Abstract
Objectives: Colonoscopy is the first-line modality to examine the colon even in the very elderly but may have an increased risk of complications. This study aimed to evaluate the efficacy and safety of colonoscopy in the very elderly. Methods: Patients ≥85y old, who underwent colonoscopy between September 2010 and August 2012 in two tertiary-care hospitals in Japan were enrolled. Main outcome measures were cecal intubation rate, detection rate of adenomas and cancers, treatment, adverse events, and long-term outcomes. Results: A total of 207 colonoscopies were performed in 177 patients (females 72, males 105; maximum age 95 years). Of these, 202 attempted to reach the cecum, with success in 92%. Excluding patients with known colorectal neoplasms, invasive cancers were detected in 12%, including T1 lesions in 2% and T2 or deeper in 9%. No cancers were detected in patients referred for surveillance or mild abdominal symptoms. Cancers were found in 25% of patients with positive fecal immunochemical tests, 22% with altered bowel habits, 21% with anemia, and 18% with hematochezia. Treatment of 29 patients with cancer included surgery in 22, endoscopic resection in two and no treatment (due to comorbidities) in five. There were no complications. During 730 days (mean) of follow up, 27 patients died but only three died from recurrent colorectal cancer. Conclusions: Colonoscopy for patients aged ≥85 years is safe. A relatively high detection rate of cancers was found, and most were treatable and even curable. (UMIN000018575)
Keywords: colonoscopy, colorectal cancer, elderly patients
Introduction
Colorectal cancer (CRC) is a second leading cause of cancer deaths in Japan. Colon screening with colonoscopy has been shown to prevent the development of CRC by allowing the excision of pre-malignant lesions and subsequently reduced the number of deaths due to colon cancer1,2). Therefore, average risk adults are recommended to undergo colon screening test beginning at age 50 in the US3,4). In contrast, cancer screening in the elderly is controversial. Since the incidence of CRC increases with age5-7), a greater number of CRCs would be detected if patients aged 85 years or older undergo colonoscopy. However, studies have demonstrated that the increase in life expectancy is much lower in the very elderly than in younger individuals8,9). Colonoscopy in the very elderly has lower efficacy for increasing life expectancy, and the benefits may be outweighed by increased risk. This is partially attributed to the fact that the elderly are more likely to die of other causes before dying from CRC. Indeed, 46% patients with a positive fecal occult blood test without follow-up colonoscopy died of other causes within five years10).
Due to a long redundant colon, total colonoscopy in the very elderly is difficult. Subsequently, a significant concern when performing colonoscopy in the very elderly is a potentially increased risk of complications11,12). Cardiopulmonary complications are the most common adverse events and the incidence is related to the level of sedation, presence of comorbidities, and prolonged procedure time. In addition, both perforation and bleeding are reported to be more common in the elderly11,12). In contrast, some reports have shown that colonoscopy in the very elderly can be performed safely and successfully13-15). CT colonography has shown to be similar detection rate for advanced neoplasia compared with optical colonoscopy16). From the viewpoint of safety, CT colonography is an alternative to colonoscopy in the very elderly17), but a significant weakness of this modality is the inability to resect colorectal polyps during the procedure. Therefore, elderly patients with polyps must undergo colonoscopy for resection, or to confirm the diagnosis.
According to the latest statistics (2014) published by the Japanese Government, life expectancy at age 85 is 6.24 years in men and 8.35 years in women18). In the US, the life expectancy at 85 year of age was 5.9 years in men and 6.9 years in women19). It is somewhat surprising that life expectancy at age 85 is greater than five years in both Japan and in the US. These data suggest that colonoscopy may be beneficial even in the very elderly, provided that short-term outcomes, such as the occurrence of adverse events relating to colonoscopy is as low as that in younger patients. To evaluate the efficacy and safety of colonoscopy in patients aged 85 years and older, we conducted a multicenter retrospective study in Japan.
Methods
Trial design
This is a retrospective review of patient data from two tertiary-care hospitals (Aizu Medical Center, Medical Center East) in Japan. Prior to commencement, the study was approved by the Institutional Review Board of Fukushima Medical University (No. 1987) and was registered with the University Hospital Medical Information Network (UMIN000018575). All the data were collected by December 2015. The STROBE (Strengthening the Reporting of Observational Study in Epidemiology) guidelines were followed in reporting this study.
Subjects
Patients aged 85 years and older who underwent colonoscopy from September 2010 to August 2012 in two tertiary-care hospitals were enrolled. Information, including long-term outcomes was obtained from medical records and telephone interviews in November 2014. In all the three hospitals, the first-line screening test for the colon in symptomatic patients, including positive FIT, was colonoscopy with lavage bowel preparation. Patients with a performance status20) of 0 or 1 were included. For bowel preparation, patients normally took 2 liters of polyethylene glycol solution or 1.8 liters of magnesium citrate solution on the day of the examination, after receiving sodium picosulfate on the previous day. An overwhelming majority of the elderly patients took polyethylene glycol in the hospital, and were monitored for adverse events related to the bowel preparation. Patients who underwent emergency colonoscopy and did not undergo bowel preparation were not included in this study.
Colonoscopy
Due to the risk of respiratory or cardiovascular complications, most of the patients did not undergo sedation during colonoscopy. When the patients complained of severe abdominal pain or discomfort during colonoscopy, diazepam or midazolam was administered with routine continuous monitoring of electrocardiogram and arterial saturation. Standard instruments with narrow band imaging or blue laser imaging are usually used; however, the pediatric colonoscope is used for patients of small stature, patients with post-operative adhesions or multiple diverticula. Chromoendoscopy was applied if necessary.
Colonoscopic procedures were started in the left lateral position. To prevent intestinal peristalsis, scopolamine butylbromide or glucagon was administered. During insertion, position change and abdominal compression were applied if the instrument failed to advance or became a loop. Cecal intubation was defined as passage of the tip of the colonoscope to a point proximal to the ileocecal valve with adequate visualization of the cecum and the appendix orifice.
Outcome measures
The outcome measures assessed include cecal intubation rate, reasons for incomplete colonoscopy, occurrence of adverse events related to colonoscopy, detection rate of adenomas and cancers, treatment, and long-term outcomes. Adverse events related to colonoscopy were rated by the occurrence of perforation, bleeding or cardiopulmonary complications (e.g., myocardial infarction, pneumonia) within 2 weeks after the procedure. For histologic classification, so-called “cancer in situ (Tis)” was defined as “adenoma with high grade dysplasia.” All the data were retrospectively collected from patient records.
Statistical analysis
The detection rate was calculated along with 95% confidence intervals (CI) using the Clopper-Pearson method and expressed as “proportion [95% CI lower, 95% CI upper].” When calculating the detection rates for adenomas and cancers, patients referred with the diagnosis of colorectal cancer were excluded from the analysis. The overall survival was calculated as the period from the date of initial colonoscopy until the date of death due to any cause, or last follow-up. The overall survival times were estimated by the Kaplan-Meier method. All the statistical analysis was performed with Stata 13.0 (Stata Corp., TX, US).
Results
Patient demographics
A total of 7438 colonoscopy procedures were performed in two tertiary-care hospitals. Procedures on patients 84 years old and younger were excluded from the analysis, and the remaining 211 (2.8%, 211/7438) procedures reviewed. Four emergency procedures in patients with sigmoid volvulus were excluded, and 207 procedures analyzed (Figure 1). A total of 207 procedures were performed in 177 patients. Twenty-one patients underwent colonoscopy twice, two underwent colonoscopy three times, and three patients underwent the procedure four times. Table 1 shows patient demographics at the time of undergoing colonoscopy for the 207 procedures. The mean age was 86.9 years and the oldest age was 95 years. The majority of patients were American Society of Anesthesiologists (ASA) physical status class 2, and 21% were class 3. Surveillance colonoscopy after previous resection by endoscopy or surgery accounted for 31% of the procedures. Examination for mild abdominal symptoms (e.g., abdominal distention, bloating, pain), positive FIT, hematochezia or anemia found during routine evaluation accounted for over 50% of procedures, and the remainder were done for various reasons, including previous endoscopic therapy or examination prior to surgery.
Figure 1.

Flow diagram of study participants.
Table 1.
Patent Demographics at Colonoscopy.
| Gender | N |
| Female | 81 (39%) |
| Male | 126 (61%) |
| Age, years | |
| Mean ± standard deviation | 86.9 ± 2.0 |
| 85-89 | 188 (91%) |
| 90-94 | 17 (8%) |
| ≥95 | 2 (1%) |
| ASA physical status | |
| Class 1 | 26 (13%) |
| Class 2 | 137 (66%) |
| Class 3 | 44 (21%) |
| Indication for colonoscopy | |
| Surveillance after resection | 67 (32%) |
| Mild abdominal symptoms | 30 (14%) |
| Altered bowel habits | 25 (12%) |
| Positive FIT | 25 (12%) |
| Hematochezia | 20 (10%) |
| Anemia | 20 (10%) |
| Examination prior to surgery | 11 (5%) |
| Endoscopic treatment | 9 (4%) |
Twenty-one patients underwent colonoscopy twice. Two underwent colonoscopy three times, and three underwent colonoscopy four times.
ASA: American Society of Anesthesiologists
FIT: fecal immunochemical test
Colonoscopy
A total of 144 (70%) procedures were performed without sedation, and 63 procedures were performed with conscious sedation. Of the 207 procedures, 202 were intended to reach the cecum. Cecal intubation was achieved in 185 (92%) procedures and failed in the remaining 17. Reasons for incomplete colonoscopy were poor bowel preparation in six, stenosis due to cancer in five, long redundant colon with fixation in three, and severe diverticulosis in three. Complication rate related to colonoscopy was 0.0% (0/207).
Adenoma/cancer detection
Twenty-one patients received multiple times of colonoscopy. The detection rates were calculated based upon the most significant lesion detected in the initial colonoscopy. In addition, patients referred for treatment of colorectal neoplasia were excluded from the calculation. In a total of 163 patients, adenoma detection rate was 48% [40, 56], cancer detection rate was 12% [7, 17], and total detection rate, including adenoma and cancer was 59% [51, 67]. Table 2 shows the adenoma/cancer detection rates according to the indications. Relatively high detection rates of adenomas were observed for all the indications although adenomas with high grade dysplasia were detected only in patients with positive FIT, altered bowel habits, or anemia.
Table 2.
Adenoma/Cancer Detection Rates by Indication.
| Indication | Adenoma | Cancer | Overall (adenoma+cancer) |
||||
|---|---|---|---|---|---|---|---|
| All | LGD | HGD | All | T1 | T2 or deeper | ||
| All indications (n=161) | 48% [40, 56] | 44% [36, 52] | 4% [1, 8] | 12% [7, 17] | 2% [1, 6] | 9% [5, 14] | 59% [51, 67] |
| Surveillance after resection (n=53) | 51% [37, 65] | 51% [37, 65] | 0% | 0% | 0% | 0% | 51% [37, 65] |
| Mild abdominal symptoms (n=25) | 60% [39, 79] | 60% [39, 79] | 0% | 0% | 0% | 0% | 60% [39, 79] |
| Positive FIT (n=24) | 54% [33, 74] | 42% [22, 63] | 13% [3, 32] | 25% [10, 47] | 8% [1, 27] | 17% [5, 37] | 79% [58, 93] |
| Altered bowel habits (n=23) | 39% [20, 61] | 35% [16, 57] | 4% [0, 22] | 22% (5/23) | 4% [0, 22] | 17% [5, 39] | 61% [39, 80] |
| Anemia (n=19) | 42% [20, 67] | 32% [13, 57] | 11% [1, 33] | 21% [6, 46] | 0% | 21% [6, 46] | 63% [38, 84] |
| Hematochezia (n=17) | 29% [10, 56] | 29% [10, 56] | 0% | 18% [4, 43] | 6% [0, 29] | 12% [1, 36] | 47% [23, 72] |
LGD: low grade dysplasia; HGD: high grade dysplasia; FIT: fecal immunochemical test
Detection rates were calculated based upon the most significant lesion detected in the initial colonoscopy. In addition, patients referred for treatment of colorectal neoplasia were excluded from the calculation.
In contrast, there was an apparent trend in the cancer detection rate. No cancer was detected in patients who underwent colonoscopy for surveillance or mild abdominal symptoms; however, cancer was detected in approximately 20% of patients who had a positive FIT, altered bowel habits, anemia or hematochezia, in almost equal proportions. The overall detection rates, including adenomas and cancer were similar (approximately 50% or greater) regardless of the indication.
Treatment
Of the 207 procedures performed, endoscopic resection was performed for low-grade adenoma in 33, high-grade adenoma in 15 and T1 cancer in two. Regarding low-grade adenoma, cold biopsy (n=10, median size 3 mm), hot snare resection (n=20, median size 7 mm, range 5-15 mm), and endoscopic mucosal resection (n=3, size 11-15 mm) were carried out. Regarding high-grade adenoma, hot snare polypectomy (n=6, median size 5 mm, range 3-10 mm) and endoscopic mucosal resection (n=9, median size 16 mm, range 8-26 mm) were performed. Regarding T1 cancers, endoscopic mucosal resection was performed for a polypoid polyp measuring 10 mm, and endoscopic submucosal dissection was performed for a laterally spreading tumors measuring 31 mm. There were no complications related to endoscopic resection. Surgical intervention was performed for T1 cancer in three and T2 or deeper in 19, with no complications related to surgical resection. The remaining five cancers (T1: 1, T2: 4) were not treated, because these five patients had significant comorbidities (ASA physical status class 3) that were finally judged to preclude surgical resection.
Long-term outcomes
The mean observation time was 730 days (range 34-1531 days). Figure 2 shows the cumulative incidence of overall survival. The survival rate at 12 mo was 92.1%, and at 24 mo was 88.2%. Of the 177 patients in the study, 27 (15.3%) died during follow-up. Table 3 shows detailed causes of death. There were five CRC related deaths. Two of the patients had untreated CRC that progressed. Three patients died of recurrent CRC after resection. Ten patients died of extra-colonic malignancies, including lung cancer, hematologic malignancies, pancreas, bile duct, stomach and liver cancers. The remaining 12 patients died of other diseases, including heart disease, pneumonia, and old age.
Figure 2.
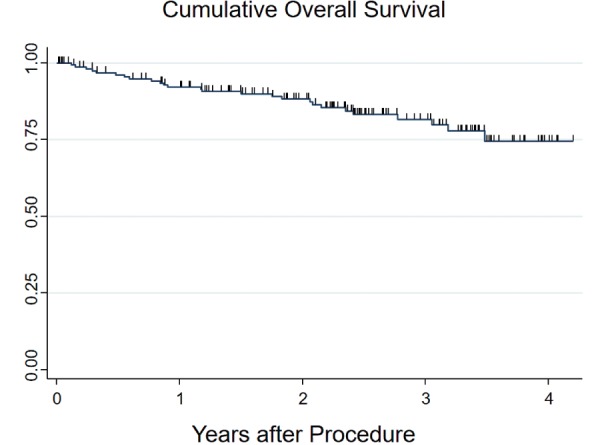
The Kaplan-Meier method is used to calculate the cumulative incidence of overall survival. Ticks denote censored cases. The survival rate at 12 mo was 92.1%, and at 24 mo was 88.2%.
Table 3.
Cause of Death.
| Cause | N |
|---|---|
| All | 27 (100%) |
| Colorectal cancer | 5* (19%) |
| Extra-colonic malignancy | 10 (37%) |
| Lung | 3 |
| Bone marrow | 2 |
| Pancreas | 2 |
| Biliary duct | 1 |
| Stomach | 1 |
| Liver | 1 |
| Others | 12 (44%) |
| Heart disease | 5 |
| Pneumonia | 2 |
| Old age | 2 |
| Unknown | 3 |
*Three patients had recurrent colorectal cancer after surgery; two were untreated due to poor health.
Discussion
In this study, no complications related to colonoscopy occurred in over 200 procedures and invasive CRCs were detected in approximately 12% of patients, higher than in previous reports13,14). The majority of CRCs detected in this study were treatable, and even curable. These results demonstrate that colonoscopy for patients over 85 years of age is safe and effective.
To the best of our knowledge, this is the largest review to date of patients aged 85 years or more who underwent colonoscopy. In a retrospective study of 157 patients aged ≥85 years with indications similar to that in the present study14), eight cancers (5.1%) were detected but all invaded beyond the muscularis propria. Another retrospective study reviewing over 1000 patients ≥80 years showed a 3.7% yield of CRCs (T stage not given)13). In contrast, the yield of CRC in the present series was nearly double. This higher result may be attributed to the fact that approximately 30% of invasive CRCs in this series were stage T1. In the latest retrospective study of 76 extremely patients aged ≥90 years, 14.9% of patients had cancer or high grade dysplasia by colonoscopy15), which is consistent with the present study.
In the subgroup analysis according to the indications for colonoscopy, invasive CRC was detected in approximately 20% of patients with positive FIT, altered bowel habits, anemia or hematochezia, in almost equal proportions. This suggests that the very elderly manifesting these symptoms are at increased risk for CRC. The CRC detection rate in the very elderly with positive FIT was almost as high as in patients with altered bowel habits, hematochezia or anemia. FIT may be effective as an initial screening test for asymptomatic patients aged 85 years or older. Duncan et al13) reported that 7% of patients ≥80 years with a positive FIT were found to have CRC and Kistler10) et al. reported CRC in 5.7% (12/212) of patients ≥ 70 years. In comparison with these two reports, in this study the CRC detection rate in the very elderly with positive FIT was relatively high. The higher incidence of CRC may at least in part be attributed to the fact that participants in the present study are older than in previous studies. Conversely, cancer was not detected in patients who underwent colonoscopy for routine surveillance or mild abdominal symptoms. This suggests that colonoscopy may not be indicated in patients over age 85 with these indications.
Some reports have shown that colonoscopy in the very elderly can be performed safely and successfully13,14). However, in the latest retrospective study of extremely elderly patients (≥90 years) undergoing colonoscopy under general anesthesia 5.3% of patients had cardiopulmonary events15). In the present series, where 70% of procedures were performed without sedation, and 30% of patients underwent colonoscopy with conscious sedation, there were no complications or adverse events, even in patients who underwent therapeutic colonoscopy. According to the latest guidelines regarding endoscopic practice for the elderly, one means of minimizing risk in elderly patients is to perform endoscopy with minimal or no sedation21). To minimize the incidence of adverse events during colonoscopy for the elderly, sedation is a key issue.
In the present series, 21% of patients were ASA physical status class 3. Of these, five patients found to have invasive CRC were unable to undergo surgical resection due to significant comorbidities although therapeutic colonoscopy was successfully performed without complications. These observations suggest that therapeutic colonoscopy is safe even in the very elderly with significant comorbidities. Surgical intervention may be impossible even if colonoscopy is possible. Practitioners should be aware that patients with ASA physical status class 3 include patients with various comorbidities.
The colonoscopy completion rate (92%) to reach the cecum is equivalent to previous reports11,22) but lower than that reported in young adults23). The most common reason for incomplete colonoscopy was poor bowel preparation5,12). Even in the very elderly, adequate bowel cleansing is vital to achieve total colonoscopy, suggesting the necessity for developing a bowel preparation regimen designed for the elderly. However, detailed information on the level of the bowel, completion rate of lavage ingestion and mild to moderate adverse events relating to bowel preparation were not collected in this study. A future study is warranted to establish an appropriate bowel preparation regimen for the elderly.
This study has some acknowledged limitations. First, this is a retrospective study, which could introduce selection bias. Second, the generalizability of these findings may be limited, since the present study was performed in Japan where colonoscopy is often performed without sedation. In our previous study comparing pain levels using the pediatric and ultra-thin colonoscope24), only one of 40 patients who underwent endoscopy with the pediatric colonoscope needed sedation. Third, the mean observation period (730 days) may be insufficient to adequately analyze long-term outcomes, regardless of age. Fourth, analysis of bowel preparation adequacy and methods was insufficient because the relevant information was not collected.
In conclusion, colonoscopy for patients aged 85 years and older is safe and has a good diagnostic yield for identifying malignancies. However, colonoscopy in the elderly may not be effective to identify malignancies when used for surveillance or screening for mild abdominal symptom.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Ms. Jinko Kobayashi, Ms. Sanae Tanaka and Ms. Sumie Suzuki for the administrative support.
References
- 1.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long - term prevention of colorectal-cancer deaths. N Engl J Med. 2012 Feb; 366(8): 687-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013 Sep; 369(12): 1106-14. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Nov; 149(9): 627-37. [DOI] [PubMed] [Google Scholar]
- 4.Qaseem A, Denberg TD, Hopkins RH, et al. Screening for colorectal cancer: A guidance statement from the American College of Physicians. Ann Intern Med. 2012 Mar; 156(5): 378-86. [DOI] [PubMed] [Google Scholar]
- 5.Day LW, Walter LC, Velayos F. Colorectal cancer screening and surveillance in the elderly patient. Am J Gastroenterol. 2011 Jul; 106(7): 1197-206. [DOI] [PubMed] [Google Scholar]
- 6.Loffeld RJ, Liberov B, Dekkers PE. Yearly diagnostic yield of colonoscopy in patients age 80 years or older, with a special interest in colorectal cancer. Geriatr Gerontol Int. 2012 Apr; 12(2): 298-303. [DOI] [PubMed] [Google Scholar]
- 7.Harewood GC, Lawlor GO, Larson MV. Incident rates of colonic neoplasia in older patients: When should we stop screening? J Gastroenterol Heaptol. 2006 Jun; 21(6): 1021-25. [DOI] [PubMed] [Google Scholar]
- 8.Inadomi JM, Sonnerberg A. The impact of colorectal cancer screening on life expectancy. Gastrointest Endosc. 2000 May; 51(5): 517-23. [DOI] [PubMed] [Google Scholar]
- 9.Lin OS, Kozarek RA, Schembre DB, et al. Screening colonoscopy in very elderly patients. Prevalence of neoplasia and estimated impact of life expectancy. JAMA. 2006 May; 295(20): 2357-65. [DOI] [PubMed] [Google Scholar]
- 10.Kistler CE, Kirby KA, Lee D, et al. Long-term outcomes following positive fecal occult blood test results in older adults. Benefits and Burden. Arch Intern Med. 2011 Aug; 171(15): 1344-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day LW, Kwon A, Inadomi JM, et al. Adverse events in older patients undergoing colonoscopy: A systematic review and meta-analysis. Gastrointest Endosc. 2011 Oct; 74(4): 885-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travis AC, Pievsky D, Saltzman JR. Endoscopy in the elderly. Am J Gastroenterol. 2012 Oct; 107(10): 1495-501. [DOI] [PubMed] [Google Scholar]
- 13.Duncan JE, Sweeney WB, Trudel JL, et al. Colonoscopy in the elderly: Low risk, low yield in asymptomatic patients. Dis Colon Rectum. 2006 May; 49(5): 646-51. [DOI] [PubMed] [Google Scholar]
- 14.Zerey MZ, Paton BL, Khan DK et al. Colonoscopy in the very elderly: A review of 157 cases. Surg Endosc. 2007 Oct; 21(10): 1806-9. [DOI] [PubMed] [Google Scholar]
- 15.Cha JM, Kozarek RA, La Selva D, et al. Risks and benefits of colonoscopy in patients 90 years or older, compared with younger patients. Clin Gastroenterol Hepatol. 2016 Jan; 14(1): 80-6. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007 Oct 4; 357(14): 1403-12. [DOI] [PubMed] [Google Scholar]
- 17.The Japanese Society of Gastroenterology. Evidence-based Clinical Practice Guidelines for Colonic Polyp 2014-[cited 2016 Aug29]. Available from: https://www.jsge.or.jp/files/uploads/CPGL2_re.pdf. Japanese [Google Scholar]
- 18.Ministry of Health, Labor and Welfare, Japan, 2014-[cited 2017 Jan 9]. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/life/life14/dl/life14-02.pdf. Japanese
- 19.Arias E. United States Life Table 2011. National Vital Statistics Report-[cited 2016 Oct 10]. 2015 Sep; 64(11). Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_11.pdf [PubMed]
- 20.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982 Dec; 5(6): 649-55. [PubMed] [Google Scholar]
- 21.Qureshi WA, Zuckerman MJ, Adler DG, et al. ASGE guideline: modifications in endoscopic practice for the elderly. Gastrointest Endosc. 2006 Apr; 63(4): 566-69. [DOI] [PubMed] [Google Scholar]
- 22.Lukens FJ, Loeb DS, Machicao VI, et al. Colonoscopy in octogenarians: a prospective outpatient study. Am J Gastroenterol. 2002 Jul; 97(7): 1722-25. [DOI] [PubMed] [Google Scholar]
- 23.Nelson DB, McQuaid KR, Bond JH, et al. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc. 2002 Mar; 55(3): 307-14. [DOI] [PubMed] [Google Scholar]
- 24.Nemoto D, Utano K, Endo S, et al. Ultrathin versus pediatric instruments for colonoscopy in older female patients: A randomized trial. Dig Endosc. 2017 Mar; 29(2): 168-74. [DOI] [PubMed] [Google Scholar]


