Abstract
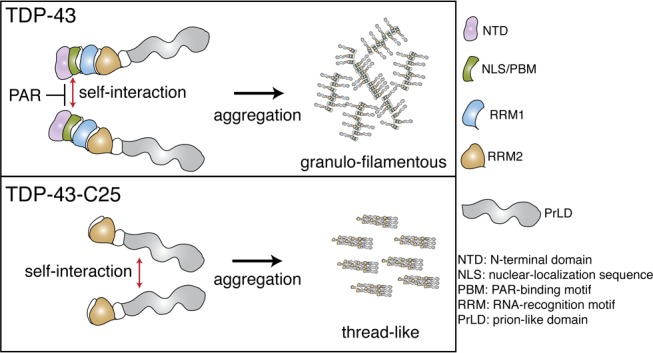
TAR DNA-binding protein of 43 kDa (TDP-43) forms granulo-filamentous aggregates in affected brain regions of >95% of patients with ALS and ∼50% of patients with frontotemporal degeneration (FTD). Furthermore, in disease, TDP-43 becomes N-terminally truncated resulting in protein deposits that are mainly composed of the C-terminal prion-like domain (PrLD). The PrLD is inherently aggregation-prone and is hypothesized to drive protein aggregation of TDP-43 in disease. Here, we establish that the N-terminal region of the protein is critical for rapid TDP-43 granulo-filamentous aggregation. We show that the biopolymer poly(ADP-ribose), or PAR, inhibits granulo-filamentous aggregation of TDP-43 by engaging PAR-binding motifs (PBMs) embedded in the TDP-43 nuclear-localization sequence. We demonstrate that progressive N-terminal truncation of TDP-43 can decelerate aggregation kinetics and promote formation of thread-like filaments. Thus, the N-terminal region and the PBMs of TDP-43 promote rapid granulo-filamentous aggregation and antagonize formation of thread-like fibrils. These findings illustrate the complexity of TDP-43 aggregation trajectories.
Amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) are two fatal neurodegenerative disorders characterized by the presence of insoluble aggregates of TAR DNA-binding protein of 43 kDa (TDP-43) in affected brain regions.1,2 To date, most of the disease-causing mutations in TDP-43 occur in the C-terminal prion-like domain (PrLD).3 PrLDs are intrinsically disordered regions that can switch from unfolded states to self-templating fibril forms such as the amyloid-like cross-β fibrils.3−7 The majority of TDP-43 aggregates in ALS/FTLD-U patients have the appearance of granular filaments, but a subset have amyloid-like qualities.8−11 Full-length TDP-43 forms granulo-filamentous aggregates in vitro that can transition into thread-like fibrils.4,12,13 This transition is promoted by certain disease-linked mutations in the PrLD, including Q331K.12 An emerging hypothesis is that the PrLD of TDP-43 may drive the protein aggregation observed in disease.12
PrLDs have also been implicated in liquid–liquid phase separation (LLPS), a process by which proteins condense into reversible liquid droplets.14−16 Of interest are the ALS-linked proteins hnRNPA1, FUS, and TDP-43 which undergo LLPS in vitro.4,16−20 We uncovered that the biopolymer poly(ADP-ribose) (PAR) potently promotes TDP-43 LLPS in vitro(20) and that PAR is elevated in ALS motor neuron nuclei.21 PAR is generated by poly(ADP-ribose) polymerases (PARPs),22 and inhibitors of various PARPs (PARP-1, PARP-2, PARP-5a, and PARP-5b) mitigate cytoplasmic aggregation of TDP-43 and TDP-43-associated toxicity to primary neurons and in Drosophila.20,21 These findings raised the possibility that PAR may directly regulate TDP-43 aggregation.
To determine if PAR could impact TDP-43 aggregation, we purified full-length human TDP-43 with a His6-SUMO solubility tag23 (Figures S1A and S2A). At physiological concentrations of TDP-43 protein,24 cleavage of the His6-SUMO tag with ubiquitin-like specific protease (Ulp1) induced TDP-43 aggregation over a 200 min period (Figure 1A). The addition of PAR to His6-SUMO-TDP-43-WT significantly reduced TDP-43-WT aggregation (Figure 1A, Figure S2B,C), while mono(ADP-ribose) had no effect (Figure 1B). Our previous studies established that LLPS of TDP-43 can occur in the presence of a crowding reagent and is promoted by PAR.20 We examined TDP-43-WT by differential interference contrast (DIC) microscopy; before cleavage with and without PAR, the protein remained diffuse and did not form any visible micron-sized aggregates (Figure S3A). However, 30 min after Ulp-1 cleavage, we observed the formation of spherical droplets that appeared to coalesce into solid structures after a further 30 min (Figure S3B). Our present data indicate that under conditions that lack a crowding reagent, PAR reduces filamentous aggregation of TDP-43.
Figure 1.
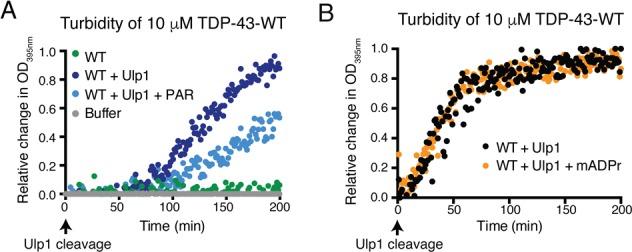
PAR inhibits TDP-43 aggregation. (A) Ulp1-cleavage of His6-SUMO-TDP-43-WT increased optical density (OD). Co-incubation with 6 μM PAR reduced the optical density of TDP-43-WT. (B) Mono(ADP-ribose) (mADPr, 6 μM) had no effect on the optical density of TDP-43-WT.
The nuclear-localization sequence (NLS) of TDP-43 is a region of intrinsic disorder25 (Figure S1B) and is critical for physically binding to PAR and as well as LLPS of TDP-43 in vitro.20 In contrast to cleaved His6-SUMO-TDP-43-WT, cleaved His6-SUMO-TDP-43-ΔPAR-binding motif (PBM) (Figure S4A) exhibited decelerated aggregation kinetics (Figure 2A) and took over 18 h to aggregate (Figure 2B). The addition of PAR had no effect on the aggregation of TDP-43-ΔPBM (Figure 2B and Figure S4B). Examination of TDP-43-ΔPBM before cleavage revealed no preformed micron-sized aggregates (Figure S3A). Thus, the N-terminal region of TDP-43, and specifically the PBMs, enables rapid aggregation of TDP-43, and PAR engages PBMs within the NLS to reduce TDP-43 aggregation.
Figure 2.
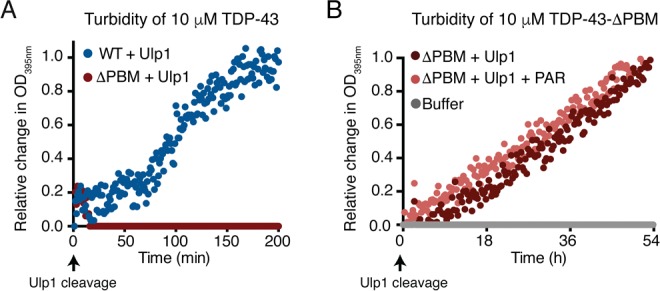
PAR-binding motifs enable rapid TDP-43 aggregation. (A) Compared to TDP-43-WT, the TDP-43-ΔPAR-binding motif (PBM) did not aggregate in the same time frame. (B) TDP-43-ΔPBM aggregated over 54 h. PAR (6 μM) had no effect on the optical density of TDP-43-ΔPBM.
Transmission electron microscopy (TEM) revealed that cleavage of the His6-SUMO tag from both TDP-43-WT and TDP-43-ΔPBM led to the formation of granulo-filamentous aggregates (Figure 3A), consistent with previous TEM studies and of TDP-43 aggregates in human tissue.8,10,12 PAR did not drastically alter the structure of the TDP-43-WT or TDP-43-ΔPBM aggregates (Figure 3A). However, PAR significantly reduced the overall size of the TDP-43-WT aggregates, while having no effect on the size of the TDP-43-ΔPBM aggregates (Figure 3B). Indeed, PAR promoted retention of TDP-43-WT in the supernatant fraction after low-speed centrifugation (Figure 3C and Figure S5). Thus, we propose that PAR reduces granulo-filamentous aggregation of TDP-43 via an interaction with PBMs embedded within the NLS.
Figure 3.
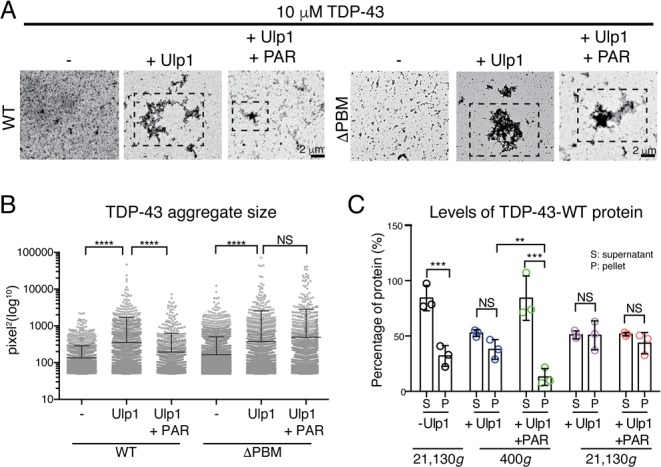
PAR reduces TDP-43 aggregation. (A) Ulp1 cleavage of HIS6-SUMO-TDP-43-WT and HIS6-SUMO-TDP-43-ΔPBM led to granulo-filamentous aggregation (hatched boxes). PAR (6 μM) reduced aggregate size of TDP-43-WT and had no effect on TDP-43-ΔPBM (hatched boxes). (B) Quantification of aggregate size. Mean (±SD), one-way ANOVA (P < 0.0001), and Kruskal–Wallis test. (C) PAR (6 μM) reduced the amount of TDP-43-WT in the pellet fraction at 400g (Figure S5). Mean (±SD), two-way ANOVA, and Tukey’s test.
In ALS and FTLD-U, splicing defects and proteolytic cleavage can elicit formation of TDP-43 C-terminal fragments that contain the PrLD.26−28 As the C-terminal fragments of TDP-43 either contain a partial PAR-binding region (TDP-43-C35) or lack the PAR-binding region (TDP-43-C25) (Figure S1A), we examined the aggregation kinetics of these two C-terminal fragments. Strikingly, the ability of TDP-43-C35 and TDP-43-C25 to form turbid aggregates was, like TDP-43-ΔPBM, reduced compared to TDP-43-WT (Figure 4A). Examination by TEM revealed that TDP-43-C35 formed granulo-filamentous aggregates, whereas TDP-43-C25 formed granulo-filamentous aggregates and thread-like fibrils (Figure 4B). The TDP-43-C25 aggregates were unreactive to the amyloid diagnostic dye Thioflavin T (Figure S6). Combined, these data reveal that the N-terminal portion of TDP-43 contributes to granulo-filamentous aggregation and antagonizes the transition into thread-like oligomers.
Figure 4.
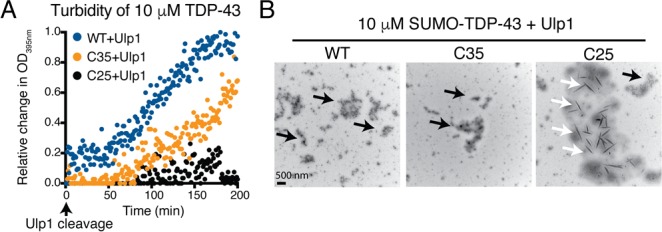
C-terminal fragments of TDP-43 have altered aggregation properties. (A) The increase in optical density of TDP-43-C35 and TDP-43-C25 was reduced compared to TDP-43-WT. (B) TDP-43-WT, TDP-43-C35, and TDP-43-C25 formed granulo-filamentous protein aggregates (black arrows). TDP-43-C25 also formed thread-like aggregates (white arrows).
Here, we show that N-terminal portions of TDP-43 contribute to granulo-filamentous aggregation. Our data indicate that PAR interacts with PBMs embedded within the NLS of TDP-43 to reduce granulo-filamentous aggregation. Defining the mechanism by which PAR binding reduces TDP-43 aggregation will require further study. Regions within the N-terminal domain of TDP-43 regulate self-oligomerization.25,29−32Thus, PAR-binding to the NLS adjacent to the N-terminal domain may physically block interactions that contribute toward aggregation. In disease, TDP-43 aggregates appear to be predominantly granulo-filamentous. Thus, agents that antagonize contributions from the N-terminal region of TDP-43 could have therapeutic utility. However, as oligomerization is essential for TDP-43 function,25,29−32 agents that prevent this functional oligomerization could be detrimental. Understanding under what circumstances functional versus toxic TDP-43 assemblies form,33 how they differ, and how they are resolved will help develop therapeutic strategies to selectively target toxic assemblies.
Acknowledgments
We thank members of the Shorter and Bonini Laboratories for insightful comments. We thank Kelvin Luk for providing the α-synuclein fibrils used in these studies.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem.8b00910.
TDP-43 protein domains, protein purification analysis, turbidity assay, analysis of the sedimentation assay, and detailed materials and methods (PDF)
Author Contributions
§ L.M., E.G., and L.G. contributed equally. All authors have given approval to the final version of the manuscript.
This work was funded by the Ellison Medical Foundation, American Federation for Aging Research, Alzheimer’s Association (to L.G.); Life Extension Foundation, ALS Association, Department of Biochemistry and Biophysics Pilot Grant, Packard Center for ALS Research at Johns Hopkins, NIH R01GM099836, R21NS090205 (J.S.); Target ALS (J.S. and N.M.B.); and the Glenn Foundation, NIH 5R01NS073660, R35NS097275 (N.M.B.).
The authors declare no competing financial interest.
Supplementary Material
References
- Lee E. B.; Lee V. M.; Trojanowski J. Q. (2012) Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 13, 38–50. 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L.; Shorter J. (2017) Biology and Pathobiology of TDP-43 and Emergent Therapeutic Strategies. Cold Spring Harbor Perspect. Med. 7, a024554. 10.1101/cshperspect.a024554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. F.; Shorter J. (2017) RNA-binding proteins with prion-like domains in health and disease. Biochem. J. 474, 1417–1438. 10.1042/BCJ20160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L.; Kim H. J.; Wang H.; Monaghan J.; Freyermuth F.; Sung J. C.; O’Donovan K.; Fare C. M.; Diaz Z.; Singh N.; Zhang Z. C.; Coughlin M.; Sweeny E. A.; DeSantis M. E.; Jackrel M. E.; Rodell C. B.; Burdick J. A.; King O. D.; Gitler A. D.; Lagier-Tourenne C.; Pandey U. B.; Chook Y. M.; Taylor J. P.; Shorter J. (2018) Nuclear-Import Receptors Reverse Aberrant Phase Transitions of RNA-Binding Proteins with Prion-like Domains. Cell 173, 677–692. 10.1016/j.cell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M.; Han T. W.; Xie S.; Shi K.; Du X.; Wu L. C.; Mirzaei H.; Goldsmith E. J.; Longgood J.; Pei J.; Grishin N. V.; Frantz D. E.; Schneider J. W.; Chen S.; Li L.; Sawaya M. R.; Eisenberg D.; Tycko R.; McKnight S. L. (2012) Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767. 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. T.; Kato M.; Lin Y.; Thurber K. R.; Hung I.; McKnight S. L.; Tycko R. (2017) Structure of FUS Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains. Cell 171, 615–627. 10.1016/j.cell.2017.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S.; Halfmann R.; King O.; Kapila A.; Lindquist S. (2009) A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 137, 146–158. 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. L.; Geser F.; Stieber A.; Umoh M.; Kwong L. K.; Van Deerlin V. M.; Lee V. M.; Trojanowski J. Q. (2013) TDP-43 skeins show properties of amyloid in a subset of ALS cases. Acta Neuropathol. 125, 121–131. 10.1007/s00401-012-1055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigio E. H.; Wu J. Y.; Deng H. X.; Bit-Ivan E. N.; Mao Q.; Ganti R.; Peterson M.; Siddique N.; Geula C.; Siddique T.; Mesulam M. (2013) Inclusions in frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) and amyotrophic lateral sclerosis (ALS), but not FTLD with FUS proteinopathy (FTLD-FUS), have properties of amyloid. Acta Neuropathol. 125, 463–465. 10.1007/s00401-013-1089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe J. R.; Tang H.; Atherton J.; Cairns N. J. (2008) Fine structural analysis of the neuronal inclusions of frontotemporal lobar degeneration with TDP-43 proteinopathy. J. Neural Transm (Vienna) 115, 1661–1671. 10.1007/s00702-008-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y. S.; Tsai K. J.; Chang Y. J.; Kao P.; Woods R.; Kuo P. H.; Wu C. C.; Liao J. Y.; Chou S. C.; Lin V.; Jin L. W.; Yuan H. S.; Cheng I. H.; Tu P. H.; Chen Y. R. (2014) Full-length TDP-43 forms toxic amyloid oligomers that are present in frontotemporal lobar dementia-TDP patients. Nat. Commun. 5, 4824. 10.1038/ncomms5824. [DOI] [PubMed] [Google Scholar]
- Johnson B. S.; Snead D.; Lee J. J.; McCaffery J. M.; Shorter J.; Gitler A. D. (2009) TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J. Biol. Chem. 284, 20329–20339. 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackrel M. E.; DeSantis M. E.; Martinez B. A.; Castellano L. M.; Stewart R. M.; Caldwell K. A.; Caldwell G. A.; Shorter J. (2014) Potentiated Hsp104 variants antagonize diverse proteotoxic misfolding events. Cell 156, 170–182. 10.1016/j.cell.2013.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A.; Weber C. A.; Julicher F. (2014) Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58. 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Brangwynne C. P. (2013) Phase transitions and size scaling of membrane-less organelles. J. Cell Biol. 203, 875–881. 10.1083/jcb.201308087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E.; Shorter J. (2018) The molecular language of membraneless organelles. J. Biol. Chem. 1. 10.1074/jbc.TM118.001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.; Brangwynne C. P. (2017) Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382. 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- Wang A.; Conicella A. E.; Schmidt H. B.; Martin E. W.; Rhoads S. N.; Reeb A. N.; Nourse A.; Ramirez Montero D.; Ryan V. H.; Rohatgi R.; Shewmaker F.; Naik M. T.; Mittag T.; Ayala Y. M.; Fawzi N. L. (2018) A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing. EMBO J. 37, e97452 10.15252/embj.201797452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conicella A. E.; Zerze G. H.; Mittal J.; Fawzi N. L. (2016) ALS Mutations Disrupt Phase Separation Mediated by alpha-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain. Structure 24, 1537–1549. 10.1016/j.str.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk L.; Gomes E.; Guo L.; Mojsilovic-Petrovic J.; Tran V.; Kalb R. G.; Shorter J.; Bonini N. M. (2018) Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Mol. Cell 71, 703–717. 10.1016/j.molcel.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk L.; Mojsilovic-Petrovic J.; Van Deerlin V. M.; Shorter J.; Kalb R. G.; Lee V. M.; Trojanowski J. Q.; Lee E. B.; Bonini N. M. (2018) Nuclear poly(ADP-ribose) activity is a therapeutic target in amyotrophic lateral sclerosis. Acta neuropathologica communications 6, 84–95. 10.1186/s40478-018-0586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson B. A.; Kraus W. L. (2012) New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 13, 411–424. 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- Molliex A.; Temirov J.; Lee J.; Coughlin M.; Kanagaraj A. P.; Kim H. J.; Mittag T.; Taylor J. P. (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133. 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S. C.; Albuquerque C. P.; Han J. S.; Lagier-Tourenne C.; Tokunaga S.; Zhou H.; Cleveland D. W. (2010) ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc. Natl. Acad. Sci. U. S. A. 107, 13318–13323. 10.1073/pnas.1008227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. K.; Wu T. H.; Wu C. Y.; Chiang M. H.; Toh E. K.; Hsu Y. C.; Lin K. F.; Liao Y. H.; Huang T. H.; Huang J. J. (2012) The N-terminus of TDP-43 promotes its oligomerization and enhances DNA binding affinity. Biochem. Biophys. Res. Commun. 425, 219–224. 10.1016/j.bbrc.2012.07.071. [DOI] [PubMed] [Google Scholar]
- Tsuji H.; Arai T.; Kametani F.; Nonaka T.; Yamashita M.; Suzukake M.; Hosokawa M.; Yoshida M.; Hatsuta H.; Takao M.; Saito Y.; Murayama S.; Akiyama H.; Hasegawa M.; Mann D. M.; Tamaoka A. (2012) Molecular analysis and biochemical classification of TDP-43 proteinopathy. Brain 135, 3380–3391. 10.1093/brain/aws230. [DOI] [PubMed] [Google Scholar]
- Xiao S.; Sanelli T.; Chiang H.; Sun Y.; Chakrabartty A.; Keith J.; Rogaeva E.; Zinman L.; Robertson J. (2015) Low molecular weight species of TDP-43 generated by abnormal splicing form inclusions in amyotrophic lateral sclerosis and result in motor neuron death. Acta Neuropathol. 130, 49–61. 10.1007/s00401-015-1412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani F.; Obi T.; Shishido T.; Akatsu H.; Murayama S.; Saito Y.; Yoshida M.; Hasegawa M. (2016) Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains. Sci. Rep. 6, 23281. 10.1038/srep23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afroz T.; Hock E. M.; Ernst P.; Foglieni C.; Jambeau M.; Gilhespy L. A. B.; Laferriere F.; Maniecka Z.; Pluckthun A.; Mittl P.; Paganetti P.; Allain F. H. T.; Polymenidou M. (2017) Functional and dynamic polymerization of the ALS-linked protein TDP-43 antagonizes its pathologic aggregation. Nat. Commun. 8, 45. 10.1038/s41467-017-00062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L. L.; Xue W.; Hong J. Y.; Zhang J. T.; Li M. J.; Yu S. N.; He J. H.; Hu H. Y. (2017) The N-terminal dimerization is required for TDP-43 splicing activity. Sci. Rep. 7, 6196. 10.1038/s41598-017-06263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano V.; Quadri Z.; Baralle F. E.; Buratti E. (2015) The structural integrity of TDP-43 N-terminus is required for efficient aggregate entrapment and consequent loss of protein function. Prion 9, 1–9. 10.1080/19336896.2015.1011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. J.; Caulfield T.; Xu Y. F.; Gendron T. F.; Hubbard J.; Stetler C.; Sasaguri H.; Whitelaw E. C.; Cai S.; Lee W. C.; Petrucelli L. (2013) The dual functions of the extreme N-terminus of TDP-43 in regulating its biological activity and inclusion formation. Hum. Mol. Genet. 22, 3112–3122. 10.1093/hmg/ddt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler T. O.; Wheeler J. R.; Nguyen E. D.; Hughes M. P.; Britson K. A.; Lester E.; Rao B.; Betta N. D.; Whitney O. N.; Ewachiw T. E.; Gomes E.; Shorter J.; Lloyd T. E.; Eisenberg D. S.; Taylor J. P.; Johnson A. M.; Olwin B. B.; Parker R. (2018) TDP-43 and RNA form amyloid-like myo-granules in regenerating muscle. Nature 563, 508. 10.1038/s41586-018-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


