Abstract
Transanal total mesorectal excision (taTME) has been developed to overcome the difficulty of laparoscopic dissection and transection in the deep pelvis. TaTME has several clinical benefits over laparoscopic surgery, such as better exposure of the distal rectum and direct determination of distal resection margin. Although evidence demonstrating the true benefits of taTME over laparoscopic TME (LapTME) is still insufficient, accumulating data have revealed that, as compared with LapTME, taTME is associated with shorter operative time and a lower conversion rate without jeopardizing other short-term outcomes. However, taTME is a technically demanding procedure with specific complications such as urethral injury, and so sufficient experience of LapTME and step-by-step acquisition of the skills needed for this procedure are requisite. The role of transanal endoscopic surgery is expected to change, along with the recent progress in the treatment of rectal cancer, such as robotic surgery and the watch-and-wait strategy. Optimization of treatment will be needed in the future in terms not only of oncological but also of functional outcomes.
Keywords: rectal cancer, laparoscope, transanal TME, surgery
Introduction: Why the Transanal Approach?
The laparoscopic approach has gradually gained acceptance in rectal cancer surgery. However, recent clinical trials from western countries comparing the laparoscopic and open approach for rectal cancer surgery, high rate of conversion to open surgery (around 10%), and some concerns about margin status have been reported1,2). For some difficult cases such as narrow pelvis or bulky tumor, these results might be due to the difficulty of laparoscopic rectal dissection and transection in the deep pelvis.
The transanal approach (under direct vision) was reported to be beneficial in terms of margin status3,4), but the limited visibility under direct vision has hampered the widespread adoption of it. With the recent advances in minimally invasive surgery, such as transanal endoscopic microsurgery5) and single-port surgery, a combination of the transanal and minimally invasive approaches was introduced and is referred to as transanal TME (taTME)6). In this paper, we will review the current status and future prospects of taTME.
Operative Procedure
Several operative procedures are performed in the transanal endoscopic approach for treating rectal cancer (Figure 1). In this review study, we will focus on the transanal TME/ISR and transperineal APR.
Figure 1.
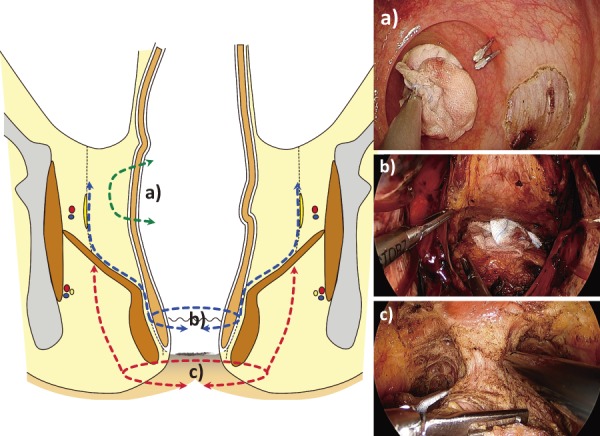
Operations performed using transanal endoscopic approach.
a) Local excision (also referred to as transanal minimally-invasive surgery “TAMIS”).
b) Intersphincteric resection (ISR) / total mesorectal excision (TME).
c) Abdominoperineal resection (APR) (also referred to as “transperineal” APR).
Typically, the transanal approach is used with the laparoscopic approach, simultaneously (two-team approach) or sequentially (one-team approach). The extent of dissection in each approach usually differs from case to case, however, rectal transection and dissection of the extra-peritoneal part of the rectum is performed transanally. There are several potential benefits of the two-team approach over the one-team approach, including assistance with exposure of the operative field and/or enhanced comprehension of the surgical anatomy7). However, the two-team approach requires extra human and device resources that might not always be available with the exception of in specialized centers.
The anastomotic method depends on the height of anastomosis. Conventional hand-sewing is performed for low anastomosis and stapled anastomosis is perfomed for high anastomosis. Stapled anastomosis is performed in a single staple manner, using a purse-string suture around the distal rectal stump8). This method is relatively technically demanding, as it is not always easy to make a full-thickness circumferential purse-string suture.
Abdominoperineal resection (APR) is another indication for this approach, and is often referred to as “transperineal APR”9,10). This approach gives the option, depending on the extent of the tumor, of several perianal dissection lines, including intersphincteric, extralevator, and ischioanal11). Although this approach offers good surgical exposure of the anterior aspect through minimal skin incision around the anus, which is the most dangerous area for positive circumferential resection margin (CRM)12), the risk of urethral injury is not negligible because of the complex anatomy of this area13).
Some aggressive surgeons have reported an absolute transanal approach without laparoscopic assistance or abdominal scar14). Although this concept of “no-scar surgery” or natural orifice transluminal endoscopic surgery (NOTES) is attractive, transanal mobilization of the splenic flexure/sigmoid colon and division of the inferior mesenteric vessels is still difficult using current operative instruments15).
Operative Devices (Setup)
Several types of energy devices are used for transanal dissection, which include ultrasonic scissors, vessel sealing devices, and electrocautery (hook or spatula). Many surgeons seem to prefer electrocautery as the energy device of use, with its the main benefit being that it facilitates identification of the striated muscles such as the external anal sphincter and levator ani muscle by electrical stimulation, which are crucial surgical landmarks in taTME.
Several types of transanal platforms have been used, including rigid type (i.e., TEO) or single-port devices16). Currently, the most popular platform is the GelPOINT path transanal access platform (Applied Medical, Ranco Santa Margarita, CA), which allows for better instrumental triangulation in the narrow operative field.
One of the technical problems specific to taTME is associated with CO2 insufflation and smoke evacuation. The transanal approach is performed through a very a narrow operative field causing unstable perirectal pressure called “bellowing”. Although the use of a pressure-sensitive insufflator such as the AirSealⓇ system is recommended for prevention of bellowing and for smoke evacuation from a surgical field, several effective methods have been reported even without such a costly device17).
The arrangement of the operating room with respect to devices is important, especially for the two-team approach, so surgeons in the laparoscopic and transanal teams can see both operative images; an example setup of the operating room is shown in Figure 2.
Figure 2.
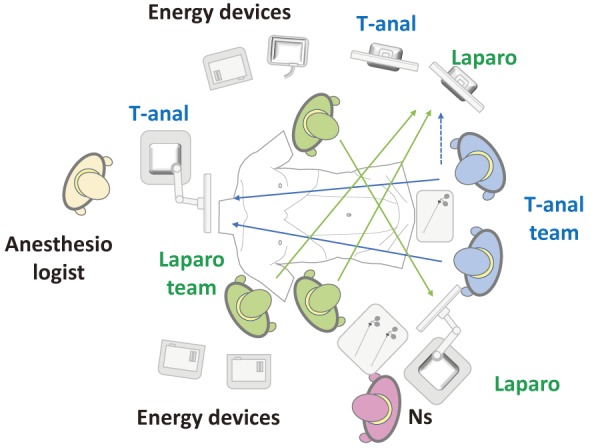
Example of set up of operative room.
Potential Advantages of taTME
There are several potential advantages and disadvantages of taTME as compared with laparoscopic surgery. Compared with the transabdominal approach, secure determination of the distal margin under direct (endoscopic) vision can be performed in the transanal approach. There are two important technical points for the determination of the distal margin. Firstly, circumferential marking with adequate distance from the tumor should be done before closure of the rectum. Secondly, because of the restriction of the direction of the instrument, incision of the rectal wall tends to go obliquely and that might threaten the CRM. Instead, rectal incision should be performed perpendicularly.
Theoretically, the transanal approach offers an in-line vantage point to the distal rectum, which facilitates the dissection of the distal rectum, especially for cases with narrow pelvis or bulky tumor18). This good accessibility to the distal rectum will facilitate identification and preservation of the pelvic autonomic splanchnic nerves, better specimen quality, proper CRM, and low conversion rate to open surgery. Reduction of operative time, especially when a two-team approach is used, is another benefit.
Disadvantages and Specific Complications of taTME
In this approach as compared with the abdominal approach, the dissection line tends to move laterally, so there is a possibility of damage to structures, such as the urethra or pelvic autonomic nerves, which are not usually damaged during conventional open and laparoscopic surgery.
Among these, urethral injury is one of the most serious and specific complications of this procedure. The complex surgical anatomy around the anal canal, especially in male patients, is another possible cause of this complication. Anatomically, urethral injury does not occur in cases with relatively high-lying tumor in which dissection starts above the inferior border of the prostate. According to a review by Atallah19), the risk factors for urethral injury are as follows: prostatic hypertrophy, history of radiation therapy for prostate cancer, and prior history of surgery for prostate and anal pathology. To avoid this severe complication, other measures, such as intraoperative identification of the prostate or membranous urethra by digital examination, lighted urethral stent placement, ultrasound guidance, and stereotactic navigation are recommended19).
Purse-string rupture is another significant complication specific to this procedure and might lead to implantation of tumor cells and bacterial contamination20). Adequate training for correctly making a secure purse-string suture is necessary before performing this procedure.
Also, transanal specimen extraction is attractive from a cosmetic perspective. However, extracting a bulky tumor through the anus without a protector might implant cancer cells around the anal canal, as in port-site recurrence in the early stages of the development of laparoscopic surgery for colorectal cancer21).
There are some concerns about the effect of dilatation of the anal canal on anal function when placing the device22,23). As compared with rigid anoscope like TEO/TEM, applying single port device into anus for local excision might be a comparable procedure for anal function24,25). However, its effect in patients who underwent TaTME, in which long-time application of transanal device is needed, has not yet been fully demonstrated and should be carefully evaluated26).
Clinical Evidence
As this procedure is relatively new, data evaluating the efficacy of this approach as compared with laparoscopic surgery is scarce.
The largest set of data currently available is the International taTME Registry reported in 2016 and 201827,28). The number of registered patients increased from 720 in 2016 to 1,594 in 2018, concurrent with the widespread adoption of this approach (Table 1). Although this registry is international, the majority of patients included in this registry are from western countries. The reported positive CRM rate and specimen quality are promising as compared with those of recent clinical trials from western countries comparing laparoscopic and open surgery1,2). Nevertheless, it should be noted with caution that the rates of positive CRM and anastomotic leakage have increased with the widespread use of this procedure in comparison with the previous survey in 2016.
Table 1.
Results of International Registry of taTME.
| 2016 | 2018* | |
|---|---|---|
| Nations | 23 | 29 |
| No of patients (cancer) | 720 (634) | 1594 (1540) |
| Sex (male; %) | 68 | 68 |
| BMI | 26.5 | 26.3 |
| Tumor height (mean; cm) | 6 | 6 |
| ≥cT3 (%) | 67 | 69 |
| cN+ (%) | 57 | 56 |
| Operation time (perineal) | 128 | 123 |
| Operation (%) | ||
| HAR | 5 | 8 |
| LAR | 86 | 92 |
| APR | 3.2 | |
| (inter-sphincteric) | 26.2 | 20.0 |
| (purse-string) | 62.5 | 72.5 |
| Anastomosis (Manual / Stapled: %) | 45 /55 | 34 / 66 |
| Two-team approach (%) | 32.5 | 41.7 |
| Conversion (Abdominal / Perineal: %) | 6 / 2.8 | 4.3 / 1.5 |
| Intraop.adverse events (%) | ||
| Wrong dissection plane | 7.8 | 5.7 |
| Pelvic bleeding | 6.9 | 4.2 |
| Visceral injury | 1.5 | 1.8 |
| Urethra | 0.7 | 0.8 |
| Rectum | 0.3 | 0.4 |
| Postope adverse events (%) | ||
| Anastomotic leak (early/delay) | 5.4 / 1.3 | 7.8 / 2.0 |
| Pelvic abscess | 2.4 | 4.7 |
| Clavien Dindo ≥ III | 11.4 | 13.2 |
| Pathological findings | ||
| Quality of specimen (Intact + minor defect: %) | 96 | 97 |
| DM (median: mm) | 15 | 16 |
| CRM (median: mm) | 8 | 10 |
| CRM+ (≤1mm: %) | 2.4 | 4.1 |
*only cases with anastomosis
Although there are no randomized controlled studies comparing taTME and laparoscopic TME (LapTME), several comparative retrospective studies have been reported. A meta-analysis combining the available data has revealed that taTME is associated with longer CRM, distant metastasis, and a lower rate of positive CRM, but there was no significant difference in other pathological parameters, such as number of harvested lymph nodes and quality of resected mesorectum29). Hu et al. examined short-term clinical outcomes and found that taTME was associated with lower conversion rate and shorter operative time, despite there being no difference in other parameters, including rate of postoperative complications30).
Furthermore, data on long-term functional and oncological outcomes are not satisfactory, and these are most important for evaluating the quality of surgery. According to reports in the study by Veltcamp and Koedam, quality of life including anal function after taTME was comparable with that after laparoscopic surgery31,32). There are two ongoing large randomized controlled trials comparing taTME and LapTME, namely the COLOR III and GRECCAR 11 studies33,34). Results of these studies, including long-term outcomes, are awaited for accurately evaluating the efficacy of taTME.
Indications for taTME
The selection of surgical approach depends on patient body habitus (obese, narrow pelvis), tumor status (location and extent), and surgeon preference and experience. As mentioned previously, this technique has been developed because of the limitations in the deep pelvis of laparoscopic surgery, especially in western countries. Therefore, surgeons tend to use this approach for cases in which they expect to be presented with difficulty in dissection and transection of the rectum in the deep pelvis. There are also some differences in indications for taTME between Japan and western countries.
Tumor location is one of the most important factors. According to recent results of the International taTME Registry, where the majority of patients were from western countries, the proportion of high-lying tumor has increased over the past two years27,28). On the contrary, in eastern countries like Japan and Korea, as compared with western countries, patient body habitus and anatomical restriction are not so severe, and the benefit of taTME over laparoscopic surgery is not as prominent. Indeed, the reported outcomes of laparoscopic rectal cancer surgery seem fairly satisfactory, having low conversion rate and equivalent oncologic outcomes as compared with open surgery in Japan and Korea35-38). Therefore, many Japanese surgeons prefer the laparoscopic approach, which is technically familiar.
With regard to the extent of the tumor, theoretically advanced tumor might be a good indication for taTME because of its greater tendency to gain a more radial margin than laparoscopic surgery. For example, a bulky tumor located in the mid rectum hampers exposure and dissection of the rectum distal to the tumor via the transabdominal approach alone. In such cases, bi-directional dissection from above and below might be a reasonable option to obtain better exposure and subsequent good pathological or oncological outcomes.
Education and Training
taTME is a technically demanding procedure, and so appropriate education and training for performing this procedure is vital for safe adoption of this technique39). The International TaTME Education Collaborative has published training guidelines40). The guidelines recommend step-by-step acquisition of the technique skills as follows: self-learning; training; proctorship; and then independent practice. Adequate knowledge of the surgical anatomy of the anal canal and lower rectum, especially from below, is also mandatory. The development of taTME has been accompanied with further accumulation of knowledge of the surgical anatomy of the anal canal41-44).
Currently, cadaver training is the most effective method available for mastery of this procedure45-47). Trainees can learn some key steps of this procedure, including purse-string suture for rectal closure, full thickness rectal incision, bottom-to-up dissection, and purse-string suture for stapled anastomosis. The use of human cadaver models reportedly facilitates the acquisition of vital skills for rectal cancer surgery because the detailed anatomy of the complex human pelvis can only be represented by a cadaveric model48). However, the major problem is their limited availability.
Although the learning curve of taTME has not been fully clarified7,49), the skills are believed to be difficult to acquire and care should be taken to avoid serious complications, especially in the early learning stages50). Deijen et al. in their study performed pooled-analysis and compared clinical outcomes between low-volume (< 30 cases) and high-volume (≥ 30 cases) centers. They stated there might be trends for better outcomes in high-volume centers with regard to conversion rate (4.3% vs. 2.7%), major complications (12.2 vs. 10.5%), complete TME specimens (80.5% vs. 89.7%), and CRM involvement (4.8% vs. 4.5%)51).
Future Prospects
There is another solution for difficult cases in conventional laparoscopic surgery. Robotic surgery has several advantages over conventional laparoscopic surgery, such as high-dexterity EndoWrist instruments, tremor filtering, and 3D high-definition imaging. Several reports have also demonstrated the clinical benefits of robotic surgery over laparoscopic surgery, especially for male patients, obesity, or low-lying tumors52,53). Thus, transanal and robotic approaches aim at almost the same targets in rectal cancer surgery and there is an argument over which approach is better, robotic TME or taTME for patients with rectal cancer having challenging features54,55). Robotic surgery also has several drawbacks such as high cost, longer operative time, and lack of tactile sensation53,56,57). Interestingly, there are regional differences in the selection of surgical treatments for rectal cancer, for example, robotic surgery is popular in the US while taTME is popular in other western countries58).
Several retrospective studies comparing robotic surgery and taTME for rectal cancer have revealed that they are equivalent as per short-term outcomes and/or histopathological outcomes59,60). According to a recent paper in the study by Lee et al. comparing the outcomes of robotic and taTME for mid- and low-rectal cancer (≤ 10 cm from the anal verge) using coarsened exact matching, short-term postoperative outcomes and pathological outcomes were closely comparable (Table 2)59). It should be noted that distal margin tumor involvement was observed more frequently in the taTME group (1.8% vs. 0.3%; P = 0.051) as opposed to the robotic group, despite the longer length to distal margin (16.9 mm vs. 15.1 mm; P = 0.097).
Table 2.
Comparison of Transanal TME and Robotic TME.
| Characteristic | Transanal (226) | Robo (370) |
|---|---|---|
| Age (mean; year-old) | 62.1 | 62.5 |
| Sex (male; %) | 62.8 | 63.5 |
| BMI | 26.1 | 25.8 |
| Clinical T-stage (cT1-2/cT3/cT4) | 22/68/10 | 21/68/11 |
| cCRM positive (%) | 30 | 29.2 |
| Distance from a.v. (-5cm / 6-10cm; %) | 52/48 | 53/47 |
| Tumor size (cm) | 2.8 | 3 |
| Preoperative RT or CRT (yes; %) | 70.7 | 69.2 |
| Operative time (mean; min) | 190 | 189 |
| Anastomosis (None/Stapled/Hand-sewn; %) | 3.5/57/39 | 6.5/64/30 |
| Diverting stoma (yes; %) | 94 | 81 |
| Conversion (%) | 1.3 | 1.1 |
| 30d-postop. complications (%) | 33 | 35 |
| Anastomotic leakage (%) | 11 | 9.5 |
| Reoperation (%) | 7.5 | 6.2 |
| Lymph nodes harvests | 16.1 | 16.8 |
| Pathological CRM+ (%) | 6.3 | 6.2 |
| low rectal tumors | 5.4 | 6.3 |
| mid rectal tumors | 5.7 | 5.5 |
| Distal margin + (%) | 1.8 | 0.3 |
| low rectal tumors | 2.7 | 0.9 |
| mid rectal tumors | 0.9 | 0 |
| Distal margin (length; mm) | 16.9 | 15.1 |
| low rectal tumors | 12.2 | 9.6 |
| mid rectal tumors | 22 | 21.3 |
| TME grade (Complete/Near complete/Incomplete; %) |
92.5/6.6/0.9 | 95.4/3.8/0.8 |
| low rectal tumors | 91/8.5/0.9 | 94/4.6/1.5 |
| mid rectal tumors | 94.5/4.6/0.6 | 97.1/2.9/0 |
| High quality specimen (complete + near complete/CRM, DM-; %) |
93.1 | 93.2 |
| low rectal tumors | 93.3 | 92.1 |
| mid rectal tumors | 92.8 | 94.5 |
We believe that, at any rate, the significance of the transanal approach will endure, particularly in cases with difficulty via the transabdominal approach, whether open, laparoscopic, or robotic54). Recently, transanal use of robotic platforms has been reported to reduce the limitations of the ergonomics of single-port surgery61-64). Furthermore, with the advent of robotic platforms designed for single-port surgery, robotic transanal surgery has been expected to overcome the limitations of single-port surgery61,62,64). While these approaches might not be mutually exclusive, a combination of the modalities might lead to better outcomes, including NOTES.
Although not directly related to TME, the importance of the transanal endoscopic approach might become more prominent in the near future. With the widespread adoption of the watch-and-wait strategy in the treatment of rectal cancer65,66), local excision following chemoradiotherapy (CRT) to remove residual tumor and evaluate the effect of CRT has gained ground67,68). The role of local excision will thus be more important in the treatment of rectal cancer and this approach will become essential procedure for colorectal surgeons.
Conclusions
taTME might have several benefits over laparoscopic surgery, especially for cases in which dissection and transection of the rectum is expected to be difficult. Data demonstrating the true safety and efficacy of this approach is limited. Furthermore, the operative procedure is difficult and is associated with several complications specific to this procedure. There are now multiple surgical approaches available as treatment options for rectal cancer, each with specific benefits and drawbacks. Nonetheless, it is most important to do good surgery, based on the principles of surgical oncology, regardless of surgical approach.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. Jama. 2015 Oct; 314(13): 1346-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. Jama. 2015 Oct; 314(13): 1356-63. [DOI] [PubMed] [Google Scholar]
- 3.Marks JH, Frenkel JL, D'Andrea AP, Greenleaf CE. Maximizing rectal cancer results: TEM and TATA techniques to expand sphincter preservation. Surg Oncol Clin North Am. 2011 Jul; 20(3): 501-20, viii-ix. [DOI] [PubMed] [Google Scholar]
- 4.Denost Q, Adam JP, Rullier A, Buscail E, Laurent C, Rullier E. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg. 2014 Dec; 260(6): 993-9. [DOI] [PubMed] [Google Scholar]
- 5.Buess G, Theiss R, Gunther M, Hutterer F, Pichlmaier H. Endoscopic surgery in the rectum. Endoscopy. 1985 Jan; 17(1): 31-5. [DOI] [PubMed] [Google Scholar]
- 6.Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010 May; 24(5): 1205-10. [DOI] [PubMed] [Google Scholar]
- 7.Koedam TWA, Veltcamp Helbach M, van de Ven PM, et al. Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol. 2018 Apr; 22(4): 279-87. [DOI] [PubMed] [Google Scholar]
- 8.Penna M, Knol JJ, Tuynman JB, Tekkis PP, Mortensen NJ, Hompes R. Four anastomotic techniques following transanal total mesorectal excision (TaTME). Tech Coloproctol. 2016 Jan; 20(3): 185-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa S, Okada T, Hida K, Kawada K, Sakai Y. Transperineal minimally invasive approach for extralevator abdominoperineal excision. Surg Endosc. 2016 Oct; 30(10): 4620-1. [DOI] [PubMed] [Google Scholar]
- 10.Yasukawa D, Hori T, Kadokawa Y, Kato S, Aisu Y, Hasegawa S. Trans-perineal minimally invasive surgery during laparoscopic abdominoperineal resection for low rectal cancer. Surg Endosc. 2018 Jul. [DOI] [PubMed] [Google Scholar]
- 11.Holm T. Controversies in abdominoperineal excision. Surg Oncol Clin North Am. 2014 Jan; 23(1): 93-111. [DOI] [PubMed] [Google Scholar]
- 12.Simillis C, Baird DL, Kontovounisios C, et al. A systematic review to assess resection margin status after abdominoperineal excision and pelvic exenteration for rectal cancer. Ann Surg. 2017 Feb; 265(2): 291-9. [DOI] [PubMed] [Google Scholar]
- 13.Stelzner S, Holm T, Moran BJ, et al. Deep pelvic anatomy revisited for a description of crucial steps in extralevator abdominoperineal excision for rectal cancer. Dis Colon Rectum. 2011 Aug; 54(8): 947-57. [DOI] [PubMed] [Google Scholar]
- 14.Leroy J, Barry BD, Melani A, Mutter D, Marescaux J. No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery. JAMA Surg. 2013 Mar; 148(3): 226-30; discussion 31. [DOI] [PubMed] [Google Scholar]
- 15.Marks JH, Lopez-Acevedo N, Krishnan B, Johnson MN, Montenegro GA, Marks GJ. True NOTES TME resection with splenic flexure release, high ligation of IMA, and side-to-end hand-sewn coloanal anastomosis. Surg Endosc. 2016 Oct; 30(10): 4626-31. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa S, Takahashi R, Hida K, Kawada K, Sakai Y. Transanal total mesorectal excision for rectal cancer. Surg Today. 2016 Jun; 46(6): 641-53. [DOI] [PubMed] [Google Scholar]
- 17.Loong TH, Liu HM, Fong SS. Stable pneumorectum using an inline glove - a cost-effective technique to facilitate transanal total mesorectal excision. Colorectal Dis. 2018 May; 20(5): O119-22. [DOI] [PubMed] [Google Scholar]
- 18.Atallah S, Martin-Perez B, Albert M, et al. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol. 2014 May; 18(5): 473-80. [DOI] [PubMed] [Google Scholar]
- 19.Atallah S, Mabardy A, Volpato AP, Chin T, Sneider J, Monson JRT. Surgery beyond the visible light spectrum: theoretical and applied methods for localization of the male urethra during transanal total mesorectal excision. Tech Coloproctol. 2017 Jun; 21(6): 413-24. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Perez B, Otero-Pineiro A, Lacy AM. Purse-string rupture: pitfalls of transanal total mesorectal excision (Cecil approach). Tech Coloproctol. 2018 May; 22(5): 393-4. [DOI] [PubMed] [Google Scholar]
- 21.Perdawood SK. A case of local recurrence following transanal total mesorectal excision: a new form of port-site metastasis? Tech Coloproctol. 2018 Apr; 22(5): 319-20. [DOI] [PubMed] [Google Scholar]
- 22.Mendes CRS, Araujo SEA, Perez R, Cecconello I, DÁlbuquerque LAC. Continence changes following transanal endoscopic microsurgery result from the impact on rectal capacity: clinical and functional evaluation before and after surgical treatment. J Coloproctol. 2018 Jul/Sept; 38(3): 227-32. [Google Scholar]
- 23.Mora Lopez L, Serra Aracil X, Hermoso Bosch J, Rebasa P, Navarro Soto S. Study of anorectal function after transanal endoscopic surgery. Int J Surg (London, England). 2015 Jan; 13: 142-7. [DOI] [PubMed] [Google Scholar]
- 24.Clermonts S, van Loon YT, Schiphorst AHW, Wasowicz DK, Zimmerman DDE. Transanal minimally invasive surgery for rectal polyps and selected malignant tumors: caution concerning intermediate-term functional results. Int J Colorectal Dis. 2017 Dec; 32(12): 1677-85. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Florez LJ, Otero-Diez JL, Encinas-Muniz AI, Sanchez-Dominguez L. Indications and outcomes from 32 consecutive patients for the treatment of rectal lesions by transanal minimally invasive surgery. Surg Innov. 2017 Aug; 24(4): 336-42. [DOI] [PubMed] [Google Scholar]
- 26.Jakubauskas M, Jotautas V, Poskus E, et al. Fecal incontinence after transanal endoscopic microsurgery. Int J Colorectal Dis. 2018 Apr; 33(4): 467-72. [DOI] [PubMed] [Google Scholar]
- 27.Penna M, Hompes R, Arnold S, et al. Transanal total mesorectal excision: International registry results of the first 720 cases. Ann Surg. 2017 Jul; 266(1): 111-7. [DOI] [PubMed] [Google Scholar]
- 28.Penna M, Hompes R, Arnold S, et al. Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the International TaTME Registry. Ann Surg. 2018 Jan. [DOI] [PubMed] [Google Scholar]
- 29.Jiang HP, Li YS, Wang B, et al. Pathological outcomes of transanal versus laparoscopic total mesorectal excision for rectal cancer: a systematic review with meta-analysis. Surg Endosc. 2018 Jun; 32(6): 2632-42. [DOI] [PubMed] [Google Scholar]
- 30.Hu D, Jin P, Hu L, et al. The application of transanal total mesorectal excision for patients with middle and low rectal cancer: a systematic review and meta-analysis. Medicine. 2018 Jul; 97(28): e11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koedam TW, van Ramshorst GH, Deijen CL, et al. Transanal total mesorectal excision (TaTME) for rectal cancer: effects on patient-reported quality of life and functional outcome. Tech Coloproctol. 2017 Jan; 21(1): 25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veltcamp Helbach M, Koedam TWA, Knol JJ, et al. Quality of life after rectal cancer surgery: differences between laparoscopic and transanal total mesorectal excision. Surg Endosc. 2018 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deijen CL, Velthuis S, Tsai A, et al. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016 Aug; 30(8): 3210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lelong B, de Chaisemartin C, Meillat H, et al. A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer. 2017 Apr; 17(1): 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii S, Yamamoto S, Ito M, et al. Short-term outcomes of laparoscopic intersphincteric resection from a phase II trial to evaluate laparoscopic surgery for stage 0/I rectal cancer: Japan Society of Laparoscopic Colorectal Surgery Lap RC. Surg Endosc. 2012 Nov; 26(11): 3067-76. [DOI] [PubMed] [Google Scholar]
- 36.Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014 Jun; 15(7): 767-74. [DOI] [PubMed] [Google Scholar]
- 37.Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010 Jul; 11(7): 637-45. [DOI] [PubMed] [Google Scholar]
- 38.Hida K, Okamura R, Sakai Y, et al. Open versus laparoscopic surgery for advanced low rectal cancer: a large, multicenter, propensity score matched cohort study in Japan. Ann Surg. 2018 Aug; 268(2): 318-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atallah SB, DuBose AC, Burke JP, et al. Uptake of transanal total mesorectal excision in north america: initial assessment of a structured training program and the experience of delegate surgeons. Dis Colon Rectum. 2017 Oct; 60(10): 1023-31. [DOI] [PubMed] [Google Scholar]
- 40.Francis N, Penna M, Mackenzie H, Carter F, Hompes R. Consensus on structured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc. 2017 Jul; 31(7): 2711-9. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe J, Ishibe A, Suwa Y, et al. Surgical techniques for identification of the prostate gland using the autonomic nerve as a landmark during transanal total mesorectal excision: secure dissection of the male rectourethral muscle. Dis Colon Rectum. 2018 Aug; 61(8): 999-1000. [DOI] [PubMed] [Google Scholar]
- 42.Muro S, Tsukada Y, Harada M, Ito M, Akita K. Spatial distribution of smooth muscle tissue in the male pelvic floor with special reference to the lateral extent of the rectourethralis muscle: application to prostatectomy. Clin Anat (New York, NY). 2018 Aug. [DOI] [PubMed] [Google Scholar]
- 43.Tsukada Y, Ito M, Watanabe K, et al. Topographic anatomy of the anal sphincter complex and levator ani muscle as it relates to intersphincteric resection for very low rectal disease. Dis Colon Rectum. 2016; 59: 426-33. [DOI] [PubMed] [Google Scholar]
- 44.Atallah S, Gonzalez P, Chadi S, Hompes R, Knol J. Operative vectors, anatomic distortion, fluid dynamics and the inherent effects of pneumatic insufflation encountered during transanal total mesorectal excision. Tech Coloproctol. 2017 May; 21(5): 783-94. [DOI] [PubMed] [Google Scholar]
- 45.Wynn GR, Austin RCT, Motson RW. Using cadaveric simulation to introduce the concept and skills required to start performing transanal total mesorectal excision. Colorectal Dis. 2018 Jun; 20: 496-501. [DOI] [PubMed] [Google Scholar]
- 46.Penna M, Whiteford M, Hompes R, Sylla P. Developing and assessing a cadaveric training model for transanal total mesorectal excision: initial experience in the UK and USA. Colorectal Dis. 2017 May; 19(5): 476-84. [DOI] [PubMed] [Google Scholar]
- 47.Telem DA, Han KS, Kim MC, et al. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc. 2013 Jan; 27(1): 74-80. [DOI] [PubMed] [Google Scholar]
- 48.Foster JD, Gash KJ, Carter FJ, et al. Development and evaluation of a cadaveric training curriculum for low rectal cancer surgery in the English LOREC National Development Programme. Colorectal Dis. 2014 Sep; 16(9): O308-19. [DOI] [PubMed] [Google Scholar]
- 49.Lee L, Kelly J, Nassif GJ, deBeche-Adams TC, Albert MR, Monson JRT. Defining the learning curve for transanal total mesorectal excision for rectal adenocarcinoma. Surg Endosc. 2018 Jul. [DOI] [PubMed] [Google Scholar]
- 50.Mege D, Hain E, Lakkis Z, Maggiori L, Prost AlDJ, Panis Y. Is trans-anal total mesorectal excision really safe and better than laparoscopic total mesorectal excision with a perineal approach first in patients with low rectal cancer? A learning curve with case-matched study in 68 patients. Colorectal Dis. 2018 Jun; 20(6): O143-51. [DOI] [PubMed] [Google Scholar]
- 51.Deijen CL, Tsai A, Koedam TW, et al. Clinical outcomes and case volume effect of transanal total mesorectal excision for rectal cancer: a systematic review. Tech Coloproctol. 2016 Nov; 20(12): 811-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jayne D, Pigazzi A, Marshall H, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR Randomized Clinical Trial. Jama. 2017 Oct; 318(16): 1569-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Essani R, Bergamaschi R. Robotic colorectal surgery: advance or expense? Adv Surg. 2016 Sep; 50(1): 157-71. [DOI] [PubMed] [Google Scholar]
- 54.Kuo LJ, Ngu JC, Chen CC. Transanal total mesorectal excision: is it necessary in the era of robots? Int J Colorectal Dis. 2018 Mar; 33(3): 341-3. [DOI] [PubMed] [Google Scholar]
- 55.Wexner SD, Berho M. Transanal total mesorectal excision of rectal carcinoma: evidence to learn and adopt the technique. Ann Surg. 2015 Feb; 261(2): 234-6. [DOI] [PubMed] [Google Scholar]
- 56.Cheng CL, Rezac C. The role of robotics in colorectal surgery. BMJ. 2018 Feb; 360: j5304. [DOI] [PubMed] [Google Scholar]
- 57.Park EJ, Baik SH. Robotic surgery for colon and rectal cancer. Curr Oncol Rep. 2016 Jan; 18(1): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pellino G, Warusavitarne J. Medium-term adoption trends for laparoscopic, robotic and transanal total mesorectal excision (TaTME) techniques. Tech Coloproctol. 2017 Dec; 21(12): 911-3. [DOI] [PubMed] [Google Scholar]
- 59.Lee L, de Lacy B, Gomez Ruiz M, et al. A multicenter matched comparison of transanal and robotic total mesorectal excision for mid and low-rectal adenocarcinoma. Ann Surg. 2018 Jun: 1. [DOI] [PubMed] [Google Scholar]
- 60.Law WL, Foo DCC. Comparison of early experience of robotic and transanal total mesorectal excision using propensity score matching. Surg Endosc. 2018 Jul. [DOI] [PubMed] [Google Scholar]
- 61.Paull JO, Pudalov N, Obias V. Medrobotics FLEX transanal excision of a rectal GIST: First video of Transanal Flex robot used in a human - video vignette. Colorectal Dis. 2018 Aug. [DOI] [PubMed] [Google Scholar]
- 62.Atallah S, Hodges A, Larach SW. Direct target NOTES: prospective applications for next generation robotic platforms. Tech Coloproctol. 2018 May; 22(5): 363-71. [DOI] [PubMed] [Google Scholar]
- 63.Liu S, Suzuki T, Murray BW, et al. Robotic transanal minimally invasive surgery (TAMIS) with the newest robotic surgical platform: a multi-institutional North American experience. Surg Endosc. 2018 Jul: 1-6. [DOI] [PubMed] [Google Scholar]
- 64.Atallah S. Assessment of a flexible robotic system for endoluminal applications and transanal total mesorectal excision (taTME): Could this be the solution we have been searching for? Tech Coloproctol. 2017 Oct; 21(10):809-14. [DOI] [PubMed] [Google Scholar]
- 65.van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018 Jun; 391(10139): 2537-45. [DOI] [PubMed] [Google Scholar]
- 66.Dattani M, Heald RJ, Goussous G, et al. Oncological and survival outcomes in watch and wait patients with a clinical complete response after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and pooled analysis. Ann Surg 2018 May. [DOI] [PubMed] [Google Scholar]
- 67.Rombouts AJM, Al-Najami I, Abbott NL, et al. Can we Save the rectum by watchful waiting or TransAnal microsurgery following (chemo) Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC study)?: protocol for a multicentre, randomised feasibility study. BMJ open. 2017 Dec; 7(1): e019474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verseveld M, de Graaf EJ, Verhoef C, et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg. 2015 Jun; 102(7): 853-60. [DOI] [PubMed] [Google Scholar]


