Abstract
Ligand binding can induce shifts in protein conformation. In the case of tubulin, these drug-induced confirmational changes can prevent or stabilize microtubule polymerization. 5′,5′-Dithiobis(2-nitrobenzoate) (DTNB) reacts with free and accessible sulfhydryls and stoichiometrically produces a detectable product, which allows an exact measurement of reacted thiols. Since binding of small ligands may alter conformational dynamics, it may also affect the reactivity of thiols on tubulin. Differences in DTNB reactivity with thiols upon ligand binding can therefore be used to deduce binding characteristics. We will describe two methods that use tubulin cysteine reactivity with DTNB in the presence of drug to define ligand-binding characteristics.
I. Introduction and Rationale
Tubulin is a sulfhydryl-rich heterodimer with 20 cysteine residues distributed across both subunits in mammalian brain tubulin. While it is unclear exactly how tubulin cysteine residues directly contribute to microtubule assembly and function, it has been shown that the oxidation of cysteines can poison polymerization of tubulin to microtubules and that direct reaction with cysteines contributes to the toxicity of numerous antimitotic compounds (reviewed in Sackett, 2008). All 20 cysteine residues are reduced (-SH) and are reactive with sulfhydryl-directed reagents. Reactivity is not solely dependent on tubulin surface exposure as some exposed cysteines are not particularly reactive and some more buried ones are quite reactive (Britto et al., 2005). This presumably reflects the dynamics of the dimer, such that partial unfolding, or breathing, of the dimer transiently opens access to nonsurface-exposed cysteines.
Tubulin binds a large number of structurally varied small molecules. Each has an effect upon the tubulin dimer: some regulate (guanosine triphosphate/guanosine diphosphate) or inhibit polymerization (colchicine, vinblastine, and many others), while others hyperstabilize the polymer, inhibiting depolymerization (taxol, epothilones, and others). Binding of small molecules to tubulin is often assigned to one of four binding sites (colchicine, vinblastine, guanine nucleotide (GXP), taxol), though new sites have been recently described, such as for peloruside (Huzil et al., 2008), and others may well exist. In principle, the binding of any ligand may be measured with a radioactive label, but this is often not available. Alternatively, the binding of some ligands may be measured by changes in absorbance or fluorescence of the drug, like colchicine (Bane et al., 2007). Other small compounds may not have a useful optical signature of their own, but may induce a change in the fluorescence of tubulin upon binding, which may be used to quantitate binding.
Many ligands cause a change in the dynamics of the protein to which they are bound and tubulin ligands are no exception. Hence, these changes in dynamics can be used to detect the binding of a small molecule to the protein. It has been shown that binding of many ligands changes the reactivity of particular tubulin cysteines, using mass spectrometry to localize the residues so altered (Kim et al., 2004). Similarly, sulfhydryl reagents iodoacetamide and N,N′-ethylenebis-(iodoacetamide) have demonstrated an ability to detect conformational effects of numerous ligands upon binding to tubulin, particularly colchicine and podophyllotoxin (Luduena and Roach, 1991). Ligand-induced lowering of cysteine reactivity has been reported with a number of tubulin-binding drugs, using optical measures of sulfhydryl reactivity (Roychowdhury et al., 2000). Colchicine has been shown to modify the tubulin structure and can shield cysteine residues from 5′,5′-dithiobis(2-nitrobenzoate) (DTNB) likely through a conformational change (Passarella et al., 2008). Cysteine reactivity has long been known to be lower with polymerized tubulin than with soluble dimers (only four cysteine residues are accessible in taxol-stabilized microtubules MT), presumably reflecting the reduction in dynamics imposed by incorporation into the microtubules lattice.
We will demonstrate how ligand binding can be detected and measured through tubulin reactivity with the sulfhydryl reagent DTNB. DTNB is an aryl disulfide that easily reacts with free thiols in a thiol-disulfide exchange at neutral pH. In the presence of a native or unfolded protein with accessible thiols, the reaction yields a mixed protein disulfide and a 2-nitro-5-thiobenzoate (TNB) dianion. The latter has a high absorbance at 412nm (ε = 14.15 mM−1 cm−1), which allows a direct measurement of free thiols on the protein due to its stoichiometric and nonreversible formation (Eyer et al., 2003). With the addition of denaturants like guanidine hydrochloride (GuHCl) or urea, a rapid measurement of the total thiol content of a protein can be established. Due to the natural breathing dynamics of the tubulin dimer, all cysteines become solvent exposed and hence, a reaction of tubulin with excess DTNB will eventually (tens of minutes) produce enough TNB to indicate total thiol content. A small number of residues react very rapidly (in the first minute or two) and the rest react in the following tens of minutes (Britto et al., 2002). It is these cysteines whose reactivity is altered by ligands. Kinetic studies examining the reaction of the slower-reacting cysteine residues following ligand binding showed a reduction in DTNB reactivity dependent on the nature of the drug and also showed that reactivity with these slow-reacting sulfhydryls depends on the “breathing” of tubulin as demonstrated by reduced DTNB reactivity in the presence of glycerol (Roychowdhury et al., 2000). Because of this, thiol reactivity may be used to characterize the binding of any drug or ligand that affects the breathing dynamics of tubulin.
To demonstrate the potential of DTNB reactivity in measuring binding kinetics, we chose the aryltetralin ligand podophyllotoxin (reviewed in Desbène and Giorgi-Renault, 2002; Sackett, 1993). Podophyllotoxin binds at the colchicine site near the αβ interface and prevents microtubule assembly, leading to cell cycle arrest in prometaphase. Podophyllotoxin binds to tubulin with a reported Kd of ~0.6 μM and has been shown to bind faster and more reversibly than colchicine (Cortese et al., 1977). Since podophyllotoxin binding is a well-characterized and relevant tubulin-ligand interaction, it is a useful ligand for a demonstration of the use of tubulin thiol reactivity with DTNB in the presence of ligand through two versions of the reaction method. These versions only differ in the choice of a cuvet or microplate for reactions. Two methods of analyzing the kinetic data of the DTNB reaction are described. Both analysis methods yield similar EC50 (half-maximal effective concentration) for podophyllotoxin.
Binding characteristics are inferred through reductions in intermolecular reaction rates that are coupled to ligand-induced reduction in intramolecular (tubulin) dynamics. Due to the indirect nature of the data (i.e., nondirect coupling between ligand binding and alteration of the measureable variable), we characterize the binding by EC50, rather than by attempting to deduce an actual Kd for ligand binding. We note that EC50 is higher than Kd, and variably so. These considerations are further examined in Section III. Nonetheless, the DTNB method provides a simple, versatile method to detect and characterize the binding of many ligands to tubulin.
II. Methods
We describe two versions of the method. One uses cuvets and the other uses 96-well microplates. The cuvet assay has the advantage of a known and unvarying optical path length, but typically analyzes one sample at a time. The microplate assay has the advantage of processing multiple samples simultaneously, but has a varying optical path length due to the sample meniscus and the vertical light path.
We have used both 50 and 250 μl cuvets in the assay. The 50 μl cuvets have the obvious advantage of requiring less material, but at the cost of loss in sensitivity due to a 3 mm versus a 10 mm path length in the 250 μl cuvet. The larger volume required in the 250 μl cuvet is almost offset by the ability to use lower concentrations of tubulin (due to the longer path length). In the microplate format, we have found it useful to use plates with half-area wells, which afford a path length of ~3 mm for a 50 μl sample.
A. Materials
Solutions:
PM Buffer: 0.1 M Pipes supplemented with 0.5 mM MgCl2, pH 7.0
DTNB in 0.1 M Pipes buffer with Mg+
5 M GuHCl in diH2O
100 μM (10 g/l) purified brain tubulin in 0.1 M Pipes/Mg+, pH 7.0
Drug compound in dimethyl sulfoxide (DMSO)
Materials:
Quartz cuvets (3 or 10 mm path length, 50 or 250 ul). We obtain these from Hellma (Plainview NY), but other sources are readily available.
96-well half-area Costar microplates (Lowell, MA)
Microplate reader set to 412 nm, preferably capable of kinetic mode.
Multichannel pipet for multiple reactions.
B. Cuvet Method—Protocol
The volumes given here are for a 250 μl cuvet and should be scaled appropriately for other sized cuvets. It is assumed that the stock solution of tubulin is 10 g/l and that test drugs are in DMSO solution. All reactions contain tubulin at 2 μM (0.2 g/l), which corresponds to 40 μM cysteine sulfhydryls, and hence a potential final A412 of ~0.55 after reaction with DTNB.
Set up the spectrophotometer. All measurements are made at room temperature and are read against a blank of PM buffer. Allow the PM to warm to room temperature before beginning.
Prepare the no-drug control. Dilute 5 μl of tubulin (10 g/l) with 245 μl of room temperature PM buffer. Keep the tubulin stock on ice.
Record A280 versus a buffer blank to confirm tubulin concentration (ε280 = 0.116 μM−1 cm−1) (Andreu, 2007). We typically scan from 260 to 320 nm for this step. This step is an optional confirmation that may be performed on each sample, on the no-drug control only, or may be skipped entirely, although if a measure of total sulfhydryl per tubulin is required, this step should be included. Note that if this step is included with samples that contain drugs or DMSO, then the A280 should be recorded before adding the drug and/or DMSO (see Section IID, Note 1).
Set the spectrophotometer to kinetic mode and begin recording at 412 nm versus the PM buffer blank. After recording the baseline for a short time, add DTNB to 1 mM by adding 1 μl of 25 mM DTNB in PM. Mix by pipetting up and down quickly but carefully to avoid bubbles.
Continue recording A412 versus time for 30 min. Often we run only the no-drug control for 30 min. The maximum velocity of the reaction, the period following the initial rapid reaction, which is the parameter needed (see Section IIE), is usually measurable in a run of ~10 min, so multiple samples containing different drug concentrations may be run for these shorter times.
Add 125 μl of 5M guanidine hydrochloride to unfold the tubulin and obtain a measure of total cysteine. Mix by pipetting up and down. Measure A412 after 2 min. Note that the additional volume of the GuHCl will require correction for dilution in the calculation of total sulfhydryl content (see D, Note 1).
During the recording of the first sample (step 5 above), prepare the next sample. We typically allow ~5 min for tubulin and drug/DMSO mixture to equilibrate before adding the DTNB. If you are reading the A280 on each sample, do so before adding the drug/DMSO. This and all subsequent drug-containing samples are composed of tubulin 5 μl of 10 g/l stock, PM buffer 235 μl, and drug/DMSO-combined additions to equal 10 μl (added last) (see D, Note 2).
In addition to running the no-drug control and all of the desired drug concentrations, it is advisable to run one sample that does not contain tubulin, i.e., a buffer/DMSO control. This will assure that there are no components in the solvents that cause cleavage of DTNB.
Although this protocol only measures one sample at a time, the protocol is quite easy to incorporate into other daily work, since most of the time is taken by step 5. It is not difficult to run 20 samples in a day and obtain high-quality recordings with good control coverage.
C. Microplate Method—Protocol
With a microplate reader, multiple reactions can be monitored at once, easily examining a gradient of ligand concentrations within 40 min. As with the cuvet method, we assume here that stock solutions of tubulin are 10 g/l and drug compounds are dissolved in DMSO. All reactions will contain 2 μM (0.2 g/l) of tubulin, equivalent to 40 μM of cysteine sulfhydryls. All reactions may be set up in the microplate (see Table I).
Table I.
Microplate Reaction Volumes
| Component | Stock | Volume added (μl) | Total volume (μl) | Final |
|---|---|---|---|---|
| Brain tubulin | 100 μM | 1 | 2 μM | |
| Pipes buffer | 0.1 M | 47 | 0.1 M | |
| Drug (in PM Buffer) | Varied | Up to 2 | 4% v/va | |
| DMSO | 100% | 2 minus drug volumea | 4% v/va | |
| Initial volume | – | – | 50b | – |
| DTNB | 3 mM | 5 | 0.3 mM | |
| Total reaction volume | – | – | 55c | – |
| GuHCl | 5 M | 25 | 80d | 1.6 M |
DMSO, dimethyl sulfoxide; DTNB, 5′,5′-dithiobis(2-nitrobenzoate); GuHCl, guanidine hydrochloride.
Total DMSO + ligand should be 4% of total volume. Add additional DMSO to bring total DMSO + ligand up to 2 μl if drug addition is below 2 μl.
Tubulin and drug/DMSO mixture should be allowed to equilibrate for 10 min at room temperature.
After DTNB addition, mix carefully and begin gathering A412 for 40 min.
GuHCl can be added after completing A412 step to ensure equal protein concentration (through reacted thiol content) across each reaction.
Set up the spectrophotometer to gather absorbance values at 412 nm in kinetic mode. Since measurements will be made at room temperature, all reaction buffers and working solutions should be warmed to room temperature. Tubulin stock solutions (10 g/l) should be kept on ice.
-
The absorbance versus time of a blank solution containing 0.3 mM DTNB with 4% v/v DMSO in PM buffer at 412 nm should be collected before proceeding. This blank solution can be run as a negative control to ensure DTNB reactivity is limited to thiols on tubulin, similar to step 8 in Section IIB. It is equally important to consistently maintain DMSO at 4% v/v across reactions so that accurate comparisons in reactivity can be made.
This zero value can also be used to correct absorbance values when determining cysteine concentrations after completing the DTNB reaction.
Create a working solution of tubulin in PM buffer that contains 47 μl of PM buffer plus 1 μl of stock tubulin solution (10 g/l) multiplied by the number of wells to be examined. For example, if you are performing eight DTNB reactions, prepare a 384 μl stock containing 376 μl of PM buffer and 8 μl of tubulin. As with all working solutions, it is preferable to prepare additional solution (for instance, if your experiment involves eight reactions, prepare enough working solution for nine reactions).
Prepare reactions in the microplate. Add 48 μl of the working solution of tubulin (which contains 1 μl of tubulin plus 47 μl of PM buffer) to each reaction well for a final tubulin concentration of 0.2 g/l (2 μM). See D, NOTE 3.
Add 2 μl of DMSO to the no-drug control well.
Add drug compounds to each reaction well. Since drug compounds are usually dissolved in DMSO, drug stock concentrations should be used that limit volume additions to 2 μl (4% v/v in a 50 μl reaction) to achieve final desired concentration of both drug and DMSO. If total drug addition in a reaction is less than 2 μl, DMSO should be added to bring total drug + DMSO volume to 2 μl. Mix carefully by pipetting up and down (see D, Note 4).
Allow the tubulin and drug/DMSO mixtures to equilibrate for 5 min at room temperature before adding DTNB. During this time, the spectrophotometer should be set up in kinetic mode to gather absorbance values at 412 nm every 30 s for 40 min.
Using a 3 mM stock DTNB solution, quickly add 5 μl to each reaction well and quickly mix through pipet aspiration being sure to avoid bubbles (see Section IID, Notes 4 and 5).
Quickly place the microplate into the microplate reader. Begin collecting A412 values at 30-s intervals for 40 min.
After the 40-min data collection, add 25 μl of 5 M GuHCl to each reaction well for a final concentration of 1.6 M. Mix well through pipet aspiration. After 2 min, a final absorbance reading at 412 nm should be gathered to ensure total thiol content across all reactions was equal (see D, Note 3).
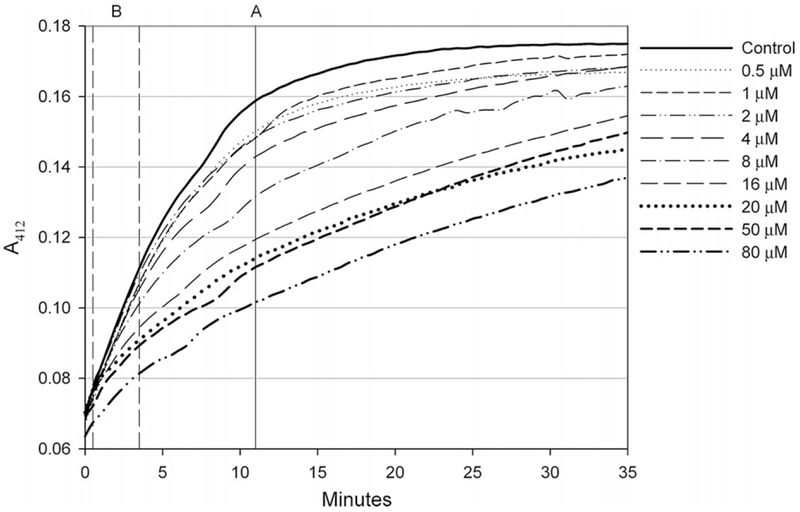
Plot the data A412 versus time. An example with podophyllotoxin can be seen in Fig. 1.
Fig. 1.
Raw absorbance values of microplate reactions collected at 412nm over 35 min. Each reaction contained 2 μM rat brain tubulin preincubated with increasing concentrations of podophyllotoxin and was initiated upon dithiobis(2-nitrobenzoate) addition.
D. Notes
In order to measure the total number of sulfhydryls per tubulin dimer, it is essential that a measure be made of the exact tubulin concentration in the solution in the cuvet. Small differences in concentration make large differences in sulfhydryl due to the 20 sulfhydryls per tubulin ratio.
It is not necessary to make each cuvet solution separately. One can make a working solution of tubulin containing 5 μl of tubulin stock (10 g/l) and 235 μl of PM buffer per reaction anticipated. Note that this solution once made should be kept on ice and used within a few hours, with aliquots removed when needed, and with a no-drug control at the beginning and end. Furthermore, each aliquot of the solution should be allowed to warm to room temperature in the cuvet before adding the drug/DMSO and DTNB.
Due to limitations of the microplate format (undefined path length due to meniscus and vertical light path, and the high UV absorbance of plastic microplates), a measurement to confirm protein concentration from A280 cannot be reliably gathered as described previously in step 3 of the cuvet method.
Avoid bubbles. This is more important in the microplate method than the cuvet due to the vertical path length used in microplate readers.
If performing multiple reactions, a multichannel pipet should be used in order to begin all reactions simultaneously. Additionally, when adding DTNB reagent through a multichannel pipet, it is important to avoid bubbles while ensuring proper mixing of the reagent into the reaction volume. To do this while aspirating 5 μL up and down with the multichannel pipet, gently stir each reaction volume with the pipet tips.
E. Data Analysis
Absorbance values at 412 nm obtained during a DTNB reaction from either method (such as the microplate absorbance values shown in Fig. 1) can be evaluated in several ways. Typically, absorbance values are first corrected using baseline values (A412 of blank solutions of PM buffer or PM with 4% v/v DMSO). These corrected values can be used to directly calculate reacted thiol content using the extinction coefficient (14.15 mM−1 cm−1). It is also important to note that the concentration of tubulin thiols reacted with DTNB will be 20× the total concentration of tubulin since there are 20 cysteine residues across both monomers. While it is possible to directly measure and confirm the concentration of tubulin in the cuvet method by the absorbance at 280 nm, such a measurement is not possible using the microplate method due to absorbance limitations of the microplate. However, the thiol content and thus the total protein concentration in each reaction can be indirectly verified from the absorbance value obtained after GuHCl-induced protein unfolding.
Evaluation of data from either the cuvet or the microplate method follows the same path. First, the corrected absorbance values at 412 nm are plotted versus time. Then either (1) a specific time point is found where the reaction is maximally different between control and highest ligand or (2) the region is found in which the slope of the reaction is maximally different between control and highest ligand. In either case, the difference from control is calculated for each reaction and the resulting set of differences plotted versus the ligand concentration. Fitting this data to a binding model yields the EC50.
-
Absorbance values at a specific time point
When analyzing absorbance values at a specific time point, you may use either corrected absorbance values or reacted thiol concentration values. The difference between these two parameters will not affect curve-fitting. When plotted against drug/ligand concentration, these values can reveal if the presence of drug or ligand has altered thiol reactivity at a given time point. Choosing an adequate time point for this analysis is important since most DTNB reactions will eventually go to completion regardless of ligand concentration. Raw absorbance values should be examined to find the time point where the absorbance of the sample containing the highest drug concentration is most different from the control (no ligand) absorbance value. Typically, this will occur within the first 10–15 min.: (for example, “Line A” on Fig. 1). Once a point has been selected, the absorbance values of each drug/ligand reaction should be recorded. Each absorbance value is then subtracted from the control value, producing a value that is the difference in absorbance or thiol concentration from the control. These values should increase as ligand concentration is increased if DTNB reactivity has been affected by the addition of ligand. These difference values are then divided by the control absorbance value obtained from the reaction containing no ligand:(1) By plotting these absorbance differences versus the drug concentrations and fitting the data to a binding model using appropriate data analysis software, EC50 values for the ligand being tested can be determined. See below for a demonstration with podophyllotoxin.
-
Kinetic values obtained over a range of time points
As an alternative to absorbance values at a single time point, reaction slopes may be used to measure the effect of ligand binding upon thiol reactivity. By dividing the difference in absorbance values at two time points by the time difference of those two time points, a reaction rate (slope) can be determined. As was done with the raw absorbance values, it is important to choose a range that demonstrates the greatest difference in slope between the control reaction (containing no ligand) and the reaction with the highest ligand concentration. The time range that supplies the largest difference in slope is usually within the first 5–10 min of a DTNB reaction. Previously, we discussed the existence of both fast-reacting and slow-reacting cysteines in tubulin, the latter being more affected by ligand binding. It is important to be aware of this initial jump in reactivity when determining a reactivity slope range used for further analysis and comparison even though some spectrophotometer software may allow a fast determination of the reaction slope. Choosing a range within the first minute may reduce or eliminate differences in reactivity caused by the presence of ligand due to those fast-reacting residues. For our evaluation, we used the absorbance change of each reaction over the 3-min period between 30 and 210 s after the first reading. The reason we allowed this range was the inherent delay caused by using our plate-reading spectrophotometer, typically around 30 s after DTNB addition until the spectrophotometer gathers the first absorbance reading. By noting the time typically required for steps 8 and 9, you can avoid incorporating the fastreacting cysteines into the comparison range. (In Fig. 1, you can see a region just before our designated comparison range (B) where there is little difference in reactivity, likely due to the fast-reacting cysteines.) The need for using a multichannel pipet for DTNB addition becomes apparent since it is necessary to use identical time points in order to make a clear comparison of the kinetic data.
The slope of a DTNB reaction with tubulin pretreated with ligand is compared to the slope obtained from a control reaction containing no ligand. If the binding of a ligand has an effect upon DTNB reactivity, the slope will decrease. Slope values from ligand-treated reactions can be then converted into a percentage reduction in comparison to the control slope, in the same way as with the single-point data above, using the following equation:(2) These values can be then analyzed by fitting the data to a binding model to determine EC50 as described above and shown below for data with podophyllotoxin.
-
Fitting data to determine EC50
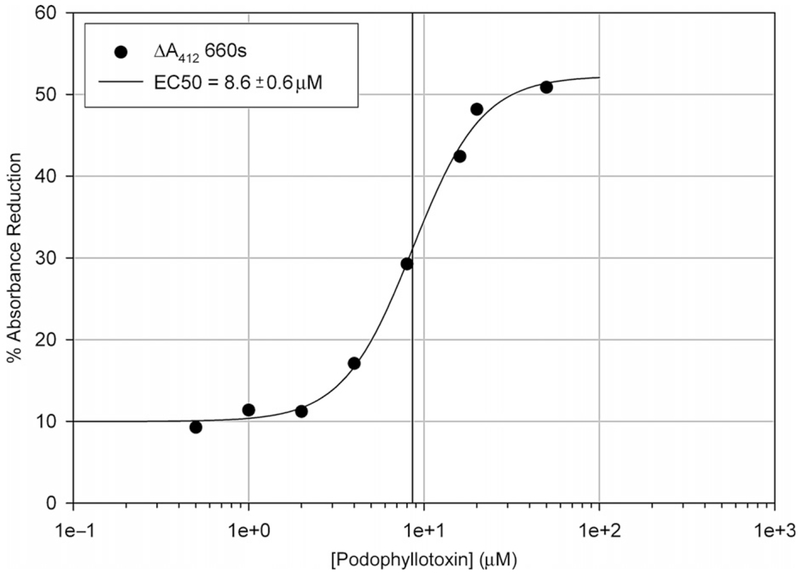
Figure 2 depicts a data set with increasing concentrations of podophyllotoxin derived from the microplate raw absorbance data shown in Fig. 1. The largest difference in absorbance between the control (no podophyllotoxin) and the highest tested podophyllotoxin concentration (80 μM) was at 660 s after the first absorbance reading (the line marked “A” on Fig. 1). The raw absorbance values at 660 s were recorded and analyzed through Eq. (1) to determine the percentage reduction in absorbance at each podophyllotoxin concentration. These values were then plotted against podophyllotoxin concentration. Using data analysis software, binding constants can be determined from these values by fitting the data to a binding model described by the following equation:(3)
Fig. 2.
Dithiobis(2-nitrobenzoate) reactivity with tubulin preincubated with podophyllotoxin derived from the difference in A412 at 660 s (line “A” seen in Fig. 1). Data were modified using Eq. (1) and fit to a nonlinear regression curve using SigmaPlot 8.0 to calculate an EC50.
We used SigmaPlot 8.0 to perform the nonlinear fit, which yielded the half-maximal effective concentration (EC50) of the ligand upon thiol reactivity. The EC50 of podophyllotoxin upon DTNB reactivity of tubulin determined through differences in absorbance was 8.6 ±0.6 μM.
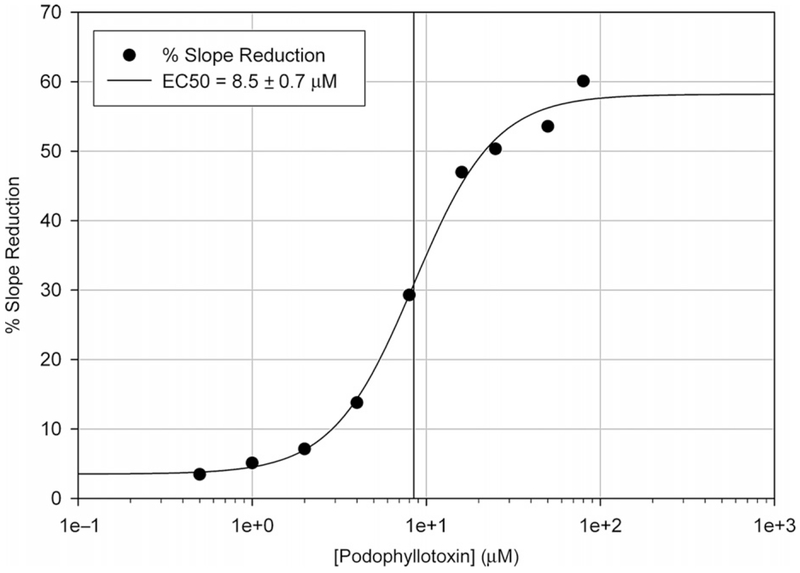
Figure 3 depicts a data set with increasing concentrations of podophyllotoxin using the slope values calculated from the raw absorbance data shown in Fig. 1. To begin data analysis, an adequate time range that demonstrates the greatest difference in slope needs to be determined. Although this can be determined visually, it is helpful to use spectrophotometer software capable of evaluating kinetic data to determine the greatest difference in slope. Using the SoftMax Pro 5 software bundled with our spectrophotometer, the slope (in milliunits per minute) was determined from the difference in absorbance between time points of 30 and 210 s shown as range B in Fig. 1. This range was chosen as it had the largest difference in slope between the control (no ligand) and the highest podophyllotoxin concentration (80 μM). The slope values between reactions just before range B in Fig. 1 show little variation, likely a result of the fast-reacting cysteines on tubulin. It is directly after this point that we begin to see differences in reactivity due to podophyllotoxin addition (range B). The % slope reduction values for each reaction were calculated using Eq. (2) and plotted against podophyllotoxin concentration. Using SigmaPlot 8.0 in the same fitting procedure as above we obtained an EC50 of 8.5 ± 0.7 μM for podophyllotoxin action on tubulin-DTNB reactivity, determined from the slopes in range B (30–210 s) in Fig. 1.
Fig. 3.
Dithiobis(2-nitrobenzoate) (DTNB) reactivity with tubulin preincubated with podophyllotoxin derived from the reaction slopes in range “B” seen in Fig. 1. Slope values were obtained from the absorbance difference between 30 and 210 s. Using Eq. (2), these slopes were converted to a slope reduction percentage and plotted against drug concentration. An EC50 of podophyllotoxin on DTNB reactivity was determined using a nonlinear regression fit.
III. Discussion
The use of DTNB reactivity described above is a simple method for detecting ligand binding to tubulin that does not rely on having available a radioactive or fluorescent version of the ligand in question. The method relies on the inherent conformational dynamics of tubulin and their restriction upon ligand binding. Conformational dynamics of proteins have been documented and examined in a number of contexts other than tubulin, especially insofar as these motions contribute to enzyme catalysis (Hammes-Schiffer and Benkovic, 2006). Different ligands may restrict conformational dynamics to differing extents, even when their binding site is fully saturated. Therefore the % reduction in reactivity at saturation may be different for different drugs, so determining an EC50 for a given drug requires examining a number of concentrations of compound and fitting the results. It is possible that a ligand could bind to tubulin in such a way that it may alter cysteine reactivity only locally, thereby affecting a particular residue, but not affecting the global reaction course, as was reported for 1-anilino-8-naphthalene sulfonate (ANS) binding (Roychowdhury et al., 2000). In this case, it would appear that the compound did not bind since there would be little effect on the global reaction. Other, more detailed methods would be necessary in this situation. In our experience, this is an unusual case—most ligands do alter the global reaction course in the way we have described.
The EC50 obtained for a ligand in question is a measure of the binding of that ligand to tubulin, but is not the same as Kd for binding. In fact it should be higher than the Kd. This is because the difference between EC50 and Kd originates in the coupling of ligand binding per se and DTNB reaction. DTNB reaction with most of tubulin’s cysteines requires reversible opening (breathing) of the protein structure to afford access to the nonsurface sulfhydryls. Hence the structure must open up sufficiently and for a sufficiently long period of time for DTNB to have access to, and to react with, those nonsurface sulfhydryls. Ligand binding restricts conformational dynamics and hence slows DTNB reaction. Ligands with a rapid off-rate may not hold the protein in a restrained, less-reactive form as long as ligands with slow off-rates. Therefore ligands with rapid off-rates will likely show EC50 values that differ from (are higher than) their binding Kd by a greater amount than ligands with slow off-rates. This is likely the explanation for the difference between the measured EC50 for podophyllotoxin (~10 μM) obtained with the DTNB method and the published Kd (~1 μM).
These considerations counsel care in quantitative interpretation of the EC50 values obtained with this method. However, for many applications of ligand screening, the ability to quickly demonstrate tubulin interaction without the need for labeled ligand, and the ability to do this in a way easily amenable to large numbers of samples, makes this method attractive. Furthermore, for quantitative studies of ligand binding, these considerations of kinetic effects may prove to be valuable in themselves.
Acknowledgments
This work was supported by Intramural Research Funds of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD.
References
- Andreu JM (2007). Large scale purification of brain tubulin with the modified Weisenberg procedure In “Microtubule Protocols” (Zhou J, ed.), Vol. 137, pp. 17–28. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- Bane SL, Ravindra R, and Zaydman AA (2007). High-throughput screening of microtubule-interacting drugs In “Microtubule Protocols” (Zhou J, ed.), Vol. 137, pp. 281–288. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- Britto PJ, Knipling L, McPhie P, and Wolff J (2005). Thiol-disulphide interchange in tubulin: Kinetics and the effect on polymerization. Biochem. J 389, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto PJ, Knipling L, and Wolff J (2002). The local electrostatic environment determines cysteine reactivity of tubulin. J. Biol. Chem 277(32), 29018–29027. [DOI] [PubMed] [Google Scholar]
- Cortese F, Bhattacharyya B, and Wolff J (1977). Podophyllotoxin as a probe for colchicine binding at the site of tubulin. J. Biol. Chem 252, 1134–1140. [PubMed] [Google Scholar]
- Desbène S, and Giorgi-Renault S (2002). Drugs that inhibit tubulin polymerization: The particular case of podophyllotoxin and analogues. Curr. Med. Chem. Anticancer Agents 2(1), 71–90. [DOI] [PubMed] [Google Scholar]
- Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, and Reiner E (2003). Molar absorption coefficients for the reduced Ellman reagent: Reassessment. Anal. Biochem 312, 224–227. [DOI] [PubMed] [Google Scholar]
- Hammes-Schiffer S, and Benkovic SJ (2006). Relating protein motion to catalysis. Annu. Rev. Biochem 75, 519–541. [DOI] [PubMed] [Google Scholar]
- Huzil JT, Chik JK, Slysz GW, Freedman H, Tuszynski J, Taylor RE, Sackett DL, and Schriemer DC (2008). A unique mode of microtubule stabilization induced by peloruside A. J. Mol. Biol 378(5), 1016–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Pannell LK, and Sackett DL (2004). Mass spectrometric measurement of differential reactivity of cysteine to localize protein-ligand binding sites. Application to tubulin-binding drugs. Anal. Biochem 332(2), 376–383. [DOI] [PubMed] [Google Scholar]
- Luduena RF, and Roach MC (1991). Tubulin sulfhydryl groups as probes and targets for antimitotic and antimicrotubule agents. Pharmacol. Ther 49(1-2), 133–152. [DOI] [PubMed] [Google Scholar]
- Passarella D, Giardini A, Peretto B, Fontana G, Sacchetti A, Silvani A, Ronchi C, Cappelletti G, Cartelli D, Borlak J, and Danieli B (2008). Inhibitors of tubulin polymerization: Synthesis and biological evaluation of hybrids of vindoline, anhydrovinblastine and vinorelbine with thiocolchicine, podophyllotoxin and baccatin III. Bioorg. Med. Chem 16(11), 6269–6285. [DOI] [PubMed] [Google Scholar]
- Roychowdhury M, Sarkar N, Manna T, Bhattacharyya S, Sarkar T, Basusarkar P, Roy S, and Bhattacharyya B (2000). Sulfhydryls of tubulin. A probe to detect conformational changes of tubulin. Eur. J. Biochem 267(12), 3469–3476. [DOI] [PubMed] [Google Scholar]
- Sackett DL (2008). Antimicrotubule agents that bind covalently to tubulin In “The Role of Microtubules in Cell Biology, Neurobiology, and Oncology” (Fojo T, ed.), pp. 281–306. Humana Press, Totowa, NJ. [Google Scholar]
- Sackett DL (1993). Podophyllotoxin, steganacin and combretastatin: Natural products that bind at the colchicine site of tubulin. Pharmacol. Ther 59, 163–228. [DOI] [PubMed] [Google Scholar]