycle exercise at low (<50%) and more conventional training workloads (70%−80% peak VO2). Diastolic wall stress was significantly reduced at the lower than at the conventional training rates in these patients.
Several recent studies have analyzed the impact of exercise training in patients with HF with preserved ejection fraction (HFpEF). Kitzman et al. showed that exercise training improved peak oxygen uptake compared to the control group and increased peak power output, exercise time, 6-min-walk distance and the ventilatory anaerobic threshold (90). These findings have been confirmed by Edelmann et al. who showed that exercise training improves exercise capacity and physical dimensions of quality of life in HFpEF. This benefit was associated with atrial reverse remodeling and improved left ventricular diastolic function (49).
Skeletal muscle mass is also reduced in chronic HF and could lead to early skeletal muscle fatigue and decreased exercise capacity (131). Physical deconditioning may contribute to wasting, especially of the leg muscles. The specific role of physical training on increasing leg muscle bulk versus function has yet to be determined but may well contribute to the increased exercise tolerance seen after training.
The effect of aerobic training on endothelial function has also been studied. Isolated forearm training using handgrip exercise resulted in improved flow-dependent dilatation (75). L-NMMA attenuated this improvement implying that the normalization of flow-dependent dilatation with training resulted from enhanced endothelial release of NO. This is an important finding in that it may indicate that with training there is an improvement in skeletal muscle perfusion. Also improvement in the endothelial function of large conduit vessels may decrease impedance to the failing left ventricle and thus improve left ventricular ejection fraction. At this point, other investigators have demonstrated increases in skeletal muscle leg perfusion and cardiac output at maximal but not during submaximal activity.
Modulation of “sympathetic overdrive” is another beneficial effect of exercise training. Training increases the parasympathetically mediated component of heart rate variability as well as prolonged exercise duration and increased peak VO2 (88). Similarly, the effect of training on autonomic tone assessed by heart rate variability and radiolabeled norepinephrine spillover demonstrated a shift from sympathetic to enhanced vagal activity (35). Other investigators have demonstrated a decrease in serum catecholamine levels both at rest and during submaximal exercise in these patients following aerobic training (71,84).
In summary, exercise capacity in patients with HF is limited not only by changes which affect the ability to increase cardiac output but also by changes which occur in the blood vessels, the muscles, and the lungs as a consequence of their disease.
Impact of exercise training on respiratory function in HF
Modification of respiratory muscle function with an appropriate shift in perceived dyspnea could demonstrate the importance of respiratory muscle function in the development of dyspnea. If respiratory muscles were a key modulator of the sensation of dyspnea than selective respiratory muscle training should attenuate exertional dyspnea and may also improve exercise performance particularly in patients limited by dyspnea. Accordingly, the effect of selective respiratory muscle training on exertional dyspnea and exercise capacity has been examined in HF. In one study, we examined the effect of respiratory muscle training in HF. Supervised respiratory muscle training was performed for 3 months. Maximal sustainable ventilatory capacity, maximal mouth pressures, pulmonary function tests and submaximal and maximal exercise capacity were measured before and after training. The training protocol included a variety of elements including isocapnic hyperpnea at a predetermined maximal sustainable ventilatory level, strength training with repetitive maximal inspiratory and expiratory mouth pressures, pulmonary rehabilitation exercises, and resistive breathing using a commercial available device. Thus, the impact of both strength and endurance training of the respiratory muscles in HF patients was investigated. Respiratory muscle endurance was significantly improved with training as evidenced by increases in maximal sustainable ventilatory capacity by 57%. Respiratory muscle strength, submaximal and maximal exercise capacity (6-min walk, peak VO2) were significantly improved with selective respiratory muscle training. Dyspnea was subjectively improved in the majority of trained patients. In contrast, no significant improvements were observed in the controls (127).
Other investigators (38,100,102,205) have studied respiratory muscle training in these patients using predominantly resistive pressure threshold load devices or computer controlled biofeedback trainers. Training has been performed at a wide range of inspiratory pressure loads (15%−60% of Pimax). Even though the training regimens have varied, the overall results consistently demonstrated an improvement of respiratory muscle strength, peak VO2 and 6-min-walk tests. The consistency of the improvement in overall peak VO2 has lead to studies to identify the mechanism for the enhanced exercise capacity (Table 3).
Table 3.
Clinical Trials of Respiratory Muscle Training in Heart Failure
| Investigator | n | NYHA | LVEF% | Protocol | Outcome |
|---|---|---|---|---|---|
| MANCINI | 14 | I-IV | 22 ± 9 | Isocapnic hyperpnea Resistive breathing at 30% Pimax Strength training |
Increase Pimax Increase MVV Increase 6 min walk Increase VO2max |
| WEINER | 20 | II-III | <30 | Resistive breathing at 15%–60% Pimax | Increase Pimax Increase resp muscle endurance Increase 12 min walk test No change peak VO2 |
| LAOUTARIS | 37 | II-III | 24 ± 1 | 60% Pimax | Increase Pimax Increase VO2max Increase 6 min walk |
| DALL’AGO | 32 | <45 | 30% Pimax | Increase Pimax Increase VO2max Increase 6 min walk |
|
| LAOUTARIS | 38 | II-III | 28 ± 1 | 60%Pimaxvs. 15% Pimax (control) | Increase Pimax Increase peak VO2 Increase 6 min walk |
| LAOUTARIS | 23 | II-III | 29 ± 1 | 60% Pimax 15% Pi |
Increase Pimax Increase peak VO2 Increase 6 min walk |
Abbreviations: Pimax, max. inspiratory pressure; VO2max, max. aerobic capacity; MVV, max. voluntary ventilation.
Chiappa et al. hypothesized that the improvement in peak VO2 may result from a redistribution of blood from the lungs to the locomotor muscles (30). Inspiratory muscle loading results in a reduction of blood flow to resting and exercising muscle which following respiratory muscle training is alleviated in patients with HF and respiratory muscle weakness. Borghi Silva et al. (23) also have demonstrated that unloaded ventilation improves submaximal exercise and skeletal muscle perfusion in HF.
Conversely, if skeletal muscle metaboreceptors modulate the sensation of dyspnea then selectively improving leg muscles may similarly alleviate dyspnea. As aerobic training results in training of both the leg and respiratory muscles, selective low-level leg training was performed in 17 HF patients. Low-level bicycle and treadmill exercise where minute ventilation was below 25 L/min was performed along with leg exercises. Perceived dyspnea during submaximal exercise was reduced in the patients with low-level training suggesting that skeletal muscle function impacts this sensation (19).
The HF-ACTION trial
The HF-ACTION trial was a large multicenter, randomized study examining the effects of exercise training on patients with HF (2331 patients with an ejections fraction <35% enrolled over 4 years). Its primary hypothesis was based on the multiple studies demonstrating beneficial effects of aerobic exercise training in patients with HF. The primary endpoint of the study was overall mortality. The study did not meet its primary endpoint. The substudy analysis, which adjusted for mortality predictors, did show an exercise dose-dependent benefit in QOL, oxygen uptake, and functional capacity. All patients were treated under recent pharmacotherapeutic standards. The observed exercise effect was very modest with an increase of peak VO2 or only 0.3 mL/min/kg. The study showed that exercise training was safe in patients with HF over an extended period of time in a closely supervised setting with frequent follow-up. Unlike previous studies suggesting that women and older patients may not respond well to exercise training, the HF-ACTION substudy demonstrated that exercise-related benefits are consistent across sex, race, age, and other subgroups (153).
The modest effect of exercise training on peak functional capacity in the HF-ACTION trial also argues against disuse playing an important role in the pathogenesis of muscle alterations in HF. However whether these findings simply reflect the difficulty with adherence to exercise training in a large study population is unclear. The findings of the HF-ACTION trial do not invalidate the previously well documented beneficial effects of exercise training on functional capacity and muscle mass in smaller populations of HF patients.
Potential limitations of exercise training in HF
Many questions remain regarding the value of exercise training in patients with HF. Currently, it is unclear which mode of training is optimal for these patients and whether training has effects on long term morbidity and mortality.
Initial studies by Letac (109), Lee (107), Williams (207), and Conn (37) established the safety of training in this population. They also demonstrated that patients with reduced left ventricular function can demonstrate the hallmarks of training, that is, improvement in work capacity with a concomitant decline in heart rate. In contrast, a study by Jugdutt (84) suggests that a 12-week-exercise training program in patients after extensive anterior infarctions can further distort left ventricular shape, increase infarct expansion, reduce scar thickness, and reduce ejection fraction. Endurance training in rats after large myocardial infarctions resulted in reduced survival and left ventricular dilation (56). However, in the exercise in anterior myocardial infarction trial on patients with EF <40% postmyocardial infarction, exercise training did not produce greater ventricular dilatation or reduction in ejection fraction compared to the control group. In a subgroup analysis on patients with ejection fractions below 40%, no echocardiographic evidence of deterioration of LV function was observed. However in this group mean LVEF was still quite high averaging 35% and only a small percent of patients were maintained on ACE inhibitors.
To circumvent the potential deleterious effect on high-intensity aerobic training, a few studies have focused on either low-intensity or specific-muscle training. Studies which focus on small muscle mass training which does not stress the cardiovascular system may have the added advantage of inducing peripheral skeletal muscle changes without adverse cardiac effects.
Aerobic training does not invariably result in improved exercise capacity. Compliance with the training regimen is the most frequently cited marker to predict success. However, some studies suggest that the initial cardiac output response to maximal exercise may determine the response to exercise training. In a study on patients with HF with hemodynamic measurements during treadmill exercise prior to enrolling in a rehabilitation program, patients were divided the patients into normal and reduced cardiac output response to exercise using the formula CO = 0.5 VO2 + 3 (209). Patients with a “normal” cardiac output response to exercise tolerated training and achieved therapeutic benefit with training defined as > 10% increase in both peak VO2 and anaerobic threshold. In contrast, the patients with a reduced cardiac output response had a high drop-out rate during training due to exhaustion.
Conclusion
Exercise training appears to have therapeutic benefits for individuals with CHF. Improvements in regional blood flow, skeletal muscle histology, and biochemistry, and improvements in the autonomic imbalance likely account for the increase in exercise capacity and delay in fatigue that the trained group experiences. The beneficial effects therefore appear to be primarily related to peripheral rather than cardiac mechanisms. Improved peripheral extraction or use of oxygen during exercise leads to the increased maximal oxygen consumption seen in trained patients. However, the survival implications of enhanced maximal oxygen consumption due to training are unknown. Future studies investigating the clinical benefit of low intensity exercise training and/or regional muscle group training in patients with HF appear warranted. These studies will elucidate the optimal regimen and the long-term effects of exercise training in patients with HF.
References
- 1.Aaronson K CTMD. Demonstration of the continuous nature of peak VO2 for predicting survival in ambulatory patients evaluated for transplant. J Heart Lung Transplant 15: s66, 1996. [Google Scholar]
- 2.Adamopoulos S, Coats AJ, Brunotte F, Arnolda L, Meyer T, Thompson CH, Dunn JF, Stratton J, Kemp GJ, Radda GK. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J Am CollCardiol 21: 1101–1106, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosino N, Opasich C, Crotti P, Cobelli F, Tavazzi L, Rampulla C. Breathing pattern, ventilatory drive and respiratory muscle strength in patients with chronic heart failure. EurRespirJ 7: 17–22, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 349: 1050–1053, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J 151: 851–813, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. Development of a ventilatory classification system in patients with heart failure. Circulation 115: 2410–2417, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Arena R, Myers J, Abella J, Pinkstaff S, Brubaker P, Moore B, Kitzman D, Peberdy MA, Bensimhon D, Chase P, Guazzi M. The partial pressure of resting end-tidal carbon dioxide predicts major cardiac events in patients with systolic heart failure. Am Heart J 156: 982–988, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: An evidence-based review. Heart FailRev 13: 245–269, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong RB, Horton HS, Terjung RL. Muscle fiber recruitment patterns and their metabolic correlates Exercise, Nutrition, and Energy Metabolism page 9–26, 1988. New York: Macmillan Publishing Company. [Google Scholar]
- 10.Arnold JM, Ribeiro JP, Colucci WS. Muscle blood flow during forearm exercise in patients with severe heart failure. Circulation 82: 465–472, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Arora NS, Rochester DF. Effect of body weight and muscularity on human diaphragm muscle mass, thickness, and area. J Appl Physiol 52: 64–70, 1982. [DOI] [PubMed] [Google Scholar]
- 12.Aubier M, Farkas G, De TA, Mozes R, Roussos C. Detection of diaphragmatic fatigue in man by phrenic stimulation. J Appl Physiol 50: 538–544, 1981. [DOI] [PubMed] [Google Scholar]
- 13.Aukrust P, Ueland T, Gullestad L, Yndestad A. Testosterone: A novel therapeutic approach in chronic heart failure? J Am Coll Cardiol 54: 928–929, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Balady G American heart association scientific statement: A clinician’s guide to cardiopulmonary exercise testing in adults. Am Heart Assoc Circulation Jul 13;122(2): 191–225, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guany-late cyclase expression: Role of enhanced vascular superoxide production. Circulation 100: 29–298, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Beckendorf L, Linke WA. Emerging importance of oxidative stress in regulating striated muscle elasticity. JMuscle Res Cell Motil 36: 25–36, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belardinelli R, Georgiou D, Scocco V, Barstow TJ, Purcaro A. Low intensity exercise training in patients with chronic heart failure. J Am CollCardiol 26: 975–982, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Bellinger AM, Mongillo M, Marks AR. Stressed out: The skeletal muscle ryanodine receptor as a target of stress. J ClinInvest 118: 445–453, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beniaminovitz A, Lang CC, LaManca J, Mancini DM. Selective low-level leg muscle training alleviates dyspneain patients with heart failure. J Am CollCardiol 40: 1602–1608, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle. J Endocrinol 170: 27–38, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 270: 1702–1707, 1993. [PubMed] [Google Scholar]
- 22.Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. AnnuRevPhysiol 45: 169–189, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Borghi-Silva A, Carrascosa C, Oliveira CC, Barroco AC, Berton DC, Vilaca D, Lira-Filho EB, Ribeiro D, Nery LE, Neder JA. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. AJP - Heart and Circulatory Physiology 294: H2465–H2472, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Breitbart A, Auger-Messier M, Molkentin JD, Heineke J. Myostatin from the heart: Local and systemic actions in cardiac failure and muscle wasting. Am J Physiol Heart Circ Physiol 300: H1973–H1982, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M, Mammi C, Piepoli M, Fini M, Rosano GM. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am CollCardiol 54: 919–927, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Campbell EJ, Gandevia SC, Killian KJ, Mahutte CK, Rigg JR. Changes in the perception of inspiratory resistive loads during partial curarization. J Physiol 309: 93–100, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell EJ, Howell JB. The sensation of breathlessness. BrM ed Bull 19: 36–40, 1963. [DOI] [PubMed] [Google Scholar]
- 28.Chati Z, Zannad F, Jeandel C, Lherbier B, Escanye JM, Robert J, Aliot E. Physical deconditioning may be a mechanism for the skeletal muscle energy phosphate metabolism abnormalities in chronic heart failure. Am Heart J 131:56–566, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Chati Z, Zannad F, Robin-Lherbier B, Escanye JM, Jeandel C, Robert J, Aliot E. Contribution of specific skeletal muscle metabolic abnormalities to limitation of exercise capacity in patients with chronic heart failure: A phosphorus 31 nuclear magnetic resonance study. Am Heart J 128:781–792, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Chiappa GR, Roseguini BT, Vieira PJ, Alves CN, Tavares A, Winkel-mann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol 51: 1663–1671, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Chomsky DB, Lang CC, Rayos GH, Shyr Y, Yeoh TK, Pierson RN, III, Davis SF, Wilson JR. Hemodynamic exercise testing. A valuable tool in the selection of cardiac transplantation candidates. Circulation 94:3176–3183, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Chua TP, Anker SD, Harrington D, Coats AJ. Inspiratory muscle strength is a determinant of maximum oxygen consumption in chronic heart failure. BrHeart J 74: 381–385, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: Central role of the periphery. J Am Coll Cardiol 28: 1092–1102, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Coats AJ, Adamopoulos S, Meyer TE, Conway J, Sleight P. Effects of physical training in chronic heart failure. Lancet 335: 63–66, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 85: 2119–2131, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Cohen-Solal A, Laperche T, Morvan D, Geneves M, Caviezel B, Gourgon R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure. Analysis with gas exchange measurements and NMR spectroscopy. Circulation 91: 2924–2932, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Conn EH, Williams RS, Wallace AG. Exercise responses before and after physical conditioning in patients with severely depressed left ventricular function. Am J Cardiol 49: 296–300, 1982. [DOI] [PubMed] [Google Scholar]
- 38.Dall’ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: A randomized trial. J Am CollCardiol 47: 757–763, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Davies S Respiratory muscle failure on exercise in chronic heart failure. Circulation 82: III–24, 1990. [Google Scholar]
- 40.De SE, Veksler V, Bigard X, Mateo P, Serrurier B, Ventura-Clapier R. Dual influence of disease and increased load on diaphragm muscle in heart failure. J MolCell Cardiol 33: 699–710, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Demopoulos L, Bijou R, Fergus I, Jones M, Strom J, Le Jemtel TH. Exercise training in patients with severe congestive heart failure: Enhancing peak aerobic capacity while minimizing the increase in ventricular wall stress. J Am Coll Cardiol 29: 597–603, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Doehner W, von Haehling S, Anker SD. Insulin resistance in chronic heart failure. J Am Coll Cardiol 52: 239, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Dominguez JF, Howell S. Compartmental analysis of steady-state diaphragm Ca2 +kinetics in chronic congestive heart failure. Cell Calcium 33: 163–174, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Drexler H, Hayoz D, Munzel T, Hornig B, Just H, Brunner HR, Zelis R. Endothelial function in chronic congestive heart failure. Am J Cardiol 69: 1596–1601, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J ClinInvest 113: 115–123,2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: A contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol 33: 1956–1963, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Duscha BD, Schulze PC, Robbins JL, Forman DE. Implications of chronic heart failure on peripheral vasculature and skeletal muscle before and after exercise training. Heart Fail Rev 13: 21–37, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction results of the Ex-DHF (exercise training in diastolic heart failure) pilot study. J Am Coll Cardiol 58: 1780–1791, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Elmariah S, Goldberg LR, Allen MT, Kao A. Effects of gender on peak oxygen consumption and the timing of cardiac transplantation. J Am CollCardiol 47: 2237–2242, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, Cassani G, Visioli O. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation 92: 1479–1486, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Fishman AP, Ledlie JF. Dyspnea. Bull Eur Physiopathol Respir Sep-Oct; 15(5): 789–804, 1979. [PubMed] [Google Scholar]
- 53.Fixler DE, Atkins JM, Mitchell JH, Horwitz LD. Blood flow to respiratory, cardiac, and limb muscles in dogs during graded exercise. Am J Physiol 231: 1515–1519, 1976. [DOI] [PubMed] [Google Scholar]
- 54.Frankenstein L, Meyer FJ, Sigg C, Nelles M, Schellberg D, Remppis A, Katus HA, Zugck C. Is serial determination of inspiratory muscle strength a useful prognostic marker in chronic heart failure? Eur J Cardiovasc Prev Rehabil 15: 156–161, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Fregosi RF, Seals DR. Hypoxic potentiation of the ventilatory response to dynamic forearm exercise. J Appl Physiol 74: 2365–2372, 1993. [DOI] [PubMed] [Google Scholar]
- 56.Gaudron P, Hu K, Schamberger R, Budin M, Walter B, Ertl G. Effect of endurance training early or late after coronary artery occlusion on left ventricular remodeling, hemodynamics, and survival in rats with chronic transmural myocardial infarction. Circulation 89: 402–412, 1994. [DOI] [PubMed] [Google Scholar]
- 57.George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, Sweeney HL, Maybaum S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail 12: 444–453, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giannuzzi P, Tavazzi L, Temporelli PL, Corra U, Imparato A, Gattone M, Giordano A, Sala L, Schweiger C, Malinverni C. Long-term physical training and left ventricular remodeling after anterior myocardial infarction: Results of the Exercise in Anterior Myocardial Infarction (EAMI) trial. EAMI Study Group. J Am CollCardiol 22: 1821–1829, 1993. [DOI] [PubMed] [Google Scholar]
- 59.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am CollCardiol 42: 861–868, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, Schneider S, Schwarz A, Senges J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 106: 3079–3084, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Golberg LR J M. Stage B heart failue management of asymptomatic left ventricular systtolic dysfunction. Circulation 113: 2851–2860, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Goletti S, Gruson D. Personalized risk assessment of heart failure patients: More perspectives from transforming growth factor superfamily members. Clin ChimActa 443: 94–99, 2015. [DOI] [PubMed] [Google Scholar]
- 63.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. P rocN atlA cadSciU SA 98: 14440–14445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: Underlying mechanisms and therapy perspectives. Am J Clin Nutr 71: 1033–1047, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Green P, Lund LH, Mancini D. Comparison of peak exercise oxygen consumption and the Heart Failure Survival Score for predicting prognosis in women versus men. Am J Cardiol 99: 399–403, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Griffin BP, Shah PK, Ferguson J, Rubin SA. Incremental prognostic value of exercise hemodynamic variables in chronic congestive heart failure secondary to coronary artery disease or to dilated cardiomyopathy. Am J Cardiol 67: 848–853, 1991. [DOI] [PubMed] [Google Scholar]
- 67.Gruson D, Ahn SA, Ketelslegers JM, Rousseau MF. Increased plasma myostatin in heart failure. Eur J Heart Fail 13: 734–736, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Guazzi M, Arena R, Ascione A, Piepoli M, Guazzi MD. Exercise oscillatory breathing and increased ventilation to carbon dioxide production slope in heart failure: An unfavorable combination with high prognostic value. Am Heart J 153: 859–867, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 43: 12–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 98: 2709–2715, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, Riede U, Schlierf G, Kubler W, Schuler G. Physical training in patients with stable chronic heart failure: Effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am CollCardiol 25: 1239–1249, 1995. [DOI] [PubMed] [Google Scholar]
- 72.Hammond MD, Bauer KA, Sharp JT, Rocha RD. Respiratory muscle strength in congestive heart failure. Chest 98: 1091–1094, 1990. [DOI] [PubMed] [Google Scholar]
- 73.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J Am Coll Cardiol 49: 2329–2336, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 121:419–425,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation 93: 210–214, 1996. [DOI] [PubMed] [Google Scholar]
- 76.Isnard R, Lechat P, Kalotka H, Chikr H, Fitoussi S, Salloum J, Golmard JL, Thomas D, Komajda M. Muscular blood flow response to submaximal leg exercise in normal subjects and in patients with heart failure. J Appl Physiol 81: 2571–2579, 1996. [DOI] [PubMed] [Google Scholar]
- 77.Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M, Anker SD, Banasiak W, Poole-Wilson PA, Ponikowski P. Anabolic deficiency in men with chronic heart failure: Prevalence and detrimental impact on survival. Circulation 114: 1829–1837, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, Decramer M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation 111: 996–1005, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119: 1977–2016, 2009. [DOI] [PubMed] [Google Scholar]
- 80.Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol 460: 385–405, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jondeau G, Katz SD, Toussaint JF, Dubourg O, Monrad ES, Bourdarias JP, LeJemtel TH. Regional specificity of peak hyperemic response in patients with congestive heart failure: Correlation with peak aerobic capacity. J Am Coll Cardiol 22: 1399–1402, 1993. [DOI] [PubMed] [Google Scholar]
- 82.Jondeau G, Katz SD, Zohman L, Goldberger M, McCarthy M, Bourdarias JP, Le Jemtel TH. Active skeletal muscle mass and cardiopulmonary reserve. Failure to attain peak aerobic capacity during maximal bicycle exercise in patients with severe congestive heart failure. Circulation 86: 1351–1356, 1992. [DOI] [PubMed] [Google Scholar]
- 83.Jones GL, Killian KJ, Summers E, Jones NL. Inspiratory muscle forces and endurance in maximum resistive loading. J Appl Physiol 58: 1608–1615, 1985. [DOI] [PubMed] [Google Scholar]
- 84.Jugdutt BI, Michorowski BL, Kappagoda CT. Exercise training after anterior Q wave myocardial infarction: Importance of regional left ventricular function and topography. J Am Coll Cardiol 12: 362–372, 1988. [DOI] [PubMed] [Google Scholar]
- 85.Kaiser L, Spickard RC, Olivier NB. Heart failure depresses endothelium-dependent responses in canine femoral artery. Am J Physiol 256: H962–H967, 1989. [DOI] [PubMed] [Google Scholar]
- 86.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, Solomon S, Nikolic SD, Forman R, Le Jemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol 19: 918–925, 1992. [DOI] [PubMed] [Google Scholar]
- 87.Katz SD, Maskin C, Jondeau G, Cocke T, Berkowitz R, LeJemtel T. Near-maximal fractional oxygen extraction by active skeletal muscle in patients with chronic heart failure. J Appl Physiol 88: 2138–2142, 2000. [DOI] [PubMed] [Google Scholar]
- 88.Kiilavuori K, Toivonen L, Naveri H, Leinonen H. Reversal of autonomic derangements by physical training in chronic heart failure assessed by heart rate variability. Eur Heart J 16: 490–495, 1995. [DOI] [PubMed] [Google Scholar]
- 89.Killian KJ, Jones NL. Respiratory muscles and dyspnea. Clinics in Chest Medicine 9: 237–248, 1988. [PubMed] [Google Scholar]
- 90.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. Circ Heart Fail 3: 659–667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kleber FX, Vietzke G, Wernecke KD, Bauer U, Opitz C, Wensel R, Sperfeld A, Glaser S. Impairment of ventilatory efficiency in heart failure: Prognostic impact. Circulation 101: 2803–2809, 2000. [DOI] [PubMed] [Google Scholar]
- 92.Koelling TM, Joseph S, Aaronson KD. Heart failure survival score continues to predict clinical outcomes in patients with heart failure receiving beta-blockers. J Heart Lung Transplant 23: 1414–1422,2004. [DOI] [PubMed] [Google Scholar]
- 93.Kokkinos PF, Narayan P, Papademetriou V. Exercise as hypertension therapy. Cardiol Clin 19: 507–516, 2001. [DOI] [PubMed] [Google Scholar]
- 94.Kubo Sh, Rector TS, Bank AJ, Raij L, Kraemer MD, Tadros P, Beard-slee M, Garr MD. Lack of contribution of nitric oxide to basal vasomotor tone in heart failure. Am J Cardiol 74: 1133–1136, 1994. [DOI] [PubMed] [Google Scholar]
- 95.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation 84: 1589–1596, 1991. [DOI] [PubMed] [Google Scholar]
- 96.Lang C Peak Cardiac Power Output measured non-invasively, is a powerful predictor of outcome in chronic heart failure. Circ Heart Fail 2: 33–38, 2009. [DOI] [PubMed] [Google Scholar]
- 97.Lang CC, Agostoni P, Mancini DM. Prognostic significance and measurement of exercise-derived hemodynamic variables in patients with heart failure. J Card Fail 13: 672–679, 2007. [DOI] [PubMed] [Google Scholar]
- 98.Lang CC, Chomsky DB, Rayos G, Yeoh TK, Wilson JR. Skeletal muscle mass and exercise performance in stable ambulatory patients with heart failure. J Appl Physiol 82: 257–261, 1997. [DOI] [PubMed] [Google Scholar]
- 99.Lang CC, Karlin P, Haythe J, Tsao L, Mancini DM. Ease of noninvasive measurement of cardiac output coupled with peak VO2 determination at rest and during exercise in patients with heart failure. Am J Cardiol 99: 404–405, 2007. [DOI] [PubMed] [Google Scholar]
- 100.Laoutaris I, Dritsas A, Brown MD, Manginas A, Alivizatos PA, Cokkinos DV. Inspiratory muscle training using an incremental endurance test alleviates dyspnea and improves functional status in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 11: 489–496, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Laoutaris ID, Dritsas A, Brown MD, Manginas A, Kallistratos MS, Chaidaroglou A, Degiannis D, Alivizatos PA, Cokkinos DV. Effects of inspiratory muscle training on autonomic activity, endothelial vasodilator function, and N-terminal pro-brain natriuretic peptide levels in chronic heart failure. J Cardiopulm Rehabil Prev 28: 99–106, 2008. [DOI] [PubMed] [Google Scholar]
- 102.Laoutaris ID, Dritsas A, Brown MD, Manginas A, Kallistratos MS, Degiannis D, Chaidaroglou A, Panagiotakos DB, Alivizatos PA, Cokkinos DV. Immune response to inspiratory muscle training in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 14: 679–685, 2007. [DOI] [PubMed] [Google Scholar]
- 103.Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 49: 171–180, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Lecarpentier Y, Chemla D, Blanc FX, Pourny JC, Joseph T, Riou B, Coirault C. Mechanics, energetics, and crossbridge kinetics of rabbit diaphragm during congestive heart failure. FASEBJ 12: 981–989, 1998. [DOI] [PubMed] [Google Scholar]
- 105.Lecarpentier Y, Coirault C, Langeron O, Blanc FX, Salmeron S, Attal P, Riou B, Chemla D. Impaired load dependence of diaphragm relaxation during congestive heart failure in the rabbit. J Appl Physiol 87: 1339–1345, 1999. [DOI] [PubMed] [Google Scholar]
- 106.Lecarpentier Y, Pery N, Coirault C, Scalbert E, Desche P, Suard I, Lambert F, Chemla D. Intrinsic alterations of diaphragm muscle in experimental cardiomyopathy. Am Heart J 126: 770–776, 1993. [DOI] [PubMed] [Google Scholar]
- 107.Lee AP, Ice R, Blessey R, Sanmarco ME. Long-term effects of physical training on coronary patients with impaired ventricular function. Circulation 60: 1519–1526, 1979. [DOI] [PubMed] [Google Scholar]
- 108.LeJemtel TH, Maskin CS, Lucido D, Chadwick BJ. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation 74: 245–251, 1986. [DOI] [PubMed] [Google Scholar]
- 109.Letac B, Cribier A, Desplanches JF. A study of left ventricular function in coronary patients before and after physical training. Circulation 56: 375–378, 1977. [DOI] [PubMed] [Google Scholar]
- 110.Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann JP, Baud L. The role of calpains in myocardial remodelling and heart failure. Cardiovasc Res 96: 38–45, 2012. [DOI] [PubMed] [Google Scholar]
- 111.Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation 110: 3049–3054, 2004. [DOI] [PubMed] [Google Scholar]
- 112.Li X, Moody MR, Engel D, Walker S, Clubb FJ Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102: 1690–1696, 2000. [DOI] [PubMed] [Google Scholar]
- 113.Lindsay DC, Lovegrove CA, Dunn MJ, Bennett JG, Pepper JR, Yacoub MH, Poole-Wilson PA. Histological abnormalities of muscle from limb, thorax and diaphragm in chronic heart failure. Eur Heart J 17: 1239–1250, 1996. [DOI] [PubMed] [Google Scholar]
- 114.Lipkin DP, Jones DA, Round JM, Poole-Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol 18: 187–195, 1988. [DOI] [PubMed] [Google Scholar]
- 115.Longhurst J, Capone RJ, Zelis R. Evaluation of skeletal muscle capillary basement membrane thickness in congestive heart failure. Chest 67: 195–198, 1975. [DOI] [PubMed] [Google Scholar]
- 116.Lopes FS, Carvalho RF, Campos GE, Sugizaki MM, Padovani CR, Nogueira CR, Cicogna AC, Pai-Silva MD. Down-regulation of MyoD gene expression in rat diaphragm muscle with heart failure. Int J Exp Pathol 89: 216–222, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lund LH, Aaronson KD, Mancini DM. Predicting survival in ambulatory patients with severe heart failure on beta-blocker therapy. Am J Cardiol 92: 1350–1354,2003. [DOI] [PubMed] [Google Scholar]
- 118.Lund LH, Aaronson KD, Mancini DM. Validation of peak exercise oxygen consumption and the Heart Failure Survival Score for serial risk stratification in advanced heart failure. Am J Cardiol 95: 734–741, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Lunde PK, Dahlstedt AJ, Bruton JD, Lannergren J, Thoren P, Sejersted OM, Westerblad H. Contraction and intracellular Ca(2+) handling in isolated skeletal muscle of rats with congestive heart failure. Circ Res 88: 1299–1305, 2001. [DOI] [PubMed] [Google Scholar]
- 120.Lunde PK, Sjaastad I, Schiotz Thorud HM, Sejersted OM. Skeletal muscle disorders in heart failure. Acta Physiol Scand 171: 277–294, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Mahy IR, Tooke JE. Peripheral microvascular function in human heart failure. Clin Sci (Lond) 88: 501–508, 1995. [DOI] [PubMed] [Google Scholar]
- 122.Mancini D, Katz S, Donchez L, Aaronson K. Coupling of hemodynamic measurements with oxygen consumption during exercise does not improve risk stratification in patients with heart failure. Circulation 94:2492–2496, 1996. [DOI] [PubMed] [Google Scholar]
- 123.Mancini DM, Coyle E, Coggan A, Beltz J, Ferraro N, Montain S, Wilson JR. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation 80: 1338–1346, 1989. [DOI] [PubMed] [Google Scholar]
- 124.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 83: 778–786, 1991. [DOI] [PubMed] [Google Scholar]
- 125.Mancini DM, Ferraro N, Nazzaro D, Chance B, Wilson JR. Respiratory muscle deoxygenation during exercise in patients with heart failure demonstrated with near-infrared spectroscopy. J Am Coll Cardiol 18: 492–498, 1991. [DOI] [PubMed] [Google Scholar]
- 126.Mancini DM, Ferraro N, Tuchler M, Chance B, Wilson JR. Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus-31 nuclear magnetic resonance. Am J Cardiol 62: 1234–1240, 1988. [DOI] [PubMed] [Google Scholar]
- 127.Mancini DM, Henson D, La MJ, Donchez L, Levine S. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation 91: 320–329, 1995. [DOI] [PubMed] [Google Scholar]
- 128.Mancini DM, Henson D, LaManca J, Levine S. Evidence of reduced respiratory muscle endurance in patients with heart failure. J Am Coll Cardiol 24: 972–981, 1994. [DOI] [PubMed] [Google Scholar]
- 129.Mancini DM, Henson D, LaManca J, Levine S. Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation 86: 909–918, 1992. [DOI] [PubMed] [Google Scholar]
- 130.Mancini DM, Schwartz M, Ferraro N, Seestedt R, Chance B, Wilson JR. Effect of dobutamine on skeletal muscle metabolism in patients with congestive heart failure. Am J Cardiol 65: 1121–1126, 1990. [DOI] [PubMed] [Google Scholar]
- 131.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 85:1364–1373, 1992. [DOI] [PubMed] [Google Scholar]
- 132.Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ Res 91: 988–998, 2002. [DOI] [PubMed] [Google Scholar]
- 133.Mann DL, Reid MB. Exercise training and skeletal muscle inflammation in chronic heart failure: Feeling better about fatigue. J Am Coll Cardiol 42: 869–872, 2003. [DOI] [PubMed] [Google Scholar]
- 134.Maskin CS, Forman R, Sonnenblick EH, Frishman WH, Le Jemtel TH. Failure of dobutamine to increase exercise capacity despite hemodynamic improvement in severe chronic heart failure. Am J Cardiol 51: 177–182, 1983. [DOI] [PubMed] [Google Scholar]
- 135.Massie B, Conway M, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G, Rajagopalan B. Skeletal muscle metabolism in patients with congestive heart failure: Relation to clinical severity and blood flow. Circulation 76: 1009–1019, 1987. [DOI] [PubMed] [Google Scholar]
- 136.McCully K, Mancini D, Levine S. Nuclear magnetic resonance spectroscopy: Its role in providing valuable insight into diverse clinical problems. Chest 116: 1434–1441, 1999. [DOI] [PubMed] [Google Scholar]
- 137.McParland C, Krishnan B, Wang Y, Gallagher CG. Inspiratory muscle weakness and dyspnea in chronic heart failure. Am Rev Respir Dis 146: 467–472, 1992. [DOI] [PubMed] [Google Scholar]
- 138.Metra M, Faggiano P, D’Aloia A, Nodari S, Gualeni A, Raccagni D, Dei CL. Use of cardiopulmonary exercise testing with hemodynamic monitoring in the prognostic assessment of ambulatory patients with chronic heart failure. J Am Coll Cardiol 33: 943–950, 1999. [DOI] [PubMed] [Google Scholar]
- 139.Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol 38: 947–954, 2001. [DOI] [PubMed] [Google Scholar]
- 140.Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol 38: 947–954, 2001. [DOI] [PubMed] [Google Scholar]
- 141.Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kubler W, Haass M. Respiratory muscle dysfunction in congestive heart failure: Clinical correlation and prognostic significance. Circulation 103: 2153–2158,2001. [DOI] [PubMed] [Google Scholar]
- 142.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated muscle mechanoreflex control of reflex renal vasoconstriction in heart failure. J Appl Physiol 90: 1714–1719,2001. [DOI] [PubMed] [Google Scholar]
- 143.Miller MS, Van Buren P, Le Winter MM, Braddock JM, Ades PA, Maughan DW, Palmer BM, Toth MJ. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans. J Physiol 588: 4039–4053, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller MS, Vanburen P, Lewinter MM, Lecker SH, Selby DE, Palmer BM, Maughan DW, Ades PA, Toth MJ. Mechanisms underlying skeletal muscle weakness in human heart failure: Alterations in single fiber myosin protein content and function. Circ Heart Fail 2: 700–706, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Minotti JR, Christoph I, Oka R, Weiner MW, Wells L, Massie BM. Impaired skeletal muscle function in patients with congestive heart failure. Relationship to systemic exercise performance. J Clin Invest 88: 2077–2082, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mourkioti F, Rosenthal N. NF-kappaB signaling in skeletal muscle: Prospects for intervention in muscle diseases. J Mol Med 86: 747–759, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Musch TI. Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. AJP - Heart and Circulatory Physiology 265: H1721–H1726, 1993. [DOI] [PubMed] [Google Scholar]
- 148.Musch TI, Moore RL, Leathers DJ, Bruno A, Zelis R. Endurance training in rats with chronic heart failure induced by myocardial infarction. Circulation 74: 431–441, 1986. [DOI] [PubMed] [Google Scholar]
- 149.Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, Kangawa K. Treatment of cachexia with ghrelin in patients with COPD. Chest 128: 1187–1193, 2005. [DOI] [PubMed] [Google Scholar]
- 150.Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 110: 3674–3679, 2004. [DOI] [PubMed] [Google Scholar]
- 151.Nanas S, Anastasiou-Nana M, Dimopoulos S, Sakellariou D, Alex-opoulos G, Kapsimalakou S, Papazoglou P, Tsolakis E, Papazachou O, Roussos C, Nanas J. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int J Cardiol 110: 393–400, 2006. [DOI] [PubMed] [Google Scholar]
- 152.Nishimura Y, Maeda H, Tanaka K, Nakamura H, Hashimoto Y, Yokoyama M. Respiratory muscle strength and hemodynamics in chronic heart failure. Chest 105: 355–359, 1994. [DOI] [PubMed] [Google Scholar]
- 153.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTiOn randomized controlled trial. JAMA 301: 1439–1450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 111: 2313–2318, 2005. [DOI] [PubMed] [Google Scholar]
- 155.Oelberg DA, Evans AB, Hrovat MI, Pappagianopoulos PP, Patz S, Systrom DM. Skeletal muscle chemoreflex and pHi in exercise ventilatory control. J Appl Physiol 84: 676–682, 1998. [DOI] [PubMed] [Google Scholar]
- 156.Okada Y, Toth MJ, VanBuren P. Skeletal muscle contractile protein function is preserved in human heart failure. J Appl Physiol 104: 952–957, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Opasich C, Aquilani R, Dossena M, Foppa P, Catapano M, Pagani S, Pasini E, Ferrari R, Tavazzi L, Pastoris O. Biochemical analysis of muscle biopsy in overnight fasting patients with severe chronic heart failure. EurH eartJ 17: 1686–1693, 1996. [DOI] [PubMed] [Google Scholar]
- 158.Osada N, Chaitman BR, Miller LW, Yip D, Cishek MB, Wolford TL, Donohue TJ. Cardiopulmonary exercise testing identifies low risk patients with heart failure and severely impaired exercise capacity considered for heart transplantation. J Am Coll Cardiol 31: 577–582, 1998. [DOI] [PubMed] [Google Scholar]
- 159.Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172: 200–205, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parikh MN, Lund LH, Goda A, Mancini D. Usefulness of peak exercise oxygen consumption and the heart failure survival score to predict survival in patients >65 years of age with heart failure. Am J Cardiol 103:998–1002, 2009. [DOI] [PubMed] [Google Scholar]
- 161.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008. [DOI] [PubMed] [Google Scholar]
- 162.Peters DG, Mitchell HL, McCune SA, Park S, Williams JH, Kandarian SC. Skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase gene expression in congestive heart failure. Circ Res 81: 703–710, 1997. [DOI] [PubMed] [Google Scholar]
- 163.Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 328: 189,2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Piepoli MF, Kaczmarek A, Francis DP, Davies LC, Rauchhaus M, Jankowska EA, Anker SD, Capucci A, Banasiak W, Ponikowski P. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation 114: 126–134, 2006. [DOI] [PubMed] [Google Scholar]
- 165.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: Involvement of muscle myofilaments. Am J Respir Crit Care Med 166: 479–484, 2002. [DOI] [PubMed] [Google Scholar]
- 166.Ribeiro JP, Chiappa GR, Neder JA, Frankenstein L. Respiratory muscle function and exercise intolerance in heart failure. Curr Heart Fail Rep 6: 95–101, 2009. [DOI] [PubMed] [Google Scholar]
- 167.Robbins M, Francis G, Pashkow FJ, Snader CE, Hoercher K, Young JB, Lauer MS. Ventilatory and heart rate responses to exercise: Better predictors of heart failure mortality than peak oxygen consumption. Circulation 100: 2411–2417, 1999. [DOI] [PubMed] [Google Scholar]
- 168.Rostagno C, Olivo G, Comeglio M, Boddi V, Banchelli M, Galanti G, Gensini GF. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: Comparison with other methods of functional evaluation. Eur J Heart Fail 5: 247–252, 2003. [DOI] [PubMed] [Google Scholar]
- 169.Roul G, Germain P, Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J 136: 449–457, 1998. [DOI] [PubMed] [Google Scholar]
- 170.Roul G, Moulichon ME, Bareiss P, Gries P, Koegler A, Sacrez J, Germain P, Mossard JM, Sacrez A. Prognostic factors of chronic heart failure in NYHA class II or III: Value of invasive exercise haemodynamic data. Eur Heart J 16: 1387–1398, 1995. [DOI] [PubMed] [Google Scholar]
- 171.Roussos C, Macklem PT. The respiratory muscles. N Engl J Med 307: 786–797, 1982. [DOI] [PubMed] [Google Scholar]
- 172.Sabbah HN, Shimoyama H, Sharov VG, Kono T, Gupta RC, Lesch M, Levine TB, Goldstein S. Effects of ACE inhibition and beta-blockade on skeletal muscle fiber types in dogs with moderate heart failure. Am J Physiol 270: H115–H120, 1996. [DOI] [PubMed] [Google Scholar]
- 173.Sacca L Heart failure as a multiple hormonal deficiency syndrome. Circ Heart Fail 2: 151–156, 2009. [DOI] [PubMed] [Google Scholar]
- 174.Schaufelberger M, Eriksson BO, Grimby G, Held P, Swedberg K. Skeletal muscle fiber composition and capillarization in patients with chronic heart failure: Relation to exercise capacity and central hemodynamics. J Card Fail 1: 267–272, 1995. [DOI] [PubMed] [Google Scholar]
- 175.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev 76: 371–423, 1996. [DOI] [PubMed] [Google Scholar]
- 176.Schieffer B, Wollert KC, Berchtold M, Saal K, Schieffer E, Hornig B, Riede UN, Drexler H. Development and prevention of skeletal muscle structural alterations after experimental myocardial infarction. Am J Physiol 269: H1507–H1513, 1995. [DOI] [PubMed] [Google Scholar]
- 177.Schulze PC, Fang J, Kassik KA, Gannon J, Cupesi M, MacGillivrayC, Lee RT, Rosenthal N. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Circ Res 97: 418–426, 2005. [DOI] [PubMed] [Google Scholar]
- 178.Schulze PC, Gielen S, Adams V, Linke A, Mobius-Winkler S, Erbs S, Kratzsch J, Hambrecht R, Schuler G. Muscular levels of proinflammatory cytokines correlate with a reduced expression of insulinlike growth factor-I in chronic heart failure. Basic Res Cardiol 98: 267–274, 2003. [DOI] [PubMed] [Google Scholar]
- 179.Simonini A, Long CS, Dudley GA, Yue P, McElhinny J, Massie BM. Heart failure in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circ Res 79: 128–136, 1996. [DOI] [PubMed] [Google Scholar]
- 180.Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111(16): 2056–2065, 2005. [DOI] [PubMed] [Google Scholar]
- 181.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin Il-induced skeletal muscle wasting. J Clin Invest 115: 451–458, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spruit MA, Eterman RM, Hellwig VA, Janssen PP, Wouters EF, Uszko Lencer NH. Effects of moderate-to-high intensity resistance training in patients with chronic heart failure. Heart 95: 1399–1408, 2009. [DOI] [PubMed] [Google Scholar]
- 183.Stubbing DG, Ramsdale EH, Killian KJ, Campbell EJ. Psychophysics of inspiratory muscle force. J Appl Physiol 54: 1216–1221, 1983. [DOI] [PubMed] [Google Scholar]
- 184.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 81: 518–527, 1990. [DOI] [PubMed] [Google Scholar]
- 185.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation 78: 506–515, 1988. [DOI] [PubMed] [Google Scholar]
- 186.Sun XG, Hansen JE, Beshai JF, Wasserman K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. J Am Coll Cardiol 55: 1814–1823, 2010. [DOI] [PubMed] [Google Scholar]
- 187.Szentesi P, Bekedam MA, van Beek-Harmsen BJ, van der Laarse WJ, Zaremba R, Boonstra A, Visser FC, Stienen GJ. Depression of force production and ATPase activity in different types of human skeletal muscle fibers from patients with chronic heart failure. J Appl Physiol 99: 2189–2195, 2005. [DOI] [PubMed] [Google Scholar]
- 188.Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. A m J Cardiol 55: 1037–1042, 1985. [DOI] [PubMed] [Google Scholar]
- 189.Tan LB. Cardiac pumping capability and prognosis in heart failure. Lancet 2: 1360–1363, 1986. [DOI] [PubMed] [Google Scholar]
- 190.Teerlink JR, Clozel M, Fischli W, Clozel JP. Temporal evolution of endothelial dysfunction in a rat model of chronic heart failure. J Am Coll Cardiol 22: 615–620, 1993. [DOI] [PubMed] [Google Scholar]
- 191.Testa M, Yeh M, Lee P, Fanelli R, Loperfido F, Berman JW, LeJemtel TH. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol 28: 964–971, 1996. [DOI] [PubMed] [Google Scholar]
- 192.Thomas GD, Zhang W, Victor RG. Impaired modulation ofsympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: Role of oxidative stress. Circ Res 88: 816–823, 2001. [DOI] [PubMed] [Google Scholar]
- 193.Thurlbeck WM. Diaphragm and body weight in emphysema. Thorax 33: 483–487, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation 95: 910–916, 1997. [DOI] [PubMed] [Google Scholar]
- 195.Todaka K, Wang J, Yi GH, Knecht M, Stennett R, Packer M, Burkhoff D. Impact of exercise training on ventricular properties in a canine model of congestive heart failure. Am J Physiol 272: H1382–H1390, 1997. [DOI] [PubMed] [Google Scholar]
- 196.Tristani FE, Hughes CV, Archibald DG, Sheldahl LM, Cohn JN, Fletcher R. Safety of graded symptom-limited exercise testing in patients with congestive heart failure. Circulation 76: VI54–VI58, 1987. [PubMed] [Google Scholar]
- 197.Troosters T, Gosselink R, Decramer M. Chronic obstructive pulmonary disease and chronic heart failure: Two muscle diseases? J Cardiopulm Rehabil 24: 137–145, 2004. [DOI] [PubMed] [Google Scholar]
- 198.van Hees HW, van der Heijden HF, Hafmans T, Ennen L, Heunks LM, Verheugt FW, Dekhuijzen PN. Impaired isotonic contractility and structural abnormalities in the diaphragm of congestive heart failure rats. Int J Cardiol 128: 326–335, 2008. [DOI] [PubMed] [Google Scholar]
- 199.van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, Verheugt FW, Brouwer RM, Dekhuijzen PN. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol 293: H819–H828, 2007. [DOI] [PubMed] [Google Scholar]
- 200.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154: 557–568, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ventura-Clapier R Exercise training, energy metabolism, and heart failure. Appl Physiol Nutr Metab 34: 336–339, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vescovo G, Dalla LL, Serafini F, Leprotti C, Facchin L, Volterrani M, Ceconi C, Ambrosio GB. Improved exercise tolerance after losartan and enalapril in heart failure: Correlation with changes in skeletal muscle myosin heavy chain composition. Circulation 98: 1742–1749, 1998. [DOI] [PubMed] [Google Scholar]
- 203.Wasserman K, Casaburi R. Dyspnea: Physiological and pathophysiological mechanisms. Annu Rev Med 39: 503–515, 1988. [DOI] [PubMed] [Google Scholar]
- 204.Weber KT, Janicki JS. Cardiopulmonary exercise testing: Physiologic principles and clinical applications. pp. 378, Philadelphia, USA: WB Saunders Company, 1986. [Google Scholar]
- 205.Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 22: 727–732, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wiener DH, Fink LI, Maris J, Jones RA, Chance B, Wilson JR. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: Role of reduced muscle blood flow. Circulation 73(6): 1127–36, 1986. [DOI] [PubMed] [Google Scholar]
- 207.Williams RS, McKinnis RA, Cobb FR, Higginbotham MB, Wallace AG, Coleman RE, Califf RM. Effects of physical conditioning on left ventricular ejection fraction in patients with coronary artery disease. Circulation 70: 69–75, 1984. [DOI] [PubMed] [Google Scholar]
- 208.Williams SG, Cooke GA, Wright DJ, Parsons WJ, Riley RL, Marshall P, Tan LB. Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. Eur HeartJ 22: 1496–1503, 2001. [DOI] [PubMed] [Google Scholar]
- 209.Wilson JR, Groves J, Rayos G. Circulatory status and response to cardiac rehabilitation in patients with heart failure. Circulation 94: 1567–1572, 1996. [DOI] [PubMed] [Google Scholar]
- 210.Wilson JR, Martin JL, Ferraro N. Impaired skeletal muscle nutritive flow during exercise in patients with congestive heart failure: Role of cardiac pump dysfunction as determined by the effect of dobutamine. Am J Cardiol 53: 1308–1315, 1984. [DOI] [PubMed] [Google Scholar]
- 211.Wilson JR, Rayos G, Yeoh TK, Gothard P. Dissociation between peak exercise oxygen consumption and hemodynamic dysfunction in potential heart transplant candidates. J Am Coll Cardiol 26: 429–435, 1995. [DOI] [PubMed] [Google Scholar]
- 212.Yndestad A, Ueland T, Oie E, Florholmen G, Halvorsen B, Attramadal H, Simonsen S, Froland SS, Gullestad L, Christensen G, Damas JK, Aukrust P. Elevated levels of activin A in heart failure: Potential role in myocardial remodeling. Circulation 109: 1379–1385, 2004. [DOI] [PubMed] [Google Scholar]
- 213.Zamora E, Simo R, Lupon J, Galan A, Urrutia A, Gonzalez B, Mas D, Valle V. Serum myostatin levels in chronic heart failure. Rev Esp Cardiol 63: 992–996, 2010. [DOI] [PubMed] [Google Scholar]
- 214.Zelis R, Delea CS, Coleman HN, Mason DT. Arterial sodium content in experimental congestive heart failure. Circulation 41: 213–216, 1970. [DOI] [PubMed] [Google Scholar]
- 215.Zelis R, Mason DT, Braunwald E. A comparison of the effects of vasodilator stimuli on peripheral resistance vessels in normal subjects and in patients with congestive heart failure. J Clin Invest 47: 960–970, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zelis R, Nellis SH, Longhurst J, Lee G, Mason DT. Abnormalities in the regional circulations accompanying congestive heart failure. Prog Cardiovasc Dis 18: 181–199, 1975. [DOI] [PubMed] [Google Scholar]
- 217.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science 296: 1486–1488, 2002. [DOI] [PubMed] [Google Scholar]