Abstract
We systematically reviewed literature regarding “Lewy body constipation”, i.e., constipation due to Lewy body diseases (LBD), with minimal neurologic symptoms. Epidemiology and pathology studies showed that LBD can start with constipation alone, mostly due to neuronal loss and appearance of Lewy bodies in the myenteric plexus. Because LBD significantly increases with age, “Lewy body constipation” may also increase with age. Neuroimaging methods such as metaiodobenzylguanidine (MIBG) scintigraphy and dopamine transporter (DAT) scan provide a way to detect “Lewy body constipation.” Key for “Lewy body constipation” includes minimal non-motor features such as REM sleep behavior disorder (night talking). Add-on therapy may be required to ameliorate constipation in patients. Diagnosis is not always easy; therefore, collaboration of gastroenterologists and neurologists is highly recommended to maximize patients' quality of life. In conclusion, “Lewy body constipation” might become a distinct category among geriatric constipation, regarding patients' follow-up and their management.
Keywords: Lewy body constipation, dementia with Lewy bodies, Parkinson's disease, constipation, neuroimaging
Introduction
Functional constipation is common in the geriatric population1,2), and is defined as at least two of any kinds of defecation difficulties or fewer than three spontaneous bowel movements per week (Rome IV criteria)3). Constipation increases significantly with age (affecting up to 50% among octogenerians), affects the quality of life significantly, and causes morbidity1,2). Constipation may occur after loss of dietary fiber consumption and reduction of physical exercise1,2). In children and young adults, rare causes include smooth muscle myopathy, vasculitic neuropathy, amyloidosis, mitochondrial disease, etc4). In the elderly, a recent view has emerged that there may also be a significant degenerative component for the occurrence of constipation5-7). This line of thinking started with the advent of neuroimaging, which allows us to diagnose degenerative diseases more easily than we did before. Dementia increases significantly with age (up to 33% among octogenerians), in which dementia with Lewy bodies (DLB, typically presenting dementia and parkinsonism) comprises up to 10%-20%. DLB and Parkinson's disease (PD, typically presenting motor disorder) share Lewy body pathology (Lewy body diseases, LBD). In addition, LBD produces non-motor disorders such as rapid-eye movement (REM) sleep behavior disorder (RBD, night talking), etc. The present article reviews the current concepts of “Lewy body constipation,” which connotes constipation due to LBD, with minimal neurologic symptoms.
Methods
We systematically reviewed literature regarding “Lewy body constipation.” The literature search keywords and phrases for bowel dysfunction included “bowel,” “gastrointestinal,” “incontinence,” “constipation,” “fecal,” “stercoral,” “intestinal,” “ileus,” “pseudo-obstruction,” “defecation,” “volvulus,” “introsusception,” and “anismus,” whereas those for neurological disease included “Parkinson's disease,” “Lewy body,” “dopamine,” “dementia with Lewy bodies,” “dementia,” “neurological,” and “neuropathy.” We used the current version of PubMed focusing on publications since 2000.
Method of Neuroimaging; MIBG Scintigraphy and DAT Scan
Here, we briefly describe neuroimaging useful to diagnose “Lewy body constipation” non-invasively. 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy was originally developed to assess postganglionic presynaptic cardiac sympathetic nerve endings in heart disease including: congestive heart failure, ischemic heart disease, and cardiomyopathy. Subsequently, cardiac MIBG uptake was found to be reduced in patients with LBD and is reportedly useful for differentiating LBD from other disorders such as Alzheimer's disease. MIBG scintigraphy is based on evidence that norepinephrine (NE) and MIBG have the same mechanisms for uptake, storage, and release8). There are two types of NE and MIBG uptake: uptake-1 (neuronal uptake), when the concentration is low, depends on sodium and adenosine triphosphate, and uptake-2 (extra-neuronal uptake), which takes place only when the concentration is high, represents simple diffusion. Delayed images are less dependent on uptake-2, and more accurately reflect cardiac sympathetic nerve activity as well as pathology9). The sensitivity and specificity of MIBG scintigraphy are around 90% even in very early cases10,11). According to disease progression of LBD, the yearly reduction rate of the heart to mediastinum (HM) ratio is 0.0212). When using levodopa for motor disorder in LBD, levodopa might affect MIBG uptake13) (Figure 1A, B).
Figure 1.
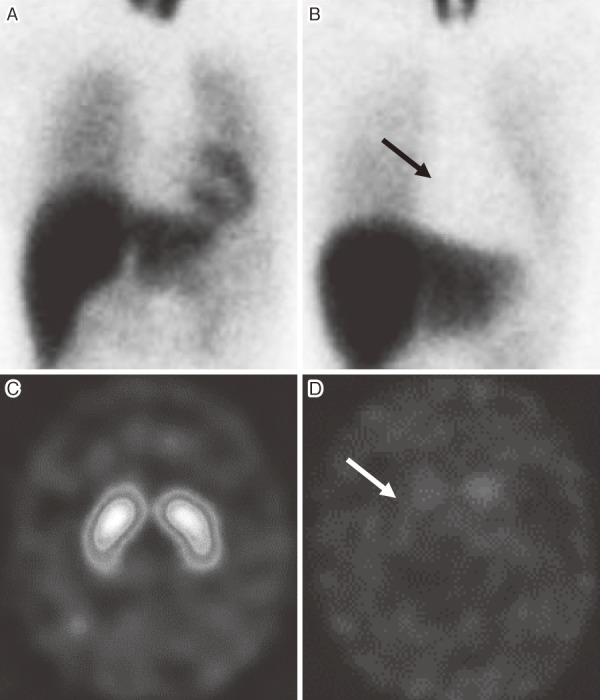
Representative cases of MIBG myocardial scintigraphy and DAT scan.
MIBG myocardial scintigraphy: A, normal, HM ratio 3.50, B, peripheral noradrenergic denervation (arrow), HM ratio 1.12. DAT scan: C, normal, SBR 4.80, D, central dopaminergic denervation (arrow), SBR 0.12. DAT: dopamine transporter, HM ratio: the heart to mediastinum ratio, normal >2.0, MIBG: metaiodobenzylguanidine, SBR: the specific binding ratio, normal >3.0.
123I-ioflupane (N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane, i.e., FP-CIT) single-photon emission computed tomography (SPECT) was developed to assess brain presynaptic dopaminergic nerve endings using dopamine transporter (DAT)14). Therefore, it is called DAT scan. It is also reportedly useful for differentiating LBD from other disorders such as Alzheimer's disease. Specificity and sensitivity of DAT scan is around 85%-90%14), (Figure 1C, D) slightly lower than that of MIBG scintigraphy. This is because DAT scan shows abnormality in other movement disorders such as multiple system atrophy. According to disease progression of LBD, the yearly reduction rate of the specific binding ratio in the striatum is 6%15).
“Lewy Body Constipation”
In 2012, Tateno and Sakakibara5) reported five elderly (age 60-81 years) men. Most of them were referred from a gastroenterology clinic for elucidation of the neurologic etiologies of constipation. Besides constipation (decreased bowel frequency and difficult defecation, lasting for 2-10 years before arrival), they had otherwise normal gait or cognitive function. The quantitative lower-gastrointestinal autonomic test (QL-GAT) revealed mild dysfunction: case 1, normal; case 2, low resting anal pressure alone; case 3, decreased rectal sensation, decreased rectal contraction, anismus, and post-defecation residual; case 4, slowed total colonic transit time (CTT); and case 5, slowed total CTT and post-defecation residual. However, in addition to constipation, four reported REM sleep behavior disorder (RBD, night talking and arm swings like fighting, loss of atonia in skeletal muscles during REM sleep by polysomnography), which started simultaneously with constipation in three, and 2 years after the onset of constipation in one. In addition, overactive bladder was observed in four, and transient mild hallucination in two. Among these, constipation, RBD, overactive bladder, and psychiatric symptoms are all regarded “non-motor” features of LBD. Furthermore, all five patients were revealed to have abnormal MIBG scintigraphy and three had occipital hypoperfusion, also suggestive of LBD. During the follow-up period of three years, one patient developed mild muscle rigidity and postural tremor unilaterally, and the diagnosis of PD was made. Constipation and other symptoms responded to the appropriate treatments. No DAT scan was performed in this report.
Succeeding to this report, Sakakibara et al. experienced an additional 18 similar cases with constipation and positive MIBG scintigraphy or DAT scan (unpublished data). They were uniformly elderly (67-86 years), had male dominance (14 men and four women), and had long histories (mean age of onset 61.0 years; mean duration before arrival 14.5 years). Their clinical manifestation were constipation/RBD in 10 (56%), constipation/RBD/pure autonomic failure (PAF, postural hypotension > 20 mmHg) in six (33%), and constipation/PAF in the remaining two patients (11%). Among them, 17 cases had an abnormal MIBG scintigraphy, whereas 10 had an abnormal DAT scan.
Because constipation is a common problem in the geriatric population, the etiology of constipation may vary considerably. Considering the above findings, “Lewy body constipation” might become a distinct category among geriatric constipation, with minimal non-motor features such as RBD.
Underlying Pathology of “Lewy Body Constipation”
Recently, it has been recognized that LBD pathology may start at the bowel. The underlying mechanisms are as follows. 1) Epidemiological studies in a Japanese-immigrant cohort in Hawaii, USA indicated a clear association between constipation and the future risk of developing motor signs (namely, PD) in LBD16). The interval between the questionnaire of constipation to the development of motor sign was more than 10-20 years in those studies. 2) Serial pathology by Braak and colleagues showed that PD pathology in the brain starts in the dorsal vagal nucleus (that might regulate bowel function) earlier than in the substantia nigra (that regulates motor function). Gelpi et al. further reported that alpha-synuclein aggregates (pathological hallmark of PD) tended to appear in the peripheral nerves earlier than in the brain: e.g., vagus nerve (86.7%), myenteric plexus (86.7%), cardiac sympathetic nerve (100%), etc18). 3) Environmental toxins (particularly pesticides in rural regions) may change microbiota (bacterial flora) in the bowel, triggering myenteric LBD pathology, and developing constipation by epidemiological19) and experimental studies20,21). 4) Once LBD pathology starts in the myenteric plexus, it is postulated that aggregated α-synuclein (a marker of LBD pathology) has the ability to transmit (like prion) from neuron to neuron, then spread to the brain through sympathetic (thoracic and lumbar sympathetic trunk) and parasympathetic (vagal and pelvic) nerves22,23).
Underlying Physiology of “Lewy Body Constipation”
Constipation in DLB might be more common than that in PD, while only limited literature is available in the elderly population. In normal conditions, the peripheral enteric nervous system (ENS) seems to regulate bowel movement more than CNS does. Experiments including knockout mice have shown that myenteric (Auerbach's) and submucous (Meisner's) plexus have a major role in controlling bowel movement (Figure 2)24,25). Peristaltic reflexes can be evoked by surface stroking or by circumferential stretch. Contraction of the longitudinal muscle via acetylcholine (ACh) is controlled positively by serotonin (mainly 5-HT4) and negatively by dopamine (D2), vasoactive intestinal peptide (VIP) and nitric oxide (NO). In PD, constipation is common mostly due to neuronal loss and appearance of Lewy bodies in the myenteric plexus. In particular, dopamine and VIP neurons are depleted, whereas few reports are available for intestinal ACh and 5-HT4 neurons.
Figure 2.
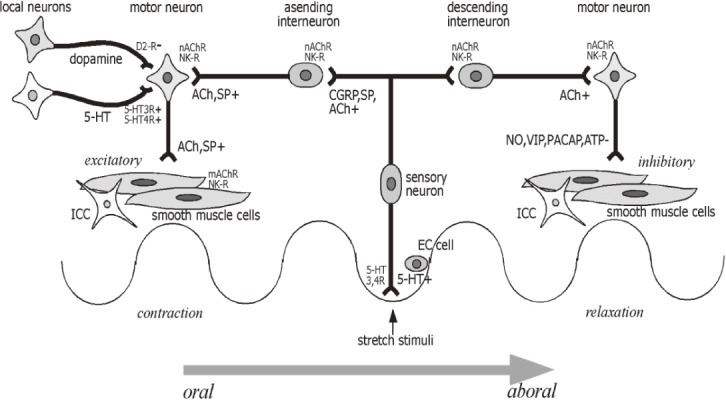
Neural circuitry relevant to bowel movement.
Enteric neural circuitry relevant to peristaltic reflex. Following mucosal stimulation, 5-HT is released from enterochromaffin cells to intrinsic primary sensory neurons (with 5-HT3 and 5-HT4 receptors) and extrinsic vagal and spinal sensory neurons (with 5-HT3 receptors). Sensory neurons release calcitonin gene-regulated peptide (CGRP), substance (SP) and acetylcholine (ACh) to interneurons. Interneurons release ACh and SP orally to excitatory motorneurons, while ACh is released aborally to inhibitory motorneurons. Excitatory motorneurons release ACh and SP to smooth muscle cells, while inhibitory motorneurons release nitric oxide (NO), vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), and adenosine triphosphate (ATP) to smooth muscle cells. 5-HT also act as an excitatory modulator on motor neurons (with 5-HT3 and 5-HT4 receptors), whereas dopamine seems to be an inhibitory modulator on motor neurons (with D2 receptor). Interstitial cells of Cajal (ICC) interact with smooth muscle cells for generating rhythmicity (with Ach, VIP, and NO receptors). See text.
Extra-enteric innervation of the bowel/anus originates from the sacral spinal cord (pelvic [left colon], hypogastric [mainly smooth sphincter], and pudendal [somatic sphincter] nerves) and the brainstem (dorsal vagal nucleus [right colon], and the Kolliker-Fuse nucleus [straining]). These sacral-brainstem structures are thought to be further regulated by Barrington's defecation center [possibly defecation], hypothalamus, basal ganglia [mostly inhibitory] and the prefrontal cortex [mostly inhibitory, and initiation of defecation]24-26). While LBD affects these areas pathologically, few reports are available clinically27,28); therefore, the exact role of these areas for LBD bowel requires further study.
In PD, control studies showed the frequency of constipation in up to 80%1,25,29), comprising both defecation difficulties (suggesting anorectal type) and fewer than three spontaneous bowel movements per week (suggesting slow transit type). Constipation significantly affects the quality of life and absorption of levodopa (L-dihydroxy- phenylalanine, a precursor of dopamine)30) in PD patients, and leads to emergency (intestinal pseudo-obstruction [paralytic ileus]31), introsusception, volvulus, malignant syndrome32), stercoral ulcer33)), and in the most advanced cases, morbidity. As described above, these dysfunctions may occur early in the course of disease. The quantitative lower-gastrointestinal autonomic test (QL-GAT) in PD patients frequently shows a combination of slow transit type (slowed colonic transit time, loss of peristaltic contractions [autonomic]) and anorectal type constipation (weak strain, anismus on defecation [somatic], and large post-defecation residuals)25) (Figure 3).
Figure 3.
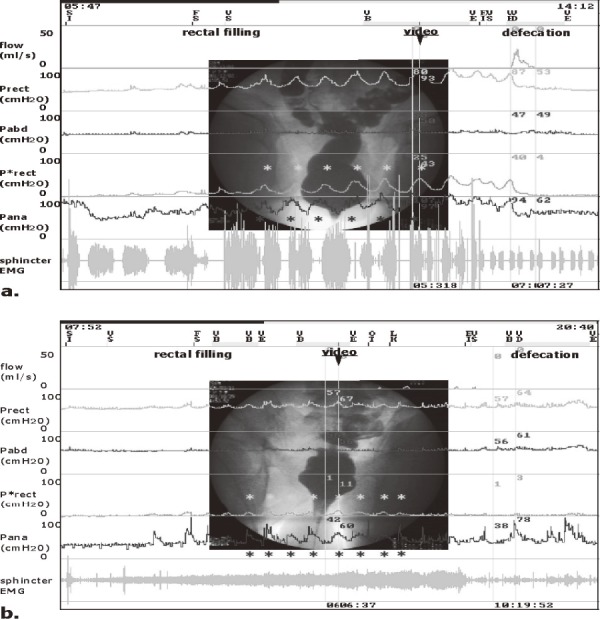
The quantitative lower-gastrointestinal autonomic test (QL-GAT): videomanometry.
(a control subject; a, and a Parkinson’s disease patient; b).
Flow: fecal flow; Prect, rectal pressure, Pabd, abdominal (bladder) pressure; P*rect, differential rectal pressure = Prect-Pabd; Pana, anal pressure; EMG, electromyography; SI, start of infusion; FS, first sensation; EI, end of infusion (maximum capacity); VD, defecation. During filling, the control subject (a) had spontaneous phasic rectal contractions (P*rect). Spontaneous phasic anal contractions were also observed (Pana). When the rectal pressure increased, the anal pressure tended to decrease together with radiographical open anal neck (video). The Parkinson’s disease patient (b) also had smaller phasic rectal contractions. However, when the rectal pressure increased, the anal pressure tended to increase with marked open anal neck (video), and this patient leaked rectal content intermittently (flow). During defecation, the first subject showed as rectal contraction-type defecation (P*rect) (A), and the second as strain-type defecation (Pabd) (B). In both subjects, anal pressure increased mild to moderately. However, the Parkinson’s disease patient (C) showed paradoxical sphincter contraction on defecation (Pana) with minimum strain (Pabd), and this patient was almost unable to defecate with large post-defecation residuals. See text.
Management of “Lewy Body Constipation”
Management of “Lewy body constipation” can follow that of general constipation. From the viewpoint of LBD, in contrast to gait difficulty, “Lewy body constipation” is not always responsive to levodopa, suggesting a complex patho-mechanism for the occurrence. Add-on therapy may therefore be needed to ameliorate constipation. Here we propose an algorism for the management of “Lewy body constipation” (Figure 4). First, check comorbid organic gastrointestinal diseases, particularly when patients have pain, black or bloody stool, or extremely difficult defecation.
Figure 4.

Treatment algorithm for Lewy body constipation.
See text.
When the patients have mild motor disorder, antiparkinsonian drugs are given by neurologists. Therefore, the effect of such drugs on constipation should be observed. The primary action of dopamine within the myenteric plexus (periphery) on GI motility is inhibitory, through D2 dopamine receptors. Therefore, dopaminergic drugs for the treatment of motor disorder in Parkinson's disease (PD) has long been believed to be inhibitory. However, recent research has shown that central dopamine may act as facilitatory on GI motility, because deep brain stimulation (DBS) for PD patients ameliorated gastric emptying. Oral levodopa does not worsen gastric emptying, and ameliorates constipation in de novo PD patients34,35). Further, transdermal formulation of dopamine receptor agonist ameliorated gastric emptying, like DBS, presumably minimizes the GI myenteric action of D2 dopamine inhibition on GI motility.
Second, bowel intervention may include exercise (bicycle manometry study showed improvement36)) and toileting devices (Asian lap-up/half-squatting posture facilitated defecation by widening recto-anal angle)2,37). Then, dietary fibers are started (not absorbed, bulking, and stimulating the bowel wall). Drugs for the bowel include: bulking agents (psyllium, polyethylene glycol 3350 [Macrogol]38), polycarbophil39)), softening agents (lubiprostone40), linaclotide, elobixibat, magnesium), and yogurt/ probiotics (microbial adjustment). However, the effects are scarce, prokinetics for slow transit type constipation are available: serotonergics (mosapride41), tegaserod42)), cholinergic (nizatidine43)), and herbal medicine (Dai-Kenchu-To [5HT3/4 stimulation]44), Rikkunshi-To [ghrelin release]45)). Approach for anorectal type constipation includes: suppository and botulinum toxin injection to relax the anal sphincter46). However, bowel tests (such as QL-GAT) should be considered in treatment-resistant patients in order to see pathophysiology. If medical treatment fails, gastrointestinal intervention should be considered. If the patients visit the emergency room because of intestinal pseudo-obstruction [paralytic ileus]31), introsusception, volvulus, malignant syndrome32), or stercoral ulcer33), immediate gastrointestinal intervention, i.e., endoscopic manual reduction of introsusception/volvulus under fluoroscopic guidance is necessary. If the patients have developed peritonitis due to perforation or severe ischemia of bowel, emergency surgery and stoma formation are necessary.
Conclusively, neuroimaging such as MIBG scintigraphy and DAT scan provides a way to detect “Lewy body constipation”, i.e., early LBD in situ within a pre-motor window. Detecting these patients may open a path to start disease-modifying therapies for LBD, and may prevent GI emergency in susceptible patients.
Conclusions
We reviewed the current concepts of “Lewy body constipation”, i.e., constipation due to Lewy body diseases (LBD), with minimal neurologic symptoms. LBD can start with constipation alone, mostly due to neuronal loss and appearance of Lewy bodies in the myenteric plexus. Since LBD significantly increases with age, “Lewy body constipation” may also increase with age. Therefore, “Lewy body constipation” might become a distinct category among geriatric constipation, regarding the follow-up and management of patients. Key for “Lewy body constipation” includes minimal non-motor features such as RBD (night talking)6,15). Arriving at a diagnosis is not always easy, since diagnostic neuroimaging is unfamiliar to gastroenterologists, while test and management of constipation is unfamiliar to neurologists. Therefore, collaboration of gastroenterologists and neurologists is recommended in order to maximize patients' quality of life.
Conflicts of Interest
There are no conflicts of interest.
Source of funding
No funding was for this case series.
Acknowledgments
We cordially thank to Dr Kenichi Kashiwabara for his valuable suggestions.
References
- 1.Cotterill N, Madersbacher H, Wyndaele JJ, Apostolidis A, Drake MJ, Gajewski J, Heesakkers J, Panicker J, Radziszewski P, Sakakibara R, Sievert KD, Hamid R, Kessler TM, Emmanuel A. Neurogenic bowel dysfunction: clinical management recommendations of the neurologic incontinence Committee of the Fifth International Consultation on Incontinence 2013. Neurourol Urodyn. 2018; 37: 46-53. [DOI] [PubMed] [Google Scholar]
- 2.Ghoshal UC. Chronic constipation in Rome IV era: The Indian perspective. Indian J Gastroenterol. 2017; 36: 163-73. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Chang L, Chey WC, Kellow J, Tack J, Whitehead WE, etd. Rome IV Functional Gastrointestinal Disorders: Disorders of Gut-Brain Interaction, Volume 1 and 2, Rome Foundation, Inc., New York, 2017. [Google Scholar]
- 4.Downes TJ, Cheruvu MS, Karunaratne TB, De Giorgio R, Farmer AD. Pathophysiology, diagnosis, and management of chronic intestinal pseudo-obstruction. J Clin Gastroenterol. 2018; 52: 477-89. [DOI] [PubMed] [Google Scholar]
- 5.Tateno F, Sakakibara R, Kishi M, Ogawa E, Takada N, Hosoe N, et al. Constipation and metaiodobenzylguanidine myocardial scintigraphy abnormality. J Am Geriatr Soc. 2012; 60: 185-7. [DOI] [PubMed] [Google Scholar]
- 6.Sakakibara R, Tateno F, Kishi M, Tsuyusaki Y, Terada H, Inaoka T. MIBG myocardial scintigraphy in pre-motor Parkinson's disease: a review. Parkinsonism Relat Disord. 2014; 20: 267-73. [DOI] [PubMed] [Google Scholar]
- 7.Tateno H, Sakakibara R, Tateno F, Tuyusaki Y, Aiba Y, Kishi M, Tateno A, Ogata T, Doi H, Inaoka T, Terada H, Suzuki Y. Metaiodobenzylguanidine myocardial scintigraphy identifies premotor parkinson's disease during a negative dopamine transporter scan. J Am Geriatr Soc. 2015; 63: 2428-30. [DOI] [PubMed] [Google Scholar]
- 8.Yamashina S, Yamazaki J. Neuronal imaging using SPECT. Eur J Nucl Med Mol Imaging 2007; 34: 939-50. [DOI] [PubMed] [Google Scholar]
- 9.Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain 2008; 131: 642-50. [DOI] [PubMed] [Google Scholar]
- 10.Nuvoli S, Palumbo B, Malaspina S, Madeddu G, Spanu A. 123I-ioflupane SPET and 123I-MIBG in the diagnosis of Parkinson's disease and parkinsonian disorders and in the differential diagnosis between Alzheimer's and Lewy's bodies dementias. Hell J Nucl Med. 2018; 21: 60-68. [DOI] [PubMed] [Google Scholar]
- 11.Treglia G, Cason E, Stefanelli A, Cocciolillo F, Di Giuda D, Fagioli G, et al. MIBG scintigraphy in differential diagnosis of Parkinsonism: a meta-analysis. Clin Auton Res. 2012; 22: 43-55. [DOI] [PubMed] [Google Scholar]
- 12.Satoh A, Serita T, Seto M, Tomita I, Satoh H, Iwanaga K, et al. Loss of 123I-MIBG uptake by the heart in Parkinson's disease: assessment of cardiac sympathetic denervation and diagnostic value. J Nucl Med 1999; 40: 371-5. [PubMed] [Google Scholar]
- 13.Brandl SJ, Braune S. Sensitivity and specificity of cardiac metaiodobenzylguanidine scintigraphy in the early diagnosis of Parkinson's disease. Clin Auton Res. 2018 Jun 5. doi: 10.1007/s10286-018-0534-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi S, Makino K, Hatakeyama S, Ishii T, Tateno M, Iwamoto T, Tsujino H, Kawasaki K, Mikuni K, Ukai W, Murayama T, Hashimoto E, Utsumi K, Kawanishi C. The usefulness of combined brain perfusion single-photon emission computed tomography, dopamine-transporter single-photon emission computed tomography, and 123 I-metaiodobenzylguanidine myocardial scintigraphy for the diagnosis of dementia with Lewy bodies. Psychogeriatrics 2017; 17: 247-55. [DOI] [PubMed] [Google Scholar]
- 15.Iranzo A, Valldeoriola F, Lomeña F, Molinuevo JL, Serradell M, Salamero M, Cot A, Ros D, Pavía J, Santamaria J, Tolosa E. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011; 10: 797-805. [DOI] [PubMed] [Google Scholar]
- 16.Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001; 57: 456-62. [DOI] [PubMed] [Google Scholar]
- 17.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson's disease. Parkinsonism and Related Disorders 2010; 16: 79-84. [DOI] [PubMed] [Google Scholar]
- 18.Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, Martí MJ, Hernández I, Valldeoriola F, Reñé R, Ribalta T. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014; 29: 1010-8. [DOI] [PubMed] [Google Scholar]
- 19.Yan D, Zhang Y, Liu L, Shi N, Yan H. Pesticide exposure and risk of Parkinson's disease: Dose-response meta-analysis of observational studies. Regul Toxicol Pharmacol. 2018; 96: 57-63. [DOI] [PubMed] [Google Scholar]
- 20.McCann H, Cartwright H, Halliday GM. Neuropathology of α-synuclein propagation and braak hypothesis. Mov Disord. 2016; 31: 152-60. [DOI] [PubMed] [Google Scholar]
- 21.FeliceVD, Quigley EM, Sullivan AM, O'Keeffe GW, O'Mahony SM. Microbiota-gut-brain signalling in Parkinson's disease: Implications for non-motor symptoms. Parkinsonism Relat Disord. 2016; 27: 1-8. [DOI] [PubMed] [Google Scholar]
- 22.Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE. 2010; 5: e8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015; 78: 522-9. [DOI] [PubMed] [Google Scholar]
- 24.Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol. Res. 2003; 52: 1-30. [PubMed] [Google Scholar]
- 25.Sakakibara R, Kishi M, Ogawa E, Tateno F, Uchiyama T, Yamamoto T, Yamanishi T. Bladder, bowel, and sexual dysfunction in Parkinson's disease. Parkinsons Dis. 2011; 2011: 924605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weng Y, Qi R, Liu C, Ke J, Xu Q, Wang F, Zhang LJ, Lu GM. Disrupted functional connectivity density in irritable bowel syndrome patients. Brain Imaging Behav. 2017; 11: 1812-22. [DOI] [PubMed] [Google Scholar]
- 27.Schaller BJ, Graf R, Jacobs AH. Pathophysiological changes of the gastrointestinal tract in ischemic stroke. Am J Gastroenterol. 2006; 101: 1655-65. [DOI] [PubMed] [Google Scholar]
- 28.Sakakibara R. Cyclic vomiting syndrome: the nervous system has the guts. Clin Auton Res. 2018; 28: 167-9. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee A, Biswas A, Das SK. Gut dysfunction in Parkinson's disease. World J Gastroenterol. 2016; 22: 5742-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bestetti A, Capozza A, Lacerenza M, Manfredi L, Mancini F. Delayed gastric emptying in advanced Parkinson disease: correlation with therapeutic doses. Clin Nucl Med. 2017; 42: 83-7. [DOI] [PubMed] [Google Scholar]
- 31.Tateno F, Sakakibara R, Kishi M, Ogawa E, Yoshimatsu Y, Takada N, Suzuki Y, Mouri T, Uchiyama T, Yamamoto T. Incidence of emergency intestinal pseudo-obstruction in Parkinson's disease. J Am Geriatr Soc. 2011; 59: 2373-5. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa E, Sakakibara R, Kishi M, Tateno F. Constipation triggered the malignant syndrome in Parkinson's disease. Neurol Sci. 2012; 33: 347-50. [DOI] [PubMed] [Google Scholar]
- 33.Tateno F, Sakakibara R, Aiba Y, Tsuyusaki Y, Kishi M, Tateno H, Ogata T. Stercoral ulcer and colonic perforation in an individual with Parkinson's disease with constipation. J Am Geriatr Soc. 2016; 64: e118-20. [DOI] [PubMed] [Google Scholar]
- 34.Tateno F, Sakakibara R, Yokoi Y, Kishi M, Ogawa E, Uchiyama T, Yamamoto T, Yamanishi T, Takahashi O. Levodopa ameliorated anorectal constipation in de novo Parkinson's disease: The QL-GAT study. Parkinsonism Relat Disord. 2011; 17: 662-6. [DOI] [PubMed] [Google Scholar]
- 35.Tateno H, Sakakibara R, Shiina S, Doi H, Tateno F, Sato M, Masaka T, Kishi M, Tsuyusaki Y, Aiba Y, Ogata T, Suzuki Y. Transdermal dopamine agonist ameliorates gastric emptying in Parkinson's disease. J Am Geriatr Soc. 2015; 63: 2416-8. [DOI] [PubMed] [Google Scholar]
- 36.Rao SS, Beaty J, Chamberlain M, Lambert PG, Gisolfi C. Effects of acute graded exercise on human colonic motility. Am J Physiol. 1999; 276(5 Pt 1): G1221-6. [DOI] [PubMed] [Google Scholar]
- 37.Takano S, Sands DR. Influence of body posture on defecation: a prospective study of “The Thinker” position. Tech Coloproctol. 2016; 20: 117-21. [DOI] [PubMed] [Google Scholar]
- 38.Zangaglia R, Martignoni E, Glorioso M, Ossola M, Riboldazzi G, Calandrella D, Brunetti G, Pacchetti C. Macrogol for the treatment of constipation in Parkinson's disease. A randomized placebo-controlled study. Mov Disord. 2007; 22: 1239-44. [DOI] [PubMed] [Google Scholar]
- 39.Sakakibara R, Yamaguchi T, Uchiyama T, Yamamoto T, Ito T, Liu Z, Odaka T, Yamaguchi C, Hattori T. Calcium polycarbophil improves constipation in primary autonomic failure and multiple system atrophy subjects. Mov Disord. 2007; 22: 1672-3. [DOI] [PubMed] [Google Scholar]
- 40.Ondo WG, Kenney C, Sullivan K, Davidson A, Hunter C, Jahan I, McCombs A, Miller A, Zesiewicz TA. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology. 2012; 78: 1650-4. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Sakakibara R, Odaka T, Uchiyama T, Uchiyama T, Yamamoto T, Ito T, Asahina M, Yamaguchi K, Yamaguchi T, Hattori T. Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients. Mov Disord. 2005; 20: 680-6. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan KL, Staffetti JF, Hauser RA, Dunne PB, Zesiewicz TA. Tegaserod (Zelnorm) for the treatment of constipation in Parkinson's disease. Mov. Disord. 2006; 21: 115-6. [DOI] [PubMed] [Google Scholar]
- 43.Sakakibara R, Doi H, Sato M, Hirai S, Masaka T, Kishi M, Tsuyusaki Y, Tateno A, Tateno F, Aiba Y, Ogata T, Suzuki Y. Nizatidine ameliorates slow transit constipation in Parkinson's disease. J Am Geriatr Soc. 2015; 63: 399-401. [DOI] [PubMed] [Google Scholar]
- 44.Sakakibara R, Odaka T, Lui Z, Uchiyama T, Yamaguchi K, Yamaguchi T, Asahina M, Yamamoto T, Ito T, Hattori T. Dietary herb extract dai-kenchu-to ameliorates constipation in parkinsonian patients (Parkinson's disease and multiple system atrophy). Mov Disord. 2005; 20: 261-2. [DOI] [PubMed] [Google Scholar]
- 45.Doi H, Sakakibara R, Sato M, Hirai S, Masaka T, Kishi M, Tsuyusaki Y, Tateno A, Tateno F, Takahashi O, Ogata T. Dietary herb extract rikkunshi-to ameliorates gastroparesis in Parkinson's disease: a pilot study. Eur Neurol. 2014; 71: 193-5. [DOI] [PubMed] [Google Scholar]
- 46.Cadeddu F, Bentivoglio AR, Brandara F, Marniga G, Brisinda G, Maria G. Outlet type constipation in Parkinson's disease: results of botulinum toxin treatment. Aliment Pharmacol. Ther. 2005; 22: 997-1003. [DOI] [PubMed] [Google Scholar]


