Abstract
Background
High-intensity focused ultrasound (HIFU) and plasma radiofrequency ablation (PRA) have been used to treat recurrent allergic rhinitis (AR); however, there is a lack of literature comparing the efficacy of these 2 methods. We assessed and compared the therapeutic effects of HIFU and PRA on recurrent AR.
Material/Methods
We enrolled 66 patients with recurrent AR at West China Hospital of Sichuan University. Visual analogue score (VAS), pain score, rhinoconjunctivitis quality of life questionnaire (RQLQ), and nasal endoscopy were performed to evaluate the therapeutic effect.
Results
Nasal endoscopy showed that HIFU and PAR reduced the volume of the inferior turbinate, whereas HIFU reduced the amount of nasal secretions in patients. VAS scores showed that HIFU and PRA nasal congestion symptoms were significantly reduced (P<0.05). The preoperative VAS scores for nasal fluid and sneezing were significantly lower in patients receiving HIFU (P<0.05) than in those receiving PRA (P>0.05). HIFU-treated patients had significantly lower postoperative pain scores than those in the PRA group (P<0.05). RQLQ showed activity, sleep, and non-nasal or ocular symptoms, and both HIFU and PRA patients had significantly lower scores (P<0.05). Nasal symptom scores, actual problems, and mood in the HIFU group were significantly worse than those in the PRA group (P<0.05). However, neither treatment had a significant effect on ocular symptoms (P>0.05).
Conclusions
Compared with PRA, HIFU can significantly reduce the nasal symptoms of AR patients, improve the quality of life, and can be used as an adjuvant therapy with better therapeutic effect.
MeSH Keywords: High-Intensity Focused Ultrasound Ablation; Pulsed Radiofrequency Treatment; Rhinitis, Allergic, Seasonal
Background
AR is a form I anaphylaxis that is mainly released by Th2 cytokines. The interaction between genes and environment plays a major role in the occurrence of the disease [1]. The abundance of local infiltrating eosinophils in nasal mucosa cause rhinorrhea, sneezing, and nasal obstruction, and quality of life and work efficiency of patients are seriously affected. Although dexamethasone has been widely used in the treatment of AR, some patients have no relief of allergic symptoms [2].
HIFU is currently mainly an effective and convenient method for tumor resection, using transient high temperature and other biological effects to kill cells when low-energy ultrasound is focused on the target area in the body [3,4]. Lang et al. reported that high-intensity focused ultrasound had a similar effect as open thyroid lobectomy, but avoided neck scarring and was associated with shorter treatment time and hospital stay [5]. von Hardenberg et al. also found that targeted MRI/TRUS fusion-guided focal HIFU is effective in tumor resection of prostate cancer, with acceptable outcomes [6].
In recent years, many reports have found that HIFU is an effective treatment for AR [7]. PRA is also used to reduce inferior turbinate hypertrophy n AR and it improves ventilation [8]. However, the difference between HIFU and PRA in treatment of AR has not been reported. Our study focused on the efficacy and complications of HIFU and PRA, providing a basis for clinical application.
Material and Methods
Patients
This study was approved by the Ethics Committee of West China Hospital (approval no. 201716A). All steps were conducted in accordance with the principles of the Declaration of Helsinki. Treatment with HIFU or PRA was assigned randomly on a 1: 1 ratio according to the date of application. These 2 groups of patients were fairly homogeneous in clinical findings. The diagnosis was built on allergy symptoms, nasal endoscopy, allergen test, and serum specific immunoglobulin E levels. Patients needed to halt any treatment of AR 30 days before receiving surgery. Inclusion criteria: male or female patients aged 18–75 years; diagnosis of AR; and treatment with glucocorticoid-based drugs lasted more than 12 weeks and was ineffective. Exclusion criteria were: asthma or upper respiratory infection; serious deviation of nasal septum; sinusitis; nasal polyps; any other rhinoplasty surgery was received before; any other treatments may influence the allergic rhinitis was received during the follow-up period; tumors, diabetes, and heart disease. Before any patient was recruited, we obtained sighed informed consent.
Surgery
All patients were told before surgery that they could choose either HIFU or PRA for treatment, and local anesthesia was performed in the nasal cavity after shielding the eyes. All procedures were performed by the same surgeon. Patients kept in supine position and received surgeries under mucosa surface anesthesia with tetracaine, and then nasal cavities were shrunk by adrenaline.
HIFU
The inferior turbinate was scanned by HIFU (Chongqing HIFU Technology Co.) as a straight line from the posterior tip to anterior tip. Beyond that, we also scanned from superior tip to the inferior tip to ensure that three-fourths of the anterior inferior turbinate was covered, and then the corresponding septum was scanned as a ‘Z’. The scan speed was controlled at 4 mm/s. The treatment power was 1500 w. The time of turbinate and septum application was controlled at 150–250 s and 25–50 s, respectively.
PRA
The inferior turbinate was divided into 2 parts. A short leaf electrode was inserted 1.5 cm into the submucosa of the inferior turbinate for 10-s ablation until the mucous membrane appeared pale. Each part of the turbinate received ablation 2–3 times.
Epinephrine was administered for 30 min after both treatments. The nasal cavity was not filled with any hemostatic material.
Follow-up
All patients were followed up by the same surgeon, who did not know which type of surgery the patients had received. Patients underwent preoperative blood examination, nasal endoscopy, and allergen examination; they received nasal endoscopy at 1 and 2 weeks after surgery to remove scabs and secretions at the surgical site; then we observed nasal mucosa recovery at 1, 3, 6, and 12 months after surgery. VAS score was used to assess the severity of nasal obstruction, nasal discharge, and sneezing (0–10 cm line). VAS score (0–10) was also used to assess pain 1 week after surgery. The quality of life survey uses the RQLQ (0–6), an evaluation system used to measure functional impairment related to allergic rhinitis, with 28 questions in 7 areas: activity, sleep, non-nasal or eye symptoms, actual problems, nasal symptoms, eye symptoms, and emotions, which has long been used in clinical evaluation of patients with allergic rhinitis [9]. Previous studies have shown that RQLQ is useful in accessing different areas of injury in patients and in fully understanding the impact of disease on patients [10]. In addition, its self-managing measurement features make clinical work easier.
Data analysis
All statistical analyses were done using SPSS 21.0 (SPSS, Inc, Chicago, IL). The paired t test was used to compare preoperative and postoperative VAS score. Mann-Whitney tests were utilized to detect differences between different groups of indicators. The differences in patient characteristics between the 2 groups were compared by chi-square test. P value <0.05 was considered to be statistically significant.
Results
The study included 66 AR patients who visited the West China Hospital of Sichuan University from June 2016 to February 2018. The mean ages of patients in the HIFU and PRA groups were 44.42±14.19 years and 42.21±12.15 years, respectively (p=0.773). The HIFU and PRA groups included 34 females (18 vs. 16 years, respectively) and 32 males (15 vs. 17 years, respectively) (p=0.622). The durations of disease in the HIFU and PRA groups were 4.61±1.71 years and 4.45±1.53 years, respectively (p=0.894), and the numbers of patients with history of treatment with traditional chinese medicine, glucocorticoid, and other therapies were 14 (6 vs. 8, p=0.547), 66 (33 vs. 33, p=1.000), and 61 (31 vs. 30, p=0.642), respectively (Table 1). None of the patients in our study had significant postoperative nasal bleeding during the follow-up period.
Table 1.
Patient characteristics.
| Variables | HIFU (33) | PRA (33) | p |
|---|---|---|---|
| Age(year) | 44.42±14.19 | 42.21±12.15 | 0.773 |
| Sex | 0.622 | ||
| Female | 18 (55.5) | 16 (48.5) | |
| Male | 15 (44.5) | 17 (51.5) | |
| Duration of disease (year) | 4.61±1.71 | 4.45±1.53 | 0.894 |
| Treatment history | |||
| Traditional chinese medicine | 6 (18.2) | 8 (24.2) | 0.547 |
| Glucocorticoid | 33 (100) | 33 (100) | 1.000 |
| Others | 31 (93.4) | 30 (90.1) | 0.642 |
HIFU – high-intensity focused ultrasound; PRA – plasma radiofrequency ablation.
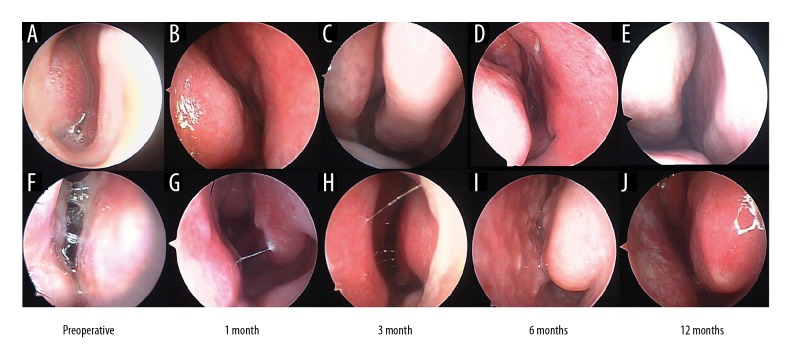
As shown in Figure 1, endoscopic sinus examination showed that HIFU and PRA reduced the volume of the inferior turbinate and increase nasal ventilation. However, the lower turbinate became more edematous with longer time of follow-up. Importantly, compared with HIFU, patients receiving PRA therapy had a larger amount of clear water-like secretions on endoscopy.
Figure 1.
Preoperative and postoperative endoscopic examination. HIFU, (A–E). PRA, (F–J). (A, F) Preoperative nasal endoscopy. (B, G) The results of the endoscopic examination showed that both HIFU and PRA significantly reduced the secretion of turbinate edema and AR at 1 month. (C, H) Nasal endoscopy of 3 months. (D, I) The PAR group showed swollen turbinate and a small amount of secretion compared with the HIFU group at 6 months. (E, J) The swollen turbinates and secretions were seen in the PRA group compared with those in the HIFU group at 12 months. HIFU – high-intensity focused ultrasound; PRA – plasma radiofrequency ablation.
The VAS scores also showed that the symptom of nasal obstruction was significantly reduced during postoperative follow-up in both groups (P<0.05) (Table 2). However, the postoperative VAS scores for nasal discharge and sneezing in the HIFU group were significantly lower than preoperative scores (P<0.05). PRA treatment had no significant effect on the above indicators, which was not significantly different from preoperative scores. Postoperative pain scores were significantly lower in the HIFU group than in the PRA group (P<0.05) (Table 3). However, the scores of both groups showed a similar trend, with the highest pain scores in the first month and decreasing with longer time of follow-up.
Table 2.
Preoperative and postoperative VAS scores.
| Variables | Groups | Preoperative | One month | Three months | Six months | Twelve months |
|---|---|---|---|---|---|---|
| Obstruction | HIFU | 7.92±2.55& | 2.16±0.52* | 2.48±0.77* | 2.84±0.87* | 3.13±1.13* |
| PRA | 7.89±2.61 | 2.28±0.69* | 2.96±0.87* | 3.04±1.12* | 3.19±1.99* | |
| Discharge | HIFU | 7.97±2.23& | 2.98±0.43*# | 3.25±0.73*# | 3.78±0.83*# | 4.04±1.56*# |
| PRA | 7.83±2.11 | 6.81±1.55* | 6.79±1.98* | 6.77±1.93* | 6.78±1.54* | |
| Sneezing | HIFU | 7.98±1.73& | 4.13±0.49*# | 4.81±1.04*# | 4.91±0.79*# | 5.12±1.34*# |
| PRA | 7.82±1.89 | 6.74±1.77* | 6.77±1.12 | 6.96±1.88$ | 7.14±1.77$ |
Compare with preoperative, P<0.05;
Compare with same stage of different groups, P<0.05;
Compare with same stage of different groups, P>0.05.
HIFU – high-intensity focused ultrasound; PRA – plasma radiofrequency ablation.
Table 3.
Preoperative and postoperative pain scores.
| Groups | One day | Two days | Three days | Four days | Five days | Six days | Seven days |
|---|---|---|---|---|---|---|---|
| HIFU | 4.03±1.55# | 2.16±1.02# | 2.08±0.77# | 1.97±1.17# | 1.92±1.03# | 1.21±0.23# | 1.02±0.19# |
| PRA | 8.89±0.75 | 7.88±0.69 | 6.46±0.81 | 5.84±0.82 | 5.72±1.99 | 5.64±0.41 | 3.73±0.53 |
Compare with same stage of different groups, P<0.05.
RQLQ results showed that postoperative activity, sleep, and non-nasal or eye symptoms were significantly lower than preoperatively in both groups (P<0.05) (Table 4). In addition, there was no significant difference in eye symptoms scores between the 2 groups during the follow-up period. It is worth noting that scores for actual problems, nasal symptoms, and emotions in the HIFU group were significantly lower than in the PRA group (P<0.05).
Table 4.
Preoperative and postoperative RQLQ scores.
| Variables | Groups | Preoperative | One month | Three months | Six months | Twelve months |
|---|---|---|---|---|---|---|
| Activity | HIFU | 3.92±0.95& | 2.46±1.24* | 2.28±1.71* | 2.51±1.64* | 2.72±1.15* |
| PRA | 3.77±1.61 | 2.28±0.91* | 2.35±1.23* | 2.38±1.81* | 3.44±1.24* | |
| Sleep | HIFU | 4.97±0.91& | 2.47±1.12* | 2.65±1.24* | 2.74±1.73* | 3.25±0.96* |
| PRA | 5.21±0.88 | 2.58±1.17 | 2.68±1.71 | 2.79±0.95 | 3.11±1.07 | |
| Non-nasal or eye symptoms | HIFU | 3.44±0.69& | 1.85±1.15* | 1.72±1.31* | 1.91±1.17* | 2.28±1.27* |
| PRA | 3.21±0.91 | 2.05±1.21* | 1.87±1.18* | 2.27±1.25* | 2.11±1.28* | |
| Actual problems | HIFU | 5.12±0.81& | 1.44±0.73*# | 2.19±1.08*# | 2.21±1.27*# | 2.51±1.28*# |
| PRA | 4.97±1.12 | 4.37±0.91 | 4.08±0.75 | 4.14±1.31 | 4.43±1.31 | |
| Nasal symptoms | HIFU | 5.12±0.83& | 2.21±0.85*# | 2.41±0.77*# | 2.68±0.65*# | 2.87±0.93*# |
| PRA | 5.01±1.08 | 3.98±0.84 | 3.98±1.01 | 3.99±0.85 | 4.21±1.15 | |
| Eye symptoms | FIFU | 5.21±0.91& | 4.01±1.29 | 4.23±0.85 | 4.31±0.69 | 4.41±0.85 |
| PRA | 4.98±1.31 | 3.91±1.44 | 4.07±0.81 | 4.15±1.14 | 4.73±0.47 | |
| Emotions | HIFU | 5.22±0.69& | 1.73±0.59*# | 1.75±0.97*# | 2.01±1.03*# | 2.21±1.19*# |
| PRA | 5.21±0.87 | 4.09±0.87 | 4.34±0.71 | 4.47±1.21 | 4.49±1.24 |
Compare with preoperative, P<0.05;
Compare with same stage of different groups, P<0.05;
Compare with same stage of different groups, P>0.05.
HIFU – high-intensity focused ultrasound; PRA – plasma radiofrequency ablation.
Discussion
AR is a local inflammatory infiltration caused by the release of a variety of inflammatory mediators caused by allergens [7,11]. Its clinical symptoms include nasal obstruction, nasal discharge, and sneezing, which seriously affect patients’ work and sleep. Invasive therapy may be an option for patients with no significant response to medications, including antihistamines, antileukotrienes, and glucocorticoids. Our study found that both HIFU and PRA significantly reduced inferior turbinate volume. We found that HIFU had greater effectiveness in reducing the symptoms of AR patients compared with PRA, and that HIFU was more effective in decreasing postoperative pain compared with PRA.
HIFU is a treatment tool that focuses low-energy ultrasound on the lesion site, causing coagulation necrosis of tissue, which could be widely use in a variety of cancers [12,13]. In recent years, HIFU has been gradually accepted in the treatment of AR. Wei et al. found that HIFU reduces symptoms of AR and reduces the recurrence rate [7]. Cheng et al. also found that HIFU has an obvious short-term curative effect on AR, and it is more convenient and less complicated than other treatment methods [14]. It has been reported that the effect of HIFU in the control of AR symptoms is similar to that of oral administration of corticosteroid nasal spray combined with cetirizine hydrochloride [15]. PRA has long been recognized as an effective treatment for nasal obstruction of turbinate hypertrophy [16,17]. In addition to effective protection of the nasal mucosa, PRA also significantly improves olfaction, decreases nasal resistance, and produces subjective benefits [18]. The results of a randomized placebo-controlled trial showed that PAR has a beneficial therapeutic effect on patients with turbinate hypertrophy [19]. Studies have shown that PAR substantially reduces the clinical symptoms of AR without affecting the cilia of the inferior turbinate [20].
However, the differences between the effects of HIFU and PRA on AR have been poorly documented. In our study, both HIFU and PRA significantly reduced the volume of the turbinate and enhanced nasal ventilation to relieve the symptoms of nasal obstruction, which is consistent with previous studies. Interestingly, our endoscopic results suggested that postoperative nasal mucosal edema and secretions in patients treated with PRA were more severe than in those treated with HIFU, especially after 6-month and 12-month follow-up. Most importantly, HIFU significantly reduces allergic symptoms of nasal secretions and sneezing compared with PRA.
Although PRA is a minimally invasive procedure, the risk of postoperative bleeding and pain is unavoidable. However, HIFU significantly reduces these complications. In our study, postoperative pain was most pronounced on the first day after surgery, and then decreased gradually over the following 6 days, which was related to the invasiveness of PRA. Due to the submucosal ablation caused by PRA, postoperative scab formation is inevitable, which also increases the time of wound cleaning and mucosal recovery. Our RQLQ results suggested that there was a significant difference in nasal symptoms between HIFU and PRA, and this treatment effect led to a similar trend in the scores of actual problems and emotions. However, these differences in treatment effects did not significantly affect patient activity, sleep, and non-nasal or eye symptoms. Our findings suggest that patients’ mood and physical problems are significantly affected by nasal mucus and sneezing, and that improvement in nasal obstruction improves sleep and activity.
The instantaneous high temperature generated by HIFU denatures the tissue, leading to coagulative necrosis, and the free radicals generated also promote the decomposition of cells at the same time. In addition, high-amplitude mechanical waves inactivate cells, and chemical reactions of chemical components in the membrane structure under the action of high-energy ultrasound also promote cell death [21–23]. When energy is concentrated in the submucosa of the nasal cavity, the death of immune cells reduces the release of cytokines and inflammatory mediators. The necrosis of glandular tissues decreases the glandular secretion of nasal mucosa; HIFU can also destroy deep parasympathetic ganglion cells and fibers, thereby reducing the excitability of cholinergic nerves and inhibiting the release of vasoactive peptide; and coagulative necrosis of the submucosal vascular network leads to vascular atresia or thrombosis, which reduces plasma exudate and edema of the nasal mucosa [7,24–27]. It should be noted that gland swelling and tissue necrosis caused by HIFU treatment may lead to an increase in short-term inflammatory responses, and blood vessel swelling may also lead to increased secretions [27].
With PRA, ablation of the turbinate increases the space for nasal ventilation and reduces nasal obstruction, which improve sleep quality. Gunhan et al. reported that radiofrequency turbinoplasty significantly reduces nasal obstruction in patients with persistent AR compared with intranasal steroids [20]. Contrary to our findings, some studies have suggested that radiofrequency turbinoplasty significantly reduces nasal and ocular hypersensitivity in AR patients [28,29]. The mechanism may be related to the reduction of turbinate volume, which reduces the surface area of allergen contact, while scar formation occludes the submucosal small blood vessels and destroys the submucosal glands [2].
It is worth noting that although HIFU has an obvious effect on allergic rhinitis, we found that the VAS scores and RQLQ scores showed an increasing trend with longer follow-up. Cheng et al. found that the effective rate of HIFU in the treatment of allergic rhinitis reached 97.2% in the follow-up period of 2–6 months, but did not indicate whether there was recurrence [14]. The results of Wei et al. also suggested that the nasal symptom scores at 1-year follow-up of AR patients were higher than at 3-month follow-up of AR patients, but no patients underwent reoperation [7]. During the 12-month follow-up period, none of the HIFU patients showed any signs of recurrence, although the long-term efficacy was not as satisfactory as the short-term outcome. These results may be related to glandular hyperplasia, capillary regeneration, and nerve repair. If patients receiving HIFU relapse, we recommend using dexamethasone and HIFU to control the symptoms of AR, which may be more satisfactory than the efficacy of HIFU alone, but these scenarios require more experimental studies in the future to confirm.
This study has some limitations. Firstly, the different methods of treatment and patient psychology may have produced subjective bias in scoring. Secondly, the limited sample size may have reduced the credibility of the results, and larger studies may be needed to confirm our results in the future. In addition, our comparisons of HIFU and PRA relied on endoscopic observation and subjective ratings, and we did not assess pathological changes, which should be evaluated in further experiments.
Conclusions
Our study showed that both HIFU and PRA alleviated the symptoms of nasal congestion, but HIFU was more effective in reducing the symptoms of runny nose and sneezing in patients with nasal congestion. In addition, HIFU was associated with lower postoperative pain than PRA. Therefore, compared with PRA, HIFU is a better choice for the treatment of refractory AR.
Acknowledgment
We appreciate Tingting Shang’s help in the follow-up work.
Footnotes
Source of support: Departmental sources
Confict of interest
None.
References
- 1.Seo JH, Kim HY, Jung YH, et al. Interactions between innate immunity genes and early-life risk factors in allergic rhinitis. Allergy Asthma Immunol Res. 2015;7(3):241–48. doi: 10.4168/aair.2015.7.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin HC, Lin PW, Friedman M, et al. Long-term results of radiofrequency turbinoplasty for allergic rhinitis refractory to medical therapy. Arch Otolaryngol Head Neck Surg. 2010;136(9):892–95. doi: 10.1001/archoto.2010.135. [DOI] [PubMed] [Google Scholar]
- 3.Xia JZ, Xie FL, Ran LF, et al. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol. 2012;38(8):1363–71. doi: 10.1016/j.ultrasmedbio.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Feng G, Hao L, Xu C, et al. High-intensity focused ultrasound-triggered nanoscale bubble-generating liposomes for efficient and safe tumor ablation under photoacoustic imaging monitoring. Int J Nanomedicine. 2017;12:4647–59. doi: 10.2147/IJN.S135391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang BHH, Wong CKH, Ma EPM, et al. A propensity-matched analysis of clinical outcomes between open thyroid lobectomy and high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Surgery. 2019;165(1):85–91. doi: 10.1016/j.surg.2018.05.080. [DOI] [PubMed] [Google Scholar]
- 6.von Hardenberg J, Westhoff N, Baumunk D, et al. Prostate cancer treatment by the latest focal HIFU device with MRI/TRUS-fusion control biopsies: A prospective evaluation. Urol Oncol. 2018;36(9):401e1–9. doi: 10.1016/j.urolonc.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Wei H, Zhang Y, Shi L, et al. Higher dosage of HIFU treatment may lead to higher and longer efficacy for moderate to severe perennial allergic rhinitis. Int J Med Sci. 2013;10(13):1914–20. doi: 10.7150/ijms.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acevedo JL, Camacho M, Brietzke SE. Radiofrequency ablation turbinoplasty versus microdebrider-assisted turbinoplasty: A systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;153(6):951–56. doi: 10.1177/0194599815607211. [DOI] [PubMed] [Google Scholar]
- 9.Juniper EF, Riis B, Juniper BA. Development and validation of an electronic version of the Rhinoconjunctivitis Quality of Life Questionnaire. Allergy. 2007;62(9):1091–93. doi: 10.1111/j.1398-9995.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 10.Juniper EF, Thompson AK, Roberts JN. Can the standard gamble and rating scale be used to measure quality of life in rhinoconjunctivitis? Comparison with the RQLQ and SF-36. Allergy. 2002;57(3):201–6. doi: 10.1034/j.1398-9995.2002.1o3306.x. [DOI] [PubMed] [Google Scholar]
- 11.Devillier P, Molimard M, Ansolabehere X, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: A retrospective database study in France. Allergy. :2018. doi: 10.1111/all.13705. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Duran-Rivera A, Montoliu Garcia A, Juan Escudero J, et al. High-intensity focused ultrasound therapy for the treatment of prostate cancer: Medium-term experience. Actas Urol Esp. 2018;42(7):450–56. doi: 10.1016/j.acuro.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhai YP, Wang Y. Effect of the combination treatment of high-intensity focused ultrasound and cryocare knife in advanced liver cancer. J BUON. 2017;22(2):495–99. [PubMed] [Google Scholar]
- 14.Cheng LJ, Liu B, Ning B, et al. High-intensity focused ultrasound for the treatment of allergic rhinitis using nasal endoscopy. Exp Ther Med. 2013;5(1):320–22. doi: 10.3892/etm.2012.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng GF, Han ZL, Wang F, et al. Comparison of high-intensity focused ultrasound therapy under nasal endoscopy guidance versus first-line drug treatment in patients with persistent allergic rhinitis. Genet Mol Res. 2015;14(3):9865–71. doi: 10.4238/2015.August.19.20. [DOI] [PubMed] [Google Scholar]
- 16.Bakshi SS, Shankar Manoharan K, Gopalakrishnan S. Comparison of the long term efficacy of radiofrequency ablation and surgical turbinoplasty in inferior turbinate hypertrophy: a randomized clinical study. Acta Otolaryngol. 2017;137(8):856–61. doi: 10.1080/00016489.2017.1294764. [DOI] [PubMed] [Google Scholar]
- 17.Akagun F, Imamoglu M, Cobanoglu HB, Ural A. Comparison of radiofrequency thermal ablation and microdebrider-assisted turbinoplasty in inferior turbinate hypertrophy: A prospective, randomized, and clinical study. Turk Arch Otorhinolaryngol. 2016;54(3):118–23. doi: 10.5152/tao.2016.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garzaro M, Pezzoli M, Pecorari G, et al. Radiofrequency inferior turbinate reduction: An evaluation of olfactory and respiratory function. Otolaryngol Head Neck Surg. 2010;143(3):348–52. doi: 10.1016/j.otohns.2010.06.908. [DOI] [PubMed] [Google Scholar]
- 19.Nease CJ, Krempl GA. Radiofrequency treatment of turbinate hypertrophy: A randomized, blinded, placebo-controlled clinical trial. Otolaryngol Head Neck Surg. 2004;130(3):291–99. doi: 10.1016/j.otohns.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Gunhan K, Unlu H, Yuceturk AV, Songu M. Intranasal steroids or radiofrequency turbinoplasty in persistent allergic rhinitis: Effects on quality of life and objective parameters. Eur Arch Otorhin-laryngol. 2011;268(6):845–50. doi: 10.1007/s00405-010-1462-1. [DOI] [PubMed] [Google Scholar]
- 21.Alkhorayef M, Mahmoud MZ, Alzimami KS, et al. High-intensity focused ultrasound (HIFU) in localized prostate cancer treatment. Pol J Radiol. 2015;80:131–41. doi: 10.12659/PJR.892341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bini F, Trimboli P, Marinozzi F, Giovanella L. Treatment of benign thyroid nodules by high intensity focused ultrasound (HIFU) at different acoustic powers: A study on in-silico phantom. Endocrine. 2018;59(3):506–9. doi: 10.1007/s12020-017-1350-1. [DOI] [PubMed] [Google Scholar]
- 23.de Castro Abreu AL, Ashrafi AN, Gill IS, et al. Contrast-enhanced transrectal ultrasound for follow-up after focal HIFU ablation for prostate cancer. J Ultrasound Med. 2019;38(3):811–19. doi: 10.1002/jum.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez C, Cardenas R, Martin D, et al. Analysis of skin testing and serum-specific immunoglobulin E to predict airway reactivity to cat allergens. Clin Exp Allergy. 2007;37(3):391–99. doi: 10.1111/j.1365-2222.2007.02659.x. [DOI] [PubMed] [Google Scholar]
- 25.Oberhuber C, Ma Y, Wopfner N, et al. Prevalence of IgE-binding to Art v 1, Art v 4 and Amb a 1 in mugwort-allergic patients. Int Arch Allergy Immunol. 2008;145(2):94–101. doi: 10.1159/000108134. [DOI] [PubMed] [Google Scholar]
- 26.Wang DY, Raza MT, Gordon BR. Control of nasal obstruction in perennial allergic rhinitis. Curr Opin Allergy Clin Immunol. 2004;4(3):165–70. doi: 10.1097/00130832-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wei H, Shi L, Zhang J, et al. High-intensity focused ultrasound leads to histopathologic changes of the inferior turbinate mucosa with allergic inflammation. Ultrasound Med Biol. 2014;40(10):2425–30. doi: 10.1016/j.ultrasmedbio.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Lin HC, Lin PW, Su CY, Chang HW. Radiofrequency for the treatment of allergic rhinitis refractory to medical therapy. Laryngoscope. 2003;113(4):673–78. doi: 10.1097/00005537-200304000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Parida PK, Santhosh K, Ganesan S, et al. The efficacy of radiofrequency volumetric tissue reduction of hypertrophied inferior turbinate in allergic rhinitis. Indian J Med Sci. 2011;65(7):269–77. [PubMed] [Google Scholar]