Abstract
The medial habenula-interpeduncular nucleus (MHb-IPN) pathway modulates negative affective states produced by nicotine withdrawal. Sex differences in the contribution of acetylcholine (ACh) systems in this pathway have not been explored. Thus, this study assessed ACh levels and gene expression of α- and β-containing nicotinic acetylcholine receptor (nAChR) subunits in the IPN of female and male rats following nicotine treatment and withdrawal. Rats were prepared with a pump that delivered nicotine for 14 days, and naïve controls received a sham surgery. In Study 1, rats were prepared with a probe in the IPN, and ACh levels were measured following saline and then mecamylamine administration. In Study 2, separate groups of naïve control or nicotine-treated rats received saline or mecamylamine and physical signs and anxiety-like behavior were assessed using elevated plus maze (EPM) procedures. The IPN was then dissected and mRNA levels were assessed using RT-qPCR methods. Nicotine treatment increased ACh levels to a larger extent in females than males. Nicotine withdrawal produced a similar increase in physical signs; however, females displayed greater anxiety-like behavior than males. In females, gene expression of α5 increased following nicotine treatment and withdrawal. In males, α7 increased following nicotine treatment and α2 and α3 increased during nicotine withdrawal. Both females and males displayed an increase in β3 and β4 during nicotine withdrawal. In females, anxiety-like behavior was correlated with α4, α5, and β2 gene expression in the IPN. These results suggest that sex differences in withdrawal are modulated via cholinergic systems in the IPN.
Keywords: nAChR, acetylcholine, sex differences, female, male, rat
1. Introduction
Tobacco use is largely motivated by experiencing the pleasurable effects of nicotine and avoiding negative affective states produced by withdrawal from nicotine (Benowitz, 2010; Picciotto and Kenny, 2013). In rodents, nicotine dependence is often induced via surgical implantation of osmotic pumps that deliver nicotine for approximately 7 days (Kenny and Markou, 2001; Malin, 2001; O’Dell et al., 2004). Nicotine withdrawal can then be assessed following removal of the pump (spontaneous withdrawal) or administration of a nicotinic receptor antagonist, such as mecamylamine (precipitated withdrawal). By either method, nicotine withdrawal produces a behavioral profile in rodents that is comprised of physical signs and negative affective states. The physical signs of nicotine withdrawal include facial fasciculations, teeth chatters, writhes, gasps, eye blinks, and ptosis (Malin, 2001; O’Dell et al., 2004; Watkins et al., 2000). The negative affective states elicited by withdrawal include anxiety-like behavior that can be assessed as an increase in time spent in the closed versus open arms of an elevated plus maze (EPM; Bruijnzeel et al., 2012; Tejeda et al., 2012; Wilmouth and Spear, 2006).
Previous studies have revealed that there are sex differences in the magnitude of negative affective states produced by nicotine withdrawal in rodents. Specifically, females display a larger aversion to an environment paired previously with nicotine withdrawal as compared to male rats (O’Dell and Torres, 2014) and mice (Kota et al., 2007; 2008). Female rats also display higher plasma levels of corticosterone and adrenocorticotropic hormone during nicotine withdrawal as compared to males (Gentile et al., 2011; Skwara et al., 2012). During nicotine withdrawal, female rats also display higher levels of anxiety-like behavior and expression of stress-associated genes in the nucleus accumbens (NAc) than males (Torres et al., 2013). Interestingly, there do not appear to be sex differences in the physical manifestations of nicotine withdrawal (O’Dell and Torres, 2014), suggesting that negative affective states and physical signs of withdrawal may be modulated via distinct neural substrates.
The behavioral effects of nicotine are modulated via nicotinic acetylcholine receptors (nAChRs) consisting of pentameric membrane proteins of homomeric or heteromeric complexes of α or β subunits. To date, 12 subunits have been identified in brain tissue (α2–α10 and β2–β4), and the various combinations of these subunits lead to differences in receptor sensitivity and channel activation in the presence of nicotine. Previous work has shown that different nAChR subunits modulate the expression of the behavioral effects of nicotine withdrawal in rodents. For example, using knockout (KO) mouse technology, previous reports have concluded that the α2, α3, α5, α7, and β4 subunits modulate physical signs, whereas α6 and β2 modulate affective states produced by nicotine withdrawal (DeBiasi and Dani, 2011; Jackson et al., 2008; Salas et al., 2009).
Recent work suggests that the behavioral effects of nicotine withdrawal are modulated via the habenula-interpeduncular nucleus (Hb-IPN) pathway (Antolin-Fontes et al., 2015; Dani et al., 2011; Jackson et al., 2015; Fowler and Kenny, 2014; Mola et al., 2017; Zhao-Shea et al., 2013 and 2015). The medial portion of the habenula (MHb) projects to the IPN via neurons that release glutamate and ACh. Pre-clinical studies have shown that microinjections of a nAChR antagonist into the MHb or IPN elicit physical signs of withdrawal in nicotine-dependent mice (Salas et al., 2009). Also, male mice lacking the ACh enzyme, choline acetyl transferase in the MHb were insensitive to the development of physical signs and negative affective states produced by nicotine withdrawal (Frahm et al., 2015). These studies suggest that the MHb-IPN pathway modulates the behavioral effects of nicotine withdrawal. However, to our knowledge, sex differences in cholinergic systems in the MHb-IPN pathway have not been compared during nicotine withdrawal. As a first step in addressing this issue, the present study compared ACh levels and nAChR subunit expression in the IPN of female and male rats during nicotine exposure and withdrawal. The rationale for examining sex differences in the expression of various nAChR subunits is based on their high concentration in the MHb-IPN pathway, and the presence of a gene cluster (CHRNA3-CHRNA5-CHRNB4) that synthesizes α3, α5, and β4 subunits and is expressed in persons who are at greater risk of developing nicotine dependence (Berretini et al., 2008; Bierut et al., 2008).
2. Materials and methods
2.1. Subjects
Female and male Wistar rats (N=79) were housed in groups of 2–3 per cage in a humidity- and temperature-controlled vivarium using a 12-hour light/dark cycle with lights off at 8:00 am. Rats had ad libitum access to standard rodent chow except during testing. All testing was done in adult rats between post-natal day 60–75. The rats were handled for 5 days prior to experimentation. All procedures were approved by the UTEP Institutional Animal Care and Use Committee and followed NIH guidelines for the care and use of laboratory animals. Separate groups of rats were used to study sex differences in ACh levels in the IPN using in vivo microdialysis procedures (Study 1; n=35) and anxiety-like behavior and gene expression of various nAChR subunits in the IPN using RT-qPCR methods (Study 2; n=44).
2.2. Study 1
This diagram depicts the experimental timeline for Study 1,
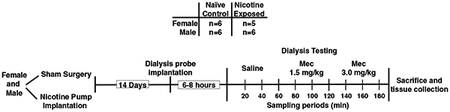 which compared sex differences in ACh levels in the IPN following saline and mecamylamine administration. The rats were first anesthetized with an isoflurane/oxygen mixture (1–3% isoflurane), and then they received a sham surgery (naïve control group) or were surgically prepared with an osmotic pump (nicotine-treated group) that delivered nicotine continuously (3.2 mg/kg/day/expressed as base; Alzet 2ML2). The groups that received a sham surgery served as naïve controls that received saline and then mecamylamine during dialysis testing. The addition of this group allowed for an assessment of sex differences in ACh levels during baseline and following mecamylamine administration. Fourteen days after the sham surgery or nicotine exposure, the rats were re-anesthetized and stereotaxically implanted with a dialysis probe with an active membrane length of 1 mm (model CMA 11, Holliston, MA). The probe was aimed at the IPN using the following stereotaxic coordinates from bregma: AP = −6.3 mm, ML = +/−2.5 mm, and DV = −9.2 mm at a 15 angle. The probe was perfused with artificial cerebral spinal fluid (aCSF; pH of 7.2–7.4) for 1 hour at a rate of 1.0 μL/minute to establish equilibrium prior to sample collection. The hemisphere that was implanted with the probe was alternated between animals to control for possible hemispheric differences in the IPN across groups. Following surgery, the rats were transferred to a test cage where food and water were freely available during dialysis testing. Approximately 6–8 hours after probe implantation, dialysate samples were collected in 20-minute intervals following saline administration in a 1-hour baseline period, and then following administration of 2 doses of mecamylamine (1.5 and 3.0 mg/kg, sc) for 2 additional 1-hour sampling periods. Given that the half-life of mecamylamine is approximately 72 minutes, the possibility exists that there were some cumulative neurochemical effects of this drug given that the first and second injections of mecamylamine were 60 minutes apart (Debruyne et al., 2003). After dialysate samples were collected, they were frozen on dry ice and stored at −80°C until analyzed. At the end of testing, the brains were extracted, frozen, and then sectioned for verification of probe placement using the Paxinos & Watson (2014) atlas. Each animals’ baseline ACh levels had to fall within a range that was less than 2 standard deviations from their respective group mean in order to be included in the final analysis. Five rats were excluded on the basis of aberrant baseline values and/or misplaced probes.
which compared sex differences in ACh levels in the IPN following saline and mecamylamine administration. The rats were first anesthetized with an isoflurane/oxygen mixture (1–3% isoflurane), and then they received a sham surgery (naïve control group) or were surgically prepared with an osmotic pump (nicotine-treated group) that delivered nicotine continuously (3.2 mg/kg/day/expressed as base; Alzet 2ML2). The groups that received a sham surgery served as naïve controls that received saline and then mecamylamine during dialysis testing. The addition of this group allowed for an assessment of sex differences in ACh levels during baseline and following mecamylamine administration. Fourteen days after the sham surgery or nicotine exposure, the rats were re-anesthetized and stereotaxically implanted with a dialysis probe with an active membrane length of 1 mm (model CMA 11, Holliston, MA). The probe was aimed at the IPN using the following stereotaxic coordinates from bregma: AP = −6.3 mm, ML = +/−2.5 mm, and DV = −9.2 mm at a 15 angle. The probe was perfused with artificial cerebral spinal fluid (aCSF; pH of 7.2–7.4) for 1 hour at a rate of 1.0 μL/minute to establish equilibrium prior to sample collection. The hemisphere that was implanted with the probe was alternated between animals to control for possible hemispheric differences in the IPN across groups. Following surgery, the rats were transferred to a test cage where food and water were freely available during dialysis testing. Approximately 6–8 hours after probe implantation, dialysate samples were collected in 20-minute intervals following saline administration in a 1-hour baseline period, and then following administration of 2 doses of mecamylamine (1.5 and 3.0 mg/kg, sc) for 2 additional 1-hour sampling periods. Given that the half-life of mecamylamine is approximately 72 minutes, the possibility exists that there were some cumulative neurochemical effects of this drug given that the first and second injections of mecamylamine were 60 minutes apart (Debruyne et al., 2003). After dialysate samples were collected, they were frozen on dry ice and stored at −80°C until analyzed. At the end of testing, the brains were extracted, frozen, and then sectioned for verification of probe placement using the Paxinos & Watson (2014) atlas. Each animals’ baseline ACh levels had to fall within a range that was less than 2 standard deviations from their respective group mean in order to be included in the final analysis. Five rats were excluded on the basis of aberrant baseline values and/or misplaced probes.
For the neurochemical analyses, calibration standards were diluted from a stock solution using aCSF to yield various concentrations of ACh (0.5, 5, 10, 50, and 100 nM). The internal standard and derivatization procedures followed the methods described in Song et al., (2012). Quantification of ACh in dialysate samples was performed using an approach involving liquid chromatography/mass spectrometry (LC/MS) methods. The neurochemical analyses were performed using a Thermo Scientific UltiMate™ 3000 Standard Quaternary System with a Waters BEH C18 column for separation (1 mm × 100 mm, 1.7 μm, 130 Å pore size). The autosampler was coupled to a TSQ Endura triple quadrupole MS. All dialysate level estimations were acquired and processed using Thermo Scientific Tracefinder™ software version 3.2.512. The peaks were visually inspected to detect artifacts. Peak areas were obtained for ACh and its internal standard, and the ratio of these measures was used to calculate the concentration of ACh in each dialysate sample.
2.3. Study 2
This diagram depicts the experimental timeline of Study 2,
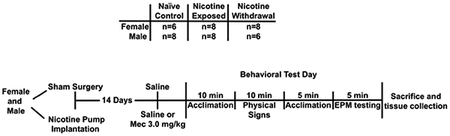 which compared sex differences in physical signs, anxiety-like behavior, and nAChR subunit gene expression in the IPN following nicotine treatment and withdrawal. A separate cohort of rats was anesthetized and then received a sham surgery or were surgically prepared with an osmotic pump that delivered nicotine continuously (3.2 mg/kg/day/expressed as base; Alzet 2ML2). The rats that received a sham surgery (naïve control group) received saline on the test day in order to assess sex differences in baseline gene expression. The experimental design also included separate groups of nicotine-treated rats that received either saline (nicotine treated) or mecamylamine (nicotine withdrawal) on the test day in order to compare sex differences in gene expression following nicotine treatment or withdrawal. In this way, rats that received chronic nicotine exposure and then saline on the test day allowed for the assessment of changes produced by nicotine alone. Fourteen days after the sham surgery or nicotine exposure, separate groups of rats received saline or mecamylamine (3.0 mg/kg, sc) administration. Ten minutes later, the rats were placed in a clear Plexiglas® container (24 × 24 × 30 cm) and physical signs were assessed for an additional 10 minutes, an observation time period that has been used previously in our laboratory (O’Dell et al., 2004; Tejeda et al., 2012). The recorded signs included blinks, writhes, body shakes, teeth chatters, and gasps. If observed, ptosis was counted only once per minute. The total number of somatic signs was defined as the sum of individual occurrences of the aforementioned signs during the entire observation period. The rats were then transported in a rectangular Plexiglass® cage to the EPM test room where they acclimated for an additional 5 minutes under red light conditions. The EPM apparatus consisted of 4 arms (10 cm × 50 cm) that were elevated to a height of 50 cm above the ground. The closed arms had 20 cm high walls around them, and the open arms did not have walls. The rats were placed in the center of the EPM facing the open arm, and time spent in the open versus closed arms was recorded for 5 minutes. The maze was thoroughly cleaned with 70% ethanol and then water between each individual test.
which compared sex differences in physical signs, anxiety-like behavior, and nAChR subunit gene expression in the IPN following nicotine treatment and withdrawal. A separate cohort of rats was anesthetized and then received a sham surgery or were surgically prepared with an osmotic pump that delivered nicotine continuously (3.2 mg/kg/day/expressed as base; Alzet 2ML2). The rats that received a sham surgery (naïve control group) received saline on the test day in order to assess sex differences in baseline gene expression. The experimental design also included separate groups of nicotine-treated rats that received either saline (nicotine treated) or mecamylamine (nicotine withdrawal) on the test day in order to compare sex differences in gene expression following nicotine treatment or withdrawal. In this way, rats that received chronic nicotine exposure and then saline on the test day allowed for the assessment of changes produced by nicotine alone. Fourteen days after the sham surgery or nicotine exposure, separate groups of rats received saline or mecamylamine (3.0 mg/kg, sc) administration. Ten minutes later, the rats were placed in a clear Plexiglas® container (24 × 24 × 30 cm) and physical signs were assessed for an additional 10 minutes, an observation time period that has been used previously in our laboratory (O’Dell et al., 2004; Tejeda et al., 2012). The recorded signs included blinks, writhes, body shakes, teeth chatters, and gasps. If observed, ptosis was counted only once per minute. The total number of somatic signs was defined as the sum of individual occurrences of the aforementioned signs during the entire observation period. The rats were then transported in a rectangular Plexiglass® cage to the EPM test room where they acclimated for an additional 5 minutes under red light conditions. The EPM apparatus consisted of 4 arms (10 cm × 50 cm) that were elevated to a height of 50 cm above the ground. The closed arms had 20 cm high walls around them, and the open arms did not have walls. The rats were placed in the center of the EPM facing the open arm, and time spent in the open versus closed arms was recorded for 5 minutes. The maze was thoroughly cleaned with 70% ethanol and then water between each individual test.
Immediately after EPM testing, the rats were sacrificed by rapid decapitation and their brains were removed. The IPN was collected using a 2 mm micro-punch pen (Electron Microscopy Sciences), and the tissue was stored at −80 C. Total RNA was isolated using an Ambion® RNAqueous®-Micro kit. A total of 200 ng of RNA was then digested with DNAse I (Invitrogen™) prior to cDNA synthesis to remove any genomic DNA contamination. Following the DNAse I digestion, RNA was quantified using a BioPhotometer® D30 (Eppendorf). The inclusion criterion for all RNA samples was an A260/280 ratio of 1.8–2.0. Also, the quality of RNA was verified using a MOPS 1% agarose gel (4% formaldehyde) using a Thermo Scientific EasyCast B1™ electrophoresis system. The characteristic 18S and 28S ribosomal RNA bands were visualized using ethidium bromide on a Bio-Rad ChemiDoc XRS+ imaging system. RNA samples were then reverse transcribed into cDNA with the AdvantageRT-for-PCR kit (Clontech) using Oligo(dT) primers, following manufacturer’s instructions. Once the cDNA was synthesized, the samples were diluted 1:10 in nuclease-free water and stored at −20°C. Specific primers for the nAChR subunits and the reference genes were obtained from Integrated DNA Technologies, Inc. (see Table 1). The expression of 4 commonly used reference genes were first compared in the IPN of female and male rats in order to assess potential candidates for a normalizing gene. The results revealed that ribosomal protein L13a (RPL13a) was the most stable reference gene across female and male rats in the IPN, as compared to the other reference genes (GAPDH, β-actin, and RPLP). Commercially available SYBR® Fast qPCR fluorescent labeling kits were used to perform RT-PCR using a CFX Connect™ Real-Time PCR Instrument (Bio-Rad, Inc.). All samples were analyzed in duplicates and amplified using the following protocol: initial denaturation at 95°C for 3 minutes, continued denaturation at 95°C for 10 seconds, and annealing at 55°C for 30 seconds. This method was repeated for a total of 40 cycles. Gene expression was normalized to RPL13A using the comparative CT method adopted from Schmittgen and Livak, (2008). The amplification specificity for each primer was tested for a single-product, as shown by a single band via TAE 1% gel electrophoresis and visualized on the ChemiDoc XRS+ system (Bio-Rad).
Table 1.
Primer sequences 5’−3’
| Gene | Forward primer | Reverse primer |
|---|---|---|
| RPL13a | GGA TCC CTC CAC CCT ATG ACA | CTG GTA CTT CCA CCC GAC CTC |
| α2 | TCA ATG TAC ACC ACC GCT CC | AGA ATC TCG CTA GCC TGG GA |
| α3 | GCC ACC ACC GTA GGG TAA AA | TGC AGA AAT AAT CCC GCC GT |
| α4 | CAA CTT TCT GCA ACC CCA CG | TGG CAA CGT ATT TCC AGT CC |
| α5 | TCA GGT ACA ACG GCA CTG TC | TGT CCA CGA GCC GAA TTT CA |
| 1 α6 | CTT TGA GTT GGC CAT CAC GC | GGA AGT CAC CGA CGG CAT TA |
| α7 | ACA TGT CTG AGT ACC CCG GA | CGC TCA TCA GCA CTG TTA TAG A |
| β2 | AAG TGA GCG ACA CTG GTC TG | GTC TGG AGC CCT CTG AGG TA |
| β3 | TGT GAA GTC CAG TGG AAC CG | CGC TGT AAA ACA AGG GCA GG |
| β4 | CAC CAA CGT GAT TGT GCG TT | TCA TAG GTC CAG GAG CGG AA |
Gene not sufficiently amplified.
One commonly used approach for presenting gene expression data is the 2(−ΔΔ Ct) method described in Livak and Schmittgen (2001). Gene expression data were normalized to an endogenous reference gene and then expressed relative to an untreated control group. This method first involved calculating ΔCT values (Ct of experimental gene – Ct of reference gene), which reflects normalized gene expression relative to the housekeeping gene. All ΔCT values are presented in supplementary Table S1, which illustrates baseline group differences in gene expression. In the next step, the ΔΔCT were calculated (average ΔCt of experimental group - average ΔCt of reference group). This step provided a normalization of gene expression data to naïve controls. The ΔΔCT values were then expressed as fold change using the following formula: 2(−ΔΔCt). This method for gene expression data has been used in previous work from our laboratory (Torres et al., 2013 and 2015).
2.4. Statistics
For all data sets, initial analyses compared baseline sex differences in naïve controls using analysis of variance (ANOVA). For the neurochemical data, the dependent variable was ACh levels. For data in Figure 1, an overall 3-way ANOVA was conducted that included sex and nicotine treatment condition (naïve control or nicotine-treated) as between-subject factors and time as a within-subjects factor (20-minute samples). For the behavioral analyses, the dependent variables were total physical signs and % time spent in the open arms. For the data in Figure 3, anxiety-like behavior was operationally defined as a decrease in % time spent in the open arms relative to controls. Total physical signs or % time spent in the open arms were analyzed using 2-way ANOVA with sex and treatment condition (saline or mecamylamine) as between-subject factors. For the gene expression data in Figures 4 and 5, the initial analysis compared baseline sex differences in ΔCt values for each nAChR subunit in naïve controls. Then, a 2-way MANOVA was conducted with sex and nicotine treatment condition as between-subject factors with all of the genes included. Then, separate 2-way ANOVAs were conducted for each nAChR subunit with sex and nicotine treatment condition as between-subject factors. For all analyses, significant interaction effects were followed by post-hoc comparisons using protected Fisher LSD tests (p ≤ 0.05). For the data in Figure 6, separate linear regression analyses assessed the relationship between anxiety-like behavior and the expression of various nAChR subunits in nicotine-treated female and male rats that received the 3.0 mg/kg dose of mecamylamine.
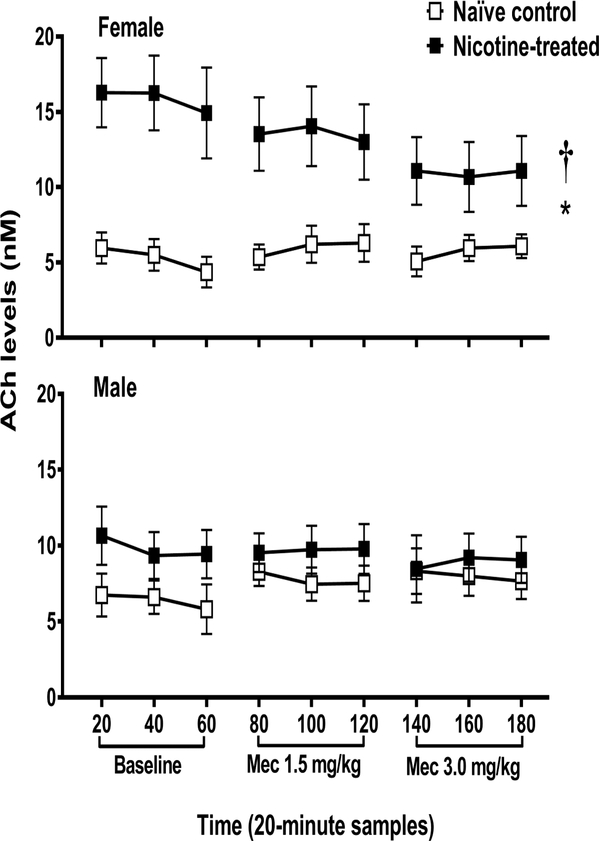
Figure 1.
ACh levels (nM ± SEM) in the IPN of female (naïve control n=6; nicotine-treated n=5) and male (naïve control n=6; nicotine-treated n=6) rats in Study 1. Each point reflects a 20-minute sampling period during baseline and then following mecamylamine administration. The asterisk (*) denotes a difference from naïve control rats and the dagger (†) denotes a sex difference in their respective treatment condition (p ≤ 0.05).
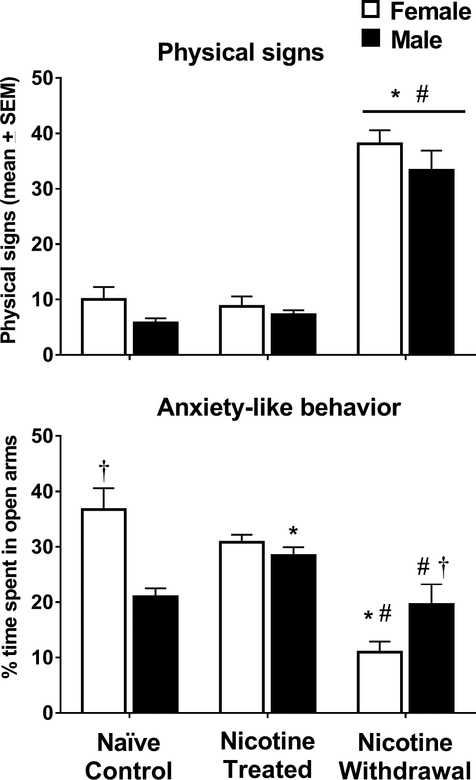
Figure 3.
Total physical signs and % time spent in the open arms of the EPM (±SEM) in female (naïve control n=6; nicotine treated n=8 and nicotine withdrawal n=8) and male (naïve control n=8; nicotine treated n=8 and nicotine withdrawal n=6) rats in Study 2. Asterisks (*) denote a difference from naïve controls, number signs (#) denote a difference from nicotine-treated rats that received saline, and the dagger (†) denotes a sex difference in their respective treatment condition (p ≤ 0.05).
Figure 4.
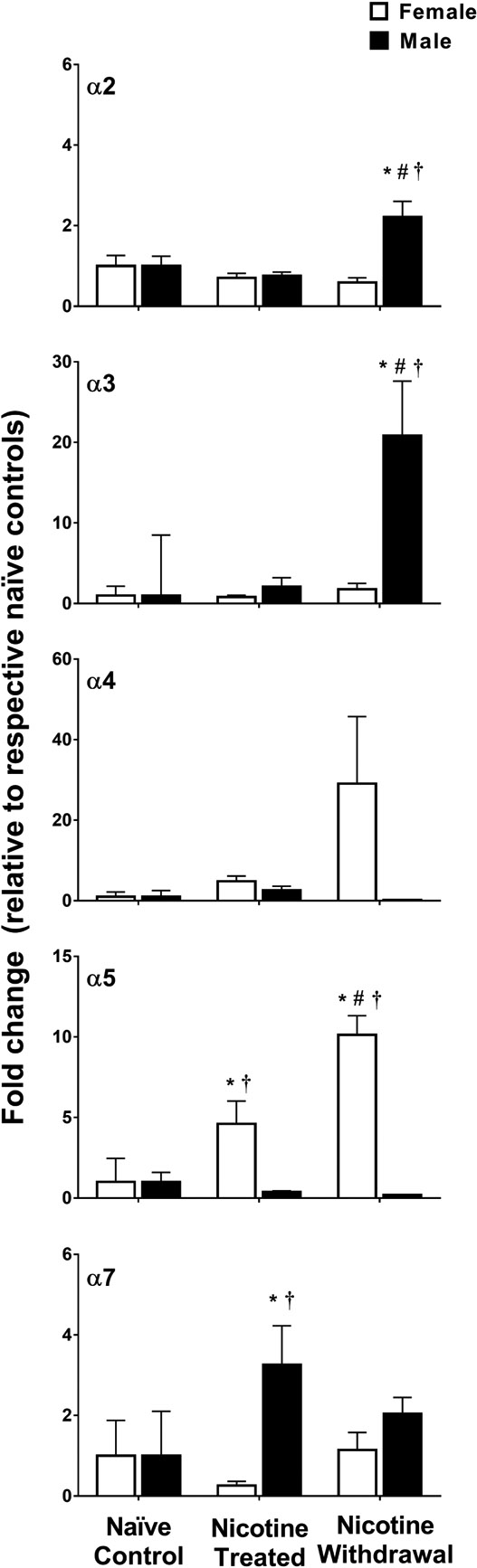
Gene expression of the α-containing nAChR subunits (α2, α3, α4, α5, and α7) in female (naïve control n=6; nicotine treated n=8 and nicotine withdrawal n=8) and male (naïve control n=8; nicotine treated n=8 and nicotine withdrawal n=6) rats in Study 2. The data are expressed as a fold change relative to naïve controls (± SEM). Asterisks (*) denote a difference from naïve controls, number signs (#) denote a difference from nicotine-treated rats that received saline, and daggers (†) denote a sex difference in their respective treatment condition (p ≤ 0.05).
Figure 5.
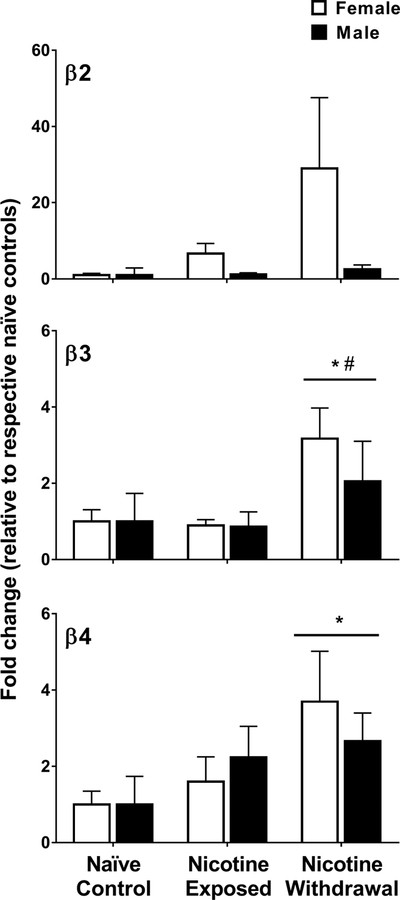
Gene expression of the β-containing nAChR subunits (β2, β3, and β4) in female (naïve control n=6; nicotine treated n=8 and nicotine withdrawal n=8) and male (naïve control n=8; nicotine treated n=8 and nicotine withdrawal n=6) rats in Study 2. The data are expressed as a fold change relative to naïve controls (± SEM). Asterisks (*) denote a difference from naïve controls, number signs (#) denote a difference from nicotine-treated rats that received saline, and daggers (†) denote a sex difference in their respective treatment condition (p ≤ 0.05).
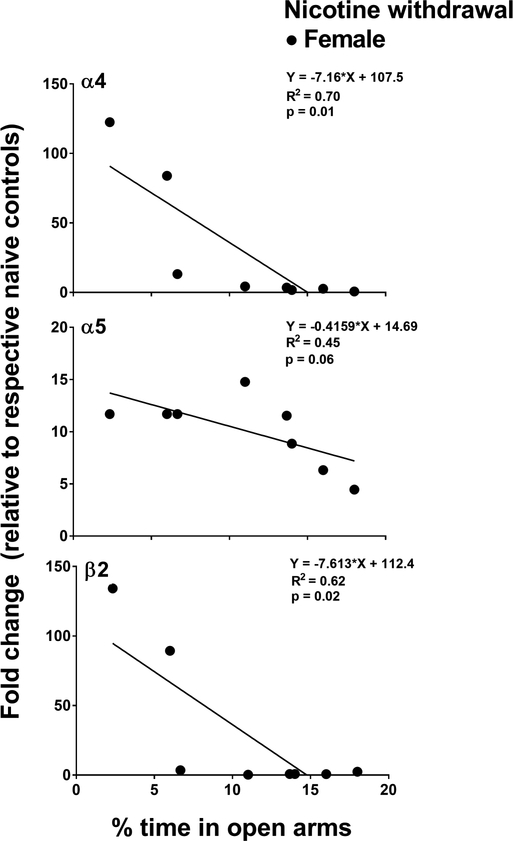
Figure 6.
This panel reflects correlational analyses between α4, α5, and β2 gene expression and anxiety-like behavior in female (naïve control n=6; nicotine treated n=8 and nicotine withdrawal n=8) and male (naïve control n=8; nicotine treated n=8 and nicotine withdrawal n=6) rats in Study 2. A negative correlation was observed between % open arm time and gene expression of α4 and α5. In addition, there was a strong trend between % open arm time and gene expression of β2. The negative correlations indicate that more anxiety-like behavior is associated with greater gene expression of these nAChR subunits.
3. Results
3.1. Study 1
Figure 1 illustrates ACh levels in naïve control and nicotine-treated female and male rats that received saline and then mecamylamine during dialysis testing. The overall 3-way analysis that included sex, nicotine treatment condition, and time was not significant [F (8, 82) = 0.63, p = 0.66], suggesting that mecamylamine did not alter ACh levels across time in a sex- or treatment-dependent manner. However, there was a significant 2-way interaction between sex and nicotine treatment condition [F (1, 19) = 5.70, p = 0.03], with female nicotine-treated rats displaying higher ACh levels throughout all sampling conditions relative to their respective naïve controls (*p = 3.4E–4) and male nicotine-treated rats (†p = 0.04).
Figure 2 illustrates that the microdialysis probe placements were located in the IPN.
Figure 2.
Schematic illustrating the placements of the 1 mm dialysis probe membranes from all rats in Study 1. The placements spanned between −6.04 and −6.72 mm posterior to bregma according to the atlas of Paxinos and Watson (1998).
Figure 3 illustrates physical signs and anxiety-like behavior in nicotine-treated female and male rats that received saline or mecamylamine on the test day. An analysis of the total physical signs revealed that there was no interaction between sex and nicotine treatment condition [F (2, 38) = 0.35, p = 0.71]. However, there was a main effect of nicotine treatment condition [F (2, 38) = 117.20, p = 5.59E–17]. Following mecamylamine administration, physical signs increased in both groups of nicotine-treated female and male rats as compared to their respective naïve controls (*p=1.97E–15) and relative to nicotine-treated rats that received saline on the test day (#p = 2.41E–16). There were no sex differences in the magnitude of physical signs across treatment conditions.
An analysis of % time spent in the open arms revealed a significant interaction between sex and nicotine treatment condition [F (2, 38) = 19.55, p = 1.0E–6]. Time spent in the open arms was higher in naïve control female versus male rats (†p = 3.0E–6). There was an increase in time spent in the open arms that reached statistical significance in nicotine-treated male rats relative to their respective naïve controls (*p = 0.003). Following mecamylamine administration, nicotine-treated female rats spent more time in the open arms relative to their respective naïve controls (*p = 1.95E–11) and their same-sex controls that received saline on the test day (#p = 4.26E–10). Following mecamylamine administration, nicotine-treated female rats displayed less time spent in the open arms as compared to males (†p = 0.002). Following mecamylamine administration, nicotine-treated male rats spent less time spent in the open arms as compared to nicotine-treated males that received saline (#p = 0.001). An additional analysis of closed arm entries was conducted in order to examine whether our assessment of anxiety-like behavior was influenced by group differences in locomotor activity (see supplementary Table S2). The analysis revealed that there were no group by treatment differences in closed arm entries [F (2, 38) = 0.06, p = 0.94].
Figure 4 illustrates the α-containing subunits (α2, α3, α4, α5, and α7) in the IPN of nicotine-treated female and male rats that received saline or mecamylamine. A MANOVA analysis that included all of the genes revealed a significant interaction between sex and nicotine treatment condition [F (16, 62) = 4.76, p = 4.0E–6; Wilk’s Λ = 0.20]. The initial analysis of α2 revealed that there were no baseline differences between naïve control female (average ΔCt = 14.02 ± 0.36) and male (average ΔCt = 14.34 ± 0.31) rats (p = 0.53; see supplementary Table S3). A subsequent analysis of the data expressed as fold change relative to respective naïve controls revealed a significant interaction between sex and nicotine treatment condition [F (2, 38) = 15.99, p = 1.0E–5]. Following mecamylamine administration, nicotine-treated males displayed an increase in α2 relative to their respective naïve controls (*p = 7.0E–6), their same-sex naïve controls that received saline (#p = 2.65E–7), and females (†p = 2.89E–8).
Similarly, the initial analysis of α3 revealed there were no baseline differences between naïve control female (average ΔCt = 10.72 ± 0.33) and male (average ΔCt = 12.16 ± 1.56) rats (p = 0.40; see supplementary Table S1). A subsequent analysis of the data expressed as fold change relative to respective naïve controls revealed an interaction between sex and nicotine treatment condition [F (2, 38) = 8.82, p = 0.001). Following mecamylamine administration, nicotine-treated males displayed an increase in α3 relative to their respective naïve controls (*p = 3.0E–6), their same-sex controls that received saline (#p = 8E–6), and females (†p = 6.0E–6).
The initial analysis of α4 revealed that there were no baseline differences between naïve control female (average ΔCt = 14.55±0.79) and male (average ΔCt = 11.91±1.13) rats (p = 0.10; see supplementary Table S1). A subsequent analysis of the data expressed as fold change relative to respective naïve controls revealed that there was no interaction between sex and treatment condition [F (2, 38) = 2.19, p = 0.12]. Also, there was no main effect of sex [F (2, 38) = 2.81, p = 0.10] or treatment condition [F (2, 38) = 1.75, p = 0.19].
The initial analysis of α5 revealed baseline differences between naïve control female (average ΔCt = 11.34 ± 0.91) and male (average ΔCt = 8.39 ± 0.60) rats (p = 0.02; supplementary Table S1). A subsequent analysis of the data expressed as fold change relative to respective naïve controls revealed that there was an interaction between sex and nicotine treatment condition [F (2, 38) = 16.89, p = 6.0E–6). Following saline administration, nicotine-treated females displayed an increase in α5 relative to their respective naïve controls (*p = 0.005) and nicotine-treated males (†p = 0.001). Following mecamylamine administration, nicotine-treated females displayed an increase in α5 relative to their respective naïve controls (*p = 4.84E–9), their same-sex controls that received saline (#p = 1.7E–6), and males (†p=6.47E–10).
The initial analysis of α7 revealed that there were no baseline differences between naïve control female (average ΔCt = 12.30 ± 0.44) and male (average ΔCt = 13.23 ± 0.55) rats (p = 0.24; supplementary Table S1). A subsequent analysis of the data expressed as fold change relative to respective naïve controls revealed that there was an interaction between sex and nicotine treatment condition [F (2, 38) = 4.88, p = 0.01). Following saline administration, α7 was higher in nicotine-treated males as compared to their respective naïve controls (*p = 0.002) and nicotine-treated females (†p = 7.4E–6).
Figure 5 illustrates the β-containing nAChR subunits (β2, β3, and β4) in the IPN of nicotine-treated female and male rats that received saline or mecamylamine. The initial analysis of β2 revealed that there were no baseline differences between naïve control female (average ΔCt = 16.53 ± 0.59) and male (average ΔCt = 14.68 ± 0.77) rats (p = 0.10; supplementary Table S1). The subsequent analysis of the data expressed as fold change relative to respective naïve controls revealed that there was no interaction between sex and nicotine treatment condition [F (2, 38) = 1.29, p = 0.29]. Also, there were no main effects of sex [F (2, 38) = 2.34, p = 0.13] or nicotine treatment condition [F (2, 38) = 1.64, p = 0.21].
The initial analysis of β3 revealed that there were baseline differences between naïve control female (average ΔCt = 14.11 ± 0.49) and male (average ΔCt = 8.54 ± 0.70) rats (p = 5.4E–5; supplementary Table S1). A subsequent analysis of the data expressed as % of naïve controls revealed that there was no interaction between sex and nicotine treatment condition [F (2, 38) = 0.63, p = 0.53]. However, there was a significant main effect of nicotine treatment condition [F (2, 38) = 5.92, p = 0.006]. Following mecamylamine administration, β3 was higher in both groups of nicotine-treated female and male as compared to naïve controls (*p = 0.007) and nicotine-treated rats that received saline (#p = 0.003).
The initial analysis of β4 revealed that there were no baseline differences between naïve control female (average ΔCt =13.91 ± 0.49) and male (average ΔCt =12.67 ± 0.77) rats (p = 0.20; supplementary Table S1). A subsequent analysis revealed that there was no interaction between sex and nicotine treatment condition [F(2, 38) = 0.56, p = 0.57]. However, there was a significant main effect of nicotine treatment condition [F(2, 38) = 3.52, p = 0.03]. Following mecamylamine administration, β4 was higher in both groups of nicotine-treated female and male rats as compared to naïve controls (*p = 0.012).
Figure 6 illustrates the relationship between anxiety-like behavior (% time in open arms) and gene expression of the individual nAChR subunits in nicotine-treated female rats that received the 3.0 mg/kg dose of mecamylamine. The correlational values for all the genes are shown in supplementary Table S2. Only subunits that displayed a sex-dependent increase during nicotine withdrawal were included in the regression analyses (α4, α5, β2 in females and α2, α3, α7 in males). In males, the results revealed that there was no correlation between anxiety-like behavior and gene expression of α2 (r = 0.01, p = 0.97), α3 (r = −0.64, p = 0.16), and α7 (r = −0.57, p = 0.23). In females, there was a significant correlation between anxiety-like behavior and gene expression of α4 (r = −0.83, p = 0.01) and a trend for α5 (r = −0.68, p = 0.06). Also, there was a negative correlation between anxiety-like behavior and the expression of β2 (r = −0.79, p = 0.02).
4. Discussion
4.1. Summary
Study 1 revealed that ACh levels in the IPN were higher in female versus male rats during all experimental conditions. Study 2 revealed that female and male rats displayed similar physical signs of nicotine withdrawal; however, females displayed greater anxiety-like behavior than males. There were sex differences in the expression of α-containing subunits, with females displaying an increase in gene expression of α5 during nicotine exposure and withdrawal, and males displaying an increase in gene expression of α7 during nicotine exposure and α2 and α3 during withdrawal (see inset). Female and male rats displayed a similar withdrawal-induced increase in gene expression of β3 and β4 in the IPN. A correlational analysis in females revealed a positive relationship between anxiety-like behavior and gene expression of α4, α5, and β2, suggesting that these nAChR subunits in the IPN may promote the aversive effects of withdrawal in females.
| Outcome | Exposure | Withdrawal |
|---|---|---|
| Higher in Males | α7 | α2 α3 |
| Higher in Females | α5 | α5 |
| Increased Equally | - | β3 β4 |
4.2. ACh levels
Previous work has shown that ACh projections from the MHb to the IPN are critical for the development of nicotine dependence (De Biasi and Salas, 2008; Jackson et al., 2015). A previous report identified a pace-making mechanism in the MHb that controls tonic firing frequency of cholinergic neurons in the MHb, and blockade of this pace-making mechanism elicited somatic and affective signs of withdrawal (Gorlich et al., 2013). Although ACh levels in the IPN were not altered during withdrawal in the present study, nicotine treatment increased ACh levels to a larger extent in female versus male rats. Thus, the possibility exists that heightened ACh levels in the IPN of nicotine-treated females modulates sex differences in gene expression of the various nAChR subunits. Previous work has shown that within the NAc, chronic nicotine treatment also increases baseline ACh levels, albeit to the same extent in female and male rats (Carcoba et al., 2017). Together with the present findings, it appears that cholinergic systems modulate sex differences in nicotine withdrawal in a region-specific manner.
4.3. Behavioral effects
The present study revealed that nicotine withdrawal increased physical signs to the same extent in female and male rats, consistent with previous work (O’Dell and Torres, 2014). A previous study revealed that nicotine-dependent male mice displayed physical signs of withdrawal following intra-IPN or intra-MHb administration of mecamylamine (Salas et al., 2009). The latter report also revealed that the physical signs of withdrawal were not precipitated following infusions of mecamylamine into the ventral tegmental area (VTA), cortex, or hippocampus. Although the MHb-IPN pathway appears to modulate the somatic manifestations of withdrawal, the contribution of this pathway is not sex-dependent. In contrast, the present study revealed that the withdrawal-induced increases in anxiety-like behavior was larger in females versus males, consistent with previous work (Torres et al., 2013). The lack of sex differences in physical signs and the presence of sex differences in negative affective states suggests that these behavioral manifestations of withdrawal are modulated via distinct neural pathways in the brain.
4.4. Gene expression
The present study revealed that following nicotine exposure, females displayed an increase in gene expression of α5 and males displayed an increase in α7 in the IPN. During nicotine withdrawal, females displayed an increase in gene expression of α5 and males displayed an increase in α2 and α3 in the IPN. This pattern of results suggests that sex differences produced by nicotine exposure and withdrawal may be modulated via different α-containing nAChR subtypes in the IPN, particularly since gene expression of β3 and β4 increased to the same extent in female and male rats experiencing nicotine withdrawal. It is important to note that the β3 subunit acts as an accessory subunit to form the α6β2β3 complex (Kuryatov et al., 2008). Given the lack of α6 subunits in the IPN, it is unlikely that this receptor complex contributes to the expression of the behavioral effects of nicotine withdrawal in the IPN.
In males, the present study revealed that gene expression of α2, α3, β3, and β4 subunits increased during nicotine withdrawal in the IPN. Previous work revealed that male mice lacking the α2 subunit display fewer physical signs of nicotine withdrawal relative to wild type controls (Salas et al., 2009). Together, these studies suggest that α2 subunits in the IPN play a distinct role in modulating nicotine withdrawal. A previous report also revealed that β3 and β4 subunits appear in the α3β3β4 configuration in the IPN (Grady et al., 2009). The present finding that gene expression of α3, β3, β4 all increased in males during nicotine withdrawal warrants future work examining the role of this receptor complex in the IPN in modulating nicotine withdrawal in males.
In females, the present study revealed that gene expression of α5, β3, and β4 subunits increased during nicotine withdrawal. The present study also revealed that the magnitude of anxiety-like behavior was positively correlated with gene expression of α4, α5, and β2 in the IPN. The latter finding provides a unique contribution to the literature with regard to the nAChR subunits that modulate nicotine withdrawal in females. Importantly, the α5 subunit only yields functional receptors when co-expressed with the α4β2, α3β2, or α3β4 subunit clusters (Jackson et al., 2010). Thus, the possibility exists that the α4β2α5 nAChR complex in the IPN is a significant modulator of negative affective states produced by withdrawal in female rats. Indeed, the combination of α4β2α5 subunits appears in the brain, and activation of this nAChR complex has been shown to enhance nicotine-induced dopamine release in the striatum (Salminen et al., 2004). With regard to a mechanism, previous work has revealed that the α5 subunit is expressed on inhibitory gamma-aminobutyric acid (GABA) interneurons in the IPN (Hsu et al., 2013). Thus, in females it may be the case that heightened function of the α4α5β2 complex might enhance GABA release in the IPN, which may promote the decrease in ACh levels in the IPN during withdrawal. In males, the possibility exists that a lack of changes in α4β2α5 subunits that regulate GABA might explain the smaller decrease in ACh levels in the IPN during withdrawal. Future studies are needed to explore amino acid regulation of cholinergic systems in the IPN in order to further understand the complex neural circuits that modulate sex differences in nicotine withdrawal.
4.5. Additional considerations
There are some remaining issues to consider for future work examining the role of the mHb-IPN pathway in modulating sex differences in the behavioral effects of nicotine. First, the present results utilized gene expression methods that reflect a pre-translational marker that signals a change at the mRNA level of each nAChR subunit. With the development of more selective antibodies, future studies might examine whether the pre-translational changes observed here are translated at the protein level. Second, the present study focused on the IPN, a relatively small structure in the brain that is anatomically close to the VTA. The VTA has been shown to play an important role in modulating the behavioral effects of nicotine and withdrawal from this drug (de Kloet et al., 2015). Thus, a potential concern is that the present results may be attributed to the VTA. To address this issue, we assessed changes in ACh levels in the VTA of male rats (n=3) during nicotine withdrawal. Our results revealed that the pattern of neurochemical changes in the IPN and VTA were distinct, as nicotine treatment produced a nearly 2-fold increase in ACh levels in the VTA as compared to the IPN where only a modest increase in ACh levels was detected. Importantly, baseline levels of ACh in these regions were similar to previous reports in the VTA (8 ± 2 nM; Song et al., 2012). With regard to gene expression, previous work has shown that there is a high concentration of the α6 subunit in the VTA (Azam et al., 2002; Yang et al., 2011). However, in the present study, we were unable to amplify this nAChR subunit, suggesting that our tissue punch was largely isolated to the IPN and that the α6 subunit in the IPN does not likely contribute to the expression of the behavioral effects of nicotine withdrawal.
Supplementary Material
Highlights.
Following nicotine exposure, female rats displayed higher levels of ACh in the IPN than males.
Following nicotine exposure, female rats displayed an increase in gene expression of α5 and males displayed an increase in α7 in the IPN.
During nicotine withdrawal, female rats display an increase in gene expression of α5, β3 and β4, and males displayed an increase in α2, α3, β3 and β4 in the IPN.
In females, the magnitude of anxiety-like behavior was correlated with the expression of α4, α5, and β2 in the IPN.
Acknowledgements
This research was supported by the National Institutes of Health (R01-DA021274 and R25-DA033613). Dr. Victor Correa was funded via a post-doctoral training contract from the National Institute on Drug Abuse (HHSN271201600057C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antolin-Fontes B, Ables JL, Görlich A, Ibañez-Tallon I, 2015. The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology. 96, 213–22. https://doi:10.1016/j.neuropharm.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, and Leslie FM, 2002. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J. Comp. Neurol 444, 260–274. doi: 10.1002/cne.10138 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, 2010. Nicotine addiction. N Engl J Med. 362 (24), 2295–303. https://doi:10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V, 2008. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 13(4), 368–73. https://doi:10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J Jr., Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate A,M, 2008. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 65(9), 1163–71. https://doi:10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, Alexander JC, 2012. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacol Biochem Behav. 101(1), 62–8. https://doi:10.1016/j.pbb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcoba LM, Flores RJ, Natividad LA, O’Dell LE, 2017. Amino acid modulation of dopamine in the nucleus accumbens mediates sex differences in nicotine withdrawal. Addict Biol. 23(5), 1046–1054.https://doi:10.1111/adb.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Jenson D, Broussard JI, De Biasi M, 2011. Neurophysiology of nicotine addiction. J Addict Res Ther.;20S1(1) https://doi:10.4172/2155-6105.S1-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Dani JA, 2011. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 34, 105–30. https://doi:10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Salas R, 2008. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med (Maywood). 233(8), 917–29. https://doi:10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- de Kloet SF, Mansvelder HD, De Vries TJ, 2015. Cholinergic modulation of dopamine pathways through nicotinic acetylcholine receptors. Biochem Pharmacol. 15;97(4):425–438. doi: 10.1016/j.bcp.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barré L, 2003. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J Pharm Sci. 92(5):1051–7. DOI: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ, 2014, Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 76 Pt B:533–44. doi: 10.1016/j.neuropharm.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Antolin-Fontes B, Görlich A, Zander JF, Ahnert-Hilger G, Ibañez-Tallon I, 2015. An essential role of acetylcholine-glutamate synergy at habenular synapses in nicotine dependence. Elife. 4:e11396 https://doi:10.7554/eLife.11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME, 2011. Sexually diergic hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Research Bulletin. 85:145–152. https://doi:10.1016/j.brainresbull.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C, 2009. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 29(7), 2272–82. https://doi:10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich A, Antolin-Fontes B, Ables JL, Frahm S, Slimak MA, Dougherty JD, Ibañez-Tallon I, 2013. Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. Proc Natl Acad Sci U S A. 110(42), 17077–82. https://doi:10.1073/pnas.1313103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YW, Tempest L, Quina LA, Wei AD, Zeng H, Turner EE, 2013. Medial habenula output circuit mediated by α5 nicotinic receptor-expressing GABAergic neurons in the interpeduncular nucleus.J Neurosci. 33(46),18022–35. doi: 10.1523/JNEUROSCI.2927-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, Damaj M,I, 2010. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 334(1), 137–46. https://doi:10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI, 2008. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 325(1), 302–12. https://doi:10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Muldoon PP, De Biasi M, Damaj MI, 2015. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology. 96(Pt B), 223–34. https://doi:10.1016/j.neuropharm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A, 2001. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 70(4), 531–49. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj M, 2008. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology. 198, 201–120. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI, 2007. Nicotine dependence and reward differ between adolescent and adult male mice. The Journal of Pharmacology and Experimental Therapeutics. 322, 399–407. https://doi:10.1124/jpet.107.121616 [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J, 2008. Roles of accessory subunits in α4β2* nicotinic receptors. Molecular Pharmacology 74(1), 132–143. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–8. DOI: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Malin DH, 2001. Nicotine dependence: studies with a laboratory model. Pharmacol Biochem Behav.2001 70(4), 551–9. [DOI] [PubMed] [Google Scholar]
- Mola S, DeGroot SR, Zhao-Shea R, Tapper AR, 2017. Anxiety and nicotine dependence: Emerging role of the habenulo-interpeduncular axis. Trends Pharmacol Sci. 38(2), 169–180. doi: 10.1016/j.tips.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF, 2004. Nicotine withdrawal in adolescent and adult rats. Annals of the New York Academy of Sciences. 1021, 167–174. https://doi:10.1196/annals.1308.022 [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, 2014. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 76 Pt B:566–80. doi: 10.1016/j.neuropharm.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, and Watson C, 2014. The Rat Brain in Stereotaxic Coordinates. 7th Edition, Academic Press, San Diego. [Google Scholar]
- Picciotto MR, Kenny P, 2013. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med. 3(1), a012112 https://doi:10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M, 2009. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 29(10), 3014–8. https://doi:10.1523/JNEUROSCI.4934-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC., Grady SR, 2004. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 65(6), 1526–35. https://doi:10.1124/mol.65.6.1526 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ, 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 3(6), 1101–8. [DOI] [PubMed] [Google Scholar]
- Skwara AJ, Karwosk TE, Czambel RK, Rubin RT, Rhodes ME, 2012. Influence of environmental enrichment on hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine by osmotic mini-pumps, and nicotine withdrawal by mecamylamine in male and female rats. Behavioral Brain Research. 234, 1–10. https://doi:10.1016/j.bbr.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Mabrouk OS, Hershey ND, Kennedy RT, (2012). In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Anal Chem 3,412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda H,A, Natividad LA, Orfila JE, Torres OV, O’Dell LE, 2012. Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology (Berl). 224(2), 289–301. https://doi:10.1007/s00213-012-2752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE, 2013. Behavioral, Biochemical, and Molecular Indices of Stress are Enhanced in Female Versus Male Rats Experiencing Nicotine Withdrawal. Front Psychiatry. 20;4, 38. doi: 10.3389/fpsyt.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Pipkin JA, Ferree P, Carcoba LM, O’Dell LE, (2015). Nicotine withdrawal increases stress-associated genes in the nucleus accumbens of female rats in a hormone-dependent manner. Nicotine Tob Res. 17(4):422–30. doi: 10.1093/ntr/ntu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A, 2000. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2(1), 19–37. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP, 2006. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 85(3), 648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Buhlman L, Khan GM, Nichols RA, Jin G, Mcintosh JM, et al. , (2011). Functional nicotinic acetylcholine receptors containing alpha6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons. J. Neurosci 31, 2537–2548. doi: 10.1523/JNEUROSCI.3003-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, Gao G, Rando OJ, Martin GE, George O, Gardner PD, Tapper AR, 2015. Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun. 6, 6770 https://doi:10.1038/ncomms7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Pang X, Gardner PD, Tapper AR, 2013. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr Biol. 23(23), 2327–35. https://doi:10.1016/j.cub.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.