Abstract
INTRODUCTION
Tobacco is one of the most important risk factors for premature death globally. More than 60 toxic chemicals in tobacco can invade the body’s various systems. Oral squamous cell carcinoma (OSCC) is a pathological type of oral cancer, accounting for over 90% of oral cancers. A vast quantity of scientific, clinical and epidemiological data shows that tobacco is associated with the development of oral squamous cell carcinoma, and its carcinogenic pathways may be complicated.
METHODS
We conducted a thorough electronic search by Cochrane, EMBASE and PubMed to identify relevant studies. Studies published up to the end of October 2018 were included. After assessing and selecting articles based on eligibility criteria, studies were classified and elaborated according to the pathogenesis.
RESULTS
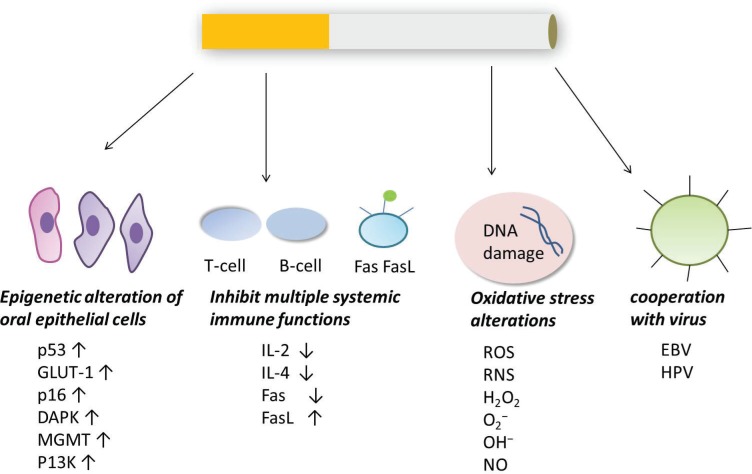
Tobacco as an important risk factor can cause epigenetic alteration of oral epithelial cells, inhibit multiple systemic immune functions of the host, and its toxic metabolites can cause oxidative stress on tissues and induce OSCC. In addition, some specific viruses such as EBV and HPV are thought to play a role in the development of OSCC.
CONCLUSIONS
Oral cancer ranks eighth among the most common causes of cancer-related deaths worldwide, and tobacco is one the most important carcinogenic factors of OSCC. This review of the literature attempts to provide directions and ideas for future related research, and emphasizes the need for efforts to reduce tobacco consumption.
Keywords: tobacco, smoking, oral squamous cell carcinoma, carcinogenic pathways
INTRODUCTION
It is well known that tobacco is one of the most important risk factors for premature death globally1. It is reported that there are more than 1.3 billion smokers worldwide2. The World Health Organization (WHO) estimates that tobacco causes nearly 6.4 million deaths and hundreds of billions of dollars of economic damage worldwide each year3. If current trends continue, by 2030 tobacco will kill more than 8 million people worldwide each year, most of which will occur in developing countries with lower incomes4. Although many people are aware that tobacco harms their health, most still accept smoking as part of their daily life, unaware that more than 60 toxic chemicals including carcinogens and cancer-promoting substances5,6, in tobacco can invade the body’s various systems7. Each cigarette is made of many ingredients, and some tobacco companies may use certain flavor additives to make their tobacco products more attractive, which may also be harmful to health8. Not only can these original components cause harm, but the intermediate metabolites play an unavoidable role in the process during smoking.
Oral squamous cell carcinoma (OSCC) is a pathological type of oral cancer, accounting for over 90% of oral cancers9. Oral cancer ranks eighth among the most common causes of cancer-related deaths worldwide10. Oral and oropharyngeal cancers are reported to account for approximately 220000 new cases per year (5% of all cancers) worldwide11. According to the recent epidemiology of OSCC, the incidence in lower/middle income countries or developing countries tends to be higher than that of developed countries12. The data show that the risk factors that attribute to OSCC are age, sex, race, gender, tobacco, alcohol, betel nut, diet and nutrition13. Among them the most common is tobacco. Many epidemiological studies have demonstrated a clear dose-response relationship between tobacco use and the risk of oral cancer or potentially malignant oral disease. Early in 1994, a study14 analyzed 454 patients with oral carcinoma and found that 60% of those with oral carcinoma smoked and over 95% of neoplasms were squamous cell carcinoma, while another study15 in 1999 stressed the significance of tobacco in the progress of oral epithelial dysplasia (OED) in a large number of European patients.
More than 180000 cases of oral cancer occur every year in South-East Asia; approximately 90% of which are due to smoking and chewing habits. Depending on the different products, tobacco may contain more than 60 established or potential carcinogens that can increase the relative risk of cancer through different mechanisms, including oxidative stress on tissues, persistent reactive oxygen species, lipids, carbohydrates and DNA to disrupt cell cycle-regulated mutations or through effects on the immune system16.
It is widely accepted that tobacco is one the most important carcinogenic factors of OSCC, and its carcinogenic pathways may be multifaceted. The purpose of this review is to summarize the possible mechanisms of tobacco that promote the development of OSCC, on the basis of relevant research, so as to provide directions and ideas for future related research.
METHODS
Eligibility criteria
The eligibility criteria for studies were: 1) research articles that studied the pathogenesis of oral squamous cell carcinoma (SCC) caused by smoking, and 2) articles in English. Epidemiological investigations, reports, literature reviews, comments and letters to the editor were excluded.
Search strategy and studies selection
We conducted a thorough electronic search by Cochrane, EMBASE and PubMed to identify relevant studies. Studies published up to the beginning of December 2018 were included. The search terms were: [Mouth Neoplasms OR Oral carcinoma OR Oral squamous cell carcinoma OR Oral Sprays OR OSCC] AND [Tobacco OR Smoking OR Tobacco Products OR Tobacco smoking OR Cigarette OR Cigarette smoking OR Cigar]. No data restrictions were applied in searching.
RESULTS
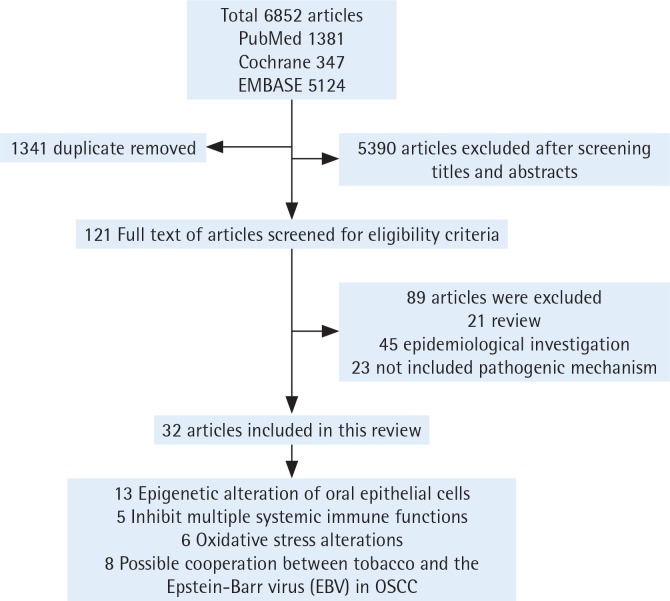
We first excluded duplicate articles. Then, two authors independently assessed the titles and abstracts of the studies on the basis of the theme of this review. Next, the full text of the remaining studies was evaluated and articles without conclusions of pathogenesis were excluded. Finally, studies were classified and elaborated according to the pathogenesis. When the two authors’ opinions were not uniform, consensus was reached through discussion along the process (Figure 1).
Figure 1.
Flow chart of studies selection process
DISCUSSION
Possible carcinogenic pathways
The possible carcinogenic pathways are summarized in Figure 2.
Figure 2.
The possible carcinogenic pathways
Epigenetic alteration of oral epithelial cells
Many studies have shown that tobacco can cause the abnormal expression of p53, GLUT-1, p16, DAPK, MGMT, P13K and other genes in oral epithelium, which is associated with the occurrence of OSCC.
The p53 cancer suppressor gene is the most universally identified mutated gene in human malignancies. The protein encoded by it is a transcriptional factor that controls the start of the cell cycle17. The p53-mediated cellular signal transduction pathway plays an important role in regulating normal cell life activities. After mutation, the p53 gene loses its regulatory effects on cell growth, apoptosis and DNA repair, and transforms from a tumor suppressor gene into an oncogene18. In 199419, a study in India investigated the expression of p53 protein in premalignant oral lesions and observed that p53 aberrations are an inchoate change in the development of oral carcinoma. They found that the proportion of p53 protein overexpression was high in premalignant and malignant oral lesions in patients who were heavily consuming tobacco. Later, further studies20-23 verified that tobacco was associated with the overexpression of the p53 gene in epithelial cells.
Glucose transporters (GLUTs) are a protein family that mediates glucose transport through the cell membrane. Glucose metabolism depends on the uptake of glucose by cells. However, glucose cannot freely enter cells through the lipid bilayer structure of the cell membrane. Glucose uptake by cells requires glucose transporters on the cell membrane. The expression of GLUT-1 is upregulated in malignant cells, which suggests increased proliferative activity, energy requirements, aggressive behaviour and poor radiation response24. GLUT-1 expression correlates significantly with histological grade and pathology Tumor Node Metastasis (pTNM) staging of OSCC25-27. A recent study showed that GLUT-1 significantly correlates with tobacco-related human oral carcinoma28. In that study, involving 50 samples, the tobacco addiction group showed a larger proportion of cells displaying GLUT-1 immunostaining (79.2%) compared with the non-tobacco group (52%), which was statistically significant.
In addition, p16 (MTS, multiple tumor suppressor 1), DAPK (death-associated protein kinase), MGMT (O6-methylguanine-DNA methyltransferase), PI3K (the phosphatidylinositol 3-kinase), c-myc and other genes were investigated in oral tobacco-related tumor tissues and cancer associated adjacent tissues21,29-32. The epigenetic alteration of these genes is a common event in oral malignancy, and is an inchoate change discovered in oral mucosa of these patients. It indicates that epigenetic alteration is of vital importance in tobacco associated oral carcinogenesis. To validate the findings, further studies are needed that comprise larger sample sizes.
Inhibition of multiple systemic immune functions
Immune dysfunction plays an important role in the escape of cancer cells from effector immunological functions, leading to the occurrence, establishment and development of the cancer. The incidence of malignancy in immunocompromised patients is 100 times higher than in normal ones33.
IL-4 is an anti-inflammatory cytokine and various in vitro studies have documented its anti-tumor activity on breast and colon cancer34. It directly modulates proliferation of various cancer cell types including gastric and renal cancers by increasing expression of p21WAFI and interferon regulating factor (IRF-1) and decreasing cyclin-dependent kinase (CDK)-2 activities besides facilitating the infiltration of inflammatory cells such as macrophages, eosinophils, and neutrophils35. A study36 in 2010 investigated the systemic immunity and the expression of IL-4 and IL-2 in T-cell subsets from peripheral blood of tobacco-related OSCC patients, on the basis of major lymphocyte subsets. They found that those with oral malignancy showed obviously decreased CD4+ and CD3+ T-cell subsets with a lower CD4/ CD8 percentage in comparison to the normal controls. The proportion of CD4+ IL-2+ was obviously lower while CD8+ IL-4+ and CD3+ IL-4+ T cells were significantly higher in them in comparison to the normal controls. Decreased expression of IL-2 in both CD8+ and CD4+ subsets was connected with the late stage of the neoplasm. The tobacco-related oral cancer is likely to be connected with multiple systemic immune impairs, especially defected CD4+ and CD3+ T cells and a differential regulation of IL-4 and IL-2 in CD8+ and CD4+ T-cell subsets in the peripheral blood. In addition, some scientists assessed the relationship between IL-4 promoter and IL-6 functional genetic polymorphisms in Asian Indians and tobacco-related oral cancer. In the study37, IL-4 genotype seemed to be susceptible in patients, while IL-6 genotype seemed to be protective. It is suggested that tobacco can decrease the transcription rate of IL-4 gene as this may have anti-tumor effects.
Fas receptor and Fas Ligand (FasL) system are associated with the suppression of apoptosis, insensitivity to chemotherapy, and with providing immune privilege to a majority of the tumours via the Fas mediated apoptosis of tumour-specific lymphocytes38-40. The decreased expression of Fas and/or increased expression of FasL avails tumour transformation and malignant progression41. A study42 in 2011 used DNA flow cytometry for cell cycle parameters and immunohistochemistry for Fas and FasL on 10 normal samples and 41 paraffin embedded tumours. The results showed that low Fas expression was observed only in 2 of 41 (5%) oral tumours while FasL immunoreactivity was observed in 26 of 41 (63.4%) tumours on the cell membrane. In contrast, all 10 normal oral tissues performed strong cytoplasmic and membrane Fas receptor immunoreactivity but without FasL staining. Up-regulation of FasL and downregulation of Fas receptor is likely to be an important character of tobacco-related OSCC.
From the above, the immunosuppressive effects may exist in tobacco-related OSCC. The IL-4 promoter and IL-6 functional genetic polymorphisms, Fas and FasL system etc. are supposed to be used as important prognostic variables in tobacco-related OSCC patients. Further, the IL-1β-511 C/T polymorphism43, interferon(IFN)44 may also play a role. Future studies that include larger sample sizes are needed to validate these findings.
Oxidative stress alterations
Tobacco, which is a foreign substance45, has been shown to stimulate46 the body to produce more free radicals that are endogenously produced in various cellular metabolic activities and which play a role in preventing microbial pathogen invasion at low concentrations. However, as their concentration rises, they may damage cellular components, ultimately leading to denaturation or mutation, which can be seen in parasitic infections, inflammatory diseases and cancers47. It has been proven that oral cancer is related to oxidative stress48,49. Free radicals include reactive oxygen species (ROS), reactive nitrogen species (RNS) and reactive oxygen metabolites such as hydrogen peroxide (H2O2), superoxide anions (O2-), hydroxyl radicals (OH-), nitric oxide (NO) and malondialdehyde. They can induce several DNA damages including strand breakage, DNA-protein cross-linkage and base modification. They can form lipid peroxides and react with cell membrane fatty acids45. For instance, ROS and RNS participates in the initiation and promotion of carcinogenesis through DNA damage50,51. NO-mediated base excision inhibits DNA repair, which may aggravate oxidative DNA damage in cells, which is possibly related to carcinogenesis52,53. They can also affect antioxidant systems. For example, GSH54 levels are a key factor in protecting organisms from toxicity and disease, as they provide reduced power for several reactions and play an important role in the detoxification of hydrogen peroxide and other free radicals. Higher oxidants and lower antioxidant activities in blood of cancer cases suggest their importance in progression of disease55,56. They can also decrease the normal effects of the superoxide dismutase (SOD).
A study57 in 2005 researched tobacco habits and alterations in enzymatic antioxidant system in oral cancer. They found that the risk of oral cancer development in smokers was significantly higher than non-smokers on the basis of erythrocytic glutathione reductase (GR), SOD, catalase (CAT) and plasma thiol. In addition, updated studies58-62 have proven the association between tobacco related OSCC and oxidative stress alterations. More research is needed to validate these findings.
Possible cooperation between tobacco and the Epstein– Barr virus (EBV) in OSCC
Epstein-Barr virus (EBV), also known as human herpes virus 4 (HHV-4)63, is a type of herpes virus64. EBV causes lifelong persistent infections in more than 90% of the world’s population. The virus has an incubation period in healthy individuals carrying the virus. Under ambient pressure, the virus can be reactivated periodically during the lifetime of the individual. EBV is often associated with a variety of malignancies such as Burkitt’s lymphoma, Hodgkin’s disease, stomach cancer, and nasopharyngeal carcinoma (NPC)65.
Tobacco is an important risk factor, which through its toxic metabolites, can cause DNA damage that induces OSCC. In addition, some specific viruses are thought to play a role in the development of OSCC66. For example, a study showed that there is a possible interaction between tobacco and HPV16 in inducing OSCC67. Some published controlled studies provide indirect evidence of a significant epidemiological association between EBV and OSCC68. Furthermore, several EBV proteins have been found to be expressed in OSCC tissues and are associated with tumor phenotypes69, which indicates that there is a strong correlation between EBV and OSCC. As mentioned above, the relevant pathogenesis of NPC is similar. Some authors have noticed the association between tobacco and EBV through epidemiological investigations, and they believe that tobacco may play a role in the carcinogenesis of NPC by inducing EBV reactivation. In a study70 in the Guangdong province of China, it was shown that cigarette smoke extract (CSE) promotes EBV replication in Akata and B95-8 cells and enhances the expression of the EBV transcriptional factors, Zta and Rta, which may be the latent-to-lytic switch of EBV. It confirmed that tobacco may act as an inducer of recurrent EBV reactivation71, which has been shown to promote genome instability and enhance NPC progression. However, few studies have mentioned the interaction of EBV and tobacco in the development of OSCC72, or in other words, provided sufficient evidence to explain the relationship between them. The results of one study showed that in OSCC patients, the difference in EBV prevalence between the smoking control group and the non-smoking control group was not significant. We believe that EBV is present in oral diseases such as OSCC and OLP. Smoking, drinking or age does not appear to be a risk factor for EBV infection. However, another study in Yemen73 showed a strong correlation between the rate of exposure to shama (tobacco) and the positive rate of EBV in OSCC patients. This has never been reported before. In conclusion, it can be assumed that tobacco induces the occurrence of OSCC by inducing EBV reactivation, similar to NCP. This carcinogenic pathway may be another potential mechanism. Further epidemiological and experimental studies are necessary to confirm the interaction between tobacco use and EBV positivity in oral cancer and to reveal potential mechanisms.
CONCLUSIONS
It has been proven that the use of tobacco is associated with the development of OSCC. Based on existing research, tobacco can cause epigenetic alteration of oral epithelial cells, inhibit multiple systemic immune functions of the host, and through its toxic metabolites cause oxidative stress on tissues to induce OSCC. In addition, some specific viruses such as EBV and HPV are thought to play a role in the development of OSCC. To validate these findings, further studies are needed comprising larger sample sizes. Meanwhile, with the development of research on this topic, more possible mechanisms remain to be studied. As the treatment of OSCC is difficult and the prognosis is poor, further research on this topic will be helpful for early diagnosis or prevention of tobacco-related oral carcinoma through efforts for cessation of tobacco consumption.
ACKNOWLEDGEMENTS
We thank the National Natural Science Foundation of China for supporting this work.
CONFLICTS OF INTEREST
Authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none was reported.
FUNDING
This work is partially supported by the National Natural Science Foundation of China (NSFC 31800114).
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer reviewed.
REFERENCES
- 1.Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. The Lancet. 2017;389(10082):1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walt G. WHO’s World Health Report 2003: Shaping the future depends on strengthening health systems. Bmj British Medical Journal. 2004;328(7430):6. doi: 10.1136/bmj.328.7430.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samim D, Méan M, Clair C, Marques-Vidal P. A 10-year observational study on the trends and determinants of smoking status. PLoS One. 2018;13(7):e0200010. doi: 10.1371/journal.pone.0200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cederbye F, Norberg R. WHO Report on the Global Tobacco Epidemic 2011: Warning about the dangers of tobacco. 3. Vol. 34. Geneva, Switzerland: World Health Organization; 2008. pp. 581–581. https://www.who.int/tobacco/global_report/2011/en/. Accessed December 28, 2018. [Google Scholar]
- 5.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362(9387):847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 6.Roe FJC. Role of 3,4-Benzopyrene in Carcinogenesis by Tobacco Smoke Condensate. Nature. 1962;194(4833):1089–1090. doi: 10.1038/1941089a0. [DOI] [PubMed] [Google Scholar]
- 7.Proctor RN. The Global Smoking Epidemic: A History and Status Report. Clin Lung Cancer. 2004;5(6):371–376. doi: 10.3816/clc.2004.n.016. [DOI] [PubMed] [Google Scholar]
- 8.Lisko JG, Stanfill SB, Watson CH. Quantitation of ten flavor compounds in unburned tobacco products. Anal Methods. 2014;6(13):4698–4704. doi: 10.1039/C4AY00271G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson NW, Jayasekara P, Amarasinghe AA. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontol. 2011;57(1):19–37. doi: 10.1111/j.1600-0757.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 10.Patel RS, Clark JR, Dirven R, Wyten R, Gao K, O’Brien CJ. Prognostic factors in the surgical treatment of patients with oral carcinoma. ANZ J Surg. 2010;79(1-2):19–22. doi: 10.1111/j.1445-2197.2008.04791.x. [DOI] [PubMed] [Google Scholar]
- 11.Abram MH, van Heerden WF, Rheeder P, Girdler-Brown BV, van Zyl AW. Epidemiology of oral squamous cell carcinoma. SADJ. 2012;67(10):550–553. https://www.ncbi.nlm.nih.gov/pubmed/?term=23957093. Accessed December 28, 2018. [PubMed] [Google Scholar]
- 12.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.McDowell JD. An Overview of Epidemiology and Common Risk Factors for Oral Squamous Cell Carcinoma. Otolaryngol Clin North Am. 2006;39(2):277–294. doi: 10.1016/j.otc.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Llewelyn J, Mitchell R. Smoking, alcohol and oral cancer in south east Scotland: a 10-year experience. Br J Oral Maxillofac Surg. 1994;32(3):146–152. doi: 10.1016/0266-4356(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 15.Jaber MA, Porter SR, Gilthorpe MS, et al. Risk factors for oral epithelial dysplasia--the role of smoking and alcohol. Oral Oncol. 1999;35(2):151–156. doi: 10.1016/S1368-8375(98)00106-7. [DOI] [PubMed] [Google Scholar]
- 16.Yuan JM, Yuan JM, Stepanov I, et al. Abstract 4347: A randomized phase 2 clinical trial of PEITC on detoxification of tobacco-specific and non-specific carcinogens and toxicants. Cancer Research. 2016;76(Suppl 14):4347–4347. doi: 10.1158/1538-7445.AM2016-4347. [DOI] [Google Scholar]
- 17.Fischer M, Quaas M, Steiner L, Engeland K. The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res. 2016;44(1):164–174. doi: 10.1093/nar/gkv927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams AB, Schumacher B. p53 in the DNA-Damage-Repair Process. Cold Spring Harb Perspect Med. 2016;6(5):a026070. doi: 10.1101/cshperspect.a026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur J, Srivastava A, Ralhan R. Overexpression of p53 protein in betel- and tobacco-related human oral dysplasia and squamous-cell carcinoma in India. Int J Cancer. 1994;58(3):340–345. doi: 10.1002/ijc.2910580305. [DOI] [PubMed] [Google Scholar]
- 20.Baral R, Patnaik S, Das BR. Co-overexpression of p53 and c-myc proteins linked with advanced stages of betel-and tobacco-related oral squamous cell carcinomas from eastern India. Eur J Oral Sci. 2010;106(5):907–913. doi: 10.1046/j.0909-8836.1998.eos106502.x. [DOI] [PubMed] [Google Scholar]
- 21.Wong YK, Liu TY, Chang KW, Lin SC, Chao TW, Li PL, Chang CS. p53 alterations in betel quid- and tobacco-associated oral squamous cell carcinomas from Taiwan. J Oral Pathol Med. 2010;27(6):243–248. doi: 10.1111/j.1600-0714.1998.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 22.Chakrobarty B, Roy JG, Majumdar S, Uppala D. Relationship among tobacco habits, human papilloma virus (HPV) infection, p53 polymorphism/mutation and the risk of oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2014;18(2):211–216. doi: 10.4103/0973-029X.140752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Gimenezconti IB, Cunningham JE, et al. Alterations of p53, cyclin D1, Rb, and H-ras in human oral carcinomas related to tobacco use. Cancer. 2015;83(2):204–212. doi: 10.1002/(SICI)1097-0142(19980715)83:2<204::AID-CNCR2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Azad N, Kumari Maurya M, Kar M, et al. Expression of GLUT-1 in oral squamous cell carcinoma in tobacco and non-tobacco users. J Oral Biol Craniofac Res. 2016;6(1):25–31. doi: 10.1016/j.jobcr.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikdar N, Paul RR, Roy B. Glutathione S-transferase M3 (A/A) genotype as a risk factor for oral cancer and leukoplakia among Indian tobacco smokers. Int J Cancer. 2004;109(1):95–101. doi: 10.1002/ijc.11610. [DOI] [PubMed] [Google Scholar]
- 26.Harshani JM, Yeluri S, Guttikonda VR. Glut-1 as a prognostic biomarker in oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2014;18(3):372–378. doi: 10.4103/0973-029X.151318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li CX, Sun JL, Gong ZC, et al. Prognostic value of GLUT-1 expression in oral squamous cell carcinoma: A prisma-compliant meta-analysis. Medicine (Baltimore) 2016;95(45):e5324. doi: 10.1097/md.0000000000005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azad N, Kumari MM, Kar M, et al. Expression of GLUT-1 in oral squamous cell carcinoma in tobacco and non-tobacco users. J Oral Biol Craniofac Res. 2016;6(1):25–31. doi: 10.1016/j.jobcr.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni V, Saranath D. Concurrent hypermethylation of multiple regulatory genes in chewing tobacco associated oral squamous cell carcinomas and adjacent normal tissues. Oral Oncol. 2004;40(2):145–153. doi: 10.1016/S1368-8375(03)00143-X. [DOI] [PubMed] [Google Scholar]
- 30.Garg R, Kapoor V, Mittal M, Singh MK, Shukla NK, Das SN. Abnormal expression of PI3K isoforms in patients with tobacco-related oral squamous cell carcinoma. Clin Chim Acta. 2013;416:100–106. doi: 10.1016/j.cca.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Smitha T, Mohan CV, Hemavathy S. Prevalence of human papillomavirus16 DNA and p16INK4aprotein in oral squamous cell carcinoma: A systematic review and meta-analysis. J Oral Maxillofac Pathol. 2017;21(1):76–81. doi: 10.4103/jomfp.JOMFP_248_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakrabarti S, Multani S, Dabholkar J, Saranath D. Whole genome expression profiling in chewing-tobacco-associated oral cancers: a pilot study. Med Oncol. 2015;32(3):60. doi: 10.1007/s12032-015-0483-4. [DOI] [PubMed] [Google Scholar]
- 33.Kersey JH, Spector BD, Good RA. Primary immunodeficiency diseases and cancer: the immunodeficiency-cancer registry. Int J Cancer. 2010;12(2):333–347. doi: 10.1002/ijc.2910120204. [DOI] [PubMed] [Google Scholar]
- 34.Toi M, Bicknell R, Harris AL. Inhibition of colon and breast carcinoma cell growth by interleukin-4. Cancer. 1992;52(2):275–279. doi: 10.1002/1097-0142(19920115)69:2<603::AIDCNCR2820690256>3.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Yu SJ, Kim HS, Cho SW, Sohn J. IL-4 inhibits proliferation of renal carcinoma cells by increasing the expression of p21WAF1 and IRF-1. Exp Mol Med. 2004;36(4):372–379. doi: 10.1038/emm.2004.49. [DOI] [PubMed] [Google Scholar]
- 36.Manchanda P, Sharma SC, Das SN. Differential regulation of IL-2 and IL-4 in patients with tobacco-related oral squamous cell carcinoma. Oral Dis. 2010;12(5):455–462. doi: 10.1111/j.1601-0825.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- 37.Gaur P, Mittal M, Mohanti BK, Das SN. Functional variants of IL4 and IL6 genes and risk of tobacco-related oral carcinoma in high-risk Asian Indians. Oral Dis. 2011;17(7):720–726. doi: 10.1111/j.1601-0825.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 38.Hahne M, Rimoldi D, Schroter M, et al. Melanoma Cell Expression of Fas(Apo-1/CD95) Ligand: Implications for Tumor Immune Escape. Science. 1996;274(5291):1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184(3):1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichmann E. The biological role of the Fas/FasL system during tumor formation and progression. Semin Cancer Biol. 2002;12(4):309–315. doi: 10.1016/S1044-579X(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 41.Müschen M, Warskulat U, Beckmann MW. Defining CD95 as a tumor suppressor gene. J Mol Med. 2000;78(6):312–325. doi: 10.1007/s001090000112. [DOI] [PubMed] [Google Scholar]
- 42.Das SN, Khare P, Singh MK, Sharma SC. Fas receptor (CD95) & Fas ligand (CD178) expression in patients with tobacco-related intraoral squamous cell carcinoma. Indian J Med Res. 2011;134(1):54–60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3171918/. Accessed December 28, 2018. [PMC free article] [PubMed] [Google Scholar]
- 43.Lakhanpal M, Yadav DS, Devi TR, et al. Association of interleukin-1β –511 C/T polymorphism with tobacco-associated cancer in northeast India: a study on oral and gastric cancer. Cancer Genet. 2014;207(1-2):1–11. doi: 10.1016/j.cancergen.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal A, Rani M, Saha GK, Valarmathi TM, Bahadur S, Mohanti BK, Das Satya N. Disregulated expression of the Th2 cytokine gene in patients with intraoral squamous cell carcinoma. Immunol Invest. 2003;32(1-2):17. doi: 10.1081/IMM-120019205. [DOI] [PubMed] [Google Scholar]
- 45.Zhao YH, Zhang M, Yan F, Casto BC, Tang XF. Nicotine-induced upregulation of antioxidant protein Prx 1 in oral squamous cell carcinoma. Chin Sci Bull. 2013;58(16):1912–1918. doi: 10.1007/s11434-013-5779-1. [DOI] [Google Scholar]
- 46.Peterson LA. Formation, Repair, and Genotoxic Properties of Bulky DNA Adducts Formed from Tobacco-Specific Nitrosamines. J Nucleic Acids. 2010;2010(14):284935. doi: 10.4061/2010/284935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar A, Pant MC, Singh HS, Khandelwal S. Assessment of the redox profile and oxidative DNA damage (8-OHdG) in squamous cell carcinoma of head and neck. J Cancer Res Ther. 2012;8(2):254–259. doi: 10.4103/0973-1482.98980. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava KC, Austin RD, Shrivastava D. Evaluation of oxidant-antioxidant status in tissue samples in oral cancer: A case control study. Dent Res J. 2016;13(2):181–187. doi: 10.4103/1735-3327.178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel BP, Rawal UM, Dave TK, et al. Lipid Peroxidation, Total Antioxidant Status, and Total Thiol Levels Predict Overall Survival in Patients With Oral Squamous Cell Carcinoma. Integr Cancer Ther. 2007;6(4):365–372. doi: 10.1177/1534735407309760. [DOI] [PubMed] [Google Scholar]
- 50.Korde SD, Basak A, Chaudhary M, Goyal M, Vagga A. Enhanced Nitrosative and Oxidative Stress with Decreased Total Antioxidant Capacity in Patients with Oral Precancer and Oral Squamous Cell Carcinoma. Oncology. 2011;80(5-6):382–389. doi: 10.1159/000329811. [DOI] [PubMed] [Google Scholar]
- 51.Lin WJ, Jiang RS, Wu SH, Chen FJ, Liu SA. Smoking, alcohol, and betel quid and oral cancer: a prospective cohort study. J Oncol. 2011;2011:525976. doi: 10.1155/2011/525976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel JB, Shah FD, Shukla SN, Shah PM, Patel PS. Role of nitric oxide and antioxidant enzymes in the pathogenesis of oral cancer. J Cancer Res Ther. 2009;5(4):247–253. doi: 10.4103/0973-1482.59898. [DOI] [PubMed] [Google Scholar]
- 53.Beevi SS, Rasheed AM, Geetha A. Evaluation of oxidative stress and nitric oxide levels in patients with oral cavity cancer. Jpn J Clin Oncol. 2004;34(7):379–385. doi: 10.1093/jjco/hyh058. [DOI] [PubMed] [Google Scholar]
- 54.Fiaschi AI, Cozzolino A, Ruggiero G, Giorgi G. Glutathione, ascorbic acid and antioxidant enzymes in the tumor tissue and blood of patients with oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2005;9(6):361–367. [PubMed] [Google Scholar]
- 55.Bhagat SS, Ghone RA, Suryakar AN, Hundekar PS. Lipid peroxidation and antioxidant vitamin status in colorectal cancer patients. Indian J Physiol Pharmacol. 2011;55(1):72–76. [PubMed] [Google Scholar]
- 56.Battisti V, Maders LD, Bagatini MD, et al. Oxidative stress and antioxidant status in prostate cancer patients: relation to Gleason score, treatment and bone metastasis. Biomed Pharmacother. 2011;65(7):516–524. doi: 10.1016/j.biopha.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Patel BP, Rawal UM, Shah PM, Prajapati JA, Rawal RM, Dave TK, Patel PS. Study of Tobacco Habits and Alterations in Enzymatic Antioxidant System in Oral Cancer. Oncology. 2005;68(4-6):511–519. doi: 10.1159/000086995. [DOI] [PubMed] [Google Scholar]
- 58.Yadav DS, Chattopadhyay I, Verma A. A pilot study evaluating genetic alterations that drive tobacco-and betel quid-associated oral cancer in Northeast India. Tumour Biol. 2014;35(9):9317–9330. doi: 10.1007/s13277-014-2222-4. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava KC, Austin RD, Shrivastava D, Sethupathy S, Rajesh S. A Case control study to evaluate oxidative stress in plasma samples of oral malignancy. Contemp Clin Dent. 2012;3(3):271–276. doi: 10.4103/0976-237X.103617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elena B, Galina Z, Svetlana G, et al. Biomarkers of oxidative stress and smoking in cancer patients. J Cancer Res Ther. 2010;6(1):47–53. doi: 10.4103/0973-1482.63569. [DOI] [PubMed] [Google Scholar]
- 61.Patel BP, Rawal UM, Rawal RM, Shukla SN, Patel PS. Tobacco, Antioxidant Enzymes, Oxidative Stress, and Genetic Susceptibility in Oral Cancer. Am J Clin Oncol. 2008;31(5):454–459. doi: 10.1097/coc.0b013e31816a61da. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava KC, Austin RD, Shrivastava D. Evaluation of oxidant-antioxidant status in tissue samples in oral cancer: A case control study. Dent Res J. 2016;13(2):181–187. doi: 10.4103/1735-3327.178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metgud R, Astekar M, Verma M, Sharma A. Role of viruses in oral squamous cell carcinoma. Oncology Reviews. 2012;6(2):164–170. doi: 10.4081/oncol.2012.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersson J. An Overview of Epstein-Barr Virus: from Discovery to Future Directions for Treatment and Prevention. Herpes. 2000;7(3):76–82. [PubMed] [Google Scholar]
- 65.Shimakage M, Horii K, Tempaku A, Kakudo K, Shirasaka T, Sasagawa T. Association of Epstein-Barr virus with oral cancers. Hum Pathol. 2002;33(6):608–614. doi: 10.1053/hupa.2002.129786. [DOI] [PubMed] [Google Scholar]
- 66.Jalouli J, Jalouli MM, Sapkota D, Ibrahim SO, Larsson PA, Sand L. Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res. 2012;32(2):571–580. http://ar.iiarjournals.org/content/32/2/571.long Accessed December 28, 2018. [PubMed] [Google Scholar]
- 67.Polz-Gruszka D, Morshed K, Stec A, Polz-Dacewicz M. Prevalence of Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in oral and oropharyngeal squamous cell carcinoma in south-eastern Poland. Infect Agent Cancer. 2015;10(1):37–44. doi: 10.1186/s13027-015-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi I, Shima K, Saito I, et al. Prevalence of Epstein–Barr virus in oral squamous cell carcinoma. J Pathol. 1999;189(1):34–39. doi: 10.1002/(SICI)1096-9896(199909)189:1<34::AID-PATH391>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 69.Yen CY, Lu MC, Tzeng CC, et al. Detection of EBV Infection and Gene Expression in Oral Cancer from Patients in Taiwan by Microarray Analysis. Biomed Res Int. 2009;2009:1–15. doi: 10.1155/2009/904589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu FH, Xiong D, Xu YF, et al. An Epidemiological and Molecular Study of the Relationship Between Smoking, Risk of Nasopharyngeal Carcinoma, and Epstein-Barr Virus Activation. J Natl Cancer Inst. 2012;104(18):1396–1410. doi: 10.1093/jnci/djs320. [DOI] [PubMed] [Google Scholar]
- 71.Fang C, Lee CC, Chang Y, et al. Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int J Cancer. 2010;124(9):2016–2025. doi: 10.1002/ijc.24179. [DOI] [PubMed] [Google Scholar]
- 72.Higa M, Kinjo T, Kamiyama K, Chinen K, Iwamasa T, Arasaki A, Sunakawa H. Epstein-Barr virus (EBV)-related oral squamous cell carcinoma in Okinawa, a subtropical island, in southern Japan--simultaneously infected with human papillomavirus (HPV) Oral Oncol. 2003;39(4):405–414. doi: 10.1016/S1368-8375(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 73.Nasher AT, Al-Hebshi NN, Al-Moayad EE, Suleiman AM. Viral infection and oral habits as risk factors for oral squamous cell carcinoma in Yemen: a case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(5):566–572. doi: 10.1016/j.oooo.2014.08.005. [DOI] [PubMed] [Google Scholar]