Abstract
Objectives: One of the characteristics of colorectal cancer complicating Crohn's disease (CD) in the Japanese population is that it frequently occurs in the lower anorectal site. This study aimed to examine CD patients biopsied in the lower anorectal sites to investigate the significance and problems associated with this method of cancer surveillance. Methods: Among 116 patients with CD duration of ≥10 years, we examined patients diagnosed with cancer using histological examination of the lower anorectal site (287 times). We also evaluated the detection rates of cancer and atypical cells using this method.Results: Of the 116 patients, neoplastic lesions were detected through biopsy in 22 (19.0%), of which 18 had carcinomas and 4 had atypical cells. The clinicopathological traits of the cancer patients were early-age onset and chronic disease duration of CD before cancer diagnosis. Histologic findings were characterized by a high frequency of poorly differentiated adenocarcinoma and mucinous carcinoma. The 18 patients with cancer were assigned to groups A and B depending on the presence or absence of cancer-related symptoms, and their characteristics were compared. Of these, 5 patients whose cancer was detected without symptoms (group A) had better prognosis than those detected with symptoms (group B) based on survival curves. We next examined 103 patients for surveillance after excluding 13 patients who were diagnosed with cancer-related symptoms from the 116 patients and found a 5.8% (6 patients) detection rate of cancer and atypical cells.Conclusions: Our results suggest the effectiveness of transanal histological testing for the surveillance of anorectal cancer with CD.
Keywords: Crohn's disease, anorectal cancer, cancer surveillance
Introduction
In Japan, the number of patients with inflammatory bowel disease (IBD) has recently increased markedly, and cancers complicating IBD are becoming a serious problem in patients with chronic disease.
Because the colon should be specifically targeted in the treatment of ulcerative colitis, a cancer surveillance program has been established, which has improved prognosis by increasing the frequency of early diagnosis1-4). In contrast, the entire gastrointestinal tract is at an increased risk of being affected by cancer in Crohn's disease (CD) patients, and the disease is accompanied by fistulae and stenosis, which increase the difficulty of performing endoscopy. Thus, there is currently no effective surveillance method that is linked to the poor prognosis associated with CD-related gastrointestinal cancers5,6).
Previous studies in Western populations have noted that CD-related colorectal cancer frequently occurs in the right colon5,7). In Japan, however, CD-related colorectal cancers are characterized by a high incidence in the distal colon, particularly in the anorectal site8,9). Japanese surveillance programs are currently under investigation10).
Our team routinely conducts histological examinations during screening and procedures for anal lesions in CD patients to detect non-caseating, epithelioid granulomas and perform cancer surveillance. The current study investigated patients who underwent biopsies of the lower anorectal sites to evaluate the effectiveness of this method for the surveillance of related tumors and problems associated with this method.
Methods
This study was included 116 CD patients (287 biopsies), with a history of lesions at anorectal sites of ≥10 years, who underwent transanal lower anorectal site histological examination (i.e., biopsy, diagnostic excision, and cytodiagnosis) at Fukuoka University Chikushi Hospital between July 1985 and December 2014.
The methods of histological examination were generally performed under anesthesia (i.e., lumbar, epidural, and general anesthesia). We performed random biopsy of the anorectal tract mucosa (three sites) and fistulas (secondary opening of the fistula and in the fistulous canal) transanally. We also conducted histological analyses of symptomatic skin tags and lavage cytology of the proctodeum and performed fistulous canal lavage to investigate the detection rate of cancer and atypical epithelium. These procedures were carried out once every year. We added endoscopy and endoscopic biopsy to this method in patients, in whom endoscopy of the lower gastrointestinal tract was difficult on an outpatient basis due to anal stenosis or other reasons. We examined patients in whom endoscopy could not be performed due to pain associated with digital rectal palpation on an outpatient basis via annual transanal histological examination as a general rule and performed transanal biopsy as needed when changes in symptoms (e.g., mucus discharge, pain, or worsening symptoms of stenosis) were identified. When the patients had severe anal canal stricture, dilation using a finger or metal bougie was performed before the biopsy. Next, we observed patients who could undergo an outpatient endoscopic examination by endoscopists.
We examined clinicopathological factors associated with cancer patients and assigned the subjects into two groups depending on the presence or absence of symptoms originating from cancer to investigate these factors and prognosis. Staging of this study was done according to the Japanese Classification of Colorectal Carcinoma11). We also examined the detection rate of neoplastic lesions by performing lower anorectal site biopsy as a method of cancer surveillance. This was a retrospective study.
For statistical analysis, we performed chi-square and Mann-Whitney U-tests to examine the difference between the two groups. The cumulative survival rate was analyzed with Kaplan-Meier and log-rank tests. P < 0.05 was considered a statistically significant difference.
Results
The 116 patients who underwent anorectal site biopsies (287 times) between July 1985 and December 2014 included 65 men and 51 women who had a mean age of 37.4 years at the initial examination. CD was of the small bowel type in 19 patients, of the small and large bowel type in 80, and of the large bowel type in 17. Neoplastic lesions, including atypical cells, were detected by biopsy in 22 patients (19.0%), among which 18 had carcinomas and 4 had atypical cells.
Table 1, 2 display the clinicopathological findings of the 18 patients with anorectal cancer. The detection frequency of anorectal cancer was very high at 15.5% (13.8% for men and 17.6% for women). The age at cancer diagnosis was 46.9 (range: 30-64) years, which was lower than the susceptible age for sporadic colon cancer (http://gdb.ganjoho.jp in Japanese). The time from the onset of CD to the diagnosis of cancer was 275.4 (range: 131-504) months and was more than 10 years after the onset of CD in all patients. The type of CD involved the small and large bowel in 17 patients and the small bowel complicated with cancer at the site of the anal ulcer in 1 patient. Cancer detection methods included biopsy of the anal canal in 14 patients, biopsy of fistulas in 1, and colonoscopy in 3. Of 18, 10 patients underwent lavage cytology of the proctodeum, and 9 cases were of class III or higher (class III in 4, IV in 4, and V in 1). The sites of cancer were Ra in 1 patient, Rb in 1 patient, RbP in 8 patients, PRb in 4 patients, P in 4 patients, and anal in 16 patients. The most common macroscopic type was the infiltrating type (types 3, 4, and 5). The unclassified type (type 5) most frequently occurred. The depth of invasion was greater than T3 in 17 patients, with the exception of a patient with lower rectal cancer (Tis). Invasion to neighboring organs was identified in 6 patients. Furthermore, in terms of the histologic type, there were strong trends of poor differentiation, and 10 cases had findings of mucinous carcinoma. Progression according to the Classification of the Japanese Classification of Colorectal Carcinoma11) was stage 0 in 1 patient, stage II in 8, stage IIIa in 2, stage IIIb in 2, and stage IV in 5. Of the 18 patients, 4 had local invasion or distal metastasis and were unresectable. Resection methods for the remaining 14 patients comprised total pelvic exenteration in 3 and abdominoperineal resection in 11, 3 of whom involved combined resection of adjacent organs. Curability was classified as R0 in 12, R1 in 1, and R2 in 1. Anal lesions were present in all patients, which included fistulae and abscesses in 16, skin tags in 6, ulcerated lesions in 4, and stenosis in 2 (multiple conditions possible). There were 16 cases who had a history of intestinal tract surgery (one surgery in 7, two surgeries in 4, and three surgeries in 5), and 3 patients were complicated with cancer of the excluded anorectal site following colostomy.
Table 1.
Clinicopathological Factors of Anorectal Cancers Complicating Crohn’s Disease (CD).
| Frequency | 18/116 (15.5%) |
| Sex | |
| Men | 9/65 (13.8%) |
| Women | 9/51 (17.6%) |
| Age at cancer diagnosis (years) | 46.9 ± 9.5 (30-64) |
| Disease duration (months) | 275.4 ± 100.6 (131-504) |
| CD type | |
| Small bowel | 1/19 (5.3%) |
| Small and large bowel | 17/80 (21.3%) |
| Large bowel | 0/17 |
| Site of cancer | |
| Ra | 1 |
| Rb | 1 |
| RbP | 8 |
| PRb | 4 |
| P | 4 |
Table 2.
Clinicopathological Factors of Anorectal Cancers Complicating Crohn’s Disease (CD).
| Macroscopic type | Histology | ||
| 0 | 1 | well | 5 |
| 1 | 2 | well with muc | 1 |
| 2 | 2 | well to por with muc | 3 |
| 3 | 3 | mod to por | 2 |
| 4 | 3 | por with muc | 2 |
| 5 | 7 | muc | 4 |
| neuroendocrine | 1 | ||
| Depth of invasion | |||
| Tis | 1 | Operative method | |
| T3 | 10 | Abdominoperineal resection+ | 11 |
| T4a | 1 | Total pelvic exenteration | 3 |
| T4b | 6 | Unresectable | 4 |
| Stage | Curability | ||
| 0 | 1 | R0 | 12 |
| II | 8 | R1 | 1 |
| IIIa | 2 | R2 | 1 |
| IIIb | 2 | ||
| IV | 5 |
muc: mucinous carcinoma; por: poorly differentiated adenocarcinoma; mod: moderately differentiated adenocarcinoma; +combined resection of adjacent organs
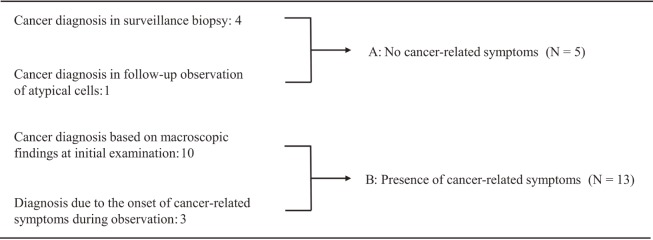
Figure 1 displays the events preceding cancer diagnosis. In 10 cases, cancer was strongly suspected on the basis of symptoms and macroscopic findings at the anal region during the initial examination (Figure 2). The onset of cancer-related symptoms during the follow-up of a CD anal lesion led to a diagnosis of cancer in 3 patients. A diagnosis was made based on transanal histological examination or endoscopy for surveillance in 4 patients, and cancer was diagnosed based on a second biopsy during the follow-up observation following the findings of atypical epithelium via histological analysis in 1. There were 2 patients in whom anal canal carcinoma was found after a diagnosis of atypical epithelium was made based on an initial transanal biopsy, of whom 1 was asymptomatically diagnosed with carcinoma by endoscopy 21 months after a follow-up of the atypical epithelium diagnosis, as mentioned above. In the other patient, cancer-related symptoms (persistent pain) appeared only four months after the detection of atypical epithelium, and a diagnosis was made based on the second biopsy (Figure 3). Aside from these patients, atypical epithelium was detected in 4, of which 2 patients underwent abdominoperineal resection for anal dysfunction due to CD; however, the resected specimen comprised regenerating epithelium, and no malignant changes were recognized. The other 2 had been regularly followed up (28 and 57 months) without the detection of atypical epithelium.
Figure 1.
The events preceding anorectal cancer diagnosis.
Figure 2.
Patients diagnosed as having anal cancer based on macroscopic findings.
A. 47-year-old male patient. Chief complaint: Anal pain. Disease duration: 288 months
B. 31-year-old female patient. Chief complaint: Anal pain. Disease duration: 150 months
Figure 3.
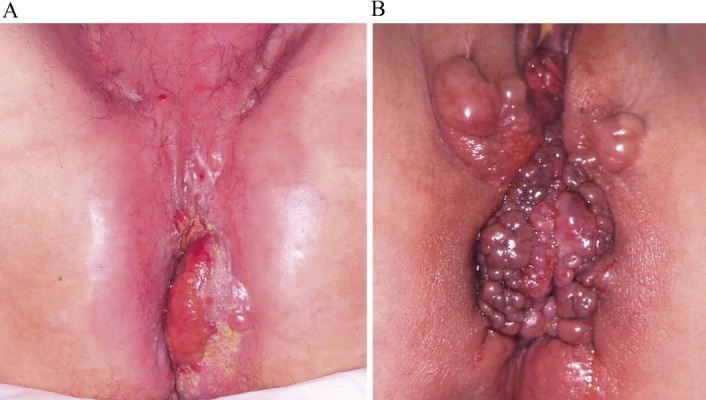
A patient with atypical cells who rapidly developed advanced cancer.
A 58-year-old female patient. Disease duration: 504 months. Chief complaint: Anal pain. Diagnosed with cancer 4 months after atypia was detected by biopsy
A. Anal findings: Ulcer on the anterior wall
B. Resected specimen (abdominoperineal resection)
C, D. Histopathological findings: Resected specimens (mucinous carcinoma)
Cancer-related symptoms were identified in 13 patients, persistent pain in 11, mucus discharge in 4, bleeding in 3, and tumor prolapse through the anus in 1 (overlap possible). The 18 anorectal cancer cases were assigned to two groups based on the presence or absence of cancer-related symptoms and their clinicopathological factors, and their prognoses were compared (Figure 1 and Table 3). Group A included cases where cancer was detected without cancer-related symptoms and regarded as the surveillance detection group. Group B included cases where cancer was diagnosed based on symptoms. There was no difference observed between the two groups regarding the patients' age, sex, or time from the onset of CD to the diagnosis of cancer. Tumor marker [carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9)] levels were within the normal range in all patients in group A without cancer-related symptoms. In contrast, an increase in the levels of these markers, particularly of CEA, was observed in 9 of the 13 patients in the symptomatic group B. In terms of imaging findings [computed tomography (CT), magnetic resonance imaging (MRI), and positon emission tomography (PET)], a cancer lesion was not detected in all patients in group A and was detected in 7 patients in group B. It was suggested that differentiation between cancer and inflammation was difficult. A comparison by progression revealed that lymph node metastasis was absent in all 5 patients in group A; it also revealed that their diagnoses of cancer were made at earlier stages than those in group B. Thus, curative resection was possible without performing composite resection of the neighboring organs in all patients. In group B, 9 of the 13 patients had stage IIIa/IV cancer, and 6 underwent non-curative resections or were unresectable. Moreover, 6 patients required composite resection of the neighboring organs, including 3 patients who required total pelvic exenteration. The post-operative observation period was 3-64 months (median: 23 months). Although group A included 1 patient who died due to CD recurrence following five intestinal surgeries that led to septicemia and who had a complication of cerebrovascular accident, all other patients followed a good course without recurrence in the 59 month post-operative observation period (range: 28-77 months). In group B, 9 patients with stage IIIa/IV cancer died (mean survival time: 13.5 months), whereas 2 of the 4 stage II patients are undergoing continued treatment for recurrence (Figure 4).
Table 3.
Comparison of Histopathological Features between Symptomatic and Asymptomatic Cases.
| Group A [5] | Group B [13] | P-value | |
|---|---|---|---|
| Age (years) | 48.0 ± 13.1 | 46.5 ± 8.8 | 0.826 |
| (34-64) | (30-59) | ||
| Sex | 0.599 | ||
| Male | 3 | 6 | |
| Female | 2 | 7 | |
| Disease duration | 290.2 ± 105.03 | 276.5 ± 106.9 | 0.811 |
| (months) | (204-441) | (131-504) | |
| CEA (ng/mL) | 0.009 | ||
| <5 | 5 | 4 | |
| >5 | 0 | 9 | |
| Depth of invasion | 0.097 | ||
| <T2 | 1 | 0 | |
| >T3 | 4 | 13 | |
| Lymph node metastasis | 0.019 | ||
| - | 5 | 5 | |
| + | 0 | 8 | |
| Distant metastasis | 0.16 | ||
| M0 | 5 | 8 | |
| M1 | 0 | 5 | |
| Curative resection | 0.063 | ||
| R0 | 5 | 7 | |
| R1, R2 | 0 | 6 | |
| Stage | 0.019 | ||
| <II | 5 | 5 | |
| >IIIa | 0 | 8 |
Figure 4.
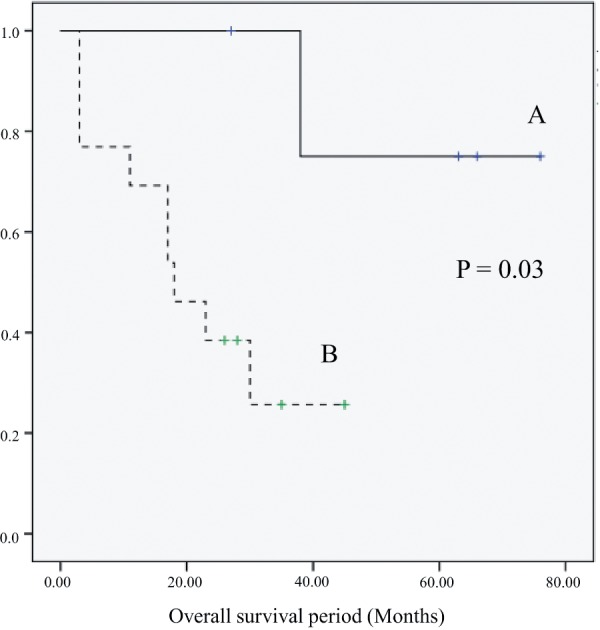
Overall survival period.
Group A had a better prognosis than group B (P = 0.03)
Next, we investigated the detection rate of neoplastic lesions via transanal histologic examination on 103 of the 116 patients after excluding 13 patients who were diagnosed following the appearance of cancer-related symptoms (Figure 5). The incidence of cancer and atypical cell detection was 5.8% (6 patients). Furthermore, when we added the 3 patients who were diagnosed by endoscopy while being observed after transanal biopsy, the detection rate of cancer and atypical cells was 8.7%.
Figure 5.
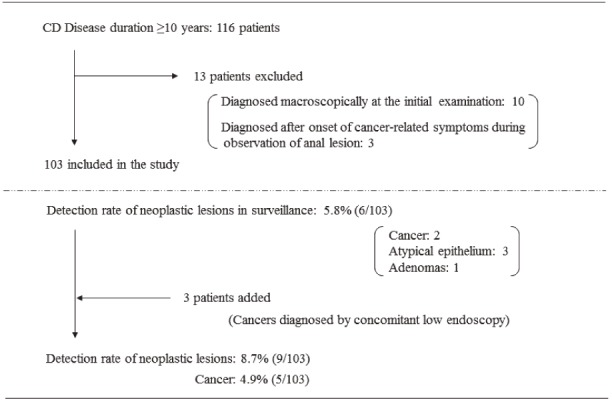
Detection of neoplastic lesions by surveillance.
The rate of cancer detection by surveillance was 4.9%
Discussion
CD is an inflammatory enteric disease with an unknown cause, and the number of CD patients has been increasing in Japan, reaching 40885 in 2014, according to the Number of Recipient Certificates Issued for Specific Disease Treatment (http://www.nanbyou.or.jp, in Japanese). In association with this increase, the number of patients with long CD duration is also increasing. Although the incidence is low, gastrointestinal malignant tumors are an emerging problem; they are an important complication of CD that affects vital prognosis.
CD-related cancers were first reported in 1948 by Warren et al.12), and the number of reports of CD-related gastrointestinal cancers is increasing in Japan. The incidence of cancer in CD patients is reportedly 0.6%-3.1% in Western countries, which is significantly higher than that in the general population13,14); in Japan, the incidence is 1%-1.4%15,16). The study conducted by von Roon et al. included a meta-analysis related to cancer development in CD patients and reported that the relative risk of colorectal cancer morbidity was 2.417). In Japan, Yano et al. examined 512 CD patients and reported the relative risk of developing colorectal cancer to be 3.2 in CD patients18). Furthermore, Canavan et al. reported the cumulative incidence of colorectal cancer in CD patients 10, 20, and 30 years after CD onset to be 2.9%, 5.6%, and 8.3%, respectively, similar to the increased risks in UC patients19).
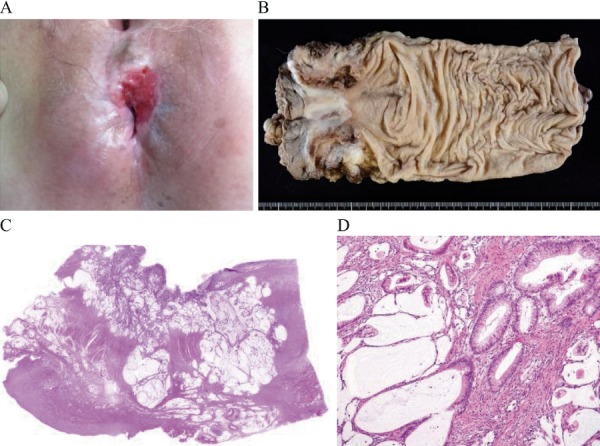
Oncogenesis in CD is generally believed to occur through developmental pathways, such as colitic cancer following a stage of dysplasia, oncogenesis of lesions (e.g., fistulae), and concurrent sporadic cancer15). Colitic cancer is referred to as colitis-associated colorectal cancer in Western countries. Its developmental pathway is characterized by the origin of a tumor in chronically inflamed mucosae due to the long disease duration, which evolves into precancerous dysplasia that eventually progresses into cancer. Environmental and genetic factors are believed to be involved in this “ginflammation-dysplasia-carcinoma sequence”20). The present study also included many patients complicated with perianal fistulae; however, carcinogenesis from the fistulae was only histopathologically suggested in 1 patient (Figure 6). Fistulous tracts cannot be identified in highly invasive cancers accompanied by perianal hardening, which were tumors visible in imaging. In the recently gradually increasing number of early diagnosed patient (1 patient with intramucosal carcinoma), the origin of the cancer is the anorectal mucosa, and the cancer is accompanied by dysplasia of the peripheral mucosa in all patients. However, as atypical cells were not found in the fistula, chronic inflammation of the anorectal site mucosa can be considered to be the most important origin of the tumor.
Figure 6.
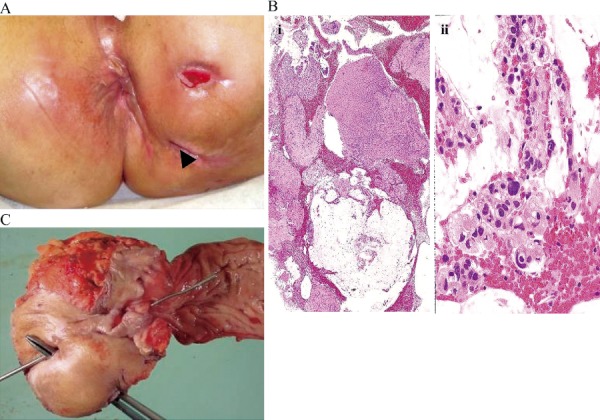
Patient with cancer diagnosed by biopsy of the fistulous tract.
A 64-year-old female patient. No changes in symptoms. Disease duration: 216 months
A. Intractable fistula: Biopsy sample collected from the arrow
B. Histological diagnosis: Mucinous carcinoma (i: low magnification, ii: high magnification)
C. Resected specimen (abdominoperineal resection)
Onset at a young age12,18), long disease duration18-20), extensive lesions in the colon12,21), and intestinal strictures22) are some of the risk factors associated with CD-related colorectal cancer. The results of our study were consistent, and the time from onset of CD to the diagnosis of cancer was ≥10 years in all cancer patients in this study.
The right colon is considered to be the more common site of cancer in Western countries5,7), but the distal colon is more common site in Japan8,9). Higashi et al. studied 122 CD patients complicated with gastrointestinal carcinoma and found that anorectal cancer was present 80% of all patients9). We also experienced 25 patients with lower gastrointestinal tract carcinomas in our department, of which 75% were lower anorectal cancers.
Among the histological types of CD-related colorectal cancer, mucinous carcinoma, poorly differentiated adenocarcinomas, and signet-ring cell carcinomas are common, occurring more frequently than sporadic cancer5,10,23). Macroscopic findings also indicate that infiltrating types of progressive cancers frequently occur; many such patients are detected in highly advanced stages and have five-year survival rate of 10.1%-46.4%, indicating a poor prognosis5,23,24). A comparison of the 72.1% five-year overall survival of colon cancer in Japan25) further reinforces the highly malignant nature of CD-related colorectal cancer. Furthermore, the age at cancer diagnosis was during the productive age of the 40s, which is 20 years less than the average age at diagnosis for sporadic colon cancers (http://gdb.ganjoho.jp). Therefore, as in the case mentioned earlier, it is important to make sure to establish a diagnosis prior to lymph node metastasis.
Various methods must be used to aim for an early diagnosis. First, diagnostic imaging (e.g., CT and MRI) are used as effective cancer diagnosis methods and are superior for diagnosing the cancer grade; however, such methods are not effective for early detection. Fluorodeoxyglucose-PET (FDG-PET) is also ineffective in differentiating between inflammation and cancer cell accumulation. Its ineffectiveness in making an early diagnosis has been demonstrated by the low FDG accumulation rate in case of the frequently occurring mucinous carcinoma26,27). Although an increase in CEA levels has been reported to be an effective tumor marker for detecting fistulas in CD28), all patients already had highly advanced cancers. Thus, CEA levels may be an effective hint for making a diagnosis, but they have poor efficacy for making an early diagnosis, which was also demonstrated through the patients we experienced. Our study suggests that transanal histology is the only method with the potential for relatively early cancer detection, although it warrants further investigation.
Previous studies on endoscopy in Western patients have included those by Friedman et al., who performed surveillance colonoscopy 663 times on 259 CD patients and reported surveillance colonoscopy to be an effective method for detecting and finding cancer and dysplasia in 16% of their patients29). In addition, Siegel et al. examined risk factors for CD onset and found that patients whose colorectal cancers were detected through surveillance colonoscopy had a better prognosis than those whose colorectal cancers were symptomatically detected30). Based on these reports, surveillance colonoscopy following the diagnosis of UC is recommended, as described in the BSG and AGA guidelines3,31). However, there is no consensus in Japan on whether colonoscopy should be performed on patients with long CD duration for the purpose of detecting cancer in the manner in which it is performed on patients with UC. This is because colonoscopy is difficult to perform in many CD patients due to characteristic complications of fistulas, stenoses, and anal lesions, compared with UC, which has established diagnostic endoscopic methods, including tumor morphology, pit patterns, and color characteristics. Furthermore, the conditions of CD lesions can make it difficult to differentiate between cancer and background mucosa; thus, making an endoscopic diagnosis is believed to be more difficult in CD than in UC. Yao et al. examined 15 cancer patients complicated with CD and found that dysplasia, which is important in diagnostic biopsies for UC, was only detected in 1 patient16); therefore, many technical and diagnostic challenges remain regarding the use of endoscopy for surveillance.
As colorectal cancers in patients complicated with CD in Japan differ from those in patients in Western countries due to them frequently being located at the anorectal site, a distinct surveillance program needs to be established for Japanese patients. Currently, the Ministry of Health, Labour and Welfare's project team for the Investigation and Research for Intractable IBD is implementing a “Pilot study for establishing a surveillance program on anorectal canal carcinomas including fistula cancers complicating CD.” This pilot study has been accumulating data on neoplastic lesions detected by transanal and endoscopic histological and cytological diagnoses of the anorectal site of CD patients with lesions (e.g., anal and rectal stenoses and fistulas, including the excluded rectum due to colostomy) for >10 years10). In the present study, we followed up 103 CD patients without cancer-related symptoms, including persistent pain or mucous and bloody stools. We succeeded in diagnosing cancer or atypical cells in 9 of these patients (8.7%), among whom 6 were diagnosed by transanal biopsy (5.8%), and 3 were diagnosed by subsequent endoscopy. Of these 9 patients, cancers were detected in 5 patients, and there was no recurrence. Although 1 patient died due to CD-related sepsis, the remaining 4 progressed favorably without recurrence for 28-77 months post-operation.
Generally, anal canal carcinoma and fistula cancers are diagnosed following the appearance of symptoms of mucus secretion or pain32); however, the cancers detected following the appearance of symptoms among our patients were all advanced with an invasion depth of ≥T3 and a low curative resection rate. As we cannot wait for the appearance of cancer-related symptoms to start treating such cancers, making a diagnosis prior to the appearance of cancer-related symptoms determines whether a good prognosis can be expected. The results of the current study support this assertion.
In terms of biopsy sites, the anorectal mucosa, which is the most important origin of the tumor, cannot be omitted. Curettage specimens of active perianal fistula canals and abscess cavities must be submitted for a pathological examination. Finally, rectal lavage cytology resulted in a classification of stage III or worse in 9 cases in which this test was performed. Therefore, this can be used as a painless method for patients in whom anal symptoms prevent receiving a digital rectal palpation comfortably in an outpatient clinic. At our hospital, we collected 10-20 mL of saline injected by inserting a thin Nelaton catheter for this cytological diagnosis.
The current study revealed some limitations to our method of randomized transanal biopsy under anesthesia. Early stage cancer in 1 patient was detected by endoscopic biopsy, with no early diagnoses made by transanal biopsy. Therefore, targeted biopsy by endoscopy is required for the early diagnosis of early stage cancers. Currently, we are attempting the routinization of performing endoscopy simultaneously with randomized transanal biopsy under anesthesia. Taking advantage of their respective strengths can also contribute to the early diagnosis of anorectal site, colon, and small bowel cancers.
Finally, we discuss the indication of rectal amputation for intractable anorectal lesions, which is a surgical treatment for cancer prevention. In CD, it is fairly common to require colostomy due to intractable anal lesions and cancers. Koganei et al. reported that 73% of CD patients who underwent temporary colostomy to alleviate symptoms followed by closure required reconstruction of colostomy33). Furthermore, Mueller et al. conducted a follow-up study on CD patients with anal lesions and found that patients with complex perianal fistulas and colorectal lesions were at a higher risk of requiring permanent colostomy34). As the chances of recovering anal functionality are low in patients with complex anal lesions (e.g., fistulas and stenosis) and are at a persistent risk of cancer, some specialists recommend considering rectal amputation33,35). The present study also included 3 patients with cancer of the excluded anorectum following colostomy. In addition to these cases, there was a patient who underwent rectal amputation due to an anal function disorder, and early stage cancer was detected in the resected specimen. Thus, considering the poor prognosis of CD-related cancers, rectal amputation for improving long-standing anal dysfunction can also be considered as a preventive treatment for cancer.
To conclude, we investigated the significance of lower anorectal site biopsy for cancer surveillance in CD and demonstrated its effectiveness as a method that enables the diagnosis of relatively early stage cancer. Additionally, performing regular observations by endoscopy can improve the rate of diagnosis even further.
Colorectal cancers complicated with CD often affect younger patients and are associated with a poor prognosis; early diagnosis is extremely important in these cancers than in general colorectal cancer. It is suggested that transanal histology is effective for the surveillance of CD-related anorectal cancer and appears to be a promising method for improving prognosis.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
We thank Crimson Interactive Pvt. Ltd. (Ulatus; www.ulatus.jp) for their assistance in manuscript translation and editing.
References
- 1.Van Assche G, Dignass A, Bokemeyer B, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3; special situations. J Crohns Coitis. 2013 Feb; 7(1): 1-33. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto T, Iwao Y, Igarashi M, et al. Endoscopic and chromoendoscopic atlas featuring dysplastic lesions in surveillance colonoscopy for patients with long-standing ulcerative colitis. Inflamm Bowel Dis. 2008 Feb; 14(2): 259-64. [DOI] [PubMed] [Google Scholar]
- 3.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010 Feb; 138(2): 738-45. [DOI] [PubMed] [Google Scholar]
- 4.Hata K, Watanabe T, Kazama S, et al. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23-year surveillance programme in the Japanese population. Br J Cancer. 2003 Oct; 6; 89(7): 1232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi PM, Zeling MP. Similarity of colorectal cancer in Crohn's disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994 Jul; 35(7): 950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen M, Mose H, Gislum M, et al. Survival after colorectal cancer in patients with Crohn's disease: A nationwide population-based Danish follow-up study. Am J Gastroenterol. 2007 Jan; 102(1): 163-7. [DOI] [PubMed] [Google Scholar]
- 7.Stahl TJ, Schoetz DJ Jr, Roberts PL, et al. Crohn's disease and carcinoma: increasing justification for surveillance? Dis Colon Rectum. 1992 Sep; 35(9): 850-6. [DOI] [PubMed] [Google Scholar]
- 8.Sinozaki M. Crohn's disease and intestinal cancer in Japan. J Jpn Soc Coloproctol. 2008 Jul; 61: 353-63. Japanese. [Google Scholar]
- 9.Higashi D, Katsuno H, Takahashi K, et al. Current state of and problems related to cancer of the intestinal tract associated with Crohn's disease in Japan. Anticancer Res. 2016 Jul; 36(7): 3761-6. [PubMed] [Google Scholar]
- 10.Sugita A, Koganei K, Tatsumi K, et al. [Gastrointestinal malignancies with Crohn's disease]. Stomach and Intestine. 2012 Sep; 47(10): 1537-44. Japanese. [Google Scholar]
- 11.Japanese Society for Cancer of the Colon and Rectum (Sugihara K edit): Japanese Classification of Colorectal Carcinoma. 2nd English Ed. Kanehara & Co. Tokyo, 2009. [Google Scholar]
- 12.Warren S, Sommers SC. Cicatrizing enteritis as a pathologic entity analysis of one hundred and twenty two cases. Am J Pathol. 1948 May; 24(3): 475-501. [PMC free article] [PubMed] [Google Scholar]
- 13.Korelitz BI. Carcinoma of the intestinal tract in Crohn's disease: results of survey conducted by the National Foundation for Ileitis and colitis. Am J Gastroenterol. 1983 Jan; 78(1): 44-6. [PubMed] [Google Scholar]
- 14.Gyde SN, Prior P, Macartney JC, et al. Malignancy in Crohn's disease. Gut. Dec; 21(12): 1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto K, Iwadare J, Kitamura S. [A study of cancerization in patients with Crohn's disease]. Stomach and Intestine. 2002 Jul; 37(8): 1023-30. Japanese. [Google Scholar]
- 16.Yao S, Iwashita A, Nishimura T, et al. [Clinicopathologic and immunohistochemical features of the colorectal carcinomas, complicating Crohn's disease]. Stomach and Intestine. 2002 Jul; 37(8): 1047-58. Japanese. [Google Scholar]
- 17.Von Roon AC, Reese G, Teare J, et al. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum. 2007 Jun; 50(6): 839-55. [DOI] [PubMed] [Google Scholar]
- 18.Yano Y, Matsui T, Uno H, et al. Risks and clinical features of colorectal cancer complicating Crohn's disease in Japanese patients. J Gastroenterol Hepatol. 2008 Nov; 1; 23(11): 1683-8. [DOI] [PubMed] [Google Scholar]
- 19.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006 Apr; 15; 23(8): 1097-104. [DOI] [PubMed] [Google Scholar]
- 20.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004 Jul; 287(1): G7-17. [DOI] [PubMed] [Google Scholar]
- 21.Maykel JA, Hagerman G, Mellgren AF, et al. Crohn's colitis: the incidence of dysplasia and adenocarcinoma in surgical patients. Dis Colon Rectum. 2006 Jul; 49(7): 950-7. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki Y, Ribeiro MB, Sachar DB, et al. Malignant colorectal stricture in Crohn's disease. Am J Gastroenterol. 1991 Jul; 86(7): 882-5. [PubMed] [Google Scholar]
- 23.Friedman S. Cancer in Crohn's disease. Gastroenterol Clin North Am. 2006 Sep; 35(3): 621-39. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro MB, Greenstein AJ, Sachar DB, et al. Colorectal adenocarcinoma in Crohn's disease. Ann Surg. 1996 Feb; 223(2): 186-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015 Apr; 20(2): 207-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeuchi H, Nakano H, Uchino M, et al. [Usefulness of FDG-PET for Crohn's disease]. J Jpn Soc Coloproctol. 2008 Jun; 61(6): 303-10. Japanese. [Google Scholar]
- 27.Delbeke D, Martin WH. PET and PET-CT for the evaluation of colorectal carcinoma. Semin Nucl Med. 2004 July; 34(3): 209-23. [DOI] [PubMed] [Google Scholar]
- 28.Sato M, Ogawa H, Shibata C, et al. [A case of anal cancer with rapidly rising CEA in longstanding perianal Crohn's disease after infliximab administration]. Journal of Japanese Society of Gastroenterology. 2010 Jun; 107(6): 885-92. Japanese. [PubMed] [Google Scholar]
- 29.Friedmann S, Rubin PH, Bodian C, et al. Screening and surveillance colonoscopy in chronic Crohn's colitis. Gastroenterology. 2001 Mar; 120(4): 820-6. [DOI] [PubMed] [Google Scholar]
- 30.Siegel CA, Sands BE. Risk factors for colorectal cancer in Crohn's colitis: a case-control study. Inflamm Bowel Dis. 2006 Jun; 12(6): 491-6. [DOI] [PubMed] [Google Scholar]
- 31.Carins SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (updated from 2002). 2010 May; 59(5): 666-89. [DOI] [PubMed] [Google Scholar]
- 32.Iwadare J. [Clinicopathological Study Fistula-cancers]. J Jpn Soc Coloproctol. 1991 Jul; 44: 461-76. Japanese. [Google Scholar]
- 33.Koganei K, Kimura H, Sugita A, et al. [Abdominoperineal resection for intractable anorectal Crohn's disease]. Jpn J Gastroenterol Surg. 2006 Apr; 39: 522-7. Japanese. [Google Scholar]
- 34.Mueller MH, Geis M, Glatzle J, et al. Risk of fecal diversion in complicated perianal Crohn's disease. J Gastrointest Surg. 2007 Apr; 11(4): 529-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ky A, Sohn N, Wenstein MA, et al. Carcinoma arising in anorectal fistulas in Crohn's disease. Dis Colon Rectum. 1998 Aug; 41(8): 992-6. [DOI] [PubMed] [Google Scholar]